Abstract
Pfizer, Inc.'s Tissue Bank, in conjunction with Pfizer's BioBank (biofluid repository), endeavored to create an overarching internal software package to cover all general functions of both research facilities, including sample receipt, reconciliation, processing, storage, and ordering. Business process flow diagrams were developed by the Tissue Bank and Informatics teams as a way of characterizing best practices both within the Bank and in its interactions with key internal and external stakeholders. Besides serving as a first step for the software development, such formalized process maps greatly assisted the identification and communication of best practices and the optimization of current procedures. The diagrams shared here could assist other biospecimen research repositories (both pharmaceutical and other settings) for comparative purposes or as a guide to successful informatics design. Therefore, it is recommended that biorepositories consider establishing formalized business process flow diagrams for their laboratories, to address these objectives of communication and strategy.
Introduction
Pfizer, Inc.'s global tissue repository is a collection of >12,000 human and animal tissue samples obtained from academic and commercial sources and from a growing number of clinical trials. Tissues are stored in a variety of fixed and frozen dispositions to meet various research needs. The tissues from the bank are used to support a wide variety of discovery and development programs in the company. On a volume basis, the majority of bank use is human tissue, though preclinical species tissues (especially rat, mouse, and primate) are of increasing importance. The bank assists in the acquisition, storage, archiving, and supply of tissue through a centralized inventory and database and offers tissue sectioning, frozen and paraffin-embedded tissue blocks, histologic quality assurance, and scientific consultations on a request-driven basis to company investigators. Additionally, the bank is dedicated to the ongoing acquisition of tissues to meet anticipated research needs. Appropriate consent and institutional review board approval is in place for all tissues and contracts, and the use of the tissues is also governed by Pfizer's human tissue policy. Pfizer's tissue repository is linked (from both informatics and strategic governance standpoints) with a separate facility (the Pfizer BioBank) for DNA and biofluids, which has high-capacity, automated storage capabilities. This biofluids facility can link separate biofluid and tissue samples when both types are received from the same patient source.
As part of the globalization and more efficient utilization of the tissue repository, it was desired to write and implement an overarching internal software package to cover all general functions of both research biobanking facilities, including sample receipt, reconciliation, processing, storage, and ordering. As a first step for that software development, business process flow diagrams for the Tissue Bank were prepared through cooperative work between the Bank and the Research Informatics Division.1 The use of such diagrams (also referred to as workflow diagrams) to model task-based laboratories or bioinformatics initiatives, for the purposes of informatics design or integration, is established in the literature.2,3 Separate flow diagrams had been previously written for Pfizer's automated biofluids facility, but because of the very different laboratory practices of the tissue repository, new and distinct diagrams were required. In general, multiple advantages were gleaned from this process. The diagrams and associated learning are the subject of this article.
In business process mapping, the main objective was to focus on business participants that add value (from both customer and business perspectives), describing what the participants do from step to step. Sometimes, these maps are called business use cases. This is to be distinguished from system use cases, which automate a business use case, thus providing detail at the system functionality level, in other words, what the system does in response to a participant's actions. Thus, the advantages of our approach was the creation of fundamental maps that focused on both business and customer value, and that would provide the foundation for system use cases and other subsequent work. The main stakeholders for the work were of 2 broad groups: (1) the Informatics team who intended to use the diagrams as a blueprint for further software development, and (2) the Tissue Bank personnel carrying out the business workflow, their scientific customers, and internal and external tissue sources that were sometimes contacted as part of the workflows. The Tissue Bank personnel were the main individuals who responded to workflow changes and/or changes in best practices.
Materials and Methods
The Tissue Bank personnel (Laboratory Lead and histotechnologists) met on multiple occasions over a period of several weeks (in person and through teleconferences) with key Research Informatics personnel within Pfizer and closely examined the existing laboratory practices. The flow diagrams were principally organized along the distinct paths corresponding to tissue storage dispositions (eg, frozen vs. fixed tissue and slides vs. blocks) and activity (ordering, processing, and tissue disbursal), because the inception of one of these paths was typically the main determinant of workflow.
For each workflow path, the Tissue Bank personnel first provided a verbal description of how work was carried out. The Informatics personnel created a draft of these descriptions using the schemes shown in Figs. 1–5. The Tissue Bank personnel then reconsidered the processes with the workflow drafts in front of the group, identifying gaps and areas for refinement. Attention was given to the typical, expected workflow path and its deviations that were important or frequent enough to warrant inclusion. Particular attention was given to the identification of decision points in the pathways and the points where different pathways intersected or yielded to one another, because these features would be important for informatics design.
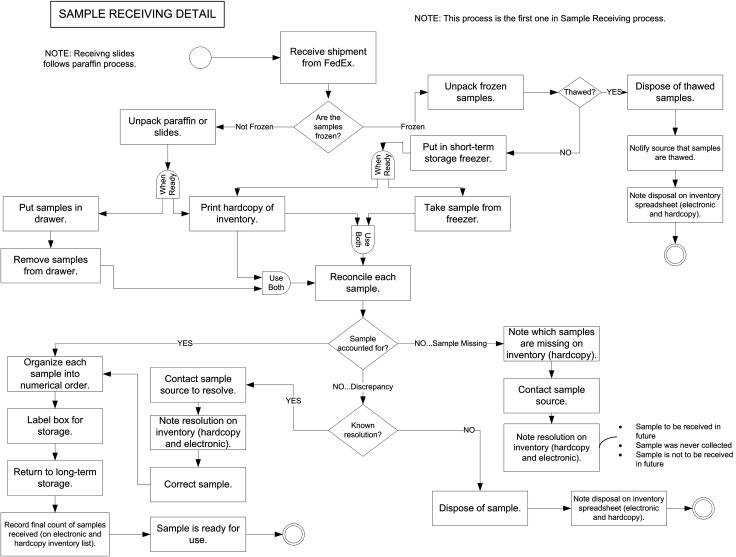
FIG. 1.
Business process flow diagram for sample receiving. Single circle at top represents the entry point; double circles at bottom represent exit points. PI, primary investigator (the person ordering tissues from the bank for research purposes); QC, quality control; TB, tissue bank; H&E, hematoxylin- and eosin-stained slide, used for QC evaluation of tissues; Lab Lead, the tissue bank manager and primary strategic decision maker.
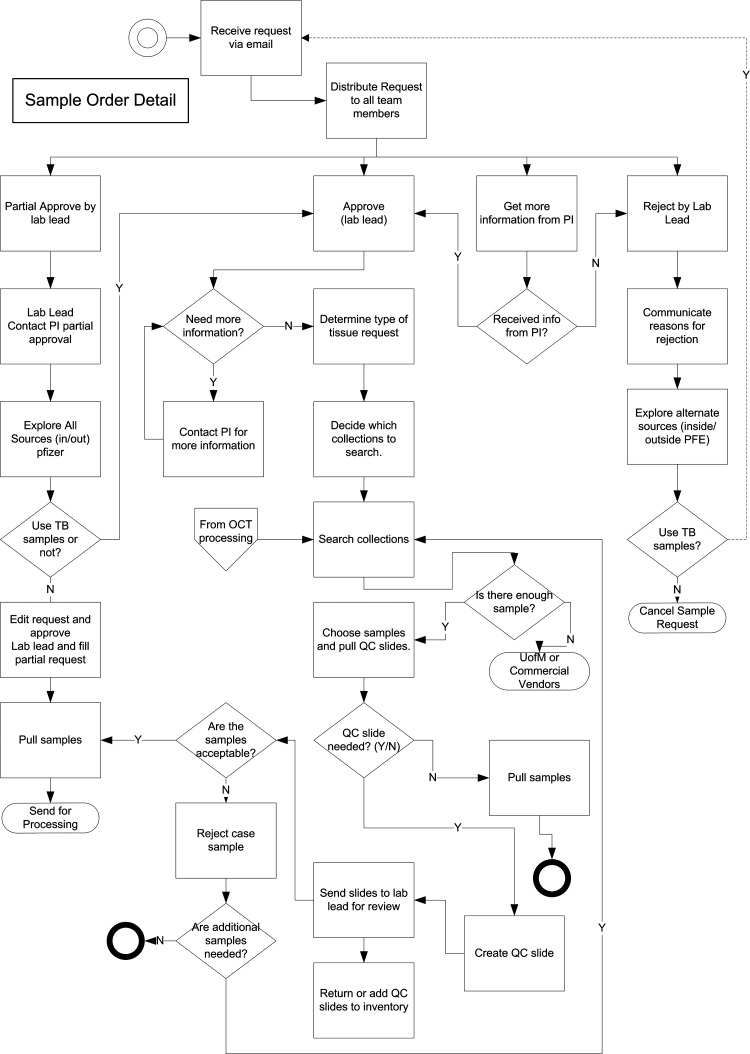
FIG. 5.
Business process flow diagram for specimen orders. Entry points are the double circle at the top, and the polygon labeled “From OCT processing” in the middiagram. Exit points are the thick circles at bottom, and the 2 ovals on the left and right labeled “Send for processing” and “Cancel sample request,” respectively.
The discussions thus continued iteratively until the participants reached a consensus that all key paths and decisions were captured in the diagrams. The Tissue Bank personnel reviewed all of their core activities for the prior 3 years to make sure they had been captured in at least 1 diagram. After discussions were complete, the diagrams were formalized using the vector graphics capabilities of the Microsoft Office Visio 2007TM program.
The approach here was consistent with the latest version of the National Cancer Institute's Best Practices for Biospecimen Repositories, section B.6.4.1,4 which concerns the development of informatics management systems for biorepositories.
Results
Figures 1–5 show the business process flow diagrams. These respectively cover (1) sample receipt, including procedures for missing or otherwise problematic samples, (2) sample processing for paraffinized tissue (both blocks and histologic slides) and optimal cutting temperature compound (OCT)-embedded tissue, (3) sample processing for snap-frozen tissue, both that intended to be released as snap-frozen, unembedded tissue, and that desired to be converted to an OCT block before release, (4) sample processing for inventoried glass slides, and (5) handling and approving sample orders and selecting tissues to fulfill orders. As is customary with such diagrams, diamond-shaped boxes indicate decision points, rectangular boxes indicate workflow steps, and circles or polygons show starting points or endpoints or the places where 1 workflow path joins or leads to another.
Discussion
The feedback from the Informatics Design team at Pfizer, Inc., on receipt of the diagrams, was that the diagrams were quite helpful as a first-step blueprint for the informatics system design covering Pfizer, Inc.'s Tissue Bank and BioBank. Similarly, for the readership, it is expected that the diagrams here may serve as a useful guide to biorepository or informatics design or as a comparative guide to best practices. By examining workflows at the level of depth required to build the diagrams, best practices can be more readily identified. Also, areas for improvement and key features required for a good informatics solution can be identified as well. The following discussion highlights the salient points stemming from the business process flow diagrams.
Identification of separate but interconnected workflow paths
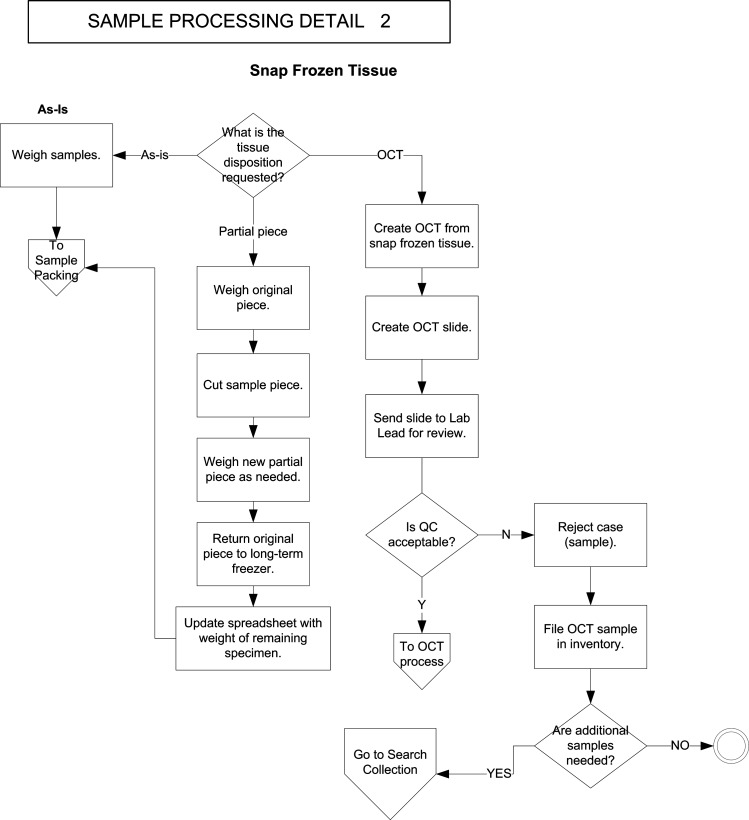
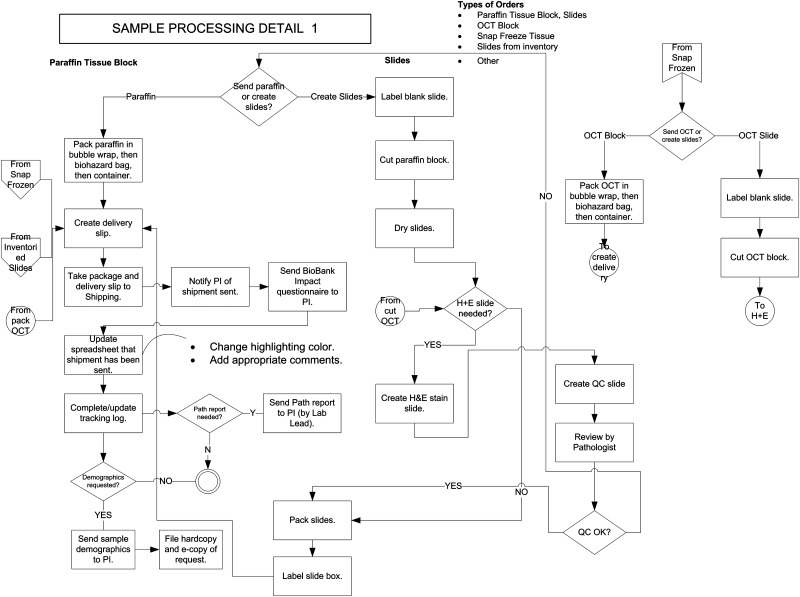
Different tissue storage dispositions and intended uses clearly require distinct flow diagram paths. This principle was important to the success of the project. Probably, the most fundamental decisions in a research tissue repository are those between paraffin blocks and frozen tissue, which meet different research needs, and between snap-frozen and OCT-embedded tissue, which also address different needs. However, several areas were identified, where different paths intersected or yielded to one another. Examples include the need for an OCT block created from a snap-frozen tissue piece (Fig. 3), yielding to the process flow for OCT block processing (Fig. 2), and the failure of quality control (QC) for a slide in the processing pathway (Fig. 3), returning the user to the search collections pathway in the order flow diagram (Fig. 5). Another commonly encountered one is the sectioning of slides from paraffin blocks or OCT blocks (Figs. 2 and 3), which later rejoin the main processing/delivery pathway for paraffin blocks (also Fig. 2, left). These interconnections were helpful in designing efficient informatics solutions that would use and return to the basic pathways when needed, without undue redundancy.
FIG. 3.
Business process flow diagram for sample processing, part 2 (snap-frozen tissue). Entry point is the upper diamond (“What is the tissue disposition requested?”). Exit points are the double circle at lower right, and the polygon at left labeled “To sample packing.”
FIG. 2.
Business process flow diagram for sample processing, part 1 (paraffin tissue blocks and OCT blocks). Multiple entry points are present here, including diamond at upper left (“Send paraffin or create slides”), and the polygons and circles at left and upper right that start with “From” represent entry points from other flow diagrams. Exit points are the double circle at lower left, and the circles at right that begin with “To” represent entry to other flow diagrams. OCT, optimal cutting temperature compound.
Pathway juxtapositions in the diagrams were also helpful in the strategic positioning of lab equipment during facility moves, which took place shortly after the diagrams were established; for example, a freezer was placed near the specimen receipt area to accommodate specimens needing reconciliation or short-term storage (Fig. 1), and frequently used paraffin blocks and slides were localized close to the processing areas so they could be readily incorporated into the workflow (Fig. 5 for sample selection process, followed by Figs. 2–4 for processing).
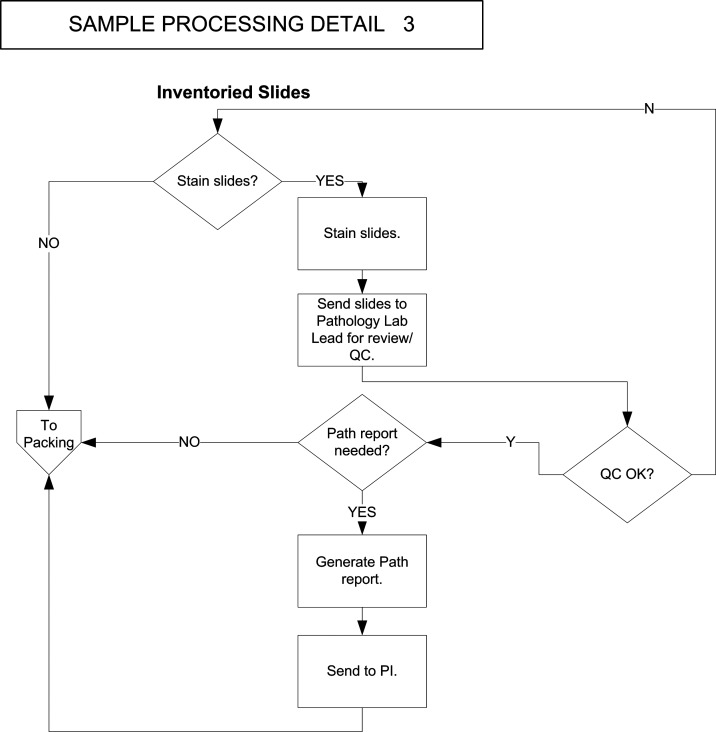
FIG. 4.
Business process flow diagram for sample processing, part 3 (inventoried slides). Entry point is the diamond labeled “Stain slides?” Exit point is the polygon labeled “To packing.”
Procedures for missing or otherwise problematic samples
This is a common problem for samples received from outside sources. The lower half of Fig. 1 shows how this reconciliation process is handled, subsequent to the process of receipt covered in the top half of the figure. Note that the workflow paths for receipt of frozen and fixed samples eventually unify as they proceed to reconciliation in the middiagram. The key actions here are the contacts with the sample source to attempt to resolve the discrepancy and the incorporation of such information into both the hardcopy and electronic rosters. This flow diagram was helpful in the informatics design and strategic positioning of the reconciliation process, including the electronic rosters and how corrections on them were documented (eg, missing sample and discrepant sample are handled differently, and the measures for resolution are distinct within each path).
Inclusion of sample QC decision points in workflow
Histologic QC of specimens in the research tissue repository is critically important for assuring that disbursed samples are of the type intended and that they are well preserved enough to support their intended laboratory use. The pathways incorporate several locations where the quality of specimens is assessed, including the creation of an OCT block from a snap-frozen specimen and then the review of a slide cut from the OCT block (Fig. 3), the review of inventoried slides following their retrieval and, if needed, their staining (Fig. 4), and review and subsequent choice of the best specimens to fulfill incoming orders from investigators (Fig. 5). Identifying the workflow locations where these QC assessments were being made or used was crucial to the correct informatics design—in particular, selecting the right times for these assessments and their entrance into the system, and optimizing the retrieval of QC reviews when they were needed to help select specimens from the inventory.
Quality, feasibility, and tracking issues for specimens from an outside source
Although samples can be received from within the company, many samples are obtained from outside sources. These could include academic collaborations, commercial vendors, and clinical trials. Figure 1 covers these processes as far as receipt, processing, identification, and discard of frozen samples found to have thawed in transit. Outside samples that satisfy quality assessment criteria can then be routed through the paths in Fig. 5 (see discussion above) when it is time to use them for requests.
Customer communication practices
Customer communications are a key part of an investigator-focused research tissue repository, and the workflow paths incorporate several junctures where they are carried out, both for the purposes of influencing decisions within the repository (typically regarding specimen selection or processing options) and communicating information of research benefit to the investigator. As previously mentioned, outside sources are contacted regarding incoming problematic specimens (Fig. 1). Note also the customer-centered approach used for delivery of pathology and clinical data (lower parts of Figs. 2 and 4). Also, note in Fig. 5 that one of the options at the disposal of the laboratory lead is to contact the investigator for more information regarding a request, both before and after an order approval is made. Figure 5 shows how this information is then funneled into an order approval or rejection or into the path where specimens are selected from the inventory.
Centralized decision making
The laboratory lead (also known as the tissue bank manager) is the centralized point of order approval and decision making in these flow diagrams. Recognition of this feature was crucial in the informatics design, for capture of order approvals and specimen selection, and the correct placement of these in the software algorithms. Regarding the lead's approval options for orders, there are 3 separate but intersecting paths: full approval, partial approval, and order rejection (Fig. 5). As shown in this figure, an interactive customer-centered approach is important for the latter 2 paths, to resolve the order in a way that is maximally beneficial. One other workflow feature in Fig. 5 is the option to consider external tissue sources to fulfill an order.
Conclusion
In conclusion, optimized business process flow diagrams contribute to the success of several endeavors in pharmaceutical research tissue banking, including best practice identification and sharing, informatics development, and stakeholder communications. Using modern software, the diagrams can be readily updated to reflect changing needs or business practices. Therefore, research biorepositories should consider establishing these flow diagrams for their own settings. Although described here in a pharmaceutical industrial setting, the diagrams would be useful for repositories in other types of institutions also.
Acknowledgments
The authors acknowledge the support of Pfizer, Inc., Drug Safety Research & Development (DSRD) Division, St. Louis and Groton Laboratories, the sites where the work and principles described here were developed and practiced.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.McDonald SA. Ilasi N. Velasco E. Identification of best practices in a pharmaceutical research tissue bank: business process flow diagrams. ISBER Annual Meeting (HSR #3); Portland, OR. May 12–14;2009 . [Google Scholar]
- 2.Goodman N. Rozen S. Stein LD. The LabFlow system for workflow management in large scale biology research laboratories. Proc Int Conf Intell Syst Mol Biol. 1998;6:69–77. [PubMed] [Google Scholar]
- 3.Tiwari A. Sekhar AK. Workflow based framework for life science informatics. Comput Biol Chem. 2007;31:305–319. doi: 10.1016/j.compbiolchem.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 4.National Cancer Institute (NCI) Best Practices for Biospecimen Repositories, 2010 Revised Version. Office of Biorepositories and Biospecimen Research, National Institutes of Health, U.S. Department of Health and Human Services. http://biospecimens.cancer.gov http://biospecimens.cancer.gov