Abstract
The continued occurrence of refractory seizures in at least one-third of children and adults with epilepsy, despite the availability of almost 15 conventional and novel anticonvulsant drugs, speaks to a dire need to develop novel therapeutic approaches. Cellular metabolism, the critical pathways by which cells access and utilize energy, is critical for normal neuronal function. Furthermore, mounting evidence suggests direct links between energy metabolism and cellular excitability. The high-fat, low-carbohydrate ketogenic diet has been used as a treatment for drug-refractory epilepsy for almost a century. Yet, the multitude of alternative therapies to target aspects of cellular metabolism and hyperexcitability is almost untapped. Approaches discussed in this review offer a wide diversity of therapeutic targets that might be exploited by investigators in the search for safer and more effective epilepsy treatments.
Keywords: epilepsy, metabolism, dietary treatments, ketogenic diet, calorie restriction, 2-deoxy-D-glucose, mTOR, anaplerosis
1. Introduction
Epilepsy is one of the most common neurological diseases but despite major advances in drug development, 20-30% of patients have seizures that are not controlled by the first two medications prescribed [1-4]. This gap in medication efficacy is a significant challenge in clinical epilepsy care and indicates the need for new strategies to better treat the underlying pathological process of epilepsy [5]. For patients who do not have a lesion that can be resected surgically (i.e., multifocal epilepsy or certain epilepsy syndromes of childhood), dietary therapy remains an underutilized but highly effective option.
The beneficial effect of fasting on seizure control was noted at the time of Hippocrates [6]. In order to mimic the beneficial effects of fasting on seizures, a high-fat, low-carbohydrate ketogenic diet was developed at the beginning of the last century [6]. To this day, the ketogenic diet still is used to treat patients with epilepsy that is refractory to medicines (i.e., “drug-resistant” or “medically intractable”). Despite its clinical success (discussed below), there remains a lack of a clear mechanistic understanding of how metabolism can be manipulated to prevent recurrent unprovoked seizures. Over time, a number of other dietary and pharmacologically-based metabolism interventions have been developed in an attempt to mimic the effects of the ketogenic diet while providing an easier regimen for patients and their families to follow. A mechanistic understanding of how the ketogenic diet works also would allow clinicians to optimize the diet’s efficacy while minimizing adverse consequences (Table 1). Implementation and maintenance of the ketogenic diet require the involvement of a dietician familiar with its management, and such expertise may not be available in some medical centers. Increasing the ease of use of the diet also might increase its accessibility to patients. From a scientific perspective, elucidating these mechanisms also would likely improve our understanding of the role of how seizures recur and may provide key insights into neuronal pathophysiology. Finally, mechanism-based studies also may identify new pharmacological antiseizure targets.
Table 1.
Reasons to explore the mechanism of metabolism-based antiseizure therapies
| Optimize efficacy |
| Minimize adverse consequences Increase convenience |
| Increase accessibility |
| Improve understanding of epilepsy |
| Identify new anticonvulsant targets |
2. Specific metabolic treatments for epilepsy
2.1 Ketogenic diet
2.1.1. Background
Patients consuming a high-fat, low-carbohydrate diet metabolize the fats via mitochondrial fatty acid oxidation into the main ketone bodies (i.e., β-hydroxybutyrate, acetoacetate, and acetone), which then are filtered by the kidneys, resulting in ketonuria [7]. Blood-borne ketone bodies circulate to regions of high energy demand (e.g., brain, muscle), supplying these tissues with an alternative energy source to carbohydrates. Despite the ketogenic diet’s name, ketone bodies are not necessarily the primary mechanism of its antiseizure action (see below) [6].
2.1.2. Clinical efficacy
There are three main indications for clinical use of the ketogenic diet. First, patients with mutations in the SLC2A1 gene have a decreased ability to transport glucose from the blood into the cerebrospinal fluid (i.e., GLUT-1 deficiency), resulting in seizures, movement disorders, and developmental delays of varying severity [8]. The ketogenic diet is thought to circumvent this ‘block’ by providing 2-carbon equivalents to the tricarboxylic acid (TCA) or Krebs cycle, allowing nonglycolytic production of ATP [9]. Second, patients with pyruvate dehydrogenase deficiency can have seizures and benefit from the ketogenic diet for analogous reasons [10]. Third, as a treatment for epilepsy, the ketogenic diet has been used for nearly a century in patients with medically intractable seizures but reports of its efficacy for this indication were largely anecdotal until approximately 20 years ago. Case series (some prospectively assembled) were summarized in a series of meta-analyses demonstrating the efficacy of a ketogenic diet. Methodological concerns about case series have been outlined elsewhere; it is particularly challenging to blind observers involved in ketogenic diet clinical studies [11]. The first meta-analysis showed that 16% of children had a complete cessation of seizures and 56% of children had a >50% reduction in seizures, comparable to most standard medicines (keeping in mind that most patients consuming a ketogenic diet have failed >5 medications before trying the diet) [12]. A different meta-analysis showed an odds ratio of 2.25 (95% confidence interval 1.69-2.98) for a >50% reduction in seizures [13]. The most convincing data demonstrating the efficacy of the ketogenic diet come from a randomized controlled study that showed a >50% reduction in seizures in 38% of children on the ketogenic diet vs. 6% in the control group (who continued their anticonvulsants) over a 3-month period [14]. Most of these studies were based on ketogenic diets using primarily long-chain fatty acids but diets based on highly ketogenic medium chain triglycerides have shown similar efficacy [15-17]. The main difference between long-chain and medium-chain fat diets is the occurrence of gastrointestinal-related side effects, with constipation being especially common with long-chain fat diets and diarrhea a frequent adverse effect of medium-chain fat diets [11]. To summarize, the clinical antiseizure efficacy of ketogenic diets has been established. Another trial used a novel strategy (reversal of ketosis with an oral glucose solution) in a randomized, blinded controlled study [18]. The primary outcome measure, seizure frequency, did not achieve significance, probably due to an underestimation of the amount of glucose needed to reverse ketosis.
While the ketogenic diet is effective in a broad spectrum of drug-resistant epilepsies, the question has arisen as to whether it might afford particular benefit in specific epilepsy syndromes, which could then lead to mechanistic hypotheses about the how the diet works [19]. One relevant example is Dravet syndrome [20-23]. Most cases of Dravet syndrome are associated with mutations in the SCN1A gene, which encodes a subunit of the sodium channel [24]. The mutation seems to preferentially affect inhibitory interneurons, leading to reduction of inhibition in neuronal circuits prone to seizure generation [25]. Clinically, Dravet syndrome typically begins with a prolonged febrile seizure in the first year of life, followed later in childhood by medically intractable seizures, developmental delays, and movement disorders including myoclonus, with further neurological deterioration into adulthood [26]. In case series, a ketogenic diet led to improved seizure control in slightly over half of patients with Dravet syndrome, with some patients becoming seizure-free [20, 21, 23]. Similar findings have been shown in mice harboring mutations in SCN1A, in which two weeks of ketogenic diet treatment led to an increased latency to onset of generalized convulsions induced by flurothyl [27]. These experiments create an opportunity for mechanism-based studies in Dravet syndrome.
2.1.3. Putative mechanisms
2.1.3.1. Approaches to studying mechanisms
A wide variety of experimental approaches have been utilized to study how the ketogenic diet and other dietary therapies work. Broadly, these can be divided into in vitro systems using brain slices, cultured neurons or other reduced preparations, and whole animals. In vitro systems allow investigation of direct effects of compounds on cellular excitability, while whole animals are used to assess the effect of ingested or administered agents on seizure threshold as well as safety issues. Each approach has advantages and disadvantages, depending on the specific information sought [28]. In the sections that follow, studies of epilepsy diet mechanism that employ both approaches are considered, but first, we discuss an alternative test of seizure susceptibility to address the question: Does the ketogenic diet have a similar profile to standard anticonvulsant drugs on acute seizure tests [7]?
Currently, candidate anticonvulsants currently are tested in a panel of tests in the Anticonvulsant Screening Program (ASP) at the National Institutes of Health (NIH) [29]. Until recently, the initial screen consisted of the GABAA receptor antagonist pentylenetetrazol (PTZ) and the maximal electroshock test (MES). The PTZ and MES tests are used to screen anticonvulsant compounds against generalized tonic-clonic and myoclonic seizure susceptibility, via chemical and electrical means, respectively. Generally speaking, drugs with similar mechanisms of action have a similar pattern of protection against certain convulsants and lack of protection against others (i.e., comparable acute seizure test profiles). For example, phenytoin and lamotrigine inhibit activity of voltage-gated sodium channels; both work in the MES test but not in the PTZ test [30-32].
In a series of experiments designed to test the effects of the ketogenic diet in rodents, mice (rather than rats) were chosen as the experimental subjects based on the goal of eventually testing the paradigm using genetically-modified animals. Although the ketogenic diet protects against seizures induced by PTZ in rats, it does not protect against PTZ-induced convulsions in mice [33-36]. Results using MES have been mixed, partly because this test is performed differently with a dietary treatment (i.e., with a limited dose range), versus an injectable drug (i.e., in which doses are widely varied in order to determine an ED50). Thus, there was a need for an easily-administered screening test with the capability of rapid throughput that would demonstrate the ketogenic diet’s anticonvulsant effects in mice – an effect that has been already demonstrated clinically. The 6 Hz electroshock test was used in the 1950s but lost favor as a screening tool because of its inability to demonstrate the anticonvulsant effects of phenytoin [32]. Nonetheless, the 6 Hz electroshock test was recently re-introduced into the ASP because it demonstrated anticonvulsant effects of levetiracetam, whereas this drug did not exert protective effects in the PTZ or MES tests [29, 32]. The 6 Hz electroshock test also demonstrated the anticonvulsant effects of a ketogenic diet in mice [37], a finding that has been confirmed by other investigators [34]. Furthermore, the ketogenic diet had an acute seizure test profile that was distinct from other anticonvulsants, suggesting that its mechanism of action is unique.
2.1.3.2. Inhibitory neurotransmission
Ultimately, changes in neuronal excitability are due to alterations in excitatory or inhibitory neurotransmission, which is the rationale for investigating the role of neurotransmitters in ketogenic diet mechanisms. Ketogenic diets may exert their antiseizure effects by increasing neuronal inhibition. This effect may occur via a variety of mechanisms, including alteration of synaptic γ-aminobutyric acid (GABA) levels, GABA metabolism, GABA receptors, or some other alteration in GABAergic neuron function. One study of children with epilepsy consuming a ketogenic diet showed an increase in cerebrospinal fluid levels of the inhibitory neurotransmitter GABA [38]. Interestingly, patients who responded best to the ketogenic diet had higher cerebrospinal fluid (CSF) levels of GABA at baseline, which raises the possibility that CSF GABA levels may serve as a biomarker that would predict successful treatment with the diet. Ketone bodies increase the GABA content in rat brain synaptosomes [39]. However, differences in GABA levels in rodent brains have not been demonstrated during ketogenic diet treatment [40-44]. Measurement of regional GABA concentrations might mask more subtle increases in GABA synthesis during ketogenic diet consumption; such increases in GABA synthesis were demonstrated using isotope-labeled amino acids [42]. However, ketone bodies do not appear to change GABA receptor currents in cultured neurons and magnetic resonance spectroscopy studies of brain GABA in patients have been inconclusive [45-47]. Direct application of ketone bodies at physiologically relevant concentrations did not alter GABA-evoked inhibitory currents in cultured hippocampal neurons [45]. GABAA receptors expressed in Xenopus oocytes show increased activity when incubated with 10 mM β– hydroxybutyrate or 50 mM acetone but not at lower ketone body concentrations seen typically in children on a ketogenic diet [32, 33]. In one study, rats consuming a ketogenic diet showed elevated levels of the GABA-synthesizing enzyme glutamic acid decarboxylase (both mRNA and protein) but the translational relevance of this finding is unclear because the increase was noted only in regions not thought to be involved in the generation of seizures [48]. Given their importance in neonatal brain metabolism [49], ketone bodies may play a role in neonatal GABA-mediated neurotransmission. However, the specifics of such an interaction and their applicability to ketogenic diets in older children are debated (discussed in [50]). The role of GABA neurotransmission in the ketogenic diet remains an area of active investigation.
Another way in which ketone bodies may directly dampen neuronal excitability is by activation of inwardly rectifying ATP-sensitive potassium (KATP) channels. These channels contribute to neuronal hyperpolarization when intracellular ATP levels fall. In GABAergic neurons of the substantia nigra pars reticulata, considered to be a “gate” that prevents seizure generalization, application of ketone bodies slowed neuronal firing rate, an effect reversed by GABAB receptor antagonists [32, 33]. Therefore, it is possible that ketone bodies exert inhibitory effects by altering GABAB receptor function. Other factors that influence metabolism may affect KATP channels, as well. Dentate granule neuron KATP channel activity is increased when the protein BAD (Bcl-2-associated death promoting protein), which plays roles in both apoptosis and metabolism, is modified or deleted, resulting in decreased glucose metabolism [51]. Metabolism of β-hydroxybutyrate is increased in neurons and astrocytes in these genetically modified mice. The same modifications of BAD increase resistance to seizures induced by pentylenetetrazol and kainic acid. KATP channels also are an attractive target to study because they serve as a link between metabolism (via ATP levels) and neuronal firing. Data on brain ATP levels have been somewhat conflicting, with some studies showing no change after ketogenic diet treatment and other studies demonstrating an increase in levels [44, 52-55]. Importantly, KATP channels usually are thought to be activated by low, rather than high, levels of ATP [56]. However, in hippocampal CA3 neurons cultured in low glucose media, KATP channels may be activated via adenosine A1 receptors, which in turn are activated by adenosine released from the dephosphorylation of ATP released by pannexin-1 hemichannels [57]. The translational relevance of pannexin-1 hemichannel-mediated KATP activity is somewhat unclear because patients consuming a ketogenic diet have normal fasting morning blood glucose levels, while blood glucose levels in rodent models have shown varied results levels [34, 58, 59]. Nonetheless, adenosine receptors appear to be necessary for the ability of the ketogenic diet to inhibit spontaneous electrographic seizures in adenosine A1 receptor knockout mice [60]. Similarly, mice lacking dopamine-β-hydroxylase (i.e., an enzyme in the inhibitory norepinephrine synthesis pathway) are not protected from seizures induced by the volatile convulsant flurothyl [61]. The necessity of norepinephrine synthesis also has been seen with other anticonvulsants such as phenobarbital[62]. Although multiple inhibitory neurotransmitters appear to be necessary for seizure protection during a ketogenic diet, the specific contribution made by each neurotransmitter system (and possibly, an interaction between them) remains unclear.
2.1.3.3. Excitatory neurotransmission
Patients in the study of cerebrospinal fluid neurotransmitters did not have significantly lower cerebrospinal fluid levels of aspartate and glutamate (compared to the pre-diet baseline), although these levels were significant in children under the age of 5.5 years [38]. Decreased levels of excitatory amino acids are unlikely to be the sole mechanism of the ketogenic diet’s anticonvulsant action, since only some rodent studies have shown decreases in aspartate or glutamate [41, 42, 55, 63]. Ketone bodies did not exert an effect on NMDA or AMPA receptor synaptic currents in hippocampal slices or cultured cortical neurons [45, 47]. The ketogenic diet does not alter levels of the glutamate transporter EAAC1 in rat neocortex or hippocampus, making it less likely that increased glutamate clearance decreases seizures in rodents consuming the diet [64]. A novel potential mechanism for the effect of ketone bodies on excitatory neurotransmission was shown by the ability of acetoacetate to compete with chloride ions for allosteric regulation of glutamate uptake into synaptic vesicles (mediated by the glutamate transporter, VGLUT) [65]; therefore, ketone bodies might alter the metabolic state of the neuron and thus its ability to release glutamate. Acetoacetate also inhibited glutamate release in cultured neurons and hippocampal slices, as well as in rats exposed to the potassium channel blocker 4-aminopyridine. However, the concentration of acetoacetate found to be most effective in the animal experiments was 10 mM, which is nearly two orders of magnitude higher than cerebrospinal fluid levels of acetoacetate in children consuming a ketogenic diet (in vitro data showed that submillimolar concentrations of acetoacetate altered neuronal firing, however) [66]. In summary, ketogenic diets do not appear to directly induce a significant decrease in levels of excitatory neurotransmitters but ketone bodies at high concentrations may decrease glutamate levels in the synaptic cleft. The effects of the ketogenic diet on excitatory neurotransmitter receptor subunit expression, trafficking, and physiology remain underexplored.
2.1.3.4. Mitochondria and metabolism
Changes in metabolism pathways that reside in the mitochondrial matrix (i.e., TCA or Krebs cycle) may serve as a link between metabolism and neurotransmitters [67]. Metabolism of fats to ketone bodies may increase activity in the initial reactions of the Krebs cycle, which may in turn increase levels of α–ketoglutarate [54]. In turn, increased α–ketoglutarate should increase glutamate synthesis (catalyzed by aspartate transaminase), which has been demonstrated experimentally [42]. Interestingly, levels of total brain glutamate are not elevated, implying that glutamate metabolism is increased [42, 55]. Therefore, mitochondria may provide a link between ketogenic diet effects on both excitatory and inhibitory neurotransmission [68].
In specific disease models associated with mitochondrial degeneration or depletion, ketogenic diets increase mitochondrial biomass or function [52, 69]. Ketogenic diets also increase the transcription of subunits involved in oxidative phosphorylation (OXPHOS), although it is unclear whether this directly leads to an increase in ATP production [55]. In considering mitochondrial electron flow through OXPHOS proteins, it is interesting to note that ketogenic diets also increase the expression of brain mitochondrial uncoupling proteins [70], implying that an electron “leak” may be an alternative explanation in studies showing no difference in ATP levels (discussed previously).
2.2 Intermittent fasting and calorie restriction
As noted previously, the ketogenic diet was designed to mimic the beneficial effects of fasting on seizure control. This has led to the commonly-held belief that calorie restriction and ketogenic diets have identical anticonvulsant mechanisms [71, 72]. Daily calorie restriction protects against seizures in one mixed genetic/environmental model of epilepsy, EL mice [73]. Another form of calorie restriction, intermittent fasting (i.e., alternating ad lib feeding days with fasting days), protects against cell death after exposure to systemic or intrahippocampally-delivered kainic acid [74-76]. The efficacy of the ketogenic diet requires some degree of calorie restriction in some rodent models [77, 78] and there is no difference in the expression of glutamic acid decarboxylase (-65 & -67 isoforms) between rats fed a ketogenic diet and those that had calories restricted [48]. However, differences were noted between a calorie-restricted ketogenic diet and calorie-restricted normal rodent chow in the maximal dentate activation test (an electrophysiological test that assesses the excitability or burst-firing propensity of dentate granule neurons) [79, 80]. Importantly, both of those diets involved some form of calorie restriction. Thus, it was unclear whether the antiseizure effect of the ketogenic diet was simply due to restriction of calorie intake. To address this question, the ketogenic diet was compared to intermittent fasting (for 11-14 days) in a battery of acute seizure tests based on those used in the ASP. If the two treatments had identical mechanisms of action, it would be predicted that the treatments would protective in the same tests. Surprisingly, the two interventions had opposite effects in the acute seizure tests. On the 6 Hz electroshock test, mice that were fasted intermittently had lower seizure thresholds than those treated with the ketogenic diet. Upon exposure to kainic acid to induce status epilepticus, mice fasted intermittently had lower overall seizure scores than mice fed normal chow ad lib or a ketogenic diet, despite being matched for body weight [81]. These data challenge the assumption that the ketogenic diet and intermittent fasting share identical anticonvulsant mechanisms and suggest that while both exert anticonvulsant actions, the two dietary protocols need to be considered as distinct entities. We are not aware of any modern literature examining the clinical efficacy of intermittent fasting.
2.3 Modified Atkins Diet and Low Glycemic Index Treatment
The modified Atkins diet (MAD) and low glycemic index treatment (LGIT) are alternative dietary regimens that have proven useful in the management of refractory seizures in patients in whom the classic ketogenic diet is not feasible. The MAD differs from the classic ketogenic diet in that it is less restrictive with regard to protein and calorie limitations, with its efficacy thought to be related to the high-fat intake and more modest carbohydrate restriction. The LGIT employs foods that raise blood glucose less abruptly and to a lesser degree than a typical diet. Further details about the clinical use and efficacy of these regimens are available elsewhere [82, 83]. As of this writing, the MAD and LGIT have not been studied in animal models, though additional mechanistic insights could be obtained by examining the effect of increasing protein content (i.e., similar to MAD) or altering the carbohydrate content in a more conventional diet to mimic the LGIT.
2.4 Glycolytic Inhibition (2-deoxy-D-glucose and fructose-1,6-bisphosphate) and Anaplerosis
Since ingestion of a small amount of carbohydrate by a patient on the ketogenic diet results in rapid reversal of seizure control [84], the hypothesis arose that carbohydrate restriction could protect against seizures [85, 86]. The ketogenic diet bypasses glycolysis by utilizing ketones as the main energy source. Therefore, it was hypothesized that glycolytic inhibition might protect against seizures.
2-Deoxy-D-glucose (2DG) is a glucose analog that differs from glucose only by substitution of a single oxygen atom in the 2′-position. 2DG cannot be metabolized and inhibits glycolysis by blocking the enzyme phosphoglucose isomerase, thereby preventing the conversion of glucose-6-phosphate to fructose-6-phosphate. 2DG has potent anticonvulsant and antiepileptic actions in several animal models, including kindling, audiogenic seizures in Fring’s mice, and the 6 Hz electroshock test [87, 88]. In vitro, 2DG reduces hyperexcitability in hippocampal CA3 neurons in slices exposed to elevated extracellular K+, bicuculline, 4-aminopyridine or metabotropic Group 1 agonists [89].
Both acute and chronic anticonvulsant effects of 2DG have been documented. Acute anticonvulsant effects of 2DG in vitro and in vivo against both ictal and interictal activity suggest that 2DG may act directly at the synaptic or membrane levels through mechanisms independent of altered gene expression [88]. Acute effects of 2DG could be related to rapid-onset metabolic or electrophysiological consequences of glycolytic inhibition leading to reduction of network synchronization. For example, 2DG could influence neuronal excitability by alteration of systemic lipid metabolism, mitochondrial function, or the phosphorylation state of GABAA receptor subunits [90]. In whole-cell recordings of hippocampal CA3 neurons, acute application of 2DG suppressed spontaneous excitatory postsynaptic currents (EPSCs) after transient epileptic activity induced by elevated extracellular potassium or metabotropic Group 1 agonists, but not in normal slices, suggesting activity-dependent effects of glycolytic inhibition [91]. These and other potential acute mechanisms are currently under study.
Chronic antiepileptic effects of 2DG are related to the molecular regulation of genes for brain-derived neurotrophic factor (BDNF) and its receptor, tyrosine kinase B (trkB). Repression of both BDNF and trkB expression are required for kindling progression [92]. 2DG suppresses seizure-induced increases in BDNF and trkB, mediated by the transcriptional repressor neuron restrictive silencing factor (NRSF) and its nicotinamide adenine dinucleotide (NADH)-sensitive co-repressor carboxy-terminal binding protein (CtBP). NRSF and CtBP act at the promoter regions of BDNF and trkB genes [87]. Glycolysis and glucose production are increased during seizures, leading to elevated levels of NADH. NADH elevation causes dissociation of CtBP from NRSF, decreasing transcriptional repression and resulting in increased expression of BDNF and trkB. In the presence of 2DG, which reduces NADH levels as a consequence of glycolytic inhibition, the NRSF-CtBP complex maintains repression of BDNF and trkB, and kindling progression is slowed [87, 93]. It is likely that the ketogenic diet and 2DG work by different mechanisms, since the ketogenic diet retards kindling development in mice with NRSF conditionally knocked out [94].
These chronic anticonvulsant and antiepileptic effects of 2DG make it a viable candidate for clinical trials with the potential for modification of both seizure susceptibility and disease progression, assuming 2DG can be shown to be nontoxic. A favorable safety profile has been reported with regard to cognitive performance [86, 92], though a recent pathological evaluation of chronic oral 2DG ingestion in two rat strains reported cardiotoxic effects with vacuolization of cardiac myocytes and reduced lifespans [95]. While it is difficult to compare oral and parenteral 2DG doses, as well as interspecies differences, further studies are needed to assess reversibility of histological effects observed with 2DG treatment.
Another glycolytic intermediate, fructose-1,6-bisphosphate (FBP), exerts acute anticonvulsant activity in several seizure models in adult rats including kainate, pilocarpine, pentylenetetrazol, and kindling [96, 97]. In fact, FBP exhibited greater anticonvulsant efficacy than 2DG, KD, and valproate. FBP increases glucose flux from glycolysis into the pentose phosphate pathway (PPP). NADPH generated in the PPP reduces glutathione, which possesses anticonvulsant activity. Thus, FBP may exert an endogenous anticonvulsant (and perhaps anti-oxidant) action [98], though the precise mechanism has not yet been determined.
A final dietary approach to epilepsy relates to the observation that seizures cause a deficiency in TCA intermediates (particularly α-ketoglutarate and oxaloacetate), leading to increased excitability and possibly to further seizures. It has been conjectured that “refilling” these deficient compounds, a process called “anaplerosis”, might oppose seizure generation. An example of an anaplerotic compound is triheptanoin, which has recently been investigated in both acute and chronic seizure models [99]. Mice fed triheptanoin exhibited resistance to development of seizures by corneal kindling, and triheptanoin feeding increased PTZ seizure threshold in chronically epileptic mice that had undergone status epilepticus 3 weeks before PTZ testing [99]. Therefore, like 2DG, anaplerotic compounds alter both acute and chronic seizure susceptibility. Anaplerosis represents a novel approach that expands the potential metabolic modifications that could be anticonvulsant or antiepileptic. Together, results from studies of the KD, 2DG, FBP, and anaplerosis suggest that modification of metabolic pathways such as glycolysis could represent a novel mechanism for epilepsy management.
2.5 mTOR Inhibition
2.5.1. Clinical efficacy
Tuberous sclerosis complex is a genetic disorder of cell growth and differentiation that involves dysplasia or benign tumor formation in several organs including kidney, heart, and brain. Specific neuropathological findings include cortical tubers, which are hamartomatous collections of abnormal neurons and glia and subependymal giant cell nodules that have a propensity to become astrocytomas. Neurological consequences of tuberous sclerosis complex include seizures (frequently medically intractable), autism and related behavior problems, and intellectual disability [100-103]. Tuberous sclerosis complex is caused by mutations in the TSC1 or TSC2 gene, which encode key proteins upstream of the serine-threonine protein kinase mammalian target of rapamycin (mTOR) [102]. This pathway is one of the master integrators of metabolic signaling, with upstream regulation by the insulin receptor and glucose levels (via adenosine monophosphate kinase) and downstream effects on translation and lipid metabolism [104]. Patients with mutations in TSC1 or TSC2 have increased mTOR activity, leading to decreased constraint on cell growth and tumor formation in multiple tissues. Everolimus, an inhibitor of mTOR activity, decreases seizure frequency in patients with tuberous sclerosis and limits progression of the size of subependymal giant cell astrocytomas [105]. The ketogenic diet is another effective treatment for intractable epilepsy in patients with tuberous sclerosis [103].
2.5.2. Putative mechanisms
Rapamycin, the canonical mTOR inhibitor, inhibits seizures in a mouse model of tuberous sclerosis [106]. Rapamycin also inhibits recurrent seizures after status epilepticus induced by kainic acid or pilocarpine in rats that do not harbor mutations in the mTOR pathway [107, 108]. Rapamycin treatment also suppressed behavioral spasms in the doxorubicin/lipopolysaccharide/p-chlorophenylalanine model of infantile spasms [109] and decreased susceptibility to kainic acid-induced seizures in a juvenile rat hypoxia model [110]. These data raise the possibility that mTOR plays a wider role in seizure perpetuation, beyond tuberous sclerosis complex. However, mTOR’s effect may not be universal because rapamycin (or other rapamycin analogs, known as “rapalogs”) does not prevent recurrent seizures after pilocarpine-induced status epilepticus in mice, nor does it prevent acute seizures in the rat hypoxia model (without kainic acid exposure) [110, 111].
The specific mechanism by which mTOR inhibitors prevent seizures is not entirely clear because in vitro studies of rapamycin have not shown a consistent decrease in baseline or provoked neuronal firing [112, 113]. However, mTOR activity is necessary for synaptic plasticity, including long term potentiation and long term depression [114-117]. This requirement may be a reflection of the role played by mTOR activity in the development and maintenance of dendrite morphology [112, 118-121].
Interestingly, ketogenic diets decrease mTOR activity in rats [122]. Ketogenic diets differ from rapamycin in their acute antiseizure test profiles but it is unknown whether they have similar antiseizure mechanisms in models of chronic epilepsy [123]. Nonetheless, the studies outlined here suggest that the role of mTOR inhibition in seizure prevention deserves further study.
3. Concluding thoughts
Despite the availability of at least ten new anticonvulsant medications in the past two decades, the percentage of patients with medically refractory epilepsy has not changed. Even in the present day, up to one-third of epilepsy sufferers continue to have seizures as well as disabling co-morbidities such as cognitive dysfunction and neuropsychiatric disorders. Therefore, the need for alternative and effective epilepsy treatments is dire. The metabolic approach to seizure management represents is both novel and compelling, with several such therapies providing seizure control as well as improved quality of life and fewer side effects (Fig. 1). These considerations make understanding of the mechanisms by which dietary and metabolic approaches work a major research priority [124]. Studies such as those outlined above are just beginning to touch the surface of the panoply of mechanisms that could improve epilepsy through alteration of energy utilization.
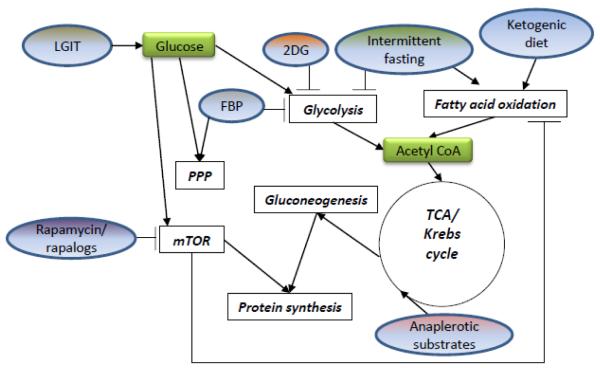
Figure 1. Summary of metabolism-based therapies and their sites of action.
Different types of metabolism-based antiseizure treatments (dietary and pharmacological) affect metabolic pathways at different sites. Some processes are affected by more than one type of treatment (i.e., glycolysis, fatty acid oxidation). “Rapalogs” are analogs of rapamycin, modified to decrease toxicity and/or increase specific activity. Specific interventions are shown in ovals; metabolic processes are shown in clear rectangles; and specific metabolites are shown in green rectangles. FBP, fructose-1,6-bisphosphate; LGIT, low glycemic index treatment; mTOR, mammalian target of rapamycin; PPP, pentose phosphate pathway; TCA, tricarboxylic acid cycle; 2DG, 2-deoxy-D-glucose.
Highlights for review.
New data suggest direct links between energy metabolism and neuronal excitability.
Therapy targeting cellular metabolism and hyperexcitability is relatively untapped.
Approaches discussed here offer a variety of potential therapeutic targets.
Acknowledgements
This work was supported by K08NS070931, Johns Hopkins University School of Medicine Clinician-Scientist Award, Passano Foundation, Pakula Family (ALH), The Charlie Foundation, and Wisconsin Alumni Research Foundation (CES).
Footnotes
Disclaimer The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Institutes of Health.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Adam L. Hartman, Department of Neurology, Johns Hopkins Medicine Department of Molecular Microbiology and Immunology, Johns Hopkins Bloomberg School of Public Health Baltimore, MD.
Carl E. Stafstrom, Departments of Neurology and Pediatrics University of Wisconsin Madison, WI.
References
- [1].Russ SA, Larson K, Halfon N. A national profile of childhood epilepsy and seizure disorder. Pediatrics. 2012;129:256–64. doi: 10.1542/peds.2010-1371. [DOI] [PubMed] [Google Scholar]
- [2].Hirtz D, Thurman DJ, Gwinn-Hardy K, Mohamed M, Chaudhuri AR, Zalutsky R. How common are the “common” neurologic disorders? Neurology. 2007;68:326–37. doi: 10.1212/01.wnl.0000252807.38124.a3. [DOI] [PubMed] [Google Scholar]
- [3].Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med. 2000;342:314–9. doi: 10.1056/NEJM200002033420503. [DOI] [PubMed] [Google Scholar]
- [4].Berg AT, Levy SR, Testa FM, D’Souza R. Remission of epilepsy after two drug failures in children: a prospective study. Ann Neurol. 2009;65:510–9. doi: 10.1002/ana.21642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kelley MS, Jacobs MP, Lowenstein DH. The NINDS epilepsy research benchmarks. Epilepsia. 2009;50:579–82. doi: 10.1111/j.1528-1167.2008.01813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bailey EE, Pfeifer HH, Thiele EA. The use of diet in the treatment of epilepsy. Epilepsy Behav. 2005;6:4–8. doi: 10.1016/j.yebeh.2004.10.006. [DOI] [PubMed] [Google Scholar]
- [7].Hartman AL, Gasior M, Vining EP, Rogawski MA. The neuropharmacology of the ketogenic diet. Pediatr Neurol. 2007;36:281–92. doi: 10.1016/j.pediatrneurol.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Brockmann K. The expanding phenotype of GLUT1-deficiency syndrome. Brain Dev. 2009 doi: 10.1016/j.braindev.2009.02.008. [DOI] [PubMed] [Google Scholar]
- [9].Klepper J. Glucose transporter deficiency syndrome (GLUT1DS) and the ketogenic diet. Epilepsia. 2008;49(Suppl 8):46–9. doi: 10.1111/j.1528-1167.2008.01833.x. [DOI] [PubMed] [Google Scholar]
- [10].Wexler ID, Hemalatha SG, McConnell J, Buist NR, Dahl HH, Berry SA, Cederbaum SD, Patel MS, Kerr DS. Outcome of pyruvate dehydrogenase deficiency treated with ketogenic diets. Studies in patients with identical mutations. Neurology. 1997;49:1655–61. doi: 10.1212/wnl.49.6.1655. [DOI] [PubMed] [Google Scholar]
- [11].Hartman AL, Vining EP. Clinical aspects of the ketogenic diet. Epilepsia. 2007;48:31–42. doi: 10.1111/j.1528-1167.2007.00914.x. [DOI] [PubMed] [Google Scholar]
- [12].Lefevre F, Aronson N. Ketogenic diet for the treatment of refractory epilepsy in children: A systematic review of efficacy. Pediatrics. 2000;105:E46. doi: 10.1542/peds.105.4.e46. [DOI] [PubMed] [Google Scholar]
- [13].Henderson CB, Filloux FM, Alder SC, Lyon JL, Caplin DA. Efficacy of the ketogenic diet as a treatment option for epilepsy: meta-analysis. J Child Neurol. 2006;21:193–8. doi: 10.2310/7010.2006.00044. [DOI] [PubMed] [Google Scholar]
- [14].Neal EG, Chaffe H, Schwartz RH, Lawson MS, Edwards N, Fitzsimmons G, Whitney A, Cross JH. The ketogenic diet for the treatment of childhood epilepsy: a randomised controlled trial. Lancet Neurol. 2008;7:500–6. doi: 10.1016/S1474-4422(08)70092-9. [DOI] [PubMed] [Google Scholar]
- [15].Neal EG, Chaffe H, Schwartz RH, Lawson MS, Edwards N, Fitzsimmons G, Whitney A, Cross JH. A randomized trial of classical and medium-chain triglyceride ketogenic diets in the treatment of childhood epilepsy. Epilepsia. 2009;50:1109–17. doi: 10.1111/j.1528-1167.2008.01870.x. [DOI] [PubMed] [Google Scholar]
- [16].Schwartz RM, Boyes S, Aynsley-Green A. Metabolic effects of three ketogenic diets in the treatment of severe epilepsy. Dev Med Child Neurol. 1989;31:152–60. doi: 10.1111/j.1469-8749.1989.tb03973.x. [DOI] [PubMed] [Google Scholar]
- [17].Huttenlocher PR, Wilbourn AJ, Signore JM. Medium-chain triglycerides as a therapy for intractable childhood epilepsy. Neurology. 1971;21:1097–103. doi: 10.1212/wnl.21.11.1097. [DOI] [PubMed] [Google Scholar]
- [18].Freeman JM, Vining EP, Kossoff EH, Pyzik PL, Ye X, Goodman SN. A blinded, crossover study of the efficacy of the ketogenic diet. Epilepsia. 2009;50:322–5. doi: 10.1111/j.1528-1167.2008.01740.x. [DOI] [PubMed] [Google Scholar]
- [19].Hartman AL. Does the effectiveness of the ketogenic diet in different epilepsies yield insights into its mechanisms? Epilepsia. 2008;49(Suppl 8):53–6. doi: 10.1111/j.1528-1167.2008.01835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Nabbout R, Copioli C, Chipaux M, Chemaly N, Desguerre I, Dulac O, Chiron C. Ketogenic diet also benefits Dravet syndrome patients receiving stiripentol: a prospective pilot study. Epilepsia. 2011;52:e54–7. doi: 10.1111/j.1528-1167.2011.03107.x. [DOI] [PubMed] [Google Scholar]
- [21].Korff C, Laux L, Kelley K, Goldstein J, Koh S, Nordli D., Jr. Dravet syndrome (severe myoclonic epilepsy in infancy): a retrospective study of 16 patients. J Child Neurol. 2007;22:185–94. doi: 10.1177/0883073807300294. [DOI] [PubMed] [Google Scholar]
- [22].Fejerman N, Caraballo R, Cersosimo R. Ketogenic diet in patients with Dravet syndrome and myoclonic epilepsies in infancy and early childhood. Adv Neurol. 2005;95:299–305. [PubMed] [Google Scholar]
- [23].Kang HC, Kim YJ, Kim DW, Kim HD. Efficacy and safety of the ketogenic diet for intractable childhood epilepsy: Korean multicentric experience. Epilepsia. 2005;46:272–9. doi: 10.1111/j.0013-9580.2005.48504.x. [DOI] [PubMed] [Google Scholar]
- [24].Claes L, Del-Favero J, Ceulemans B, Lagae L, Van Broeckhoven C, De Jonghe P. De novo mutations in the sodium-channel gene SCN1A cause severe myoclonic epilepsy of infancy. Am J Hum Genet. 2001;68:1327–32. doi: 10.1086/320609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Yu FH, Mantegazza M, Westenbroek RE, Robbins CA, Kalume F, Burton KA, Spain WJ, McKnight GS, Scheuer T, Catterall WA. Reduced sodium current in GABAergic interneurons in a mouse model of severe myoclonic epilepsy in infancy. Nat Neurosci. 2006;9:1142–9. doi: 10.1038/nn1754. [DOI] [PubMed] [Google Scholar]
- [26].Catarino CB, Liu JY, Liagkouras I, Gibbons VS, Labrum RW, Ellis R, Woodward C, Davis MB, Smith SJ, Cross JH, Appleton RE, Yendle SC, McMahon JM, Bellows ST, Jacques TS, Zuberi SM, Koepp MJ, Martinian L, Scheffer IE, Thom M, Sisodiya SM. Dravet syndrome as epileptic encephalopathy: evidence from long-term course and neuropathology. Brain. 2011;134:2982–3010. doi: 10.1093/brain/awr129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Dutton SB, Sawyer NT, Kalume F, Jumbo-Lucioni P, Borges K, Catterall WA, Escayg A. Protective effect of the ketogenic diet in Scn1a mutant mice. Epilepsia. 2011;52:2050–6. doi: 10.1111/j.1528-1167.2011.03211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Rho JM, Stafstrom CE. The ketogenic diet: What has science taught us? Epilepsy Res. 2012;100:210–7. doi: 10.1016/j.eplepsyres.2011.05.021. [DOI] [PubMed] [Google Scholar]
- [29].Smith M, Wilcox KS, White HS. Discovery of antiepileptic drugs. Neurotherapeutics. 2007;4:12–7. doi: 10.1016/j.nurt.2006.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Dalby NO, Nielsen EB. Comparison of the preclinical anticonvulsant profiles of tiagabine, lamotrigine, gabapentin and vigabatrin. Epilepsy Res. 1997;28:63–72. doi: 10.1016/s0920-1211(97)00031-4. [DOI] [PubMed] [Google Scholar]
- [31].Klitgaard H, Matagne A, Gobert J, Wulfert E. Evidence for a unique profile of levetiracetam in rodent models of seizures and epilepsy. Eur J Pharmacol. 1998;353:191–206. doi: 10.1016/s0014-2999(98)00410-5. [DOI] [PubMed] [Google Scholar]
- [32].Barton ME, Klein BD, Wolf HH, White HS. Pharmacological characterization of the 6 Hz psychomotor seizure model of partial epilepsy. Epilepsy Res. 2001;47:217–27. doi: 10.1016/s0920-1211(01)00302-3. [DOI] [PubMed] [Google Scholar]
- [33].Uhlemann ER, Neims AH. Anticonvulsant properties of the ketogenic diet in mice. J Pharmacol Exp Ther. 1972;180:231–8. [PubMed] [Google Scholar]
- [34].Samala R, Willis S, Borges K. Anticonvulsant profile of a balanced ketogenic diet in acute mouse seizure models. Epilepsy Res. 2008;81:119–27. doi: 10.1016/j.eplepsyres.2008.05.001. [DOI] [PubMed] [Google Scholar]
- [35].Bough KJ, Eagles DA. A ketogenic diet increases the resistance to pentylenetetrazole-induced seizures in the rat. Epilepsia. 1999;40:138–43. doi: 10.1111/j.1528-1157.1999.tb02066.x. [DOI] [PubMed] [Google Scholar]
- [36].Bough KJ, Gudi K, Han FT, Rathod AH, Eagles DA. An anticonvulsant profile of the ketogenic diet in the rat. Epilepsy Res. 2002;50:313–25. doi: 10.1016/s0920-1211(02)00086-4. [DOI] [PubMed] [Google Scholar]
- [37].Hartman AL, Lyle M, Rogawski MA, Gasior M. Efficacy of the ketogenic diet in the 6-Hz seizure test. Epilepsia. 2008;49:334–9. doi: 10.1111/j.1528-1167.2007.01430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Dahlin M, Elfving A, Ungerstedt U, Amark P. The ketogenic diet influences the levels of excitatory and inhibitory amino acids in the CSF in children with refractory epilepsy. Epilepsy Res. 2005;64:115–25. doi: 10.1016/j.eplepsyres.2005.03.008. [DOI] [PubMed] [Google Scholar]
- [39].Erecinska M, Nelson D, Daikhin Y, Yudkoff M. Regulation of GABA level in rat brain synaptosomes: fluxes through enzymes of the GABA shunt and effects of glutamate, calcium, and ketone bodies. J Neurochem. 1996;67:2325–34. doi: 10.1046/j.1471-4159.1996.67062325.x. [DOI] [PubMed] [Google Scholar]
- [40].Appleton DB, DeVivo DC. An animal model for the ketogenic diet. Epilepsia. 1974;15:211–27. doi: 10.1111/j.1528-1157.1974.tb04943.x. [DOI] [PubMed] [Google Scholar]
- [41].Yudkoff M, Daikhin Y, Nissim I, Lazarow A. Brain amino acid metabolism and ketosis. J Neurosci Res. 2001;66:272–81. doi: 10.1002/jnr.1221. [DOI] [PubMed] [Google Scholar]
- [42].Yudkoff M, Daikhin Y, Nissim I, Horyn O, Lazarow A, Luhovyy B, Wehrli S. Response of brain amino acid metabolism to ketosis. Neurochem Int. 2005;47:119–28. doi: 10.1016/j.neuint.2005.04.014. [DOI] [PubMed] [Google Scholar]
- [43].Melo TM, Nehlig A, Sonnewald U. Neuronal-glial interactions in rats fed a ketogenic diet. Neurochem Int. 2006;48:498–507. doi: 10.1016/j.neuint.2005.12.037. [DOI] [PubMed] [Google Scholar]
- [44].Al-Mudallal AS, LaManna JC, Lust WD, Harik SI. Diet-induced ketosis does not cause cerebral acidosis. Epilepsia. 1996;37:258–61. doi: 10.1111/j.1528-1157.1996.tb00022.x. [DOI] [PubMed] [Google Scholar]
- [45].Thio LL, Wong M, Yamada KA. Ketone bodies do not directly alter excitatory or inhibitory hippocampal synaptic transmission. Neurology. 2000;54:325–31. doi: 10.1212/wnl.54.2.325. [DOI] [PubMed] [Google Scholar]
- [46].Wang ZJ, Bergqvist C, Hunter JV, Jin D, Wang DJ, Wehrli S, Zimmerman RA. In vivo measurement of brain metabolites using two-dimensional double-quantum MR spectroscopy--exploration of GABA levels in a ketogenic diet. Magn Reson Med. 2003;49:615–9. doi: 10.1002/mrm.10429. [DOI] [PubMed] [Google Scholar]
- [47].Donevan SD, White HS, Anderson GD, Rho JM. Voltage-dependent block of N-methyl-D-aspartate receptors by the novel anticonvulsant dibenzylamine, a bioactive constituent of L-(+)-beta-hydroxybutyrate. Epilepsia. 2003;44:1274–9. doi: 10.1046/j.1528-1157.2003.07203.x. [DOI] [PubMed] [Google Scholar]
- [48].Cheng CM, Hicks K, Wang J, Eagles DA, Bondy CA. Caloric restriction augments brain glutamic acid decarboxylase-65 and -67 expression. J Neurosci Res. 2004;77:270–6. doi: 10.1002/jnr.20144. [DOI] [PubMed] [Google Scholar]
- [49].Nehlig A. Age-dependent pathways of brain energy metabolism: the suckling rat, a natural model of the ketogenic diet. Epilepsy Res. 1999;37:211–21. doi: 10.1016/s0920-1211(99)00073-x. [DOI] [PubMed] [Google Scholar]
- [50].McNally MA, Hartman AL. Ketone bodies in epilepsy. J Neurochem. 2012;121:28–35. doi: 10.1111/j.1471-4159.2012.07670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Gimenez-Cassina A, Martinez-Francois JR, Fisher JK, Szlyk B, Polak K, Wiwczar J, Tanner GR, Lutas A, Yellen G, Danial NN. BAD-Dependent Regulation of Fuel Metabolism and K(ATP) Channel Activity Confers Resistance to Epileptic Seizures. Neuron. 2012;74:719–30. doi: 10.1016/j.neuron.2012.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Nylen K, Velazquez JL, Sayed V, Gibson KM, Burnham WM, Snead OC., 3rd The effects of a ketogenic diet on ATP concentrations and the number of hippocampal mitochondria in Aldh5a1(−/−) mice. Biochim Biophys Acta. 2009;1790:208–12. doi: 10.1016/j.bbagen.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Nakazawa M, Kodama S, Matsuo T. Effects of ketogenic diet on electroconvulsive threshold and brain contents of adenosine nucleotides. Brain Dev. 1983;5:375–80. doi: 10.1016/s0387-7604(83)80042-4. [DOI] [PubMed] [Google Scholar]
- [54].DeVivo DC, Leckie MP, Ferrendelli JS, McDougal DB., Jr. Chronic ketosis and cerebral metabolism. Ann Neurol. 1978;3:331–37. doi: 10.1002/ana.410030410. [DOI] [PubMed] [Google Scholar]
- [55].Bough KJ, Wetherington J, Hassel B, Pare JF, Gawryluk JW, Greene JG, Shaw R, Smith Y, Geiger JD, Dingledine RJ. Mitochondrial biogenesis in the anticonvulsant mechanism of the ketogenic diet. Ann Neurol. 2006;60:223–35. doi: 10.1002/ana.20899. [DOI] [PubMed] [Google Scholar]
- [56].Benarroch EE. Potassium channels: brief overview and implications in epilepsy. Neurology. 2009;72:664–9. doi: 10.1212/01.wnl.0000343739.72081.4e. [DOI] [PubMed] [Google Scholar]
- [57].Kawamura M, Jr., Ruskin DN, Masino SA. Metabolic autocrine regulation of neurons involves cooperation among pannexin hemichannels, adenosine receptors, and KATP channels. J Neurosci. 2010;30:3886–95. doi: 10.1523/JNEUROSCI.0055-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Bergqvist AG, Schall JI, Richard EL, Gallagher PR, Stallings VA. Predictive power of first morning glucose and the ketogenic diet. Neuropediatrics. 2007;38:193–6. doi: 10.1055/s-2007-992816. [DOI] [PubMed] [Google Scholar]
- [59].Hartman AL, Zheng X, Bergbower E, Kennedy M, Hardwick JM. Seizure tests distinguish intermittent fasting from the ketogenic diet. Epilepsia. 2010;51:1395–1402. doi: 10.1111/j.1528-1167.2010.02577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Masino SA, Li T, Theofilas P, Sandau US, Ruskin DN, Fredholm BB, Geiger JD, Aronica E, Boison D. A ketogenic diet suppresses seizures in mice through adenosine A(1) receptors. J Clin Invest. 2011;121:2679–83. doi: 10.1172/JCI57813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Szot P, Weinshenker D, Rho JM, Storey TW, Schwartzkroin PA. Norepinephrine is required for the anticonvulsant effect of the ketogenic diet. Brain Res Dev Brain Res. 2001;129:211–4. doi: 10.1016/s0165-3806(01)00213-9. [DOI] [PubMed] [Google Scholar]
- [62].Fischer W, Muller M. Pharmacological modulation of central monoaminergic systems and influence on the anticonvulsant effectiveness of standard antiepileptics in maximal electroshock seizure. Biomed Biochim Acta. 1988;47:631–45. [PubMed] [Google Scholar]
- [63].Melo TM, Sonnewald U, Touret M, Nehlig A. Cortical glutamate metabolism is enhanced in a genetic model of absence epilepsy. J Cereb Blood Flow Metab. 2006;26:1496–506. doi: 10.1038/sj.jcbfm.9600300. [DOI] [PubMed] [Google Scholar]
- [64].Bough KJ, Paquet M, Pare JF, Hassel B, Smith Y, Hall RA, Dingledine R. Evidence against enhanced glutamate transport in the anticonvulsant mechanism of the ketogenic diet. Epilepsy Res. 2007;74:232–6. doi: 10.1016/j.eplepsyres.2007.03.002. [DOI] [PubMed] [Google Scholar]
- [65].Juge N, Gray JA, Omote H, Miyaji T, Inoue T, Hara C, Uneyama H, Edwards RH, Nicoll RA, Moriyama Y. Metabolic control of vesicular glutamate transport and release. Neuron. 2010;68:99–112. doi: 10.1016/j.neuron.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Nordli DR, Jr., De Vivo DC. The ketogenic diet revisited: back to the future. Epilepsia. 1997;38:743–9. doi: 10.1111/j.1528-1157.1997.tb01460.x. [DOI] [PubMed] [Google Scholar]
- [67].Yudkoff M, Daikhin Y, Horyn O, Nissim I. Ketosis and brain handling of glutamate, glutamine, and GABA. Epilepsia. 2008;49(Suppl 8):73–5. doi: 10.1111/j.1528-1167.2008.01841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Milder J, Patel M. Modulation of oxidative stress and mitochondrial function by the ketogenic diet. Epilepsy Res. 2012;100:295–303. doi: 10.1016/j.eplepsyres.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Ahola-Erkkila S, Carroll CJ, Peltola-Mjosund K, Tulkki V, Mattila I, Seppanen-Laakso T, Oresic M, Tyynismaa H, Suomalainen A. Ketogenic diet slows down mitochondrial myopathy progression in mice. Hum Mol Genet. 2010;19:1974–84. doi: 10.1093/hmg/ddq076. [DOI] [PubMed] [Google Scholar]
- [70].Sullivan PG, Rippy NA, Dorenbos K, Concepcion RC, Agarwal AK, Rho JM. The ketogenic diet increases mitochondrial uncoupling protein levels and activity. Ann Neurol. 2004;55:576–80. doi: 10.1002/ana.20062. [DOI] [PubMed] [Google Scholar]
- [71].Maalouf M, Rho JM, Mattson MP. The neuroprotective properties of calorie restriction, the ketogenic diet, and ketone bodies. Brain Res Rev. 2009;59:293–315. doi: 10.1016/j.brainresrev.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Stafstrom CE. Dietary approaches to epilepsy treatment: old and new options on the menu. Epilepsy Curr. 2004;4:215–22. doi: 10.1111/j.1535-7597.2004.46001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Greene AE, Todorova MT, McGowan R, Seyfried TN. Caloric restriction inhibits seizure susceptibility in epileptic EL mice by reducing blood glucose. Epilepsia. 2001;42:1371–8. doi: 10.1046/j.1528-1157.2001.17601.x. [DOI] [PubMed] [Google Scholar]
- [74].Bruce-Keller AJ, Umberger G, McFall R, Mattson MP. Food restriction reduces brain damage and improves behavioral outcome following excitotoxic and metabolic insults. Ann Neurol. 1999;45:8–15. [PubMed] [Google Scholar]
- [75].Contestabile A, Ciani E. Dietary restriction differentially protects from neurodegeneration in animal models of excitotoxicity. Brain Res. 2004;1002:162–6. doi: 10.1016/j.brainres.2004.01.005. [DOI] [PubMed] [Google Scholar]
- [76].Youssef FF, Ramchandani J, Manswell S, McRae A. Adult-onset calorie restriction attenuates kainic acid excitotoxicity in the rat hippocampal slice. Neurosci Lett. 2008;431:118–22. doi: 10.1016/j.neulet.2007.11.064. [DOI] [PubMed] [Google Scholar]
- [77].Bough KJ, Valiyil R, Han FT, Eagles DA. Seizure resistance is dependent upon age and calorie restriction in rats fed a ketogenic diet. Epilepsy Res. 1999;35:21–8. doi: 10.1016/s0920-1211(98)00125-9. [DOI] [PubMed] [Google Scholar]
- [78].Mantis JG, Centeno NA, Todorova MT, McGowan R, Seyfried TN. Management of multifactorial idiopathic epilepsy in EL mice with caloric restriction and the ketogenic diet: role of glucose and ketone bodies. Nutr Metab (Lond) 2004;1:11. doi: 10.1186/1743-7075-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Bough KJ, Schwartzkroin PA, Rho JM. Calorie restriction and ketogenic diet diminish neuronal excitability in rat dentate gyrus in vivo. Epilepsia. 2003;44:752–60. doi: 10.1046/j.1528-1157.2003.55502.x. [DOI] [PubMed] [Google Scholar]
- [80].Nadler JV, Zhan RZ. Properties of dentate granule cells and their relevance to seizures. In: Schwartzkroin PA, editor. Encyclopedia of Basic Epilepsy Research. Elsevier; Amsterdam: 2009. pp. 472–476. [Google Scholar]
- [81].Hartman AL, Zheng X, Bergbower E, Kennedy M, Hardwick JM. Seizure tests distinguish intermittent fasting from the ketogenic diet. Epilepsia. 2010;51:1395–402. doi: 10.1111/j.1528-1167.2010.02577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Muzykewicz DA, Lyczkowski DA, Memon N, Conant KD, Pfeifer HH, Thiele EA. Efficacy, safety, and tolerability of the low glycemic index treatment in pediatric epilepsy. Epilepsia. 2009;50:1118–26. doi: 10.1111/j.1528-1167.2008.01959.x. [DOI] [PubMed] [Google Scholar]
- [83].Lee PR, Kossoff EH. Dietary treatments for epilepsy: management guidelines for the general practitioner. Epilepsy Behav. 2011;21:115–21. doi: 10.1016/j.yebeh.2011.03.008. [DOI] [PubMed] [Google Scholar]
- [84].Huttenlocher PR. Ketonemia and seizures: Metabolic and anticonvulsant effects of two ketogenic diets in childhood epilepsy. Pediatric Research. 1976;10:536–540. doi: 10.1203/00006450-197605000-00006. [DOI] [PubMed] [Google Scholar]
- [85].Greene AE, Todorova MT, Seyfried TN. Perspectives on the metabolic management of epilepsy through dietary reduction of glucose and elevation of ketone bodies. J Neurochem. 2003;86:529–537. doi: 10.1046/j.1471-4159.2003.01862.x. [DOI] [PubMed] [Google Scholar]
- [86].Yamada KA. Calorie restriction and glucose regulation. Epilepsia. 2008;49(Suppl. 8):94–96. doi: 10.1111/j.1528-1167.2008.01847.x. [DOI] [PubMed] [Google Scholar]
- [87].Garriga-Canut M, Schoenike B, Qazi R, Bergendahl K, Daley TJ, Pfender RM, Morrison JF, Ockuly J, Stafstrom C, Sutula T, Roopra A. 2-Deoxy-D-glucose reduces epilepsy progression by NRSF-CtBP-dependent metabolic regulation of chromatin structure. Nature Neurosci. 2006;9:1382–1387. doi: 10.1038/nn1791. [DOI] [PubMed] [Google Scholar]
- [88].Stafstrom CE, Ockuly JC, Murphree L, Valley MT, Roopra A, Sutula TP. Anticonvulsant and antiepileptic actions of 2-deoxy-D-glucose in epilepsy models. Ann Neurol. 2009;65:435–447. doi: 10.1002/ana.21603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Pan YZ, Ockuly JC, Rutecki PA, Sutula TP. Activity-dependent effects of 2DG influencing synaptic transmission, LTP, LTD, and epileptogenesis. Soc Neurosci Abstr. 2008;147 6/W29. [Google Scholar]
- [90].Pumain R, Ahmed MS, Kurcewicz I, Trottier S, Louvel J, Turak B, Devaux B, Laschet J. Lability of GABAA receptor function in human partial epilepsy: possible relationship to hypometabolism. Epilepsia. 2008;49(Suppl 8):87–90. doi: 10.1111/j.1528-1167.2008.01845.x. [DOI] [PubMed] [Google Scholar]
- [91].Pan YZ, Devinney MJ, Rutecki PA, Sutula TP. Effect of glycollytic metabolism on synaptic currents in CA3 pyramidal neurons. Soc Neurosci. Abstr 2009;331.10/16. [Google Scholar]
- [92].He XP, Kotloski R, Nef S, Luikart BW, Parada LF, McNamara JO. Conditional deletion of TrkB but not BDNF prevents epileptogenesis in the kindling model. Neuron. 2004;43:31–42. doi: 10.1016/j.neuron.2004.06.019. [DOI] [PubMed] [Google Scholar]
- [93].Huang YZ, McNamara JO. Inhibiting glycolysis to reduce seizures: how it might work. Nat Neurosci. 2006;9:1351–1352. doi: 10.1038/nn1106-1351. [DOI] [PubMed] [Google Scholar]
- [94].Hu XL, Cheng X, Fei J, Xiong ZQ. Neuron-restrictive silencer factor is not required for the antiepileptic effect of the ketogenic diet. Epilepsia. 2011;52:1609–16. doi: 10.1111/j.1528-1167.2011.03171.x. [DOI] [PubMed] [Google Scholar]
- [95].Minor RK, Smith DL, Jr., Sossong AM, Kaushik S, Poosala S, Spangler EL, Roth GS, Lane M, Allison DB, de Cabo R, Ingram DK, Mattison JA. Chronic ingestion of 2-deoxy-D-glucose induces cardiac vacuolization and increases mortality in rats. Toxicol Appl Pharmacol. 2010;243:332–9. doi: 10.1016/j.taap.2009.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Lian X-Y, Khan FA, Stringer JL. Fructose-1,6-biphosphate has anticonvulsant activity in models of acute seizures in adult rats. J Neurosci. 2007;27:12007–12011. doi: 10.1523/JNEUROSCI.3163-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Ding Y, Wang S, Zhang MM, Guo Y, Yang Y, Weng SQ, Wu JM, Qiu X, Ding MP. Fructose-1,6-diphosphate inhibits seizure acquisition in fast hippocampal kindling. Neurosci Lett. 2010;477:33–36. doi: 10.1016/j.neulet.2010.04.030. [DOI] [PubMed] [Google Scholar]
- [98].Stringer JL, Xu K. Possible mechanisms for the anticonvulsant activity of fructose-1,6-diphosphate. Epilepsia. 2008;49(Suppl. 8):101–103. doi: 10.1111/j.1528-1167.2008.01849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Willis S, Stoll J, Sweetman L, Borges K. Anticonvulsant effects of a triheptanoin diet in two mouse chronic seizure models. Neurobiol Dis. 2010;40:565–572. doi: 10.1016/j.nbd.2010.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Connolly MB, Hendson G, Steinbok P. Tuberous sclerosis complex: a review of the management of epilepsy with emphasis on surgical aspects. Childs Nerv Syst. 2006;22:896–908. doi: 10.1007/s00381-006-0130-7. [DOI] [PubMed] [Google Scholar]
- [101].Coppola G, Klepper J, Ammendola E, Fiorillo M, della Corte R, Capano G, Pascotto A. The effects of the ketogenic diet in refractory partial seizures with reference to tuberous sclerosis. Eur J Paediatr Neurol. 2006;10:148–51. doi: 10.1016/j.ejpn.2006.03.001. [DOI] [PubMed] [Google Scholar]
- [102].Orlova KA, Crino PB. The tuberous sclerosis complex. Ann N Y Acad Sci. 2010;1184:87–105. doi: 10.1111/j.1749-6632.2009.05117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Kossoff EH, Thiele EA, Pfeifer HH, McGrogan JR, Freeman JM. Tuberous sclerosis complex and the ketogenic diet. Epilepsia. 2005;46:1684–6. doi: 10.1111/j.1528-1167.2005.00266.x. [DOI] [PubMed] [Google Scholar]
- [104].Laplante M, Sabatini DM. mTOR signaling at a glance. J Cell Sci. 2009;122:3589–94. doi: 10.1242/jcs.051011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Krueger DA, Care MM, Holland K, Agricola K, Tudor C, Mangeshkar P, Wilson KA, Byars A, Sahmoud T, Franz DN. Everolimus for subependymal giant-cell astrocytomas in tuberous sclerosis. N Engl J Med. 2010;363:1801–11. doi: 10.1056/NEJMoa1001671. [DOI] [PubMed] [Google Scholar]
- [106].Zeng LH, Xu L, Gutmann DH, Wong M. Rapamycin prevents epilepsy in a mouse model of tuberous sclerosis complex. Ann Neurol. 2008;63:444–53. doi: 10.1002/ana.21331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Zeng LH, Rensing NR, Wong M. The mammalian target of rapamycin signaling pathway mediates epileptogenesis in a model of temporal lobe epilepsy. J Neurosci. 2009;29:6964–72. doi: 10.1523/JNEUROSCI.0066-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Huang X, Zhang H, Yang J, Wu J, McMahon J, Lin Y, Cao Z, Gruenthal M, Huang Y. Pharmacological inhibition of the mammalian target of rapamycin pathway suppresses acquired epilepsy. Neurobiol Dis. 2010;40:193–9. doi: 10.1016/j.nbd.2010.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Raffo E, Coppola A, Ono T, Briggs SW, Galanopoulou AS. A pulse rapamycin therapy for infantile spasms and associated cognitive decline. Neurobiol Dis. 2011;43:322–9. doi: 10.1016/j.nbd.2011.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Talos DM, Sun H, Zhou X, Fitzgerald EC, Jackson MC, Klein PM, Lan VJ, Joseph A, Jensen FE. The interaction between early life epilepsy and autistic-like behavioral consequences: a role for the mammalian target of rapamycin (mTOR) pathway. PLoS One. 2012;7:e35885. doi: 10.1371/journal.pone.0035885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Buckmaster PS, Lew FH. Rapamycin suppresses mossy fiber sprouting but not seizure frequency in a mouse model of temporal lobe epilepsy. J Neurosci. 2011;31:2337–47. doi: 10.1523/JNEUROSCI.4852-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Ruegg S, Baybis M, Juul H, Dichter M, Crino PB. Effects of rapamycin on gene expression, morphology, and electrophysiological properties of rat hippocampal neurons. Epilepsy Res. 2007;77:85–92. doi: 10.1016/j.eplepsyres.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Daoud D, Scheld HH, Speckmann EJ, Gorji A. Rapamycin: brain excitability studied in vitro. Epilepsia. 2007;48:834–6. doi: 10.1111/j.1528-1167.2006.00976.x. [DOI] [PubMed] [Google Scholar]
- [114].Tang SJ, Reis G, Kang H, Gingras AC, Sonenberg N, Schuman EM. A rapamycin-sensitive signaling pathway contributes to long-term synaptic plasticity in the hippocampus. Proc Natl Acad Sci U S A. 2002;99:467–72. doi: 10.1073/pnas.012605299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Ran I, Laplante I, Bourgeois C, Pepin J, Lacaille P, Costa-Mattioli M, Pelletier J, Sonenberg N, Lacaille JC. Persistent transcription- and translation-dependent long-term potentiation induced by mGluR1 in hippocampal interneurons. J Neurosci. 2009;29:5605–15. doi: 10.1523/JNEUROSCI.5355-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Hou L, Klann E. Activation of the phosphoinositide 3-kinase-Akt-mammalian target of rapamycin signaling pathway is required for metabotropic glutamate receptor-dependent long-term depression. J Neurosci. 2004;24:6352–61. doi: 10.1523/JNEUROSCI.0995-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Auerbach BD, Osterweil EK, Bear MF. Mutations causing syndromic autism define an axis of synaptic pathophysiology. Nature. 2011;480:63–8. doi: 10.1038/nature10658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Kumar V, Zhang MX, Swank MW, Kunz J, Wu GY. Regulation of dendritic morphogenesis by Ras-PI3K-Akt-mTOR and Ras-MAPK signaling pathways. J Neurosci. 2005;25:11288–99. doi: 10.1523/JNEUROSCI.2284-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Campbell DS, Holt CE. Chemotropic responses of retinal growth cones mediated by rapid local protein synthesis and degradation. Neuron. 2001;32:1013–26. doi: 10.1016/s0896-6273(01)00551-7. [DOI] [PubMed] [Google Scholar]
- [120].Jaworski J, Spangler S, Seeburg DP, Hoogenraad CC, Sheng M. Control of dendritic arborization by the phosphoinositide-3′-kinase-Akt-mammalian target of rapamycin pathway. J Neurosci. 2005;25:11300–12. doi: 10.1523/JNEUROSCI.2270-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Chow DK, Groszer M, Pribadi M, Machniki M, Carmichael ST, Liu X, Trachtenberg JT. Laminar and compartmental regulation of dendritic growth in mature cortex. Nat Neurosci. 2009;12:116–8. doi: 10.1038/nn.2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].McDaniel SS, Rensing NR, Thio LL, Yamada KA, Wong M. The ketogenic diet inhibits the mammalian target of rapamycin (mTOR) pathway. Epilepsia. 2011;52:e7–11. doi: 10.1111/j.1528-1167.2011.02981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Hartman AL, Santos P, Dolce A, Hardwick JM. The mTOR inhibitor rapamycin has limited acute anticonvulsant effects in mice. PLoS One. 2012 doi: 10.1371/journal.pone.0045156. (accepted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Stafstrom CE, Rho JM. The ketogenic diet as a treatment paradigm for diverse neurological disorders. Front Pharmacol. 2012;3:59. doi: 10.3389/fphar.2012.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]