Abstract
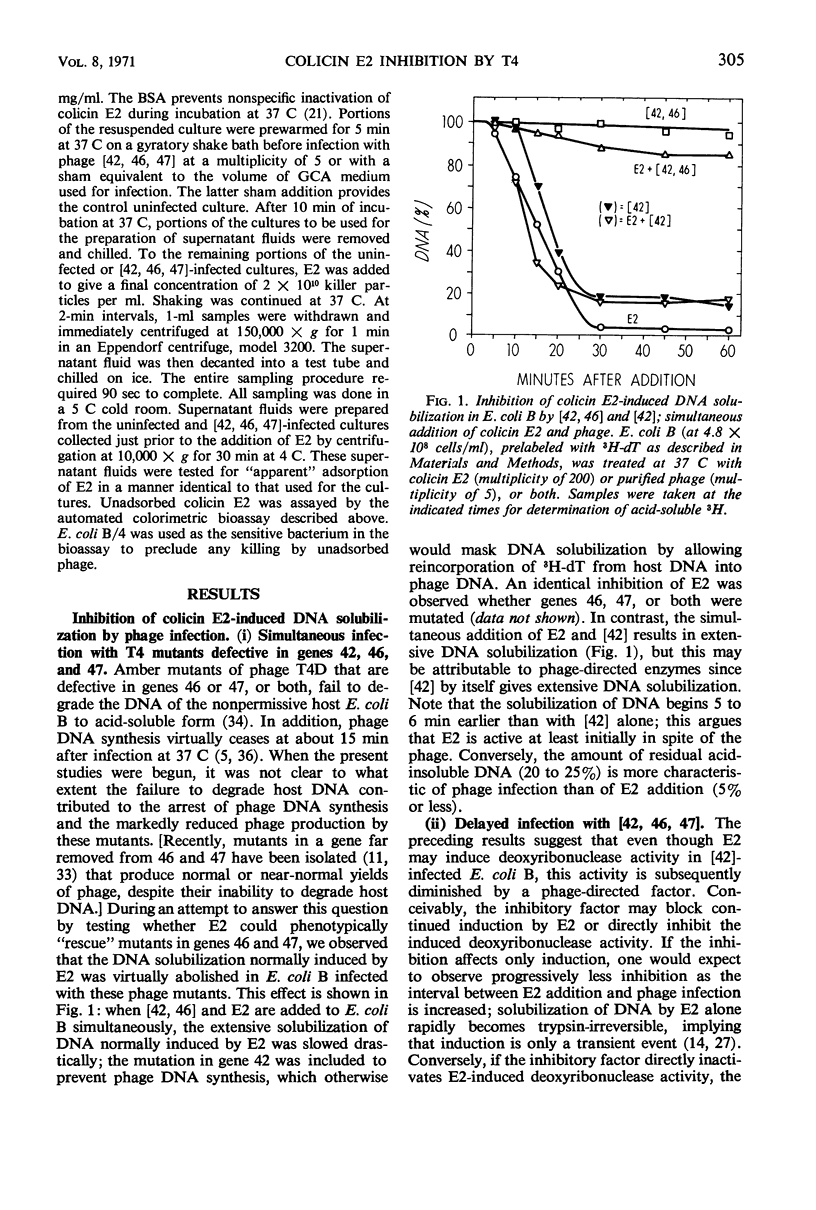
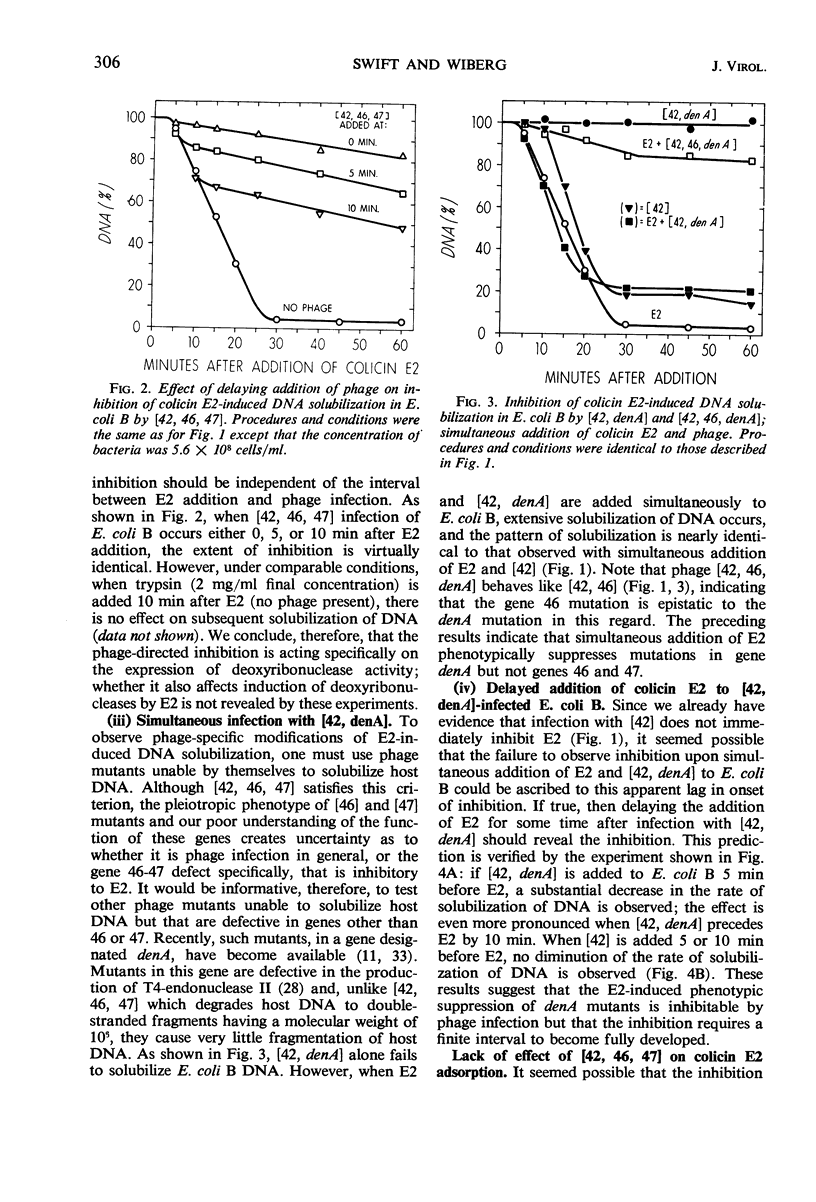
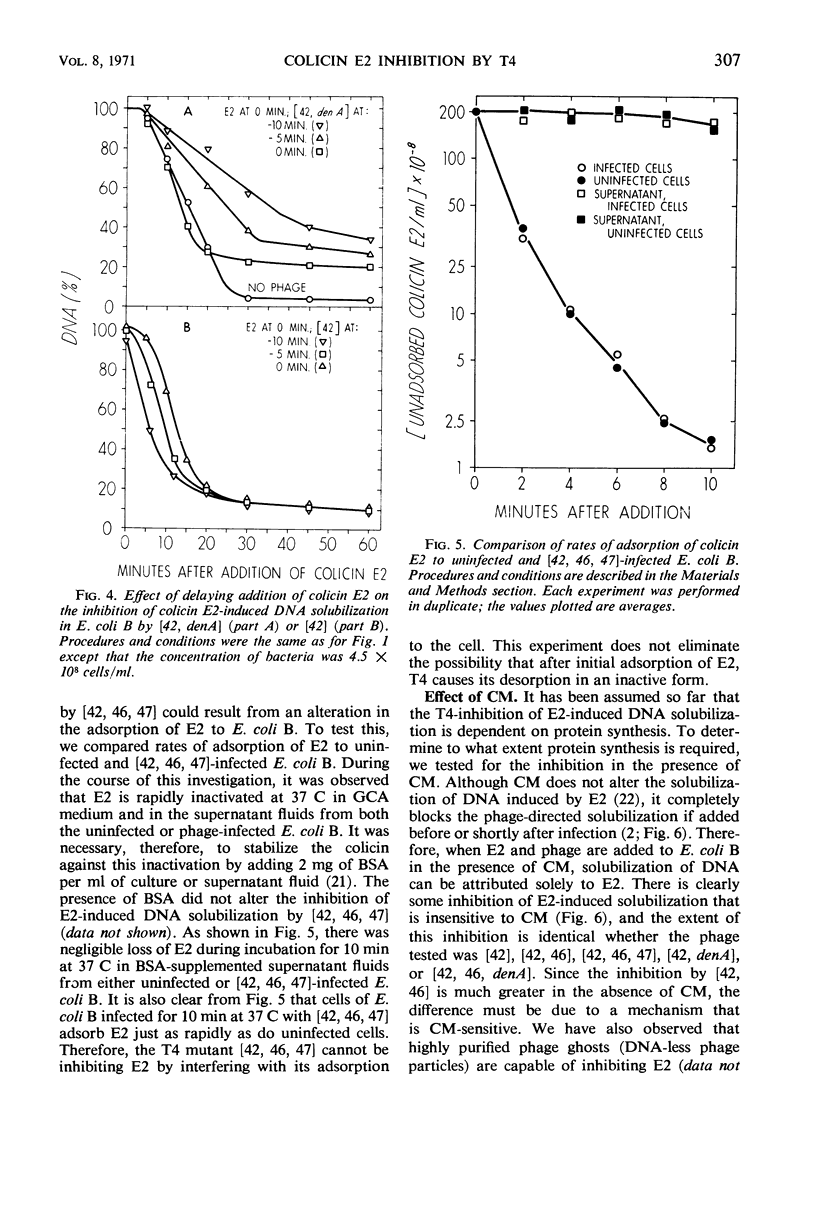
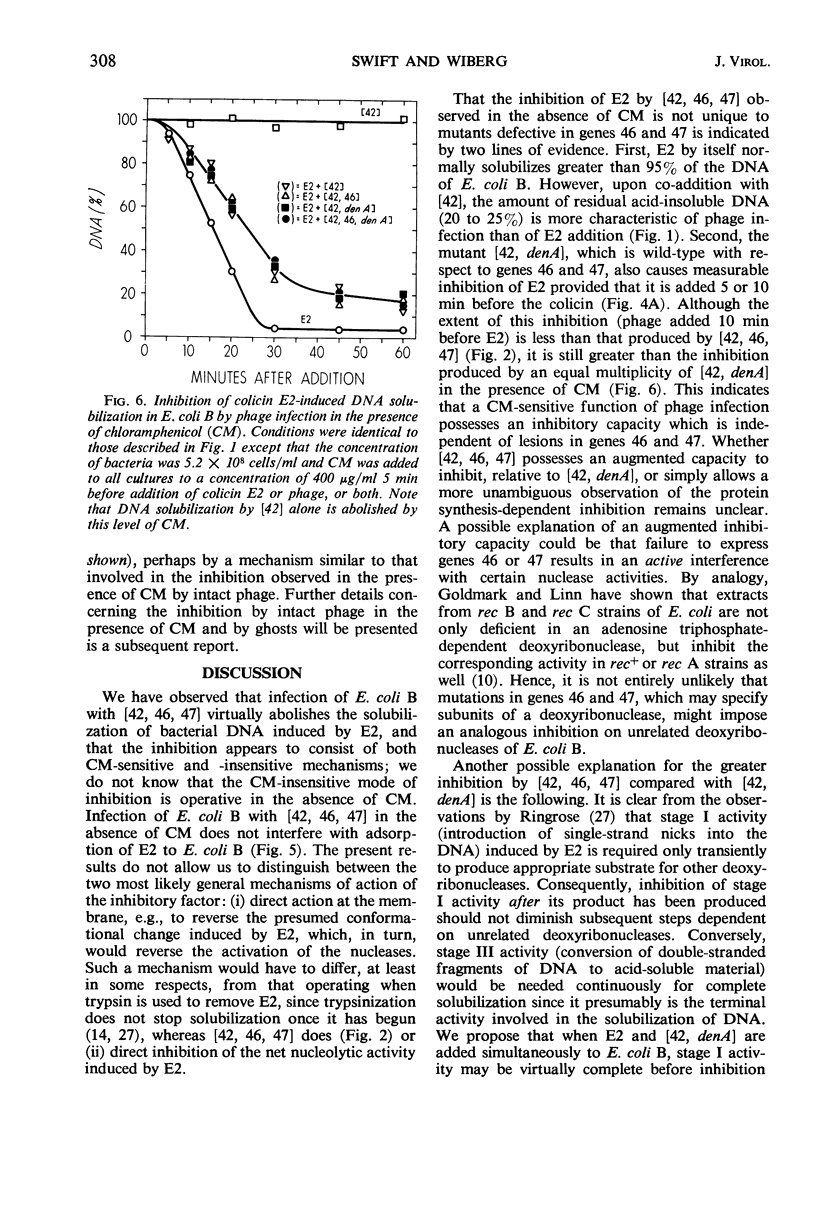
The deoxyribonucleic acid (DNA) of Escherichia coli B is converted by colicin E2 to products soluble in cold trichloroacetic acid; we show that this DNA degradation (hereafter termed solubilization) is subject to inhibition by infection with bacteriophage T4. At least two modes of inhibition may be differentiated on the basis of their sensitivity to chloramphenicol. The following observations on the inhibition of E2 by phage T4 in the absence of chloramphenicol are described: (i) Simultaneous addition to E. coli B of E2 and a phage mutated in genes 42, 46, and 47 results in a virtually complete block of the DNA solubilization normally induced by E2; the mutation in gene 42 prevents phage DNA synthesis, and the mutations in genes 46 and 47 block a late stage of phage-induced solubilization of host DNA. (ii) This triple mutant inhibits equally well when added at any time during the E2-induced solubilization. (iii) Simultaneous addition to E. coli B of E2 and a phage mutated only in gene 42 results in extensive DNA solubilization, but the amount of residual acid-insoluble DNA (20 to 25%) is more characteristic of phage infection than of E2 addition (5% or less). (iv) denA mutants of phage T4 are blocked in an early stage (endonuclease II) of degradation of host DNA; when E2 and a phage mutated in both genes 42 and denA are added to E. coli B, extensive solubilization of DNA occurs with a pattern identical to that observed upon simultaneous addition of E2 and the gene 42 mutant. (v) However, delaying E2 addition for 10 min after infection by this double mutant allows the phage to develop considerable inhibition of E2. (vi) Adsorption of E2 to E. coli B is not impaired by infection with phage mutated in genes 42, 46, and 47. In the presence of chloramphenicol, the inhibition of E2 by the triple-mutant (genes 42, 46, and 47) still occurs, but to a lesser extent.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOLLUM F. J. Thermal conversion of nonpriming deoxyribonucleic acid to primer. J Biol Chem. 1959 Oct;234:2733–2734. [PubMed] [Google Scholar]
- Bose S. K., Warren R. J. Bacteriophage-induced inhibition of host functions. II. Evidence for multiple, sequential bacteriophage-induced deoxyribonucleases responsible for degradation of cellular deoxyribonucleic acid. J Virol. 1969 Jun;3(6):549–556. doi: 10.1128/jvi.3.6.549-556.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changeux J. P., Thiéry J. On the mode of action of colicins: a model of regulation at the membrane level. J Theor Biol. 1967 Nov;17(2):315–318. doi: 10.1016/0022-5193(67)90175-0. [DOI] [PubMed] [Google Scholar]
- Duckworth D. H., Bessman M. J. Assay for the Killing Properties of T2 Bacteriophage and Their "Ghosts". J Bacteriol. 1965 Sep;90(3):724–728. doi: 10.1128/jb.90.3.724-728.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRASER D., JERREL E. A. The amino acid composition of T3 bacteriophage. J Biol Chem. 1953 Nov;205(1):291–295. [PubMed] [Google Scholar]
- Fields K. L., Luria S. E. Effects of colicins E1 and K on cellular metabolism. J Bacteriol. 1969 Jan;97(1):64–77. doi: 10.1128/jb.97.1.64-77.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields K. L., Luria S. E. Effects of colicins E1 and K on transport systems. J Bacteriol. 1969 Jan;97(1):57–63. doi: 10.1128/jb.97.1.57-63.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldmark P. J., Linn S. An endonuclease activity from Escherichia coli absent from certain rec- strains. Proc Natl Acad Sci U S A. 1970 Sep;67(1):434–441. doi: 10.1073/pnas.67.1.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hercules K., Munro J. L., Mendelsohn S., Wiberg J. S. Mutants in a nonessential gene of bacteriophage T4 which are defective in the degradation of Escherichia coli deoxyribonucleic acid. J Virol. 1971 Jan;7(1):95–105. doi: 10.1128/jvi.7.1.95-105.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herschman H. R., Helinski D. R. Purification and characterization of colicin E2 and colicin E3. J Biol Chem. 1967 Nov 25;242(22):5360–5368. [PubMed] [Google Scholar]
- Hirata H., Fukui S., Ishikawa S. Initial events caused by colicin K infection--cation movement and depletion of ATP pool. J Biochem. 1969 May;65(5):843–847. doi: 10.1093/oxfordjournals.jbchem.a129088. [DOI] [PubMed] [Google Scholar]
- Holland E. M., Holland I. B. Induction of DNA breakdown and inhibition of cell division by colicin E2. Nature of some early steps in the process and properties of the E-2-specific nuclease system. J Gen Microbiol. 1970 Dec;64(2):223–239. doi: 10.1099/00221287-64-2-223. [DOI] [PubMed] [Google Scholar]
- JACOB F., SIMINOVITCH L., WOLLMAN E. Sur la biosynthèse d'une colicine et sur son mode d'action. Ann Inst Pasteur (Paris) 1952 Sep;83(3):295–315. [PubMed] [Google Scholar]
- Konisky J., Nomura M. Interaction of colicins with bacterial cells. II. Specific alteration of Escherichia coli ribosomes induced by colicin E3 in vivo. J Mol Biol. 1967 Jun 14;26(2):181–195. doi: 10.1016/0022-2836(67)90290-2. [DOI] [PubMed] [Google Scholar]
- Kutter E. M., Wiberg J. S. Biological effects of substituting cytosine for 5-hydroxymethylcytosine in the deoxyribonucleic acid of bacteriophage T4. J Virol. 1969 Oct;4(4):439–453. doi: 10.1128/jvi.4.4.439-453.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutter E. M., Wiberg J. S. Degradation of cytosin-containing bacterial and bacteriophage DNA after infection of Escherichia coli B with bacteriophage T4D wild type and with mutants defective in genes 46, 47 and 56. J Mol Biol. 1968 Dec;38(3):395–411. doi: 10.1016/0022-2836(68)90394-x. [DOI] [PubMed] [Google Scholar]
- LURIA S. E. ON THE MECHANISMS OF ACTION OF COLICINS. Ann Inst Pasteur (Paris) 1964 Nov;107:SUPPL–SUPPL:73. [PubMed] [Google Scholar]
- Maeda A., Nomura M. Interaction of colicins with bacterial cells. I. Studies with radioactive colicins. J Bacteriol. 1966 Feb;91(2):685–694. doi: 10.1128/jb.91.2.685-694.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsui E., Mizuno D. Stabilization of colicin E2 by bovine serum albumin. J Bacteriol. 1969 Nov;100(2):1136–1137. doi: 10.1128/jb.100.2.1136-1137.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura M. Colicins and related bacteriocins. Annu Rev Microbiol. 1967;21:257–284. doi: 10.1146/annurev.mi.21.100167.001353. [DOI] [PubMed] [Google Scholar]
- REYNOLDS B. L., REEVES P. R. Some observations on the mode of action of colicin F. Biochem Biophys Res Commun. 1963 Apr 23;11:140–145. doi: 10.1016/0006-291x(63)90081-0. [DOI] [PubMed] [Google Scholar]
- Reynolds B. L., Reeves P. R. Kinetics of adsorption of colicin CA42-E2 and reversal of its bactericidal activity. J Bacteriol. 1969 Oct;100(1):301–309. doi: 10.1128/jb.100.1.301-309.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringrose P. Sedimentation analysis of DNA degradation products resulting from the action of colicin E2 on Escherichia coli. Biochim Biophys Acta. 1970 Aug 8;213(2):320–334. doi: 10.1016/0005-2787(70)90040-7. [DOI] [PubMed] [Google Scholar]
- Sadowski P. D., Warner H. R., Hercules K., Munro J. L., Mendelsohn S., Wiberg J. S. Mutants of bacteriophage T4 defective in the induction of T4 endonuclease II. J Biol Chem. 1971 May 25;246(10):3431–3433. [PubMed] [Google Scholar]
- Senior B. W., Kwasniak J., Holland I. B. Colicin E3-directed changes in ribosome function and polyribosome metabolism in Escherichia coli K12. J Mol Biol. 1970 Oct 28;53(2):205–220. doi: 10.1016/0022-2836(70)90295-0. [DOI] [PubMed] [Google Scholar]
- Shannon R., Hedges A. J. A colorimetric bioassay method for colicins. J Appl Bacteriol. 1970 Sep;33(3):555–565. doi: 10.1111/j.1365-2672.1970.tb02234.x. [DOI] [PubMed] [Google Scholar]
- Tanner D., Oishi M. The effect of bacteriophage T4 infection on an ATP-dependent deoxyribonuclease in Escherichia coli. Biochim Biophys Acta. 1971 Feb 11;228(3):767–769. doi: 10.1016/0005-2787(71)90747-7. [DOI] [PubMed] [Google Scholar]
- WIBERG J. S., DIRKSEN M. L., EPSTEIN R. H., LURIA S. E., BUCHANAN J. M. Early enzyme synthesis and its control in E. coli infected with some amber mutants of bacteriophage T4. Proc Natl Acad Sci U S A. 1962 Feb;48:293–302. doi: 10.1073/pnas.48.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner H. R., Snustad P., Jorgensen S. E., Koerner J. F. Isolation of bacteriophage T4 mutants defective in the ability to degrade host deoxyribonucleic acid. J Virol. 1970 Jun;5(6):700–708. doi: 10.1128/jvi.5.6.700-708.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiberg J. S. Amber mutants of bacteriophage T4 defective in deoxycytidine diphosphatase and deoxycytidine triphosphatase. On the role of 5-hydroxymethylcytosine in bacteriophage deoxyribonucleic acid. J Biol Chem. 1967 Dec 25;242(24):5824–5829. [PubMed] [Google Scholar]
- Wiberg J. S. Mutants of bacteriophage T4 unable to cause breakdown of host DNA. Proc Natl Acad Sci U S A. 1966 Mar;55(3):614–621. doi: 10.1073/pnas.55.3.614. [DOI] [PMC free article] [PubMed] [Google Scholar]



