Abstract
A persistent hurdle in the field of tissue regeneration is to produce tissues with biochemical and biomechanical properties robust enough to meet the aggressive physiological demands of the native milieu. In an effort to improve these properties, tissues grown in vitro are often subjected to mechanical stimuli that aim to recapitulate in vivo physiology. These mechanical stimuli are thought to produce downstream alterations in intracellular ion concentrations, which ultimately give rise to increased biosynthesis. There is mounting evidence that these perturbations in the cellular microenvironment are regulated by the Ca(2+)-permeable transient receptor potential vanilloid 4 (TRPV4) channel. In this study, we examined the effects of targeted TRPV4 activation on self-assembled articular cartilage constructs. The objectives of this study were 1) to determine whether TRPV4 activation would enhance self-assembled constructs, 2) to identify an optimal treatment time window for TRPV4 activation, and 3) to compare TRPV4 activation to Na(+)/K(+) pump inhibition, which has been shown previously to improve construct tensile properties. This study employed a two-phased approach. In Phase I, self-assembled constructs were grown for 4 weeks and subjected to treatment with the TRPV4 agonist 4alpha-phorbol-12,13-didecanoate (4alpha-PDD) during three treatment time windows: t=6-10 days, t=10-14 days, and t=14-18 days. Treatment during t=10-14 days produced an 88% increase in collagen and a 153% increase in tensile stiffness. This treatment window was carried forward to Phase II. In Phase II, we performed a head-to-head comparison between TRPV4 activation using 4alpha-PDD and Na(+)/K(+) pump inhibition using ouabain. Treatment with 4alpha-PDD produced improvements on par with ouabain (91% to 107% increases in tensile stiffness). The results of this study demonstrate the effectiveness of ion channel modulation as a strategy for improving engineered tissues. To our knowledge, this is the first study to examine TRPV4 channel activation in tissue engineering.
Keywords: TRPV4, cartilage tissue engineering, self-assembly, biomechanics
Introduction
Many tissue types lack an intrinsic physiological capacity to heal in response to injury or degeneration. Tissues that do recover after acute injury are often replaced with physiologically inferior replacement tissue. This poses a significant clinical burden, particularly in the field of cartilage regeneration. Injury to the cartilage found at the articulating surfaces of diarthrodial joints is irreversible and leads inescapably to pain and disability [1]. Tissue engineering aims to replace damaged articular cartilage by producing biologic replacements in vitro for eventual in vivo implantation. A persistent challenge in cartilage tissue engineering is to produce biomaterials with biochemical and biomechanical properties robust enough to meet the aggressive physiological demands of the native joint [2]. To address this challenge, our laboratory has developed a self-assembly process for engineering cartilage constructs [3]. Self-assembly involves seeding chondrocytes at high density into pre-fabricated, non-adherent, cylindrical molds. Cells condense into disc-shaped constructs and, over time, synthesize an extracellular matrix (ECM) rich in collagen and sulfated glycosaminoglycans (GAG), components that give the tissue its tensile and compressive integrity [4]. To date, however, native tissue functional properties remain elusive.
A variety of mechanical stimulation modalities have been examined for the potential to affect chondrocyte physiology. These stimuli have been informed by the dynamic physiologic loading conditions experienced by native cartilage in the intact joint. By studying the effects of these mechanical stimuli on chondrocyte physiology, targeted strategies for enhanced cartilage engineering may be devised. Example stimuli include dynamic compression [5-10], fluid shear [11-13], hydrostatic pressure [14-19], and osmotic stress [20-23]. Underlying these studies is the idea that changes in the macroscopic environment of the tissue can give rise to beneficial perturbations in the in situ cellular microenvironment. Dynamic changes at the cellular level manifest physiologically as transient alterations in intracellular ion concentrations. For example, hydrostatic pressure inhibits the action of the Na+/K+ pump [16], an ATPase that pumps ions against a concentration gradient to maintain a higher intracellular concentration of K+ than Na+. Thus, by inhibiting the Na+/K+ pump, hydrostatic pressure produces increased levels of intracellular Na+. A recent study fom our group [24] showed that the selective inhibition of the Na+/K+ pump using 20 μM ouabain in self-assembled cartilage constructs was able to produce significant increases in collagen content and tensile properties, a result that recapitulated our group’s previous success with hydrostatic pressure [25].
Another example of a dynamic tissue-level stimulus giving rise to changes at the cellular level is cyclic deformational loading. During joint motion, compressive loading of cartilage causes fluid expulsion, which can create a microenvironment of temporary hyper-osmotic stress or increased charge density for chondrocytes within the tissue [26]. This kind of hyper-osmotic stress – for example, from 310 mOsm to 550 mOsm [20] –has been shown to produce transient increases in intracellular Ca2+ [20, 21, 27], which can drive gene expression toward ECM biosynthesis [28, 29]. The precise mechanism underlying this osmoregulation in cartilage remains unclear. However, there is mounting evidence that the chondrocyte response to osmotic stress may be regulated by the transient receptor vanilloid 4 (TRPV4) channel [30, 31], a Ca2+-permeable membrane protein found across many tissue types [32]. Although a handful of recent papers have examined the molecular and cellular physiology of the TRPV4 channel in chondrocytes, no study to date has selectively targeted the TRPV4 channel for use in a tissue engineering strategy.
Encouraged by results from the literature that suggest that the TRPV4 channel plays a vital role in chondrocyte physiology, we decided to examine the effects of TRPV4 activation on self-assembled articular cartilage constructs. The objectives of this study were 1) to determine whether TRPV4 activation would enhance the biochemical and biomechanical properties of self-assembled constructs, 2) to identify an optimal treatment time window for TRPV4 activation, and 3) to compare TRPV4 activation to Na+/K+ pump inhibition. This study employed a two-phased approach. In Phase I, constructs were self-assembled from bovine chondrocytes and subjected to treatment with the TRPV4 agonist 4α-phorbol-12,13-didecanoate (4α-PDD) during three treatment time windows: t=6-10 days, t=10-14 days, and t=14-18 days. These treatment periods were selected based on previous work that showed time-dependent differences in construct properties [25, 33]. Constructs were grown until t=28 days, at which time they were evaluated morphologically, biochemically, and biomechanically. The optimal 4α-PDD treatment time window was then carried forward to Phase II. In Phase II, we performed a head-to-head comparison between TRPV4 activation using 4 α-PDD and Na+/K+ pump inhibition using ouabain; we also examined the combination of 4α-PDD and ouabain. It was hypothesized that 1) TRPV4 activation would improve construct properties, 2) an optimal treatment time window exists for which constructs undergo greatest improvement, and 3) activation of TRPV4 would produce results comparable to those observed with inhibition of the Na+/K+ pump. Assessments included gross morphology, biochemical analysis for GAG and collagen, and biomechanical testing.
Materials and Methods
Chondrogenic medium
This study employed a chemically defined medium termed “chondrogenic medium,” which has been used previously by our group [4, 24, 34] and contains the following components: DMEM with 4.5 mg/mL of glucose and L-glutamine (Invitrogen); 100 nM dexamethasone (Sigma); 0.1 mM non-essential amino acids (Invitrogen); 1% ITS+ (insulin, human transferrin, and selenous acid; BD Biosciences); 1% penicillin-streptomycin-fungizone (BioWhittaker); 50 μg/mL ascorbate-2-phosphate; 40 μg/mL L-proline; and 100 μg/mL sodium pyruvate (Fisher Scientific). Chondrogenic medium contains 151 mM Na+, 5.2 mM K+, and 1.7 mM Ca2+. Medium osmolarity was assessed using a VAPRO 5520 vapor pressure osmometer (Wescor) and was determined to be ~347 mOsm.
Chondrocyte isolation
Cartilage was harvested from the distal femurs and patellofemoral grooves of week-old male calves (Research 87, Inc.) shortly after slaughter, then digested in 0.2% collagenase type II (Worthington) for 24 h. To normalize variability among animals, each leg came from a different animal, and cells from 8 legs were pooled to create a mixture of chondrocytes. Separate harvests were conducted for each phase of this study. Cells were counted using a hemocytometer and then frozen at −80°C in DMEM containing 20% FBS and 10% DMSO.
Preparation of agarose wells for construct self-assembly
Cylindrical, non-adherent wells were prepared using a technique adapted from previous work [3, 4]. Briefly, a stainless steel mold consisting of 5 mm diameter cylindrical prongs was placed into sterile, molten 2% agarose in a 48-well plate. The agarose solidified at room temperature for 1 h, and the stainless steel mold was carefully removed. Two changes of chondrogenic medium were used to completely saturate the agarose well by the time of cell seeding.
Self-assembly of cartilage constructs
Chondrocytes were thawed and counted within 5 days of being isolated and frozen. After thawing, cell viability was >90%. To create each construct, a suspension of 5.5 million cells in 100 μL of chondrogenic media was deposited into each pre-formed cylindrical agarose well, followed by addition of 400 μL chondrogenic media after 4 h. Cells settled and condensed into free-floating cylindrical disc-shaped constructs; t=1 day was defined as 24 h after seeding. All constructs were cultured in the agarose wells until t=10 days, at which point they were gently unconfined and transferred to 48-well plates unrestricted by circumferential confinement. Constructs received 500 μL medium change every 24 h and remained in culture until t=28 days. All culture was performed at 37°C and 10% CO2, simulating hypoxic conditions that have produced consistent biosynthetic results previously with self-assembled cartilage constructs in our laboratory [24].
Phase I: Evaluation of treatment time windows for TRPV4 channel activation
In Phase I, we tested the hypothesis that TRPV4 channel activation can improve the biochemical and biomechanical properties of tissue engineered cartilage. We further sought to determine the optimal time window for performing this stimulation. Self-assembled constructs were treated with a TRPV4 channel agonist, 4α-PDD (Enzo Life Sciences), during three treatment windows: t=6-10 days, t=10-14 days, and t=14-18 days. During treatment, constructs were cultured in petri dishes for 1 h with ~4 mL chondrogenic medium containing 10 μM 4α-PDD. Control constructs were also moved to petri dishes containing chondrogenic medium during this time. Treatment was followed by a 30 min wash step in chondrogenic medium without 4α-PDD before the constructs were returned to their wells. Treatment occurred at the same time every day over the course of 5 days.
Phase II: TRPV4 activation versus Na+/K+ pump inhibition
A previous study from our group showed that inhibition of the Na+/K+ pump improved the tensile properties of tissue engineered cartilage [24]. In Phase II, we performed a head-to-head comparison between TRPV4 activation and Na+/K+ pump inhibition. We further sought to determine the effects of the combination of these two stimuli. The regimen for TRPV4 activation was chosen from the most effective treatment time window determined in Phase I: 10 μM 4α-PDD during t=10-14 days (see Results section for details). The regimen for Na+/K+ pump inhibition was selected from previous work done by our group [24]: 20 μM ouabain during t=10-14 days. During t=10-14 days, constructs were cultured in petri dishes for 1 h with ~4 mL chondrogenic medium containing either 10 μM 4α-PDD, 20 μM ouabain (Sigma), or both agents. Control constructs were also moved to petri dishes containing chondrogenic medium during this time. Treatment was followed by a 30 min wash step in chondrogenic medium before the constructs were returned to their wells. Treatment occurred at the same time every day over the course of 5 days.
Gross morphology and specimen portioning
At t=28 days, constructs were removed from culture. Photographs were taken, and dimensions were measured from photographs using ImageJ software (National Institutes of Health). Wet weights (WW) were recorded, and constructs were portioned for analysis. A 3 mm diameter punch was taken from the construct’s center for indentation testing. The remaining outer ring was divided into two semilunar portions, one for biochemical analysis and one for tensile testing.
Biochemical analysis
Biochemical samples were weighed wet, frozen, and lyophilized. Samples were digested with 125 μg/mL papain (Sigma) for 18 h at 65°C. Total DNA content was assessed with the Quant-iT PicoGreen dsDNA Assay Kit (Invitrogen), and cell number was estimated assuming 7.8 pg DNA per cell. Sulfated GAG content was quantified using the Blyscan Glycosaminoglycan Assay (Biocolor). Following hydrolysis with 4 N sodium hydroxide for 20 min at 110°C, total collagen content was quantified with a modified chloramine-T hydroxyproline assay [3, 35]. Sircol collagen standard (Biocolor) was used such that the standard curve reflected collagen amount, eliminating the need to convert hydroxyproline to collagen. Total collagen and sulfated GAG were normalized to WW and cell number for making comparisons.
Tensile testing
Each semilunar specimen designated for tensile testing was cut into a dog-bone shape. To prepare the dog-bone shape, a 3 mm diameter punch was used to cut a semicircle from the curved, uncut edge of the semilunar specimen. Care was taken to ensure a 1 mm gauge length, which was confirmed with ImageJ software. Specimen thickness and width were also measured from photographs using ImageJ software. Specimens were then affixed with glue to paper tabs outside the gauge length, and these tabs were gripped during testing (for more details, including photographs of the process, please see reference [24]). A uniaxial materials testing system (Instron Model 5565) was employed to determine tensile properties. Tensile tests were performed until failure at a strain rate of 1% of the gauge length per second. Force-displacement curves were generated, and stress-strain curves were calculated by normalizing to specimen dimensions. Young’s modulus, a measure of tensile stiffness, was determined by least squares fitting of the linear region of the stress-strain curve. The ultimate tensile strength (UTS) was determined as the maximum stress reached during a test.
Creep indentation testing
A creep indentation apparatus was used to determine the compressive behavior of each construct [36]. Each 3 mm sample was affixed to a stainless steel surface and equilibrated for 20 min in PBS. A 0.7 g (0.007 N) mass was applied with a 0.8 mm diameter flat, porous indenter tip, and specimens crept until equilibrium. Specimen thickness was measured from photographs using ImageJ software. Aggregate modulus, a measure of compressive stiffness, was calculated using a semi-analytical, semi-numeric, linear biphasic model [36].
Statistical analysis
All quantitative biochemical and biomechanical assessments were made using n=6-8; that is, each group for every quantitative test had a minimum of 6 samples and a maximum of 8 samples. Data are presented as means ± standard deviations. Single factor ANOVA was employed in each phase of the study to assess for differences among experimental groups. Statistical significance was defined as p<0.05. If significant differences were observed, a Tukey’s HSD post hoc test was performed to determine specific differences among groups. All statistical analyses were performed using JMP 9.0.2 (SAS Institute).
Results
Phase I: Evaluation of treatment time windows for TRPV4 channel activation
In Phase I, we studied the effects of treating self-assembled articular cartilage constructs with 4α-PDD, an agonist of the TRPV4 channel. We examined the use of 10 μM 4α-PDD during three different treatment time windows: t=6-10 days, t=10-14 days, and t=14-18 days. Gross morphological measurements of all constructs at t=28 days are presented in Table 1. No differences were found in construct diameter, thickness, or wet weight (WW) among groups.
Table 1.
Diameter, thickness, and wet weight (WW) of tissue engineered constructs. In Phase I, no differences were found in construct diameter, thickness, or WW among groups. In Phase II, constructs treated with ouabain or with both 4α-PDD and ouabain had significantly reduced diameters (0.83x and 0.86x control, respectively), thicknesses (0.51x and 0.67x control), and WW (0.40 and 0.43x control). Data are presented as means ± standard deviations. Lowercase letters denote significant differences within a column; groups not connected by the same letter are considered significantly different (p<0.05).
| Group | Diameter (mm) | Thickness (mm) | WW (mg) |
|---|---|---|---|
| Phase I (10 μM 4α-PDD) | |||
| Control | 6.29 ± 0.17 | 0.62 ± 0.05 | 36.7 ± 3.1 |
| Treatment on days 6-10 | 6.27 ± 0.16 | 0.63 ± 0.05 | 37.2 ± 2.8 |
| Treatment on days 10-14 | 6.31 ± 0.18 | 0.63 ± 0.05 | 36.2 ± 2.4 |
| Treatment on days 14-18 | 6.26 ± 0.16 | 0.62 ± 0.06 | 37.2 ± 2.8 |
| Phase II | |||
| Control | 6.35 ± 0.06 a | 0.68 ± 0.10 a | 38.3 ± 1.5 a |
| 10 μM 4α-PDD | 6.30 ± 0.13 a | 0.63 ± 0.10 a | 39.6 ± 2.3 a |
| 20 μM ouabain | 5.29 ± 0.04 b | 0.35 ± 0.08 b | 15.2 ± 0.6 b |
| Combo | 5.45 ± 0.18 b | 0.45 ± 0.08 b | 16.3 ± 1.3 b |
Biochemical analyses were conducted to quantify construct cellularity, collagen content, and GAG content. Construct biochemical data are provided in Table 2. No differences were found in cell numbers across treatment times. Collagen/WW was highest in constructs treated with 4α-PDD during days 10-14 (88% increase over control), followed by constructs treated during days 14-18 (40% increase over control). When normalized to cell number, these differences in collagen content were also seen, with approximately the same increases in magnitude compared to control. There were no differences observed in GAG content across treatment time windows.
Table 2.
Biochemical content of tissue engineered constructs. In Phase I, collagen/WW was highest in constructs treated with 4α-PDD during days 10-14 (1.88x control), followed by constructs treated during days 14-18 (1.40x control). These differences in collagen content were upheld when normalized to cell number. There were no differences observed in GAG content in Phase I. In Phase II, treatment with 4α-PDD, ouabain, or their combination resulted in significant increases in collagen/WW compared to control (1.80x, 2.18x, and 1.93x control, respectively), but no differences between each other. Collagen production per cell was greatest in constructs treated with 4α-PDD (1.85x control). GAG production per cell was significantly decreased in constructs treated with ouabain (0.40x control) and the combination of 4α-PDD and ouabain (0.43x control). No differences in cell number were observed in Phase I or Phase II. Data are presented as means ± standard deviations. Lowercase letters denote significant differences within a column; groups not connected by the same letter are considered significantly different (p<0.05).
| Group | Total cells (× 106) |
Collagen (% WW) |
GAG (% WW) |
Collagen (μg/106 cells) |
GAG (μg/106 cells) |
|---|---|---|---|---|---|
| Phase I (10 μM 4α-PDD) | |||||
| Control | 5.64 ± 0.33 | 5.9 ± 0.7 c | 3.6 ± 0.5 | 383 ± 60 c | 232 ± 44 |
| Treatment on days 6-10 | 5.73 ± 0.37 | 6.2 ± 1.1 bc | 3.7 ± 0.4 | 401 ± 82 bc | 245 ± 39 |
| Treatment on days 10-14 | 5.71 ± 0.43 | 11.1 ± 2.3 a | 3.4 ± 0.9 | 694 ± 97 a | 212 ± 46 |
| Treatment on days 14-18 | 5.67 ± 0.46 | 8.3 ± 1.1 b | 3.7 ± 0.7 | 548 ± 123 ab | 242 ± 62 |
| Phase II | |||||
| Control | 5.73 ± 0.24 | 5.5 ± 0.7 b | 4.0 ± 0.8 | 363 ± 37 b | 268 ± 50 a |
| 10 μM 4α-PDD | 5.79 ± 0.29 | 9.8 ± 1.8 a | 4.5 ± 1.1 | 669 ± 127 a | 305 ± 74 a |
| 20 μM ouabain | 5.55 ± 0.20 | 11.9 ± 2.5 a | 4.0 ± 1.1 | 325 ± 67 b | 108 ± 28 b |
| Combo | 5.71 ± 0.26 | 10.5 ± 2.7 a | 4.1 ± 1.3 | 296 ± 63 b | 116 ± 39 b |
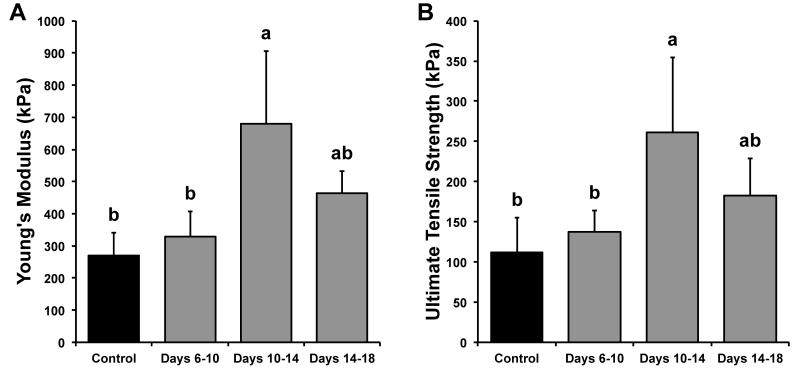
Uniaxial tensile and creep indentation tests were performed to determine construct tensile and compressive properties. Tensile stiffness and strength data for Phase I are shown in Figure 1. Young’s moduli for control, treatment on days 6-10, treatment on days 10-14, and treatment on days 14-18 were 269 ± 73, 328 ± 80, 681 ± 224, and 464 ± 69 kPa, respectively. Constructs treated with 4α-PDD during days 10-14 had the highest Young’s moduli (153% increase over control). UTS values for control, treatment on days 6-10, treatment on days 10-14, and treatment on days 14-18 were 112 ± 43, 138 ± 27, 261 ± 94, and 182 ± 46 kPa, respectively. Constructs treated with 4α-PDD during days 10-14 had the highest UTS (133% increase over control). With respect to compressive stiffness, the aggregate moduli for control, treatment on days 6-10, treatment on days 10-14, and treatment on days 14-18 were 75 ± 19, 72 ± 21, 82 ± 20, and 76 ± 25 kPa, respectively; no differences were found in aggregate moduli across groups.
Figure 1.
Phase I tensile properties of tissue engineered constructs. (A) Tensile stiffness for all groups. Constructs treated with 4α-PDD during days 10-14 had the highest Young’s moduli (153% greater than control). (B) Tensile strength for all groups. Constructs treated with 4α-PDD during days 10-14 had the highest ultimate tensile strength (UTS) (133% greater than control). Data are presented as means + standard deviations. Lowercase letters denote significant differences; groups not connected by the same letter are considered significantly different (p<0.05).
Altogether, treatment with 4α-PDD during days 10-14 provided the greatest increases in collagen content and tensile properties. Based on these results, this treatment regimen was carried forward into Phase II.
Phase II: TRPV4 activation versus Na+/K+ pump inhibition
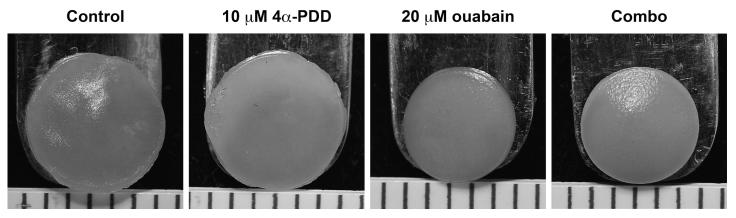
In Phase II, we compared the effects of TRPV4 activation to the effects of Na+/K+ pump inhibition, and we further studied whether the combination of these two stimuli would outperform their individual use. Self-assembled articular cartilage constructs were treated with either 10 μM 4α-PDD, 20 μM ouabain, or a combination of the two during t=10-14 days. Constructs were grown in culture to t=28 days. At the end of culture, constructs treated with ouabain or with the combination of 4α-PDD and ouabain were visibly smaller than control constructs or constructs treated with 4α-PDD alone (Figure 2). Diameter, thickness, and WW values are provided in Table 1. Constructs treated with ouabain or with both agents had significantly smaller diameters (17% and 14% decreases from control, respectively), thicknesses (49% and 33% decreases), and wet weights (60% and 57% decreases).
Figure 2.
Phase II gross morphology of tissue engineered constructs at 4 weeks. From left to right: representative photographs of constructs from the control group, treated with 10 μM 4α-PDD, treated with 20 μM ouabain, and treated with both with 10 μM 4α-PDD and 20 μM ouabain. Constructs treated with ouabain or with both 4α-PDD and ouabain were visibly smaller than control constructs or constructs treated with 4α-PDD alone. Scale markings are spaced 1 mm apart.
Construct biochemical data for Phase II are provided in Table 2. Treatment with 4α-PDD, ouabain, or their combination resulted in significant increases in collagen/WW compared to control (increases of 80%, 118%, and 93%, respectively), but no differences between each other. Collagen production per cell was greatest in constructs treated with 4α-PDD (85% increase over control), with no differences among control, ouabain, or the combination 4α-PDD and ouabain. GAG/WW was not different across groups, but GAG production per cell was significantly decreased in constructs treated with ouabain (60% decrease from control) and the combination of 4α-PDD and ouabain (57% decrease from control). No differences were observed in cell number across groups.
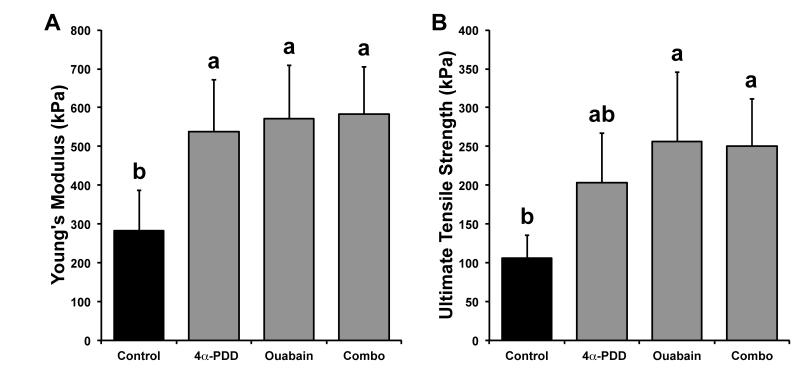
Biomechanical properties were again assessed with uniaxial tensile and creep indentation testing. Tensile stiffness and strength data for Phase II are shown in Figure 3. Young’s moduli for control, treatment with 4α-PDD, treatment with ouabain, and combined treatment were 282 ± 105, 538 ± 133, 572 ± 136, and 583 ± 121 kPa, respectively. Treatment with 4α-PDD, ouabain, and their combination resulted in significant increases in Young’s moduli compared to control (91%, 103%, and 107% increases, respectively), but no differences between each other. UTS values for control, treatment with 4α-PDD, treatment with ouabain, and combined treatment were 106 ± 29, 203 ± 64, 256 ± 89, and 251 ± 61 kPa, respectively. Treatment with ouabain or with the combination of 4α-PDD and ouabain produced the greatest improvements in construct UTS (141% and 136% increases over control, respectively), followed by treatment with 4α-PDD (91% increase over control, but not statistically significant). In terms of compressive properties, aggregate moduli for control, treatment with 4α-PDD, treatment with ouabain, and combined treatment were 67 ± 14, 73 ± 18, 67 ± 19, and 74 ± 23 kPa, respectively; no differences were found in aggregate moduli across groups.
Figure 3.
Phase II tensile properties of self-assembled cartilage constructs. (A) Tensile stiffness for all groups. Treatment with 4α-PDD, ouabain, and their combination resulted in significant increases in Young’s moduli compared to control (91%, 103%, and 107% increases, respectively), but no differences between each other. (B) Tensile strength for all groups. Treatment with ouabain or with the combination of 4α-PDD and ouabain improved construct UTS (141% and 136% increases over control, respectively), followed by treatment with 4α-PDD (91% increase over control, but not statistically significant). Data are presented as means + standard deviations. Lowercase letters denote significant differences; groups not connected by the same letter are considered significantly different (p<0.05).
Functionality index
A previously developed functionality index (FI) was used to quantify the similarity between self-assembled constructs and native tissue in Phase II. As shown in Equation 1, the FI equally weights the compressive stiffness (EC), tensile stiffness (ET), GAG content (G), and collagen content (C). The subscripts ‘nac’ and ‘sac’ represent values for native and self-assembled cartilage, respectively.
| (Eq.1) |
Native values previously determined by our laboratory for the purpose of FI calculation were 12.1 MPa, 0.2 MPa, 5.5%, and 15% for Young’s modulus, aggregate modulus, GAG/WW, and collagen/WW, respectively [37, 38]. The FI yields a score of 1 when construct properties are equivalent to those of native tissue. FI values were 0.41, 0.63, 0.70, and 0.69 for control, treatment with 4α-PDD, treatment with ouabain, and combined treatment, respectively.
Discussion
This study employed a two-phased approach to evaluate the effects of TRPV4 channel activation on tissue engineered articular cartilage. Experimental results supported the hypotheses motivating this study: 1) TRPV4 activation resulted in significant improvements in construct biochemical and biomechanical properties; 2) culture days 10-14 were identified as the optimal treatment time window to produce the greatest improvements in constructs; and 3) activation of TRPV4, a Ca2+-permeable channel, produced results comparable to Na+/K+ pump inhibition. To our knowledge, this is the first study to examine TRPV4 channel activation in tissue engineering. The results of this investigation demonstrate that direct chemical modulation of intracellular ion concentrations can be a powerful tool in tissue engineering.
In Phase I, it was found that the optimal time window for TRPV4 activation in self-assembled articular cartilage constructs is during culture days 10-14. Compared to control, treatment with the TRPV4 channel agonist 4α-PDD during days 10-14 led to significant improvements in collagen content (88% increase), tensile stiffness (153% increase), and tensile strength (130% increase). However, constructs were not improved by treatment during days 6-10 or 14-18, thereby highlighting the importance of timing during in vitro tissue development. The beneficial effects of treatment during days 10-14 are corroborated by previous work showing that other stimuli also produce their maximal effects during this time window [24, 25]. To understand why this time period is so crucial, it is important to consider the developmental milestones of constructs during self-assembly, a process that has been shown to resemble in vivo cartilage development [4]. During self-assembly, collagen production peaks between days 10-14 of culture, while GAG production continues indiscriminately. This rapid synthesis of GAG with no new collagen secretion contributes to pre-stress within the nascent ECM, thereby compromising the engineered tissue’s tensile properties [39, 40]. Directly modulating this imbalance between GAG and collagen has been shown to improve the tensile properties of self-assembled constructs [39, 41]. Thus, during days 10-14, before collagen production tapers and GAG production ramps up, cells within the developing construct may be more susceptible to interventions that induce new collagen biosynthesis. Based on the results from Phase I, the optimal treatment time window of t=10-14 days was carried forward to Phase II.
In Phase II, TRPV4 activation using 4α-PDD was compared to Na+/K+ pump inhibition using ouabain. A previous study from our group showed that inhibition of the Na+/K+ pump using 20 μM ouabain during days 10-14 of culture improved the collagen content and tensile properties of self-assembled articular cartilage constructs [24], results that were corroborated by the present study. It was found that 10 μM 4α-PDD produced improvements in construct tensile properties that were comparable to 20 μM ouabain, with no added benefit when the two stimuli were combined. Specifically, application of either 4α-PDD or ouabain led to an approximately 2-fold increase in tensile stiffness. Though each agent produced an identical net enhancement in tensile stiffness, it is clear from the differences in construct sizes and biochemical content that the precise physiological responses to these agents, and therefore the mechanisms underlying tensile improvements, vary considerably. Moreover, combined treatment resulted in the same changes in size and biochemical content observed for treatment with ouabain alone, implying that Na+/K+ pump inhibition predominates over TRPV4 activation in producing effects at the cell and tissue levels. This observation is similar to a previous observation in which constructs treated with a combination of ouabain and ionomycin, a Ca2+ ionophore, did not outperform individual application either agent [24].
Notably, treatment with ouabain significantly reduced GAG production on a per-cell basis. Lower GAG levels are associated with decreased size and wet weight in cartilage [42], and the subsequent reduction in size in ouabain-treated constructs led to an increase in the percentage of collagen per wet weight, even though the per-cell production of collagen did not change. These phenomena suggest that ouabain treatment promotes a maturational growth phenotype, in which the tissue maintains a uniform size during ECM synthesis and remodeling, rather than an expansive growth phenotype, in which the tissue experiences a volumetric increase in size during ECM deposition [39, 42, 43]. Unlike ouabain, treatment of constructs with 4α-PDD resulted in increased collagen production per cell, with no change in GAG production per cell. This net increase in collagen deposition at a steady level of GAG production is likely responsible for the improved tensile stiffness of constructs treated with 4α-PDD.
Further work is necessary to determine how ion channel modulators elicit changes in chondrocyte ECM synthesis. Ion channels are thought to be involved in the cellular response to dynamic compression [5-7], fluid shear [11-13], hydrostatic pressure [14-17], and osmotic stress [20-23]. Specifically, ion channels play a role in the chondrocyte response to changes in volume; for example, a decrease in cell volume activates the Na+/K+/2Cl− cotransporter to increase the tonicity of the intracellular compartment, thereby encouraging the cell to return to its initial state [20, 44]. Similarly, the Na+/K+ pump is an ATPase that pumps ions against a concentration gradient to keep intracellular K+ higher than Na+; inhibition of this pump, as with ouabain in the present study, leads to increased intracellular Na+ [24]. Other channels, such as the Na+/H+ transporter and Ca2+ permeable channels, are also involved in volume regulation, among other roles, in response to mechanical or osmotic loading. In particular, the TRPV4 channel has been shown to play a central role in regulating the chondrocyte response to osmotic stress [30, 31], as well as in promoting chondrogenic differentiation [45]. TRPV4 may also be implicated in osmotic stress-related pathogenesis of osteoarthritis [46]. The present study demonstrates that TRPV4 activation in engineered cartilage constructs can produce observable, tissue-level changes. Because of the osmosensitivity of TRPV4, it will be important in the future to examine the combined effects of osmotic stress and TRPV4 modulation on tissue engineered cartilage. Future studies that involve confocal imaging of intact self-assembled constructs may provide a better understanding of the importance of TRPV4 during in vitro tissue development.
In summary, this study investigated whether activation of the Ca2+-permeable TRPV4 channel would alter the biochemical and biomechanical properties of tissue engineered articular cartilage. It was shown that TRPV4 activation improved construct tensile properties, that the effects of TRPV4 activation were time-dependent, and that net improvements were on par with those produced by inhibiting the Na+/K+ pump. To our knowledge, this is the first study to examine TRPV4 channel activation in tissue engineering. The results of this study demonstrate the effectiveness of ion channel modulation as a strategy for improving the functional properties of engineered tissues. Further investigation of the role of TRPV4 in self-assembled constructs should be undertaken at both a mechanistic level (e.g., examine cell volume regulation and calcium transients in situ) and at a functional engineering level (e.g., assessment of different durations of TRPV4 activation, or combining TRPV4 activation or inhibition with hyper-osmotic or hypo-osmotic stimulation).
Acknowledgments
Grants This work was funded by the following grants from the National Institutes of Health: NIAMS R01AR053286 and NIGMS T32GM007730.
Footnotes
Disclosures There are no conflicts of interest to report.
Author Contributions SVE and KAA conceived and designed the experiments. SVE acquired the data. SVE and KAA reviewed the data. SVE and KAA wrote and edited the paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Buckwalter JA. Articular cartilage: injuries and potential for healing. J Orthop Sports Phys Ther. 1998;28:192–202. doi: 10.2519/jospt.1998.28.4.192. [DOI] [PubMed] [Google Scholar]
- [2].Darling EM, Athanasiou KA. Biomechanical strategies for articular cartilage regeneration. Annals of biomedical engineering. 2003;31:1114–24. doi: 10.1114/1.1603752. [DOI] [PubMed] [Google Scholar]
- [3].Hu JC, Athanasiou KA. A self-assembling process in articular cartilage tissue engineering. Tissue Eng. 2006;12:969–79. doi: 10.1089/ten.2006.12.969. [DOI] [PubMed] [Google Scholar]
- [4].Ofek G, Revell CM, Hu JC, Allison DD, Grande-Allen KJ, Athanasiou KA. Matrix development in self-assembly of articular cartilage. PloS one. 2008;3:e2795. doi: 10.1371/journal.pone.0002795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Chowdhury TT, Knight MM. Purinergic pathway suppresses the release of NO and stimulates proteoglycan synthesis in chondrocyte/agarose constructs subjected to dynamic compression. J Cell Physiol. 2006;209:845–53. doi: 10.1002/jcp.20768. [DOI] [PubMed] [Google Scholar]
- [6].Mouw JK, Imler SM, Levenston ME. Ion-channel regulation of chondrocyte matrix synthesis in 3D culture under static and dynamic compression. Biomech Model Mechanobiol. 2007;6:33–41. doi: 10.1007/s10237-006-0034-1. [DOI] [PubMed] [Google Scholar]
- [7].Hunter CJ, Mouw JK, Levenston ME. Dynamic compression of chondrocyte-seeded fibrin gels: effects on matrix accumulation and mechanical stiffness. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2004;12:117–30. doi: 10.1016/j.joca.2003.08.009. [DOI] [PubMed] [Google Scholar]
- [8].Likhitpanichkul M, Guo XE, Mow VC. The effect of matrix tension-compression nonlinearity and fixed negative charges on chondrocyte responses in cartilage. Mol Cell Biomech. 2005;2:191–204. [PubMed] [Google Scholar]
- [9].Lima EG, Bian L, Ng KW, Mauck RL, Byers BA, Tuan RS, et al. The beneficial effect of delayed compressive loading on tissue-engineered cartilage constructs cultured with TGF-beta3. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2007;15:1025–33. doi: 10.1016/j.joca.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Mauck RL, Nicoll SB, Seyhan SL, Ateshian GA, Hung CT. Synergistic action of growth factors and dynamic loading for articular cartilage tissue engineering. Tissue Eng. 2003;9:597–611. doi: 10.1089/107632703768247304. [DOI] [PubMed] [Google Scholar]
- [11].Edlich M, Yellowley CE, Jacobs CR, Donahue HJ. Cycle number and waveform of fluid flow affect bovine articular chondrocytes. Biorheology. 2004;41:315–22. [PubMed] [Google Scholar]
- [12].Edlich M, Yellowley CE, Jacobs CR, Donahue HJ. Oscillating fluid flow regulates cytosolic calcium concentration in bovine articular chondrocytes. Journal of biomechanics. 2001;34:59–65. doi: 10.1016/s0021-9290(00)00158-5. [DOI] [PubMed] [Google Scholar]
- [13].Yellowley CE, Jacobs CR, Donahue HJ. Mechanisms contributing to fluid-flow-induced Ca2+ mobilization in articular chondrocytes. J Cell Physiol. 1999;180:402–8. doi: 10.1002/(SICI)1097-4652(199909)180:3<402::AID-JCP11>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- [14].Wilkins RJ, Browning JA, Urban JP. Chondrocyte regulation by mechanical load. Biorheology. 2000;37:67–74. [PubMed] [Google Scholar]
- [15].Urban JP, Hall AC, Gehl KA. Regulation of matrix synthesis rates by the ionic and osmotic environment of articular chondrocytes. J Cell Physiol. 1993;154:262–70. doi: 10.1002/jcp.1041540208. [DOI] [PubMed] [Google Scholar]
- [16].Hall AC. Differential effects of hydrostatic pressure on cation transport pathways of isolated articular chondrocytes. J Cell Physiol. 1999;178:197–204. doi: 10.1002/(SICI)1097-4652(199902)178:2<197::AID-JCP9>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- [17].Elder BD, Athanasiou KA. Hydrostatic pressure in articular cartilage tissue engineering: from chondrocytes to tissue regeneration. Tissue engineering Part B, Reviews. 2009;15:43–53. doi: 10.1089/ten.teb.2008.0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Elder SH, Sanders SW, McCulley WR, Marr ML, Shim JW, Hasty KA. Chondrocyte response to cyclic hydrostatic pressure in alginate versus pellet culture. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2006;24:740–7. doi: 10.1002/jor.20086. [DOI] [PubMed] [Google Scholar]
- [19].Smith RL, Lin J, Trindade MC, Shida J, Kajiyama G, Vu T, et al. Time-dependent effects of intermittent hydrostatic pressure on articular chondrocyte type II collagen and aggrecan mRNA expression. J Rehabil Res Dev. 2000;37:153–61. [PubMed] [Google Scholar]
- [20].Erickson GR, Alexopoulos LG, Guilak F. Hyper-osmotic stress induces volume change and calcium transients in chondrocytes by transmembrane, phospholipid, and G-protein pathways. Journal of biomechanics. 2001;34:1527–35. doi: 10.1016/s0021-9290(01)00156-7. [DOI] [PubMed] [Google Scholar]
- [21].Erickson GR, Northrup DL, Guilak F. Hypo-osmotic stress induces calcium-dependent actin reorganization in articular chondrocytes. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2003;11:187–97. doi: 10.1053/s1063-4584(02)00347-3. [DOI] [PubMed] [Google Scholar]
- [22].Sanchez JC, Danks TA, Wilkins RJ. Mechanisms involved in the increase in intracellular calcium following hypotonic shock in bovine articular chondrocytes. Gen Physiol Biophys. 2003;22:487–500. [PubMed] [Google Scholar]
- [23].Sanchez JC, Wilkins RJ. Changes in intracellular calcium concentration in response to hypertonicity in bovine articular chondrocytes. Comp Biochem Physiol A Mol Integr Physiol. 2004;137:173–82. doi: 10.1016/j.cbpb.2003.09.025. [DOI] [PubMed] [Google Scholar]
- [24].Natoli RM, Skaalure S, Bijlani S, Chen KX, Hu J, Athanasiou KA. Intracellular Na(+) and Ca(2+) modulation increases the tensile properties of developing engineered articular cartilage. Arthritis and rheumatism. 2010;62:1097–107. doi: 10.1002/art.27313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Elder BD, Athanasiou KA. Effects of temporal hydrostatic pressure on tissue-engineered bovine articular cartilage constructs. Tissue engineering Part A. 2009;15:1151–8. doi: 10.1089/ten.tea.2008.0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Gu WY, Lai WM, Mow VC. A mixture theory for charged-hydrated soft tissues containing multi-electrolytes: passive transport and swelling behaviors. Journal of biomechanical engineering. 1998;120:169–80. doi: 10.1115/1.2798299. [DOI] [PubMed] [Google Scholar]
- [27].Yellowley CE, Hancox JC, Donahue HJ. Effects of cell swelling on intracellular calcium and membrane currents in bovine articular chondrocytes. J Cell Biochem. 2002;86:290–301. doi: 10.1002/jcb.10217. [DOI] [PubMed] [Google Scholar]
- [28].Palmer GD, Chao Ph, Raia F, Mauck RL, Valhmu WB, Hung CT. Time-dependent aggrecan gene expression of articular chondrocytes in response to hyperosmotic loading. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2001;9:761–70. doi: 10.1053/joca.2001.0473. PH. [DOI] [PubMed] [Google Scholar]
- [29].Tew SR, Peffers MJ, McKay TR, Lowe ET, Khan WS, Hardingham TE, et al. Hyperosmolarity regulates SOX9 mRNA posttranscriptionally in human articular chondrocytes. Am J Physiol Cell Physiol. 2009;297:C898–906. doi: 10.1152/ajpcell.00571.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Phan MN, Leddy HA, Votta BJ, Kumar S, Levy DS, Lipshutz DB, et al. Functional characterization of TRPV4 as an osmotically sensitive ion channel in porcine articular chondrocytes. Arthritis and rheumatism. 2009;60:3028–37. doi: 10.1002/art.24799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Guilak F, Leddy HA, Liedtke W. Transient receptor potential vanilloid 4: The sixth sense of the musculoskeletal system? Ann N Y Acad Sci. 2010;1192:404–9. doi: 10.1111/j.1749-6632.2010.05389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Hung CT. Transient receptor potential vanilloid 4 channel as an important modulator of chondrocyte mechanotransduction of osmotic loading. Arthritis and rheumatism. 2010;62:2850–1. doi: 10.1002/art.27617. [DOI] [PubMed] [Google Scholar]
- [33].Eleswarapu SV, Chen JA, Athanasiou KA. Temporal assessment of ribose treatment on self-assembled articular cartilage constructs. Biochem Biophys Res Commun. 2011;414:431–6. doi: 10.1016/j.bbrc.2011.09.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Elder BD, Athanasiou KA. Effects of confinement on the mechanical properties of self-assembled articular cartilage constructs in the direction orthogonal to the confinement surface. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2008;26:238–46. doi: 10.1002/jor.20480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Woessner JF., Jr. The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys. 1961;93:440–7. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- [36].Athanasiou KA, Agarwal A, Muffoletto A, Dzida FJ, Constantinides G, Clem M. Biomechanical properties of hip cartilage in experimental animal models. Clin Orthop Relat Res. 1995;316:254–66. [PubMed] [Google Scholar]
- [37].Elder BD, Athanasiou KA. Systematic assessment of growth factor treatment on biochemical and biomechanical properties of engineered articular cartilage constructs. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2009;17:114–23. doi: 10.1016/j.joca.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Responte DJ, Arzi B, Natoli RM, Hu JC, Athanasiou KA. Mechanisms underlying the synergistic enhancement of self-assembled neocartilage treated with chondroitinase-ABC and TGF-beta1. Biomaterials. 2012;33:3187–94. doi: 10.1016/j.biomaterials.2012.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Natoli R, Revell CM, Athanasiou K. Chondroitinase ABC Treatment Results in Increased Tensile Properties of Self-Assembled Tissue Engineered Articular Cartilage. Tissue engineering Part A. 2009;15:3119–28. doi: 10.1089/ten.tea.2008.0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Schwartz MH, Leo PH, Lewis JL. A microstructural model for the elastic response of articular cartilage. Journal of biomechanics. 1994;27:865–73. doi: 10.1016/0021-9290(94)90259-3. [DOI] [PubMed] [Google Scholar]
- [41].Natoli RM, Responte DJ, Lu BY, Athanasiou KA. Effects of multiple chondroitinase ABC applications on tissue engineered articular cartilage. Journal of Orthopaedic Research. 2009 doi: 10.1002/jor.20821. 10.1002/jor.20821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Asanbaeva A, Masuda K, Thonar EJ, Klisch SM, Sah RL. Mechanisms of cartilage growth: modulation of balance between proteoglycan and collagen in vitro using chondroitinase ABC. Arthritis and rheumatism. 2007;56:188–98. doi: 10.1002/art.22298. [DOI] [PubMed] [Google Scholar]
- [43].Huey DJ, Athanasiou KA. Maturational growth of self-assembled, functional menisci as a result of TGF-beta1 and enzymatic chondroitinase-ABC stimulation. Biomaterials. 2011;32:2052–8. doi: 10.1016/j.biomaterials.2010.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Hall AC, Horwitz ER, Wilkins RJ. The cellular physiology of articular cartilage. Exp Physiol. 1996;81:535–45. doi: 10.1113/expphysiol.1996.sp003956. [DOI] [PubMed] [Google Scholar]
- [45].Muramatsu S, Wakabayashi M, Ohno T, Amano K, Ooishi R, Sugahara T, et al. Functional gene screening system identified TRPV4 as a regulator of chondrogenic differentiation. J Biol Chem. 2007;282:32158–67. doi: 10.1074/jbc.M706158200. [DOI] [PubMed] [Google Scholar]
- [46].Clark AL, Votta BJ, Kumar S, Liedtke W, Guilak F. Chondroprotective role of the osmotically sensitive ion channel transient receptor potential vanilloid 4: age- and sex-dependent progression of osteoarthritis in Trpv4-deficient mice. Arthritis and rheumatism. 2010;62:2973–83. doi: 10.1002/art.27624. [DOI] [PMC free article] [PubMed] [Google Scholar]