Summary
Basement membranes (BM) are specialized extracellular matrices that are essential for epithelial structure and morphogenesis. However, little is known about how BM proteins are delivered to the basal cell surface, or how this process is regulated during development. Here, we identify a mechanism for polarized BM secretion in the Drosophila follicle cells. BM proteins are synthesized in a basal ER compartment from localized mRNAs, and are then exported through Tango1-positive ER exit sites to basal Golgi clusters. Next, Crag targets Rab10 to structures in the basal cytoplasm where it restricts protein delivery to the basal surface. These events occur during egg chamber elongation, a morphogenetic process that depends on follicle cell planar polarity and BM remodeling. Significantly, Tango1 and Rab10 are also planar polarized at the basal epithelial surface. We propose that the spatial control of BM production along two tissue axes promotes exocytic efficiency, BM remodeling and organ morphogenesis.
Introduction
Basement membranes (BMs) are an ancient form of extracellular matrix found at the basal surface of all epithelia (Yurchenco, 2011). Though often overlooked as passive scaffolds, these complex protein lattices provide many essential functions to neighboring cells. In addition to providing tissue support, BMs act as signaling platforms for cell polarization, stem cell regulation, and migrating organ primordia (Arnaoutova et al., 2012; Bunt et al., 2010; Mirouse et al., 2009; O’Brien et al., 2001; Schneider et al., 2006; Wang et al., 2008). They also play central roles in organ morphogenesis and physiology (Fata et al., 2004; Miner, 2011; Miner and Yurchenco, 2004; Pastor-Pareja and Xu, 2011; Urbano et al., 2009). The mis-regulation of BM structure can be a hallmark of tumor progression (Valastyan and Weinberg, 2011). Despite these critical functions, we know relatively little about the molecular control of BM assembly.
BMs are primarily composed of Type IV Collagen (Col IV), Laminin, Nidogen, and heparan sulfate proteoglycans such as Perlecan or Agrin. Among these components Col IV predominates, representing up to 50% of BM proteins (Kalluri, 2003). Despite its abundance, the pathway for Col IV production is complex. Each Col IV molecule is composed of three polypeptides, two α1 chains and one α2 chain, that initiate contact at their C-termini and then assemble into a triple helix, roughly 400 nm long (Khoshnoodi et al., 2008). Col IV folding requires a suite of ER-resident enzymes and chaperones, many of which are collagen-specific (Myllyharju and Kivirikko, 2004). These include lysyl- and prolyl-hydroxylase enzymes that modify the α-chains both during and after translation. Special mechanisms also facilitate Col IV export. Newly synthesized proteins typically leave the ER in COPII-coated vesicles that are too small to accommodate the extended Col IV trimer (Fromme and Schekman, 2005; Jin et al., 2012; Malhotra and Erlmann, 2011). The trans-membrane cargo receptor Tango1 is required at transitional ER (tER) sites to help package Collagens into enlarged Golgi-bound vesicles (Saito et al., 2009; Venditti et al., 2012; Wilson et al., 2011).
Following their synthesis, new BM proteins must be targeted to the proper membrane domain for secretion. Polarized epithelial cells have distinct apical, junctional, lateral and basal membrane domains, each of which contains a unique complement of lipids and proteins. These domains are established and maintained by polarized vesicle traffic that delivers newly synthesized secreted and trans-membrane proteins to the appropriate cell surface (Mellman and Nelson, 2008; Rodriguez-Boulan et al., 2005). Although much is known about the trafficking pathways that target trans-membrane proteins to the combined basal and lateral surfaces, there have long been indications that BM proteins reach the basal surface through a distinct mechanism (Boll et al., 1991; Caplan et al., 1987; Cohen et al., 2001; De Almeida and Stow, 1991). The first molecular evidence for this assertion came from genetic studies in Drosophila that identified the DENN domain protein, Crag, and the protease-like protein, Scarface (Scaf), as selective regulators of polarized BM deposition (Denef et al., 2008; Eastburn and Mostov, 2010; Sorrosal et al., 2010). Loss of either gene causes BM proteins to accumulate on both the basal and apical epithelial surfaces without obvious effects on other exocytic cargo. Currently, the mechanisms by which Crag and Scaf promote polarized BM secretion are unknown.
The Drosophila egg chamber provides a highly tractable system for the study of BM biology in the context of a developing organ. Egg chambers are multicellular structures within fly ovaries that will each give rise to a single egg. They are composed of an inner germ cell cluster surrounded by an outer epithelial layer of follicle cells. The apical follicle cell surfaces face the germ cells, while the basal surfaces contact the BM (Figure 1A). The BM, in turn, forms the egg chamber’s outer most layer. To our knowledge, the follicle cells are the only fly epithelium that synthesizes all major BM components (Mirre et al., 1988; Pastor-Pareja and Xu, 2011; Yasothornsrikul et al., 1997).
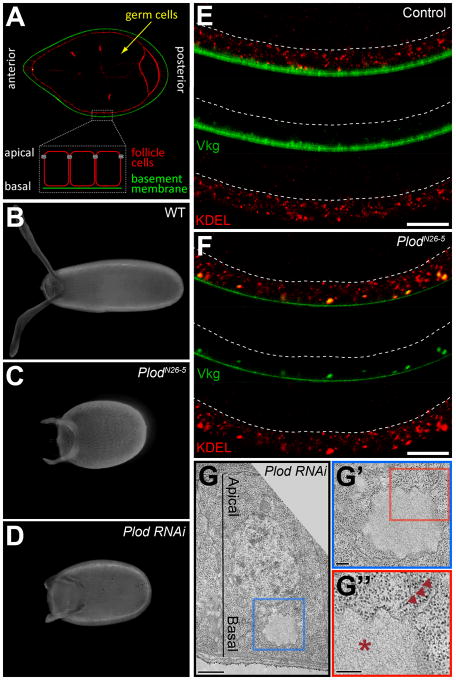
Figure 1. PlodN26–5 disrupts the secretion of Col IV.
(A) Overview of egg chamber structure at stage 8. Actin (red), Vkg-GFP (green). (B–D) Representative egg shapes for (B) wild type, (C) PlodN26-5, and (D) Plod-RNAi. (E) In wild type, proteins with the KDEL ER retention signal are throughout the cell, while Vkg-GFP is in the BM. (F) In PlodN26-5, Vkg-GFP punctae overlap with KDEL near the basal surface, indicating ER accumulation. (E and F) Dashed lines mark the apical surface, and scale bars are 10 μm. (G) TEM of a single Plod-RNAi follicle cell showing a distended ER region in the basal cytoplasm. Scale bar is 1 μm. (G′ and G″) Magnifications of the image in (G). Ribosomes decorate the distended ER cisterna (asterisk) and adjacent, normal ER membranes (arrowheads). Scale bars are 200 nm. (E–G) Experiments performed at stages 7–8. See also Figure S1.
Interestingly, the follicle cell BM plays a critical role in shaping the fly egg. Though initially spherical, egg chambers lengthen along their anterior-posterior (AP) axes as they develop. This morphogenesis depends on an unusual form of planar polarity, in which actin filaments at the basal cell surface become aligned orthogonal to the AP axis (Gutzeit, 1990). Once oriented, the follicle cells undergo a directed migration along the inner BM surface, a process that causes the entire egg chamber to rotate inside the stationary matrix (Haigo and Bilder, 2011). Importantly, the migrating follicle cells also secrete new BM proteins. Through a process that is still poorly understood, the combination of cell movement and matrix secretion creates fibril-like structures in the BM, perpendicular to the elongation axis (Gutzeit et al., 1991; Haigo and Bilder, 2011; Schneider et al., 2006) (Figure S1A). It has been proposed that this fibrillar matrix then constrains isometric egg chamber growth to promote AP elongation, but many questions remain about how this unusual BM structure is built.
Here, we identify a mechanism for polarized BM secretion in the follicle cells. Starting from a mutation that traps Col IV in the cell, we show that BM proteins are locally synthesized in a basal ER compartment, and are exported through Tango1-positive ER exit sites to basal Golgi clusters. We then introduce Rab10 as a regulator of polarized BM secretion, and show that Crag regulates Rab10 in this context. Finally, we show that the BM exocytic machinery is also planar polarized at the basal epithelial surface. Our data suggest that BM proteins are secreted from the trailing edge of each migrating follicle cell, a process that may contribute to the formation of BM fibrils, and egg chamber elongation. We therefore propose that the spatial regulation of BM production along two tissues axes promotes both polarized secretion and matrix remodeling during organ morphogenesis.
Results
Col IV accumulates in a basal ER region in Plod mutant follicle cells
We have previously reported a genetic screen for mutations that block egg chamber elongation and produce round eggs (Horne-Badovinac et al., 2012). The RE-H complementation group from this screen contained a single allele, N26-5 (Figures 1B–1C). We used deficiency mapping to localize N26-5 to a chromosomal region containing the procollagen lysyl hydroxylase (Plod) gene. Sequencing the Plod coding regions in N26-5 mutant larvae identified a lesion, which is predicted to change a highly conserved glycine to aspartic acid in one of the protein’s catalytic domains (Figure S1B). RNAi knockdown of Plod in the follicle cells also produces round eggs (Figures 1D). We now refer to this mutation as PlodN26-5.
Plod is an ER-resident enzyme that co- and post-translationally modifies lysine residues on Col IV α-chains (Myllyla et al., 2007). Previous work in Drosophila and other organisms has shown that Plod depletion leads to Col IV retention in the ER (Bunt et al., 2011; Norman and Moerman, 2000; Rautavuoma et al., 2004; Schneider and Granato, 2006; Sipila et al., 2007). Drosophila have only one Col IV isoform, in which the α1 and α2 chains are Collagen gene at 25C (Cg25C) and Viking (Vkg), respectively (Natzle et al., 1982; Yasothornsrikul et al., 1997). To determine whether Col IV is retained in the ER of PlodN26-5 cells, we used a GFP protein trap in the vkg locus (Vkg-GFP) (Buszczak et al., 2007). When wild-type follicle cells are viewed along their apical-basal axes, Vkg-GFP is typically only visible within the BM (Figure 1E). As expected, PlodN26-5 follicle cells show intracellular Vkg-GFP accumulation, as well as reduced Vkg-GFP in the BM. However, we were surprised to find that the intracellular Col IV is always located near the basal surface, while the distribution of KDEL ER-retention signal and the ER membrane dye ER-tracker both reveal that this organelle extends throughout the cytoplasm (Figures 1E, 1F, S1C and S1D). By performing transmission electron microscopy (TEM) on follicle cells expressing Plod-RNAi, we found that the ER structure is normal except for unusually distended basal ER cisternae where the Col IV is presumably trapped (Figure 1G). These data indicate that Col IV accumulates in a basal ER compartment in Plod-depleted cells.
BM proteins are locally synthesized in a basal ER compartment
The pattern of Col IV accumulation in PlodN26-5 cells led us to hypothesize that Col IV may be synthesized near the basal surface. Indeed, approximately 70% of Cg25C and vkg mRNAs localize to the basal half of the cytoplasm, making it likely that the Col IV α-chains are translated directly into the basal ER (Figures 2A, 2B and S2A). Col IV protein is also basally enriched in wild-type cells (Figure S2B and S2C). Plod and Prolyl-4-hydroxylase-alpha EFB (PH4αEFB) enzymes modify Col IV α-chains as they are translocated across the ER membrane (Myllyharju and Kivirikko, 2004). Significantly, the mRNAs for these proteins show a similar localization (Figure 2C, 2D and S2A). Moreover, PH4αEFB-RNAi causes Col IV to become trapped in the basal ER, similar to loss of Plod (Figure 2E and S2D). Basal enrichment is not a general property of follicle cell mRNAs, as we and others have also identified ER-associated transcripts that localize to the apical cytoplasm (Horne-Badovinac and Bilder, 2008; Konsolaki and Schupbach, 1998; Li et al., 2008). Together, these data indicate that Col IV is predominantly synthesized in a basal ER compartment in wild-type follicle cells.
Figure 2. Col IV is synthesized in the basal ER.
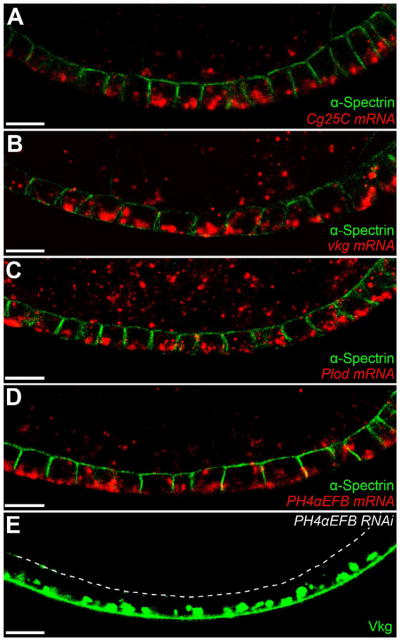
(A–E) mRNAs encoding the Col IV α-chains (A) Cg25C and (B) Vkg, and the Col IV biosynthetic enzymes (C) Plod and (D) PH4αEFB are all enriched in the basal cytoplasm. α-Spectrin marks the apical and lateral epithelial surfaces. (E) PH4αEFB-RNAi causes Vkg-GFP to accumulate in the basal ER. The dashed line marks the apical surface. Experiments performed at stages 7–8. Scale bars are 10 μm. See also Figure S2.
Col IV production in the basal ER raises the possibility that local synthesis promotes polarized secretion to the BM. Unlike mammalian cells in which the Golgi often forms a singular organelle called the Golgi ribbon, Drosophila cells have a distributed system in which small Golgi clusters are found throughout the cytoplasm. Each cluster is associated with a transitional ER (tER) site, from which newly synthesized proteins exit the ER. This organization of the early secretory pathway makes it possible for proteins synthesized in a sub-region of the ER to be dispatched through the tER-Golgi units closest to the targeted plasma membrane (Kondylis et al., 2009; Kondylis and Rabouille, 2009).
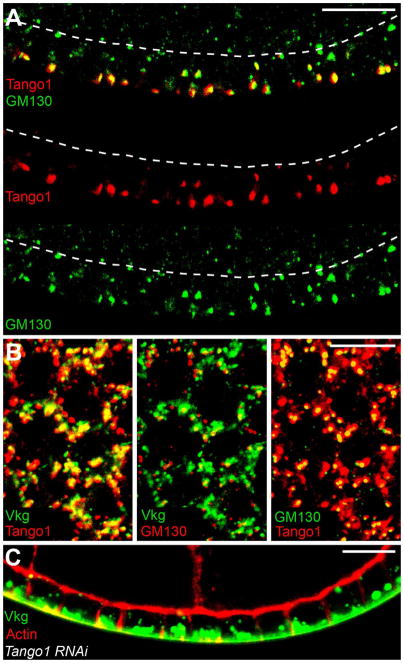
To determine whether Col IV exits the ER from the basal region where it is synthesized, we investigated the function of the Tango1 cargo receptor, which helps package Col IV into enlarged Golgi-bound vesicles (Saito et al., 2009; Wilson et al., 2011). For these studies, we raised an antibody against Tango1 and validated its specificity (Figure S3A). This protein localizes to approximately 90% of tER-Golgi units, as shown by co-localization with the CopII-associated GTPase Sar1 and the cis-Golgi marker GM130 (Figures 3A, S3B and S3C). Although tER-Golgi units are evenly distributed along the apical-basal axis, the Tango1 signal is much stronger at basal tER sites (Figures 3A, S3B and S3D). Significantly, Vkg-GFP also accumulates at the basal Tango1-positive tER sites in wild-type cells (Figure 3B), and Tango1-RNAi causes most Vkg-GFP to become trapped in the basal ER (Figure 3C and S3E). It therefore appears that most Col IV exits the ER though basally-localized tER-Golgi units.
Figure 3. Tango1 mediates Col IV ER exit predominantly at basal tER sites.
(A) Tango1 is strongly enriched at basal tER-Golgi units, as indicated by the cis-Golgi marker, GM130. Dashed lines mark the apical surface. (B) An optical section through the basal cytoplasm showing co-localization between Tango1, GM130 and Vkg-GFP. (C) Tango1-RNAi causes Vkg-GFP to accumulate in the basal ER. Experiments performed at stages 7–8. Scale bars are 10 μm. See also Figure S3.
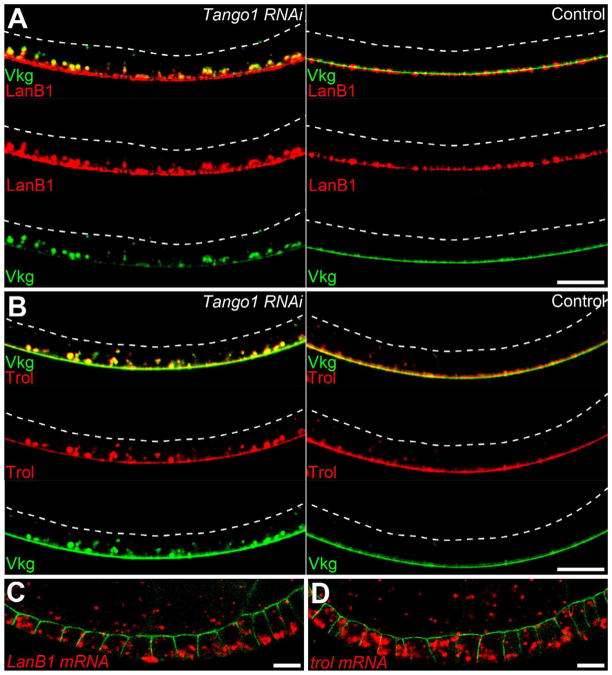
We next asked whether other BM proteins are also synthesized in the basal ER. Quantification of intracellular Laminin and Perlecan (Trol in Drosophila) levels revealed that these proteins are also enriched in the basal cytoplasm (Figures S4A–S4D). Moreover, Tango1-RNAi causes both proteins to accumulate in the basal ER with Col IV (Figures 4A and 4B). To investigate whether these proteins are translated directly into the basal ER, we again examined mRNA localization. Similar to the Col IV-encoding mRNAs, Laminin B1 transcripts are enriched in the basal cytoplasm (Figures 4C and S4E). Conversely, trol transcripts show no basal bias (Figure 4D and S4E), suggesting that this protein’s enrichment in the basal ER occurs by a separate mechanism. These data suggest that Laminin is also synthesized in the basal ER, and that other BM proteins may exit with Col IV through Tango1-positive tER sites.
Figure 4. Laminin and Trol are also enriched in the basal cytoplasm.
(A and B) Tango1-RNAi causes (A) LanB1 and (B) Trol to accumulate in the basal ER with Vkg-GFP. Dashed lines mark the apical surface. (C) LanB1 mRNA is basally enriched. (D) trol mRNA does not show a basal bias. (C and D) α-Spectrin marks the apical and lateral epithelial surfaces. Experiments performed at stages 7–8. Scale bars are 10 μm. See also Figure S4.
Crag regulates Rab10 to promote polarized BM secretion
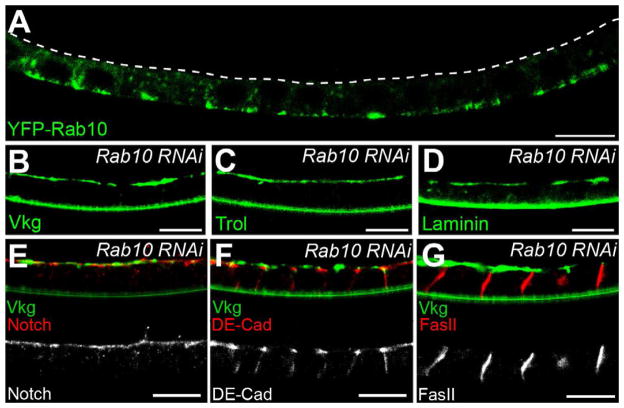
If BM proteins predominantly traffic through basal tER-Golgi units, how then do they reach the basal cell surface? Rab proteins are small GTPases that function as master regulators of intracellular membrane traffic (Hutagalung and Novick, 2011). Using a transgenic collection of YFP-tagged Rabs (Zhang et al., 2007), we found that Rab10 has an intriguing localization pattern. In addition to general cytoplasmic staining, there is a strong YFP-Rab10 signal near the basal follicle cell surface (Figures 5A and S5A). Significantly, Rab10-RNAi causes large BM proteins like Col IV, Perlecan and Laminin to accumulate on both the basal and apical surfaces (Figures 5B–5D); this phenotype is seen to a lesser extent with the smaller BM protein Nidogen (Figure S5B). In contrast, Rab10-RNAi has no effect on the localization of 4 other proteins that normally undergo polarized trafficking in the follicle cells (Figures 5E–5G and S5C). The basal enrichment of BM-encoding mRNAs and Tango1 is also normal under these conditions (Figures S5D–S5F). Thus, Rab10 is required downstream of the basal bias in BM synthesis to ensure that BM proteins are delivered exclusively to the basal cell surface.
Figure 5. Rab10 promotes polarized BM secretion.
(A) YFP-Rab10 is enriched near the basal follicle cell surface. The dashed line marks the apical surface. (B–D) Rab10-RNAi mis-targets (B) Col IV (Vkg-GFP), (C) Perlecan (Trol-GFP) and (D) Laminin to the apical surface. (E-G) Rab10-RNAi does not affect the localization of (E) apical (Notch), (F) junctional (DE-Cadherin), or (G) lateral (FasII) trans-membrane proteins. Rab10 knockdown is shown by apical Vkg-GFP. Experiments performed at stages 7–8, except (C and D), which are stage 9. Scale bars are 10 μm. See also Figure S5.
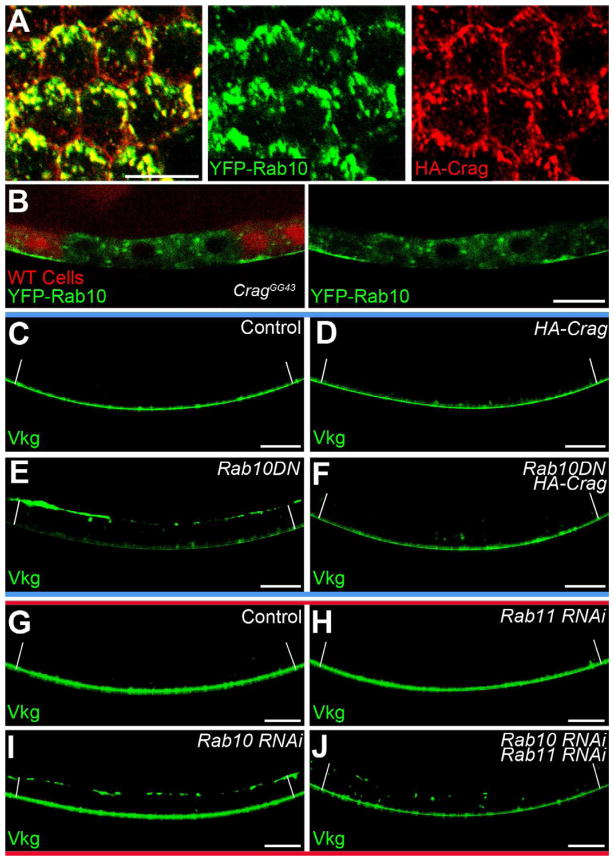
The apical accumulation of BM proteins in Rab10-depleted cells is remarkably similar to the phenotype first described for Crag (Denef et al., 2008). We therefore investigated the relationship between these two proteins. Although Crag is broadly distributed along the follicle cell apical-basal axis (Denef et al., 2008), an optical section near the basal surface revealed that Crag and Rab10 co-localize in this region (Figures 6A and S6A). Moreover, in CragGG43 follicle cell clones, the strong YFP-Rab10 signal largely disappears from the basal surface, and is redistributed to the apical cytoplasm (Figures 6B and S6B). Rab10 mRNA shows a 60% basal enrichment, which is unchanged under Crag-RNAi (Figure S6C). It therefore appears that Crag is required for the post-translational targeting of Rab10 to structures near the basal cell surface.
Figure 6. Crag regulates Rab10 during polarized BM secretion.
(A) Optical section near the basal surface showing YFP-Rab10 and HA-Crag co-localization. (B) A CragGG43 follicle cell clone shows a redistribution of YFP-Rab10 away from the basal surface. (C–F) Interaction between UAS-HA-Crag and UAS-Rab10.T23N. (C–D) Vkg-GFP localization is normal in (C) wild-type and (D) UAS-HA-Crag follicle cells. (E) A UAS-Rab10.T23N dominant negative transgene causes Vkg-GFP to accumulate on the apical surface. (F) Co-expression of UAS-HA-Crag with UAS-Rab10.T23N suppresses this phenotype. (E–F) A YFP on Rab10.T23N contributes to the fluorescent signal in the cytoplasm. (G–J) Interaction between Rab10-RNAi and Rab11-RNAi. (G and H) Vkg-GFP localization is normal in (G) wild-type and (H) Rab11-depleted cells. (I) Rab10 depletion causes Vkg-GFP to accumulate on the apical surface. (J) Co-depletion of Rab10 and Rab11 eliminates the apical Vkg-GFP and increases the cytoplasmic signal. (C–J) White lines extend between the apical and basal epithelial surfaces. Experiments performed at stages 7–8. Scale bars are 10 μm. See also Figure S6.
Recently, Crag’s mammalian homologs have been shown to function as highly specific Rab10 guanine nucleotide exchange factors (GEFs) in vitro (Yoshimura et al., 2010). Rab proteins with mutations that block GTP binding are thought to act as dominant negatives, in part, by sequestering GEFs (Li and Stahl, 1993; Stenmark et al., 1994). If Crag is a Rab10 GEF in this system, over-expressing Crag together with a Rab10.T23N dominant negative transgene may suppress the dominant negative phenotype (Zhu et al., 2007). Similar to Rab10-RNAi, expression of UAS-Rab10.T23N causes Vkg-GFP to accumulate on the apical surface (Figure 6E). Importantly, co-expressing UAS-HA-Crag with UAS-Rab10.T23N rescues this defect (Figures 6C–6F). We have also confirmed that Crag and Rab10 physically interact through co-immunoprecipitation experiments (Figure S6D). Combined with the mammalian data, these results argue that Crag functions as a Rab10 GEF to promote polarized BM secretion.
Denn domain proteins can bind multiple Rabs through interactions both within and outside their GEF domain. Crag has been previously shown to co-localize with Rab5 and Rab11 in the follicle cells (Denef et al., 2008). Rab5 depletion has no effect on polarized BM secretion (Figure S6E). Interestingly, however, we did observe Rab11 punctae interspersed with the Crag and Rab10 signals in the basal cytoplasm (Figure S6F). To investigate whether Rab11 regulates BM secretion, we followed Vkg-GFP localization under a series of RNAi conditions. Unlike the situation with Rab10-RNAi (Figures 5B and 6I), the Vkg-GFP localization in Rab11-depleted cells is similar to wild-type controls (Figures 6G and 6H). However, when we simultaneously depleted Rab10 and Rab11, Vkg-GFP was no longer found on the apical surface, but often showed a punctate intracellular distribution, in addition to its BM localization (Figure 6J). This phenotype was also observed with simultaneous depletion of Crag and Rab11 (Figures S6G–S6J). We conclude that Rab11 does not regulate BM secretion under normal conditions, but that in the absence of Crag or Rab10, BM proteins are inappropriately targeted to the apical surface through a Rab11-dependent mechanism.
Planar polarization of the BM exocytic machinery during egg chamber elongation
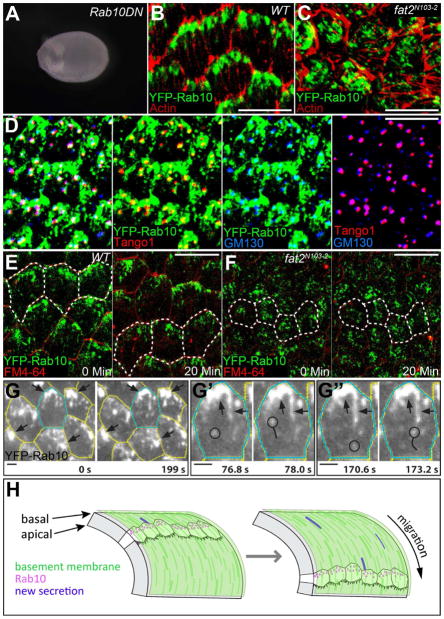
All experiments described above were performed during oogenic stages 6–8, which is the peak period of Col IV production in the egg chamber (Haigo and Bilder, 2011). Significantly, this is also the time when planar polarization of the follicle cell epithelium first drives egg chamber elongation. We noticed that Rab10.T23N expression in the follicle cells produces round eggs (Figure 7A). Moreover, YFP-Rab10 shows a striking planar polarization at the basal epithelial surface, when viewed at the level of the basal actin filaments (Figure 7B). Tango1, GM130 and the Translocon protein Sec61α are also planar polarized (Figures 7D, S7A and S7B). Consistent with Rab10’s role in mediating late stages of protein traffic to the plasma membrane (Cao et al., 2008; Chen et al., 2012; Sano et al., 2007; Schuck et al., 2007), most of the YFP-Rab10 signal lies between the Tango1-positive tER-Golgi units and a basal region of the lateral plasma membrane (Compare Figure 7D with 7B and 7E). Rab10 also partially overlaps with the tER-Golgi units (Figure 7D), but is not required for their planar polarization (Figure S7B). This unexpected positioning of the BM exocytic machinery suggests that BM deposition may be polarized along both the apical-basal and planar axes during egg chamber elongation.
Figure 7. The BM exocytic machinery is planar polarized at the basal epithelial surface.
(A) Rab10.T23N expression produces round eggs. (B) YFP-Rab10 is planar polarized at the level of the basal actin filaments. (C) YFP-Rab10’s planar polarization is lost in fat2N103-2 epithelia. (D) Tango1 and GM130 are also planar polarized, and partially overlap with YFP-Rab10. (E) Live imaging reveals that YFP-Rab10 is enriched at the trailing edge of each migrating cell. (F) fat2N103–2 epithelia fail to migrate and YFP-Rab10 is mis-polarized. (B–F) Scale bars are 10 μm. (E and F) FM4–64 marks cell membranes. Dashed lines mark the same 3–4 cells at each time point. (G) TIRF microscopy reveals that larger Rab10-positive structures are largely immobile (arrows), while smaller Rab10-positive structures (circles in G′ and G″) move rapidly through the cytoplasm. Scale bars are 2 μm. (H) Speculative model for how planar polarization of BM secretion would synergize with follicle cell migration to create the unusual BM fibrils associated with egg chamber elongation. Illustration adapted from (Bilder and Haigo, 2012). Experiments performed at stages 7–8. See also Figure S7 and Movies S1–S3.
How does Rab10 become localized along the planar axis? The atypical cadherin Fat2 is a key regulator of follicle cell planar polarity (Viktorinova et al., 2009). In fat2N103-2 epithelia, YFP-Rab10 is polarized normally along the apical-basal axis, but is mis-localized within the epithelial plane (Figures 7C, S7C and S7D). This effect is post-translational, as Rab10 mRNA is not polarized along the planar axis (Figure S7E). Interestingly, this pattern is the opposite of Crag loss of function, where Rab10’s apical-basal polarization is disrupted, but the protein still present at the basal surface is planar polarized (Figures 6B, S6B, and S7F). Thus, Rab10’s polarization along the apical-basal and planar axes is controlled by two different mechanisms, one requiring Crag and the other requiring Fat2.
Follicle cell planar polarity is intimately linked with a directed epithelial migration that creates fibril-like structures in the BM. Live imaging of wild-type epithelia revealed that the intense YFP-Rab10 signal corresponds to the trailing edge of each migrating cell (Figure 7E and Movie S1). Conversely, fat2N103-2 epithelia do not migrate, and BM structure is highly disrupted (Figures 7F and S7G, and Movie S2). Near-TIRF imaging (Konopka and Bednarek, 2008; Tokunaga et al., 2008) of wild-type cells at higher temporal resolution shows that the large, Rab10-positive structures at the trailing edge are surprisingly stable (on the order of minutes), while smaller Rab10-positive structures move at speeds of up to 1 μm/second though the basal cytoplasm (Figure 7G and Movie S3). Although we do not yet know the identity of these Rab10-positive compartments, these observations suggest that newly synthesized BM proteins are secreted from the trailing edge of each follicle cell as it migrates, which may have important implications for BM remodeling during elongation morphogenesis.
Discussion
The BM is essential for epithelial structure and morphogenesis; yet little is known about how the component proteins are secreted to the basal surface, or how this process is regulated during development. Here, we have shown that the synthesis and trafficking of BM proteins is largely restricted to the basal cytoplasm in the Drosophila follicle cells. We have also identified Rab10 as a key regulator of polarized BM secretion, and shown that Crag likely functions as a Rab10 GEF. Surprisingly, the BM exocytic machinery shows a striking planar polarity at the basal epithelial surface, specifically during the time when the BM is being remodeled to promote egg chamber elongation. We therefore propose that spatial control of BM production along two tissue axes may promote exocytic efficiency, BM remodeling and organ morphogenesis.
The polarized deposition of the two major BM components, Col IV and Laminin, appears to begin with the targeting of their transcripts to the basal cytoplasm. For mRNA localization to enhance polarized membrane traffic requires that the cell have distributed tER-Golgi units, as opposed to a single Golgi ribbon (Kondylis et al., 2009; Kondylis and Rabouille, 2009). With this modular organization, localized mRNAs can be translated into a sub-region of the ER, and newly synthesized proteins quickly dispatched through the tER-Golgi units closest to the target cell surface. Elegant studies in Drosophila have documented the use of this strategy for the polarized secretion of the TGFα homolog Gurken in the oocyte, and for the apical secretion of Wingless in the embryo (Herpers and Rabouille, 2004; Simmonds et al., 2001). Moreover, the targeting of mRNAs encoding secreted and trans-membrane proteins to neuronal dendrites containing Golgi outposts (structures analogous to tER-Golgi units) suggests that this distributed exocytic program is conserved in at least some vertebrate cells (Ramirez and Couve, 2011). We propose that the basal targeting of the Col IV- and Laminin-encoding mRNAs may similarly enhance the delivery of these proteins to the basal surface.
The local synthesis of BM components may also address several challenges that these complex proteins pose to the ER. For example, Col IV requires dedicated machinery to mediate both trimer formation and ER exit. Concentrating these functions into a smaller ER region is therefore likely to increase Col IV biosynthetic efficiency. We have shown that the mRNAs encoding Plod and PH4αEFB are also enriched in the basal cytoplasm, and that loss of either protein causes Col IV to accumulate in the basal ER. Tango1 function is also higher at basal ER exit sites. Interestingly, the ECM component Aggrecan is synthesized in a restricted ER region in avian chondrocytes (Vertel et al., 1989). Thus, ER compartmentalization may be a conserved strategy for ECM biosynthesis.
While local synthesis likely facilitates polarized BM secretion, additional downstream mechanisms are required to restrict protein delivery to the basal surface. The first evidence for this regulation came from Denef et al. (2008), who showed that loss of Crag causes BM proteins to accumulate on both the basal and apical follicle cell surfaces. The presence of the DENN domain made it likely that Crag was a direct regulator of polarized membrane traffic. Consistent with this notion, Crag localized to Rab5- and Rab11-positive endosomes, and along the apical and lateral plasma membranes (Denef et al., 2008). However, the diversity of the protein’s distribution made it difficult to pinpoint Crag’s function in polarized BM secretion.
Here, we have identified Rab10 as a second critical component in this system. Importantly, this protein shows a more restricted localization, with the strongest Rab10 signal being tightly associated with the basal cell surface. Crag co-localizes with Rab10 in this region and is required for Rab10’s basal enrichment. Moreover, Rab10 interacts with Crag both genetically and physically, and Rab10 depletion phenocopies Crag’s BM defect. Given that Crag’s mammalian homologs function as Rab10 GEFs in vitro (Yoshimura et al., 2010), we now propose that Crag functions as a Rab10 GEF in vivo to control polarized BM secretion.
It is likely, however, that Crag also has Rab10-independent functions. For instance, Crag mutant cells show defects in apical-basal polarity (Denef et al., 2008), while Rab10-depleted cells do not (data not shown). This phenotypic difference could be due to incomplete Rab10 knockdown, but it is also consistent with Crag regulating more than one trafficking pathway. DENN domain proteins can bind multiple Rabs through interactions outside their GEF domain (Marat et al., 2011). Since Crag also co-localizes with Rab5 and Rab11, both of which are required for follicle cell apical-basal polarity (Lu and Bilder, 2005; Xu et al., 2011), Crag may play a second role with these proteins controlling epithelial architecture.
How then does Rab10 control polarized BM secretion? We have shown that Rab10 has a complex distribution in the basal cytoplasm, where it associates both with Tango1-positive tER-Golgi units and the plasma membrane. Rab10 is a known exocytic regulator in multiple organisms and cell types; however, the exact compartment(s) to which it localizes vary with cargo and cell polarization state (Babbey et al., 2006; Babbey et al., 2010; Chen et al., 2006; Sano et al., 2007; Schuck et al., 2007; Wang et al., 2011). A recent proteomic analysis in MDCK cells found Rab10 on exocytic vesicles bound for a basal region of the lateral plasma membrane (Cao et al., 2008). We propose that Rab10 may localize to similarly targeted exocytic vesicles in the follicle cells. Interestingly, Rab10 is not absolutely required for basal secretion, as a significant fraction of BM proteins do reach the basal surface when Rab10 is depleted. However, it is required to stop BM proteins from taking an alternate, Rab11-dependent route to the apical surface. Thus, local synthesis may provide sufficient information for the basal targeting of some BM vesicles, but Rab10 is required to ensure robust polarized secretion.
In addition to identifying mechanisms directing BM secretion to the basal surface, this work also provides a strong foundation for future studies of BM remodeling during egg chamber elongation. We have shown that the BM exocytic machinery is enriched at the trailing edge of each migrating follicle cell in a Fat2-dependent manner, a surprising observation given that many migrating cells orient their Golgi toward the front (Sutterlin and Colanzi, 2010; Yadav and Linstedt, 2011). It has been proposed, however, that the entire purpose of follicle cell migration is to create the unusual fibril-like structures in the BM that promote egg chamber elongation (Haigo and Bilder, 2011). We have not yet identified the exact location where BM proteins exit the cell; however, Rab10’s known function as a late exocytic regulator evokes the following model. We envision that newly synthesized BM proteins are quickly transported from basal tER-Golgi units to the trailing plasma membrane through a Rab10-dependent mechanism. Planar polarized secretion then synergizes with cell movement to produce the fibril-like structures in the BM (Figure 7H). Whether Rab10 directly regulates planar secretion is not yet clear, as the BM material that reaches the basal surface under Rab10 depletion still shows the fibril-like morphology (data not shown). However, incomplete depletion and/or partial redundancy with closely related Rabs may obscure this aspect of Rab10’s function (Schuck et al., 2007; Shi et al., 2010). The follicle cells thus provide a powerful system to investigate the dynamic regulation of BM secretion and remodeling during organ morphogenesis.
Experimental Procedures
Drosophila genetics
Follicle cell clones were generated using e22c-Gal4 to drive expression of the FLP recombinase. UAS transgenes were expressed with TubP-Gal4 or traffic jam-Gal4 (tj-Gal4) for full follicle cell expression, or hs-FLP; Act5c≫Gal4 for clones. Most lines are from the Bloomington Drosophila stock center, with exceptions listed below. RNAi lines for Plod, Tango1 and Rab5 are from the Vienna Drosophila RNAi Center. Rab11-RNAi is from (Satoh et al., 2005). tj-Gal4 (NP1624) is from the Drosophila Genetic Resource Center, Kyoto. vkg-GFP (CC00791), trol-GFP (CA06698), Sar1-GFP (CA07674) and Sec61α-GFP (CC00735) are from (Buszczak et al., 2007). CragGG43, FRT19A and UAS-HA-Crag are from (Denef et al., 2008). fat2N103-2, FRT80 is from (Horne-Badovinac et al., 2012). w1118 typically served as a wild-type control. Most crosses were raised, and experiments performed at 25°C. Full genotypes for each experiment and unusual temperature conditions can be found in Supplemental Experimental Procedures.
Fluorescent in situ hybridization
For probe synthesis, a single coding exon was PCR-amplified from w1118 genomic DNA, using a reverse primer with the T7 promoter sequence (see Supplemental Experimental Procedures). The PCR products were purified by Qiaquick gel purification (Qiagen) and antisense probes synthesized using a DIG RNA labeling kit (Roche). For trol mRNA, we used a probe against GFP on tissue expressing the Trol-GFP protein trap. The antisense eGFP probe was synthesized from the pBS-eGFPA vector, by linearizing with KpnI and transcribing with the T7 polymerase. Probes were detected using an HRP-conjugated sheep anti-DIG antibody (1:1000, Roche), followed by TSA Cyanine 3 staining (Perkin Elmer). Ovaries were then stained with mouse anti-α-Spectrin (1:50, DSHB, concentrate). Samples were mounted in SlowFade Antifade (Invitrogen). Images were obtained using a Zeiss LSM 510 confocal microscope, and processed with Photoshop.
Immuno-fluorescence and Staining
Ovaries were dissected in S2 medium and fixed for 15 minutes in 4% EM-grade formaldehyde (Polysciences). Antibody stains were performed in PBS with 0.1% Triton, and detected using AlexaFluor-conjugated secondary antibodies (1:200, Invitrogen). TRITC-Phalloidin (1:200, Sigma) or AlexaFluor-647 Phalloidin (1:50, Invitrogen) labeled cell cortices. ER-Tracker Red (Invitrogen) was diluted in PBS with 4% EM grade formaldehyde (Polysciences) and the staining reaction was incubated at 37°C for 20 minutes. Egg chambers were mounted in SlowFade Antifade (Invitrogen) and visualized using a Zeiss LSM 510 confocal microscope. Commercial antibodies include: mouse α-KDEL (10C3, 1:25, Calbiochem), rabbit α-GM130 (1:200, Abcam), rabbit α-HA (1:200, Rockland), mouse α-Rab11 (1:20, BD Biosciences), and rabbit α-LanB1 (1:100, Abcam). DSHB Antibodies include: mouse α-Notch (C458.2H,1:20), rat α-DE-Cadherin (DCAD2, 1:20), mouse α-FasII (1D4, 1:10), and mouse α-FasIII (7G10, 1:10). Rabbit α-Laminin (1:100), rabbit α-Pcan (1:1000) and rabbit α-Nidogen (1:1000) are from (Fessler et al., 1987; Friedrich et al., 2000; Wolfstetter et al., 2009). Guinea pig α-Tango1 (1:200), this study.
Live imaging
Live imaging of follicle cell migration was performed as described (Prasad et al., 2007), with the following modifications. Dissected egg chambers were placed onto a pad of 0.4% NuSieve GTG low melt agarose (Lonza) in live imaging medium, and follicle cell membranes were marked with 6.6 μM FM4-64FX (Invitrogen). The cover slip was cushioned with vacuum grease at each corner, and then sealed with halocarbon oil 27 (Sigma). Confocal movies were taken on a Zeiss LSM 510 microscope, and processed using LSM image browser. TIRF movies were taken on an Olympus IX-50 microscope equipped with an iXon EMCCD camera (Andor) and a 100x objective fitted with through-the-objective TIRF illumination, and processed using ImageJ.
Supplementary Material
Highlights.
Basement membrane (BM) proteins are made in and exit from a basal ER compartment
Crag promotes Rab10 localization to structures near the basal cell surface
Rab10 restricts the delivery of BM proteins to the basal cell surface
Planar polarization of exocytic machinery may control BM structure and organ shape
Acknowledgments
Trudi Schüpbach, Lisa Fessler, Stefan Baumgartner and Anne Holz generously provided reagents. We are grateful to Amanda Neisch for help with antibody production, Pam Vanderzalm for advice on Co-IPs, Guillermina Ramirez-San Juan for MATLAB assistance, and Nick Badovinac for illustrations. We also thank Chip Ferguson, Ben Glick, Lucy O’Brien and members of the S.H-B. lab for comments on the manuscript. This work was supported by NSF predoctoral fellowships to D.W.L. and A.J.I., and a Basil O’Connor Starter Scholar Award (5-FY10-50) from the March of Dimes and NIH grant (R01GM094276) to S.H-B.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arnaoutova I, George J, Kleinman HK, Benton G. Basement membrane matrix (BME) has multiple uses with stem cells. Stem Cell Rev. 2012;8:163–169. doi: 10.1007/s12015-011-9278-y. [DOI] [PubMed] [Google Scholar]
- Babbey CM, Ahktar N, Wang E, Chen CC, Grant BD, Dunn KW. Rab10 regulates membrane transport through early endosomes of polarized Madin-Darby canine kidney cells. Mol Biol Cell. 2006;17:3156–3175. doi: 10.1091/mbc.E05-08-0799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babbey CM, Bacallao RL, Dunn KW. Rab10 associates with primary cilia and the exocyst complex in renal epithelial cells. Am J Physiol Renal Physiol. 2010;299:F495–506. doi: 10.1152/ajprenal.00198.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilder D, Haigo SL. Expanding the morphogenetic repertoire: perspectives from the Drosophila egg. Dev Cell. 2012;22:12–23. doi: 10.1016/j.devcel.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boll W, Partin JS, Katz AI, Caplan MJ, Jamieson JD. Distinct pathways for basolateral targeting of membrane and secretory proteins in polarized epithelial cells. Proc Natl Acad Sci U S A. 1991;88:8592–8596. doi: 10.1073/pnas.88.19.8592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunt S, Denholm B, Skaer H. Characterisation of the Drosophila procollagen lysyl hydroxylase, dPlod. Gene Expr Patterns. 2011;11:72–78. doi: 10.1016/j.gep.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunt S, Hooley C, Hu N, Scahill C, Weavers H, Skaer H. Hemocyte-secreted type IV collagen enhances BMP signaling to guide renal tubule morphogenesis in Drosophila. Dev Cell. 2010;19:296–306. doi: 10.1016/j.devcel.2010.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buszczak M, Paterno S, Lighthouse D, Bachman J, Planck J, Owen S, Skora AD, Nystul TG, Ohlstein B, Allen A, et al. The carnegie protein trap library: a versatile tool for Drosophila developmental studies. Genetics. 2007;175:1505–1531. doi: 10.1534/genetics.106.065961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Z, Li C, Higginbotham JN, Franklin JL, Tabb DL, Graves-Deal R, Hill S, Cheek K, Jerome WG, Lapierre LA, et al. Use of fluorescence-activated vesicle sorting for isolation of Naked2-associated, basolaterally targeted exocytic vesicles for proteomics analysis. Mol Cell Proteomics. 2008;7:1651–1667. doi: 10.1074/mcp.M700155-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan MJ, Stow JL, Newman AP, Madri J, Anderson HC, Farquhar MG, Palade GE, Jamieson JD. Dependence on pH of polarized sorting of secreted proteins. Nature. 1987;329:632–635. doi: 10.1038/329632a0. [DOI] [PubMed] [Google Scholar]
- Chen CC, Schweinsberg PJ, Vashist S, Mareiniss DP, Lambie EJ, Grant BD. RAB-10 is required for endocytic recycling in the Caenorhabditis elegans intestine. Mol Biol Cell. 2006;17:1286–1297. doi: 10.1091/mbc.E05-08-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Wang Y, Zhang J, Deng Y, Jiang L, Song E, Wu XS, Hammer JA, Xu T, Lippincott-Schwartz J. Rab10 and myosin-Va mediate insulin-stimulated GLUT4 storage vesicle translocation in adipocytes. J Cell Biol. 2012;198:545–560. doi: 10.1083/jcb.201111091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen D, Musch A, Rodriguez-Boulan E. Selective control of basolateral membrane protein polarity by cdc42. Traffic. 2001;2:556–564. doi: 10.1034/j.1600-0854.2001.20805.x. [DOI] [PubMed] [Google Scholar]
- De Almeida JB, Stow JL. Disruption of microtubules alters polarity of basement membrane proteoglycan secretion in epithelial cells. Am J Physiol. 1991;261:C691–700. doi: 10.1152/ajpcell.1991.261.1.C691. [DOI] [PubMed] [Google Scholar]
- Denef N, Chen Y, Weeks SD, Barcelo G, Schupbach T. Crag regulates epithelial architecture and polarized deposition of basement membrane proteins in Drosophila. Dev Cell. 2008;14:354–364. doi: 10.1016/j.devcel.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastburn DJ, Mostov KE. Laying the foundation for epithelia: insights into polarized basement membrane deposition. EMBO Rep. 2010;11:329–330. doi: 10.1038/embor.2010.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fata JE, Werb Z, Bissell MJ. Regulation of mammary gland branching morphogenesis by the extracellular matrix and its remodeling enzymes. Breast Cancer Res. 2004;6:1–11. doi: 10.1186/bcr634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fessler LI, Campbell AG, Duncan KG, Fessler JH. Drosophila laminin: characterization and localization. J Cell Biol. 1987;105:2383–2391. doi: 10.1083/jcb.105.5.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich MV, Schneider M, Timpl R, Baumgartner S. Perlecan domain V of Drosophila melanogaster. Sequence, recombinant analysis and tissue expression. Eur J Biochem. 2000;267:3149–3159. doi: 10.1046/j.1432-1327.2000.01337.x. [DOI] [PubMed] [Google Scholar]
- Fromme JC, Schekman R. COPII-coated vesicles: flexible enough for large cargo? Curr Opin Cell Biol. 2005;17:345–352. doi: 10.1016/j.ceb.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Gutzeit HO. The microfilament pattern in the somatic follicle cells of mid-vitellogenic ovarian follicles of Drosophila. Eur J Cell Biol. 1990;53:349–356. [PubMed] [Google Scholar]
- Gutzeit HO, Eberhardt W, Gratwohl E. Laminin and basement membrane-associated microfilaments in wild-type and mutant Drosophila ovarian follicles. J Cell Sci. 1991;100(Pt 4):781–788. doi: 10.1242/jcs.100.4.781. [DOI] [PubMed] [Google Scholar]
- Haigo SL, Bilder D. Global tissue revolutions in a morphogenetic movement controlling elongation. Science. 2011;331:1071–1074. doi: 10.1126/science.1199424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herpers B, Rabouille C. mRNA localization and ER-based protein sorting mechanisms dictate the use of transitional endoplasmic reticulum-golgi units involved in gurken transport in Drosophila oocytes. Mol Biol Cell. 2004;15:5306–5317. doi: 10.1091/mbc.E04-05-0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne-Badovinac S, Bilder D. Dynein regulates epithelial polarity and the apical localization of stardust A mRNA. PLoS Genet. 2008;4:e8. doi: 10.1371/journal.pgen.0040008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne-Badovinac S, Hill J, Gerlach G, 2nd, Menegas W, Bilder D. A screen for round egg mutants in Drosophila identifies tricornered, furry, and misshapen as regulators of egg chamber elongation. G3 (Bethesda) 2012;2:371–378. doi: 10.1534/g3.111.001677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutagalung AH, Novick PJ. Role of Rab GTPases in membrane traffic and cell physiology. Physiol Rev. 2011;91:119–149. doi: 10.1152/physrev.00059.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin L, Pahuja KB, Wickliffe KE, Gorur A, Baumgartel C, Schekman R, Rape M. Ubiquitin-dependent regulation of COPII coat size and function. Nature. 2012;482:495–500. doi: 10.1038/nature10822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalluri R. Basement membranes: structure, assembly and role in tumour angiogenesis. Nat Rev Cancer. 2003;3:422–433. doi: 10.1038/nrc1094. [DOI] [PubMed] [Google Scholar]
- Khoshnoodi J, Pedchenko V, Hudson BG. Mammalian collagen IV. Microsc Res Tech. 2008;71:357–370. doi: 10.1002/jemt.20564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondylis V, Pizette S, Rabouille C. The early secretory pathway in development: a tale of proteins and mRNAs. Semin Cell Dev Biol. 2009;20:817–827. doi: 10.1016/j.semcdb.2009.03.012. [DOI] [PubMed] [Google Scholar]
- Kondylis V, Rabouille C. The Golgi apparatus: lessons from Drosophila. FEBS Lett. 2009;583:3827–3838. doi: 10.1016/j.febslet.2009.09.048. [DOI] [PubMed] [Google Scholar]
- Konopka CA, Bednarek SY. Variable-angle epifluorescence microscopy: a new way to look at protein dynamics in the plant cell cortex. Plant J. 2008;53:186–196. doi: 10.1111/j.1365-313X.2007.03306.x. [DOI] [PubMed] [Google Scholar]
- Konsolaki M, Schupbach T. windbeutel, a gene required for dorsoventral patterning in Drosophila, encodes a protein that has homologies to vertebrate proteins of the endoplasmic reticulum. Genes Dev. 1998;12:120–131. doi: 10.1101/gad.12.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Stahl PD. Structure-function relationship of the small GTPase rab5. J Biol Chem. 1993;268:24475–24480. [PubMed] [Google Scholar]
- Li Z, Wang L, Hays TS, Cai Y. Dynein-mediated apical localization of crumbs transcripts is required for Crumbs activity in epithelial polarity. J Cell Biol. 2008;180:31–38. doi: 10.1083/jcb.200707007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Bilder D. Endocytic control of epithelial polarity and proliferation in Drosophila. Nat Cell Biol. 2005;7:1232–1239. doi: 10.1038/ncb1324. [DOI] [PubMed] [Google Scholar]
- Malhotra V, Erlmann P. Protein export at the ER: loading big collagens into COPII carriers. EMBO J. 2011;30:3475–3480. doi: 10.1038/emboj.2011.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marat AL, Dokainish H, McPherson PS. DENN domain proteins: regulators of Rab GTPases. J Biol Chem. 2011;286:13791–13800. doi: 10.1074/jbc.R110.217067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman I, Nelson WJ. Coordinated protein sorting, targeting and distribution in polarized cells. Nat Rev Mol Cell Biol. 2008;9:833–845. doi: 10.1038/nrm2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner JH. Glomerular basement membrane composition and the filtration barrier. Pediatr Nephrol. 2011;26:1413–1417. doi: 10.1007/s00467-011-1785-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner JH, Yurchenco PD. Laminin functions in tissue morphogenesis. Annu Rev Cell Dev Biol. 2004;20:255–284. doi: 10.1146/annurev.cellbio.20.010403.094555. [DOI] [PubMed] [Google Scholar]
- Mirouse V, Christoforou CP, Fritsch C, St Johnston D, Ray RP. Dystroglycan and perlecan provide a basal cue required for epithelial polarity during energetic stress. Dev Cell. 2009;16:83–92. doi: 10.1016/j.devcel.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Mirre C, Cecchini JP, Le Parco Y, Knibiehler B. De novo expression of a type IV collagen gene in Drosophila embryos is restricted to mesodermal derivatives and occurs at germ band shortening. Development. 1988;102:369–376. doi: 10.1242/dev.102.2.369. [DOI] [PubMed] [Google Scholar]
- Myllyharju J, Kivirikko KI. Collagens, modifying enzymes and their mutations in humans, flies and worms. Trends Genet. 2004;20:33–43. doi: 10.1016/j.tig.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Myllyla R, Wang C, Heikkinen J, Juffer A, Lampela O, Risteli M, Ruotsalainen H, Salo A, Sipila L. Expanding the lysyl hydroxylase toolbox: new insights into the localization and activities of lysyl hydroxylase 3 (LH3) J Cell Physiol. 2007;212:323–329. doi: 10.1002/jcp.21036. [DOI] [PubMed] [Google Scholar]
- Natzle JE, Monson JM, McCarthy BJ. Cytogenetic location and expression of collagen-like genes in Drosophila. Nature. 1982;296:368–371. doi: 10.1038/296368a0. [DOI] [PubMed] [Google Scholar]
- Norman KR, Moerman DG. The let-268 locus of Caenorhabditis elegans encodes a procollagen lysyl hydroxylase that is essential for type IV collagen secretion. Dev Biol. 2000;227:690–705. doi: 10.1006/dbio.2000.9897. [DOI] [PubMed] [Google Scholar]
- O’Brien LE, Jou TS, Pollack AL, Zhang Q, Hansen SH, Yurchenco P, Mostov KE. Rac1 orientates epithelial apical polarity through effects on basolateral laminin assembly. Nat Cell Biol. 2001;3:831–838. doi: 10.1038/ncb0901-831. [DOI] [PubMed] [Google Scholar]
- Pastor-Pareja JC, Xu T. Shaping cells and organs in Drosophila by opposing roles of fat body-secreted Collagen IV and perlecan. Dev Cell. 2011;21:245–256. doi: 10.1016/j.devcel.2011.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad M, Jang AC, Starz-Gaiano M, Melani M, Montell DJ. A protocol for culturing Drosophila melanogaster stage 9 egg chambers for live imaging. Nat Protoc. 2007;2:2467–2473. doi: 10.1038/nprot.2007.363. [DOI] [PubMed] [Google Scholar]
- Ramirez OA, Couve A. The endoplasmic reticulum and protein trafficking in dendrites and axons. Trends Cell Biol. 2011;21:219–227. doi: 10.1016/j.tcb.2010.12.003. [DOI] [PubMed] [Google Scholar]
- Rautavuoma K, Takaluoma K, Sormunen R, Myllyharju J, Kivirikko KI, Soininen R. Premature aggregation of type IV collagen and early lethality in lysyl hydroxylase 3 null mice. Proc Natl Acad Sci U S A. 2004;101:14120–14125. doi: 10.1073/pnas.0404966101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Boulan E, Kreitzer G, Musch A. Organization of vesicular trafficking in epithelia. Nat Rev Mol Cell Biol. 2005;6:233–247. doi: 10.1038/nrm1593. [DOI] [PubMed] [Google Scholar]
- Saito K, Chen M, Bard F, Chen S, Zhou H, Woodley D, Polischuk R, Schekman R, Malhotra V. TANGO1 facilitates cargo loading at endoplasmic reticulum exit sites. Cell. 2009;136:891–902. doi: 10.1016/j.cell.2008.12.025. [DOI] [PubMed] [Google Scholar]
- Sano H, Eguez L, Teruel MN, Fukuda M, Chuang TD, Chavez JA, Lienhard GE, McGraw TE. Rab10, a target of the AS160 Rab GAP, is required for insulin-stimulated translocation of GLUT4 to the adipocyte plasma membrane. Cell Metab. 2007;5:293–303. doi: 10.1016/j.cmet.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Satoh AK, O’Tousa JE, Ozaki K, Ready DF. Rab11 mediates post-Golgi trafficking of rhodopsin to the photosensitive apical membrane of Drosophila photoreceptors. Development. 2005;132:1487–1497. doi: 10.1242/dev.01704. [DOI] [PubMed] [Google Scholar]
- Schneider M, Khalil AA, Poulton J, Castillejo-Lopez C, Egger-Adam D, Wodarz A, Deng WM, Baumgartner S. Perlecan and Dystroglycan act at the basal side of the Drosophila follicular epithelium to maintain epithelial organization. Development. 2006;133:3805–3815. doi: 10.1242/dev.02549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider VA, Granato M. The myotomal diwanka (lh3) glycosyltransferase and type XVIII collagen are critical for motor growth cone migration. Neuron. 2006;50:683–695. doi: 10.1016/j.neuron.2006.04.024. [DOI] [PubMed] [Google Scholar]
- Schuck S, Gerl MJ, Ang A, Manninen A, Keller P, Mellman I, Simons K. Rab10 is involved in basolateral transport in polarized Madin-Darby canine kidney cells. Traffic. 2007;8:47–60. doi: 10.1111/j.1600-0854.2006.00506.x. [DOI] [PubMed] [Google Scholar]
- Shi A, Chen CC, Banerjee R, Glodowski D, Audhya A, Rongo C, Grant BD. EHBP-1 functions with RAB-10 during endocytic recycling in Caenorhabditis elegans. Mol Biol Cell. 2010;21:2930–2943. doi: 10.1091/mbc.E10-02-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds AJ, dosSantos G, Livne-Bar I, Krause HM. Apical localization of wingless transcripts is required for wingless signaling. Cell. 2001;105:197–207. doi: 10.1016/s0092-8674(01)00311-7. [DOI] [PubMed] [Google Scholar]
- Sipila L, Ruotsalainen H, Sormunen R, Baker NL, Lamande SR, Vapola M, Wang C, Sado Y, Aszodi A, Myllyla R. Secretion and assembly of type IV and VI collagens depend on glycosylation of hydroxylysines. J Biol Chem. 2007;282:33381–33388. doi: 10.1074/jbc.M704198200. [DOI] [PubMed] [Google Scholar]
- Sorrosal G, Perez L, Herranz H, Milan M. Scarface, a secreted serine protease-like protein, regulates polarized localization of laminin A at the basement membrane of the Drosophila embryo. EMBO Rep. 2010;11:373–379. doi: 10.1038/embor.2010.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenmark H, Parton RG, Steele-Mortimer O, Lutcke A, Gruenberg J, Zerial M. Inhibition of rab5 GTPase activity stimulates membrane fusion in endocytosis. EMBO J. 1994;13:1287–1296. doi: 10.1002/j.1460-2075.1994.tb06381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutterlin C, Colanzi A. The Golgi and the centrosome: building a functional partnership. J Cell Biol. 2010;188:621–628. doi: 10.1083/jcb.200910001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokunaga M, Imamoto N, Sakata-Sogawa K. Highly inclined thin illumination enables clear single-molecule imaging in cells. Nat Methods. 2008;5:159–161. doi: 10.1038/nmeth1171. [DOI] [PubMed] [Google Scholar]
- Urbano JM, Torgler CN, Molnar C, Tepass U, Lopez-Varea A, Brown NH, de Celis JF, Martin-Bermudo MD. Drosophila laminins act as key regulators of basement membrane assembly and morphogenesis. Development. 2009;136:4165–4176. doi: 10.1242/dev.044263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147:275–292. doi: 10.1016/j.cell.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venditti R, Scanu T, Santoro M, Di Tullio G, Spaar A, Gaibisso R, Beznoussenko GV, Mironov AA, Mironov A, Jr, Zelante L, et al. Sedlin controls the ER export of procollagen by regulating the Sar1 cycle. Science. 2012;337:1668–1672. doi: 10.1126/science.1224947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertel BM, Velasco A, LaFrance S, Walters L, Kaczman-Daniel K. Precursors of chondroitin sulfate proteoglycan are segregated within a subcompartment of the chondrocyte endoplasmic reticulum. J Cell Biol. 1989;109:1827–1836. doi: 10.1083/jcb.109.4.1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viktorinova I, Konig T, Schlichting K, Dahmann C. The cadherin Fat2 is required for planar cell polarity in the Drosophila ovary. Development. 2009;136:4123–4132. doi: 10.1242/dev.039099. [DOI] [PubMed] [Google Scholar]
- Wang T, Liu Y, Xu XH, Deng CY, Wu KY, Zhu J, Fu XQ, He M, Luo ZG. Lgl1 activation of rab10 promotes axonal membrane trafficking underlying neuronal polarization. Dev Cell. 2011;21:431–444. doi: 10.1016/j.devcel.2011.07.007. [DOI] [PubMed] [Google Scholar]
- Wang X, Harris RE, Bayston LJ, Ashe HL. Type IV collagens regulate BMP signalling in Drosophila. Nature. 2008;455:72–77. doi: 10.1038/nature07214. [DOI] [PubMed] [Google Scholar]
- Wilson DG, Phamluong K, Li L, Sun M, Cao TC, Liu PS, Modrusan Z, Sandoval WN, Rangell L, Carano RA, et al. Global defects in collagen secretion in a Mia3/TANGO1 knockout mouse. J Cell Biol. 2011;193:935–951. doi: 10.1083/jcb.201007162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfstetter G, Shirinian M, Stute C, Grabbe C, Hummel T, Baumgartner S, Palmer RH, Holz A. Fusion of circular and longitudinal muscles in Drosophila is independent of the endoderm but further visceral muscle differentiation requires a close contact between mesoderm and endoderm. Mech Dev. 2009;126:721–736. doi: 10.1016/j.mod.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Xu J, Lan L, Bogard N, Mattione C, Cohen RS. Rab11 is required for epithelial cell viability, terminal differentiation, and suppression of tumor-like growth in the Drosophila egg chamber. PLoS One. 2011;6:e20180. doi: 10.1371/journal.pone.0020180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav S, Linstedt AD. Golgi positioning. Cold Spring Harb Perspect Biol. 2011:3. doi: 10.1101/cshperspect.a005322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasothornsrikul S, Davis WJ, Cramer G, Kimbrell DA, Dearolf CR. viking: identification and characterization of a second type IV collagen in Drosophila. Gene. 1997;198:17–25. doi: 10.1016/s0378-1119(97)00274-6. [DOI] [PubMed] [Google Scholar]
- Yoshimura S, Gerondopoulos A, Linford A, Rigden DJ, Barr FA. Family-wide characterization of the DENN domain Rab GDP-GTP exchange factors. J Cell Biol. 2010;191:367–381. doi: 10.1083/jcb.201008051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurchenco PD. Basement membranes: cell scaffoldings and signaling platforms. Cold Spring Harb Perspect Biol. 2011:3. doi: 10.1101/cshperspect.a004911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Schulze KL, Hiesinger PR, Suyama K, Wang S, Fish M, Acar M, Hoskins RA, Bellen HJ, Scott MP. Thirty-one flavors of Drosophila rab proteins. Genetics. 2007;176:1307–1322. doi: 10.1534/genetics.106.066761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Zhu G, Liu J, Liang Z, Zhang XC, Li G. Rabaptin-5-independent membrane targeting and Rab5 activation by Rabex-5 in the cell. Mol Biol Cell. 2007;18:4119–4128. doi: 10.1091/mbc.E07-02-0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.