Abstract
Delivery of vaccine antigens with an appropriate adjuvant can trigger potential immune responses against cancer leading to reduced tumor growth and improved survival. In this study, various formulations of a bioerodible amphiphilic polyanhydride copolymer based on 1,8-bis(p-carboxyphenoxy)-3,6-dioxaoctane (CPTEG) and 1,6-bis(p-carboxyphenoxy) hexane (CPH) with inherent adjuvant properties were evaluated for antigen-loading properties, immunogenicity, and antitumor activity. Mice were vaccinated with 50:50 CPTEG:CPH microparticles encapsulating a model tumor antigen, ovalbumin (OVA), in combination with the Toll-like receptor-9 agonist, CpGoligonucleotide 1826 (CpG ODN). Mice treated with OVA-encapsulated CPTEG:CPH particles elicited the highest CD8+ T cell responses on day 14 and day 20 when compared to other treatment groups. This treatment group also displayed the most delayed tumor progression and the most extended survival times. Particles encapsulating OVA and CpG ODN generated the highest anti-OVA IgG1 antibody responses in mice serum but these mice did not show significant tumor protection. These results suggest that antigen-loaded CPTEG:CPH microparticles can stimulate antigen-specific cellular responses and could therefore potentially be used to promote antitumor responses in cancer patients.
Keywords: Polyanhydride, anti-tumor immune response, microparticles, biodegradable polymer, antigen, CpG ODN
1. Introduction
Cancer is responsible for one in every four deaths in the United States and is still not effectively managed therapeutically [1]. The current paradigm of chemotherapy and surgery requires improvements so as to enhance overall survival of cancer patients, and to limit toxic side effects of current chemotherapeutic approaches. Therapeutic cancer vaccines have received substantial impetus through a succession of findings over the past two decades that include: (i) the discovery of tumor-associated antigens (TAAs) that potentially flag the presence of tumor cells to the host’s immune system [2]; (ii) the finding that dendritic cells (DC) orchestrates the course of immune responses [3]; and (iii) the observation that pathogen-associated molecular patterns derived from microbes are strong inducers of DC maturation and resultant cellular immune responses [4]. Most recently, FDA approval of the first cancer vaccine, Sipuleucel-T (Provenge™), has given the field of cancer immunotherapy a further boost [5].
The delivery of a cancer vaccine through the use of nano- and micro-particle based vectors is showing promise in both clinical and preclinical settings [6]. Ideally such vectors should possess a number of favorable traits that include: biocompatibility; being capable of efficient co-delivery of immunogen (e.g., TAA) and bacterial adjuvants to DC; possessing adjuvant properties; and being capable of being stably stored and inexpensively manufactured [7–9]. In this study we investigated the potential of amphiphilic polyanhydride microparticles based on 1,8-bis(p-carboxyphenoxy)-3,6-dioxaoctane (CPTEG) and 1,6-bis(p-carboxyphenoxy) hexane (CPH) to be used as a cancer vaccine delivery vehicle. We used 50:50 CPTEG:CPH (Figure 1) microparticles, which have previously shown adjuvant properties in generating robust antigen-specific humoral responses and preferential uptake and activation of DC and macrophages [10–14]. In addition, CPTEG:CPH copolymers have been shown to be non-toxic, stabilizing to antigens, and biodegradable with CPTEG:CPH particles providing a burst release of 10 to 30% of encapsulated antigen followed by zero order release for 10 to 30 days [11, 15–17]. Thus, these polyanhydride vectors possess many of the traits desirable for a cancer vaccine vehicle. When combined with a potent antigen delivery vehicle, the presence of a bacterial or viral adjuvant has shown to increase antigenicity. Many unmethylated CpG motifs of bacterial DNA act as immune stimulants which can bias immune responses to a Th1 type. The synthetic CpG-B oligonucleotide 1826 (CpG ODN) induces DC maturation and B cell activation, through interaction with the Toll-like receptor 9, which leads to enhanced activation of cytotoxic T cell responses [18].
Figure 1.
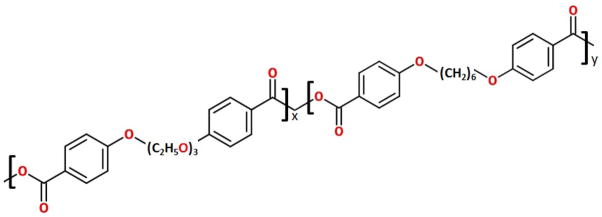
Chemical structure of CPTEG:CPH polymer.
In this study, we report on the successful fabrication of CPTEG:CPH microparticles encapsulating the model TAA, ovalbumin (OVA), either alone (CPTEG:CPH-OVA), or co-encapsulated with CpG ODN (CPTEG:CPH-OVA/CpG). We also further demonstrate the immunogenicity and anti-cancer potential of these microparticles in a prophylactic mouse tumor model.
2. Materials and Methods
2.1 Polymer synthesis
Synthesis of CPTEG:CPH copolymer was carried out by melt polycondensation as described previously [19]. In brief, CPTEG and CPH monomers were mixed in a round-bottom flask at a 50:50 molar ratio to complete a total of 2 g of monomers. 100 mL of acetic anhydride were added to the monomer mixture and reacted for 30 min at 125°C. The acetic anhydride was removed in the rotary evaporator, and the resulting viscous liquid was polymerized in an oil bath at 140°C, under vacuum (<0.03 torr) for 90 min. The resulting polymer was dissolved in methylene chloride and isolated by precipitation into cold hexane in a 1:15 ratio. Purity of the polymer and number average molecular weights were verified and estimated using 1H nuclear magnetic resonance (1H NMR) spectra obtained from a Varian VXR-300 MHz NMR spectrometer (Varian Inc., Palo Alto, CA). In addition, gel permeation chromatography (GPC) (Waters HPLC 277 System, Milford, MA using Varian Inc. GPC columns) was performed to determine polymer molecular weight.
2.2. Fabrication and characterization of microparticles loaded with CpG ODN and OVA
Microparticles were prepared using a double emulsion solvent evaporation method derived from Intra et al [20]. Briefly, purified endotoxin free chicken egg white ovalbumin (OVA) (Sigma, St Louis, MO) and endotoxin-free CpG ODN (Integrated DNA Technologies, Coralville, IA) were dissolved in 100 μL of 1% poly(vinyl alcohol) (PVA) (Mowiol®; Sigma, Allentown, PA) solution. This solution was sonicated for 30 seconds on power setting #10, (Sonic Dismembrator Model 100, Fisher Scientific, Pittsburgh, PA) in 1.5 mL of dichloromethane (DCM) containing 200 mg of 50:50 CPTEG:CPH copolymer. This primary emulsion was then sonicated into 8 mL of 1% PVA solution to generate a secondary emulsion, which was then added to 22 mL of 1% PVA solution. Secondary emulsions were stirred in a fume hood for 2 h to allow evaporation of DCM. Microparticles were centrifuged at 2880 x g for 5 min. Pellets obtained were washed twice with distilled water followed by freeze drying using FreeZone 4.5 (Labconco Corporation, Kansas City, MO). Particles were stored in sealed containers at −20 °C. Size distribution and zeta potential were measured using a Zetasizer Nano ZS (Malvern, Southborough, MA).
To estimate the loading of OVA and CpG ODN, 20 mg of microparticles from each batch was incubated with 0.2 N NaOH for approximately 12 h at room temperature or until microparticles had fully degraded. This solution was then neutralized using 1N HCl and loading was calculated using equation 1. Percentage encapsulation efficiency (EE) of the fabrication process was calculated as described in equation 2.
| Eq. 1 |
| Eq. 2 |
Here, Loading = μg OVA or CpG ODN encapsulated per mg of particles, Concentration = calculated concentration of OVA or CpG from standard curve (μg/mL), and Volume = volume of OVA or CpG ODN solution (mL).
The surface morphology and shape of microparticles was examined using scanning electron microscopy (SEM). Briefly, a suspension of particles was plated onto a silicon wafer mounted on a SEM stub. This was then coated with gold-palladium by an argon beam K550 sputter coater (Emitech Ltd., Kent, England). Images were captured using a Hitachi S-4800 SEM (Hitachi High-Technologies, Ontario, Canada) at 5 kV accelerating voltage.
2.3. Prophylactic murine tumor model
Eight to twelve week-old male wild type C57BL/6 mice (Jackson Laboratory, Bar Harbor, Maine) (n = 4/group) were treated with intraperitoneal injections of the following six groups of treatments: (i) OVA and CpG ODN encapsulated in 50:50 CPTEG:CPH microparticles (CPTEG:CPH-OVA/CpG); (ii) OVA encapsulated in 50:50 CPTEG:CPH microparticles (CPTEG:CPH-OVA); (iii) OVA encapsulated in 50:50 CPTEG:CPH microparticles with soluble CpG ODN; (iv) Blank 50:50 CPTEG:CPH microparticles at an equivalent dose to the OVA particles; (v) Soluble OVA and CpG ODN; (vi) Naïve (untreated). For mice treated with soluble CpG, the particles or solution of OVA was admixed with CpG solution immediately prior to injections. Each mouse was primed on day 0 and similarly boosted on day 7 with the indicated treatments. Doses of 100 μg of OVA and 50 μg of CpG ODN per mouse were consistently used. On day 21 OVA-specific CD8+ CD3+ T lymphocyte levels were determined from peripheral blood harvested by submandibular bleeds (see section 2.4). On day 28 OVA-specific IgG2a and IgG1 antibody titers were measured in serum harvested by submandibular bleeds (see section 2.5). On day 35, mice were subcutaneously challenged with 2 × 106 OVA-expressing E.G7 cells (American Type Culture Collection, Manassas, VA)) and tumor volumes were monitored over time using equation 3. All animal experiments were carried out in accordance with current institutional guidelines for the care and use of experimental animals.
| [ 21] | Eq. 3 |
2.4. Tetramer staining on peripheral blood lymphocytes
The frequency of OVA-specific CD3+ CD8+ T lymphocytes was determined by tetramer staining and direct immunofluorescence, as previously described [22]. The tetramer used was the H-2Kb SIINFEKL Class I iTAg™ MHC Tetramer (Kb-OVA257) labeled with PE (Beckman Coulter, Fullerton, CA). Surface CD8 and CD3 were stained with FITC-labeled rat anti-mouse CD8 (eBioscience, San Diego, CA) and PE-Cy5-labeled hamster anti-mouse CD3 (eBioscience, San Diego, CA) antibodies respectively. Samples were acquired using a FACScan flow cytometer (Becton Dickinson, NJ) and analyzed with FlowJo software (TreeStar, OR).
2.5. Estimation of anti-OVA antibodies in peripheral blood using the enzyme-linked immuno-sorbent assay (ELISA)
Measuring antigen-specific IgG1 and IgG2a levels can provide information not only with respect to the degree of humoral stimulation but also as to the type (Th1 or Th2) of antigen-specific immune response generated through the determination of IgG2a:IgG1 ratios [23]. High IgG2a:IgG1 ratios tend to indicate potential for a Th1 type or cellular immune response whilst a low IgG2a:IgG1 ratio is more indicative of a Th2 type or primarily humoral response. OVA-specific IgG1 and IgG2a antibodies in peripheral blood were quantified using a modification of a standard ELISA protocol that has been described in detail previously [24]. Briefly, serum samples were collected via submandibular bleeding and serial dilutions of the serum samples were incubated in wells of Immulon® 2HB 96-well high binding polystyrene microtiter plates (Thermo, Milford, MA) that had been previously coated with 5 μg/mL of OVA solution in PBS. Plates were washed with PBS-tween, followed by incubation with alkaline phosphatase conjugated goat anti-mouse IgG antibodies (Southern Biotech, Birmingham, AL). Excess antibody was washed away followed by addition of p-nitrophenylphosphate (Sigma, St Louis, MO) in the dark. Absorbance was measured after 2 hours at 405 nm using a SpectraMax® Plus384 microplate reader (Molecular Devices LLC, Sunnyvale, California).
2.6 Statistical Analysis
Groups were compared by one-way analysis of variance (ANOVA) followed by Tukey post-test to compare all pairs of treatments. Analysis of survival curves was performed using log-rank (Mantel-Cox) test (GraphPad Prism, La Jolla, CA). Results presented are representative of 2–3 repeats.
3. Results
3.1. Polymer characterization
The purity of the 50:50 CPTEG:CPH copolymer was verified using 1H NMR spectroscopy. The NMR spectra indicated that the actual composition of the copolymer was in agreement with the molar feed ratio (data not shown). GPC analysis showed that the 50:50 CPTEG:CPH copolymer had a weight average molecular weight of 8500 g mol−1 with a polydispersity index of 1.7. These results are consistent with previously published data [14, 25].
3.2. Characterization of 50:50 CPTEG:CPH microparticles loaded with CpG ODN and OVA or OVA alone
The 50:50 CPTEG:CPH microparticles were prepared using a double emulsion solvent evaporation process as described in materials and methods. When OVA alone was used, loading efficiencies of >78% were obtained, yielding 11.8 μg of OVA encapsulated per mg of microparticles (Table 1). When CpG ODN and OVA were co-encapsulated, the loading efficiency of OVA was only 12%, but the amount of OVA in the microparticles was adequate to be used in subsequent in vivo studies. The mean diameter of all microparticle preparations was between 1 to 3 μm. The zeta potential of blank microparticles was -0.63 mV, which did not change significantly upon encapsulation of OVA and CpG ODN into the microparticles (Table 2). SEM revealed the various microparticle preparations to possess smooth morphology (Figure 2).
Table 1.
Loading efficiency and loading mass of OVA and CpG encapsulated CPTEG:CPH microparticles
| Group | % Loading efficiency | Loading mass (μg/mg of Polymer) |
|---|---|---|
| OVA loaded CPTEG:CPH | 78.6% | OVA:11.8 |
| OVA and CpG loaded CPTEG:CPH | OVA: 12.0% | OVA:1.8 |
| CpG: 21.0% | CpG: 0.2 |
Table 2.
Particle size and zeta potential of CPTEG:CPH microparticles as measured by zetasizer Nano ZS
| Particle size (μm) | Polydispersity index (PDI) | Zeta potential (mV) | |
|---|---|---|---|
| Blank Particles | 3.08 | 0.25 | −0.63 ± 3.81 |
| OVA loaded CPTEG:CPH | 2.02 | 0.54 | −3.50 ± 10.10 |
| OVA and CpG loaded CPTEG:CPH | 1.57 | 0.52 | −5.24 ± 7.24 |
Figure 2. SEM microphotographs of different CPTEG:CPH microparticle formulations.
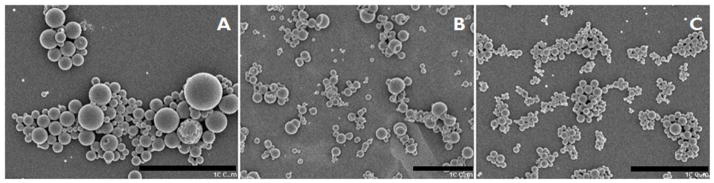
SEM images of A: Blank CPTEG:CPH microparticles; B: CPTEG:CPH microparticles encapsulating OVA; C: CPTEG:CPH microparticles encapsulating OVA and CpG-ODN. Size bar represents 10 μm.
3.2 Immunogenicity of different CPTEG:CPH formulations
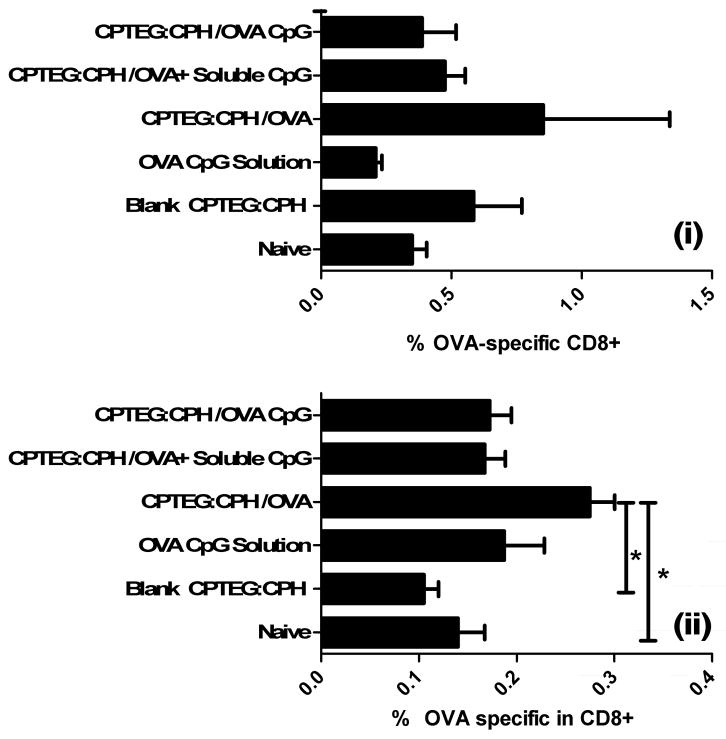
Immunocompetent C57BL/6 mice were given prime/boost vaccinations with CPTEG:CPH microparticles encapsulating OVA plus/minus soluble or co-encapsulated CpG ODN. On day 28 after the priming vaccination serum titers of OVA-specific IgG1 and IgG2a antibodies were measured using ELISA. Results revealed that mice receiving CPTEG:CPH-OVA/CpG generated significantly higher OVA-specific antibody responses (both IgG1 and IgG2a) than all other formulations (Figure 3). Using a tetramer binding assay, OVA-specific T lymphocytes in the peripheral blood from mice on days 14 and 20 revealed no significant changes with the exception of the group vaccinated with CPTEG:CPH-OVA, which displayed the greatest levels of OVA-specific T lymphocytes on both days. In addition, the levels of OVA-specific T lymphocytes in the group vaccinated with CPTEG:CPH-OVA were found to be significantly increased by day 20 (Figure 4).
Figure 3. Comparative serum titers of IgG2a and IgG1 OVA-specific antibodies after vaccination with different CPTEG:CPH microparticle formulations.
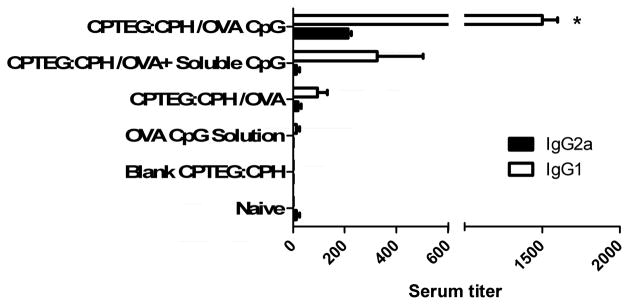
On day 28 post primary vaccination with indicated treatments, OVA-specific IgG1 and IgG2a titers were measured in sera using ELISA (as described in materials and methods section). All groups were compared using ANOVA followed by Tukey post-test (*p < 0.01). CPTEG:CPH/OVA CpG group showed significantly higher serum titers for IgG1 when compared with all groups.
Figure 4. Analysis of OVA-specific T cell frequency in PBLs of mice vaccinated with different CPTEG:CPH microparticle formulations.
Mice were primed (day 0) and boosted (day 7) with indicated formulations. PBLs were stained using a fluorescently tagged tetramer, designed to bind to OVA-specific CD8+ T cells, on day 14 (i) and day 20 (ii) post primary vaccination with indicated microparticle formulations. All groups were statistically compared using ANOVA followed by Tukey post-test (*p < 0.05).
3.3 Tumor protection studies
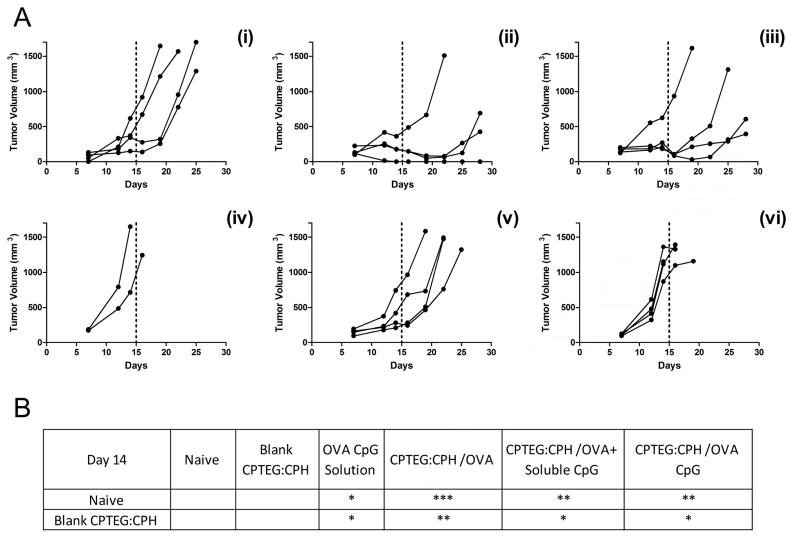
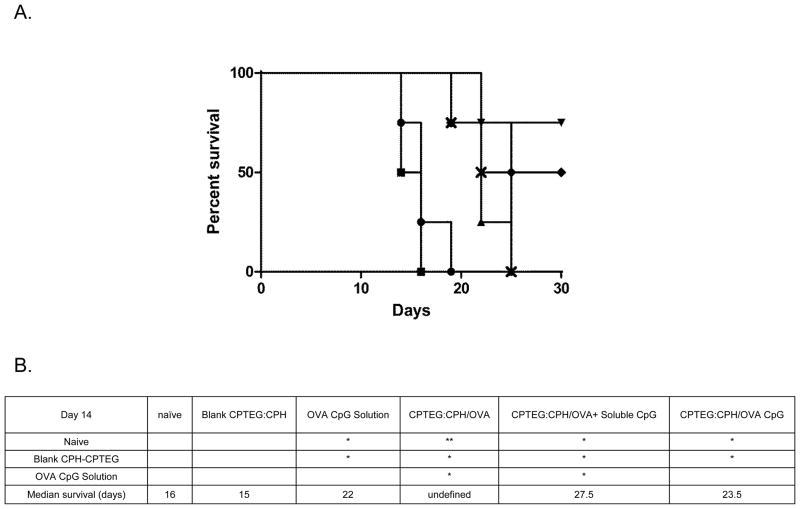
Thirty-five days after the initial boost with the various microparticle formulations C57BL/6 mice were challenged with a lethal dose of an OVA-expressing tumor cell line (E.G7). Tumor volumes were monitored over the subsequent 28 days and revealed all formulations to have significantly enhanced protective effects when comparing tumor volumes on day 14 to untreated (naïve) mice and mice treated with blank microparticles (Figure 5). Analysis of survival revealed that mice treated with CPTEG:CPH-OVA or CPTEG:CPH-OVA + soluble CpG ODN had significantly (p < 0.05) improved survival over all other groups (Figure 6 b) on day 14. At the termination (day 28 post tumor challenge) of the study, 75 % of mice survived in the CPTEG:CPH-OVA group whilst 50 % of mice survived in the group treated with CPTEG:CPH-OVA + soluble CpG ODN. All but one of the surviving mice had slow growing tumors. As calculated from the log-rank test the median survival for the CPTEG:CPH-OVA group was undefined whilst for the CPTEG:CPH-OVA + soluble CpG ODN group the median survival was 27.5 days.
Figure 5. The prophylactic anti-tumor effect of vaccinating mice with different CPTEG:CPH microparticle formulations.
Thirty-five days prior to subcutaneous challenge with E.G7-OVA tumor cells, mice were vaccinated with the following microparticle formulations on day 0 (prime) and day 7 (boost): (i) OVA and CpG-ODN encapsulated in CPTEG:CPH; (ii) OVA encapsulated in CPTEG:CPH; (iii) OVA encapsulated in CPTEG:CPH with soluble CpG-ODN; (iv) Blank CPTEG:CPH particles; (v) Soluble OVA and CpG-ODN; (vi) Naive. A. Tumor volumes were recorded and each curve represents the tumor growth for each individual mouse. Tumor volumes from each treatment group were statistically compared using ANOVA followed by tukey post-test. (***p < 0.001; **p < 0.01; *p < 0.05). B. Summary of those groups that were significantly different from Naïve and Blank CPTEG:CPH groups. All other group pairings showed no significant differences.
Figure 6. Survival curve of mice bearing E.G7-OVA tumors.
Mice were primed (day 0) and boosted (day 7) with (
 ) OVA and CpG-ODN encapsulated in CPTEG:CPH microparticles; (
) OVA and CpG-ODN encapsulated in CPTEG:CPH microparticles; (
 ) OVA encapsulated in CPTEG:CPH microparticles; (
) OVA encapsulated in CPTEG:CPH microparticles; (
 ) OVA encapsulated in CPTEG:CPH microparticles with soluble CpG-ODN; (
) OVA encapsulated in CPTEG:CPH microparticles with soluble CpG-ODN; (
 ) blank CPTEG:CPH microparticles; (
) blank CPTEG:CPH microparticles; (
 ) Soluble OVA and CpG-ODN. A. Survival of mice in each treatment group was recorded along with (
) Soluble OVA and CpG-ODN. A. Survival of mice in each treatment group was recorded along with (
 ) naïve group. (n = 4). B. Survival curves were analyzed using the log-rank (Mantel-Cox) test and the groups with significant differences between them displayed. (**p < 0.01; *p < 0.05).
) naïve group. (n = 4). B. Survival curves were analyzed using the log-rank (Mantel-Cox) test and the groups with significant differences between them displayed. (**p < 0.01; *p < 0.05).
4. Discussion
To date, studies with biodegradable polyanhydride particles have involved the encapsulation of pathogen-derived antigens, which have subsequently demonstrated protection against infectious pathogens such as tetanus [26] and Yersinia pestis [27]. However, the potential for the amphiphilic CPTEG:CPH microparticles, specifically, to be used as cancer vaccines has yet to be explored. In this study, we investigated the cancer vaccine potential of CPTEG:CPH microparticles encapsulating a model TAA. Development of cancer vaccines are often challenging since there is a need to invoke a cell-mediated immune response instead of, or as well as, a humoral response. Delivery of antigen in a particulate, rather than soluble, form is required for the successful generation of an adaptive tumor-specific cytotoxic T lymphocyte response capable of eradicating the primary tumor and, more importantly, its metastatic lesions [28]. If a particulate cancer vaccine delivery system is to be successful it must fulfill a number of requirements [6], including: (i) protection of the TAA; (ii) efficient delivery to dendritic cells; and (iii) concomitant stimulation of dendritic cells leading to subsequent stimulation of an immune response capable of eradicating the tumor [6]. Microparticles made of CPTEG:CPH have shown to stabilize encapsulated antigens [29, 30] and their ability to activate dendritic cells is comparable to that of LPS [16, 27, 31]. It has been speculated that the structural similarity of 50:50 CPTEG:CPH particulates to LPS [32] leads to effective immune activation resulting in long term antibody production [19]. Thus, 50:50 CPTEG:CPH particles fulfill many of the traits required for a potentially successful cancer vaccine vector.
Amphiphilic CPTEG:CPH particles have previously shown high encapsulation efficiency of different model proteins using various particle preparation methods [16, 27]. In this study, OVA was shown to be efficiently encapsulated in CPTEG:CPH microparticles using a double emulsion solvent evaporation method. However, co-encapsulation of CpG ODN and OVA into CPTEG:CPH microparticles (CPTEG:CPH-OVA/CpG) was found to be much less efficient. High concentrations of CpG ODN in the water phase led to precipitation of polymer-DNA aggregates from the primary emulsion. Destabilization of the primary emulsion was curtailed by decreasing the CpG ODN:polymer ratio from 0.015 to 0.001. This alteration did not affect microparticle size but did result in low loading for CpG ODN and OVA. The possibility that the presence of nucleic acid is responsible for the destabilization of the primary emulsion was confirmed by the observation that microparticle aggregation also occurred when herring sperm DNA was used instead of CpG ODN (data not shown).
We found that certain CPTEG:CPH-based formulations could provide significant, albeit incomplete, prophylactic protection against tumor-challenge. Mice vaccinated with CPTEG:CPH-OVA afforded the greatest protection. What was particularly unexpected was that these mice showed both increased survival and significantly decreased tumor volumes (day 14) compared to mice vaccinated with CPTEG:CPH-OVA/CpG. It has been shown previously that a higher proportion of IgG2a antibodies relative to IgG1 antibodies favors the production of antigen-specific cytotoxic T cells [23], which is key to eradicating cancer [33]. However, we found that ratios of IgG2a:IgG1 antibodies were small (< 0.25) for all particulate treatment groups, suggesting Th2-biased immune responses. The observed Th2 response by the CPTEG:CPH-OVA formulation was not surprising since CPTEG:CPH particles encapsulating different antigens have previously been shown to generate Th2 responses. However, when CpG ODN was co-encapsulated (CPTEG:CPH-OVA/CpG), or co-delivered in soluble form, the IgG2a:IgG1 ratio did not increase. In other words, CpG ODN did not push the response toward a Th1, or even, Th0 response. This was unexpected since CpG ODN has been previously reported to promote Th1 immune responses [34, 35]. One possible explanation for this observation is that the adjuvant properties of 50:50 CPTEG:CPH particles resulted in abrogation of the normal effect of CpG ODN. In other words, 50:50 CPTEG:CPH particles have been shown previously to possess strong adjuvant properties [11] and therefore, when added in relatively high doses compared to the CpG ODN, could result in a phenotypic dominance. It has also been reported that LPS can abrogate effects of CpG ODN [36] and, since 50:50 CPTEG:CPH particles are LPS-like in their effect on dendritic cell activation [13], it is possible that 50:50 CPTEG:CPH particles have a similar dominating influence over dendritic cells and therefore subsequent immune responses. The OVA-specific CTL responses were marginally, but significantly, increased in mice vaccinated with CPTEG:CPH-OVA whilst none of the other treatment groups displayed significant increases relative to the naïve group. This may explain why the CPTEG:CPH-OVA group exhibited the greatest tumor protection and improved survival (p < 0.05). This further supports development of novel CPTEG:CPH polymeric carriers as antigen delivery systems for cancer vaccines.
Conclusions
To date, the use of biodegradable microparticles in tumor immunotherapy has primarily involved PLGA microparticles [6]. Here, microparticles composed of the bioerodible amphiphilic polyanhydride, 50:50 CPTEG:CPH, provided significant protection against tumor challenge without additional adjuvants, making it a promising vaccine delivery system that requires further evaluation of the mechanism of action. In addition, the fact that these polyanhydrides are tunable systems indicates there is potential for modifications that can lead to more cellular (or Th1-biased) immune responses.
Acknowledgments
A.K. Salem gratefully acknowledges support from the American Cancer Society (RSG-09-015-01-CDD) and the National Cancer Institute at the National Institutes of Health (1R21CA128414-01A2/UI Mayo Clinic Lymphoma SPORE/2P50CA097274-11). B. Narasimhan gratefully acknowledges the Vlasta Klima Balloun Professorship.
Footnotes
The authors declare no conflict of interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–36. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 2.Giresand O, Seliger B. Tumor-Associated Antigens: Identification, Characterization and Clinical Applications. Weinheim, Germany: Wiley-Blackwell; 2009. [Google Scholar]
- 3.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 4.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–76. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 5.Higano CS, Small EJ, Schellhammer P, Yasothan U, Gubernick S, Kirkpatrick P, et al. Sipuleucel-T. Nat Rev Drug Discov. 2010;9:513–4. doi: 10.1038/nrd3220. [DOI] [PubMed] [Google Scholar]
- 6.Krishnamachari Y, Geary SM, Lemke CD, Salem AK. Nanoparticle delivery systems in cancer vaccines. Pharmaceutical research. 2011;28:215–36. doi: 10.1007/s11095-010-0241-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang X-Q, Dahle CE, Weiner GJ, Salem AK. A comparative study of the antigen-specific immune response induced by co-delivery of CpG ODN and antigen using fusion molecules or biodegradable microparticles. Journal of Pharmaceutical Sciences. 2007;96:3283–92. doi: 10.1002/jps.20978. [DOI] [PubMed] [Google Scholar]
- 8.Zhang XQ, Dahle CE, Baman NK, Rich N, Weiner GJ, Salem AK. Potent antigen-specific immune responses stimulated by codelivery of CpG ODN and antigens in degradable microparticles. J Immunother. 2007;30:469–78. doi: 10.1097/CJI.0b013e31802fd8c6. [DOI] [PubMed] [Google Scholar]
- 9.Krishnamachari Y, Salem AK. Innovative strategies for co-delivering antigens and CpG oligonucleotides. Advanced Drug Delivery Reviews. 2009;61:205–17. doi: 10.1016/j.addr.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ulery BD, Petersen LK, Phanse Y, Kong CS, Broderick SR, Kumar D, et al. Rational design of pathogen-mimicking amphiphilic materials as nanoadjuvants. Sci Rep. 2011;1:198. doi: 10.1038/srep00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petersen LK, Ramer-Tait AE, Broderick SR, Kong CS, Ulery BD, Rajan K, et al. Activation of innate immune responses in a pathogen-mimicking manner by amphiphilic polyanhydride nanoparticle adjuvants. Biomaterials. 2011;32:6815–22. doi: 10.1016/j.biomaterials.2011.05.063. [DOI] [PubMed] [Google Scholar]
- 12.Chavez-Santoscoy AV, Roychoudhury R, Pohl NLB, Wannemuehler MJ, Narasimhan B, Ramer-Tait AE. Tailoring the immune response by targeting C-type lectin receptors on alveolar macrophages using “pathogen-like” amphiphilic polyanhydride nanoparticles. Biomaterials. 2012;33:4762–72. doi: 10.1016/j.biomaterials.2012.03.027. [DOI] [PubMed] [Google Scholar]
- 13.Ulery BD, Petersen LK, Phanse Y, Kong CS, Broderick SR, Kumar D, et al. Rational Design of Pathogen-Mimicking Amphiphilic Materials as Nanoadjuvants. Sci Rep-Uk. 2011:1. doi: 10.1038/srep00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carrillo-Conde B, Song EH, Chavez-Santoscoy A, Phanse Y, Ramer-Tait AE, Pohl NLB, et al. Mannose-Functionalized “Pathogen-like” Polyanhydride Nanoparticles Target C-Type Lectin Receptors on Dendritic Cells. Mol Pharmaceut. 2011;8:1877–86. doi: 10.1021/mp200213r. [DOI] [PubMed] [Google Scholar]
- 15.Adler AF, Petersen LK, Wilson JH, Torres MP, Thorstenson JB, Gardner SW, et al. High Throughput Cell-Based Screening of Biodegradable Polyanhydride Libraries. Comb Chem High T Scr. 2009;12:634–45. doi: 10.2174/138620709788923764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lopac SK, Torres MP, Wilson-Welder JH, Wannemuehler MJ, Narasimhan B. Effect of Polymer Chemistry and Fabrication Method on Protein Release and Stability From Polyanhydride Microspheres. J Biomed Mater Res B. 2009;91B:938–47. doi: 10.1002/jbm.b.31478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Torres MP, Determan AS, Anderson GL, Mallapragada SK, Narasimhan B. Amphiphilic polyanhydrides for protein stabilization and release. Biomaterials. 2007;28:108–16. doi: 10.1016/j.biomaterials.2006.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krieg AM. CpG motifs in bacterial DNA and their immune effects. Annual Review of Immunology. 2002;20:709–60. doi: 10.1146/annurev.immunol.20.100301.064842. [DOI] [PubMed] [Google Scholar]
- 19.Torres MP, Vogel BM, Narasimhan B, Mallapragada SK. Synthesis and characterization of novel polyanhydrides with tailored erosion mechanisms. J Biomed Mater Res A. 2006;76:102–10. doi: 10.1002/jbm.a.30510. [DOI] [PubMed] [Google Scholar]
- 20.Intra J, Salem AK. Fabrication, characterization and in vitro evaluation of poly(D, L-lactide-co-glycolide) microparticles loaded with polyamidoamine-plasmid DNA dendriplexes for applications in nonviral gene delivery. J Pharm Sci. 2010;99:368–84. doi: 10.1002/jps.21840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geary SM, Lemke CD, Lubaroff DM, Salem AK. Tumor immunotherapy using adenovirus vaccines in combination with intratumoral doses of CpG ODN. Cancer Immunol Immun. 2011;60:1309–17. doi: 10.1007/s00262-011-1038-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karan D, Krieg AM, Lubaroff DM. Paradoxical enhancement of CD8 T cell-dependent anti-tumor protection despite reduced CD8 T cell responses with addition of a TLR9 agonist to a tumor vaccine. Int J Cancer. 2007;121:1520–8. doi: 10.1002/ijc.22873. [DOI] [PubMed] [Google Scholar]
- 23.Schnare M, Barton GM, Holt AC, Takeda K, Akira S, Medzhitov R. Toll-like receptors control activation of adaptive immune responses. Nat Immunol. 2001;2:947–50. doi: 10.1038/ni712. [DOI] [PubMed] [Google Scholar]
- 24.Cohen PL, Maldonado MA. Current Protocols in Immunology. John Wiley & Sons, Inc; 2001. Animal Models for SLE. [DOI] [PubMed] [Google Scholar]
- 25.Carrillo-Conde B, Schiltz E, Yu J, Minion FC, Phillips GJ, Wannemuehler MJ, et al. Encapsulation into amphiphilic polyanhydride microparticles stabilizes Yersinia pestis antigens. Acta Biomater. 2010;6:3110–9. doi: 10.1016/j.actbio.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 26.Kipper MJ, Wilson JH, Wannemuehler MJ, Narasimhan B. Single dose vaccine based on biodegradable polyanhydride microspheres can modulate immune response mechanism. J Biomed Mater Res A. 2006;76:798–810. doi: 10.1002/jbm.a.30545. [DOI] [PubMed] [Google Scholar]
- 27.Ulery BD, Kumar D, Ramer-Tait AE, Metzger DW, Wannemuehler MJ, Narasimhan B. Design of a protective single-dose intranasal nanoparticle-based vaccine platform for respiratory infectious diseases. PLoS One. 2011;6:e17642. doi: 10.1371/journal.pone.0017642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O’Hagan DT, Jeffery H, Davis SS. Long-term antibody responses in mice following subcutaneous immunization with ovalbumin entrapped in biodegradable microparticles. Vaccine. 1993;11:965–9. doi: 10.1016/0264-410x(93)90387-d. [DOI] [PubMed] [Google Scholar]
- 29.Petersen LK, Phanse Y, Ramer-Tait AE, Wannemuehler MJ, Narasimhan B. Amphiphilic Polyanhydride Nanoparticles Stabilize Bacillus anthracis Protective Antigen. Mol Pharmaceut. 2012;9:874–82. doi: 10.1021/mp2004059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petersen LK, Sackett CK, Narasimhan B. High-throughput analysis of protein stability in polyanhydride nanoparticles. Acta Biomater. 2010;6:3873–81. doi: 10.1016/j.actbio.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 31.Torres MP, Wilson-Welder JH, Lopac SK, Phanse Y, Carrillo-Conde B, Ramer-Tait AE, et al. Polyanhydride microparticles enhance dendritic cell antigen presentation and activation. Acta Biomater. 2011;7:2857–64. doi: 10.1016/j.actbio.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Storni T, Kundig TM, Senti G, Johansen P. Immunity in response to particulate antigen-delivery systems. Advanced drug delivery reviews. 2005;57:333–55. doi: 10.1016/j.addr.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 33.Bremers AJA, Parmiani G. Immunology and immunotherapy of human cancer: present concepts and clinical developments. Crit Rev Oncol Hemat. 2000;34:1–25. doi: 10.1016/s1040-8428(99)00059-1. [DOI] [PubMed] [Google Scholar]
- 34.Brazolot Millan CL, Weeratna R, Krieg AM, Siegrist CA, Davis HL. CpG DNA can induce strong Th1 humoral and cell-mediated immune responses against hepatitis B surface antigen in young mice. Proc Natl Acad Sci U S A. 1998;95:15553–8. doi: 10.1073/pnas.95.26.15553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krieg AM. CpG motifs in bacterial DNA and their immune effects. Annu Rev Immunol. 2002;20:709–60. doi: 10.1146/annurev.immunol.20.100301.064842. [DOI] [PubMed] [Google Scholar]
- 36.Gould MP, Greene JA, Bhoj V, DeVecchio JL, Heinzel FP. Distinct modulatory effects of LPS and CpG on IL-18-dependent IFN-gamma synthesis. J Immunol. 2004;172:1754–62. doi: 10.4049/jimmunol.172.3.1754. [DOI] [PubMed] [Google Scholar]