Abstract
Pulmonary surfactant is essential for life and is comprised of a complex lipoprotein-like mixture that lines the inner surface of the lung to prevent alveolar collapse at the end of expiration. The molecular composition of surfactant depends on highly integrated and regulated processes involving its biosynthesis, remodeling, degradation, and intracellular trafficking. Despite its multicomponent composition, the study of surfactant phospholipid metabolism has focused on two predominant components, disaturated phosphatidylcholine that confers surface-tension lowering activities, and phosphatidylglycerol, recently implicated in innate immune defense. Future studies providing a better understanding of the molecular control and physiological relevance of minor surfactant lipid components are needed.
Keywords: surfactant, apoprotein, phospholipid
1. SURFACTANT FUNCTION
Alveoli represent the basic units for gas exchange and both the number and size of alveoli expand during early extrauterine life. The enormous surface-area imposed by alveolar structures is intrinsically linked to very high surface tension at the air-water interface that opposes lung inflation. These surface forces are opposed by pulmonary surfactant that reduces surface tension. Left unopposed, high surface tension leads to pulmonary edema, where intra-alveolar fluid accumulates leading to marked impairment of gas exchange and lung mechanical disturbances. Surfactant deficiency is the primary cause of the neonatal respiratory distress syndrome (RDS), but reduced levels of this material also partake in the pathobiology of adult lung disease. Surfactant supplementation in premature infants with RDS leads to a significant reduction in mortality associated with this syndrome, although beneficial effects of exogenous surfactant therapy on survival in adult lung disorders such as acute lung injury have not yet been demonstrated [1–4].
Lung surfactant is synthesized in the endoplasmic reticulum (ER) of the alveolar type II epithelial cell before it is transported and stored in lamellar bodies (LB), an intracellular storage form. Lung surfactant is secreted via exocytosis from type II cells involving fusion of LB with the plasma membrane in response to extracellular signals. Surfactant secretion can occur via constitutive and regulated pathways [5]. Once secreted from type II cells, the extracellular pool of phospholipid within LB transforms into a surfactant film that lines the alveolar surface [6]. Moreover, the phospholipid fraction obtained from bronchoalveolar lavage contains a number of different morphological and biochemical forms of surfactant including densely-packed multilamellar structures, lattice-like structures called tubular myelin, multilamellar and unilamellar vesicles [5]. Secreted extracellular surfactant acts as a vehicle in the removal of foreign material and sloughed cells from the distal lung. Importantly, extracellular (alveolar) surfactant is well recognized to play a key role in innate immune defense.
2. SURFACTANT COMPOSITION
Pulmonary surfactant contains several classes of lipids, including phospholipids, triglycerides, cholesterol, and fatty acids. Surfactant also contains the key surfactant proteins A, B, C, and D (SP-A, SP-B, SP-C, and SP-D) (Fig 1). The phospholipid composition of surfactant is highly conserved among mammals [7, 8]. Phosphatidylcholine (PC) is the major phospholipid comprising 80% of surfactant lipids. Phospholipid analysis using different techniques has confirmed that surfactant PC generally contains a disaturated PC (DSPC) species, 16:0/16:0-PC. Approximately 60% of surfactant PC is present in this disaturated form, as dipalmitoylphosphatidylcholine (DPPC), representing the major surface-active component [9, 10]. Besides 16:0/16:0-PC, mammalian surfactant contains about 10% of 16:0/14:0-PC, 16:0/16:1-PC (30%), and 16:0/18:1-PC and 16:0/18:2-PC species at lower concentrations. The second most abundant phospholipid in surfactant is phosphatidylglycerol (PG) that is present at 7–15% of the total phospholipid with each of remaining phospholipids generally accounting for less than 5% of the total. The precise biological role of PG is unknown but it may play a role in alveolar stability and recent studies suggest that it regulates the innate immune response [11, 12]. The other minor phospholipids such as phosphatidylethanolamine (PE), sphingomyelin (SM), phosphatidylinositol (PI), and phosphatidylserine (PS) comprise the remainder of the phospholipid pool. Aside from phospholipids, surfactant contains 5–10% surfactant-associated proteins that interact extensively with phospholipids. These interactions regulate structure and properties of the lipid film [13]. Of the four surfactant proteins, SP-B and SP-C are hydrophobic and SP-A and SP-D are hydrophilic. SP-B and SP-C are critical for regulation of surfactant film formation and stability [14], while SP-A and SP-D are critically involved in innate immunity and the inflammatory response [15]. SP-D may also be involved in maintenance of surfactant metabolism and pulmonary homeostasis [16].
Fig. 1. Cartoon illustrating the composition of the pulmonary surfactant.
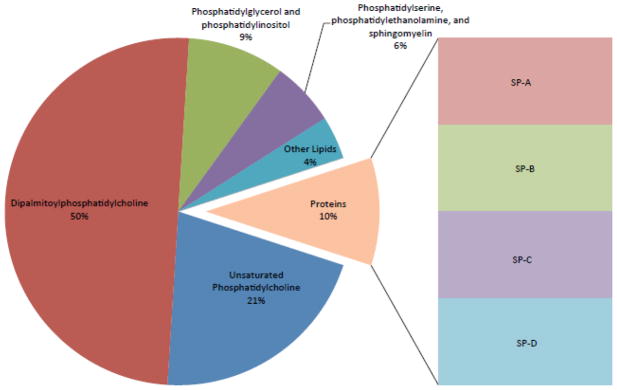
The major surface-active phospholipid component is dipalmitoylphosphatidylcholine that interacts with key surfactant-associated proteins to lower surface tension. Phosphatidylglycerol is the second most abundant phospholipid that also modulates surface activity and may regulate innate immunity.
3. SYNTHESIS AND REGULATION OF SURFACTANT PHOSPHOLIPIDS
3.1 Synthesis and regulation of surfactant phospholipid precursors
3.1.1 Fatty acid synthesis and regulation
Surfactant phospholipids produced by the de novo pathway are highly dependent on the availability of fatty acids (FA) in type II cells [17]. The FA in these cells is supplied by the circulation in the form of free FA or triacylglycerols within lipoproteins. FA uptake from the circulation is mediated by membrane FA binding protein [9, 18] or internalized from alveoli after phospholipid hydrolysis. Alternatively, de novo synthesized FA are able to use lactate as a precursor. Lactate is the preferential carbon source for FA de novo synthesis in mature type II cells while glycogen is an important precursor in the late fetal period. As endogenously synthesized FA are an important substrate for surfactant phospholipid production, it is not surprising that the activities and mRNA levels of the key enzymes of FA synthesis such as acetyl CoA carboxylase, fatty acid synthase (FAS), and citrate lyase are upregulated during surfactant lipogenesis in the lung. These enzymes are stimulated by keratinocyte growth factor (KGF) that regulates the type II cell phenotype. Thus, the differentiation of cells affects FA biosynthesis required for surfactant production in the lung [19]. Hormonal or physiologic factors can also stimulate de novo FA synthesis by increasing immunoreactive levels of FAS [20–23]. FAS is activated by glucocorticoids in fetal lung while thyroid hormone and transforming growth factor β1 (TGF-β1) antagonize this effect [24, 25]. Hormonal regulation of FAS in human fetal lung was detected selectively in type II alveolar epithelial cells [26]. The contribution of plasma free FA and de novo synthesized FA to lung surfactant is age dependent [27]. De novo synthesis of FA is sufficient to produce DPPC in the fetal lung when FA availability is limiting, but not in adult type II cells [28], and is characterized by gene activation of key biosynthetic enzymes [19]. The transcription of these enzymes is regulated by sterol-response element-binding proteins and CCAAT/enhancer-binding protein transcription factors [29]. Data regarding regulatory control of FA synthesis in the fetal and adult type II cells is still fragmented and the mechanisms require further study.
3.1.2. Phosphatidic acid synthesis and regulation
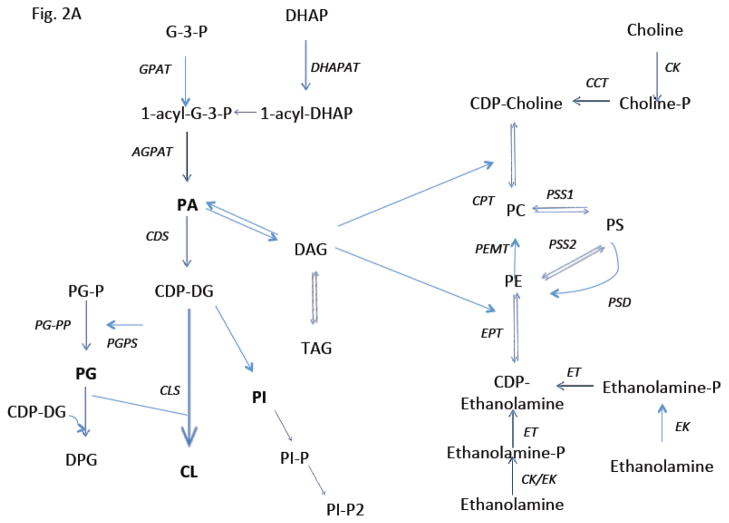
Phosphatidic acid (PA) is a key intermediate in phospholipid biosynthesis and an integral precursor for surfactant synthesis. PA de novo biosynthesis utilizes dihydroxyacetone phosphate (DHAP) derived from glucose or glycogen that is then processed into PA by two different pathways both of which are active in the lung [30, 31]. It was shown that fetal type II cell precursors accumulate significant amounts of glycogen that are used as a carbon source, providing chemical energy and serving as an important precursor for PA and surfactant synthesis [32]. Glucose and glycogen are metabolized via the glycolytic pathway to DHAP or acetate and hence to FA. The two de novo pathways for PA synthesis include the glycerol-3 phosphate (G3P) pathway and the dihydroacetone phosphate (DHAP) pathway that occur in the mitochondria and ER or the ER and the peroxisomes, respectively. The initial step in the G3P pathway involves generation of G3P by glycerol-3 phosphate dehydrogenase from DHAP in a reaction that requires NADH, or conversion of glycerol directly to G3P. Glycerol converted to G3P by glycerol kinase is present in the lung [33]. The first acylation step in this pathway is catalyzed by G3P acyltransferase that leads to formation of monoacylglycerol phosphate or lysophosphatidic acid (lyso-PA). The second pathway for PA synthesis utilizes the peroxisomal enzyme, DHAP acyltransferase, that generates acyl-DHAP from DHAP. This intermediate is then converted to lyso-PA in a NADPH-dependent reaction catalyzed by acyl-DHAP reductase. It was demonstrated that 60% of PA may be synthesized via this pathway [34]. Finally, lyso-PA synthesized via G3P or DHAP pathways is then acylated to PA in a reaction catalyzed by 1-acylglycerol-3-phosphate acyltransferase that is predominantly localized in the ER of adult type II cells. Two human isoforms of 1-acylglycerol-3-phosphate acyltransferase have been cloned [35]. The FA required for this acylation step are first converted to acyl-CoAs by acyl-CoA synthase. Several different forms of acyl-CoA synthetases are also identified in lung ER or mitochondria-associated membranes and cytosol [36]. The multiple sites of PA biosynthesis could be related to access to specific substrates, linked to eventual destiny of newly synthesized phospholipids, or based on the needs of the cell. It is suggested that membrane phospholipids are synthesized in the ER, while secreted lipids are originally synthesized within mitochondria or peroxisomes [37]. Further, acylating activities presented in mitochondria, peroxisomes or lipid particles provide a rapid and efficient mechanism for generation of PA. PA and lyso-PA are synthesized on the outer surface of the mitochondrial outer membrane and a pool of PA transmigrates to the inner membrane apparently for cardiolipin formation [38]. Mitochondrial 1-acylglycerol-3-phosphate acyltransferase and peroxisomal DHAP acyltransferase have a preference for saturated acyl-CoAs in contrast to 1-acylglycerol-3-phosphate acyltransferase in the ER that utilizes saturated, monounsaturated, and polyunsaturated acyl-CoAs. Aside from de novo synthesis, PA can be generated by additional mechanisms: 1) by the phosphorylation of diacylglycerol (DAG) by DAG kinase (DAGK), 2) by actions of phospholipase D (PLD), via the hydrolysis of PC yielding PA and choline, and 3) by acylation of lysoPA by lysoPA-acyltransferase [37, 39]. PA phosphatase (PAP) is also a key enzyme in the regulation of lung phospholipid synthesis. This enzyme catalyzes magnesium+-dependent dephosphorylation of PA, yielding DAG that is a substrate for de novo PC synthesis [40].
3.2 SYNTHESIS OF MAJOR SURFACTANT PHOSPHOLIPIDS
3.2.1. Phosphatidylcholine (PC) de novo synthesis and regulation
The de-novo biosynthesis of surfactant PC, the most abundant and functionally important phospholipid component of surfactant, occurs by the cytidine diphosphocholine (CDP-choline) or Kennedy pathway that is predominate in most tissues (Fig 2A). In this pathway, FA that are synthesized de novo by the lung are incorporated into newly synthesized PC [41]. Choline that is not generated by animal cells is obtained primarily from the diet or from phospholipase D-driven hydrolysis of PC [42, 43]. Cellular uptake of dietary choline is mediated by choline transporters [44, 45]. Upon entry into cells, choline is phosphorylated to cholinephosphate which represents the first committed step catalyzed by choline kinase (CK) [46] (Fig. 2A). In mammals, the choline kinase family consists of CKα and CKβ that are encoded by two separate genes [47]. These two isoforms of CK demonstrate different enzymatic and physiologic behavior in phospholipid metabolism [43]. Genetic deletion of murine CKα is embryonic lethal, whereas disruption of murine CKβ results in forelimb bone deformities and muscular dystrophy [48]. Choline uptake and CKα levels and activities are increased in tumor-derived cell lines and correlate with worse outcomes in lung, breast, and bladder tumors [49]. Further, phosphocholine produced by CKα may serve as an essential metabolic signal for the amplification of pro-survival pathways that impact tumor growth [50]. By contrast, CKβ may not directly influence tumor growth [43]. Nonetheless, much of the molecular and physiologic understanding of choline kinases as they relate to surfactant metabolism remains limited.
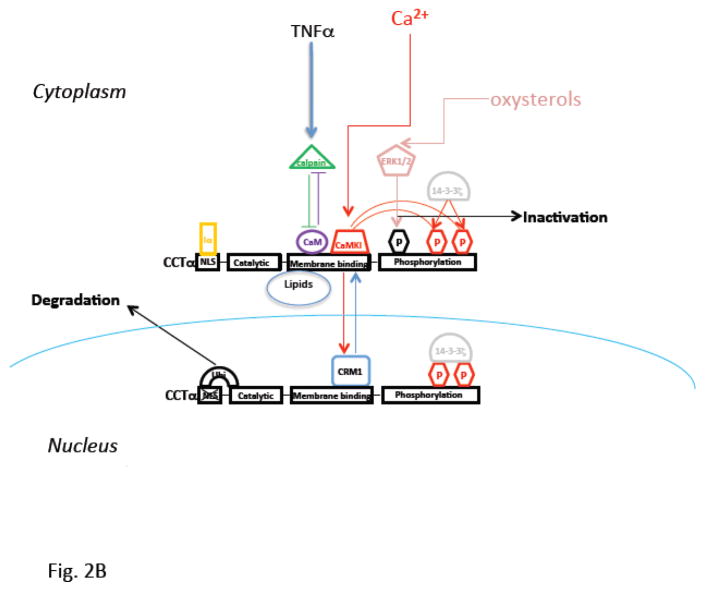
Fig 2. Cartoon illustrating biosynthetic pathways for pulmonary surfactant.
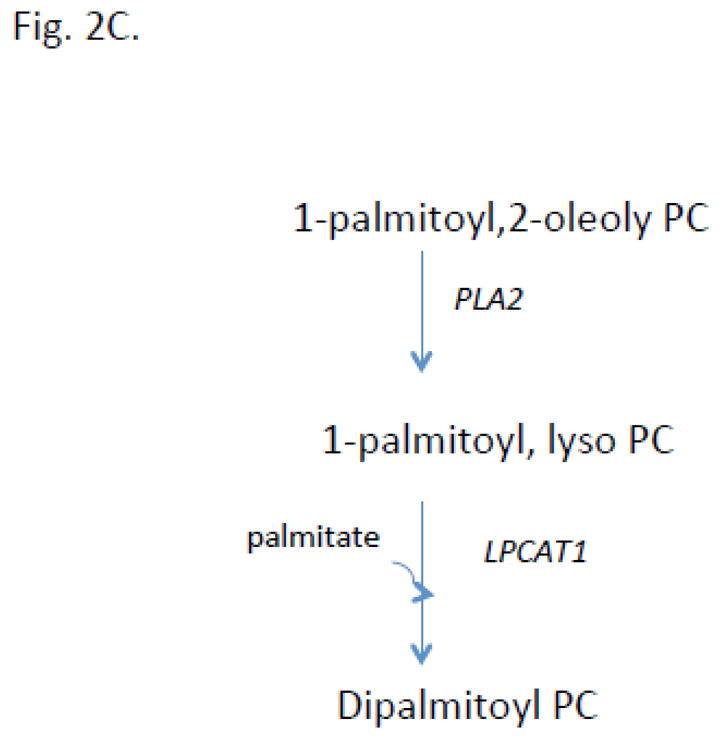
A. Pathways for the major phospholipid components are shown including the de novo pathway for phosphatidylcholine (PC) synthesis. B. Cartoon illustrating modes of regulation and functional domains of cholinephosphate cytidylyltransferase α (CCTα), the key enzyme required for PC synthesis. CCTα is phosphorylated and its activity inhibited by extracellular signal regulated kinase (ERK1/2). CCTα is also a protein that translocates between the cytoplasm and nucleus. This cytoplasmic/nuclear shuttling occurs via binding to the scaffolding protein, 14-3-3ζ. In this scenario, calmodulin kinase I (CaMKI) (red) phosphorylates CCTα thereby providing a phosphorylation dock site for 14-3-3ζ binding to the enzyme to facilitate nuclear import. Nuclear CCTα export is controlled by exportin 1 (CRM1) (blue). CCTα is also sensitive to proteolysis as it is monoubiquitinated (Ubi) at a molecular site juxtaposed near its nuclear localization signal (NLS). This post-translational mechanism limits the enzyme’s interaction with importin-α (Iα) thereby targeting the enzyme for degradation. Calpains also target CCTα for degradation, an effect antagonized by calmodulin (CaM). Many of these intermediate mechanisms are activated in cells by stimuli (TNFα, oxysterols, exogenous calcium). Last, CCTα regulated by both activating and inhibitory lipids. C. The remodeling pathway involves phospholipase A2 hydrolysis of an unsaturated PC yielding lyso-PC. This substrate is then re-acylated by LPCAT1 using palmitoyl-CoA generating dipalmitoylphosphatidylcholine. Adapted and modified from M. Hermansson et all/Progress in Lipid Research 50 (2011), 240–257.
The second reaction in the Kennedy pathway is the conversion of choline phosphate to CDP-choline utilizing CTP [51] (Fig. 2A). This reaction is catalyzed by CTP: phosphocholine cytidylyltransferase (CCT), an enzyme derived from two different genes encoding the CCTα isoform [52–54] and CCTβ isoform [55, 56] located on distinct chromosomes in humans [52]. There has been intense interest in the enzymatic and physiological behavior of CCTα because of its rate-limiting and rate-regulatory role in mammalian cells [57]. CCTα, the predominant species in lung epithelia, is essential for proliferation and survival [58, 59] and is comprised of four functional domains, including an NH2-terminal domain with a nuclear localization signal (NLS), a catalytic core, an α-helical membrane-binding domain, and C-terminal phosphorylation domain (Fig. 2B)[51]. CCTα is mostly cytoplasmic and largely associated with the ER in alveolar epithelia whereas in several other cell types it is nuclear [60]. Nevertheless, it was recently shown that CCTα is escorted by 14-3-3ζ, a scaffolding protein, to the nucleus to preserve PC homeostasis during calcium-induced stress [61]. This cytoplasmic/nuclear shuttling is regulated by calmodulin kinase I and exportin 1 via binding to a nuclear export signal within the CCTα membrane binding domain (Fig. 2B)[62]. Further, CCTα is sensitive to proteolysis as it is monoubiquitinated at a molecular site juxtaposed near its NLS [63]. This is a post-translational mechanism of significance as this limits the enzyme’s interaction with importin-α thereby targeting CCTα for lysosomal disposal [63]. In essence this mode of control appears to regulate CCTα concentrations in the nucleus (Fig. 2B)[63]. The calcium sensor, calmodulin also serves as a stabilizing ligand for CCTα that antagonizes the actions of ubiquitin E3 ligases and the proteinase, calpain [63, 64]. CCTα is also a phosphoenzyme and its phosphorylation antagonizes membrane binding [65, 66]. CCTα phosphorylation is restricted to 16 serines within the C-terminal domain of the enzyme and this has an important inhibitory effect on enzymatic activity [67, 68]. CCTα is a target for extracellular-signal-regulated kinases, resulting in inhibition of PC synthesis in lung epithelia [65](Fig. 2B). In addition to control at the post-translational level, CCTα activity is extensively regulated by both activating and inhibitory lipids [65, 69]. Other work has shown CCTα transcriptional regulation governed by Sp1, Sp3, Rb, TEF4, Ets-1 and E2F [70–72] and CCTα mRNA synthesis is developmentally regulated in the lung [73]. These transcription factors may also link CCTα to the cell cycle, cell growth, and differentiation in lung epithelia. Physiologically, CCTα activity increases in response to hormones with pulmonary maturation, resulting in increased PC synthesis for pulmonary surfactant [74–76]. For example, glucocorticoids increase synthesis of lipid-cofactors [77] and can regulate CCTα activity at the level of mRNA stability or transcriptional level in the lung [78, 79]. CCTα is also critical for proper LB formation and surfactant protein homeostasis in lung epithelia [80].
The terminal step of the Kennedy pathway involves the formation of PC from DAG synthesized from its precursor, PA, that reacts with CDP-choline (Fig. 2A). The conversion of PA into essential DAG intermediates is catalyzed by PAP (or phosphatidate phosphohydrolase, or phosphatidate phosphatase). The existence of two different isoforms of PAP, PAP-1 and PAP-2 was detected in the lung [81]. These enzymes, localize in mitochondria and microsomes in type II cells, are Mg2+-dependent and heat labile, and may play a role in signal transduction and surfactant synthesis [40, 82]. It was recently shown that phosphorylation of recombinant PAP1 regulates its activity, localization and function [83]. Both PAP isoforms are also developmentally and postnatally regulated [84, 85] and linked to increased PC synthesis and release of lung surfactant [81]. Thus, stimulation of surfactant biosynthesis requires an adequate supply of PA-derived DAG for increased PC synthesis. This final step of the de-novo synthesis pathway for PC is catalyzed by choline phosphotransferase (CPT), the membrane-bound enzyme localized primarily in the ER (Fig. 2A). Two CPT cDNAs have been identified in humans [70, 86]. CPT plays an important role in regulating the acyl group composition of PC in the lung and other mammalian tissues [87]. This enzyme is able to utilize both endogenous and exogenous DAGs for PC synthesis in the lung and its activity is also dependent on CDP-choline availability [88]. The two forms of CPT are localized in ER/microsomes and mitochondria in the fetal lung and type II epithelial cells that differ by their substrate specificity based on preference for unsaturated or saturated DAGs, Km values, and sensitivity to inhibitors [89, 90]. CPT levels appear also to be regulated at the level of protein stability [91] and by inhibitory lipids such as ceramides [90, 92]. CPT activity is upregulated just prior to birth, decreasing in the adult lung [93]. Conversely, mitochondrial CPT activity is high and increases with age [94]. Thyroid hormone upregulates lung mitochondrial and microsomal CPT activity [95].
3.2.2. Disaturated phosphatidylcholine and phosphatidylcholine remodeling
The PC in lung contains a large proportion of disaturated molecular species where DPPC, the main surface-active component, is primarily responsible for the ability of pulmonary surfactant to decrease lung surface tension to very low levels. Although the CDP-choline (de novo) pathway is clearly important for PC biosynthesis, prior data suggest that the DPPC synthesized via this pathway is not predominant [96–98]. Direct utilization of disaturated DAG [96] by CPT will generate DPPC. However, if newly generated PC is contains unsaturated fatty acyl species, the molecule undergoes remodeling. It is estimated that ~45% of surfactant DPPC is formed via de novo synthesis, while the other 55%–75% is generated by remodeling [9, 99, 100]. This remodeling pathway is catalyzed by a series of enzymatic reactions involving phospholipase A2 (PLA2) mediated hydrolysis of unsaturated fatty acids at the sn-2-position of PC generating lyso-PC followed by re-acylation with saturated (16:0) species yielding DPPC [7, 101, 102] (Fig. 2C). The PLA2 mediated hydrolysis appears to be catalyzed by a lysosomal-type enzyme that is expressed at high levels in the lung to regulate surfactant composition [103]. The reacylation of resultant 1-palmitoyl-lyso-PC is catalyzed by acyl CoA:lysophosphatidylcholine acyltransferase (LPCAT1) [104, 105]. Type II alveolar epithelial cells are enriched with this key enzyme that exhibits calcium-independent activity and demonstrates a preference for saturated fatty acyl-CoAs as an acyl donor, and 1-myristoyl- or 1-palmitoyl-LPC as an acceptor [101, 104, 106, 107]. LPCAT1 has three putative transmembrane domains and a conserved cysteine (Cys211) that plays a crucial role in its activity [101]. LPCAT1 expression is upregulated during differentiation of type II cells [107, 108] and newborn mice bearing a hypomorphic allele of LPCAT1 exhibit varying degrees of prenatal mortality from respiratory failure from reduced saturated PC content [109]. LPCAT1 in lung epithelia is targeted for polyubiquitination and proteasomal degradation [110]. Interestingly, LPCAT1 also catalyzes O-palmitoylation of histones to regulate pro-inflammatory gene expression [111]. Thus, LPCAT1 appears critical for surfactant DPPC generation but also appears to display unique enzymatic behavior within epigenetic programs.
3.2.3 Phosphatidylglycerol synthesis and regulation
Phosphatidylglycerol (PG) is the second most abundant surfactant phospholipid with palmitoyl-oleoyl phosphatidylglycerol (POPG) as the dominant molecular species [112, 113] that comprises approximately ~10 mole % of phospholipids. This concentration of PG in the lung is unusually high in comparison with the other mammalian tissues. PG is involved in the adsorption and spreading of surfactant over the epithelial surface. Surfactant deficiency in premature infants is associated with a lack of PG [114–116]. The microsomes and mitochondria both serve as sites for de novo PG formation destined for incorporation into surfactant [115]. PG is synthesized from CDP-diacylglycerol produced from PA and then converted by glycerophosphate phosphatidyltransferase to phosphatidylglycerolphosphate followed by its dephosphorylation to PG catalyzed by phosphatidylglycerophosphatase (Fig 2A). Like DPPC, deacylation/reacylation also serves as a remodeling mechanism involved in formation of surfactant dipalmitoyl-PG. The dipalmitoyl species in surfactant PG ranges from 17% to 38% [1, 117]. Recent studies demonstrate that PG plays an important role in regulating innate immunity and viral infection [12, 112, 118, 119]. For example, POPG acts as a strong antiviral biomolecule against Influenza A virus via attenuation of interleukin-8 production and POPG strongly suppresses the development of inflammatory cell infiltrates [118]. PG also suppresses pathogen-induced eicosanoid production in human and mouse macrophages after Mycoplasma pneumoniae infection [112] and this phospholipid attenuates respiratory syncytial virus -induced inflammation [12]. High concentrations of PG reduce numbers of alveolar macrophages in the alveolar space impacting lung function [120]. Additional work is needed to ascertain the in vivo biologic and translational significance of PG to humans.
3.3. Minor phospholipids in surfactant
Phosphatidylethanolamine (PE), the third most abundant phospholipid in pulmonary surfactant, is formed via a separate, distinct branch of the Kennedy pathway (Fig 2A). The first step of this pathway, the conversion of ethanolamine to phosphoethanolamine is not rate-limiting and catalyzed by ethanolamine kinase (EK) that exists in two forms, EK1 and EK2 encoded by the Etnk1 and Etnk2 mammalian genes [121, 122]. The second enzyme in PE synthesis is CTP:phosphoethanolamine cytidylyltransferase (ECT) encoded by a single gene (Pcyt2) in mammalian tissues. ECT catalyzes the conversion of phosphoethanolamine to cytidine-diphosphoethanolamine utilizing CTP [123, 124] and this enzyme is rate-limiting and transcriptionally regulated by the early growth response protein 1 [125]. The final step of PE formation is catalyzed by ethanolaminephosphotransferase (EPT). Two enzymes with EPT activity have been identified in mammals, CEPT that utilizes both CDP-ethanolamine and CDP-choline as substrates, and EPT, which is selective for CDP-ethanolamine [126, 127]. CEPT was found in ER and nuclear membranes, but little is known of EPT [128]. Surfactant PE, in contrast to surfactant PC, contains very little disaturated species [9]. The alveolar type II cells presumably produce a separate pool of DAG containing very little dipalmitoyl species for PE formation. The remodeling pathway for the formation of dipalmitoylphosphatidylethanolamine by acylation of 1-palmitoyl-lysophosphatidylethanolamine with palmitoyl-CoA has very low activity in type II cells [9]. The synthesis of PE predominantly localizes on the inner membrane of mitochondria. Defects in mitochondrial PE production are responsible for mitochondrial abnormalities that cause embryonic lethality in mice [129, 130]. PE is also required for optimal activity in the respiratory complexes and proper ubiquinone function [130, 131].
Another minor surfactant lipid, phosphatidylinositol (PI) is synthesized initially via the formation of CDP-diacylglycerol (CDP-DAG) from PA and CTP catalyzed by ER membrane-associated CDP-diacylglycerol synthase (CDS) (Fig 2A). This enzyme exists as two forms, CDS1 and CDS2, in mammals and is highly expressed in lung, brain and kidney [126, 132]. The second step of PI synthesis utilizes myo-inositol and CDP-DAG in a reaction catalyzed by CDP-diacylglycerol:inositol-3-phosphatidyltransferase or PI synthase that is also localized in the ER of type II cells [126, 133]. A remodeling mechanism is also involved in surfactant PI formation [9]. Information regarding the regulation of PI synthesis in mammalian tissue is limited and controversial. It was shown that phosphoinositide-specific phospholipase C (PI-PLC) can regulate PI levels in lung surfactant [134]. Six isoforms of PI-PLC were identified in lung tissue with one species, PLCβ3, highly expressed in adult rat type II cells [135, 136]. PI-PLC activated by ligand-stimulation of cell surface receptors hydrolyzes phosphatidylinositol 4,5-bisphosphate generating inositol 1,4,5-triphosphate and 1,2-DAG, two secondary messengers that regulate cellular differentiation, proliferation, and immune responses [134, 137]. A phosphatidylinositol transfer protein is also involved in phosphatidylinositide metabolism and might mediate exocytosis in lung cells [138]. PI may be involved in the stabilization of the surfactant monolayer and its levels are altered during acute lung injury [134, 139–141].
Phosphatidylserine (PS) first identified in rat lung is synthesized by base-exchange from PC or PE via PS synthase (PSS1 and PSS2) localized in ER and/or mitochondrial membranes in lung type II cells [126, 142] (Fig 2A). PSS1 replaces the head group of PC with L-serine while PSS2 replaces the head group of PE with L-serine in a calcium dependent reaction [126, 143, 144]. PSS1 and PSS2 also catalyze the reverse reaction where the serine group is exchanged to PC or PE. The mechanisms regulating PS synthesis and degradation are largely unknown [126, 145]. Studies using PSS1- and PSS2-deficient mice have demonstrated that normal activity of PSS1 might compensate for the lack of PSS2 and vice versa [126, 146]. PS also regulates the activities of nitric oxide synthase [147], diglyceride kinase [148] and c-Raf-1 protein kinase [149] involved in cell signaling. It was shown that PS can modulate ligand:receptor interactions [150, 151]. Phagocytes lacking the phosphatidylserine receptor (PSR) are defective in removing apoptotic cells [152]. This receptor may also be involved in respiratory distress [152] and has an essential function in terminal differentiation of the lung and other vital organs [153]. PS can also be involved in late apoptosis via binding to human SP-A [154]. A specific PS-dependent phospholipase A1 is highly expressed in the lung and is able to hydrolyze PS and 2-lysoPS and the products of this hydrolysis participate in cell signaling [155, 156]. It was also demonstrated that a calcium PS-dependent protein kinase C is involved in surfactant synthesis and secretion in fetal, neonatal, and adult rabbit lung and isolated LB [157]. In aggregate, the precise physiological role of PS in lung function and surfactant metabolism requires additional investigation.
Cardiolipin (CL) is a very minor component of surfactant but it may have important roles in lung homeostasis. CL is formed via condensation of PG and CDP-DAG in a reaction catalyzed by cardiolipin synthase [126, 158, 159] localized exclusively in the inner mitochondrial membranes [126, 160–162] (Fig 2A). Mammalian CL contains 18:2 acyl chains obtained from PC and/or PE donors in a transacylation or remodeling reaction mediated by tafazzin protein [158]. Abnormalities of CL remodeling process can compromise the normal function of mitochondria as observed in Barth syndrome where defects of tafazzin exist [126, 163]. Pulmonary lavage fluid normally contains only minor amounts of CL and CL comprises only 1–2% of alveolar surfactant [164]. However, high amounts of CL are detected in alveolar fluid in humans with pneumonia where CL acts as a extracellular damage signal released from dying host cells, profoundly antagonizing surfactant activity [165]. ATP8b1, a P-type ATPase protein is highly expressed in the apical lung epithelial membranes that translocates CL from the outer to the inner membranes in lung cells thereby regulating the abundance of CL in the lung fluid [165]. These results suggest that studies understanding the molecular regulation of CL metabolism may be important to surfactant metabolism and lung inflammation [165].
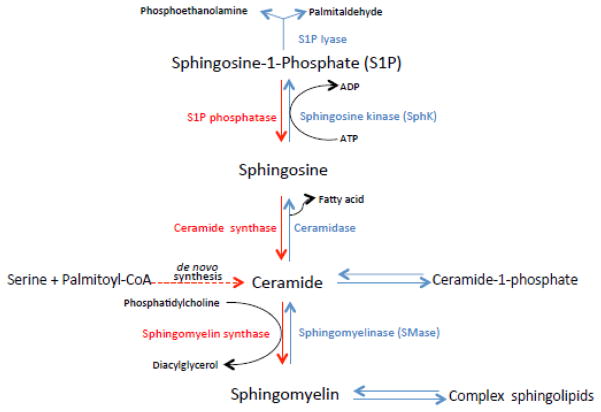
Sphingomyelin (SM) is another minor phospholipid surfactant component but some of its hydrolysis products and precursors have potentially important biological roles in lung structure and function. The first step of de novo SM synthesis is a condensation reaction utilizing L-serine and palmitoyl-CoA generating 3-ketosphinganine that is catalyzed by serine palmytoyltransferase [166, 167] localized in microsomes (Fig 3). Serine palmitoyltransferases are highly expressed in the lung relative to other tissues [168]. The 3-ketosphinganine product can be reduced to sphinganine that becomes amide-linked generating ceramide or undergoes oxidation to sphingosine, or long-chain bases [166, 167]. The final step of this pathway, the transfer of a phosphocholine headgroup from PC to ceramide yielding SM, is catalyzed by sphingomyelin synthase (SMS)(phosphatidylcholine: ceramide cholinephosphotransferase). Two species of SMS have been identified in humans, SMS1 localized in trans-Golgi, and SMS2 predominantly associated with plasma membranes. SM is developmentally regulated in the rat lung through activation of SMS [167]. SMS activity correlates with the severity of lung injury through mitogen-activated protein kinases [169]. SM levels associated with surfactant in alveolar lavage are typically low but increase in acute or chronic lung injury [170–172]. Total surface-active phospholipid/sphingomyelin ratio is used as an index of fetal lung maturity. SM also undergoes hydrolysis by sphingomyelinases in response to external stimuli, generating the biologically-active breakdown products, ceramide and sphingosine [167, 173]. Ceramide can be deacylated to sphingosine by ceramidase, or phosphorylated to ceramide 1-phosphate by ceramide kinase [167]. These products of SM degradation are involved as secondary messengers in a variety of important cellular processes and have a special relevance to lung pathophysiology as a potential mediators of pulmonary edema and acute lung injury [167, 174–176]. Ceramides are also implicated in playing a role in the pathogenesis of emphysema [177].
Fig. 3. Cartoon illustrating sphingolipid metabolism.
Biosynthesis is shown in red, degradation is shown in blue.
4. SURFACTANT PROTEINS
Pulmonary surfactant contains four major proteins that elicit critical functions (Fig. 1). SP-A is a highly conserved and most abundant surfactant-associated protein synthesized in alveolar type II epithelial cells. The protein monomer has a molecular mass of ~28–36 kDa, while in lung lavage this protein exists as a 650 kDa oligomeric glycoprotein [178, 179]. SP-A belongs to the collectin family and is a multifunctional protein. SP-A interacts strongly with surfactant phospholipids and promotes the conversion of LB into tubular myelin in the presence of calcium providing rapid formation of the surface film [164]. SP-A also mediates recycling of secreted surfactant components via a high affinity receptor on the type II cell membrane [164, 180]. SP-A also acts in a negative feedback manner by inhibiting surfactant phospholipid secretion [181]. SP-A critically interacts with diverse pathogens and immune effector cells facilitating their clearance and providing host defense [182]. SP-A also inhibits secreted PLA2 activity, thereby potentially playing a protective role to maintain surfactant integrity during lung injury [183]. SP-B and SP-C, in contrast to SP-A, are extremely hydrophobic polypeptides associated with surfactant phospholipids. SP-B exists as an 18kDa homodimer and is expressed in type II cells. This protein associates with phospholipids to decrease surface tension by facilitating rapid lipid insertion into the air-liquid monolayer [184]. SP-B is also involved in surfactant secretion and tubular myelin formation [185, 186]. Congenital deficiency of SP-B gene is lethal in humans and results in disruption of normal processing of surfactant components and LB. The absence of SP-B also blocks processing of SP-C, reduces surfactant function, and alters PG degradation [187]. SP-C is a 4.2 kDa non-polar α-helical protein exclusively expressed in lung type II alveolar cells. SP-C is secreted with surfactant phospholipids into the alveolar space and like SP-B is critically involved in the formation and maintenance of the surfactant film. SP-D is a 43kDa glycoprotein consisting of four homotrimeric units produced and secreted by type II cells. SP-D regulates surfactant metabolism in type II cells and may have affinity to PI [188, 189]. SP-D like SP-A, is an innate immune defense protein. SP-D promotes uptake of pathogenic bacteria by epithelial cells [190]. SP-D stimulates phagocytosis, chemotaxis, regulates cytokine and free radical production by multiple immune cells [182]. Similar to SP-A, SP-D also possesses antimicrobial activities and can elicit anti-inflammatory effects. Targeted disruption of the SP-D gene induces emphysema in mice [191]. As a whole, surfactant associated proteins are intimately associated with surfactant phospholipids to modulate surface activity but also play key roles in regulating inflammation, innate immune defense, and microbial pathogenesis.
5. LAMELLAR BODY FORMATION
Pulmonary surfactant is synthesized, packaged, and stored in alveolar type II epithelial cells as LB which are 1.0–2.0 μM lysosome-related organelles secreted into the alveolar space via exocytosis that transform into the surfactant film [192]. The glycogen stores of fetal type II cells are a site for surfactant PC synthesis and LB formation [193]. Secreted surfactant is internalized by type II cells that can be incorporated back to LB for recycling or degradation by alveolar macrophages [194]. As a lysosome-related organelle, LB is characterized by an acidic interior that contains lysosomal enzymes (acid phosphatase and cathepsins C and H) and proteins (CD63/LAMP3, LAMP1)[195, 196]. These organelles contain concentric and tightly packed lamellae comprised of DPPC. SP-B plays a central role in packaging of surfactant phospholipids into LB since in SP-B null mice LB are disorganized and lack ability to form a surfactant film [197, 198]. Both SP-B and SP-C processed in the Golgi are transported to LB through multivesicular bodies via proteolytic processing. In contrast, phospholipids such as PC, DPPC, and PG newly synthesized in the ER are transported directly to LB [1]. These surfactant-associated phospholipids are transported from the ER to LB via phospholipid transfer proteins and then transverse the LB membrane [32]. This process can be facilitated by ATP-binding cassette (ABC) transporters such as ATP-binding cassette A3 (ABCA3) [199]. ABCA3 is a lipid transport protein that is required for normal synthesis and storage of pulmonary surfactant in alveolar type II cells. An alternative mechanism for surfactant PL incorporation into LB might be via autophagy, a pathway by which cells incorporate organelles or part of the cytoplasm into the lysosome for degradation during nutrient deprivation [192]. However, there is no direct evidence for the involvement of this pathway in surfactant PL transport in alveolar epithelium. In total over 500 proteins were recently detected in rat lung LB that might help to identify novel molecular pathways involved in surfactant homeostasis and pathogenesis [194].
6. SURFACTANT SECRETION AND REGULATION
The secretory mechanisms of pulmonary surfactant are still not fully characterized. A burst in secretion of surfactant occurs at birth with the first breath, and then after, the requirement for synthesis of surfactant becomes lower. Surfactant secretion is a relatively slow and yet highly regulated process. This process consists of two steps: 1) transport of phospholipids from the ER to the LB in type II cells, and 2) translocation of phospholipids across the LB membrane at the type II cell surface (Fig 4). The mechanisms involved in regulation of pathways implicated in the first step are still not clear. It is suggested that Golgi apparatus (GA)-dependent and GA-independent pathways could be involved in phospholipid trafficking to LB [200]. The function of PC transporters and mechanisms responsible for lipid packaging into LB are not confirmed in vivo [32, 192]. One of the possible mechanisms for phospholipid delivery to LB is autophagy [201]. The second step of surfactant secretion occurs through the fusion of the limiting membrane of LB with the apical plasma membrane, followed by extrusion of the LB contents into the alveolar space. In the initial step, phospholipid translocation across the membrane is facilitated by ABC transporters; in particular, ABCA3 was identified in the outer membrane of the LB of type II cells [32, 202, 203]. Mutations in ABCA3 disrupt surfactant processing that perturbs lung homeostasis [199]. Two types of ABCA3 mutations have been described, one involving defects in lipid trafficking to LB, and the other impairs LB function [203]. These ABCA3 gene mutations are characterized by abnormal subcellular localization, glycosylation, ATP binding and ATP hydrolysis activities resulting in fatal surfactant deficiency in newborns [204]. Ultrastructural analysis of ABCA3 null mice lung shows an absence of surfactant from lung lavage and loss of mature LB. ABCA3 null mice have a dramatic reduction in PG levels, and selective reductions in PC species containing short acyl chains demonstrating a unique and critical role of ABCA3 in surfactant metabolism [199]. When ABCA3 was conditionally deleted in type II cells, the mice with this defect died shortly after birth from surfactant deficiency. Phospholipid content and composition was altered in lung tissue of these mice and transcription factors regulating the genes of lipid metabolism were markedly decreased [205]. ABCA3 mutations potentiated by RSV infection induce loss of type II cell differentiation [203, 206]. Another member of the ABC transporter family, ABCA5, may play a role in LB biogenesis, whereas ABCA1 can modulate the transport of surfactant phospholipid from the basolateral membrane of type II cells [32, 207, 208]. The secretion of surfactant phospholipids can be regulated by physiological and non-physiological agents. Labor and hyperventilation are important physiologic triggers for surfactant secretion in vivo [209–211]. A number of surfactant secretagogues including terbutaline, forskolin, phorbol esters, calcium ionophore, ATP and others initiate various signal transduction pathways. In response to terbutaline, cAMP increases and activates protein kinase A, whereas activation of protein kinase C, phosphoinositide-specific phospholipase C (PPI-PLC) and elevated intracellular calcium trigger surfactant secretion by an ATP mechanism [164]. ATP also increases inositol triphosphate and DAG production via activation of purinergic P2Y2 receptors [209, 212]. The purinergic P2X7 receptor localizes in type II lung epithelial cells to mediate surfactant secretion in response to extracellular ATP released from type I cells via a paracrine mechanism [209]. Further, stress-induced calcium oscillation in type I cells induces calcium shuttling to type II cells via gap junctions that also contributes to surfactant secretion [192, 213]. LB-binding proteins annexin V [214] and annexin II may be involved in surfactant secretion via reorganization of the cytoskeleton in type II cells during this process [215]. Annexin VII also plays a role in membrane fusion during exocytosis in type II cells [216]. Stimulation of surfactant secretion by some agents increase membrane association of annexin VII and its colocalization with ABCA3 [217]. Further, the lipid raft marker, flotillin-2, regulates surfactant secretion via cytoskeletal rearrangement as flotillin-2 depletion in cells blocks the G-protein coupled receptor-mediated activation of p38 mitogen-activated protein kinases [218]. Besides these stimulatory factors, surfactant phospholipid secretion can be regulated by inhibitory factors. Secretagogue-induced surfactant secretion in type II cells is inhibited by SP-A via its binding to a specific receptor on the surface of type II cells [219]. SP-A regulates surfactant secretion via pathways involving tyrosine phosphorylation, insulin-receptor substrate-1, and activation of phosphoinositide 3-kinase [220]. Vacuolar ATPase (V-ATPase) is the enzyme responsible for pumping H+ into LB and is required for processing of surfactant proteins and the packaging of surfactant lipids. The association of V-ATPase with type II cell lipid rafts and LB were revealed by proteomic analysis [221]. Bafilomycine A1, a V-ATPase inhibitor, induces pulmonary surfactant secretion that is blocked by the intracellular calcium chelator, BAPTA-AM, and PKC inhibitor, staurosporine, and a calmodulin kinase II inhibitor [221]. Bafilomycine A1 also stimulates calcium release from isolated LB [221]. The disassembly of the V-ATPase complex by surfactant secretagogues indicates the physiological relevance of the V-ATPase-mediated surfactant secretion mechanism in type II cells. V-ATPase can regulate surfactant secretion via calcium mobilization from LB and the ER, and activation of PKC and calmodulin kinase II [221]. Recently, micro-RNA 375 appears to be involved as a negative regulator of surfactant secretion [222]. Overexpression of micro-RNA 375 inhibits lung surfactant secretion without effects on surfactant synthesis or LB formation [222]. In aggregate, the biochemical and physiologic regulation of surfactant secretion and putative modulators of this process are diverse and highly relevant as they might be applicable in future work related to surfactant-deficient lung disorders.
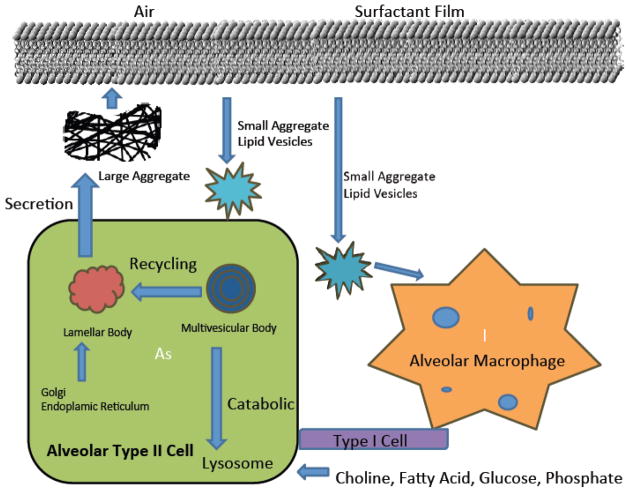
Fig. 4. Cartoon illustrating surfactant secretion, recycling, and catabolism.
Surfactant is synthesized and secreted from distal lung alveolar type II epithelial cells using cellular substrates or from circulation. The newly synthesized surfactant phospholipids are packaged into a storage form, termed lamellar bodies. The secreted surface-active lipid material rapidly transforms into tubular myelin (not shown) that serves as a precursor to the monolayer film at the air-surface interface. During respiration, small and physiologically active large aggregates are formed that can be internalized and catabolized by alveolar cells, including macrophages. A significant portion of surfactant lipid is re-utilized by type II cells. Adapted and modified from M. Ikegami/Respirology 11 (2006), S24–S27.
7. REGULATION OF SURFACTANT DEGRADATION AND RECYCLING
Alveolar surfactant is cleared by distinct pathways. First, surfactant components are reutilized by type II cells that internalize alveolar phospholipids destined for re-incorporation into LB for secretion [27]. Additionally, intra-alveolar or extracellular surfactant is degraded [27, 181]. Surfactant recycling occurs by re-internalization of a substantial portion of surfactant (25%–95%) in type II cells that is promoted by SP-A via interaction with its high-affinity receptor present on the cell surface [223]. There are several candidates for the SP-A receptor detected on type II epithelial cells including p63, a reversibly palmitoylated transmembrane protein, identified initially in the ER and Golgi apparatus [224]. The receptors for the other surfactant proteins that are also recycled are not yet identified [16]. Phospholipids and SP-A are initially internalized together and sorted to early endosomes via clathrin-coated vesicles accompanied by actin polymerization [225, 226]. However, inhibition of clathrin-mediated endocytosis has no effect on ABCA3-mediated LB marker reuptake, demonstrating that some LB components can be recycled by clathrin-independent pathways [32, 227]. Transport of internalized phospholipids to the LB occurs in a calmodulin-dependent manner and is bafilomycine A1-sensitive [226]. A portion of internalized SP-A is rapidly recycled to the cell surface via a Rab4-associated and calmodulin-sensitive pathway, while another SP-A fraction is targeted for actin-dependent degradation [225, 226]. The mechanisms of sorting of phospholipids and SP-A for recycling or degradation are not yet fully defined [32]. Some studies have demonstrated that alveolar macrophages, rather than type II cells, are involved in phospholipid degradation [228, 229]. However, using radiolabeled components, it was recently demonstrated that equal amounts of saturated PC and SP-A were associated with the macrophages and type II cells [230]. Surfactant components taken up by type II cells are recycled or degraded, while surfactant that is internalized by macrophages is largely degraded. The efficiency of recycling is age and species dependent [231, 232]. Estimates suggest 90% recycling efficiency in young pigs, >90% in newborn rabbits, and 50% in adult rabbits [233, 234]. With regard to mechanisms of recycling, in granulocyte-macrophage colony-stimulating factor (GM-CSF) deficient, granulocyte-macrophage common receptor β-chain deficient, and acid sphingomyelinase deficient mice, the levels of surfactant PC are increased up to eight fold in the lung, while PC synthetic rates are unaltered [230]. These observations suggest that increases in surfactant pool size in these mice might occur by a defect in surfactant catabolism within alveolar macrophages. Recently, it was demonstrated that peroxisome proliferator-activated receptor γ (PPARγ) promotes surfactant catabolism through regulation of the ATP-binding cassette lipid transporter, ABCG1 [235, 236]. The lipid analysis in macrophage-specific PPARγ mice demonstrates significant increases in phospholipid and cholesterol pools in alveolar macrophages and bronchoalveolar lavage fluid accompanied by decreased ABCG1 expression. Based on these studies, PPARγ might regulate surfactant homeostasis via ABCG1 [235, 236]. The role of secretory phospholipases (e.g. sPLA2) in surfactant degradation and lung inflammation is well known and these lipases also add another layer of complexity to surfactant balance; secretory lipases also mediate surfactant dysfunction and augment acute lung injury [237].
8. EXTRACELLULAR SURFACTANT PHOSPOLIPID METABOLISM
After secretion from type II cells into the alveolar space, LB undergoes a structural and metabolic transformation (Fig. 4). In the airspace, secreted LB combines with SP-A and rapidly transforms into tubular myelin (TM) that at the air-fluid interface rapidly forms the surface-tension-reducing surface film [238, 239]. TM is a unique lattice-like structure isolated with the large aggregates (LA). The important components that are required for in vitro TM structure are SP-B, 16:0/16:0-PC, and PG. The formation and function of this structure is still not fully understood. Extensive in vitro studies have demonstrated that isolated LB are transformed into TM-like structures in the presence of exogenous SP-A in a calcium-dependent manner to provide stability to LA structures [1]. The LA are a mixture of TM, LB, and multi-LB containing all the surfactant phospholipids and surfactant proteins, SP-A, SP-B and SP-C. The other structures, detected in alveolar fluid are the small aggregates (SA) [1]. The SA represent the small unilamellar vesicles containing a small amount of surfactant proteins and other components similar to LA. However, SA have limited ability to reduce surface tension [1, 240]. The LA are converted into SA during surface area cycling with respiration. During an increase in lung surface area, LA adsorb to the air liquid interface, and during the following decrease in surface area with expiration this aggregate can be converted to SA. The adsorption rate depends on the concentration of the components that form a surface film and their diffusion [241]. The conversion of LA to SA requires SP-A and possibly activity of an enzyme responsible for subtype conversion [1, 242]. LA and the active surfactant film is highly enriched with 16:0/16:0-PC that results from repeated compression:expansion events with “squeeze-out” of nonsurfactant molecular species [1, 242]. However, much of the understanding of extracellular surfactant processing was obtained from in vitro studies, and thus relevance to biological processes in humans is less clear.
9. MODULATORS OF SURFACTANT PRODUCTION IN LUNG DISEASE
Deficiencies of surfactant phospholipids occur in neonatal RDS but also acute and chronic adult lung disease [243, 244]. Deficiencies of surfactant production may have a dramatic effect on lung function leading to respiratory illness, sometimes with fatal consequences. However, there are multiple regulatory factors that physiologically and non-physiologically control production of surfactant that can impact lung homeostasis. Lung maturation and surfactant production is stimulated by glucocorticoids, thyroid hormones, estrogen, and other growth factors [245, 246]. Endogenous glucocorticoids directly affect fetal lung maturation via glucocorticoid receptors identified in the type II alveolar cells [8, 247, 248]. Glucocorticoids also exert stimulatory effects on regulatory enzymes (CTP, CPT, PAP, glycerophosphate phosphatidyltransferase and phosphatidylglycerophosphatase) involved in phospholipid biosynthesis [9, 31]. Thyroid hormone also accelerates lung maturation and increases surfactant PC biosynthesis [8, 31, 249]. Estrogens upregulate maturation of the fetal lung via increasing CCT activity and lysolecithin acyltransferase activity, and these hormones impact postnatal lung development and lung cancer progression [31, 250, 251]. In contrast, androgens and insulin have been implicated as inhibitory signals for surfactant phospholipid synthesis [9, 31, 252]. A fibroblast-pneumocyte factor stimulates PC, DPPC and PG synthesis in the fetal type II cells [9]. β-adrenergic agents and cyclic AMP stimulate surfactant synthesis and surfactant release and the levels of β-receptor expression increase at the end of gestation to facilitate increased surfactant secretion [31, 253]. Prostaglandins synthesis increases during gestation and can also regulate surfactant production in neonatal lung and perhaps in adult lung [254]. In fact, increased surfactant release during birth and labor appears to be mediated by many of the mediators described above. At the cellular level, lung fibroblasts secrete substances that induce surfactant synthesis in type II cells via the growth factor neuregulin -1 [255].
If the proper balance between physiological regulators is disturbed or inhibitory factors released as occurs during lung inflammation, surfactant deficiency can significantly contribute to impairment of lung mechanics. During both acute and chronic respiratory illness, surfactant levels can be reduced. In the prototypical illness, neonatal RDS, there is a lack of surfactant production and secretion that has led to surfactant replacement therapy [256]. However, inflammatory illness, such as pneumonia, is also associated with surfactant dysfunction. In the adult, pneumonia and sepsis are the leading causes of the acute respiratory distress syndrome (ARDS) or a less severe form, acute lung injury, devastating disorders also characterized by surfactant deficiency [257]. However, the pathobiology of this illness is more complex than RDS and surfactant replacement therapy has not been shown yet to improve mortality in ARDS [258]. Sepsis also produces a decrease in surfactant PG and PA and increases PS and PI levels and also is associated with alterations in the FA c[259]omposition of phospholipids [170]. Thyroid hormone may have a significant effect in the preserving lung function via maintenance of surfactant homeostasis during sepsis [260]. Glutathione replacement also preserves the functional surfactant phospholipid pool size and it may lessen the severity of lung injury during sepsis in animal models [261]. Abnormal surfactant function is also seen with respiratory infections, in cystic fibrosis, chronic obstructive lung disease, and asthma [259, 262]. In respiratory infections, altered surfactant composition occurs via surfactant degradation and inhibition of surfactant biosynthesis or impairment of surfactant secretion [263–267]. For example, P. aeruginosa impairs surfactant function via degradation of SP-A and SP-D or by inhibiting CCTα gene transcription [267]. Lipopolysaccharide inhibits phospholipid synthesis and secretion whereas adenovirus induces alteration in surfactant phospholipid composition [267]. The adverse effects of microbial pathogens on surfactant production may also be indirect via release of host cell cytokines. Recent studies have demonstrated that anionic phospholipids have an important role in alteration of the immune response during viral infection [268]. Aside from pathogens, a variety of other toxic agents can affect surfactant phospholipids. For example, tobacco smoke contains between 2,000 and 4,000 compounds including organic and inorganic toxins that may exert toxic effects on the lung by altering surfactant [186]. The components of smoke alter the surface tension lowering capabilities of pulmonary surfactant, reduce DPPC, and increase PE and SM levels. Tobacco smoke also can effect lung development, synthesis and secretion of surfactant [186]. Genetic disorders can also significantly disrupt normal surfactant metabolism and increase mortality and morbidity causing acute respiratory distress and failure in full-term neonates, and interstitial lung disease in older infants, children, and adults [2]. Most of these genetic disorders are linked to genes encoding SP- B and SP-C and the phospholipid transporter, ABCA3. Mutations in the SP-B gene are associated with fatal RDS in neonates [269]. SP-C mutations are linked to interstitial lung disease [270–272]. Mutations in ABCA3 are associated with both phenotypes [2, 273]. Thus, the importance of phospholipids to mammalian life is absolute and abnormalities in surfactant phospholipid metabolism contribute pathobiologically to a diverse array of respiratory illnesses.
CONCLUDING REMARKS
In spite of significant progress in understanding mechanisms that regulate surfactant phospholipid homeostasis, newer insight will be gained using more sophisticated approaches such as deep sequencing of the human genome and lipidomics. The mechanisms of action of many lipogenic enzymes, their physiological roles, and integrated regulation with various arms involved in surfactant metabolism are still not fully understood. In particular, the identification of the proteins involved in surfactant phospholipid trafficking and regulation of phospholipid biosynthetic enzymes are an essential part of maintenance of surfactant homeostasis. Additional studies are needed to better understand how the molecular and biochemical events in these pathways in human lung disease might cause dysfunction of pulmonary surfactant. By understanding these mechanisms, novel strategies might emerge to optimize phospholipid balance during respiratory illness.
Highlights.
Surfactant is comprised of key phospholipids and proteins.
Surfactant lipid components control lung stability and immunity.
Availability of surfactant is reduced in acute and chronic lung disease.
Studies are needed to understand the molecular regulation of phospholipid enzymes.
Acknowledgments
The authors wish to thank Leah Kaercher for editorial assistance with the manuscript and preparation of the Figures. This material is based upon work supported, in part, by the US Department of Veterans Affairs, Veterans health Administration, Office of Research and Development, Biomedical Laboratory Research and Development. This work was supported by a Merit Review Award from the US Department of Veterans Affairs and National Institutes of Health RO1 grants HL096376, HL097376 and HL098174 (to R.K.M.).
The abbreviations used are
- ABC
ATP-binding cassette transporter
- ARDS
acute respiratory distress syndrome
- CCT, CTP
phosphocholine cytidylyltransferase
- CDP-choline
cytidine diphosphocholine
- CDP-DAG
CDP-diacylglycerol
- CDS
CDP-diacylglycerol synthase
- CK
choline kinase
- CL
cardiolipin
- CPT
choline phosphotransferase
- DAG
diacylglycerol
- DAGK
DAG kinase
- DHAP
dihydroxyacetone phosphate
- DSPC
disaturated PC
- DPPC
dipalmitoylphosphatidylcholine
- ECT, CTP
phosphoethanolamine cytidylyltransferase
- EK
ethanolamine kinase
- EPT
ethanolaminephosphotransferase
- ER
endoplasmic reticulum
- FA
fatty acids
- FAS
fatty acid synthase
- GA
Golgi apparatus
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- G3P
glycerol-3 phosphate
- LPCAT1
lysophosphatidylcholine acyltransferase 1
- Lyso-PA
lysophosphatidic acid
- KGF
keratinocyte growth factor
- LA
large aggregates
- LB
lamellar bodies
- NES
nuclear export signal
- NLS
nuclear localization signal
- PA
phosphatidic acid
- PAP
PA phosphatase
- PC
phosphatidylcholine
- PE
phosphatidylethanolamine
- PG
phosphatidylglycerol
- PI
phosphatidylinositol
- PI-PLC
phosphoinositide-specific phospholipase C
- PLA2
phospholipase A2
- PLD
phospholipase D
- PL
phospholipids
- POPG
palmitoyl-oleoyl phosphatidylglycerol
- PPARγ
peroxisome proliferator-activated receptor γ
- PPI-PLC
phosphoinositide-specific phospholipase C
- PSR
phosphatidylserine receptor
- PSS
PS synthase
- SA
small aggregates
- SMS
sphingomyelin synthase
- SM
sphingomyelin
- SP-A
surfactant protein A
- SP-B
surfactant protein B
- SP-C
surfactant protein C
- SP-D
surfactant protein D
- TGF-β1
transforming growth factor β1
- TM
tubular myelin
- V-ATPase
vacuolar ATPase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Veldhuizen R, Possmayer F. Phospholipid metabolism in lung surfactant. Subcell Biochem. 2004;37:359–88. doi: 10.1007/978-1-4757-5806-1_11. [DOI] [PubMed] [Google Scholar]
- 2.Wert SE, Whitsett JA, Nogee LM. Genetic disorders of surfactant dysfunction. Pediatr Dev Pathol. 2009;12(4):253–74. doi: 10.2350/09-01-0586.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lynch RG. Surfactant and RDS in premature infants. FASEB J. 2004;18(13):1624. [PubMed] [Google Scholar]
- 4.Anzueto A, et al. Aerosolized surfactant in adults with sepsis-induced acute respiratory distress syndrome. Exosurf Acute Respiratory Distress Syndrome Sepsis Study Group. N Engl J Med. 1996;334(22):1417–21. doi: 10.1056/NEJM199605303342201. [DOI] [PubMed] [Google Scholar]
- 5.Wright JR, Clements JA. Metabolism and turnover of lung surfactant. Am Rev Respir Dis. 1987;136(2):426–44. doi: 10.1164/ajrccm/136.2.426. [DOI] [PubMed] [Google Scholar]
- 6.Schurch S, et al. The surface-associated surfactant reservoir in the alveolar lining. Biol Neonate. 1995;67(Suppl 1):61–76. doi: 10.1159/000244207. [DOI] [PubMed] [Google Scholar]
- 7.Hook GE. Alveolar proteinosis and phospholipidoses of the lungs. Toxicol Pathol. 1991;19(4 Pt 1):482–513. doi: 10.1177/019262339101900416. [DOI] [PubMed] [Google Scholar]
- 8.Sullivan LC, Orgeig S, Daniels CB. Control of the development of the pulmonary surfactant system in the saltwater crocodile, Crocodylus porosus. Am J Physiol Regul Integr Comp Physiol. 2002;283(5):R1164–76. doi: 10.1152/ajpregu.00009.2002. [DOI] [PubMed] [Google Scholar]
- 9.Batenburg JJ. Surfactant phospholipids: synthesis and storage. Am J Physiol. 1992;262(4 Pt 1):L367–85. doi: 10.1152/ajplung.1992.262.4.L367. [DOI] [PubMed] [Google Scholar]
- 10.Holm BA, et al. Content of dipalmitoyl phosphatidylcholine in lung surfactant: ramifications for surface activity. Pediatr Res. 1996;39(5):805–11. doi: 10.1203/00006450-199605000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Hallman M, Enhorning G, Possmayer F. Composition and surface activity of normal and phosphatidylglycerol-deficient lung surfactant. Pediatr Res. 1985;19(3):286–92. doi: 10.1203/00006450-198503000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Numata M, et al. Pulmonary surfactant phosphatidylglycerol inhibits respiratory syncytial virus-induced inflammation and infection. Proc Natl Acad Sci U S A. 2010;107(1):320–5. doi: 10.1073/pnas.0909361107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu SH, Possmayer F. Comparative studies on the biophysical activities of the low-molecular-weight hydrophobic proteins purified from bovine pulmonary surfactant. Biochim Biophys Acta. 1988;961(3):337–50. doi: 10.1016/0005-2760(88)90081-1. [DOI] [PubMed] [Google Scholar]
- 14.Weaver TE, Conkright JJ. Function of surfactant proteins B and C. Annu Rev Physiol. 2001;63:555–78. doi: 10.1146/annurev.physiol.63.1.555. [DOI] [PubMed] [Google Scholar]
- 15.Crouch E, Wright JR. Surfactant proteins a and d and pulmonary host defense. Annu Rev Physiol. 2001;63:521–54. doi: 10.1146/annurev.physiol.63.1.521. [DOI] [PubMed] [Google Scholar]
- 16.Herbein JF, Savov J, Wright JR. Binding and uptake of surfactant protein D by freshly isolated rat alveolar type II cells. Am J Physiol Lung Cell Mol Physiol. 2000;278(4):L830–9. doi: 10.1152/ajplung.2000.278.4.L830. [DOI] [PubMed] [Google Scholar]
- 17.Haagsman HP, van Golde LM. Synthesis and assembly of lung surfactant. Annu Rev Physiol. 1991;53:441–64. doi: 10.1146/annurev.ph.53.030191.002301. [DOI] [PubMed] [Google Scholar]
- 18.Maniscalco WM, Stremmel W, Heeney-Campbell M. Uptake of palmitic acid by rabbit alveolar type II cells. Am J Physiol. 1990;259(4 Pt 1):L206–12. doi: 10.1152/ajplung.1990.259.4.L206. [DOI] [PubMed] [Google Scholar]
- 19.Mason RJ. Biology of alveolar type II cells. Respirology. 2006;11(Suppl):S12–5. doi: 10.1111/j.1440-1843.2006.00800.x. [DOI] [PubMed] [Google Scholar]
- 20.Mashima T, Seimiya H, Tsuruo T. De novo fatty-acid synthesis and related pathways as molecular targets for cancer therapy. Br J Cancer. 2009;100(9):1369–72. doi: 10.1038/sj.bjc.6605007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cogo PE, et al. Metabolic precursors of surfactant disaturated-phosphatidylcholine in preterms with respiratory distress. J Lipid Res. 2009;50(11):2324–31. doi: 10.1194/jlr.M800514-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maniscalco WM, Finkelstein JN, Parkhurst AB. De novo fatty acid synthesis in developing rat lung. Biochim Biophys Acta. 1982;711(1):49–58. doi: 10.1016/0005-2760(82)90008-x. [DOI] [PubMed] [Google Scholar]
- 23.Wang MC, Meng HC. Synthesis of phospholipids and phospholipid fatty acids by isolated perfused rat lung. Lipids. 1974;9(2):63–7. doi: 10.1007/BF02532126. [DOI] [PubMed] [Google Scholar]
- 24.Lu Z, Gu Y, Rooney SA. Transcriptional regulation of the lung fatty acid synthase gene by glucocorticoid, thyroid hormone and transforming growth factor-beta 1. Biochim Biophys Acta. 2001;1532(3):213–22. doi: 10.1016/s1388-1981(01)00135-4. [DOI] [PubMed] [Google Scholar]
- 25.Pope TS, Smart DA, Rooney SA. Hormonal effects on fatty-acid synthase in cultured fetal rat lung; induction by dexamethasone and inhibition of activity by triiodothyronine. Biochim Biophys Acta. 1988;959(2):169–77. doi: 10.1016/0005-2760(88)90028-8. [DOI] [PubMed] [Google Scholar]
- 26.Wagle S, et al. Hormonal regulation and cellular localization of fatty acid synthase in human fetal lung. Am J Physiol. 1999;277(2 Pt 1):L381–90. doi: 10.1152/ajplung.1999.277.2.L381. [DOI] [PubMed] [Google Scholar]
- 27.Zimmermann LJ, et al. Surfactant metabolism in the neonate. Biol Neonate. 2005;87(4):296–307. doi: 10.1159/000084877. [DOI] [PubMed] [Google Scholar]
- 28.Maniscalco WM, Finkelstein JN, Parkhurst AB. Effects of exogenous fatty acids and inhibition of de novo fatty acid synthesis on disaturated phosphatidylcholine production by fetal lung cells and adult type II cells. Exp Lung Res. 1989;15(3):473–89. doi: 10.3109/01902148909087872. [DOI] [PubMed] [Google Scholar]
- 29.Latasa MJ, et al. Nutritional regulation of the fatty acid synthase promoter in vivo: sterol regulatory element binding protein functions through an upstream region containing a sterol regulatory element. Proc Natl Acad Sci U S A. 2000;97(19):10619–24. doi: 10.1073/pnas.180306597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wykle RL, Malone B, Snyder F. Biosynthesis of dipalmitoyl-sn-glycero-3-phosphocholine by adenoma alveolar type II cells. Arch Biochem Biophys. 1977;181(1):249–56. doi: 10.1016/0003-9861(77)90503-3. [DOI] [PubMed] [Google Scholar]
- 31.Rooney SA. Lung surfactant. Environ Health Perspect. 1984;55:205–26. doi: 10.1289/ehp.8455205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andreeva AV, Kutuzov MA, Voyno-Yasenetskaya TA. Regulation of surfactant secretion in alveolar type II cells. Am J Physiol Lung Cell Mol Physiol. 2007;293(2):L259–71. doi: 10.1152/ajplung.00112.2007. [DOI] [PubMed] [Google Scholar]
- 33.Fisher AB, Chander A. Glycerol kinase activity and glycerol metabolism of rat granular pneumocytes in primary culture. Biochim Biophys Acta. 1982;711(1):128–33. doi: 10.1016/0005-2760(82)90018-2. [DOI] [PubMed] [Google Scholar]
- 34.Mason RJ. Importance of the acyl dihydroxyacetone phosphate pathway in the synthesis of phosphatidylglycerol and phosphatidylcholine in alveolar type II cells. J Biol Chem. 1978;253(10):3367–70. [PubMed] [Google Scholar]
- 35.Vance DE. A stimulating factor for fatty acid biosynthesis--research with Konrad Bloch: mentor and friend. Biochem Biophys Res Commun. 2002;292(5):1273–8. doi: 10.1006/bbrc.2002.2030. [DOI] [PubMed] [Google Scholar]
- 36.Lewin TM, et al. Acyl-CoA synthetase isoforms 1, 4, and 5 are present in different subcellular membranes in rat liver and can be inhibited independently. J Biol Chem. 2001;276(27):24674–9. doi: 10.1074/jbc.M102036200. [DOI] [PubMed] [Google Scholar]
- 37.Athenstaedt K, Daum G. Phosphatidic acid, a key intermediate in lipid metabolism. Eur J Biochem. 1999;266(1):1–16. doi: 10.1046/j.1432-1327.1999.00822.x. [DOI] [PubMed] [Google Scholar]
- 38.Chakraborty TR, et al. Phosphatidic acid synthesis in mitochondria. Topography of formation and transmembrane migration. J Biol Chem. 1999;274(42):29786–90. doi: 10.1074/jbc.274.42.29786. [DOI] [PubMed] [Google Scholar]
- 39.Cummings R, et al. Phospholipase D/phosphatidic acid signal transduction: role and physiological significance in lung. Mol Cell Biochem. 2002;234–235(1–2):99–109. [PubMed] [Google Scholar]
- 40.Carman GM, Han GS. Phosphatidic acid phosphatase, a key enzyme in the regulation of lipid synthesis. J Biol Chem. 2009;284(5):2593–7. doi: 10.1074/jbc.R800059200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mason RJ, Dobbs LG. Synthesis of phosphatidylcholine and phosphatidylglycerol by alveolar type II cells in primary culture. J Biol Chem. 1980;255(11):5101–7. [PubMed] [Google Scholar]
- 42.Carnero A, et al. Activation of type D phospholipase by serum stimulation and ras-induced transformation in NIH3T3 cells. Oncogene. 1994;9(5):1387–95. [PubMed] [Google Scholar]
- 43.Gallego-Ortega D, et al. Differential role of human choline kinase alpha and beta enzymes in lipid metabolism: implications in cancer onset and treatment. PLoS One. 2009;4(11):e7819. doi: 10.1371/journal.pone.0007819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu G, et al. Early embryonic lethality caused by disruption of the gene for choline kinase alpha, the first enzyme in phosphatidylcholine biosynthesis. J Biol Chem. 2008;283(3):1456–62. doi: 10.1074/jbc.M708766200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Noga AA, Zhao Y, Vance DE. An unexpected requirement for phosphatidylethanolamine N-methyltransferase in the secretion of very low density lipoproteins. J Biol Chem. 2002;277(44):42358–65. doi: 10.1074/jbc.M204542200. [DOI] [PubMed] [Google Scholar]
- 46.Reo NV, Adinehzadeh M, Foy BD. Kinetic analyses of liver phosphatidylcholine and phosphatidylethanolamine biosynthesis using (13)C NMR spectroscopy. Biochim Biophys Acta. 2002;1580(2–3):171–88. doi: 10.1016/s1388-1981(01)00202-5. [DOI] [PubMed] [Google Scholar]
- 47.Aoyama C, Ohtani A, Ishidate K. Expression and characterization of the active molecular forms of choline/ethanolamine kinase-alpha and -beta in mouse tissues, including carbon tetrachloride-induced liver. Biochem J. 2002;363(Pt 3):777–84. doi: 10.1042/0264-6021:3630777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vance DE, Vance JE. Physiological consequences of disruption of mammalian phospholipid biosynthetic genes. J Lipid Res. 2009;50(Suppl):S132–7. doi: 10.1194/jlr.R800048-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chua BT, et al. Regulation of Akt(ser473) phosphorylation by choline kinase in breast carcinoma cells. Mol Cancer. 2009;8:131. doi: 10.1186/1476-4598-8-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yalcin A, et al. Selective inhibition of choline kinase simultaneously attenuates MAPK and PI3K/AKT signaling. Oncogene. 2010;29(1):139–49. doi: 10.1038/onc.2009.317. [DOI] [PubMed] [Google Scholar]
- 51.Kent C. CTP:phosphocholine cytidylyltransferase. Biochim Biophys Acta. 1997;1348(1–2):79–90. doi: 10.1016/s0005-2760(97)00112-4. [DOI] [PubMed] [Google Scholar]
- 52.Jackowski S, Fagone P. CTP: Phosphocholine cytidylyltransferase: paving the way from gene to membrane. J Biol Chem. 2005;280(2):853–6. doi: 10.1074/jbc.R400031200. [DOI] [PubMed] [Google Scholar]
- 53.Tang W, Keesler GA, Tabas I. The structure of the gene for murine CTP:phosphocholine cytidylyltransferase, Ctpct. Relationship of exon structure to functional domains and identification of transcriptional start sites and potential upstream regulatory elements. J Biol Chem. 1997;272(20):13146–51. doi: 10.1074/jbc.272.20.13146. [DOI] [PubMed] [Google Scholar]
- 54.Karim M, Jackson P, Jackowski S. Gene structure, expression and identification of a new CTP:phosphocholine cytidylyltransferase beta isoform. Biochim Biophys Acta. 2003;1633(1):1–12. doi: 10.1016/s1388-1981(03)00067-2. [DOI] [PubMed] [Google Scholar]
- 55.Lykidis A, Baburina I, Jackowski S. Distribution of CTP:phosphocholine cytidylyltransferase (CCT) isoforms. Identification of a new CCTbeta splice variant. J Biol Chem. 1999;274(38):26992–7001. doi: 10.1074/jbc.274.38.26992. [DOI] [PubMed] [Google Scholar]
- 56.Lykidis A, Murti KG, Jackowski S. Cloning and characterization of a second human CTP:phosphocholine cytidylyltransferase. J Biol Chem. 1998;273(22):14022–9. doi: 10.1074/jbc.273.22.14022. [DOI] [PubMed] [Google Scholar]
- 57.Gehrig K, Morton CC, Ridgway ND. Nuclear export of the rate-limiting enzyme in phosphatidylcholine synthesis is mediated by its membrane binding domain. J Lipid Res. 2009;50(5):966–76. doi: 10.1194/jlr.M800632-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dennis MK, Taneva SG, Cornell RB. The intrinsically disordered nuclear localization signal and phosphorylation segments distinguish the membrane affinity of two cytidylyltransferase isoforms. J Biol Chem. 2011;286(14):12349–60. doi: 10.1074/jbc.M110.201715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang L, et al. Early embryonic lethality in mice with targeted deletion of the CTP:phosphocholine cytidylyltransferase alpha gene (Pcyt1a) Mol Cell Biol. 2005;25(8):3357–63. doi: 10.1128/MCB.25.8.3357-3363.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ridsdale R, et al. CTP:phosphocholine cytidylyltransferase alpha is a cytosolic protein in pulmonary epithelial cells and tissues. J Biol Chem. 2001;276(52):49148–55. doi: 10.1074/jbc.M103566200. [DOI] [PubMed] [Google Scholar]
- 61.Agassandian M, et al. 14-3-3zeta escorts CCTalpha for calcium-activated nuclear import in lung epithelia. FASEB J. 2010;24(4):1271–83. doi: 10.1096/fj.09-136044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Agassandian M, et al. Calcium-calmodulin kinase I cooperatively regulates nucleocytoplasmic shuttling of CCTalpha by accessing a nuclear export signal. Mol Biol Cell. 2012 doi: 10.1091/mbc.E11-10-0863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen BB, Mallampalli RK. Masking of a nuclear signal motif by monoubiquitination leads to mislocalization and degradation of the regulatory enzyme cytidylyltransferase. Mol Cell Biol. 2009;29(11):3062–75. doi: 10.1128/MCB.01824-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen BB, Mallampalli RK. Calmodulin binds and stabilizes the regulatory enzyme, CTP: phosphocholine cytidylyltransferase. J Biol Chem. 2007;282(46):33494–506. doi: 10.1074/jbc.M706472200. [DOI] [PubMed] [Google Scholar]
- 65.Agassandian M, et al. Oxysterols inhibit phosphatidylcholine synthesis via ERK docking and phosphorylation of CTP:phosphocholine cytidylyltransferase. J Biol Chem. 2005;280(22):21577–87. doi: 10.1074/jbc.M412409200. [DOI] [PubMed] [Google Scholar]
- 66.Gehrig K, Cornell RB, Ridgway ND. Expansion of the nucleoplasmic reticulum requires the coordinated activity of lamins and CTP:phosphocholine cytidylyltransferase alpha. Mol Biol Cell. 2008;19(1):237–47. doi: 10.1091/mbc.E07-02-0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang W, Jackowski S. Lipid activation of CTP:phosphocholine cytidylyltransferase is regulated by the phosphorylated carboxyl-terminal domain. J Biol Chem. 1995;270(28):16503–6. doi: 10.1074/jbc.270.28.16503. [DOI] [PubMed] [Google Scholar]
- 68.Cornell RB, et al. Functions of the C-terminal domain of CTP: phosphocholine cytidylyltransferase. Effects of C-terminal deletions on enzyme activity, intracellular localization and phosphorylation potential. Biochem J. 1995;310(Pt 2):699–708. doi: 10.1042/bj3100699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stern W, Kovac C, Weinhold PA. Activity and properties of CTP: cholinephosphate cytidylyltransferase in adult and fetal rat lung. Biochimica et Biophysica Acta. 1976;441(2):280–93. doi: 10.1016/0005-2760(76)90171-5. [DOI] [PubMed] [Google Scholar]
- 70.Li Z, Vance DE. Phosphatidylcholine and choline homeostasis. J Lipid Res. 2008;49(6):1187–94. doi: 10.1194/jlr.R700019-JLR200. [DOI] [PubMed] [Google Scholar]
- 71.Sugimoto H, Banchio C, Vance DE. Transcriptional regulation of phosphatidylcholine biosynthesis. Prog Lipid Res. 2008;47(3):204–20. doi: 10.1016/j.plipres.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 72.Banchio C, Schang LM, Vance DE. Phosphorylation of Sp1 by cyclin-dependent kinase 2 modulates the role of Sp1 in CTP:phosphocholine cytidylyltransferase alpha regulation during the S phase of the cell cycle. J Biol Chem. 2004;279(38):40220–6. doi: 10.1074/jbc.M406468200. [DOI] [PubMed] [Google Scholar]
- 73.McCoy DM, et al. Transcriptional regulation of lung cytidylyltransferase in developing transgenic mice. Am J Respir Cell Mol Biol. 2006;35(3):394–402. doi: 10.1165/rcmb.2005-0401OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rooney SA. The surfactant system and lung phospholipid biochemistry. Am Rev Respir Dis. 1985;131(3):439–60. doi: 10.1164/arrd.1985.131.3.439. [DOI] [PubMed] [Google Scholar]
- 75.Schurch S, et al. Pulmonary SP-A enhances adsorption and appears to induce surface sorting of lipid extract surfactant. Am J Physiol. 1992;263(2 Pt 1):L210–8. doi: 10.1152/ajplung.1992.263.2.L210. [DOI] [PubMed] [Google Scholar]
- 76.Batenburg JJ, Haagsman HP. The lipids of pulmonary surfactant: dynamics and interactions with proteins. Prog Lipid Res. 1998;37(4):235–76. doi: 10.1016/s0163-7827(98)00011-3. [DOI] [PubMed] [Google Scholar]
- 77.Sharma A, Gonzales LW, Ballard PL. Hormonal regulation of cholinephosphate cytidylyltransferase in human fetal lung. Biochim Biophys Acta. 1993;1170(3):237–44. doi: 10.1016/0005-2760(93)90005-t. [DOI] [PubMed] [Google Scholar]
- 78.Hogan M, et al. Regulation of phosphatidylcholine synthesis in maturing type II cells: increased mRNA stability of CTP:phosphocholine cytidylyltransferase. Biochem J. 1996;314(Pt 3):799–803. doi: 10.1042/bj3140799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mallampalli RK, et al. Lipid deprivation increases surfactant phosphatidylcholine synthesis via a sterol-sensitive regulatory element within the CTP:phosphocholine cytidylyltransferase promoter. Biochem J. 2002;362(Pt 1):81–8. doi: 10.1042/0264-6021:3620081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tian Y, et al. Role of phosphocholine cytidylyltransferase alpha in lung development. Mol Cell Biol. 2007;27(3):975–82. doi: 10.1128/MCB.01512-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nanjundan M, Possmayer F. Pulmonary phosphatidic acid phosphatase and lipid phosphate phosphohydrolase. Am J Physiol Lung Cell Mol Physiol. 2003;284(1):L1–23. doi: 10.1152/ajplung.00029.2002. [DOI] [PubMed] [Google Scholar]
- 82.Nanjundan M, Possmayer F. Characterization of the pulmonary N-ethylmaleimide-insensitive phosphatidate phosphohydrolase. Exp Lung Res. 2000;26(5):361–81. doi: 10.1080/019021400408317. [DOI] [PubMed] [Google Scholar]
- 83.Choi HS, et al. Pho85p-Pho80p phosphorylation of yeast Pah1p phosphatidate phosphatase regulates its activity, location, abundance, and function in lipid metabolism. J Biol Chem. 2012 doi: 10.1074/jbc.M112.346023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Douglas WH, Sommers-Smith SK, Johnston JM. Phosphatidate phosphohydrolase activity as a marker for surfactant synthesis in organotypic cultures of type II alveolar pneumonocytes. J Cell Sci. 1983;60:199–207. doi: 10.1242/jcs.60.1.199. [DOI] [PubMed] [Google Scholar]
- 85.Navarro HA, et al. Prenatal dexamethasone administration disrupts the pattern of cellular development in rat lung. Teratology. 1989;40(5):433–8. doi: 10.1002/tera.1420400504. [DOI] [PubMed] [Google Scholar]
- 86.Henneberry AL, Wistow G, McMaster CR. Cloning, genomic organization, and characterization of a human cholinephosphotransferase. J Biol Chem. 2000;275(38):29808–15. doi: 10.1074/jbc.M005786200. [DOI] [PubMed] [Google Scholar]
- 87.Ghosh A, et al. Differential expression of cholinephosphotransferase in normal and cancerous human mammary epithelial cells. Biochem Biophys Res Commun. 2002;297(4):1043–8. doi: 10.1016/s0006-291x(02)02332-x. [DOI] [PubMed] [Google Scholar]
- 88.Stals HK, Mannaerts GP, Declercq PE. Factors influencing triacylglycerol synthesis in permeabilized rat hepatocytes. Biochem J. 1992;283(Pt 3):719–25. doi: 10.1042/bj2830719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ghosh S, et al. Study of properties of cholinephosphotransferase from fetal guinea pig lung mitochondria and microsomes. Mol Cell Biochem. 1991;101(2):157–66. doi: 10.1007/BF00229532. [DOI] [PubMed] [Google Scholar]
- 90.Bladergroen BA, et al. Inhibition of phosphatidylcholine and phosphatidylethanolamine biosynthesis in rat-2 fibroblasts by cell-permeable ceramides. European Journal of Biochemistry. 1999;264(1):152–160. doi: 10.1046/j.1432-1327.1999.00589.x. [DOI] [PubMed] [Google Scholar]
- 91.Butler PL, Mallampalli RK. Cross-talk between remodeling and de novo pathways maintains phospholipid balance through ubiquitination. J Biol Chem. 2010;285(9):6246–58. doi: 10.1074/jbc.M109.017350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sinha Roy S, et al. Inhibition of cholinephosphotransferase activity in lung injury induced by 2-chloroethyl ethyl sulfide, a mustard analog. J Biochem Mol Toxicol. 2005;19(5):289–97. doi: 10.1002/jbt.20092. [DOI] [PubMed] [Google Scholar]
- 93.Hundertmark S, et al. Gestational age dependence of 11 beta-hydroxysteroid dehydrogenase and its relationship to the enzymes of phosphatidylcholine synthesis in lung and liver of fetal rat. Biochim Biophys Acta. 1994;1210(3):348–54. doi: 10.1016/0005-2760(94)90239-9. [DOI] [PubMed] [Google Scholar]
- 94.Das SK, Chakrabarti P, Mukherjee S. Effects of aging on cholinephosphotransferase activity on guinea pig lung mitochondria and microsomes. Mol Cell Biochem. 1993;121(1):31–6. doi: 10.1007/BF00928697. [DOI] [PubMed] [Google Scholar]
- 95.Chatterjee D, Mukherjee S, Das SK. Regulation of cholinephosphotransferase by thyroid hormone. Biochem Biophys Res Commun. 2001;282(4):861–4. doi: 10.1006/bbrc.2001.4662. [DOI] [PubMed] [Google Scholar]
- 96.Van Heusden GP, et al. Synthesis of disaturated phosphatidylcholine by cholinephosphotransferase in rat lung microsomes. Biochim Biophys Acta. 1981;666(3):313–21. doi: 10.1016/0005-2760(81)90289-7. [DOI] [PubMed] [Google Scholar]
- 97.Moriya K, Maekubo H, Itoh S. Turnover rate of plasma free fatty acids in cold-acclimated rats. Jpn J Physiol. 1974;24(4):419–31. doi: 10.2170/jjphysiol.24.419. [DOI] [PubMed] [Google Scholar]
- 98.Van Golde LM. Metabolism of phospholipids in the lung. Am Rev Respir Dis. 1976;114(5):977–1000. doi: 10.1164/arrd.1976.114.5.977. [DOI] [PubMed] [Google Scholar]
- 99.Mason RJ, Nellenbogen J. Synthesis of saturated phosphatidylcholine and phosphatidylglycerol by freshly isolated rat alveolar type II cells. Biochim Biophys Acta. 1984;794(3):392–402. doi: 10.1016/0005-2760(84)90005-5. [DOI] [PubMed] [Google Scholar]
- 100.den Breejen JN, Batenburg JJ, van Golde LM. The species of acyl-CoA in subcellular fractions of type II cells isolated from adult rat lung and their incorporation into phosphatidic acid. Biochim Biophys Acta. 1989;1002(3):277–82. doi: 10.1016/0005-2760(89)90341-x. [DOI] [PubMed] [Google Scholar]
- 101.Harayama T, Shindou H, Shimizu T. Biosynthesis of phosphatidylcholine by human lysophosphatidylcholine acyltransferase 1. J Lipid Res. 2009;50(9):1824–31. doi: 10.1194/jlr.M800500-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lands WE. Metabolism of glycerolipides; a comparison of lecithin and triglyceride synthesis. J Biol Chem. 1958;231(2):883–8. [PubMed] [Google Scholar]
- 103.Fisher AB, Dodia C. Lysosomal-type PLA2 and turnover of alveolar DPPC. Am J Physiol Lung Cell Mol Physiol. 2001;280(4):L748–54. doi: 10.1152/ajplung.2001.280.4.L748. [DOI] [PubMed] [Google Scholar]
- 104.Batenburg JJ, et al. Lysolecithin acyltransferase and lysolecithin: lysolecithin acyltransferase in adult rat lung alveolar type II epithelial cells. Biochim Biophys Acta. 1979;573(1):136–44. doi: 10.1016/0005-2760(79)90180-2. [DOI] [PubMed] [Google Scholar]
- 105.Voelker DR, Snyder F. Subcellular site and mechanism of synthesis of disaturated phosphatidylcholine in alveolar type II cell adenomas. J Biol Chem. 1979;254(17):8628–33. [PubMed] [Google Scholar]
- 106.Nakanishi H, et al. Cloning and characterization of mouse lung-type acyl-CoA:lysophosphatidylcholine acyltransferase 1 (LPCAT1). Expression in alveolar type II cells and possible involvement in surfactant production. J Biol Chem. 2006;281(29):20140–7. doi: 10.1074/jbc.M600225200. [DOI] [PubMed] [Google Scholar]
- 107.Chen X, et al. Identification and characterization of a lysophosphatidylcholine acyltransferase in alveolar type II cells. Proc Natl Acad Sci U S A. 2006;103(31):11724–9. doi: 10.1073/pnas.0604946103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.McDevitt TM, et al. Role of endogenous TGF-beta in glucocorticoid-induced lung type II cell differentiation. Am J Physiol Lung Cell Mol Physiol. 2007;292(1):L249–57. doi: 10.1152/ajplung.00088.2006. [DOI] [PubMed] [Google Scholar]
- 109.Bridges JP, et al. LPCAT1 regulates surfactant phospholipid synthesis and is required for transitioning to air breathing in mice. J Clin Invest. 2010;120(5):1736–48. doi: 10.1172/JCI38061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zou C, et al. LPS impairs phospholipid synthesis by triggering beta-transducin repeat-containing protein (beta-TrCP)-mediated polyubiquitination and degradation of the surfactant enzyme acyl-CoA:lysophosphatidylcholine acyltransferase I (LPCAT1) J Biol Chem. 2011;286(4):2719–27. doi: 10.1074/jbc.M110.192377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zou C, et al. Acyl-CoA:lysophosphatidylcholine acyltransferase I (Lpcat1) catalyzes histone protein O-palmitoylation to regulate mRNA synthesis. J Biol Chem. 2011;286(32):28019–25. doi: 10.1074/jbc.M111.253385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kandasamy P, et al. Pulmonary surfactant phosphatidylglycerol inhibits Mycoplasma pneumoniae-stimulated eicosanoid production from human and mouse macrophages. J Biol Chem. 2011;286(10):7841–53. doi: 10.1074/jbc.M110.170241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wright SM, et al. Altered airway surfactant phospholipid composition and reduced lung function in asthma. J Appl Physiol. 2000;89(4):1283–92. doi: 10.1152/jappl.2000.89.4.1283. [DOI] [PubMed] [Google Scholar]
- 114.Liau DF, et al. Diphosphatidylglycerol in experimental acute alveolar injury in the dog. J Lipid Res. 1984;25(7):678–83. [PubMed] [Google Scholar]
- 115.Schlame M, et al. Phosphatidylglycerol of rat lung. Intracellular sites of formation de novo and acyl species pattern in mitochondria, microsomes and surfactant. Biochem J. 1986;240(1):247–52. doi: 10.1042/bj2400247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Haumont D, et al. Modifications of surfactant phospholipid pattern in premature infants treated with curosurf: clinical and dietary correlations. Biology of the Neonate. 1992;61(Suppl 1):37–43. doi: 10.1159/000243842. [DOI] [PubMed] [Google Scholar]
- 117.Funkhouser JD, Batenburg JJ, Van Golde LM. Acylation of 1-palmitoyl-lysophosphatidylglycerol in a alveolar type II cells from rat lung. Biochim Biophys Acta. 1981;666(1):1–6. doi: 10.1016/0005-2760(81)90084-9. [DOI] [PubMed] [Google Scholar]
- 118.Numata M, et al. Phosphatidylglycerol suppresses influenza A virus infection. Am J Respir Cell Mol Biol. 2012;46(4):479–87. doi: 10.1165/rcmb.2011-0194OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kuronuma K, et al. Anionic pulmonary surfactant phospholipids inhibit inflammatory responses from alveolar macrophages and U937 cells by binding the lipopolysaccharide-interacting proteins CD14 and MD-2. J Biol Chem. 2009;284(38):25488–500. doi: 10.1074/jbc.M109.040832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Poelma DL, et al. Influence of phosphatidylglycerol on the uptake of liposomes by alveolar cells and on lung function. J Appl Physiol. 2005;98(5):1784–91. doi: 10.1152/japplphysiol.01164.2004. [DOI] [PubMed] [Google Scholar]
- 121.Lykidis A, et al. Overexpression of a mammalian ethanolamine-specific kinase accelerates the CDP-ethanolamine pathway. J Biol Chem. 2001;276(3):2174–9. doi: 10.1074/jbc.M008794200. [DOI] [PubMed] [Google Scholar]
- 122.Tian Y, et al. Placental thrombosis and spontaneous fetal death in mice deficient in ethanolamine kinase 2. J Biol Chem. 2006;281(38):28438–49. doi: 10.1074/jbc.M605861200. [DOI] [PubMed] [Google Scholar]
- 123.Bakovic M, Fullerton MD, Michel V. Metabolic and molecular aspects of ethanolamine phospholipid biosynthesis: the role of CTP:phosphoethanolamine cytidylyltransferase (Pcyt2) Biochem Cell Biol. 2007;85(3):283–300. doi: 10.1139/o07-006. [DOI] [PubMed] [Google Scholar]
- 124.Poloumienko A, et al. Genomic organization and differential splicing of the mouse and human Pcyt2 genes. Gene. 2004;325:145–55. doi: 10.1016/j.gene.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 125.Zhu L, Johnson C, Bakovic M. Stimulation of the human CTP:phosphoethanolamine cytidylyltransferase gene by early growth response protein 1. J Lipid Res. 2008;49(10):2197–211. doi: 10.1194/jlr.M800259-JLR200. [DOI] [PubMed] [Google Scholar]
- 126.Hermansson M, Hokynar K, Somerharju P. Mechanisms of glycerophospholipid homeostasis in mammalian cells. Prog Lipid Res. 2011;50(3):240–57. doi: 10.1016/j.plipres.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 127.Horibata Y, Hirabayashi Y. Identification and characterization of human ethanolaminephosphotransferase1. J Lipid Res. 2007;48(3):503–8. doi: 10.1194/jlr.C600019-JLR200. [DOI] [PubMed] [Google Scholar]
- 128.Henneberry AL, Wright MM, McMaster CR. The major sites of cellular phospholipid synthesis and molecular determinants of Fatty Acid and lipid head group specificity. Mol Biol Cell. 2002;13(9):3148–61. doi: 10.1091/mbc.01-11-0540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Steenbergen R, et al. Disruption of the phosphatidylserine decarboxylase gene in mice causes embryonic lethality and mitochondrial defects. J Biol Chem. 2005;280(48):40032–40. doi: 10.1074/jbc.M506510200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gibellini F, Smith TK. The Kennedy pathway--De novo synthesis of phosphatidylethanolamine and phosphatidylcholine. IUBMB Life. 2010;62(6):414–28. doi: 10.1002/iub.337. [DOI] [PubMed] [Google Scholar]
- 131.Mileykovskaya E, Dowhan W. Role of membrane lipids in bacterial division-site selection. Curr Opin Microbiol. 2005;8(2):135–42. doi: 10.1016/j.mib.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 132.Inglis-Broadgate SL, et al. Isolation and characterization of murine Cds (CDP-diacylglycerol synthase) 1 and 2. Gene. 2005;356:19–31. doi: 10.1016/j.gene.2005.04.037. [DOI] [PubMed] [Google Scholar]
- 133.Antonsson B. Phosphatidylinositol synthase from mammalian tissues. Biochim Biophys Acta. 1997;1348(1–2):179–86. doi: 10.1016/s0005-2760(97)00105-7. [DOI] [PubMed] [Google Scholar]
- 134.Spyridakis S, et al. A specific phospholipase C activity regulates phosphatidylinositol levels in lung surfactant of patients with acute respiratory distress syndrome. Am J Respir Cell Mol Biol. 2010;42(3):357–62. doi: 10.1165/rcmb.2009-0078OC. [DOI] [PubMed] [Google Scholar]
- 135.Suh PG, et al. Multiple roles of phosphoinositide-specific phospholipase C isozymes. BMB Rep. 2008;41(6):415–34. doi: 10.5483/bmbrep.2008.41.6.415. [DOI] [PubMed] [Google Scholar]
- 136.Ramadurai SM, et al. Cell-specific and developmental expression of phospholipase C-gamma and diacylglycerol in fetal lung. Am J Physiol Lung Cell Mol Physiol. 2003;284(5):L808–16. doi: 10.1152/ajplung.00117.2002. [DOI] [PubMed] [Google Scholar]
- 137.Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1(1):11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 138.Ibrahim AM, Funkhouser JD. Phosphatidylinositol transfer protein in lung: cellular and subcellular localization. J Histochem Cytochem. 1997;45(4):551–8. doi: 10.1177/002215549704500407. [DOI] [PubMed] [Google Scholar]
- 139.Kooijman EE, et al. Phosphatidylinositol 4,5-bisphosphate stimulates alveolar epithelial fluid clearance in male and female adult rats. Am J Physiol Lung Cell Mol Physiol. 2011;301(5):L804–11. doi: 10.1152/ajplung.00445.2010. [DOI] [PubMed] [Google Scholar]
- 140.Perez-Gil J, et al. Interfacial adsorption of simple lipid mixtures combined with hydrophobic surfactant protein from pig lung. Biochem Cell Biol. 1992;70(5):332–8. doi: 10.1139/o92-051. [DOI] [PubMed] [Google Scholar]
- 141.Casals C. Role of surfactant protein A (SP-A)/lipid interactions for SP-A functions in the lung. Pediatr Pathol Mol Med. 2001;20(4):249–68. [PubMed] [Google Scholar]
- 142.Stone SJ, Vance JE. Phosphatidylserine synthase-1 and -2 are localized to mitochondria-associated membranes. J Biol Chem. 2000;275(44):34534–40. doi: 10.1074/jbc.M002865200. [DOI] [PubMed] [Google Scholar]
- 143.Vance JE, Steenbergen R. Metabolism and functions of phosphatidylserine. Prog Lipid Res. 2005;44(4):207–34. doi: 10.1016/j.plipres.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 144.Vance JE. Phosphatidylserine and phosphatidylethanolamine in mammalian cells: two metabolically related aminophospholipids. J Lipid Res. 2008;49(7):1377–87. doi: 10.1194/jlr.R700020-JLR200. [DOI] [PubMed] [Google Scholar]
- 145.Kuge O, Nishijima M. Biosynthetic regulation and intracellular transport of phosphatidylserine in mammalian cells. J Biochem. 2003;133(4):397–403. doi: 10.1093/jb/mvg052. [DOI] [PubMed] [Google Scholar]
- 146.Bergo MO, et al. Defining the importance of phosphatidylserine synthase 2 in mice. J Biol Chem. 2002;277(49):47701–8. doi: 10.1074/jbc.M207734200. [DOI] [PubMed] [Google Scholar]
- 147.Calderon C, et al. Isolation of a nitric oxide inhibitor from mammary tumor cells and its characterization as phosphatidyl serine. J Exp Med. 1994;180(3):945–58. doi: 10.1084/jem.180.3.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sakane F, et al. Porcine 80-kDa diacylglycerol kinase is a calcium-binding and calcium/phospholipid-dependent enzyme and undergoes calcium-dependent translocation. J Biol Chem. 1991;266(11):7096–100. [PubMed] [Google Scholar]
- 149.Ghosh S, et al. The cysteine-rich region of raf-1 kinase contains zinc, translocates to liposomes, and is adjacent to a segment that binds GTP-ras. J Biol Chem. 1994;269(13):10000–7. [PubMed] [Google Scholar]
- 150.Gagne J, et al. Effect of phosphatidylserine on the binding properties of glutamate receptors in brain sections from adult and neonatal rats. Brain Res. 1996;740(1–2):337–45. doi: 10.1016/s0006-8993(96)00897-9. [DOI] [PubMed] [Google Scholar]
- 151.Mozzi R, Buratta S, Goracci G. Metabolism and functions of phosphatidylserine in mammalian brain. Neurochem Res. 2003;28(2):195–214. doi: 10.1023/a:1022412831330. [DOI] [PubMed] [Google Scholar]
- 152.Li MO, et al. Phosphatidylserine receptor is required for clearance of apoptotic cells. Science. 2003;302(5650):1560–3. doi: 10.1126/science.1087621. [DOI] [PubMed] [Google Scholar]
- 153.Bose J, et al. The phosphatidylserine receptor has essential functions during embryogenesis but not in apoptotic cell removal. J Biol. 2004;3(4):15. doi: 10.1186/jbiol10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Jakel A, Reid KB, Clark H. Surfactant protein A (SP-A) binds to phosphatidylserine and competes with annexin V binding on late apoptotic cells. Protein Cell. 2010;1(2):188–97. doi: 10.1007/s13238-010-0024-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Nagai Y, et al. An alternative splicing form of phosphatidylserine-specific phospholipase A1 that exhibits lysophosphatidylserine-specific lysophospholipase activity in humans. J Biol Chem. 1999;274(16):11053–9. doi: 10.1074/jbc.274.16.11053. [DOI] [PubMed] [Google Scholar]
- 156.Aoki J, et al. Structure and function of phosphatidylserine-specific phospholipase A1. Biochim Biophys Acta. 2002;1582(1–3):26–32. doi: 10.1016/s1388-1981(02)00134-8. [DOI] [PubMed] [Google Scholar]
- 157.Samuels ER, Scott JE. Ca(+2)-phosphatidylserine-dependent protein kinase C activity in fetal, neonatal and adult rabbit lung and isolated lamellar bodies. Life Sci. 1995;57(17):1557–68. doi: 10.1016/0024-3205(95)02131-2. [DOI] [PubMed] [Google Scholar]
- 158.Schlame M. Cardiolipin synthesis for the assembly of bacterial and mitochondrial membranes. J Lipid Res. 2008;49(8):1607–20. doi: 10.1194/jlr.R700018-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Houtkooper RH, Vaz FM. Cardiolipin, the heart of mitochondrial metabolism. Cell Mol Life Sci. 2008;65(16):2493–506. doi: 10.1007/s00018-008-8030-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Houtkooper RH, et al. Identification and characterization of human cardiolipin synthase. FEBS Lett. 2006;580(13):3059–64. doi: 10.1016/j.febslet.2006.04.054. [DOI] [PubMed] [Google Scholar]
- 161.Lu B, et al. Cloning and characterization of a cDNA encoding human cardiolipin synthase (hCLS1) J Lipid Res. 2006;47(6):1140–5. doi: 10.1194/jlr.C600004-JLR200. [DOI] [PubMed] [Google Scholar]
- 162.Chen D, Zhang XY, Shi Y. Identification and functional characterization of hCLS1, a human cardiolipin synthase localized in mitochondria. Biochem J. 2006;398(2):169–76. doi: 10.1042/BJ20060303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Xu Y, et al. The enzymatic function of tafazzin. J Biol Chem. 2006;281(51):39217–24. doi: 10.1074/jbc.M606100200. [DOI] [PubMed] [Google Scholar]
- 164.Rooney SA, Young SL, Mendelson CR. Molecular and cellular processing of lung surfactant. FASEB J. 1994;8(12):957–67. doi: 10.1096/fasebj.8.12.8088461. [DOI] [PubMed] [Google Scholar]
- 165.Ray NB, et al. Dynamic regulation of cardiolipin by the lipid pump Atp8b1 determines the severity of lung injury in experimental pneumonia. Nat Med. 2010;16(10):1120–7. doi: 10.1038/nm.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Merrill AH, Jr, Jones DD. An update of the enzymology and regulation of sphingomyelin metabolism. Biochim Biophys Acta. 1990;1044(1):1–12. doi: 10.1016/0005-2760(90)90211-f. [DOI] [PubMed] [Google Scholar]
- 167.Longo CA, Tyler D, Mallampalli RK. Sphingomyelin metabolism is developmentally regulated in rat lung. Am J Respir Cell Mol Biol. 1997;16(5):605–12. doi: 10.1165/ajrcmb.16.5.9160843. [DOI] [PubMed] [Google Scholar]
- 168.Merrill AH, Jr, Nixon DW, Williams RD. Activities of serine palmitoyltransferase (3-ketosphinganine synthase) in microsomes from different rat tissues. J Lipid Res. 1985;26(5):617–22. [PubMed] [Google Scholar]
- 169.Gowda S, et al. Sphingomyelin synthase 2 (SMS2) deficiency attenuates LPS-induced lung injury. Am J Physiol Lung Cell Mol Physiol. 2011;300(3):L430–40. doi: 10.1152/ajplung.00208.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Gregory TJ, et al. Surfactant chemical composition and biophysical activity in acute respiratory distress syndrome. J Clin Invest. 1991;88(6):1976–81. doi: 10.1172/JCI115523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.McCormack FX, et al. Idiopathic pulmonary fibrosis. Abnormalities in the bronchoalveolar lavage content of surfactant protein A. Am Rev Respir Dis. 1991;144(1):160–6. doi: 10.1164/ajrccm/144.1.160. [DOI] [PubMed] [Google Scholar]
- 172.Uhlig S, Gulbins E. Sphingolipids in the lungs. Am J Respir Crit Care Med. 2008;178(11):1100–14. doi: 10.1164/rccm.200804-595SO. [DOI] [PubMed] [Google Scholar]
- 173.Spence MW, Beed S, Cook HW. Acid and alkaline ceramidases of rat tissues. Biochem Cell Biol. 1986;64(5):400–4. doi: 10.1139/o86-056. [DOI] [PubMed] [Google Scholar]
- 174.Rauvala H, Hallman M. Glycolipid accumulation in bronchoalveolar space in adult respiratory distress syndrome. J Lipid Res. 1984;25(11):1257–62. [PubMed] [Google Scholar]
- 175.Obeid LM, Hannun YA. Ceramide: a stress signal and mediator of growth suppression and apoptosis. J Cell Biochem. 1995;58(2):191–8. doi: 10.1002/jcb.240580208. [DOI] [PubMed] [Google Scholar]
- 176.Lin WC, et al. Inhibition of neutrophil apoptosis via sphingolipid signaling in acute lung injury. J Pharmacol Exp Ther. 2011;339(1):45–53. doi: 10.1124/jpet.111.181560. [DOI] [PubMed] [Google Scholar]
- 177.Petrache I, et al. Ceramide upregulation causes pulmonary cell apoptosis and emphysema-like disease in mice. Nat Med. 2005;11(5):491–8. doi: 10.1038/nm1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.McCormack FX, et al. The Cys6 intermolecular disulfide bond and the collagen-like region of rat SP-A play critical roles in interactions with alveolar type II cells and surfactant lipids. J Biol Chem. 1997;272(44):27971–9. doi: 10.1074/jbc.272.44.27971. [DOI] [PubMed] [Google Scholar]
- 179.Hawgood S, Shiffer K. Structures and properties of the surfactant-associated proteins. Annu Rev Physiol. 1991;53:375–94. doi: 10.1146/annurev.ph.53.030191.002111. [DOI] [PubMed] [Google Scholar]
- 180.Kuroki Y, Mason RJ, Voelker DR. Alveolar type II cells express a high-affinity receptor for pulmonary surfactant protein A. Proc Natl Acad Sci U S A. 1988;85(15):5566–70. doi: 10.1073/pnas.85.15.5566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Wright JR, Dobbs LG. Regulation of pulmonary surfactant secretion and clearance. Annu Rev Physiol. 1991;53:395–414. doi: 10.1146/annurev.ph.53.030191.002143. [DOI] [PubMed] [Google Scholar]
- 182.Wright JR. Host defense functions of pulmonary surfactant. Biol Neonate. 2004;85(4):326–32. doi: 10.1159/000078172. [DOI] [PubMed] [Google Scholar]
- 183.Chabot S, et al. Inhibitory effects of surfactant protein A on surfactant phospholipid hydrolysis by secreted phospholipases A2. J Immunol. 2003;171(2):995–1000. doi: 10.4049/jimmunol.171.2.995. [DOI] [PubMed] [Google Scholar]
- 184.Odom MJ, et al. Glucocorticoid regulation of the major surfactant associated protein (SP-A) and its messenger ribonucleic acid and of morphological development of human fetal lung in vitro. Endocrinology. 1988;123(4):1712–20. doi: 10.1210/endo-123-4-1712. [DOI] [PubMed] [Google Scholar]
- 185.deMello DE, et al. Ultrastructure of lung in surfactant protein B deficiency. Am J Respir Cell Mol Biol. 1994;11(2):230–9. doi: 10.1165/ajrcmb.11.2.8049084. [DOI] [PubMed] [Google Scholar]
- 186.Scott JE. The pulmonary surfactant: impact of tobacco smoke and related compounds on surfactant and lung development. Tob Induc Dis. 2004;2(1):3–25. doi: 10.1186/1617-9625-2-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Beers MF, et al. Pulmonary surfactant metabolism in infants lacking surfactant protein B. Am J Respir Cell Mol Biol. 2000;22(3):380–91. doi: 10.1165/ajrcmb.22.3.3645. [DOI] [PubMed] [Google Scholar]
- 188.Ogasawara Y, Kuroki Y, Akino T. Pulmonary surfactant protein D specifically binds to phosphatidylinositol. J Biol Chem. 1992;267(29):21244–9. [PubMed] [Google Scholar]
- 189.Ikegami M, et al. SP-D and GM-CSF regulate surfactant homeostasis via distinct mechanisms. Am J Physiol Lung Cell Mol Physiol. 2001;281(3):L697–703. doi: 10.1152/ajplung.2001.281.3.L697. [DOI] [PubMed] [Google Scholar]
- 190.Hogenkamp A, et al. Effects of surfactant protein D on growth, adhesion and epithelial invasion of intestinal Gram-negative bacteria. Mol Immunol. 2007;44(14):3517–27. doi: 10.1016/j.molimm.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 191.Ishii T, et al. Involvement of surfactant protein D in emphysema revealed by genetic association study. Eur J Hum Genet. 2012;20(2):230–5. doi: 10.1038/ejhg.2011.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Weaver TE, Na CL, Stahlman M. Biogenesis of lamellar bodies, lysosome-related organelles involved in storage and secretion of pulmonary surfactant. Semin Cell Dev Biol. 2002;13(4):263–70. doi: 10.1016/s1084952102000551. [DOI] [PubMed] [Google Scholar]
- 193.Ridsdale R, Post M. Surfactant lipid synthesis and lamellar body formation in glycogen-laden type II cells. Am J Physiol Lung Cell Mol Physiol. 2004;287(4):L743–51. doi: 10.1152/ajplung.00146.2004. [DOI] [PubMed] [Google Scholar]
- 194.Ridsdale R, et al. Comparative proteomic analysis of lung lamellar bodies and lysosome-related organelles. PLoS One. 2011;6(1):e16482. doi: 10.1371/journal.pone.0016482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Yayoi Y, et al. Specific localization of lysosomal aminopeptidases in type II alveolar epithelial cells of the rat lung. Arch Histol Cytol. 2001;64(1):89–97. doi: 10.1679/aohc.64.89. [DOI] [PubMed] [Google Scholar]
- 196.Wasano K, Hirakawa Y. Lamellar bodies of rat alveolar type 2 cells have late endosomal marker proteins on their limiting membranes. Histochemistry. 1994;102(5):329–35. doi: 10.1007/BF00268903. [DOI] [PubMed] [Google Scholar]
- 197.Stahlman MT, et al. Lamellar body formation in normal and surfactant protein B-deficient fetal mice. Lab Invest. 2000;80(3):395–403. doi: 10.1038/labinvest.3780044. [DOI] [PubMed] [Google Scholar]
- 198.Clark JC, et al. Targeted disruption of the surfactant protein B gene disrupts surfactant homeostasis, causing respiratory failure in newborn mice. Proc Natl Acad Sci U S A. 1995;92(17):7794–8. doi: 10.1073/pnas.92.17.7794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Fitzgerald ML, et al. ABCA3 inactivation in mice causes respiratory failure, loss of pulmonary surfactant, and depletion of lung phosphatidylglycerol. J Lipid Res. 2007;48(3):621–32. doi: 10.1194/jlr.M600449-JLR200. [DOI] [PubMed] [Google Scholar]
- 200.Osanai K, Mason RJ, Voelker DR. Pulmonary surfactant phosphatidylcholine transport bypasses the brefeldin A sensitive compartment of alveolar type II cells. Biochim Biophys Acta. 2001;1531(3):222–9. doi: 10.1016/s1388-1981(01)00104-4. [DOI] [PubMed] [Google Scholar]
- 201.Lajoie P, et al. The lipid composition of autophagic vacuoles regulates expression of multilamellar bodies. J Cell Sci. 2005;118(Pt 9):1991–2003. doi: 10.1242/jcs.02324. [DOI] [PubMed] [Google Scholar]
- 202.Cheong N, et al. Functional and trafficking defects in ATP binding cassette A3 mutants associated with respiratory distress syndrome. J Biol Chem. 2006;281(14):9791–800. doi: 10.1074/jbc.M507515200. [DOI] [PubMed] [Google Scholar]
- 203.Kaltenborn E, et al. Respiratory syncytial virus potentiates ABCA3 mutation-induced loss of lung epithelial cell differentiation. Hum Mol Genet. 2012 doi: 10.1093/hmg/dds107. [DOI] [PubMed] [Google Scholar]
- 204.Matsumura Y, et al. Characterization and classification of ATP-binding cassette transporter ABCA3 mutants in fatal surfactant deficiency. J Biol Chem. 2006;281(45):34503–14. doi: 10.1074/jbc.M600071200. [DOI] [PubMed] [Google Scholar]
- 205.Besnard V, et al. Conditional deletion of Abca3 in alveolar type II cells alters surfactant homeostasis in newborn and adult mice. Am J Physiol Lung Cell Mol Physiol. 2010;298(5):L646–59. doi: 10.1152/ajplung.00409.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Kaltenborn E, et al. Respiratory syncytial virus potentiates ABCA3 mutation-induced loss of lung epithelial cell differentiation. Hum Mol Genet. 2012;21(12):2793–806. doi: 10.1093/hmg/dds107. [DOI] [PubMed] [Google Scholar]
- 207.Zhou J, et al. Upregulation of surfactant synthesis triggers ABCA1-mediated basolateral phospholipid efflux. J Lipid Res. 2004;45(9):1758–67. doi: 10.1194/jlr.M400179-JLR200. [DOI] [PubMed] [Google Scholar]
- 208.Agassandian M, et al. Oxysterols trigger ABCA1-mediated basolateral surfactant efflux. Am J Respir Cell Mol Biol. 2004;31(2):227–33. doi: 10.1165/rcmb.2004-0038OC. [DOI] [PubMed] [Google Scholar]
- 209.Mishra A, et al. Purinergic P2X7 receptor regulates lung surfactant secretion in a paracrine manner. J Cell Sci. 2011;124(Pt 4):657–68. doi: 10.1242/jcs.066977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Dietl P, Haller T. Exocytosis of lung surfactant: from the secretory vesicle to the air-liquid interface. Annu Rev Physiol. 2005;67:595–621. doi: 10.1146/annurev.physiol.67.040403.102553. [DOI] [PubMed] [Google Scholar]
- 211.Rooney SA. Regulation of surfactant secretion. Comp Biochem Physiol A Mol Integr Physiol. 2001;129(1):233–43. doi: 10.1016/s1095-6433(01)00320-8. [DOI] [PubMed] [Google Scholar]
- 212.Chander A, et al. Protein kinase C in ATP regulation of lung surfactant secretion in type II cells. Am J Physiol. 1995;268(1 Pt 1):L108–16. doi: 10.1152/ajplung.1995.268.1.L108. [DOI] [PubMed] [Google Scholar]
- 213.Ashino Y, et al. [Ca(2+)](i) oscillations regulate type II cell exocytosis in the pulmonary alveolus. Am J Physiol Lung Cell Mol Physiol. 2000;279(1):L5–13. doi: 10.1152/ajplung.2000.279.1.L5. [DOI] [PubMed] [Google Scholar]
- 214.Sohma H, et al. Binding of annexins to lung lamellar bodies and the PMA-stimulated secretion of annexin V from alveolar type II cells. J Biochem. 2001;130(3):449–55. doi: 10.1093/oxfordjournals.jbchem.a003005. [DOI] [PubMed] [Google Scholar]
- 215.Singh TK, et al. Reorganization of cytoskeleton during surfactant secretion in lung type II cells: a role of annexin II. Cell Signal. 2004;16(1):63–70. doi: 10.1016/s0898-6568(03)00089-5. [DOI] [PubMed] [Google Scholar]
- 216.Chander A, Chen XL, Naidu DG. A role for diacylglycerol in annexin A7-mediated fusion of lung lamellar bodies. Biochim Biophys Acta. 2007;1771(10):1308–18. doi: 10.1016/j.bbalip.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Gerelsaikhan T, Chen XL, Chander A. Secretagogues of lung surfactant increase annexin A7 localization with ABCA3 in alveolar type II cells. Biochim Biophys Acta. 2011;1813(12):2017–25. doi: 10.1016/j.bbamcr.2011.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Chintagari NR, Gou D, Liu L. Knockdown of flotillin-2 inhibits lung surfactant secretion by alveolar type II cells. Cell Res. 2008;18(6):701–3. doi: 10.1038/cr.2008.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Korutla L, Strayer DS. SP-A as a cytokine: surfactant protein-A-regulated transcription of surfactant proteins and other genes. J Cell Physiol. 1999;178(3):379–86. doi: 10.1002/(SICI)1097-4652(199903)178:3<379::AID-JCP12>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 220.White MK, Strayer DS. Survival signaling in type II pneumocytes activated by surfactant protein-A. Exp Cell Res. 2002;280(2):270–9. doi: 10.1006/excr.2002.5646. [DOI] [PubMed] [Google Scholar]
- 221.Chintagari NR, et al. Vacuolar ATPase regulates surfactant secretion in rat alveolar type II cells by modulating lamellar body calcium. PLoS One. 2010;5(2):e9228. doi: 10.1371/journal.pone.0009228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Zhang H, et al. Micro-RNA-375 inhibits lung surfactant secretion by altering cytoskeleton reorganization. IUBMB Life. 2010;62(1):78–83. doi: 10.1002/iub.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Schmitz G, Muller G. Structure and function of lamellar bodies, lipid-protein complexes involved in storage and secretion of cellular lipids. J Lipid Res. 1991;32(10):1539–70. [PubMed] [Google Scholar]
- 224.Gupta N, et al. Identification and characterization of p63 (CKAP4/ERGIC-63/CLIMP-63), a surfactant protein A binding protein, on type II pneumocytes. Am J Physiol Lung Cell Mol Physiol. 2006;291(3):L436–46. doi: 10.1152/ajplung.00415.2005. [DOI] [PubMed] [Google Scholar]
- 225.Jain D, et al. Pathways for clearance of surfactant protein A from the lung. Am J Physiol Lung Cell Mol Physiol. 2005;289(6):L1011–8. doi: 10.1152/ajplung.00250.2005. [DOI] [PubMed] [Google Scholar]
- 226.Wissel H, et al. Endocytosed SP-A and surfactant lipids are sorted to different organelles in rat type II pneumocytes. Am J Physiol Lung Cell Mol Physiol. 2001;281(2):L345–60. doi: 10.1152/ajplung.2001.281.2.L345. [DOI] [PubMed] [Google Scholar]
- 227.Bates SR, et al. Lamellar body membrane turnover is stimulated by secretagogues. Am J Physiol Lung Cell Mol Physiol. 2000;278(3):L443–52. doi: 10.1152/ajplung.2000.278.3.L443. [DOI] [PubMed] [Google Scholar]
- 228.Abe A, et al. Lysosomal phospholipase A2 is selectively expressed in alveolar macrophages. J Biol Chem. 2004;279(41):42605–11. doi: 10.1074/jbc.M407834200. [DOI] [PubMed] [Google Scholar]
- 229.Hiraoka M, et al. Lysosomal phospholipase A2 and phospholipidosis. Mol Cell Biol. 2006;26(16):6139–48. doi: 10.1128/MCB.00627-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Ikegami M. Surfactant catabolism. Respirology. 2006;11(Suppl):S24–7. doi: 10.1111/j.1440-1843.2006.00803.x. [DOI] [PubMed] [Google Scholar]
- 231.Poelma DL, et al. A common pathway for the uptake of surfactant lipids by alveolar cells. Am J Respir Cell Mol Biol. 2004;30(5):751–8. doi: 10.1165/rcmb.2003-0127OC. [DOI] [PubMed] [Google Scholar]
- 232.Poelma DL, et al. Distinct effects of SP-B and SP-C on the uptake of surfactant-like liposomes by alveolar cells in vivo and in vitro. Am J Physiol Lung Cell Mol Physiol. 2004;287(5):L1056–65. doi: 10.1152/ajplung.00054.2004. [DOI] [PubMed] [Google Scholar]
- 233.Martini WZ, et al. Lung surfactant kinetics in conscious pigs. Am J Physiol. 1999;277(1 Pt 1):E187–95. doi: 10.1152/ajpendo.1999.277.1.E187. [DOI] [PubMed] [Google Scholar]
- 234.Jacobs HC, et al. Reutilization of surfactant phosphatidylcholine in adult rabbits. Biochim Biophys Acta. 1985;837(1):77–84. doi: 10.1016/0005-2760(85)90087-6. [DOI] [PubMed] [Google Scholar]
- 235.Baker AD, et al. Targeted PPAR{gamma} deficiency in alveolar macrophages disrupts surfactant catabolism. J Lipid Res. 2010;51(6):1325–31. doi: 10.1194/jlr.M001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Malur A, et al. Restoration of PPARgamma reverses lipid accumulation in alveolar macrophages of GM-CSF knockout mice. Am J Physiol Lung Cell Mol Physiol. 2011;300(1):L73–80. doi: 10.1152/ajplung.00128.2010. [DOI] [PubMed] [Google Scholar]
- 237.De Luca D, Capoluongo E, Rigo V. Secretory phospholipase A2 pathway in various types of lung injury in neonates and infants: a multicentre translational study. BMC Pediatr. 2011;11:101. doi: 10.1186/1471-2431-11-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Metcalfe IL, Enhorning G, Possmayer F. Pulmonary surfactant-associated proteins: their role in the expression of surface activity. J Appl Physiol. 1980;49(1):34–41. doi: 10.1152/jappl.1980.49.1.34. [DOI] [PubMed] [Google Scholar]
- 239.Williams MC. Ultrastructure of tubular myelin and lamellar bodies in fast-frozen adult rat lung. Exp Lung Res. 1982;4(1):37–46. doi: 10.3109/01902148209039248. [DOI] [PubMed] [Google Scholar]
- 240.Brackenbury AM, et al. Evaluation of alveolar surfactant aggregates in vitro and in vivo. Eur Respir J. 2002;19(1):41–6. doi: 10.1183/09031936.02.00211202. [DOI] [PubMed] [Google Scholar]
- 241.Wustneck R, et al. Interfacial properties of pulmonary surfactant layers. Adv Colloid Interface Sci. 2005;117(1–3):33–58. doi: 10.1016/j.cis.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 242.Gross NJ. Extracellular metabolism of pulmonary surfactant: the role of a new serine protease. Annu Rev Physiol. 1995;57:135–50. doi: 10.1146/annurev.ph.57.030195.001031. [DOI] [PubMed] [Google Scholar]
- 243.Griese M, Birrer P, Demirsoy A. Pulmonary surfactant in cystic fibrosis. Eur Respir J. 1997;10(9):1983–8. doi: 10.1183/09031936.97.10091983. [DOI] [PubMed] [Google Scholar]
- 244.Lewis JF, Jobe AH. Surfactant and the adult respiratory distress syndrome. Am Rev Respir Dis. 1993;147(1):218–33. doi: 10.1164/ajrccm/147.1.218. [DOI] [PubMed] [Google Scholar]
- 245.Ballard PL. Hormones and lung maturation. Monogr Endocrinol. 1986;28:1–354. [PubMed] [Google Scholar]
- 246.Post M, van Golde LM. Metabolic and developmental aspects of the pulmonary surfactant system. Biochim Biophys Acta. 1988;947(2):249–86. doi: 10.1016/0304-4157(88)90011-1. [DOI] [PubMed] [Google Scholar]
- 247.Bolt RJ, et al. Glucocorticoids and lung development in the fetus and preterm infant. Pediatr Pulmonol. 2001;32(1):76–91. doi: 10.1002/ppul.1092. [DOI] [PubMed] [Google Scholar]
- 248.Samtani MN, et al. Modeling glucocorticoid-mediated fetal lung maturation: I. Temporal patterns of corticosteroids in rat pregnancy. J Pharmacol Exp Ther. 2006;317(1):117–26. doi: 10.1124/jpet.105.095851. [DOI] [PubMed] [Google Scholar]
- 249.Archavachotikul K, et al. Thyroid hormone affects embryonic mouse lung branching morphogenesis and cellular differentiation. Am J Physiol Lung Cell Mol Physiol. 2002;282(3):L359–69. doi: 10.1152/ajplung.00400.2000. [DOI] [PubMed] [Google Scholar]
- 250.Patrone C, et al. Regulation of postnatal lung development and homeostasis by estrogen receptor beta. Mol Cell Biol. 2003;23(23):8542–52. doi: 10.1128/MCB.23.23.8542-8552.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Weinberg OK, et al. Aromatase inhibitors in human lung cancer therapy. Cancer Res. 2005;65(24):11287–91. doi: 10.1158/0008-5472.CAN-05-2737. [DOI] [PubMed] [Google Scholar]
- 252.Martini WZ, et al. Glucose effects on lung surfactant kinetics in conscious pigs. Am J Physiol Endocrinol Metab. 2000;279(4):E920–6. doi: 10.1152/ajpendo.2000.279.4.E920. [DOI] [PubMed] [Google Scholar]
- 253.Mendelson CR, Boggaram V. Hormonal and developmental regulation of pulmonary surfactant synthesis in fetal lung. Baillieres Clin Endocrinol Metab. 1990;4(2):351–78. doi: 10.1016/s0950-351x(05)80055-2. [DOI] [PubMed] [Google Scholar]
- 254.Westover AJ, et al. Prostaglandins mediate the fetal pulmonary response to intrauterine inflammation. Am J Physiol Lung Cell Mol Physiol. 2012;302(7):L664–78. doi: 10.1152/ajplung.00297.2011. [DOI] [PubMed] [Google Scholar]
- 255.Zscheppang K, et al. ErbB4 regulates fetal surfactant phospholipid synthesis in primary fetal rat type II cells. Am J Physiol Lung Cell Mol Physiol. 2007;293(2):L429–35. doi: 10.1152/ajplung.00451.2006. [DOI] [PubMed] [Google Scholar]
- 256.Modanlou HD, et al. Comparative efficacy of exosurf and survanta surfactants on early clinical course of respiratory distress syndrome and complications of prematurity. J Perinatol. 1997;17(6):455–60. [PubMed] [Google Scholar]
- 257.Cogo PE, et al. Surfactant disaturated-phosphatidylcholine kinetics in acute respiratory distress syndrome by stable isotopes and a two compartment model. Respir Res. 2007;8:13. doi: 10.1186/1465-9921-8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Lauer S, et al. Value of surfactant replacement therapy in the treatment of acute respiratory distress syndrome. Anaesthesist. 2006;55(4):433–42. doi: 10.1007/s00101-006-0978-7. [DOI] [PubMed] [Google Scholar]
- 259.Kurashima K, et al. A pilot study of surfactant inhalation in the treatment of asthmatic attack. Arerugi - Japanese Journal of Allergology. 1991;40(2):160–3. [PubMed] [Google Scholar]
- 260.Ksenzenko SM, et al. Effect of triiodothyronine augmentation on rat lung surfactant phospholipids during sepsis. J Appl Physiol. 1997;82(6):2020–7. doi: 10.1152/jappl.1997.82.6.2020. [DOI] [PubMed] [Google Scholar]
- 261.Velasquez A, et al. Glutathione replacement preserves the functional surfactant phospholipid pool size and decreases sepsis-mediated lung dysfunction in ethanol-fed rats. Alcohol Clin Exp Res. 2002;26(8):1245–51. doi: 10.1097/01.ALC.0000024269.05402.97. [DOI] [PubMed] [Google Scholar]
- 262.Hull J, et al. Surfactant composition in infants and young children with cystic fibrosis. American Journal of Respiratory & Critical Care Medicine. 1997;156(1):161–5. doi: 10.1164/ajrccm.156.1.9609090. [DOI] [PubMed] [Google Scholar]
- 263.Alcorn JF, Wright JR. Degradation of pulmonary surfactant protein D by Pseudomonas aeruginosa elastase abrogates innate immune function. J Biol Chem. 2004;279(29):30871–9. doi: 10.1074/jbc.M400796200. [DOI] [PubMed] [Google Scholar]
- 264.Wu Y, et al. Chronic Pseudomonas aeruginosa infection reduces surfactant levels by inhibiting its biosynthesis. Cell Microbiol. 2007;9(4):1062–72. doi: 10.1111/j.1462-5822.2006.00852.x. [DOI] [PubMed] [Google Scholar]
- 265.Henderson FC, Miakotina OL, Mallampalli RK. Proapoptotic effects of P. aeruginosa involve inhibition of surfactant phosphatidylcholine synthesis. J Lipid Res. 2006;47(10):2314–24. doi: 10.1194/jlr.M600284-JLR200. [DOI] [PubMed] [Google Scholar]
- 266.Miakotina OL, et al. Human adenovirus modulates surfactant phospholipid trafficking. Traffic. 2007;8(12):1765–77. doi: 10.1111/j.1600-0854.2007.00641.x. [DOI] [PubMed] [Google Scholar]
- 267.Glasser JR, Mallampalli RK. Surfactant and its role in the pathobiology of pulmonary infection. Microbes Infect. 2012;14(1):17–25. doi: 10.1016/j.micinf.2011.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Perino J, et al. Lung surfactant DPPG phospholipid inhibits vaccinia virus infection. Antiviral Res. 2011;89(1):89–97. doi: 10.1016/j.antiviral.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 269.Nogee LM. Alterations in SP-B and SP-C expression in neonatal lung disease. Annu Rev Physiol. 2004;66:601–23. doi: 10.1146/annurev.physiol.66.032102.134711. [DOI] [PubMed] [Google Scholar]
- 270.Nogee LM, et al. Mutations in the surfactant protein C gene associated with interstitial lung disease. Chest. 2002;121(3 Suppl):20S–21S. doi: 10.1378/chest.121.3_suppl.20s. [DOI] [PubMed] [Google Scholar]
- 271.Nerelius C, et al. Mutations linked to interstitial lung disease can abrogate anti-amyloid function of prosurfactant protein C. Biochem J. 2008;416(2):201–9. doi: 10.1042/BJ20080981. [DOI] [PubMed] [Google Scholar]
- 272.Zarbock R, et al. The surfactant protein C mutation A116D alters cellular processing, stress tolerance, surfactant lipid composition, and immune cell activation. BMC Pulm Med. 2012;12:15. doi: 10.1186/1471-2466-12-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Whitsett JA. Genetic disorders of surfactant homeostasis. Paediatr Respir Rev. 2006;7(Suppl 1):S240–2. doi: 10.1016/j.prrv.2006.04.191. [DOI] [PubMed] [Google Scholar]