Abstract
Transport of phospholipids across cell membranes plays a key role in a wide variety of biological processes. These include membrane biosynthesis, generation and maintenance of membrane asymmetry, cell and organelle shape determination, phagocytosis, vesicle tranfficking, blood coagulation, lipid homeostasis, regulation of membrane protein function, apoptosis among others. P4-ATPases and ATP binding cassette (ABC) transporters are the two principal classes of membranes proteins that actively transport phospholipids across cellular membranes. P4-ATPases utilize the energy from ATP hydrolysis to flip aminophospholipids from the exocytoplasmic (extracellular/lumen) to the cytoplasmic leaflet of cell membranes generating membrane lipid asymmetry and lipid imbalance which can induce membrane curvature. Many ABC transporters play crucial roles in lipid homeostasis by actively transporting phospholipids from the cytoplasmic to the exocytoplasmic leaflet of cell membranes or exporting phospholipids to protein acceptors or micelles. Recent studies indicate that some ABC proteins can also transport phospholipids in the opposite direction. The importance of P4-ATPases and ABC transporters is evident from the findings that mutations in many of these transporters are responsible for severe human genetic diseases linked to defective phospholipid transport.
1. Introduction
Transport of phospholipids across cell membranes plays a crucial role in many biological processes. Phospholipids, and more specifically glycerophospholipids, are synthesized on the cytoplasmic side of the endoplasmic reticulum (ER) membrane. Half of the newly synthesized phospholipid must be transported to the opposing lumenal leaflet in order to maintain a balanced ER membrane bilayer. In contrast, other cell membranes including late Golgi, endosomes and plasma membrane are highly asymmetrical in their bilayer lipid distribution [1, 2]. Phosphatidylserine (PS) and phosphatidylethanolamine (PE) are preferentially found on the cytoplasmic leaflet of these membranes, while phosphatidylcholine (PC), sphingomyelin (SM) and glycolipids are enriched on the extracellular or lumen leaflet. Membrane asymmetry is generated in part by the selective transport of phospholipids across cell membranes [3, 4]. Phospholipid transport is also important in generating lipid imbalance between the two leaflets which contributes to membrane bending and curvature. Lipid asymmetry and imbalance have been implicated in such biological processes as phagocytosis, fertilization, membrane budding and vesicle trafficking, modulation of membrane protein function, blood coagulation, cell and organelle shape. The transport of phospholipids across membranes is also essential for lipid homeostasis. For example, cholesterol efflux from cells, formation of bile, nutrient transport, removal of selected toxic compounds from cells, and secretion of pulmonary surfactants require phospholipid transport. Indeed, the importance of phospholipid transport in these and other processes is evident by the finding that many severe human disorders are caused by defects in phospholipid transport [5, 6] [7].
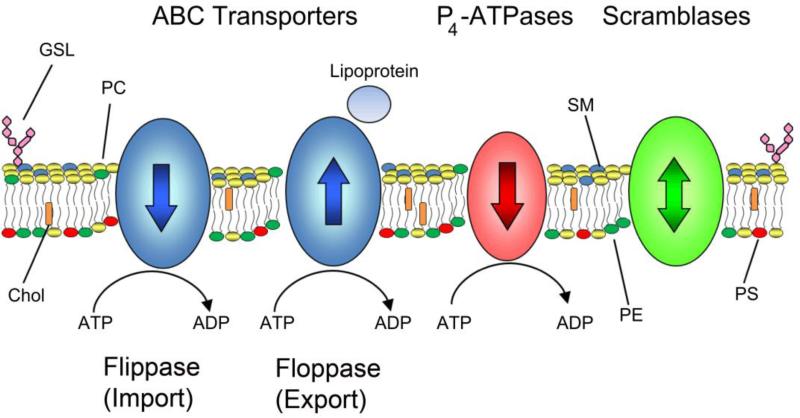
It is well-known that the phospholipid bilayer serves as an energy barrier for the free movement of hydrophilic molecules into and out of cells and subcellular organelles. As a consequence, specific transporters and channels are needed to move hydrophilic molecules across cell membranes. This is also true for amphipathic phospholipids which due to their hydrophilic, charged head group require a transporter or translocase to actively or passively facilitate their movement across membranes. Three classes of proteins have been implicated in the transport or flipping of phospholipids across cellular membranes: scramblases, P4-ATPases, and ABC transporters (Fig 1).
Fig. 1.
Regulation of lipid asymmetry in biological membranes. The distribution of lipids in biological membranes is regulated by three distinct families of membrane transporters: ABC transporters, P4-ATPases, and scramblases. Most ABC transporters catalyze the ATP-dependent transport of lipids from the cytoplasmic leaflet of the bilayer to the extracellular (lumenal) side of the membrane while P4-ATPases transport in the opposite direction. ABC transporters can efflux lipids to lipoproteins such as Apo-A1 in the case of ABCA1 or to bile micelles. Recent studies indicate that a few ABC transporters are importers or flippases transporting phospholipids in the same direction as P4-ATPases. Scramblases are energy independent transporters and act to abolish lipid asymmetry by randomizing lipid distributions. Lipids such as PC, SM, and glycolipids are found in the extracellular or lumenal leaflet while the aminophospholipids PE and PS are preferentially on the cytosolic leaflet. Abbreviations used: PC, phosphatidylcholine; SM, sphingomyelin; PE, phosphatidylethanolamine; PS, phosphatidylserine; GSL, glycosphingolipid; Chol, cholesterol.
Scramblases are energy independent, bi-directional lipid transporters. In general they have a broad substrate specificity transporting a wide variety of phospholipids and other membrane lipids across the lipid bilayer. Scramblases are important for dissipating lipid asymmetry generated during the biosynthesis of lipids in the ER and counteracting lipid asymmetry in other cell membranes to facilitate such processes as phagocytosis, apoptosis, fertilization, and blood coagulation. Scramblases in general are poorly characterized although several studies have reported the cloning and characterization of proteins which appear to function as scramblases [8-10].
P4-ATPases are a class of P-type ATPases which utilizes the energy from ATP hydrolysis to transport or flip aminophospholipids from the exocytoplasmic (extracellular/lumen) to the cytoplasmic leaflet of cellular membranes. These phospholipid flippases have been implicated in the generation and maintenance of phospholipid asymmetry found in many cellular membranes and several members have been recently linked to severe human disorders [5, 11-17].
ABC transporters comprise a large class of membrane proteins involved in the active transport of a wide variety of compounds across cell membranes. A significant number of ABC proteins are now known to transport phospholipids across cell membranes [6, 7, 18]. Most mammalian ABC transporters function as exporters or floppases transporting substrates from the cytoplasmic to the exocytoplasmic side of membranes. Recent studies, however, now indicate that some eukaryotic ABC transporters function as importers or flippases, transporting phospholipids and related derivatives lfrom the exocytoplasmic to the cytoplasmic leaflet of cells [19]. ABC proteins play a crucial role in lipid homeostasis as indicated by the finding that mutations in some ABC transporters are responsible for severe human diseases associated with defective phospholipid transport. ABC transporters may also contribute to membrane lipid asymmetry in specific biological membranes.
A number of excellent reviews have been written on membrane lipid asymmetry and its role in various cellular processes [1, 2, 4, 20, 21]. The purpose of this review is to provide an update on the molecular characterization of mammalian P4-ATPases and ABC transporters involved in phospholipid transport.
2. P-type ATPase Superfamily
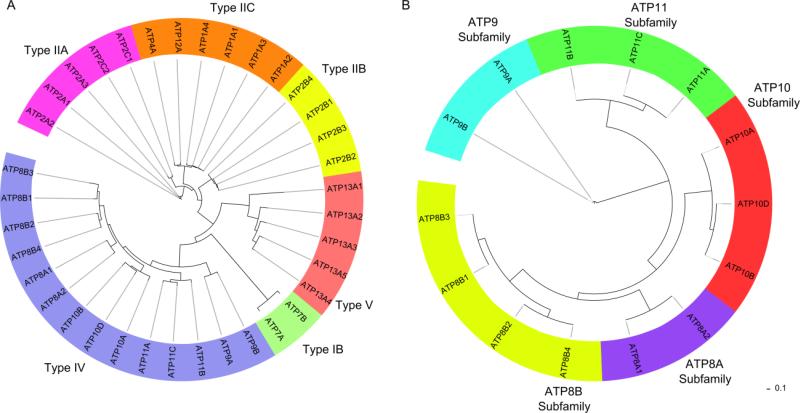
P-type ATPases comprise a large family of ion and lipid transporters (483 unique sequences, Prosite) which undergo reversible phosphorylation of the aspartic acid in the P-type signature sequence DKTGT. Interconversion of four principle conformational states termed E1, E1P, E2P, E2 (P denotes phosphorylation) drives ion translocation [22]. P-type ATPases are found in all kingdoms of life and are classified into five families based on ion specificity and sequence (Fig 2A). Type IA are bacterial K+ pumps, Type IB are heavy metal pumps, Type IIA and Type IIB are Ca2+ pumps and Type IIC are Na+/K+ or H+/K+ pumps. Type IIIA ATPases are specific to H+. Type IV and Type V ATPases are only found in eukaryotes. Type IV ATPases have recently been shown to transport phospholipids [11, 12]. The substrate specificity of Type V ATPases is unknown.
Fig. 2.
Phylogenetic tree of human P-type and P4-ATPases. (A) There are 36 different genes which encode for P-type ATPases in humans. Type IB are heavy metal pumps, Type IIA and Type IIB are Ca2+ pumps, and Type IIC are Na+/K+ or H+/K+ pumps. Type IV ATPases transport phospholipids. Type V ATPases have no assigned specificity. Type IA and Type III pumps are not found in humans. (B) There are 14 different Type IV ATPases in humans which are organized into five different subfamilies according to sequence. Trees were generated using ClustalX and visualized using the Interactive Tree of Life online tool (http://itol.embl.de/).
An extensive structural account of the reaction sequence for the Ca2+ ATPase has described many of the key events which underlie ion pumping [23]. Recent structures of the Na+,K+-ATPase, H+-ATPase, and Cu2+-ATPase have advanced our understanding of the similarities and differences between various P-type transporters of different ion specificities [24-26]. Structural information is not currently available for the Type IV and V ATPases.
3. P4-ATPases
In humans, there are 14 members of the P4-ATPase family which are organized into five subfamilies based on sequence similarity of the catalytic or α-subunit (Fig. 2B, Table 1). Most P4-ATPases are known to associate with an accessory or β-subunit known as CDC50 to form a heteromeric complex (Fig 3A). The CDC50 family consists of only three members: CDC50A, CDC50B, and CDC50C suggesting that many P4-ATPases can bind the same CDC50 β-subunit. In humans and other primates, the CDC50C gene is truncated and may result in a nonfunctional protein [27]. The interaction of various P4-ATPases with CDC50 family members have been studied primarily in cells overexpressing these proteins [28-30] (Table 1). To date there are only a few reports showing the direct interaction of mammalian P4-ATPases with CDC50 family members in normal tissues and cells [31].
Table 1.
P4-ATPase and CDC50 Families
| P4-ATPase | β-subunit | Substrate | Expression Pattern | Pathophysiology | References |
|---|---|---|---|---|---|
| ATP8A1 | CDC50A (CDC50B?) | PS, isoforms differ in PE selectivity | Ubiquitous, high in skeletal muscle, thyroid, spinal cord | Defective hippocampus-dependent learning | [15, 30, 50, 55, 66, 67] |
| ATP8A2 | CDC50A | PS, weak PE transporter | Brain, retina, testis, spinal cord | Neurological, spinal, axonal degeneration (mental retardation?) | [11, 30, 31, 68-70, 73] |
| ATP8B1 | CDC50A, CDC50B | PS, CL? | Ubiquitous, high in small intestine, pancreas | Intrahepatic cholestasis, hearing loss, pneumonia | [16, 29, 30, 50, 74, 75] |
| ATP8B2 | CDC50A, CDC50B | Unknown | Ubiquitous | Unknown | [252] |
| ATP8B3 | Unknown | PS | Testis | Defective sperm-egg interactions | [30, 82, 83] |
| ATP8B4 | CDC50A, (CDC50B?) | Unknown | Moderate levels throughout brain | Alzheimer disease? | [30, 50, 81, 253] |
| ATP9A | Not detected | Unknown | Ubiquitous, high in brain. | Unknown | [28, 254] |
| ATP9B | Not detected | Unknown | Ubiquitous, high in testis | Unknown | [28, 254] |
| ATP10A | CDC50A for ER exit | Unknown | High in brain, pancreas, kidney, lung | Angelman syndrome, obesity, diabetes | [28, 86-89, 91, 92] |
| ATP10B | CDC50A for ER exit | Unknown | Low expression, brain. | Unknown | [28, 255] |
| ATP10D | CDC50A for ER exit | Unknown | High in placenta, low kidney, undetectable in other major organs | Obesity? | [28, 90] |
| ATP11A | CDC50A | Unknown | Ubiquitous, moderate levels in liver, skeletal muscle, ovary | Unknown | [28, 256] |
| ATP11B | CDC50A | Unknown | Ubiquitous, high levels in kidney, testis, ovary | Unknown | [28, 257] |
| ATP11C | CDC50A | PS | Ubiquitous, high liver, pancreas, heart | Impaired B lymphocyte differentiation, cholestasis | [13, 28, 93, 94, 258] |
Fig. 3.
Domain structure, organization, and proposed mechanism of P4-ATPases. (A) P4-ATPases adopt a four domain structure consisting of cytosolic A (actuator), P (phosphorylation), and N (nucleotide binding) domains as well as a M (membrane) domain consisting of 10 membrane spanning segments and containing the translocation pathway. Absolutely conserved motifs are shown. P-type ATPases are phosphorylated at the aspartic acid of the invariant DKTG motif. The glutamic acid of the DGE motif in the A domain catalyzes dephosphorylation. The CDC50 β-subunit is shown containing four N-linked glycosylations and two disulfide linkages in the large E (extracellular) domain. (B) P-type ATPases exist in four primary conformations. Binding of ATP and phosphorylation of the P-domain in the E1 form converts the enzyme to E1P. During the E1P to E2P transition, the A-domain rotates allowing lipids to bind to an extracellular binding site in the M-domain in the E2P conformation. Dephosphorylation of E2P drives the translocation of the lipid through the membrane to the cytoplasmic side. The enzyme is converted back to the E1 form when the A-domain moves away from the P-domain. The role of CDC50 (green) is unknown.
3.1 Role of P4-ATPases in lipid asymmetry and vesicle trafficking
Lipid asymmetry is important for a wide variety of different physiological and cellular processes such as vesicle budding, membrane curvature, fertilization, phagocytosis, exocytosis, apoptosis, blood coagulation, regulation of the activities of membrane-associated proteins, intracellular signaling, and the maintenance of membrane integrity and impermeability [14]. One of the most widely recognized function of phosphatidylserine (PS) asymmetry occurs in apoptotic cells where exposure of PS in the outer leaflet of the plasma membrane (PM) serves as an “eat me” signal for phagocytic cells. In C. elegans, loss of the PS transporter TAT-1 leads to abnormal exposure of PS resulting in the clearance of neuronal and muscle cells [32].
Nearly all synthesis of new membranes occurs in the endoplasmic reticulum (ER). Lipids synthesized in the cytoplasmic leaflet of the ER are rapidly and symmetrically redistributed by the action of scramblases to ensure an even lipid distribution on both membrane leaflets [33]. Newly synthesized membranes and proteins travel out of the ER by budding of small vesicles which fuse with other membraneous organelles such as the Golgi complex. Lipids are sorted and further processed in the Golgi where lipid asymmetry is first generated by the combined action of flippases and floppases. Proteins are also sorted in the trans-Golgi network (TGN) and are exported to the endosomal/lysosomal pathway or sorted into cargo destined for the exocytic pathway. Clathrin dependent trafficking is a major pathway for vesicle transport from the TGN to endosomes. ARFs (ADP ribosylation factors) are GTP-binding proteins that in their active GTP-bound state regulate protein trafficking by recruiting coat proteins such as clathrin to sites of vesiculation. ArfGEFs (gaunine nucleotide exchange factors) and ArfGAPs (gaunine nucleotide activating factors) catalyze the exchange of GDP for GTP and increase the rate of Arf GTP hydrolysis, respectively.
Accumulating evidence in yeast and other cells supports a role for P4-ATPases in vesicle transport and budding, regulating many distinct protein trafficking pathways [34, 35]. Loss of TAT-1 in C. elegans results in the accumulation of large intracellular vacuoles derived from the endolysosomal pathway [36]. Loss of TAT-5, in C. elegans causes large scale shedding of extracellular vesicles disrupting cell adhesion and morphogenesis [37]. Early studies in yeast showed that knockout of Drs2p cause accumulation of abnormal membrane structures and an inability to form clathrin-coated vesicles [38, 39]. Furthermore, genetic interactions with many members of the clathrin-mediated pathway have been described. Drs2p physically interacts with ArfGEF in the Golgi [40] and binding of ArfGEF stimulates the flippase activity of Drs2p [41]. A model has been proposed for vesicle formation whereby ArfGEFs recruit activated Arfs and bind and activate P4-ATPase flippase activity thereby concentrating aminophospholipids on the cytosolic leaflet [21]. According to the bilayer couple hypothesis, a small increase in surface area of one leaflet of the bilayer and/or a decrease in the surface area of the opposing leaflet will induce bilayer curvature. Thus, expansion of the cytosolic leaflet resulting from P4-ATPase catalyzed aminophospholipid transport and the corresponding reduction in the lumenal leaflet will initiate localized membrane curvature and membrane budding. Activated-Arfs recruit coat and adaptor proteins to site of vesiculation which target the newly derived vesicles to their subcellular destinations. Evidence for P4-ATPase involvement in vesicle transport in mammalian cells is not as well understood, however, many pathophysiological features suggest that this is a conserved function for P4-ATPases across evolution. Additional experimental data is needed to more clearly define the role of P4-ATPases in mammalian vesicle trafficking mechanisms.
3.2. P4-ATPase-CDC50 Complex: Domain Structure and Mechanism
P-type ATPases associated with ion transport are integral membrane proteins which form an elongated structure consisting of four domains: a cytosolic A (“actuator”) domain, P (phosphorylation) domain, N (nucleotide binding) domain, and an M (membrane) domain consisting of ten transmembrane helices M1-10 [42]. P4-ATPases adopt a similar four domain structure based on sequence analysis (Fig. 3A). Short extracellular loops connect each transmembrane helix such that only a small portion of the structure is exposed on the lumenal or extracellular surface. The cytoplasmic C-terminal region of P4-ATPases may be involved in the regulation of phospholipid transport and/or targeting of the protein to specific cellular membranes since it displays the least sequence similarity between the various P4-ATPases. Furthermore, the C-terminal region has been shown to be a regulatory region in other P-type ATPases [43, 44].
Structural analysis of the P-ATPase ion pumps indicate that large movements of the cytosolic A-, N-, and P-domains drive the conversion of the enzyme between its four principle conformational states [42]. A flexible linker region couples these conformational changes to the M-domain, facilitating ion translocation. The transport cycle is initiated when N-domain binds ATP in the E1 conformation and rapidly phosphorylates the aspartic acid of the P-domain (Fig 3B)[45]. Many P-type ATPases require binding of a transported ion to a high affinity cytoplasmic facing site in the E1 form for phosphorylation by ATP. This is not a requirement for P4-ATPases [45]. Instead, P4-ATPases may use a charged amino acid residue as “built-in” ion as a substitute for a cation required for other P-type ATPases [26].
The phosphorylated enzyme exists in two distinct conformational states (Fig 3B). The E1P form can react with ADP producing ATP while the E2P form is resistant to ADP. During the transition from E1P to E2P, the A-domain rotates 90° inserting the TGES (DGET in P4-ATPases) motif into the space which was occupied by ADP, positioning it near the phosphorylated aspartate [42]. The consequence of A-domain rotation is the conversion of the high affinity cytoplasmic site to a low affinity extracellular facing site allowing exit of the ion. Transported aminophospholipids activate the dephosphorylation of E2P for P4-ATPases in a manner analogous to the dephosphorylation of the Na+,K+-ATPase by K+ [45, 46]. Aminophospholipids likely interact with a high affinity extracellular site in the E2P form of the enzyme similar to K+ in the Na+,K+-ATPase. This stabilizes the transition state and accelerates the rate of dephosphorylation. Binding of aminophospholipids to E2P, may trigger further movement of the glutamic acid of the DGET motif in closer proximity to the P-domain allowing it to catalyze dephosphorylation by acting as a base to remove a proton from water which carries out nucleophilic attack on the aspartyl-phosphate. In P4-ATPases, mutation of this glutamate stabilizes the E2P form and addition of PS triggers further E2P accumulation [45]. In the case of Drs2p, E2P dephosphorylation seems to be regulated by binding of phosphoinositides [41, 46]. Dephosphorylation converts the enzyme to the E2 form changing the high affinity extracellular site to a low affinity cytoplasmic site allowing the lipid to leave. The A-domain moves away from the P-domain, returning the protein to the E1 conformation.
In most P-type ATPases, transmembrane helices M1-6 are thought to form the core catalytic unit of ion transport while M7-10 support the core catalytic unit and may facilitate interactions with β-subunits. M1-6 are more flexible while M7-10 remain fairly rigid throughout the reaction cycle. P4-ATPases are unique among P-type ATPases in their ability to transport a substrate much larger than a cation. In other P-type ATPases, ion translocating pathways are not large enough to accommodate a “giant substrate” such as a phospholipid [47].
Recent studies have identified key residues which form a potential phospholipid translocation pathway [45, 48]. In the yeast PC transporter Dnf1 substitution of Phe for Tyr located at the cytosolic membrane interface of M4 alters the specificity of the transporter from PC to PS. Mutation of the equivalent Tyr for Phe in the PS transporter Drs2, essentially eliminates transport. Furthermore, substitution of a Phe in the extracellular loop connecting M3 and M4 reduces PC transport through Dnf1 [48]. Based on this work, it has been proposed that phospholipids are translocated through a non-classical pathway located along the groove of M1, M3, and M4 or alternatively M3, M4, and M5. In the mammalian ATP8A2 mutation of a lysine located in the middle of M5 produces an enzyme which is essentially devoid of PS transport displaying a large reduction in PS affinity relative to wild type [45]. The lysine is located at a “hot spot” for cation binding in the Ca2+- and Na+,K+-ATPases suggesting that lipids are transported through a classical pathway. A recent study based on the Ca2+-ATPase structure has compared the two proposed translocation pathways [49]. However, it is unclear if the pathways are compatible. These studies suggest that the phospholipid could be transported through the membrane with the headgroup forming key interactions with residues along the groove of either M1, M3, and M4 or M3, M4, and M5. The acyl chains would be free to interact with the rest of the lipid environment as it moves through the membrane similar in a concept to a credit card reader. This model potentially alleviates the “giant substrate” problem encountered for P4-ATPases [48]. Further studies are necessary to determine if these proposed pathways are compatible and what additional residues are necessary for phospholipid specificity and transport.
Most P4-ATPases form heterologous complexes with the CDC50 family of proteins (Table 1). CDC50 proteins are composed of a large glycosylated E (extracellular/lumen) domain containing three or more N-linked glycosylations, a membrane domain composed of two transmembrane helices, and two small cytoplasmic N- and C-terminal segments. In the Na+,K+-ATPase, numerous interactions of the M7 and M10 helices are made with the transmembrane helix of the β-subunit. The extracellular domain forms further interactions with the L7/8 loop of the α-subunit [24]. In P4-ATPases, both the membrane and extracellular domains of CDC50 are required for assembly with the β-subunit [31]. These subunit interactions are clearly necessary for the correct assembly and folding of P4-ATPase heteromeric complex allowing it to exit the ER [28-31, 50]. However, CDC50 proteins do not appear to be important for subcellular targeting [51]. For many P-type ATPases, complex interactions of β-subunits exert substantial changes on ion translocation kinetics [24]. Further to this, the FXYD family of single spanning membrane proteins modulate the kinetics or affinities for Na+ or K+ for the Na+,K+-ATPase in a tissue dependent manner. The CDC50 family has been proposed to behave in a similar manner as a “fusion” of the FXYD and β-subunits of the Na+,K+-ATPase [14]. In yeast, CDC50 has been shown to bind with higher affinity to the phospholipid-loaded E2P form of Drs2 suggesting that it could participate directly in lipid flipping [52]. Furthermore, the N-terminal domain of CDC50A has been shown to modulate pump kinetics of ATP8A2 [31]. Recent studies in yeast also revealed that the ectodomain is necessary for subunit specificity [53]. However, ectodomain binding was not sufficient for P4-ATPase function which required either M1 and the N-terminus or M2 and the C-terminus. In this study, two disulfide bridges in the ectodomain have also been shown to be important for subunit affinity and function. These experiments suggest that the interaction surface of the P4-ATPase-CDC50 complex is extensive and that many of these subunit interactions are necessary for subunit assembly and optimal phospholipid transport. The precise role of CDC50 proteins in the reaction cycle will require further structural and biochemical investigations.
3.3 ATP8A Subfamily
The first evidence that P4-ATPases transport aminophospholipids came from studies on the yeast Drs2p where notable deficiencies in the uptake of NBD-labeled PS were reported [54]. ATP8A1, an ortholog of Drs2p was the first P4-ATPase discovered in mammals and shown to be ubiquitously expressed throughout the body [55](Table 1). Relative to wild-type, the brains of ATP8A1 knockout mice appear morphologically normal despite PS-externalization in the hippocampus. Interestingly, these mice display hyperactivity and marked deficiencies in hippocampus-dependent learning [15]. Pioneering biochemical studies first identified a 120 kDa ATPase isolated from human red blood cells and bovine brain by conventional techniques that was robustly activated by aminophospholipids and inhibited by the phosphate analog vanadate and sulfhydryl-reactive reagents [56-59]. This ATPase was thought to be responsible for the rapid transport of spin labeled PS and PE observed in both of these systems [60, 61]. In red blood cells, phospholipid transport is thought to influence cell shape, inducing the discocyte to echinocyte change [62]. Transport stoichiometry was measured to be one ATP molecule per phospholipid [63]. Reconstitution of the ATPase from red blood cells demonstrated that it was able to catalyze the rapid flipping of PS [64]. Despite these efforts, the identity of the red blood cell ATPase still remains uncertain. Western blotting with highly specific antibodies showed that both red cells and chromaffin granules contain ATP8A1 [65, 66]. Heterologously expressed ATP8A1 from insect cells has many similar properties as the ATPase isolated from natural sources [66, 67]. ATP8A1 has been reported to bind CDC50A [30, 50] although it is possible that it can also bind CDC50B depending on experimental conditions [50]. Splice variants of ATP8A1 differ in their phospholipid selectivity and specific ATPase activity [66]. However, markedly different specific ATPase activities were observed in some studies possibly due to the absence of the appropriate CDC50A β-subunit [67]. Isolation and identification of ATP8A1 from natural sources using highly specific reagents and comparison of its enzymatic properties with earlier studies is necessary to clarify the role of ATP8A1 in these tissues.
ATP8A2 is 67% identical to ATP8A1 in sequence. ATP8A2 has a more restricted expression pattern than ATP8A1 with high level of expression in the retina, brain, spinal cord, and testis [11, 68]. In the retina, it is expressed in both rod and cone photoreceptor cells where it localizes to outer segment disc membranes, the site of phototransduction. CDC50A co-purifies with ATP8A2 from photoreceptors [31]. The ATPase activity of the purified complex is activated by the aminophospholipids PS and PE with a strong preference for PS. Reconstitution of ATP8A2 into liposomes containing NBD-labeled PS demonstrated that it is a PS-translocase [11, 31]. A patient with severe mental retardation and major hypotonia has been reported carrying a balanced translocation of chromosomes 10 and 13 disrupting the coding sequence of the ATP8A2 gene [68]. Recently, a family with four individuals carrying a missense mutation in ATP8A2 has been reported [69]. These individuals exhibit severe cerebellar ataxia, mental retardation and dysequilibrium syndrome. Additionally, wabbler-lethal mice carry mutations in the ATP8A2 gene [70]. These mice develop severe neurological abnormalities including ataxia and body tremors due to distal axonal degeneration in spinal neurons which may be a result of disrupted vesicle transport. Wabbler mice die at approximately one month of age. Knockout of both ATP8A1 and ATP8A2 results in neonatal lethality suggesting the function of ATP8A1 partially overlaps with ATP8A2. The function of ATP8A2 in photoreceptors is unknown and mutations in ATP8A2 which cause impaired vision have yet to be found. However, several functions for ATP8A2 in photoreceptors have been proposed. Rhodopsin the major membrane protein of outer segments has recently been shown to act as a phospholipid scramblase [9]. The flippase activity of ATP8A2 may maintain lipid asymmetry necessary for phototransduction in the presence of high rhodopsin concentrations. ATP8A2 may also be involved in outer segment renewal. Using microarray analysis, ATP8A2 expression was found to be downregulated in Nrl mice, potentially disrupting phospholipid organization [71]. The retinas of Nrl knockout mice only contain cone photoreceptors and large amounts of aberrant phagosomes are present at the RPE-photoreceptor interface. PS is normally localized in the cytoplasmic surface of photoreceptor plasma membranes. However, coinciding with the onset of light and phagocytosis, PS becomes exposed to the extracellular surface at the tips of photoreceptors [72]. This extracellular PS could then serve as an “eat me” signal for the RPE cells triggering phagocytosis. This would require regulation of ATP8A2 activity at the apical tips of the photoreceptor. While this is an intriguing hypothesis, ATP8A2 has yet to be shown in the plasma membrane of rod photoreceptors. ATP8A2 may also be involved in vesicular trafficking in photoreceptors and other neurons. In PC12 and hippocampal neurons, overexpressing ATP8A2 causes enhanced neurite outgrowth providing support for a role of ATP8A2 in vesicle formation or transport [73].
3.4 ATP8B Subfamily
ATP8B1 was the first P4-ATPase found to be involved in human disease. Mutations in ATP8B1 cause two forms of intrahepatic cholestasis: progressive familial intraheptic cholestasis (PFIC) and benign recurrent intrahepatic cholestasis (BFIC) [16]. These patients can also experience hearing loss and are predisposed to pneumonia [74, 75]. Cholestasis is a condition where the flow of bile from the liver is blocked. Patients often develop the disorder early in life resulting in cirrhosis within the first decade of life. ATP8B1 mutations can result in a wide spectrum of clinical severity as some patients experience cholestasis intermittently while others do not experience any symptoms [17]. In hepatocytes, ATP8B1 localizes in the canalicular membrane where its activity is thought to be important for the maintenance of membrane integrity and function of ABCB4, an ABC floppase, and consequently bile export [76-78]. ATP8B1 is necessary for apical protein expression and apical membrane organization. The loss of ATP8B1 causes substantial microvilli loss in Caco-2 cells [79]. Degeneration of stereocilia of cochlear hair cells where ATP8B1 is present is observed in knockout mice [74]. ATP8B1 can bind both CDC50A and CDC50B [29, 30, 50] but in the liver and other tissues, CDC50A is preferentially expressed and likely serves as the β-subunit for ATP8B1 in the native tissue [29, 80]. The lipid substrate that is flipped by ATP8B1 is not fully understood. In UPS-1 cells, ATP8B1 has been shown to be involved in PS translocation. In PFIC patients with severe pneumonia, cardiolipin levels were found to be elevated which correlates with higher levels of lung injury. ATP8B1 was found to bind and translocate cardiolipin via a basic residue containing motif [75]. To clarify the phospholipid substrate specificity of ATP8B1, it would be important to purify ATP8B1 from native tissue and examine its ability to transport various lipids across membranes. However, biochemical studies on purified ATP8B1 seemed to indicate that it was trapped in the E2P conformation and could not dephosphorylate [50]. It is unclear if this was due to experimental conditions, regulation, or lack of a suitable substrate. In order to determine which lipid species are transported by this important pump it will be necessary to understand what is required for dephosphorylation and completion of the reaction cycle.
To date little is known about ATP8B2 and ATP8B4. ATP8B4 is expressed throughout the brain and may be involved in Alzheimer disease [81]. Both pumps may be able to interact with CDC50A and CDC50B [50], although in the case of ATP8B4 experimental conditions may influence interaction with CDC50B [30]. ATP8B3 and its homolog ATP8B5 in mice are exclusively expressed in the testis where they are known to localize to the acrosomes of spermatids, the organelle which develops in the anterior half of the head of spermatozoa [82-84]. Knockdown of ATP8B5 has a profound effect on Golgi structure and protein secretion [84]. On the basis of cell biology and biochemical studies, ATP8B3 appears to also be a PS pump. ATP8B3 does not seem to interact with either CDC50A or CDC50B [30] however it is possible that it could interact with CDC50C based on expression. Interestingly, disruption of ATP8B3 in mice does not affect the morphology of sperm or the rate of fertilization despite sperm-egg interactions being disrupted in vitro [83].
3.5 ATP9, ATP10, and ATP11 Subfamilies
ATP9 is the only subfamily of P4-ATPases which do not appear to interact with CDC50 proteins [28]. In transfected cells, ATP9A localized to the both endosomes and the trans-Golgi network (TGN), whereas ATP9B localized only to the TGN. An N-terminal targeting sequence appears to be important for the localization for ATP9B to the TGN. The substrate(s) transported by ATP9A and ATP9B is not currently known. In yeast, loss of Neo1p, an ortholog of the ATP9 members, is lethal. Neo1p localized to endosomes and Golgi where it appears to play a role in endosomal membrane trafficking [85]. Further investigations are needed to determine if the ATP9 subfamily plays a similar role in mammalian cells.
The ATP10 subfamily harbors the least biochemically characterized subfamily of P4-ATPases. Members of the subfamily have a longer C-terminus, the function of which is unknown. CDC50A is required for exit of ATP10 proteins from ER [28]. The substrate specificities of ATP10A, ATP10B and ATP10D have not yet been defined. In mice, ATP10A and ATP10D are associated with obesity and diabetes [86-90]. There are also reports that link imprinting mutations and deletions in the region of ATP10A with Angelman syndrome, a form of autism-spectrum disorder [91, 92].
Recent work from two independent labs has shown that ATP11C is important for the proper development of B-cell lymphocytes in mice [13, 93]. ATP11C seems to be required for efficient flipping of PS in B-cells. Intrahepatic hyperbilirubinemia, an accumulation of bilirubin from hepatocellular origin and high serum levels of cholic acid indicative of cholestasis, is also observed in these animals [94]. ATP11A, ATP11B and ATP11C appear to be ubiquitously expressed and only bind CDC50A as its β-subunit [28].
4. ABC Transporters
ATP binding cassette (ABC) transporters are found in all living organisms from bacteria to mammals [95] [96]. They typically use ATP binding and hydrolysis to actively translocate a wide variety of compounds across cell membranes including amino acids, peptides, ions, metabolites, vitamins, fatty acid derivatives, steroids, organic anions, phospholipids, drugs and other compounds. Prokaryotic ABC transporters can function either as exporters or importers. Eukaryotic ABC transporters have been initially thought to only function as exporters, but several studies have now shown that some eukaryotic ABC transporters including the mammalian ABC transporter ABCA4 function as importers translocating substrates from the exocytoplasmic (extracellular/lumen) to the cytoplasmic side of biological membranes [19, 97, 98].
ABC transporters consist of four principal domains: two transmembrane domains (TMDs) containing multiple membrane-spanning segments that provide a pathway for the translocation of a substrate across the membrane and two cytoplasmic ATP-binding cassettes or nucleotide binding domains (NBDs) that provide the energy for substrate transport. Eukaryotic ABC transporters are typically synthesized as full transporters in which all four domains reside on a single polypeptide chain with a modular organization of TMD-NBC-TMD-NBD or half transporters in two polypeptides each containing a TMD and NBD assemble as homo- or hetero-dimers. Some transporters contain additional domains attached to the TMD or NBD which regulate the activity of these ABC transporters. In prokaryotes, the TMDs and NBDs can reside on individual polypeptide chains or fused together in various arrangements [96].
Eukaryotic ABC transporters typically contain 6 membrane spanning segments per core TMD. NBDs approximately 200 amino acids in length have a number of conserved structural features including Walker A and Walker B motifs found in many ATPases and the ABC signature motif (LSSGQ), D, H and Q loops characteristic of ABC proteins. Structural and biochemical studies indicate that the two NBDs dimerize in a head-to-tail manner with the two ATP molecules present at the dimer interface [99-102].
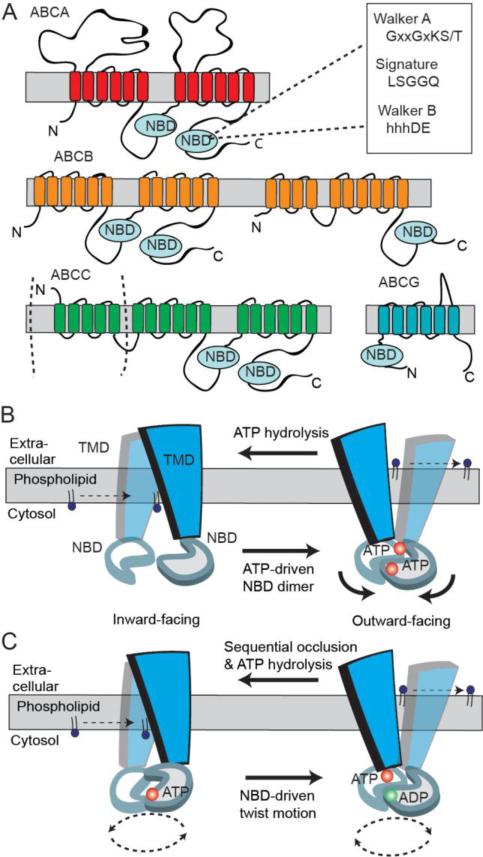
The human genome is known to contain at least 49 genes that encode ABC proteins [103]. These transporters have been organized into seven subfamily (ABCA-ABCG) based on their amino acid sequence and structural organization (Fig 4A). Two subfamilies ABCE and ABCF, which contain NBDs, but no TMDs, do not function as transporters, but instead are involved in the regulation of protein biosynthesis. Several members of various ABC subfamilies are known to transport specific membrane phospholipids across cell membranes either serving as floppases or flippases or exporting the phospholipid to an acceptor molecule [104].
Fig 4.
Predicted two-dimensional topology of lipid ABC transporters and models of ABC transporter mechanism. A. The two transmembrane domains (TMDs) and nucleotide binding domains (NBDs) arrange to form full transporter and are represented by some ABCB members and ABCA members with additional exocytosolic domains. Some ABCC family members are full transporters with an additional N-terminal TMD. Half transporters in ABCB and ABCG subfamilies have different modular organizations of a TMD and NBD. B. A simplified‘Switch Model’: In the inward-facing conformation, the NBDs are separated and nucleotide-free. Phospholipid substrate entry into the substrate binding site occurs from the cytosolic leaflet of the membrane bilayer. ATP-dependent dimerization of NBDs progresses to pull the TMDs from an inward- to outward-facing conformation. Phospholipid is translocated to the extracellular side of the membrane and ATP hydrolysis resets the transporter in the inward conformation. C. A simplified ‘Constant-contact Model’: The two NBDs remain associated in the sandwich dimer form throughout the transport cycle. Inward and outward conformations are directed via subunit conformational changes and the twisting motion of NBDs. ATP hydrolysis alternates with one site hydrolyzing nucleotide that facilitates the binding and hydrolysis in the other site.
4.1. General Mechanisms
Crystal structures of several ABC exporters (Sav1866, murine P-glycoprotein, MsbA, TM287/TM288) together with biochemical studies have provided insight into the transport mechanism of ABC proteins [105-109]. The ‘alternating-access model’ proposed for ABC transporter drug export function can be adapted for lipid translocation [106, 110]. In the absence of nucleotide, the NBDs impose separation in the TMDs. and create a large central cavity towards the cytoplasmic side of the membrane that represents an ‘open-inward’ conformation. The transport cycle is initiated when a phospholipid(s) enters the substrate binding site from the inner leaflet that is assumed to represent a high affinity binding interaction. Binding of ATP induces a conformational change in the NBDs and significant rearrangement of the TMDs resulting in a central cavity facing the extracellular/lumen side of the membrane. This is termed as the ‘outward-closed’ conformation for the ABC transporter, and is similar to that observed for Sav1866 [106]. The polarity of the TMD substrate-binding site then becomes reversed with a concomitant reduction in substrate affinity, and release of the lipid substrate to the outer leaflet. The conformational change in the TMDs may be driven either by ATP binding, in which case hydrolysis of ATP subsequently resets the ABC transporter to the inward-facing conformation or by the energy released on ATP hydrolysis. Alternatively, the transporter may only bind to the polar head group of the phospholipid similar to ion or pore channel mechanisms [111]. This model relegates the acyl chains as being shuttled across the hydrophobic interior of the bilayer as the lipid headgroup is mediated by the substrate binding pocket and transitions between the inner and outer leaflets.
The events that occur at the NBDs are also incompletely understood but two models are generalized to link substrate binding and the catalytic cycle. The ‘Switch model’ proposes that sequential ATP hydrolysis is required to destabilize the NBDs and the adjacent Walker A motifs and signature motifs in the sandwich dimer move apart before the transport cycle can begin again [112](Fig 4B). ATP binding switches from the dissociated NBD dimer to the closed dimer, reduces the substrate binding affinity, and facilitates substrate translocation via corresponding conformational changes in the TMDs. Evidence from electron paramagnetic resonance (EPR) analysis of the NBDs of MsbA, a lipid transporter, shows large conformational changes (10-20 Å) following ATP hydrolysis suggestive of NBD dissociation, and is consistent with the crystal structure conformations of MsbA [107, 113]. In contrast, the ‘Constant-contact model’ proposes that the NBD dimer is stabilized regardless of nucleotide or substrate content and that each NBD twists about enabling the exchange of nucleotide hydrolysis products (Fig 4C) [114, 115]. This effect occludes ATP in one site while the other site opens to release ADP and Pi. The transition from the inward to the outward facing conformation of the TMDs requires a twisting motion of the NBDs and a rearrangement of the consensus site, which allows binding of the second ATP. The bound lipid substrate may then be released to the outer leaflet of the membrane bilayer. Vanadate trapped complexes in P-gp support an asymmetric effect of nucleotide occluded in one site while the other remains solvent accessible consistent with the opening of one ATP site and the other one closed [116]. A cross-linking study in which the two-halves of the TMDs in Pgp were clamped in the closed conformation displayed high affinity and transport activity of drugs [117], although the open-inward conformation of the Pgp structure showed a 30 Å NBD separation bound to some substrates [105]. A recent crystal structure of the putative drug TM287/TM288 ABC transporter from T. maritima presents an inward-facing conformation with contacting NBDs [108], which is uniquely different to the ABC exporter structures of inward-facing conformations with dissociated NBDs [105, 107]. Although, ATP binding and hydrolysis may propagate conformational changes as fulfilled by the constant contact model, the importance of a highly flexible open inward conformation as part of the switch model may also be essential for efficient binding of large size physiological substrates [107, 111].
To date, only one ABC exporter, Pgp, has been resolved with a bound substrate [105]. The TMDs bear hydrophobic, aromatic and polar amino acids where multiple ligands can bind through different interactions with distinct sets of amino acids and provide multiple substrate binding sites, as found in Sav1866 [106]. The TMD cavity of MsbA has a high dielectric environment that stabilizes polar regions and hydrophobic areas in the bilayer [107]. The proposed models account for both lipid and drug efflux. In this case, translocation of lipids may be similar to that of drug substrates but partitions into the outer leaflet of the membrane bilayer, instead of being expelled into the aqueous environment. Alternatively, the transporter may promote dissociation of the lipid from the donor membrane, thereby facilitating its efflux from the cell. Such a case would require the incidental movement of lipids to a region of partial hydrophobicity on the exocytolic face of the protein. This places the substrate in an environment which energetically favors its binding and removal by an acceptor molecule like a bile salt or a docking protein such as apolipoprotein-A1 (apo-A1) [20]. This mechanism may govern the functionality in several ABCA and ABCG proteins. In the absence of additional ABC transporter structures, predicting how a lipid is flipped and/or effluxed is challenging and requires the rigorous use of homology models, molecular simulation analysis, and biochemical studies.
4.2. ABCA transporters
The A subfamily of ABC transporters consist of 12 members. All are full-length transporters organized in two tandem halves, each with a hydrophobic TMD followed by a cytoplasmic NBD. In addition, these transporters contain two large extracellular domains (ECDs) between the first and second membrane-spanning segment of each TMD [118]. Although the substrates of most ABCA members remain to be identified, disease-associated phenotypes, analysis of knockout mice, and biochemical and cell based studies suggest that most ABCA proteins play a role in phospholipid translocation and cellular homeostasis. Mutations in ABCA1 are known to cause Tangier disease and familial high density lipoprotein deficiency associated with defective cholesterol and phospholipid efflux from cells and a deficiency in the formation of high density lipoprotein (HDL) [119-123]. Defects in ABCA3 are associated with neonatal surfactant deficiency and pediatric interstitial lung disease resulting from abnormal surfactant secretion into the alveoli of the lungs and the formation of abnormal lamellar bodies [124, 125]. Disease mutations in ABCA4 cause autosomal recessive Stargardt macular degeneration and related retinal degenerative diseases associated with defective retinylidene-phosphatidylethanolamine transport [126]. Mutations in ABCA12 are associated with harlequin ichthyosis, a disease arising from defective lipid transport in the skin [127, 128]. ABCA1, ABCA2, and ABCA7 have been also linked in the etiology of Alzheimer's disease [6, 129, 130].
4.2.1 ABCA1
ABCA1 is recognized as the principal protein involved in cholesterol efflux from peripheral tissues in a process known as the reverse cholesterol transport (RCT) [122, 131]. It mediates the efflux of cellular phospholipid and cholesterol to lipid poor apolipoprotein acceptors as a key step in the production of HDL. The loss of ABCA1 function in humans leads to Tangier disease, a disorder associated with severe HDL deficiency and increased risk of atherosclerosis. To date, more than 100 mutations in the gene encoding ABCA1 have been linked to Tangier disease in which patients have absent or low circulating HDL [123]. The inverse relationship between plasma HDL levels and the risk of coronary artery disease has been reported [132], although low HDL per se is not an absolute risk factor [133].
Abca1-/- mice and chickens with dysfunctional ABCA1 also exhibit HDL deficiencies further highlighting a pivotal role of ABCA1 in the RCT pathway [134-136]. Tissue specific deletion of ABCA1 in liver and intestines of mice resulted in an 80% and 30% reduction in plasma HDL, respectively [137, 138], while ablation of ABCA1 in macrophages did not alter HDL levels [139].
ABCA1 expressed in cultured cells, mediates the secretion of PC and cholesterol when lipid-free apolipoprotein apo-A1, an extracellular lipid acceptor in the plasma, is added to the medium [140, 141]. Cell based studies of 15 disease-associated mutations in ABCA1 showed decreased phospholipid, predominantly PC, and cholesterol efflux [123, 142]. Direct interaction between apo-A1 and ABCA1 has been reported in numerous studies using cross-linking, immunoprecipitation, radiolabelling, and biotinylation techniques [143-145]. More recently, Nagao et al reported that initial binding of apo-A1 is mediated through electrostatic interactions with the extracellular domains of ABCA1 [146]. This interaction can be inhibited by heparin and poly-L-lysine treatments. Dimeric and tetrameric forms of ABCA1 represent the minimal functional unit required for apo-A1 binding and lipidation [147].
HDL constitutes a heterogeneous group of particles differing in size, density, electrophoretic mobility, lipid composition and apolipoprotein content with average diameters of ~12, 10, 7.5, and < 6nm. Electron microscopy studies suggest that the HDL particles are mostly spheroidal [148]. The HDL particles formed by the lipidation of apo-A1 via the functional activity of ABCA1 is an essential step for the formation of HDL that ultimately determines plasma HDL levels. In several studies, the formation of heterogeneous nascent pre-β-HDL was observed during incubation of apo-A1 with Chinese hamster ovary cells and THP-1 macrophages, presumably by the interaction of apo-A1 with cell surface binding sites or through retroendocytosis [149, 150]. In J774 macrophages, in which ABCA1 expression was up-regulated by cAMP, the presence of extracellular apo-A1 induced formation of 7.4-, 9.4, and 11.2-nm sizes of particles. The largest pre HDL particle carried up to 45% of total particle lipids as cholesterol and is bound by 3 apo-A1 molecules in a belt-like conformation [148]. The most abundant lipids in HDL are PC, cholesterol, and SM. The total amount of lipid also increases as the diameter of the HDL particle increases. For instance, ~26 lipid molecules reside with two apo-lipoprotein particles in the 7.4 nm HDL particle whereas ~240 lipid molecules are found along with three apo-A1 molecules in the 11.2 nm particle [148]. Interestingly, the 7.4 nm and 11.2 nm particles contain ~3:1 and 1:1 PC/free cholesterol (mol/mol), respectively. In contrast PS and SM levels remain relatively similar during HDL particle maturation.
Although it is well established that ABCA1 mediates the efflux of cholesterol and phospholipids from cells, the actual substrates transported by ABCA1 are still unclear (Table 1). Several models of ABCA1-mediated cholesterol efflux have been proposed, including a two-step model in which ABCA1 first mediates PC efflux to apo-A1, and this apo-A1 PC complex accepts cholesterol in an ABCA1 independent manner [151]. A concurrent process model in which PC and cholesterol efflux by ABCA1 to apo-A1 are coupled to each other has also been proposed [152]. A third model has been proposed in which ABCA1 generates a specific apo-A1 binding site through PS translocation to the outer leaflet of the plasma membrane with subsequent translocation of PC and cholesterol to apo-A1 [153]. More recently, Vedhachalam et al [150] and Iatan et al [154] advanced new models in which HDL particle maturation is mediated by the lateral heterogeneity of the plasma membrane (Fig 5). In this system, ABCA1-dependent activity creates a PC-rich membrane binding site for apo-A1 on the plasma membrane that has almost 10-fold higher capacity to bind apo-A1 compared with ABCA1. This is called a high-capacity binding site (HCBS) and is required for apo-A1 lipidation. In this model monomeric or oligomeric ABCA1 creates a HCBS population either associated with or excluding ABCA1 [154]. Initial interactions of apo-A1 with ABCA1 stabilize the membrane protein; reduction of intracellular degradation by calpain proteases promotes increased levels and activity of ABCA1 in the plasma membrane. In response to ATP hydrolysis, ABCA1 promotes the transbilayer transport of PC and/or PS from the inner to outer leaflet of the plasma membrane. The initial events consist of binding of a small pool of apo-A1 to ABCA1 at the PM, stimulating the net phospholipid translocation to the exofacial leaflet. ABCA1 facilitates its translocation to the HCBS populations from which apo-A1 desorbs PC, thereby becoming an efficient acceptor of cholesterol. These small PC containing particles may interact successively or simultaneously with different microdomains, including cholesterol rich rafts, allowing the formation of larger heterogeneous cholesterol containing nascent HDL particles. Alternatively, ABCA1 activity leads to unequal lateral packing densities in the two leaflets of the phospholipid bilayer, creating exovesiculated lipid domains, primarily mediated by PS. This would then result in a pool of bound apo-A1 spontaneously solubilizing phospholipids from the exovesiculated domain to create discoidal nascent HDL particles. The latter effect may be the underlying cause of apo-A1 solubilizing phospholipids and cholesterol from small 20 nm diameter unilamellar vesicles in an in vitro system [155]. More studies, however, are needed to definitively identify the lipid substrates transported by ABCA1 and the mechanism by which HDL particles are generated from apo-A1 and ABCA1.
Fig 5.
Apo-A1 lipidation via ABCA1 activity within plasma membrane microdomains. Lipid free apo-A1 initially interacts with monomeric or oligomeric ABCA1. The PC flippase activity of ABCA1 creates a High Capacity Binding Site (HCBS) within cholesterol poor microdomains. Phospholipid translocation via ABCA1 may also induce membrane bending of the bilayer to create an exovesiculated binding site. ABCA1 subsequently mediates transfer of apo-A1 to these HCBS populations, from which apo-A1 selectively microsolubilizes PC to become discoidal apo-A1 species. Further microsolubilization of cholesterol and PC generates nascent HDL with two, three, and four molecules of apo-A1.
4.2.2 ABCA2
ABCA2 is a 270 kDa protein that shares 50% sequence identity with ABCA1. It is mainly expressed in the central nervous system, ovary and macrophages [156]. Abca2-/- mice display a compact myelin sheath phenotype with reduced levels of SM and gangliosides during the early development of mice (Fig 6A). The phospholipids, PE and PS, were slightly decreased compared to the wildtype mice [157]. Recent studies have shown that ABCA2 plays a role in the trafficking of LDL-derived cholesterol and is coordinately expressed with sterol responsive genes including LDLr and HMGCoA synthase [95]. ABCA2 overexpression in neuroblastoma cells lead to a decrease in total, free, and esterified cholesterol levels. ABCA2 overexpression also decreased LDLr receptor mRNA and protein levels and increased its turnover rate. This suggests that ABCA2 may modulate cholesterol homeostasis in neuronal cells and play a regulatory role in cholesterol metabolism. Very few ABC protein encoding genes have been identified that play a role in the trafficking of LDL-derived cholesterol. In HEK293 cells, ABCA2 overexpression does not result in cholesterol efflux to apo-A1, apo-E, or apo-E disks [158] while phospholipid efflux activity remains to be investigated.
Fig 6.
A. Overview of ABC transporters involved in lipid efflux. Schematic representation of general ABC transporter localization, known acceptors and direction of transport. Vectorial transport depicted by black arrows at the plasma membrane. Vectorial transport in many ABC transporters and by intracellular ABC transporters has not been firmly established. B. Hepatocytic ABC transporters. Newly synthesized bile salts are effluxed by ABCB11, where they form micelles with PC translocated to the outer leaflet of the canalicular membrane by ABCB4. ABCG5/G8 translocates cholesterol into the bile lipid mixture.The action of PS translocation to the inner leaflet by ATP8B1 maintains the lipid asymmetry at the canalicular membrane. C. Role of ABCA4 in photoreceptor disc membranes. Photoexcitation of disc membranes in photoreceptors causes release of all-trans retinal from rhodopsin. Retinal is reduced in the visual cycle or reacts with PE in disc membranes to form N-ret PE. ABCA4 binds and translocates N-ret PE from the lumen to the cytosolic side of the disc membrane. All-trans retinal then dissociates from N-ret PE and retinal is shuttled into the visual cycle. ABCA4 also translocates PE from the lumen to the cytosolic side of the disc membrane. D. Surfactant production in alveolus. In alveolar type II cells, ABCA3 present in lamellar bodies translocates PG, PC, and PE which is then secreted into the epithelial lining of the surfactant. E. Epidermal production in skin. ABCA12 transports GlcCer and Cer into the lamellar body which is then redistributed into the cell periphery. Apo-A1/E, apolipoprotein; HDL High Density Lipoprotein; LDL, low density lipoproteins; SR-B1, Scavenger receptor Class B1 protein; Chol, cholesterol; PC, phosphatidycholine, PE, phosphatidylethanolamine; PS, phosphatidylserine; Cer, ceramine; GlcCer, glucosylceramide; N-Ret PE, N-retinyldiene PE; SM, sphingomyelin
4.2.3 ABCA3
ABCA3 is highly expressed in alveolar epithelial type II cells of the lung. In these cells, ABCA3 localizes to the limiting membrane of lamellar bodies, lipid rich organelles associated with the production, storage, and secretion of pulmonary surfactant. Surfactant is a complex mixture of 90% lipids (mostly phospholipids) and 10% surfactant-specific proteins that reduce surface tension of the air-liquid interface and prevents alveolar collapse [159]. ABCA3 is implicated in the translocation of surfactant lipids into lamellar bodies where surfactant is assembled. Mutations in the ABCA3 have been associated with neonatal surfactant deficiency and chronic interstitial lung disease [125]. Uptake of NBD-PC and NBD-PE into ABCA3 LAMP-positive vesicles in A549 cells was demonstrated (Fig 6D)[160]. Matsumura et al showed increased levels of PC, but not PE or cholesterol, in low density vesicles in A549 cells stably expressing the wild-type ABCA3 [159]. Consistent with this observation, Cheong et al showed decreased incorporation of radiolabeled PC, PG, and PE into lamellar bodies of abca3+/- mouse [161]. However, a high throughput mass spectrometry lipid profile found a significant decrease in PG and short chain PC in abca3-/- mice [162]. The exact molecular contribution of ABCA3 for surfactant production with respect to PC and PG transport remains to be established.
4.2.4 ABCA4
ABCA4 is a 250 kDa ABC transporter having a 50% amino acid sequence similarity with ABCA1/2/7. It is highly expressed in the light sensitive outer segment disc membranes of retinal rod and cone photoreceptor cells [163, 164]. Over 800 mutations in ABCA4 are known to cause Stargardt macular degeneration, a severe retinal degenerative disease characterized by progressive impairment of central vision early in life, presence of lipofuscin deposits in the central retina, and bilateral atrophy of photoreceptor cells and adjacent retinal pigment epithelial (RPE) in the macula [126, 165, 166]. Mutations in ABCA4 have also been reported to cause several related retinal degenerative diseases including cone-rod dystrophy and retinitis pigmentosa and a subset of age-related macular degeneration [167-170].
ABCA4 facilitates the clearance of retinal from rod and cone photoreceptors by functioning as a N-retinylidene-phosphatidylethanolamine (N-ret-PE) flippase [171]. Photoreceptors are subjected to a high flux of retinal as part of the visual process. In the dark, 11-cis retinal derived from the visual cycle combines with opsin in disc membranes to regenerate rhodopsin and cone opsin in the outer segment disc membranes of rod and cone cells, respectively. Vision is initiated when light photoisomerizes 11-cis retinal to all-trans retinal within the binding pocket of opsin. All-trans retinal is subsequently released from opsin and must be cleared from photoreceptor cells to prevent the formation of potentially toxic compounds. Removal of retinal can occur in part by the direct reduction of all-trans retinal to all-trans retinol, a reaction catalyzed by the retinol dehydrogenase RDH8 on the surface of photoreceptor disc membranes. All-trans retinol is subsequently converted back into 11-cis retinal via a series of reactions that comprise the visual cycle in adjacent retinal pigment epithelial (RPE) cells for the regeneration of photopigment [172]. However, all-trans retinal can also reversibly react with PE which comprises about 40% of the phospholipid in the disc membrane to form a Schiff-base adduct N-ret-PE. A fraction of N-ret-PE can be trapped on the lumen leaflet of the disc membrane [173]. ABCA4 functions in the active transport or flipping of N-ret-PE from the lumen to the cytoplasmic side of the disc membrane (Fig 6C) [19]. Upon dissociation of N-ret-PE, all-trans retinal can be reduced to retinol by RDH8 and for entry into the visual cycle, thereby facilitating the complete removal of all-trans retinal from disc membranes after photoexcitation.
Support for the role of ABCA4 as a N-ret-PE transporter has come from both biochemical studies of ABCA4 and analysis of abca4-/- mice. Purified ABCA4 reconstituted into lipid vesicles has a basal ATPase activity which is stimulated 2-5 fold by all-trans and 11-cis retinal and is dependent on PE [174, 175]. Solid-phase binding studies further demonstrated that N-ret-PE binds with high affinity to ABCA4 and is quantitatively released by the addition of ATP [176]. More recently, a direct transport assay has been developed which confirms the function of ABCA4 as an N-ret-PE flippase [19]. In this study, ABCA4 reconstituted into liposomes or present in disc membranes was observed to transport N-ret-PE from the lumen to the cytoplasmic leaflet of disc membranes. In the absence of retinoids, ABCA4 was also shown to actively flip fluorescent-labeled NBD-PE in the same ‘import’ direction. ABCA4 is the first mammalian ABC transporter known to function as an importer and more specifically as a phospholipid flippase.
Analysis of abca4–/– mice also supports the role of ABCA4 in the clearance of retinal compounds from photoreceptors [177, 178]. Initial studies reported that mice deficient in ABCA4 display elevated levels of all-trans retinal, PE, and N-ret-PE compared to wild-type (WT) mice consistent with a reduced efficiency of removal of all-trans retinal from photoreceptors. A light-dependent accumulated lipofuscin deposits containing the diretinal compound A2E was also observed in RPE cells of abca4-/- mice [177, 179]. A similar accumulation of lipofuscin deposits and A2E is also observed in patients with Stargardt disease [178, 180]. More recently, Boyer et al. confirmed the finding that abca4-/- mice show a large increase in A2E and lipofuscin compared to WT mice, but in this study the increase in A2E containing lipofuscin deposits in the abca4-/- mice was light-independent [181]. These studies suggest that ABCA4 may play a crucial role in removing excess 11-cis retinal as well as all-trans retinal from photoreceptors via its N-ret-PE transport activity. Whether ABCA4 directly transports the 11-cis isomer of N-ret-PE across the disc membrane or transports the all-trans isomer generated through PE-induced chemical isomerization of 11-cis retinal in disc membranes remains to be determined.
4.2.5 ABCA7
ABCA7 is a 220 kDa ABCA transporter sharing a ~54% sequence homology to ABCA1 [182]. The highest levels of expression are found in the brain, lung, myelo-lymphatic tissues, kidneys, macrophages, and platelets [183]. Given the homology of ABCA7 to ABCA1, it was predicted that ABCA7 may stimulate cellular phospholipid and cholesterol efflux to apo-A1 or apo-E. Initial studies confirmed ABCA7 expression promoting efflux of PC and SM, but not cholesterol to apo-A1 [184]. Interestingly, ABCA7 overexpression in HEK293 cells regulates cholesterol efflux only to apo-E disks, which resemble pre-HDL, but not lipid free apo-E or lipid-free apo-A1 (Fig 6A) [185]. The potential of ABCA7 to replicate this activity with reconstituted apo-A1 remains to be tested.
Cholesterol and phospholipid efflux levels of bone marrow-derived macrophages from abca7-/- mice were similar to that of wild-type mice [186]. However, female abca7-/- mice show a reduction in serum cholesterol, decreased HDL levels, and alteration in adipose mass. The basis of the gender specificity is unknown. A connection to an immune disorder was related to ABCA7 when an epitope of Sjogrens syndrome corresponding to a residual protein fragment of ABCA7 was detected in salivary glands of Sjogren's patients [187]. This epitope corresponds to a region from the first extracellular domain of the membrane protein and is homologous to ABCA1, ABCA2, ABCA3, and ABCA4. Sjogrens syndrome is one of a rare number of organ-specific, autoimmune diseases that is characterized by oral and ocular dryness [188]. Since, ABCA7 is expressed in myelo-lymphatic tissues, it may play an important role in lipid homeostasis in cells of the immune system.
Whereas earlier studies suggested a residual phospholipid efflux role for ABCA7, abca1-/- macrophages display no detectable apo-AI stimulated phospholipid efflux activities, inconsistent with this notion [189]. In addition, peritoneal macrophages treated with ABCA7 siRNA and from heterozygous abca7+/- mice show unaffected apo-A1 phospholipid release [189]. Recent studies point to a central role of ABCA7 in phagocytosis and engulfment of apoptotic cells (Fig 6A)[190-192]. ABCA7 mRNA and protein levels are upregulated during phagocytosis via the SREBP2 pathway, while siRNA knockdown of ABCA7 results in a decreased phagocytic activity and that abca7+/- mice exhibit a defective clearance of apoptotic cells. In ABCA1 deficient fibroblasts, both the phagocytic rate and the expression of ABCA7 are increased [190]. In J774 cells and mouse pertioneal macrophages, phagocytic activity was also stimulated two-fold by apo-AI and apo-AII [192]. In contrast, the phagocytic activity is decreased in the peritoneal cavity of abca7-/- mice. This suggests that ABCA7 forms a complex with apo-A1/HDL prior to endocytotic internalization for intracellular mediated proteolysis although a defined mechanism is still unclear. Linsel-Nitschke et al proposed that the contact between ABCA7 and apo-A1 might be restricted to specific cellular conditions [189]. In cases of cell migration or phagocytosis, it is probable that ABCA7 participates in lipid efflux activities in macrophages or blood cells. The authors also allude to the high cell surface expression of ABCA7 in kidneys where this organ plays a distinctive role of apo-A1 catabolism [189].
4.2.6 ABCA12
Mutations in ABCA12 underlie three related autosomal recessive disorders: Harlequin ichthyosis (HI), lamellar ichthyosis type 2, and non-bullous congenital ichthyosiform erythroderma [127, 128, 193]. All three diseases represent dyskeratinization disorders in which the skin develops as thick armour plates, but present with different clinical severity. It is possible that disease-causing mutations are reflected on the level of ABCA12 expression, subcellular localization, and biochemical function that correlates with the intensity of the involved disorder. For instance, a missense mutation of ABCA12 N1380S [127, 128] implicated in HI and corresponding to nucleotide binding domain 1 region: N965S (ABCA4) [19] and N935S (ABCA1) [123] have been shown to reduce PE translocation and cholesterol efflux activities in ABCA4 and ABCA1, respectively.
Extracellular lipids are thought to be essential for skin formation. Ultrastructural experiments point to the absence or malformation of lamellar granules, which physiologically contribute to assemble the skin barrier by exporting their lipid content into the extracellular space during the keratinization process [128]. ABCA12 co-localizes with glucosylceramide to lamellar granules on route from the Golgi apparatus to cell periphery [194]. In the epidermis of abca12-/- mice, increased levels of ceramides (of various acyl chain sizes), sphingosine (breakdown product of ceramide) and glucosylceramides were noted with the largest change observed in ceramides (Fig 6E)[195]. However, no differences in total levels of phospholipids (PC, PI, or PE) were noted. In mouse skin fibroblasts, a modest two-fold cholesterol efflux to apo-A1 was enhanced by LXR activation when compared to abca12-/- cells [195]. Like ABCA3, the mechanism of lipid uptake into intracellular compartments mediated by ABCA12 is unknown, as there is no characterization of lipid-protein acceptors mediated by ABC transporters within intracellular compartments.
4.3. ABCB transporter and biliary phospholipid translocation
4.3.1 ABCB1/P-glycoprotein
In addition to transporting a broad spectrum of drugs, P-glycoprotein (Pgp) also functions as a phospholipid transporter with little head group specificity. Initial studies by van Hoolvert et al showed the translocation of short chain NBD-PC and NBD-PE by Pgp across the plasma membrane of LLC-PK1 cells [196]. Using cellular fluorescence in conjunction with FACS analysis, Bosch et al demonstrated a decrease in accumulation of the NBD-PC analog in the multidrug resistant cell line (CEM/VBL300) compared to the parental cell line (CEM) [197]. There was concern that short chain lipid analogs may not reflect the physiological relevance of natural long chain lipid transport. This exception was provided by studies of Platelet Activating Factor (PAF), a naturally occurring short-chain PC and a mediator of inflammation in response to various stimuli [198]. In LLC-PK1 cells, [14C] PAF translocation to albumin was stimulated by Pgp expression and inhibited by Pgp inhibitors, PSC833 and cyclosporin A [199].
Notwithstanding these inferences, the first direct biochemical evidence of Pgp translocase activity was revealed by Romsicki and Sharom [200]. Using purified Pgp reconstituted into proteoliposomes, translocation of NBD-PC, NBD-PE, NBD-PS, NBD-SM, and a variety of NBD-glycosphingolipids was demonstrated in an ATP-dependent manner (Fig 6A)([200, 201]. Both short chain and long chain NBD-lipid analogues were translocated by Pgp, although the long chain lipid analogues were flipped at ~50% levels compared to the short chain lipid probes. This lipid translocation shared many of the fundamental characteristics of drug transport, such as energy dependence and vanadate sensitivity, and drug competition with lipid flippase activity, suggesting that membrane lipids probably followed a similar path as drug substrates through the Pgp membrane protein.
Interestingly, cholesterol exerted distinct effects on basal and verapamil-induced ATPase activity. The basal ATPase activity of Pgp reconstituted in PC vesicles increased over 5 fold in the presence of 20% cholesterol and was shown to be sterol-specific. In contrast the cholesterol effect on verapamil-induced ATPase activity was unspecific and not related to its influence on membrane fluidity and on verapamil membrane affinity [202].
4.3.2 ABCB4
Biliary PC excretion is essential for preventing bile toxicity by the formation of mixed micelles with bile acids and cholesterol. PC excretion is mediated by a PC specific translocase, ABCB4, which shares 76% identity with Pgp. Several transporters expressed on the hepatocyte canalicular membrane play important roles in canalicular bile formation (Fig 6B). Besides ABCB4, ABCB11 secretes bile salts into the canaliculus while the heterodimeric complex ABCG5/ABCG8 mediates the translocation of cholesterol into bile (Fig 6B)[203]. In addition, the P4-ATPase ATP8B1 mediates the flipping of PS from the lumen to the cytosolic side and is required to maintain the liquid crystalline nature of the canalicular membrane (Fig 6B)[203]. The clinical significance of these transporters are apparent in a condition known as progressive familial intrahepatic cholestasis (PFIC), a disorder characterized by jaundice, growth retardation, and progressive liver damage due to impaired bile flow. In order of phenotype severity, mutations in ATP8B1, ABCB11, and ABCB4 contribute to PFIC type 1, PFIC type 2, and PFIC type 3, respectively [204].
Abcb4-/- mice develop severe liver disease characterized by a lack of PC in bile [205, 206] while transgenic infusion of human ABCB4 gene into these mice rescue PC translocation activity into bile [207]. Polarized cells transfected with ABCB4 showed increased rates of NBD-PC translocation to the outer apical leaflet when compared to the absence of activity of NBD-PE and NBD-SM [196]. Smith et al demonstrate PC translocation in fibroblasts overexpressing ABCB4 by metabolic labeling with radioactive choline [207]. In this case, PC-transfer protein was used as an acceptor to monitor radioactive PC secretion. In addition, ABCB4 transfected in HEK293 cells demonstrated PC efflux in the presence of taurocholate, a mimic analog of bile salt [208]. The specificity of this assay was surprising because cholesterol efflux into bile salts was also noted along with PC efflux. In the canalicular membrane, the major cholesterol excretion is driven by the activities of the ABCG5 and ABCG8 complex (discussed below). This observation may be a side activity of taurocholate and, perhaps, using the natural lipid acceptor is not feasible as micellar concentrations of bile salts induce cytolysis in cultured cells. More recently, Groen et al illustrated the importance of ATP8B1 as being essential for cellular survival in the presence ABCB4 [209]. Transient expression of ABCB4 in HEK293 cells decreased protein levels and compromised the integrity of the cellular membrane, as noted by the leakage of lactate dehydrogenase into the culture medium. However, co-expression of ATP8B1 and ABCB4 rescued protein expression levels and displayed PC secretion in a bile salt dependent manner [209].
4.4 ABCC1
Members of the ABCC subfamily are full transporters with several members (ABCC1/2/3/6/10) containing an additional TMD on the N-terminus. The prototypical member, ABCC1, has a ubiquitous expression profile and confers resistance to a broad-spectrum of antitumor drugs, glutathione-conjugates, and sulfate-conjugates [210-212]. In membrane vesicle studies, verapamil stimulates transport of GSH via ABCC1 but conjugated oestrogen, oestrone sulfate, and vincristine are efficiently transported without reciprocal translocation of GSH suggestive of a specific allosteric or co-transport role of GSH among different substrates [213-215]. Abcc1-/- mice are healthy but have compromised inflammatory responses due to decreased secretion of leukotriene C4, an endogenous substrate of ABCC1 [216]. Red blood cells derived from abcc1-/- mice loses the ability to translocate NBD-PC and NBD-PS [217]. Active translocation of NBD-PS in wild-type erythrocyte membranes was inhibited by various antitumor drugs, glutathione-conjugates, and oxidized GSSG, indicating ABCC1 may function as a phospholipid translocase [218]. In addition, LLC-PK1 cells transfected with ABCC1 specifically flipped NBD-GlcCer and NBD-SM [219]. Direct evidence showing that ABCC1 may function as a phospholipid translocase was provided by Huang et al, whereby purified ABCC1 reconstituted into proteoliposomes demonstrated NBD-PC, but not NBD-PE, translocation (Fig 6A)[220]. Interestingly, transport of lipid was enhanced by inclusion of GSH. Further studies are required to assess whether GSH is co-transported or serves as an allosteric modulator in PC transport. In addition, a few drugs including vinblastine and doxorubicin partially inhibited NBD-PC, whereas MK-571, an inhibitor of ABCC1 protein, inhibited lipid translocation [220]. Phospholipid translocation by other homologous ABCC subfamily members remains to be investigated.
4.5. ABCG Transporters
Except for ABCG2, all members of the ABCG family function as cholesterol transporters [7, 221]. This subfamily has five members: ABCG1, ABCG2, ABCG4, ABCG5, and ABCG8. All members are half transporters in which two polypeptide chains each containing a single NBD domain followed by a TMD assemble as either homo- or hetero-dimers to form a functional complex.
4.5.1 ABCG1
ABCG1 has been demonstrated to promote cholesterol efflux to HDL, but the mechanism by which ABCG1 mediates cholesterol efflux is not well understood. Like ABCA1, ABCG1 displays similar tissue expression profiles and regulatory pathways [222-226]. In contrast to ABCA1, the efflux activity of ABCG1 is relatively nonspecific, as cholesterol efflux can be promoted to synthetic PC vesicles > LDL > HDL2 > HDL3 [223, 227]. Intriguingly, phospholipid efflux was also observed but occurred in the opposite preference to cholesterol acceptors. Targeted disruption of abcg1 in mice has no effect on plasma lipids but results in massive accumulation of both neutral lipids and phospholipids in hepatocytes and in macrophages within multiple tissues [222]. In cell cultures of HEK293 and HepG2, transfected ABCG1 altered cellular morphology and caspase-3 activation, and PS externalization events were observed [228]. Apparently, benzamil and thyroxin, effective inhibitors of ABCG1 ATPase activity, also decreased annexin V binding indicative of attenuated PS translocation. In addition, PC and SM efflux along with cholesterol has been demonstrated by ABCG1 in HEK293 cells.
Several studies in different cell types have differed on the cellular localization of ABCG1 with minor trafficking to the plasma membrane and the bulk residing in intracellular vesicles [229, 230]. More recently, Tarling and Edwards showed that ABCG1 is specifically associated with endososomal vesicles and, unlike ABCA1, is independent of LXR activation for plasma membrane localization in peritoneal macrophages [231]. Accordingly, this suggests that ABCG1 transfers sterols to the inner leaflet of endosomal vesicles before their fusion to the plasma membrane. This would result in redistribution of these sterols and phospholipids to the outer leaflet of the plasma membrane such that they can desorb in a non-specific manner to multiple acceptors including LDL and HDL.
Studies with abcg1-/- abca1-/- mice demonstrated that loss of both sterol transporters resulted in an even more striking lipid accumulation phenotype in macrophages and specific tissues than in single knockout mice [232, 233]. Thus, both transporters are important for sterol homeostasis and one cannot complement for loss of the other. Sr-B1 is a transmembrane receptor mediating bidirectional cholesterol flux, depending on concentration gradients, in the plasma membrane [221]. A systematic approach using normal and cholesterol loaded primary peritoneal macrophages from abca1-/-, abcg1-/-, or Sr-B1-/- mice was probed to determine the relative contributions of these transmembrane proteins [234]. A substantial 20% of intracellular cholesterol was mobilized/effluxed by ABCG1, ~35% by ABCA1, ~10% by SR-B1 and 35% by human serum (diffusion). In addition, phospholipid efflux analysis via mass spectrometry and TLC revealed that ABCG1 and ABCA1 effluxed several species of SM and PC, with ABCG1 transporting both SM and PC and ABCA1 preferentially transporting PC [235]. These multiple mechanisms point to the importance of maintaining cholesterol and phospholipid levels for cell maintenance and viability.
4.5.2 ABCG2
ABCG2 forms a homodimer that localizes to the plasma membrane of cells. ABCG2 is highly expressed in normal epithelial cells of the placenta, kidney, and intestine, where it has been suggested to play a role in regulating the absorption, circulation and metabolism of xenobiotics [221]. ABCG2 was shown to promote the exofacial exposure of NBD-PS and endogenous PS, as determined by Annexin V binding, in EPG85-257 human gastric carcinoma and MCF-7 breast cancer cells [236].
4.5.3 ABCG4
ABCG4 is 94% identical to ABCG1 but its tissue expression is restricted with the highest levels found in the brain, spinal cord, and testis [237]. ABCG4 also promotes cholesterol efflux to HDL [223, 238]. Vaughan et al showed that ABCG4, in addition to ABCG1, act sequentially with ABCA1 to efflux cholesterol and phospholipids and increase the formation of HDL-like particles, although the gel filtration chromatography of HDL particles for the ABCG4 or ABCG1 expressing cells showed some heterogeneity in the cholesterol distribution [239]. These findings raise the possibility that the ABCG1 and ABCG4 cholesterol export pathways remodel nascent HDL particles in slightly different ways. The observation is suggestive of the fact that these transporters can work in tandem where ABCA1 performs initial lipidation of apo-A1 generating HDL which is then used as an acceptor ABCG1/G4 transport of cholesterol.
4.5.4 ABCG5/ABCG8
The transport system contributing major cholesterol secretion into bile is the heterodimeric transporter ABCG5/G8 [240]. Mutations causing a loss of function of ABCG5 or ABCG8 are associated with β-sitosterolemia, a disorder characterized by enhanced accumulation of plant and shellfish sterols [241]. Largely present in intestines and liver, ABCG5 and ABCG5 heterodimers also transfer plant and shellfish sterols obtained from diet. In abcg5/g8-/- mice, biliary cholesterol secretion is significantly reduced [242], whereas it is significantly increased in response to overexpression in hepatocytes [243]. Wang et al developed the first biochemical sterol transfer assay expressing recombinant ABCG5/G8 heterodimer using purified proteoliposomes and “inside-outside” Sf9 membrane vesicles. Cholesterol and sitosterol, but not PC, behaved as transport substrates and were transferred in an energy dependent manner (Fig 6B)[226, 238]. Similar studies with ABCA1 would also clarify the preferred substrates transported to apo-A1 as part of the RCT pathway. In addition, a sterol binding/sensing motif (L/MxxLxxL) found in steroid/horomone receptors has been identified in the TMD of ABCG2 and is conserved among other family members, including ABCG5/G8 [244]. It will be of interest to evaluate if sterols/other classes of lipids are modulated by ABC transporters involved/implicated in cholesterol efflux.
5. Apo-E and lipid homeostasis in the brain
The brain is separated from plasma via the blood-brain-barrier and thus operates its own lipoprotein system. The cerebrospinal spinal fluid (CSF) contains lipoproteins whose composition is distinct from that in plasma. Several ABC transporters are expressed in the brain, including ABCA1 and ABCG1 which play important roles in the transfer of phospholipid and cholesterol efflux to apo-E [130]. The major apolipoprotein of HDL-like particles in the central nervous system (CNS) is apo-E which is made by astrocytes (and a few types of neurons), cells associated with the maintenance of neurons. Apo-E is secreted by glial cells and assembled with phospholipids and cholesterol into HDL-sized lipoprotein particles.
Studies on transgenic mice lacking or over-expressing ABCA1 support the view that this ABC transporter contributes to the lipidation of apo-E, although its role in astrocytes remains to be clarified. Abca1-/- mice have reduced cholesterol content and size of apo-E in CSF by 24% and reduced cholesterol concentration in the culture medium of astrocytes [245, 246]. Conversely, overexpression of ABCA1 in the mouse brain increased the size of CSF lipoproteins and enhanced the cholesterol to apo-E ratio in lipoproteins from cultured astrocytes [247]. This may signify that in the absence of ABCA1, there is enhanced catabolism of apo-E due to insufficient lipidation. Finally, absence of apo-E enhanced the transcript level of abca1 in the brain indicating that ABC transporters can compensate for the loss of apo-E-mediated cholesterol release. Astrocyte-secreted human apo-E is present in high density-like lipoproteins of three predominant sizes ranging from 8 to 15 nm in diameter. ABCA1 and ABCG1 are expressed in the brain, in both neurons and glial cells, suggesting that these transporters participate in the formation of apo-E-containing lipoproteins in the brain. Detailed characterization of phospholipid composition by electrospray ionization mass spectrometry analysis showed that PE was more abundant than PC in the apo-E particles [248]. The significance of this unusual composition remains to be established.
The residual efflux of cholesterol from astrocytes lacking ABCA1 may be mediated by ABCG transporters. Astrocytes from mice lacking both ABCG1 and ABCG4 showed reduced release of cholesterol and desmosterol onto high-density lipoprotein particles [238] and in cultured cerebellar glia, the extent of cholesterol efflux correlated with the expression level of ABCG1, but not of ABCA1 [249]. On the other hand, overexpression of ABCG1 in the CNS did not affect the apo-E content in the brain or the cholesterol efflux from cultured glial cells [250]. Interestingly, increasing the levels of ABCG1 in glial cells enhanced the ability of glia-derived lipoproteins to stimulate axonal growth in vitro [251].
In summary, there is good evidence that ABC transporters in astrocytes secrete cholesterol and phospholipids via apo-E containing lipoproteins. These lipoproteins exhibit different properties than those found in plasma, although the molecular mechanisms underlying these differences remain to be investigated.
Conclusions and Future Directions
Over the past decade significant progress has been made in understanding the molecular and cellular basis for phospholipid transport across membranes. P4-ATPases and some members of the ABC transporter family are the principal membrane proteins that carry out active phospholipid transport. Genetic, biochemical, molecular and cell biology studies using various eukaryotic cell systems including yeast, plants, mice, humans and other organisms point to the crucial role of these lipid transporters in the generation and maintenance of lipid asymmetry, lipidation of plasma proteins, cholesterol efflux, bile production, membrane budding and vesicle trafficking, vision, phagocytosis, apoptosis, membrane protein function and regulation, and membrane curvature among others.
Recent biochemical studies now directly support the function of P4-ATPases as flippases which actively transport aminophospholipids from the exocytoplasmic to the cytosolic leaflet of cell membranes. The contributions of CDC50 β-subunits on modulation of the lipid transport activity of P4-ATPases, protein stability, and cellular localization are becoming more well-defined. Mutagenesis studies in conjunction with improved protein expression systems and molecular modeling based on other P-type ATPases have identified a number of key residues important in the phospholipid translocation pathway and plausible steps in the catalytic transport cycle.
However, many fundamental issues remain to be resolved. Although sequence analysis suggests that all 14 members of the mammalian P4-ATPase family may function as phospholipid flippases, to date only one member, ATP8A2, has been purified and shown experimentally to actively flip aminophospholipids and several other members have been implicated in aminophospholipid transport on the basis of genetic and cellular studies. A future challenge is to isolate and characterize other P4-ATPases to more fully understand their phospholipid substrate specificity, define interactions with CDC50 subunit and other cellular proteins, determine the in vivo tissue distribution and subcellular localization, and elucidate regulatory mechanisms. Additionally, the role of various P4-ATPases in such cellular processes as vesicle trafficking, bile secretion, fertilization, lipid asymmetry, phagocytosis and membrane protein structure and function needs further study through the generation and characterization of transgenic and knockout animal models, analysis of protein-protein interactions, and functional studies. Although many of the structural properties of P4-ATPases appear to resemble other P-type ATPases, it will be important to determine the high resolution structure of a P4-ATPase to more fully define phospholipid recognition and transport mechanisms.
ABC transporters are known to transport a diverse set of substrates across cell membranes. Cell-based studies have implicated many ABC transporters in the movement of phospholipids across cell membranes. These include some members of the ABCA, ABCB, ABCC and ABCG subfamilies. In many cases these ABC transporters appear to serve as ‘true’ exporters in that they transport phospholipids from the cytoplasmic leaflet of a membrane to acceptor proteins or extrude the phospholipid into the extracellular medium. In some instances, however, ABC transporters appear to function as floppases or flippases, translocating phospholipids from one leaflet of the lipid bilayer to the other. The importance of ABC transporters in phospholipid translocation is apparent in the finding that mutations in genes encoding many of these lipid transporters cause severe human diseases.
Despite the emerging importance of ABC transporters in lipid transport and homeostasis, relatively few ABC transporters have been characterized at biochemical level. As a result the information on the phospholipid substrate specificity, transport mechanisms and regulatory properties are limited. Likewise, the high-resolution structures for only a few ABC transporters have been reported to date. Additional structures with and without bound phospholipid substrates and nucleotides will be needed to more fully clarify the mechanism by which ATP binding and hydrolysis promotes phospholipid transport by ABC proteins.
The next decade should witness significant advances in our understanding of phospholipid transport by P4-ATPases and ABC transporters at a molecular and cellular level. This knowledge should provide increased insight into the role of these transporters in physiological processes and facilitate the search for therapeutic treatments for diseases linked to defects in phospholipid transport.
Highlights.
Up to date review on phospholipid transport across cell membranes
Review of the role of P4-ATPases in the flipping of aminophospholipids across cell membranes
Review of the role of ABC transporters in the efflux and flipping of phospholipids across cell membranes.
Overview of the role of P4-ATases and ABC transporters in various cellular processes and disease
Table 2.
Mammalian ABC transporters, related lipid substrates, and associated genetic disorders.
| Gene | Major sites of expression | Substrates | Modulators | Acceptors | Associated genetic disorders | References |
|---|---|---|---|---|---|---|
| ABCA1 | Ubiquitous | PC, PS, Chol, SM, | Cer (stimulates) LacCer (inhibits) | Apo-A1, AII, E, | Tangier disease | [123, 151, 152, 259, 260] |
| ABCA2 | Brain | Chol, PE, PS | LDL | [157, 158] | ||
| ABCA3 | Lung, brain, heart, pancreas | PC, PG, PE | Surfactant | Neonatal surfactant lung deficiency, chronic interstitial lung disease | [125, 159-162] | |
| ABCA4 (ABCR) | Rod photoreceptors | N-ret-PE, PE | All-trans retinal 11 cis-retinal |
(Cytosolic) | Stargardt's disease, cone-rod dystrophy, retinitis pigmentosa, age-related macular degeneration | [19, 126, 167-169, 174, 175] |
| ABCA7 | Myelolymphatic system, brain, kidney, skin | PC, SM, Chol, (PS?) | Apo-AI/Apo-AII, Apo-E disc | (Sjogren's syndrome?) | [184-187] | |
| ABCA12 | Skin keratinocytes | Cer, GlcCer | Harlequin ichthyosis, lamellar ichthyosis type 2, non-bullous congenital ichthyosiform erythroderma | [127, 128, 193-195] | ||
| ABCB1 (Pgp) | Brain, liver, kidneys, GI, placenta | PC, PS, PE, Chol, SM, GlcCer, PAF | short chain PC (8:0), DHA PC (22:0) (inhibits) | [196, 197, 199-201, 261] | ||
| ABCB4 | Liver, canalicular membrane, placenta | PC, PE, SM | Bile salts | Progressive familial intrahepatic cholestasis type 3 | [196, 205-207, 262] | |
| ABCB11 | canalicular membrane | Bile salt | Progressive familial intrahepatic cholestasis type 2 | [196, 203] | ||
| ABCC1 | Ubiquitous | PC, PS, SM, GlcCer | GSH (stimulates) | [217-220] | ||
| ABCG1 | Ubiquitous | Chol, PS, PC, SM, | Thyroxin (inhibits) Benzamil (inhibits) SM (stimulates) |
HDL, LDL, PC vesicle, BSA, cyclodextrin | [231] [223, 227, 228, 235] | |
| ABCG2 | Placenta, breast, liver, GI | PC, PS | Chol (stimulates) | [236, 263, 264] | ||
| ABCG4 | Macrophage, brain, eye, spleen, liver | Chol | [223, 238, 239] | |||
| ABCG5/G8 | Liver, GI | Plant sterols, Chol | HDL, Bile salts | β-Sitosterolemia | [229, 241, 265] |
Apo, apolipoprotein; Cer, ceramide; Chol, cholesterol; DHA, docosahexaeonic acid; LacCer, Lactosylceramide; LDL, low density lipoprotein; GlcCer, glucosylceramide; GSH, glutathione, GI, gastrointestinal tract;; HDL, high-density lipoprotein; PAF, platelet activating factor
Acknowledgements
This work was supported by grants from the National Institutes of Health (EY002422) and the Canadian Institutes for Health Research (MOP-106667). JAC and FQ are supported on National Sciences and Engineering Council predoctoral studentships. RSM holds a Canada Research Chair in Vision and Macular Degeneration.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Holthuis JC, Levine TP. Lipid traffic: floppy drives and a superhighway. Nat Rev Mol Cell Biol. 2005;6:209–220. doi: 10.1038/nrm1591. [DOI] [PubMed] [Google Scholar]
- 2.van Meer G, Voelker DR, Feigenson GW. Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol. 2008;9:112–124. doi: 10.1038/nrm2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Daleke DL. Phospholipid flippases. J Biol Chem. 2007;282:821–825. doi: 10.1074/jbc.R600035200. [DOI] [PubMed] [Google Scholar]
- 4.Pomorski T, Menon AK. Lipid flippases and their biological functions. Cell Mol Life Sci. 2006;63:2908–2921. doi: 10.1007/s00018-006-6167-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Folmer DE, Elferink RP, Paulusma CC. P4 ATPases - lipid flippases and their role in disease. Biochim Biophys Acta. 2009;1791:628–635. doi: 10.1016/j.bbalip.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 6.Kaminski WE, Piehler A, Wenzel JJ. ABC A-subfamily transporters: structure, function and disease. Biochim Biophys Acta. 2006;1762:510–524. doi: 10.1016/j.bbadis.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 7.Quazi F, Molday RS. Lipid transport by mammalian ABC proteins. Essays Biochem. 2011;50:265–290. doi: 10.1042/bse0500265. [DOI] [PubMed] [Google Scholar]
- 8.Chang QL, Gummadi SN, Menon AK. Chemical modification identifies two populations of glycerophospholipid flippase in rat liver ER. Biochemistry. 2004;43:10710–10718. doi: 10.1021/bi049063a. [DOI] [PubMed] [Google Scholar]
- 9.Menon I, Huber T, Sanyal S, Banerjee S, Barre P, Canis S, Warren JD, Hwa J, Sakmar TP, Menon AK. Opsin is a phospholipid flippase. Curr Biol. 2011;21:149–153. doi: 10.1016/j.cub.2010.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou Q, Zhao J, Stout JG, Luhm RA, Wiedmer T, Sims PJ. Molecular cloning of human plasma membrane phospholipid scramblase. A protein mediating transbilayer movement of plasma membrane phospholipids. J Biol Chem. 1997;272:18240–18244. doi: 10.1074/jbc.272.29.18240. [DOI] [PubMed] [Google Scholar]
- 11.Coleman JA, Kwok MC, Molday RS. Localization, purification, and functional reconstitution of the P4-ATPase Atp8a2, a phosphatidylserine flippase in photoreceptor disc membranes. J Biol Chem. 2009;284:32670–32679. doi: 10.1074/jbc.M109.047415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou X, Graham TR. Reconstitution of phospholipid translocase activity with purified Drs2p, a type-IV P-type ATPase from budding yeast. Proc Natl Acad Sci U S A. 2009;106:16586–16591. doi: 10.1073/pnas.0904293106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yabas M, Teh CE, Frankenreiter S, Lal D, Roots CM, Whittle B, Andrews DT, Zhang Y, Teoh NC, Sprent J, Tze LE, Kucharska EM, Kofler J, Farell GC, Broer S, Goodnow CC, Enders A. ATP11C is critical for the internalization of phosphatidylserine and differentiation of B lymphocytes. Nat Immunol. 2011;12:441–449. doi: 10.1038/ni.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Puts CF, Holthuis JC. Mechanism and significance of P4 ATPase-catalyzed lipid transport: lessons from a Na+/K+-pump. Biochim Biophys Acta. 2009;1791:603–611. doi: 10.1016/j.bbalip.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 15.Levano K, Punia V, Raghunath M, Debata PR, Curcio GM, Mogha A, Purkayastha S, McCloskey D, Fata J, Banerjee P. Atp8a1 deficiency is associated with phosphatidylserine externalization in hippocampus and delayed hippocampus-dependent learning. J Neurochem. 2012;120:302–313. doi: 10.1111/j.1471-4159.2011.07543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bull LN, van Eijk MJ, Pawlikowska L, DeYoung JA, Juijn JA, Liao M, Klomp LW, Lomri N, Berger R, Scharschmidt BF, Knisely AS, Houwen RH, Freimer NB. A gene encoding a P-type ATPase mutated in two forms of hereditary cholestasis. Nat Genet. 1998;18:219–224. doi: 10.1038/ng0398-219. [DOI] [PubMed] [Google Scholar]
- 17.Klomp LW, Vargas JC, van Mil SW, Pawlikowska L, Strautnieks SS, van Eijk MJ, Juijn JA, Pabon-Pena C, Smith LB, DeYoung JA, Byrne JA, Gombert J, van der Brugge G, Berger R, Jankowska I, Pawlowska J, Villa E, Knisely AS, Thompson RJ, Freimer NB, Houwen RH, Bull LN. Characterization of mutations in ATP8B1 associated with hereditary cholestasis. Hepatology. 2004;40:27–38. doi: 10.1002/hep.20285. [DOI] [PubMed] [Google Scholar]
- 18.Borst P, Zelcer N, van Helvoort A. ABC transporters in lipid transport. Biochim Biophys Acta. 2000;1486:128–144. doi: 10.1016/s1388-1981(00)00053-6. [DOI] [PubMed] [Google Scholar]
- 19.Quazi F, Lenevich S, Molday RS. ABCA4 is an N-retinylidene-phosphatidylethanolamine and phosphatidylethanolamine importer. Nat Commun. 2012;3:925. doi: 10.1038/ncomms1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Meer G, Halter D, Sprong H, Somerharju P, Egmond MR. ABC lipid transporters: extruders, flippases, or flopless activators? FEBS Lett. 2006;580:1171–1177. doi: 10.1016/j.febslet.2005.12.019. [DOI] [PubMed] [Google Scholar]
- 21.Sebastian TT, Baldridge RD, Xu P, Graham TR. Phospholipid flippases: Building asymmetric membranes and transport vesicles. Biochim Biophys Acta. 2012;1821:1068–1077. doi: 10.1016/j.bbalip.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuhlbrandt W. Biology, structure and mechanism of P-type ATPases. Nat Rev Mol Cell Biol. 2004;5:282–295. doi: 10.1038/nrm1354. [DOI] [PubMed] [Google Scholar]
- 23.Toyoshima C. How Ca2+-ATPase pumps ions across the sarcoplasmic reticulum membrane. Biochim Biophys Acta. 2009;1793:941–946. doi: 10.1016/j.bbamcr.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 24.Toyoshima C, Kanai R, Cornelius F. First crystal structures of Na+,K+-ATPase: new light on the oldest ion pump. Structure. 2011;19:1732–1738. doi: 10.1016/j.str.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 25.Gourdon P, Liu XY, Skjorringe T, Morth JP, Moller LB, Pedersen BP, Nissen P. Crystal structure of a copper-transporting PIB-type ATPase. Nature. 2011;475:59–64. doi: 10.1038/nature10191. [DOI] [PubMed] [Google Scholar]
- 26.Pedersen BP, Buch-Pedersen MJ, Morth JP, Palmgren MG, Nissen P. Crystal structure of the plasma membrane proton pump. Nature. 2007;450:1111–1114. doi: 10.1038/nature06417. [DOI] [PubMed] [Google Scholar]
- 27.Osada N, Hashimoto K, Hirai M, Kusuda J. Aberrant termination of reproduction-related TMEM30C transcripts in the hominoids. Gene. 2007;392:151–156. doi: 10.1016/j.gene.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 28.Takatsu H, Baba K, Shima T, Umino H, Kato U, Umeda M, Nakayama K, Shin HW. ATP9B, a P4-ATPase (a putative aminophospholipid translocase), localizes to the trans-Golgi network in a CDC50 protein-independent manner. J Biol Chem. 2011;286:38159–38167. doi: 10.1074/jbc.M111.281006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paulusma CC, Folmer DE, Ho-Mok KS, de Waart DR, Hilarius PM, Verhoeven AJ, Oude Elferink RP. ATP8B1 requires an accessory protein for endoplasmic reticulum exit and plasma membrane lipid flippase activity. Hepatology. 2008;47:268–278. doi: 10.1002/hep.21950. [DOI] [PubMed] [Google Scholar]
- 30.van der Velden LM, Wichers CG, van Breevoort AE, Coleman JA, Molday RS, Berger R, Klomp LW, van de Graaf SF. Heteromeric interactions required for abundance and subcellular localization of human CDC50 proteins and class 1 P4-ATPases. J Biol Chem. 2010;285:40088–40096. doi: 10.1074/jbc.M110.139006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coleman JA, Molday RS. Critical role of the beta-subunit CDC50A in the stable expression, assembly, subcellular localization, and lipid transport activity of the P4-ATPase ATP8A2. J Biol Chem. 2011;286:17205–17216. doi: 10.1074/jbc.M111.229419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Darland-Ransom M, Wang X, Sun CL, Mapes J, Gengyo-Ando K, Mitani S, Xue D. Role of C. elegans TAT-1 protein in maintaining plasma membrane phosphatidylserine asymmetry. Science. 2008;320:528–531. doi: 10.1126/science.1155847. [DOI] [PubMed] [Google Scholar]
- 33.Buton X, Morrot G, Fellmann P, Seigneuret M. Ultrafast glycerophospholipid-selective transbilayer motion mediated by a protein in the endoplasmic reticulum membrane. J Biol Chem. 1996;271:6651–6657. doi: 10.1074/jbc.271.12.6651. [DOI] [PubMed] [Google Scholar]
- 34.Hua Z, Fatheddin P, Graham TR. An essential subfamily of Drs2p-related P-type ATPases is required for protein trafficking between Golgi complex and endosomal/vacuolar system. Mol Biol Cell. 2002;13:3162–3177. doi: 10.1091/mbc.E02-03-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pomorski T, Lombardi R, Riezman H, Devaux PF, van Meer G, Holthuis JC. Drs2p-related P-type ATPases Dnf1p and Dnf2p are required for phospholipid translocation across the yeast plasma membrane and serve a role in endocytosis. Mol Biol Cell. 2003;14:1240–1254. doi: 10.1091/mbc.E02-08-0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ruaud AF, Nilsson L, Richard F, Larsen MK, Bessereau JL, Tuck S. The C. elegans P4-ATPase TAT-1 regulates lysosome biogenesis and endocytosis. Traffic. 2009;10:88–100. doi: 10.1111/j.1600-0854.2008.00844.x. [DOI] [PubMed] [Google Scholar]
- 37.Wehman AM, Poggioli C, Schweinsberg P, Grant BD, Nance J. The P4-ATPase TAT-5 inhibits the budding of extracellular vesicles in C. elegans embryos. Curr Biol. 2011;21:1951–1959. doi: 10.1016/j.cub.2011.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gall WE, Geething NC, Hua Z, Ingram MF, Liu K, Chen SI, Graham TR. Drs2p-dependent formation of exocytic clathrin-coated vesicles in vivo. Curr Biol. 2002;12:1623–1627. doi: 10.1016/s0960-9822(02)01148-x. [DOI] [PubMed] [Google Scholar]
- 39.Chen CY, Ingram MF, Rosal PH, Graham TR. Role for Drs2p, a P-type ATPase and potential aminophospholipid translocase, in yeast late Golgi function. J Cell Biol. 1999;147:1223–1236. doi: 10.1083/jcb.147.6.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chantalat S, Park SK, Hua Z, Liu K, Gobin R, Peyroche A, Rambourg A, Graham TR, Jackson CL. The Arf activator Gea2p and the P-type ATPase Drs2p interact at the Golgi in Saccharomyces cerevisiae. J Cell Sci. 2004;117:711–722. doi: 10.1242/jcs.00896. [DOI] [PubMed] [Google Scholar]
- 41.Natarajan P, Liu K, Patil DV, Sciorra VA, Jackson CL, Graham TR. Regulation of a Golgi flippase by phosphoinositides and an ArfGEF. Nat Cell Biol. 2009;11:1421–1426. doi: 10.1038/ncb1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Palmgren MG, Nissen P. P-type ATPases. Annu Rev Biophys. 2011;40:243–266. doi: 10.1146/annurev.biophys.093008.131331. [DOI] [PubMed] [Google Scholar]
- 43.Enyedi A, Verma AK, Filoteo AG, Penniston JT. A highly active 120-kDa truncated mutant of the plasma membrane Ca2+ pump. J Biol Chem. 1993;268:10621–10626. [PubMed] [Google Scholar]
- 44.Palmgren MG, Sommarin M, Serrano R, Larsson C. Identification of an autoinhibitory domain in the C-terminal region of the plant plasma membrane H(+)-ATPase. J Biol Chem. 1991;266:20470–20475. [PubMed] [Google Scholar]
- 45.Coleman JA, Vestergaard AL, Molday RS, Vilsen B, Peter Andersen J. Critical role of a transmembrane lysine in aminophospholipid transport by mammalian photoreceptor P4-ATPase ATP8A2. Proc Natl Acad Sci U S A. 2012;109:1449–1454. doi: 10.1073/pnas.1108862109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jacquot A, Montigny C, Hennrich H, Barry R, le Maire M, Jaxel C, Holthuis J, Champeil P, Lenoir G. Phosphatidylserine stimulation of Drs2p.Cdc50p lipid translocase dephosphorylation is controlled by phosphatidylinositol-4-phosphate. J Biol Chem. 2012;287:13249–13261. doi: 10.1074/jbc.M111.313916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lenoir G, Williamson P, Holthuis JC. On the origin of lipid asymmetry: the flip side of ion transport. Curr Opin Chem Biol. 2007;11:654–661. doi: 10.1016/j.cbpa.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 48.Baldridge RD, Graham TR. Identification of residues defining phospholipid flippase substrate specificity of type IV P-type ATPases. Proc Natl Acad Sci U S A. 2012;109:E290–298. doi: 10.1073/pnas.1115725109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thogersen L, Nissen P. Flexible P-type ATPases interacting with the membrane. Curr Opin Struct Biol. 2012;22:491–499. doi: 10.1016/j.sbi.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 50.Bryde S, Hennrich H, Verhulst PM, Devaux PF, Lenoir G, Holthuis JC. CDC50 proteins are critical components of the human class-1 P4-ATPase transport machinery. J Biol Chem. 2010;285:40562–40572. doi: 10.1074/jbc.M110.139543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lopez-Marques RL, Poulsen LR, Hanisch S, Meffert K, Buch-Pedersen MJ, Jakobsen MK, Pomorski TG, Palmgren MG. Intracellular targeting signals and lipid specificity determinants of the ALA/ALIS P4-ATPase complex reside in the catalytic ALA alpha-subunit. Mol Biol Cell. 2010;21:791–801. doi: 10.1091/mbc.E09-08-0656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lenoir G, Williamson P, Puts CF, Holthuis JC. Cdc50p plays a vital role in the ATPase reaction cycle of the putative aminophospholipid transporter Drs2p. J Biol Chem. 2009;284:17956–17967. doi: 10.1074/jbc.M109.013722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Puts CF, Panatala R, Hennrich H, Tsareva A, Williamson P, Holthuis JC. Mapping functional interactions in a heterodimeric phospholipid pump. J Biol Chem. 2012 Jul 12; doi: 10.1074/jbc.M112.371088. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tang X, Halleck MS, Schlegel RA, Williamson P. A subfamily of P-type ATPases with aminophospholipid transporting activity. Science. 1996;272:1495–1497. doi: 10.1126/science.272.5267.1495. [DOI] [PubMed] [Google Scholar]
- 55.Mouro I, Halleck MS, Schlegel RA, Mattei MG, Williamson P, Zachowski A, Devaux P, Cartron JP, Colin Y. Cloning, expression, and chromosomal mapping of a human ATPase II gene, member of the third subfamily of P-type ATPases and orthologous to the presumed bovine and murine aminophospholipid translocase. Biochem Biophys Res Commun. 1999;257:333–339. doi: 10.1006/bbrc.1999.0347. [DOI] [PubMed] [Google Scholar]
- 56.Morrot G, Zachowski A, Devaux PF. Partial purification and characterization of the human erythrocyte Mg2(+)-ATPase. A candidate aminophospholipid translocase. FEBS Lett. 1990;266:29–32. doi: 10.1016/0014-5793(90)81498-d. [DOI] [PubMed] [Google Scholar]
- 57.Zimmerman ML, Daleke DL. Regulation of a candidate aminophospholipid-transporting ATPase by lipid. Biochemistry. 1993;32:12257–12263. doi: 10.1021/bi00096a040. [DOI] [PubMed] [Google Scholar]
- 58.Moriyama Y, Nelson N. Purification and properties of a vanadate- and N-ethylmaleimide-sensitive ATPase from chromaffin granule membranes. J Biol Chem. 1988;263:8521–8527. [PubMed] [Google Scholar]
- 59.Xie XS, Stone DK, Racker E. Purification of a vanadate-sensitive ATPase from clathrin-coated vesicles of bovine brain. J Biol Chem. 1989;264:1710–1714. [PubMed] [Google Scholar]
- 60.Seigneuret M, Devaux PF. ATP-dependent asymmetric distribution of spin-labeled phospholipids in the erythrocyte membrane: relation to shape changes. Proc Natl Acad Sci U S A. 1984;81:3751–3755. doi: 10.1073/pnas.81.12.3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zachowski A, Henry JP, Devaux PF. Control of transmembrane lipid asymmetry in chromaffin granules by an ATP-dependent protein. Nature. 1989;340:75–76. doi: 10.1038/340075a0. [DOI] [PubMed] [Google Scholar]
- 62.Daleke DL, Huestis WH. Erythrocyte morphology reflects the transbilayer distribution of incorporated phospholipids. J Cell Biol. 1989;108:1375–1385. doi: 10.1083/jcb.108.4.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Beleznay Z, Zachowski A, Devaux PF, Navazo MP, Ott P. ATP-dependent aminophospholipid translocation in erythrocyte vesicles: stoichiometry of transport. Biochemistry. 1993;32:3146–3152. doi: 10.1021/bi00063a029. [DOI] [PubMed] [Google Scholar]
- 64.Auland ME, Roufogalis BD, Devaux PF, Zachowski A. Reconstitution of ATP-dependent aminophospholipid translocation in proteoliposomes. Proc Natl Acad Sci U S A. 1994;91:10938–10942. doi: 10.1073/pnas.91.23.10938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Soupene E, Kuypers FA. Identification of an erythroid ATP-dependent aminophospholipid transporter. Br J Haematol. 2006;133:436–438. doi: 10.1111/j.1365-2141.2006.06051.x. [DOI] [PubMed] [Google Scholar]
- 66.Ding J, Wu Z, Crider BP, Ma Y, Li X, Slaughter C, Gong L, Xie XS. Identification and functional expression of four isoforms of ATPase II, the putative aminophospholipid translocase. Effect of isoform variation on the ATPase activity and phospholipid specificity. J Biol Chem. 2000;275:23378–23386. doi: 10.1074/jbc.M910319199. [DOI] [PubMed] [Google Scholar]
- 67.Paterson JK, Renkema K, Burden L, Halleck MS, Schlegel RA, Williamson P, Daleke DL. Lipid specific activation of the murine P4-ATPase Atp8a1 (ATPase II) Biochemistry. 2006;45:5367–5376. doi: 10.1021/bi052359b. [DOI] [PubMed] [Google Scholar]
- 68.Cacciagli P, Haddad MR, Mignon-Ravix C, El-Waly B, Moncla A, Missirian C, Chabrol B, Villard L. Disruption of the ATP8A2 gene in a patient with a t(10;13) de novo balanced translocation and a severe neurological phenotype. Eur J Hum Genet. 2010;18:1360–1363. doi: 10.1038/ejhg.2010.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Emre Onat O, Gulsuner S, Bilguvar K, Nazli Basak A, Topaloglu H, Tan M, Tan U, Gunel M, Ozcelik T. Missense mutation in the ATPase, aminophospholipid transporter protein ATP8A2 is associated with cerebellar atrophy and quadrupedal locomotion. Eur J Hum Genet. 2012 Aug 15; doi: 10.1038/ejhg.2012.170. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhu X, Libby RT, de Vries WN, Smith RS, Wright DL, Bronson RT, Seburn KL, John SW. Mutations in a p-type ATPase gene cause axonal degeneration. PLoS Genet. 2012;8:e1002853. doi: 10.1371/journal.pgen.1002853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mustafi D, Kevany BM, Genoud C, Okano K, Cideciyan AV, Sumaroka A, Roman AJ, Jacobson SG, Engel A, Adams MD, Palczewski K. Defective photoreceptor phagocytosis in a mouse model of enhanced S-cone syndrome causes progressive retinal degeneration. FASEB J. 2011;25:3157–3176. doi: 10.1096/fj.11-186767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ruggiero L, Connor MP, Chen J, Langen R, Finnemann SC. Diurnal, localized exposure of phosphatidylserine by rod outer segment tips in wild-type but not Itgb5-/- or Mfge8-/- mouse retina. Proc Natl Acad Sci U S A. 2012;109:8145–8148. doi: 10.1073/pnas.1121101109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xu Q, Yang GY, Liu N, Xu P, Chen YL, Zhou Z, Luo ZG, Ding X. P(4)-ATPase ATP8A2 acts in synergy with CDC50A to enhance neurite outgrowth. FEBS Lett. 586(2012):1803–12. doi: 10.1016/j.febslet.2012.05.018. [DOI] [PubMed] [Google Scholar]
- 74.Stapelbroek JM, Peters TA, van Beurden DH, Curfs JH, Joosten A, Beynon AJ, van Leeuwen BM, van der Velden LM, Bull L, Oude Elferink RP, van Zanten BA, Klomp LW, Houwen RH. ATP8B1 is essential for maintaining normal hearing. Proc Natl Acad Sci U S A. 2009;106:9709–9714. doi: 10.1073/pnas.0807919106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ray NB, Durairaj L, Chen BB, McVerry BJ, Ryan AJ, Donahoe M, Waltenbaugh AK, O'Donnell CP, Henderson FC, Etscheidt CA, McCoy DM, Agassandian M, Hayes-Rowan EC, Coon TA, Butler PL, Gakhar L, Mathur SN, Sieren JC, Tyurina YY, Kagan VE, McLennan G, Mallampalli RK. Dynamic regulation of cardiolipin by the lipid pump Atp8b1 determines the severity of lung injury in experimental pneumonia. Nat Med. 2010;16:1120–1127. doi: 10.1038/nm.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Eppens EF, van Mil SW, de Vree JM, Mok KS, Juijn JA, Oude Elferink RP, Berger R, Houwen RH, Klomp LW. FIC1, the protein affected in two forms of hereditary cholestasis, is localized in the cholangiocyte and the canalicular membrane of the hepatocyte. J Hepatol. 2001;35:436–443. doi: 10.1016/s0168-8278(01)00158-1. [DOI] [PubMed] [Google Scholar]
- 77.Paulusma CC, Groen A, Kunne C, Ho-Mok KS, Spijkerboer AL, Rudi de Waart D, Hoek FJ, Vreeling H, Hoeben KA, van Marle J, Pawlikowska L, Bull LN, Hofmann AF, Knisely AS, Oude Elferink RP. Atp8b1 deficiency in mice reduces resistance of the canalicular membrane to hydrophobic bile salts and impairs bile salt transport. Hepatology. 2006;44:195–204. doi: 10.1002/hep.21212. [DOI] [PubMed] [Google Scholar]
- 78.Groen A, Romero MR, Kunne C, Hoosdally SJ, Dixon PH, Wooding C, Williamson C, Seppen J, Van den Oever K, Mok KS, Paulusma CC, Linton KJ, Oude Elferink RP. Complementary functions of the flippase ATP8B1 and the floppase ABCB4 in maintaining canalicular membrane integrity. Gastroenterology. 2011;141:1927–1937. e1921–1924. doi: 10.1053/j.gastro.2011.07.042. [DOI] [PubMed] [Google Scholar]
- 79.Verhulst PM, van der Velden LM, Oorschot V, van Faassen EE, Klumperman J, Houwen RH, Pomorski TG, Holthuis JC, Klomp LW. A flippase-independent function of ATP8B1, the protein affected in familial intrahepatic cholestasis type 1, is required for apical protein expression and microvillus formation in polarized epithelial cells. Hepatology. 2010;51:2049–2060. doi: 10.1002/hep.23586. [DOI] [PubMed] [Google Scholar]
- 80.Folmer DE, Mok KS, de Wee SW, Duijst S, Hiralall JK, Seppen J, Oude Elferink RP, Paulusma CC. Cellular localization and biochemical analysis of mammalian CDC50A, a glycosylated beta-subunit for P4 ATPases. J Histochem Cytochem. 2012;60:205–218. doi: 10.1369/0022155411435705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li H, Wetten S, Li L, St Jean PL, Upmanyu R, Surh L, Hosford D, Barnes MR, Briley JD, Borrie M, Coletta N, Delisle R, Dhalla D, Ehm MG, Feldman HH, Fornazzari L, Gauthier S, Goodgame N, Guzman D, Hammond S, Hollingworth P, Hsiung GY, Johnson J, Kelly DD, Keren R, Kertesz A, King KS, Lovestone S, Loy-English I, Matthews PM, Owen MJ, Plumpton M, Pryse-Phillips W, Prinjha RK, Richardson JC, Saunders A, Slater AJ, St George-Hyslop PH, Stinnett SW, Swartz JE, Taylor RL, Wherrett J, Williams J, Yarnall DP, Gibson RA, Irizarry MC, Middleton LT, Roses AD. Candidate single-nucleotide polymorphisms from a genomewide association study of Alzheimer disease. Arch Neurol. 2008;65:45–53. doi: 10.1001/archneurol.2007.3. [DOI] [PubMed] [Google Scholar]
- 82.Gong EY, Park E, Lee HJ, Lee K. Expression of Atp8b3 in murine testis and its characterization as a testis specific P-type ATPase. Reproduction. 2009;137:345–351. doi: 10.1530/REP-08-0048. [DOI] [PubMed] [Google Scholar]
- 83.Wang L, Beserra C, Garbers DL. A novel aminophospholipid transporter exclusively expressed in spermatozoa is required for membrane lipid asymmetry and normal fertilization. Dev Biol. 2004;267:203–215. doi: 10.1016/j.ydbio.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 84.Xu P, Okkeri J, Hanisch S, Hu RY, Xu Q, Pomorski TG, Ding XY. Identification of a novel mouse P4-ATPase family member highly expressed during spermatogenesis. J Cell Sci. 2009;122:2866–2876. doi: 10.1242/jcs.047423. [DOI] [PubMed] [Google Scholar]
- 85.Wicky S, Schwarz H, Singer-Kruger B. Molecular interactions of yeast Neo1p, an essential member of the Drs2 family of aminophospholipid translocases, and its role in membrane trafficking within the endomembrane system. Mol Cell Biol. 2004;24:7402–7418. doi: 10.1128/MCB.24.17.7402-7418.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dhar MS, Sommardahl CS, Kirkland T, Nelson S, Donnell R, Johnson DK, Castellani LW. Mice heterozygous for Atp10c, a putative amphipath, represent a novel model of obesity and type 2 diabetes. J Nutr. 2004;134:799–805. doi: 10.1093/jn/134.4.799. [DOI] [PubMed] [Google Scholar]
- 87.Dhar MS, Yuan JS, Elliott SB, Sommardahl C. A type IV P-type ATPase affects insulin-mediated glucose uptake in adipose tissue and skeletal muscle in mice. J Nutr Biochem. 2006;17:811–820. doi: 10.1016/j.jnutbio.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 88.Hurst SE, Minkin SC, Biggerstaff J, Dhar MS. Transient Silencing of a Type IV P-Type ATPase, Atp10c, Results in Decreased Glucose Uptake in C2C12 Myotubes. J Nutr Metab. 2012:152902. doi: 10.1155/2012/152902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Roshwalb S, Gorman S, Hurst S, Bartges J, Agarwal S, Sommardahl C, Odoi A, Dhar MS. mRNA expression of canine ATP10C, a P4-type ATPase, is positively associated with body condition score. Vet J. 2011;190:173–175. doi: 10.1016/j.tvjl.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 90.Flamant S, Pescher P, Lemercier B, Clement-Ziza M, Kepes F, Fellous M, Milon G, Marchal G, Besmond C. Characterization of a putative type IV aminophospholipid transporter P-type ATPase. Mamm Genome. 2003;14:21–30. doi: 10.1007/s00335-002-3032-3. [DOI] [PubMed] [Google Scholar]
- 91.Meguro M, Kashiwagi A, Mitsuya K, Nakao M, Kondo I, Saitoh S, Oshimura M. A novel maternally expressed gene, ATP10C, encodes a putative aminophospholipid translocase associated with Angelman syndrome. Nat Genet. 2001;28:19–20. doi: 10.1038/ng0501-19. [DOI] [PubMed] [Google Scholar]
- 92.Hogart A, Patzel KA, LaSalle JM. Gender influences monoallelic expression of ATP10A in human brain. Hum Genet. 2008;124:235–242. doi: 10.1007/s00439-008-0546-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Siggs OM, Arnold CN, Huber C, Pirie E, Xia Y, Lin P, Nemazee D, Beutler B. The P4-type ATPase ATP11C is essential for B lymphopoiesis in adult bone marrow. Nat Immunol. 2011;12:434–440. doi: 10.1038/ni.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Siggs OM, Schnabl B, Webb B, Beutler B. X-linked cholestasis in mouse due to mutations of the P4-ATPase ATP11C. Proc Natl Acad Sci U S A. 2011;108:7890–7895. doi: 10.1073/pnas.1104631108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Davis W., Jr. The ATP-binding cassette transporter-2 (ABCA2) regulates cholesterol homeostasis and low-density lipoprotein receptor metabolism in N2a neuroblastoma cells. Biochim Biophys Acta. 2011;1811:1152–1164. doi: 10.1016/j.bbalip.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Higgins CF. ABC transporters: from microorganisms to man. Annu Rev Cell Biol. 1992;8:67–113. doi: 10.1146/annurev.cb.08.110192.000435. [DOI] [PubMed] [Google Scholar]
- 97.Shitan N, Bazin I, Dan K, Obata K, Kigawa K, Ueda K, Sato F, Forestier C, Yazaki K. Involvement of CjMDR1, a plant multidrug-resistance-type ATP-binding cassette protein, in alkaloid transport in Coptis japonica. Proc Natl Acad Sci U S A. 2003;100:751–756. doi: 10.1073/pnas.0134257100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Smriti, Krishnamurthy S, Dixit BL, Gupta CM, Milewski S, Prasad R. ABC transporters Cdr1p, Cdr2p and Cdr3p of a human pathogen Candida albicans are general phospholipid translocators. Yeast. 2002;19:303–318. doi: 10.1002/yea.818. [DOI] [PubMed] [Google Scholar]
- 99.Fetsch EE, Davidson AL. Vanadate-catalyzed photocleavage of the signature motif of an ATP-binding cassette (ABC) transporter. Proc Natl Acad Sci U S A. 2002;99:9685–9690. doi: 10.1073/pnas.152204499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chen J, Lu G, Lin J, Davidson AL, Quiocho FA. A tweezers-like motion of the ATP-binding cassette dimer in an ABC transport cycle. Mol Cell. 2003;12:651–661. doi: 10.1016/j.molcel.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 101.Zaitseva J, Jenewein S, Jumpertz T, Holland IB, Schmitt L. H662 is the linchpin of ATP hydrolysis in the nucleotide-binding domain of the ABC transporter HlyB. EMBO J. 2005;24:1901–1910. doi: 10.1038/sj.emboj.7600657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jones PM, George AM. The ABC transporter structure and mechanism: perspectives on recent research. Cell Mol Life Sci. 2004;61:682–699. doi: 10.1007/s00018-003-3336-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dean M, Annilo T. Evolution of the ATP-binding cassette (ABC) transporter superfamily in vertebrates. Annu Rev Genomics Hum Genet. 2005;6:123–142. doi: 10.1146/annurev.genom.6.080604.162122. [DOI] [PubMed] [Google Scholar]
- 104.Pohl A, Devaux PF, Herrmann A. Function of prokaryotic and eukaryotic ABC proteins in lipid transport. Biochim Biophys Acta. 2005;1733:29–52. doi: 10.1016/j.bbalip.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 105.Aller SG, Yu J, Ward A, Weng Y, Chittaboina S, Zhuo R, Harrell PM, Trinh YT, Zhang Q, Urbatsch IL, Chang G. Structure of P-glycoprotein reveals a molecular basis for poly-specific drug binding. Science. 2009;323:1718–1722. doi: 10.1126/science.1168750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dawson RJ, Locher KP. Structure of a bacterial multidrug ABC transporter. Nature. 2006;443:180–185. doi: 10.1038/nature05155. [DOI] [PubMed] [Google Scholar]
- 107.Ward A, Reyes CL, Yu J, Roth CB, Chang G. Flexibility in the ABC transporter MsbA: Alternating access with a twist. Proc Natl Acad Sci U S A. 2007;104:19005–19010. doi: 10.1073/pnas.0709388104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hohl M, Briand C, Grutter MG, Seeger MA. Crystal structure of a heterodimeric ABC transporter in its inward-facing conformation. Nat Struct Mol Biol. 2012;19:395–402. doi: 10.1038/nsmb.2267. [DOI] [PubMed] [Google Scholar]
- 109.Dawson RJP, Locher KP. Structure of the multidrug ABC transporter Sav1866 from Staphylococcus aureus in complex with AMP-PNP. FEBS Lett. 2007;581:935–938. doi: 10.1016/j.febslet.2007.01.073. [DOI] [PubMed] [Google Scholar]
- 110.Jardetzky O. Simple allosteric model for membrane pumps. Nature. 1966;211:969–970. doi: 10.1038/211969a0. [DOI] [PubMed] [Google Scholar]
- 111.Zou P, McHaourab HS. Alternating access of the putative substrate-binding chamber in the ABC transporter MsbA. J Mol Biol. 2009;393:574–585. doi: 10.1016/j.jmb.2009.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Higgins CF, Linton KJ. The ATP switch model for ABC transporters. Nat Struct Mol Biol. 2004;11:918–926. doi: 10.1038/nsmb836. [DOI] [PubMed] [Google Scholar]
- 113.Borbat PP, Surendhran K, Bortolus M, Zou P, Freed JH, McHaourab HS. Conformational motion of the ABC transporter MsbA induced by ATP hydrolysis. PLoS Biol. 2007;5 doi: 10.1371/journal.pbio.0050271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Senior AE, al-Shawi MK, Urbatsch IL. The catalytic cycle of P-glycoprotein. FEBS Lett. 1995;377:285–289. doi: 10.1016/0014-5793(95)01345-8. [DOI] [PubMed] [Google Scholar]
- 115.Senior AE, Bhagat S. P-glycoprotein shows strong catalytic cooperativity between the two nucleotide sites. Biochemistry. 1998;37:831–836. doi: 10.1021/bi9719962. [DOI] [PubMed] [Google Scholar]
- 116.Qu Q, Chu JWK, Sharom FJ. Transition state P-glycoprotein binds drugs and modulators with unchanged affinity, suggesting a concerted transport mechanism. Biochemistry. 2003;42:1345–1353. doi: 10.1021/bi0267745. [DOI] [PubMed] [Google Scholar]
- 117.Loo TW, Bartlett MC, Clarke DM. Human P-glycoprotein is active when the two halves are clamped together in the closed conformation. Biochem Biophys Res Commun. 2003;395:436–440. doi: 10.1016/j.bbrc.2010.04.057. [DOI] [PubMed] [Google Scholar]
- 118.Bungert S, Molday LL, Molday RS. Membrane topology of the ATP binding cassette transporter ABCR and its relationship to ABC1 and related ABCA transporters: identification of N-linked glycosylation sites. J Biol Chem. 2001;276:23539–23546. doi: 10.1074/jbc.M101902200. [DOI] [PubMed] [Google Scholar]
- 119.Brooks-Wilson A, Marcil M, Clee SM, Zhang LH, Roomp K, van Dam M, Yu L, Brewer C, Collins JA, Molhuizen HO, Loubser O, Ouelette BF, Fichter K, Ashbourne-Excoffon KJ, Sensen CW, Scherer S, Mott S, Denis M, Martindale D, Frohlich J, Morgan K, Koop B, Pimstone S, Kastelein JJ, Genest J, Jr., Hayden MR. Mutations in ABC1 in Tangier disease and familial high-density lipoprotein deficiency. Nat Genet. 1999;22:336–345. doi: 10.1038/11905. [DOI] [PubMed] [Google Scholar]
- 120.Bodzioch M, Orso E, Klucken J, Langmann T, Bottcher A, Diederich W, Drobnik W, Barlage S, Buchler C, Porsch-Ozcurumez M, Kaminski WE, Hahmann HW, Oette K, Rothe G, Aslanidis C, Lackner KJ, Schmitz G. The gene encoding ATP-binding cassette transporter 1 is mutated in Tangier disease. Nat Genet. 1999;22:347–351. doi: 10.1038/11914. [DOI] [PubMed] [Google Scholar]
- 121.Rust S, Rosier M, Funke H, Real J, Amoura Z, Piette JC, Deleuze JF, Brewer HB, Duverger N, Denefle P, Assmann G. Tangier disease is caused by mutations in the gene encoding ATP-binding cassette transporter 1. Nat Genet. 1999;22:352–355. doi: 10.1038/11921. [DOI] [PubMed] [Google Scholar]
- 122.Oram JF. ATP-binding cassette transporter A1 and cholesterol trafficking. Curr Opin Lipidol. 2002;13:373–381. doi: 10.1097/00041433-200208000-00004. [DOI] [PubMed] [Google Scholar]
- 123.Singaraja RR, Visscher H, James ER, Chroni A, Coutinho JM, Brunham LR, Kang MH, Zannis VI, Chimini G, Hayden MR. Specific mutations in ABCA1 have discrete effects on ABCA1 function and lipid phenotypes both in vivo and in vitro. Circ Res. 2006;99:389–397. doi: 10.1161/01.RES.0000237920.70451.ad. [DOI] [PubMed] [Google Scholar]
- 124.Ban N, Matsumura Y, Sakai H, Takanezawa Y, Sasaki M, Arai H, Inagaki N. ABCA3 as a lipid transporter in pulmonary surfactant biogenesis. J Biol Chem. 2007;282:9628–9634. doi: 10.1074/jbc.M611767200. [DOI] [PubMed] [Google Scholar]
- 125.Shulenin S, Nogee LM, Annilo T, Wert SE, Whitsett JA, Dean M. ABCA3 gene mutations in newborns with fatal surfactant deficiency. N Engl J Med. 2004;350:1296–1303. doi: 10.1056/NEJMoa032178. [DOI] [PubMed] [Google Scholar]
- 126.Allikmets R, Singh N, Sun H, Shroyer NF, Hutchinson A, Chidambaram A, Gerrard B, Baird L, Stauffer D, Peiffer A, Rattner A, Smallwood P, Li Y, Anderson KL, Lewis RA, Nathans J, Leppert M, Dean M, Lupski JR. A photoreceptor cell-specific ATP-binding transporter gene (ABCR) is mutated in recessive Stargardt macular dystrophy. Nat Genet. 1997;15:236–246. doi: 10.1038/ng0397-236. [DOI] [PubMed] [Google Scholar]
- 127.Lefevre C, Audebert S, Jobard F, Bouadjar B, Lakhdar H, Boughdene-Stambouli O, Blanchet-Bardon C, Heilig R, Foglio M, Weissenbach J, Lathrop M, Prud'homme JF, Fischer J. Mutations in the transporter ABCA12 are associated with lamellar ichthyosis type 2. Hum Mol Genet. 2003;12:2369–2378. doi: 10.1093/hmg/ddg235. [DOI] [PubMed] [Google Scholar]
- 128.Akiyama M, Sugiyama-Nakagiri Y, Sakai K, McMillan JR, Goto M, Arita K, Tsuji-Abe Y, Tabata N, Matsuoka K, Sasaki R, Sawamura D, Shimizu H. Mutations in lipid transporter ABCA12 in harlequin ichthyosis and functional recovery by corrective gene transfer. J Clin Invest. 2005;115:1777–1784. doi: 10.1172/JCI24834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Malik B, Fernandes C, Killick R, Wroe R, Usardi A, Williamson R, Kellie S, Anderton BH, Hugh Reynolds C. Oligomeric amyloid-beta peptide affects the expression of genes involved in steroid and lipid metabolism in primary neurons. Neurochem Int. 2012;61:321–333. doi: 10.1016/j.neuint.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 130.Piehler AP, Haug KB, Wenzel JJ, Kierulf PB, Kaminski WE. [ABCA-transporters: regulators of cellular lipid transport] Tidsskr Nor Laegeforen. 2007;127:2930–2933. [PubMed] [Google Scholar]
- 131.Attie AD. ABCA1: at the nexus of cholesterol, HDL and atherosclerosis. Trends Biochem Sci. 2007;32:172–179. doi: 10.1016/j.tibs.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 132.van Dam MJ, de Groot E, Clee SM, Hovingh GK, Roelants R, Brooks-Wilson A, Zwinderman AH, Smit AJ, Smelt AH, Groen AK, Hayden MR, Kastelein JJ. Association between increased arterial-wall thickness and impairment in ABCA1-driven cholesterol efflux: an observational study. Lancet. 2002;359:37–42. doi: 10.1016/S0140-6736(02)07277-X. [DOI] [PubMed] [Google Scholar]
- 133.Frikke-Schmidt R. Genetic variation in the ABCA1 gene, HDL cholesterol, and risk of ischemic heart disease in the general population. Atherosclerosis. 2010;208:305–316. doi: 10.1016/j.atherosclerosis.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 134.Attie AD, Hamon Y, Brooks-Wilson AR, Gray-Keller MP, MacDonald ML, Rigot V, Tebon A, Zhang LH, Mulligan JD, Singaraja RR, Bitgood JJ, Cook ME, Kastelein JJ, Chimini G, Hayden MR. Identification and functional analysis of a naturally occurring E89K mutation in the ABCA1 gene of the WHAM chicken. J Lipid Res. 2002;43:1610–1617. doi: 10.1194/jlr.m200223-jlr200. [DOI] [PubMed] [Google Scholar]
- 135.McNeish J, Aiello RJ, Guyot D, Turi T, Gabel C, Aldinger C, Hoppe KL, Roach ML, Royer LJ, de Wet J, Broccardo C, Chimini G, Francone OL. High density lipoprotein deficiency and foam cell accumulation in mice with targeted disruption of ATP-binding cassette transporter-1. Proc Natl Acad Sci U S A. 2000;97:4245–4250. doi: 10.1073/pnas.97.8.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mulligan JD, Flowers MT, Tebon A, Bitgood JJ, Wellington C, Hayden MR, Attie AD. ABCA1 is essential for efficient basolateral cholesterol efflux during the absorption of dietary cholesterol in chickens. J Biol Chem. 2003;278:13356–13366. doi: 10.1074/jbc.M212377200. [DOI] [PubMed] [Google Scholar]
- 137.Brunham LR, Singaraja RR, Hayden MR. Variations on a gene: rare and common variants in ABCA1 and their impact on HDL cholesterol levels and atherosclerosis. Annu Rev Nutr. 2006;26:105–129. doi: 10.1146/annurev.nutr.26.061505.111214. [DOI] [PubMed] [Google Scholar]
- 138.Timmins JM, Lee JY, Boudyguina E, Kluckman KD, Brunham LR, Mulya A, Gebre AK, Coutinho JM, Colvin PL, Smith TL, Hayden MR, Maeda N, Parks JS. Targeted inactivation of hepatic Abca1 causes profound hypoalphalipoproteinemia and kidney hypercatabolism of apoA-I. J Clin Invest. 2005;115:1333–1342. doi: 10.1172/JCI23915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhu X, Lee JY, Timmins JM, Brown JM, Boudyguina E, Mulya A, Gebre AK, Willingham MC, Hiltbold EM, Mishra N, Maeda N, Parks JS. Increased cellular free cholesterol in macrophage-specific Abca1 knock-out mice enhances pro-inflammatory response of macrophages. J Biol Chem. 2008;283:22930–22941. doi: 10.1074/jbc.M801408200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tanaka AR, Abe-Dohmae S, Ohnishi T, Aoki R, Morinaga G, Okuhira K, Ikeda Y, Kano F, Matsuo M, Kioka N, Amachi T, Murata M, Yokoyama S, Ueda K. Effects of mutations of ABCA1 in the first extracellular domain on subcellular trafficking and ATP binding/hydrolysis. J Biol Chem. 2003;278:8815–8819. doi: 10.1074/jbc.M206885200. [DOI] [PubMed] [Google Scholar]
- 141.Yokoyama S. Release of cellular cholesterol: molecular mechanism for cholesterol homeostasis in cells and in the body. Biochim Biophys Acta. 2000;1529:231–244. doi: 10.1016/s1388-1981(00)00152-9. [DOI] [PubMed] [Google Scholar]
- 142.Fitzgerald ML, Morris AL, Rhee JS, Andersson LP, Mendez AJ, Freeman MW. Naturally occurring mutations in the largest extracellular loops of ABCA1 can disrupt its direct interaction with apolipoprotein A-I. J Biol Chem. 2002;277:33178–33187. doi: 10.1074/jbc.M204996200. [DOI] [PubMed] [Google Scholar]
- 143.Chroni A, Liu T, Fitzgerald ML, Freeman MW, Zannis VI. Cross-linking and lipid efflux properties of apoA-I mutants suggest direct association between apoA-I helices and ABCA1. Biochemistry. 2004;43:2126–2139. doi: 10.1021/bi035813p. [DOI] [PubMed] [Google Scholar]
- 144.Fitzgerald ML, Morris AL, Chroni A, Mendez AJ, Zannis VI, Freeman MW. ABCA1 and amphipathic apolipoproteins form high-affinity molecular complexes required for cholesterol efflux. J Lipid Res. 2004;45:287–294. doi: 10.1194/jlr.M300355-JLR200. [DOI] [PubMed] [Google Scholar]
- 145.Denis M, Haidar B, Marcil M, Bouvier M, Krimbou L, Genest J., Jr. Molecular and cellular physiology of apolipoprotein A-I lipidation by the ATP-binding cassette transporter A1 (ABCA1) J Biol Chem. 2004;279:7384–7394. doi: 10.1074/jbc.M306963200. [DOI] [PubMed] [Google Scholar]
- 146.Nagao K, Kimura Y, Ueda K. Lysine residues of ABCA1 are required for the interaction with apoA-I. Biochim Biophys Acta. 2011;1821:530–535. doi: 10.1016/j.bbalip.2011.06.024. [DOI] [PubMed] [Google Scholar]
- 147.Denis M, Haidar B, Marcil M, Bouvier M, Krimbou L, Genest J. Characterization of oligomeric human ATP binding cassette transporter A1. Potential implications for determining the structure of nascent high density lipoprotein particles. J Biol Chem. 2004;279:41529–41536. doi: 10.1074/jbc.M406881200. [DOI] [PubMed] [Google Scholar]
- 148.Sorci-Thomas MG, Owen JS, Fulp B, Bhat S, Zhu X, Parks JS, Shah D, Jerome WG, Gerelus M, Zabalawi M, Thomas MJ. Nascent high density lipoproteins formed by ABCA1 resemble lipid rafts and are structurally organized By three ApoA-I monomers. J Lipid Res. 2012;53:1890–909. doi: 10.1194/jlr.M026674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Krimbou L, Hajj Hassan H, Blain S, Rashid S, Denis M, Marcil M, Genest J. Biogenesis and speciation of nascent apoA-I-containing particles in various cell lines. J Lipid Res. 2005;46:1668–1677. doi: 10.1194/jlr.M500038-JLR200. [DOI] [PubMed] [Google Scholar]
- 150.Vedhachalam C, Duong PT, Nickel M, Nguyen D, Dhanasekaran P, Saito H, Rothblat GH, Lund-Katz S, Phillips MC. Mechanism of ATP-binding cassette transporter A1-mediated cellular lipid efflux to apolipoprotein A-I and formation of high density lipoprotein particles. J Biol Chem. 2007;282:25123–25130. doi: 10.1074/jbc.M704590200. [DOI] [PubMed] [Google Scholar]
- 151.Fielding PE, Nagao K, Hakamata H, Chimini G, Fielding CJ. A two-step mechanism for free cholesterol and phospholipid efflux from human vascular cells to apolipoprotein A-1. Biochemistry. 2000;39:14113–14120. doi: 10.1021/bi0004192. [DOI] [PubMed] [Google Scholar]
- 152.Nagao K, Zhao Y, Takahashi K, Kimura Y, Ueda K. Sodium taurocholate-dependent lipid efflux by ABCA1: effects of W590S mutation on lipid translocation and apolipoprotein A-I dissociation. J Lipid Res. 2009;50:1165–1172. doi: 10.1194/jlr.M800597-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hamon Y, Broccardo C, Chambenoit O, Luciani MF, Toti F, Chaslin S, Freyssinet JM, Devaux PF, McNeish J, Marguet D, Chimini G. ABC1 promotes engulfment of apoptotic cells and transbilayer redistribution of phosphatidylserine. Nat Cell Biol. 2000;2:399–406. doi: 10.1038/35017029. [DOI] [PubMed] [Google Scholar]
- 154.Iatan I, Bailey D, Ruel I, Hafiane A, Campbell S, Krimbou L, Genest J. Membrane microdomains modulate oligomeric ABCA1 function: impact on apoAI-mediated lipid removal and phosphatidylcholine biosynthesis. J Lipid Res. 2011;52:2043–2055. doi: 10.1194/jlr.M016196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Vedhachalam C, Ghering AB, Davidson WS, Lund-Katz S, Rothblat GH, Phillips MC. ABCA1-induced cell surface binding sites for ApoA-I. Arterioscler Thromb Vasc Biol. 2007;27:1603–1609. doi: 10.1161/ATVBAHA.107.145789. [DOI] [PubMed] [Google Scholar]
- 156.Warren MS, Zerangue N, Woodford K, Roberts LM, Tate EH, Feng B, Li C, Feuerstein TJ, Gibbs J, Smith B, de Morais SM, Dower WJ, Koller KJ. Comparative gene expression profiles of ABC transporters in brain microvessel endothelial cells and brain in five species including human. Pharmacol Res. 2009;59:404–413. doi: 10.1016/j.phrs.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 157.Sakai H, Tanaka Y, Tanaka M, Ban N, Yamada K, Matsumura Y, Watanabe D, Sasaki M, Kita T, Inagaki N. ABCA2 deficiency results in abnormal sphingolipid metabolism in mouse brain. J Biol Chem. 2007;282:19692–19699. doi: 10.1074/jbc.M611056200. [DOI] [PubMed] [Google Scholar]
- 158.Kim WS, Rahmanto AS, Kamili A, Rye KA, Guillemin GJ, Gelissen IC, Jessup W, Hill AF, Garner B. Role of ABCG1 and ABCA1 in regulation of neuronal cholesterol efflux to apolipoprotein E discs and suppression of amyloid-beta peptide generation. J Biol Chem. 2007;282:2851–2861. doi: 10.1074/jbc.M607831200. [DOI] [PubMed] [Google Scholar]
- 159.Matsumura Y, Sakai H, Sasaki M, Ban N, Inagaki N. ABCA3-mediated choline-phospholipids uptake into intracellular vesicles in A549 cells. FEBS Lett. 2007;581:3139–3144. doi: 10.1016/j.febslet.2007.05.078. [DOI] [PubMed] [Google Scholar]
- 160.Weichert N, Kaltenborn E, Hector A, Woischnik M, Schams A, Holzinger A, Kern S, Griese M. Some ABCA3 mutations elevate ER stress and initiate apoptosis of lung epithelial cells. Respir Res. 2011;12:4. doi: 10.1186/1465-9921-12-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Cheong N, Zhang H, Madesh M, Zhao M, Yu K, Dodia C, Fisher AB, Savani RC, Shuman H. ABCA3 is critical for lamellar body biogenesis in vivo. J Biol Chem. 2007;282:23811–23817. doi: 10.1074/jbc.M703927200. [DOI] [PubMed] [Google Scholar]
- 162.Fitzgerald ML, Xavier R, Haley KJ, Welti R, Goss JL, Brown CE, Zhuang DZ, Bell SA, Lu N, McKee M, Seed B, Freeman MW. ABCA3 inactivation in mice causes respiratory failure, loss of pulmonary surfactant, and depletion of lung phosphatidylglycerol. J Lipid Res. 2007;48:621–632. doi: 10.1194/jlr.M600449-JLR200. [DOI] [PubMed] [Google Scholar]
- 163.Illing M, Molday LL, Molday RS. The 220-kDa rim protein of retinal rod outer segments is a member of the ABC transporter superfamily. J Biol Chem. 1997;272:10303–10310. doi: 10.1074/jbc.272.15.10303. [DOI] [PubMed] [Google Scholar]
- 164.Molday LL, Rabin AR, Molday RS. ABCR expression in foveal cone photoreceptors and its role in Stargardt macular dystrophy. Nat Genet. 2000;25:257–258. doi: 10.1038/77004. [DOI] [PubMed] [Google Scholar]
- 165.Molday RS. ATP-binding cassette transporter ABCA4: molecular properties and role in vision and macular degeneration. J Bioenerg Biomembr. 2007;39:507–517. doi: 10.1007/s10863-007-9118-6. [DOI] [PubMed] [Google Scholar]
- 166.Nasonkin I, Illing M, Koehler MR, Schmid M, Molday RS, Weber BH. Mapping of the rod photoreceptor ABC transporter (ABCR) to 1p21-p22.1 and identification of novel mutations in Stargardt's disease. Hum Genet. 1998;102:21–26. doi: 10.1007/s004390050649. [DOI] [PubMed] [Google Scholar]
- 167.Cremers FP, van de Pol DJ, van Driel M, den Hollander AI, van Haren FJ, Knoers NV, Tijmes N, Bergen AA, Rohrschneider K, Blankenagel A, Pinckers AJ, Deutman AF, Hoyng CB. Autosomal recessive retinitis pigmentosa and cone-rod dystrophy caused by splice site mutations in the Stargardt's disease gene ABCR. Hum Mol Genet. 1998;7:355–362. doi: 10.1093/hmg/7.3.355. [DOI] [PubMed] [Google Scholar]
- 168.Martinez-Mir A, Paloma E, Allikmets R, Ayuso C, del Rio T, Dean M, Vilageliu L, Gonzalez-Duarte R, Balcells S. Retinitis pigmentosa caused by a homozygous mutation in the Stargardt disease gene ABCR. Nat Genet. 1998;18:11–12. doi: 10.1038/ng0198-11. [DOI] [PubMed] [Google Scholar]
- 169.Maugeri A, Klevering BJ, Rohrschneider K, Blankenagel A, Brunner HG, Deutman AF, Hoyng CB, Cremers FP. Mutations in the ABCA4 (ABCR) gene are the major cause of autosomal recessive cone-rod dystrophy. Am J Hum Genet. 2000;67:960–966. doi: 10.1086/303079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Allikmets R, Shroyer NF, Singh N, Seddon JM, Lewis RA, Bernstein PS, Peiffer A, Zabriskie NA, Li Y, Hutchinson A, Dean M, Lupski JR, Leppert M. Mutation of the Stargardt disease gene (ABCR) in age-related macular degeneration. Science. 1997;277:1805–1807. doi: 10.1126/science.277.5333.1805. [DOI] [PubMed] [Google Scholar]
- 171.Molday RS, Zhang K. Defective lipid transport and biosynthesis in recessive and dominant Stargardt macular degeneration. Prog Lipid Res. 2010;49:476–492. doi: 10.1016/j.plipres.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Saari JC. Vitamin a metabolism in rod and cone visual cycles. Annu Rev Nutr. 2012;32:125–145. doi: 10.1146/annurev-nutr-071811-150748. [DOI] [PubMed] [Google Scholar]
- 173.Molday RS, Zhong M, Quazi F. The role of the photoreceptor ABC transporter ABCA4 in lipid transport and Stargardt macular degeneration. Biochim Biophys Acta. 2009;1791:573–583. doi: 10.1016/j.bbalip.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Sun H, Molday RS, Nathans J. Retinal stimulates ATP hydrolysis by purified and reconstituted ABCR, the photoreceptor-specific ATP-binding cassette transporter responsible for Stargardt disease. J Biol Chem. 1999;274:8269–8281. doi: 10.1074/jbc.274.12.8269. [DOI] [PubMed] [Google Scholar]
- 175.Ahn J, Wong JT, Molday RS. The effect of lipid environment and retinoids on the ATPase activity of ABCR, the photoreceptor ABC transporter responsible for Stargardt macular dystrophy. J Biol Chem. 2000;275:20399–20405. doi: 10.1074/jbc.M000555200. [DOI] [PubMed] [Google Scholar]
- 176.Beharry S, Zhong M, Molday RS. N-retinylidene-phosphatidylethanolamine is the preferred retinoid substrate for the photoreceptor-specific ABC transporter ABCA4 (ABCR) J Biol Chem. 2004;279:53972–53979. doi: 10.1074/jbc.M405216200. [DOI] [PubMed] [Google Scholar]
- 177.Weng J, Mata NL, Azarian SM, Tzekov RT, Birch DG, Travis GH. Insights into the function of Rim protein in photoreceptors and etiology of Stargardt's disease from the phenotype in abcr knockout mice. Cell. 1999;98:13–23. doi: 10.1016/S0092-8674(00)80602-9. [DOI] [PubMed] [Google Scholar]
- 178.Mata NL, Weng J, Travis GH. Biosynthesis of a major lipofuscin fluorophore in mice and humans with ABCR-mediated retinal and macular degeneration. Proc Natl Acad Sci U S A. 2000;97:7154–7159. doi: 10.1073/pnas.130110497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Radu RA, Mata NL, Bagla A, Travis GH. Light exposure stimulates formation of A2E oxiranes in a mouse model of Stargardt's macular degeneration. Proc Natl Acad Sci U S A. 2004;101:5928–5933. doi: 10.1073/pnas.0308302101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Delori FC, Staurenghi G, Arend O, Dorey CK, Goger DG, Weiter JJ. In vivo measurement of lipofuscin in Stargardt's disease--Fundus flavimaculatus. Invest Ophthalmol Vis Sci. 1995;36:2327–2331. [PubMed] [Google Scholar]
- 181.Boyer NP, Higbee D, Currin MB, Blakeley LR, Chen C, Ablonczy Z, Crouch RK, Koutalos Y. Lipofuscin and N-Retinylidene-N-Retinylethanolamine (A2E) Accumulate in Retinal Pigment Epithelium in Absence of Light Exposure: THEIR ORIGIN IS 11-cis-RETINAL. J Biol Chem. 2012;287:22276–22286. doi: 10.1074/jbc.M111.329235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kaminski WE, Orso E, Diederich W, Klucken J, Drobnik W, Schmitz G. Identification of a novel human sterol-sensitive ATP-binding cassette transporter (ABCA7) Biochem Biophys Res Commun. 2000;273:532–538. doi: 10.1006/bbrc.2000.2954. [DOI] [PubMed] [Google Scholar]
- 183.Kim WS, Guillemin GJ, Glaros EN, Lim CK, Garner B. Quantitation of ATP-binding cassette subfamily-A transporter gene expression in primary human brain cells. Neuroreport. 2006;17:891–896. doi: 10.1097/01.wnr.0000221833.41340.cd. [DOI] [PubMed] [Google Scholar]
- 184.Wang N, Lan D, Gerbod-Giannone M, Linsel-Nitschke P, Jehle AW, Chen W, Martinez LO, Tall AR. ATP-binding cassette transporter A7 (ABCA7) binds apolipoprotein A-I and mediates cellular phospholipid but not cholesterol efflux. J Biol Chem. 2003;278:42906–42912. doi: 10.1074/jbc.M307831200. [DOI] [PubMed] [Google Scholar]
- 185.Chan SL, Kim WS, Kwok JB, Hill AF, Cappai R, Rye KA, Garner B. ATP-binding cassette transporter A7 regulates processing of amyloid precursor protein in vitro. J Neurochem. 2008;106:793–804. doi: 10.1111/j.1471-4159.2008.05433.x. [DOI] [PubMed] [Google Scholar]
- 186.Kim WS, Fitzgerald ML, Kang K, Okuhira K, Bell SA, Manning JJ, Koehn SL, Lu N, Moore KJ, Freeman MW. Abca7 null mice retain normal macrophage phosphatidylcholine and cholesterol efflux activity despite alterations in adipose mass and serum cholesterol levels. J Biol Chem. 2005;280:3989–3995. doi: 10.1074/jbc.M412602200. [DOI] [PubMed] [Google Scholar]
- 187.Tanaka AR, Ikeda Y, Abe-Dohmae S, Arakawa R, Sadanami K, Kidera A, Nakagawa S, Nagase T, Aoki R, Kioka N, Amachi T, Yokoyama S, Ueda K. Human ABCA1 contains a large amino-terminal extracellular domain homologous to an epitope of Sjogren's Syndrome. Biochem Biophys Res Commun. 2001;283:1019–1025. doi: 10.1006/bbrc.2001.4891. [DOI] [PubMed] [Google Scholar]
- 188.Sood S, Anthony R, Pease CT. Sjogren's syndrome. Clin Otolaryngol Allied Sci. 2000;25:350–357. doi: 10.1046/j.1365-2273.2000.00412.x. [DOI] [PubMed] [Google Scholar]
- 189.Linsel-Nitschke P, Jehle AW, Shan J, Cao G, Bacic D, Lan D, Wang N, Tall AR. Potential role of ABCA7 in cellular lipid efflux to apoA-I. J Lipid Res. 2005;46:86–92. doi: 10.1194/jlr.M400247-JLR200. [DOI] [PubMed] [Google Scholar]
- 190.Iwamoto N, Abe-Dohmae S, Sato R, Yokoyama S. ABCA7 expression is regulated by cellular cholesterol through the SREBP2 pathway and associated with phagocytosis. J Lipid Res. 2006;47:1915–1927. doi: 10.1194/jlr.M600127-JLR200. [DOI] [PubMed] [Google Scholar]
- 191.Jehle AW, Gardai SJ, Li S, Linsel-Nitschke P, Morimoto K, Janssen WJ, Vandivier RW, Wang N, Greenberg S, Dale BM, Qin C, Henson PM, Tall AR. ATP-binding cassette transporter A7 enhances phagocytosis of apoptotic cells and associated ERK signaling in macrophages. J Cell Biol. 2006;174:547–556. doi: 10.1083/jcb.200601030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Tanaka N, Abe-Dohmae S, Iwamoto N, Fitzgerald ML, Yokoyama S. Helical apolipoproteins of high-density lipoprotein enhance phagocytosis by stabilizing ATP-binding cassette transporter A7. J Lipid Res. 2010;51:2591–2599. doi: 10.1194/jlr.M006049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Akiyama M, Sakai K, Hatamochi A, Yamazaki S, McMillan JR, Shimizu H. Novel compound heterozygous nonsense and missense ABCA12 mutations lead to nonbullous congenital ichthyosiform erythroderma. Br J Dermatol. 2008;158:864–867. doi: 10.1111/j.1365-2133.2008.08439.x. [DOI] [PubMed] [Google Scholar]
- 194.Sakai K, Akiyama M, Sugiyama-Nakagiri Y, McMillan JR, Sawamura D, Shimizu H. Localization of ABCA12 from Golgi apparatus to lamellar granules in human upper epidermal keratinocytes. Exp Dermatol. 2007;16:920–926. doi: 10.1111/j.1600-0625.2007.00614.x. [DOI] [PubMed] [Google Scholar]
- 195.Smyth I, Hacking DF, Hilton AA, Mukhamedova N, Meikle PJ, Ellis S, Satterley K, Collinge JE, de Graaf CA, Bahlo M, Sviridov D, Kile BT, Hilton DJ. A mouse model of harlequin ichthyosis delineates a key role for Abca12 in lipid homeostasis. PLoS Genet. 2008;4:e1000192. doi: 10.1371/journal.pgen.1000192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.van Helvoort A, Smith AJ, Sprong H, Fritzsche I, Schinkel AH, Borst P, van Meer G. MDR1 P-glycoprotein is a lipid translocase of broad specificity, while MDR3 P-glycoprotein specifically translocates phosphatidylcholine. Cell. 1996;87:507–517. doi: 10.1016/s0092-8674(00)81370-7. [DOI] [PubMed] [Google Scholar]
- 197.Bosch I, Dunussi-Joannopoulos K, Wu RL, Furlong ST, Croop J. Phosphatidylcholine and phosphatidylethanolamine behave as substrates of the human MDR1 P-glycoprotein. Biochemistry. 1997;36:5685–5694. doi: 10.1021/bi962728r. [DOI] [PubMed] [Google Scholar]
- 198.van Meer G, Vaz WL. Membrane curvature sorts lipids. Stabilized lipid rafts in membrane transport. EMBO Rep. 2005;6:418–419. doi: 10.1038/sj.embor.7400410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Raggers RJ, Vogels I, van Meer G. Multidrug-resistance P-glycoprotein (MDR1) secretes platelet-activating factor. Biochem J. 2001;357:859–865. doi: 10.1042/0264-6021:3570859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Romsicki Y, Sharom FJ. Phospholipid flippase activity of the reconstituted P-glycoprotein multidrug transporter. Biochemistry. 2001;40:6937–6947. doi: 10.1021/bi0024456. [DOI] [PubMed] [Google Scholar]
- 201.Eckford PD, Sharom FJ. The reconstituted P-glycoprotein multidrug transporter is a flippase for glucosylceramide and other simple glycosphingolipids. Biochem J. 2005;389:517–526. doi: 10.1042/BJ20050047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Belli S, Elsener PM, Wunderli-Allenspach H, Kramer SD. Cholesterol-mediated activation of P-glycoprotein: distinct effects on basal and drug-induced ATPase activities. J Pharm Sci. 2009;98:1905–1918. doi: 10.1002/jps.21558. [DOI] [PubMed] [Google Scholar]
- 203.Nicolaou M, Andress EJ, Zolnerciks JK, Dixon PH, Williamson C, Linton KJ. Canalicular ABC transporters and liver disease. J Pathol. 2011;226:300–315. doi: 10.1002/path.3019. [DOI] [PubMed] [Google Scholar]
- 204.Morotti RA, Suchy FJ, Magid MS. Progressive familial intrahepatic cholestasis (PFIC) type 1, 2, and 3: a review of the liver pathology findings. Semin Liver Dis. 2011;31:3–10. doi: 10.1055/s-0031-1272831. [DOI] [PubMed] [Google Scholar]
- 205.Voshol PJ, Havinga R, Wolters H, Ottenhoff R, Princen HM, Oude Elferink RP, Groen AK, Kuipers F. Reduced plasma cholesterol and increased fecal sterol loss in multidrug resistance gene 2 P-glycoprotein-deficient mice. Gastroenterology. 1998;114:1024–1034. doi: 10.1016/s0016-5085(98)70323-3. [DOI] [PubMed] [Google Scholar]
- 206.Smit JJ, Schinkel AH, Oude Elferink RP, Groen AK, Wagenaar E, van Deemter L, Mol CA, Ottenhoff R, van der Lugt NM, van Roon MA, et al. Homozygous disruption of the murine mdr2 P-glycoprotein gene leads to a complete absence of phospholipid from bile and to liver disease. Cell. 1993;75:451–462. doi: 10.1016/0092-8674(93)90380-9. [DOI] [PubMed] [Google Scholar]
- 207.Smith AJ, Timmermans-Hereijgers JL, Roelofsen B, Wirtz KW, van Blitterswijk WJ, Smit JJ, Schinkel AH, Borst P. The human MDR3 P-glycoprotein promotes translocation of phosphatidylcholine through the plasma membrane of fibroblasts from transgenic mice. FEBS Lett. 1994;354:263–266. doi: 10.1016/0014-5793(94)01135-4. [DOI] [PubMed] [Google Scholar]
- 208.Morita SY, Kobayashi A, Takanezawa Y, Kioka N, Handa T, Arai H, Matsuo M, Ueda K. Bile salt-dependent efflux of cellular phospholipids mediated by ATP binding cassette protein B4. Hepatology. 2007;46:188–199. doi: 10.1002/hep.21591. [DOI] [PubMed] [Google Scholar]
- 209.Groen A, Romero MR, Kunne C, Hoosdally SJ, Dixon PH, Wooding C, Williamson C, Seppen J, Van den Oever K, Mok KS, Paulusma CC, Linton KJ, Oude Elferink RP. Complementary functions of the flippase ATP8B1 and the floppase ABCB4 in maintaining canalicular membrane integrity. Gastroenterology. 2011;141:1927–1937. e1921–1924. doi: 10.1053/j.gastro.2011.07.042. [DOI] [PubMed] [Google Scholar]
- 210.Cole SP, Bhardwaj G, Gerlach JH, Mackie JE, Grant CE, Almquist KC, Stewart AJ, Kurz EU, Duncan AM, Deeley RG. Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science. 1992;258:1650–1654. doi: 10.1126/science.1360704. [DOI] [PubMed] [Google Scholar]
- 211.Leier I, Jedlitschky G, Buchholz U, Cole SP, Deeley RG, Keppler D. The MRP gene encodes an ATP-dependent export pump for leukotriene C4 and structurally related conjugates. J Biol Chem. 1994;269:27807–27810. [PubMed] [Google Scholar]
- 212.Muller M, Meijer C, Zaman GJ, Borst P, Scheper RJ, Mulder NH, de Vries EG, Jansen PL. Overexpression of the gene encoding the multidrug resistance-associated protein results in increased ATP-dependent glutathione S-conjugate transport. Proc Natl Acad Sci U S A. 1994;91:13033–13037. doi: 10.1073/pnas.91.26.13033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Loe DW, Almquist KC, Deeley RG, Cole SP. Multidrug resistance protein (MRP)-mediated transport of leukotriene C4 and chemotherapeutic agents in membrane vesicles. Demonstration of glutathione-dependent vincristine transport. J Biol Chem. 1996;271:9675–9682. doi: 10.1074/jbc.271.16.9675. [DOI] [PubMed] [Google Scholar]
- 214.Loe DW, Deeley RG, Cole SP. Verapamil stimulates glutathione transport by the 190-kDa multidrug resistance protein 1 (MRP1) J Pharmacol Exp Ther. 2000;293:530–538. [PubMed] [Google Scholar]
- 215.Qian YM, Qiu W, Gao M, Westlake CJ, Cole SP, Deeley RG. Characterization of binding of leukotriene C4 by human multidrug resistance protein 1: evidence of differential interactions with NH2- and COOH-proximal halves of the protein. J Biol Chem. 2001;276:38636–38644. doi: 10.1074/jbc.M107025200. [DOI] [PubMed] [Google Scholar]
- 216.Wijnholds J, Evers R, van Leusden MR, Mol CA, Zaman GJ, Mayer U, Beijnen JH, van der Valk M, Krimpenfort P, Borst P. Increased sensitivity to anticancer drugs and decreased inflammatory response in mice lacking the multidrug resistance-associated protein. Nat Med. 1997;3:1275–1279. doi: 10.1038/nm1197-1275. [DOI] [PubMed] [Google Scholar]
- 217.Dekkers DW, Comfurius P, Schroit AJ, Bevers EM, Zwaal RF. Transbilayer movement of NBD-labeled phospholipids in red blood cell membranes: outward-directed transport by the multidrug resistance protein 1 (MRP1) Biochemistry. 1998;37:14833–14837. doi: 10.1021/bi981011w. [DOI] [PubMed] [Google Scholar]
- 218.Kamp D, Haest CW. Evidence for a role of the multidrug resistance protein (MRP) in the outward translocation of NBD-phospholipids in the erythrocyte membrane. Biochim Biophys Acta. 1998;1372:91–9101. doi: 10.1016/s0005-2736(98)00049-2. [DOI] [PubMed] [Google Scholar]
- 219.Raggers RJ, van Helvoort A, Evers R, van Meer G. The human multidrug resistance protein MRP1 translocates sphingolipid analogs across the plasma membrane. J Cell Sci. 1999;112(Pt 3):415–422. doi: 10.1242/jcs.112.3.415. [DOI] [PubMed] [Google Scholar]
- 220.Huang Z, Chang X, Riordan JR, Huang Y. Fluorescent modified phosphatidylcholine floppase activity of reconstituted multidrug resistance-associated protein MRP1. Biochim Biophys Acta. 2004;1660:155–163. doi: 10.1016/j.bbamem.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 221.Kusuhara H, Sugiyama Y. ATP-binding cassette, subfamily G (ABCG family) Pflugers Arch. 2007;453:735–744. doi: 10.1007/s00424-006-0134-x. [DOI] [PubMed] [Google Scholar]
- 222.Kennedy MA, Barrera GC, Nakamura K, Baldan A, Tarr P, Fishbein MC, Frank J, Francone OL, Edwards PA. ABCG1 has a critical role in mediating cholesterol efflux to HDL and preventing cellular lipid accumulation. Cell Metab. 2005;1:121–131. doi: 10.1016/j.cmet.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 223.Wang N, Lan D, Chen W, Matsuura F, Tall AR. ATP-binding cassette transporters G1 and G4 mediate cellular cholesterol efflux to high-density lipoproteins. Proc Natl Acad Sci U S A. 2004;101:9774–9779. doi: 10.1073/pnas.0403506101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Laffitte BA, Repa JJ, Joseph SB, Wilpitz DC, Kast HR, Mangelsdorf DJ, Tontonoz P. LXRs control lipid-inducible expression of the apolipoprotein E gene in macrophages and adipocytes. Proc Natl Acad Sci U S A. 2001;98:507–512. doi: 10.1073/pnas.021488798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Teupser D, Kretzschmar D, Tennert C, Burkhardt R, Wilfert W, Fengler D, Naumann R, Sippel AE, Thiery J. Effect of macrophage overexpression of murine liver X receptor-alpha (LXR-alpha) on atherosclerosis in LDL-receptor deficient mice. Arterioscler Thromb Vasc Biol. 2008;28:2009–2015. doi: 10.1161/ATVBAHA.108.175257. [DOI] [PubMed] [Google Scholar]
- 226.Wang N, Ranalletta M, Matsuura F, Peng F, Tall AR. LXR-induced redistribution of ABCG1 to plasma membrane in macrophages enhances cholesterol mass efflux to HDL. Arterioscler Thromb Vasc Biol. 2006;26:1310–1316. doi: 10.1161/01.ATV.0000218998.75963.02. [DOI] [PubMed] [Google Scholar]
- 227.Sankaranarayanan S, Oram JF, Asztalos BF, Vaughan AM, Lund-Katz S, Adorni MP, Phillips MC, Rothblat GH. Effects of acceptor composition and mechanism of ABCG1-mediated cellular free cholesterol efflux. J Lipid Res. 2009;50:275–284. doi: 10.1194/jlr.M800362-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Seres L, Cserepes J, Elkind NB, Torocsik D, Nagy L, Sarkadi B, Homolya L. Functional ABCG1 expression induces apoptosis in macrophages and other cell types. Biochim Biophys Acta. 2008;1778:2378–2387. doi: 10.1016/j.bbamem.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 229.Wang J, Sun F, Zhang DW, Ma Y, Xu F, Belani JD, Cohen JC, Hobbs HH, Xie XS. Sterol transfer by ABCG5 and ABCG8: in vitro assay and reconstitution. J Biol Chem. 2006;281:27894–27904. doi: 10.1074/jbc.M605603200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Vaughan AM, Oram JF. ABCG1 redistributes cell cholesterol to domains removable by high density lipoprotein but not by lipid-depleted apolipoproteins. J Biol Chem. 2005;280:30150–30157. doi: 10.1074/jbc.M505368200. [DOI] [PubMed] [Google Scholar]
- 231.Tarling EJ, Edwards PA. ATP binding cassette transporter G1 (ABCG1) is an intracellular sterol transporter. Proc Natl Acad Sci U S A. 2011;108:19719–19724. doi: 10.1073/pnas.1113021108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Out R, Hoekstra M, Habets K, Meurs I, de Waard V, Hildebrand RB, Wang Y, Chimini G, Kuiper J, Van Berkel TJ, Van Eck M. Combined deletion of macrophage ABCA1 and ABCG1 leads to massive lipid accumulation in tissue macrophages and distinct atherosclerosis at relatively low plasma cholesterol levels. Arterioscler Thromb Vasc Biol. 2008;28:258–264. doi: 10.1161/ATVBAHA.107.156935. [DOI] [PubMed] [Google Scholar]
- 233.Yvan-Charvet L, Ranalletta M, Wang N, Han S, Terasaka N, Li R, Welch C, Tall AR. Combined deficiency of ABCA1 and ABCG1 promotes foam cell accumulation and accelerates atherosclerosis in mice. J Clin Invest. 2007;117:3900–3908. doi: 10.1172/JCI33372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Adorni MP, Zimetti F, Billheimer JT, Wang N, Rader DJ, Phillips MC, Rothblat GH. The roles of different pathways in the release of cholesterol from macrophages. J Lipid Res. 2007;48:2453–2462. doi: 10.1194/jlr.M700274-JLR200. [DOI] [PubMed] [Google Scholar]
- 235.Kobayashi A, Takanezawa Y, Hirata T, Shimizu Y, Misasa K, Kioka N, Arai H, Ueda K, Matsuo M. Efflux of sphingomyelin, cholesterol, and phosphatidylcholine by ABCG1. J Lipid Res. 2006;47:1791–1802. doi: 10.1194/jlr.M500546-JLR200. [DOI] [PubMed] [Google Scholar]
- 236.Woehlecke H, Pohl A, Alder-Baerens N, Lage H, Herrmann A. Enhanced exposure of phosphatidylserine in human gastric carcinoma cells overexpressing the half-size ABC transporter BCRP (ABCG2) Biochem J. 2003;376:489–495. doi: 10.1042/BJ20030886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Nishimura M, Naito S. Tissue-specific mRNA expression profiles of human ATP-binding cassette and solute carrier transporter superfamilies. Drug Metab Pharmacokinet. 2005;20:452–477. doi: 10.2133/dmpk.20.452. [DOI] [PubMed] [Google Scholar]
- 238.Wang N, Yvan-Charvet L, Lutjohann D, Mulder M, Vanmierlo T, Kim TW, Tall AR. ATP-binding cassette transporters G1 and G4 mediate cholesterol and desmosterol efflux to HDL and regulate sterol accumulation in the brain. Faseb J. 2008;22:1073–1082. doi: 10.1096/fj.07-9944com. [DOI] [PubMed] [Google Scholar]
- 239.Vaughan AM, Oram JF. ABCA1 and ABCG1 or ABCG4 act sequentially to remove cellular cholesterol and generate cholesterol-rich HDL. J Lipid Res. 2006;47:2433–2443. doi: 10.1194/jlr.M600218-JLR200. [DOI] [PubMed] [Google Scholar]
- 240.Graf GA, Li WP, Gerard RD, Gelissen I, White A, Cohen JC, Hobbs HH. Coexpression of ATP-binding cassette proteins ABCG5 and ABCG8 permits their transport to the apical surface. J Clin Invest. 2002;110:659–669. doi: 10.1172/JCI16000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Lutjohann D, Bjorkhem I, Beil UF, von Bergmann K. Sterol absorption and sterol balance in phytosterolemia evaluated by deuterium-labeled sterols: effect of sitostanol treatment. J Lipid Res. 1995;36:1763–1773. [PubMed] [Google Scholar]
- 242.Yu L, Gupta S, Xu F, Liverman AD, Moschetta A, Mangelsdorf DJ, Repa JJ, Hobbs HH, Cohen JC. Expression of ABCG5 and ABCG8 is required for regulation of biliary cholesterol secretion. J Biol Chem. 2005;280:8742–8747. doi: 10.1074/jbc.M411080200. [DOI] [PubMed] [Google Scholar]
- 243.Yu L, Li-Hawkins J, Hammer RE, Berge KE, Horton JD, Cohen JC, Hobbs HH. Overexpression of ABCG5 and ABCG8 promotes biliary cholesterol secretion and reduces fractional absorption of dietary cholesterol. J Clin Invest. 2002;110:671–680. doi: 10.1172/JCI16001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Velamakanni S, Janvilisri T, Shahi S, van Veen HW. A functional steroid-binding element in an ATP-binding cassette multidrug transporter. Mol Pharmacol. 2008;73:12–17. doi: 10.1124/mol.108.038299. [DOI] [PubMed] [Google Scholar]
- 245.Wahrle SE, Jiang H, Parsadanian M, Legleiter J, Han X, Fryer JD, Kowalewski T, Holtzman DM. ABCA1 is required for normal central nervous system ApoE levels and for lipidation of astrocyte-secreted apoE. J Biol Chem. 2004;279:40987–40993. doi: 10.1074/jbc.M407963200. [DOI] [PubMed] [Google Scholar]
- 246.Hirsch-Reinshagen V, Zhou S, Burgess BL, Bernier L, McIsaac SA, Chan JY, Tansley GH, Cohn JS, Hayden MR, Wellington CL. Deficiency of ABCA1 impairs apolipoprotein E metabolism in brain. J Biol Chem. 2004;279:41197–41207. doi: 10.1074/jbc.M407962200. [DOI] [PubMed] [Google Scholar]
- 247.Wahrle SE, Jiang H, Parsadanian M, Kim J, Li A, Knoten A, Jain S, Hirsch-Reinshagen V, Wellington CL, Bales KR, Paul SM, Holtzman DM. Overexpression of ABCA1 reduces amyloid deposition in the PDAPP mouse model of Alzheimer disease. J Clin Invest. 2008;118:671–682. doi: 10.1172/JCI33622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.DeMattos RB, Brendza RP, Heuser JE, Kierson M, Cirrito JR, Fryer J, Sullivan PM, Fagan AM, Han X, Holtzman DM. Purification and characterization of astrocyte-secreted apolipoprotein E and J-containing lipoproteins from wild-type and human apoE transgenic mice. Neurochem Int. 2001;39:415–425. doi: 10.1016/s0197-0186(01)00049-3. [DOI] [PubMed] [Google Scholar]
- 249.Karten B, Campenot RB, Vance DE, Vance JE. Expression of ABCG1, but not ABCA1, correlates with cholesterol release by cerebellar astroglia. J Biol Chem. 2006;281:4049–4057. doi: 10.1074/jbc.M508915200. [DOI] [PubMed] [Google Scholar]
- 250.Burgess BL, Parkinson PF, Racke MM, Hirsch-Reinshagen V, Fan J, Wong C, Stukas S, Theroux L, Chan JY, Donkin J, Wilkinson A, Balik D, Christie B, Poirier J, Lutjohann D, Demattos RB, Wellington CL. ABCG1 influences the brain cholesterol biosynthetic pathway but does not affect amyloid precursor protein or apolipoprotein E metabolism in vivo. J Lipid Res. 2008;49:1254–1267. doi: 10.1194/jlr.M700481-JLR200. [DOI] [PubMed] [Google Scholar]
- 251.Matsuo M, Campenot RB, Vance DE, Ueda K, Vance JE. Involvement of low-density lipoprotein receptor-related protein and ABCG1 in stimulation of axonal extension by apoE-containing lipoproteins. Biochim Biophys Acta. 2011;1811:31–38. doi: 10.1016/j.bbalip.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 252.Harris MJ, Arias IM. FIC1, a P-type ATPase linked to cholestatic liver disease, has homologues (ATP8B2 and ATP8B3) expressed throughout the body. Biochim Biophys Acta. 2003;1633:127–131. doi: 10.1016/s1388-1981(03)00107-0. [DOI] [PubMed] [Google Scholar]
- 253.Nagase T, Kikuno R, Ohara O. Prediction of the coding sequences of unidentified human genes. XXII. The complete sequences of 50 new cDNA clones which code for large proteins. DNA Res. 2001;8:319–327. doi: 10.1093/dnares/8.6.319. [DOI] [PubMed] [Google Scholar]
- 254.Halleck MS, Pradhan D, Blackman C, Berkes C, Williamson P, Schlegel RA. Multiple members of a third subfamily of P-type ATPases identified by genomic sequences and ESTs. Genome Res. 1998;8:354–361. doi: 10.1101/gr.8.4.354. [DOI] [PubMed] [Google Scholar]
- 255.Nagase T, Ishikawa K, Suyama M, Kikuno R, Miyajima N, Tanaka A, Kotani H, Nomura N, Ohara O. Prediction of the coding sequences of unidentified human genes. XI. The complete sequences of 100 new cDNA clones from brain which code for large proteins in vitro. DNA Res. 1998;5:277–286. doi: 10.1093/dnares/5.5.277. [DOI] [PubMed] [Google Scholar]
- 256.Kikuno R, Nagase T, Ishikawa K, Hirosawa M, Miyajima N, Tanaka A, Kotani H, Nomura N, Ohara O. Prediction of the coding sequences of unidentified human genes. XIV. The complete sequences of 100 new cDNA clones from brain which code for large proteins in vitro. DNA Res. 1999;6:197–205. doi: 10.1093/dnares/6.3.197. [DOI] [PubMed] [Google Scholar]
- 257.Nagase T, Ishikawa K, Suyama M, Kikuno R, Hirosawa M, Miyajima N, Tanaka A, Kotani H, Nomura N, Ohara O. Prediction of the coding sequences of unidentified human genes. XIII. The complete sequences of 100 new cDNA clones from brain which code for large proteins in vitro. DNA Res. 1999;6:63–70. doi: 10.1093/dnares/6.1.63. [DOI] [PubMed] [Google Scholar]
- 258.Andrew Nesbit M, Bowl MR, Harding B, Schlessinger D, Whyte MP, Thakker RV. X-linked hypoparathyroidism region on Xq27 is evolutionarily conserved with regions on 3q26 and 13q34 and contains a novel P-type ATPase. Genomics. 2004;84:1060–1070. doi: 10.1016/j.ygeno.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 259.Nagao K, Takahashi K, Hanada K, Kioka N, Matsuo M, Ueda K. Enhanced apoA-I-dependent cholesterol efflux by ABCA1 from sphingomyelin-deficient Chinese hamster ovary cells. J Biol Chem. 2007;282:14868–14874. doi: 10.1074/jbc.M611230200. [DOI] [PubMed] [Google Scholar]
- 260.Witting SR, Maiorano JN, Davidson WS. Ceramide enhances cholesterol efflux to apolipoprotein A-I by increasing the cell surface presence of ATP-binding cassette transporter A1. J Biol Chem. 2003;278:40121–40127. doi: 10.1074/jbc.M305193200. [DOI] [PubMed] [Google Scholar]
- 261.Simon S, Schubert R. Inhibitory effect of phospholipids on P-glycoprotein: Cellular studies in Caco-2, MDCKII mdr1 and MDCKII wildtype cells and P-gp ATPase activity measurements. Biochim Biophys Acta. 2012;1821:1211–1223. doi: 10.1016/j.bbalip.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 262.Frijters CM, Tuijn CJ, Ottenhoff R, Zegers BN, Groen AK, Elferink RP. The role of different P-glycoproteins in hepatobiliary secretion of fluorescently labeled short-chain phospholipids. J Lipid Res. 1999;40:1950–1958. [PubMed] [Google Scholar]
- 263.Herzog M, Storch CH, Gut P, Kotlyar D, Fullekrug J, Ehehalt R, Haefeli WE, Weiss J. Knockdown of caveolin-1 decreases activity of breast cancer resistance protein (BCRP/ABCG2) and increases chemotherapeutic sensitivity. Naunyn Schmiedebergs Arch Pharmacol. 2011;383:1–11. doi: 10.1007/s00210-010-0568-8. [DOI] [PubMed] [Google Scholar]
- 264.Storch CH, Ehehalt R, Haefeli WE, Weiss J. Localization of the human breast cancer resistance protein (BCRP/ABCG2) in lipid rafts/caveolae and modulation of its activity by cholesterol in vitro. J Pharmacol Exp Ther. 2007;323:257–264. doi: 10.1124/jpet.107.122994. [DOI] [PubMed] [Google Scholar]
- 265.Wang J, Zhang DW, Lei Y, Xu F, Cohen JC, Hobbs HH, Xie XS. Purification and reconstitution of sterol transfer by native mouse ABCG5 and ABCG8. Biochemistry. 2008;47:5194–5204. doi: 10.1021/bi800292v. [DOI] [PMC free article] [PubMed] [Google Scholar]