Abstract
Objective
The primary aim of this systematic review was to examine the evidence for a pain-sleep relationship in children with persistent pain by reviewing studies using single and mixed pediatric persistent pain samples.
Method
Electronic searches of Medline, PubMed, the Cochrane Database of Systematic Reviews, and PsycINFO were conducted to identify all relevant empirical studies. Studies were included in the review if the majority of participants were between 0-17 years and from one of the following pediatric pain populations: juvenile idiopathic arthritis, sickle cell disease, migraine/headache, functional abdominal pain, juvenile fibromyalgia syndrome, chronic musculoskeletal pain, or mixed populations including the aforementioned conditions.
Results
Research from single and mixed sample studies support the hypothesis that children and adolescents with persistent pain suffer from sleep impairment. Literature addressing factors that may influence or mediate the pain-sleep relationship and the functional outcomes of the pain-sleep relationship was reviewed, and a model of the interrelationships with pain and sleep developed.
Conclusion
Findings from this review highlight the need to assess and treat sleep problems in children presenting with persistent pain. Healthcare providers should consider conducting routine sleep screenings, including a comprehensive description of sleep patterns and behaviors obtained through clinical interview, sleep diaries, and/or the use of standardized measures of sleep. Future research focusing on investigating the mechanisms associating sleep and pediatric persistent pain and on functional outcomes of poor sleep in pediatric pain populations is needed.
Keywords: adolescent, child, persistent pain, sleep
Persistent or recurring pain is common, affecting 25-40% of community samples of children and adolescents.1 Persistent pain may be a symptom of an underlying chronic health condition, such as juvenile idiopathic arthritis (JIA) or sickle cell disease (SCD), or the problem itself, such as migraine headaches. A concerning and common comorbidity of pediatric persistent pain is poor sleep, which may be characterized by difficulty falling or staying asleep, poor subjective sleep quality, short sleep duration, poor sleep hygiene or habits, or disrupted sleep architecture. Sleep disturbances in pediatric persistent pain may be associated with underlying disease-related mechanisms (e.g. inflammation or hypoxemia), treatment regimens (e.g., medications that affect sleep, such as analgesics), or hospitalizations.2-5 Poor sleep is associated with compromised emotional, cognitive, and behavioral functioning in healthy children6-9 and has been related to reduced physical, social, and emotional function in adolescents with persistent pain,10 above and beyond the effects of pain. Evidence indicates that good quality sleep promotes immune system function, while systemic inflammation due to immune system dysfunction has been related to increased pain.11 Adult and animal studies indicate that insufficient sleep contributes to increased pain sensitivity and may initiate pain episodes.12-13 Overall, research indicates that good quality sleep is important for children with persistent pain and understanding sleep disturbances is critical to optimize health outcomes.
Two publications reviewed literature on sleep-pain relationships in adult pain populations.2-3 These reviews indicate pain is associated with insomnia (e.g, difficulty falling asleep, maintaining sleep, and reinitiating sleep;)14, sleep disturbance (e.g., changes in the distribution of REM sleep, decreased sleep efficiency, and fragmented sleep), and daytime sleepiness. Results presented by Onen et al.3 also indicated that not only does pain impact sleep, but poor sleep exacerbates pain, possibly by lowering pain thresholds. A conceptual review by Lewin and Dahl4 addressing pain, sleep, pain management, and mood in pediatric pain populations, proposed that pain disrupts sleep, and poor sleep may promote a cascade of physiological and psychosocial consequences that exacerbate pain.
Since Lewin and Dahl’s4 publication, burgeoning sleep research in pediatric pain populations, including JIA15-17 and SCD18-20, offer the opportunity to summarize the emerging literature base. In addition, Lewin and Dahl’s4 discussion, though vital for establishing the groundwork for pediatric pain-sleep research, did not provide treatment recommendations. A recent review21 focused on the reliability and validity of behavioral and physiological sleep assessments for pediatric persistent pain populations, but did not focus on investigating evidence for the pain-sleep relationship. Thus, the primary aim of this systematic review is to examine evidence for a pain-sleep relationship in children with persistent pain by reviewing studies using single and mixed pediatric pain samples. Secondary aims include development of: (1) a model describing interrelationships between pain and sleep, including factors that may influence or mediate pain-sleep relationships and functional outcomes, and (2) evidence-based recommendations for assessment and care of children with persistent pain.
METHOD
Electronic searches of Medline, PubMed, the Cochrane Database of Systematic Reviews, and PsycINFO since inception to December 2011 were conducted to identify all relevant empirical studies. Search terms included sleep, pain, and a third term including one of the following: pediatric, juvenile, adolescent, child, children, or youth. Search terms were chosen to provide the widest range of citations related to sleep in pediatric pain populations. Each list was screened for relevant titles and abstracts of all marginally relevant titles were examined. Next, two reviewers examined the reference lists and PubMed “related links” citations for each reviewed article to identify additional articles. No attempt was made to locate unpublished studies. The reviewers extracted data consisting of age range, pain populations, and findings related to sleep characteristics and pain-sleep relations from all relevant articles.
Articles in this review detailed empirical studies that included sleep assessments of participants between 0-17 years diagnosed with a pediatric persistent pain condition: JIA, SCD, migraine/headache, functional abdominal pain, juvenile fibromyalgia syndrome, chronic musculoskeletal pain, complex regional pain syndrome, or mixed populations including the aforementioned conditions. Excluded articles utilized community samples, examined fatigue only, or were not available in English. Because studies measured a variety of sleep aspects (e.g., sleep quality, duration, architecture), outcome data pooling was not viable. Therefore, results were synthesized and described systematically based upon review aims.
RESULTS
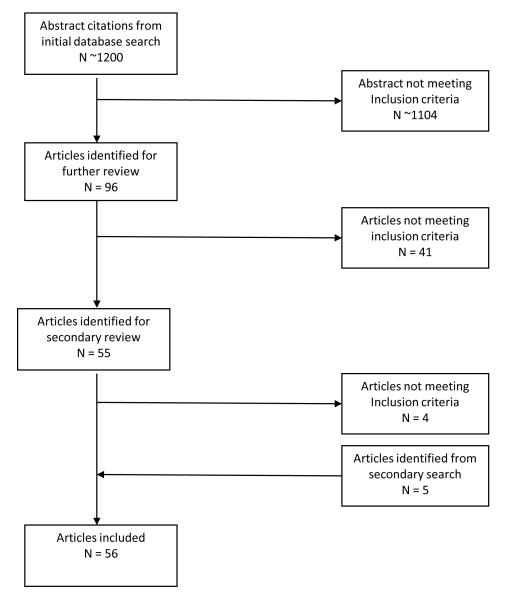
A total of 56 articles utilizing 46 independent samples met criteria and were included in the review (see Figure 1). The studies were published 1982-2011, with the majority (47/56) published after 2000. Table 1 provides an overview of the studies reviewed including presence of controls, type of sleep assessments (e.g., behavioral or physiological), and a summary of key findings.
Figure 1.
Flow Diagram of Studies Reviewed for Inclusion
Table 1.
Summary of Studies Reviewed by Pain Population and Assessment Type
| Pain Condition | # of Studies* (Combined N) |
Behavioral Assessment Findings |
Physiological Assessment Findings |
Articles Reviewed |
|---|---|---|---|---|
| Juvenile Idiopathic Arthritis | 9 (N = 310) |
7 Behavioral Studies JIA > HC for behavioral sleep problems |
5 PSG Studies JIA > HC for sleep disturbances |
15-17, 26-29, 68, 92 |
| Sickle Cell Disease | 10 (N = 360) |
8 Behavioral Studies SCD > HC for behavioral sleep problems |
3 PSG Studies Findings: No HC groups |
18-20, 32-34, 37-38, 69-70 |
| Headache | 15 (N = 1753) |
14 Behavioral Studies HA > HC for behavioral sleep problems |
2 Actigraphy Studies Mixed Findings 1 PSG Study No HC group |
43-45, 47-53, 71-73, 76, 79 |
| Mixed Pain Sample | 22 (N = 1579) |
19 Behavioral Studies MPS > HC for behavioral sleep problems |
6 Actigraphy Studies Mixed Findings 2 PSG Studies MPS > HC for sleep disturbances |
10, 54-60, 62-67,74-75, 77-78, 89, 91, 93-94 |
Note: HC = Healthy controls; HA= Headache; JIA= Juvenile idiopathic arthritis; SCD = Sickle cell disease; MPS=Mixed Pain Sample; PSG=Polysomnography
This is the total number of studies. The number of studies using behavioral assessments and objective assessments are not mutually exclusive, as about half of the studies (11/19) using objective assessments also used behavioral assessments.
Findings from Studies conducted in a Single Pediatric Pain Population
JIA
JIA is a rheumatic disease characterized by flares of joint stiffness, inflammation, and pain, which affects approximately 300,000 children in the United States under the age of 16.22 Many children with arthritis report mild pain most days and severe pain on 30% of days.23 Seven sleep studies used behavioral measures; five used polysomnography (PSG24; Table 1).
Behavioral measures found significantly more sleep problems in children with JIA than healthy controls. Two studies using the Children’s Sleep Habits Questionnaire25 (CSHQ) in children with JIA aged 6-1226-27, found parents reported more sleep-related anxiety, night wakings, parasomnias, sleep disordered breathing (SDB), and daytime sleepiness in their children with JIA than parents of healthy children. One study also found parents reported more delayed sleep onset.27 Studies using PSG in children with JIA found significantly lower sleep efficiency, higher arousal indexes, more leg movements related to arousals, less time in slow wave sleep, and more alpha/delta activity during non-REM sleep compared to healthy controls.28-29 Overall, findings from behavioral measures and PSG indicate that children with JIA experience significantly more sleep disturbances than healthy children.
Sickle Cell Disease
SCD is a family of genetic blood disorders affecting about 1 in 600 African Americans.30 Approximately 70% of individuals with SCD experience pain,30 with recurrent vaso-occlusive pain episodes occurring 10-13 times a year during childhood and adolescence.31 Eight studies utilized behavioral measures; 3 used PSG, but did not include healthy comparisons (Table 1).
Behavioral measures indicate children with SCD experience significant sleep problems.18,32-33 Prospectively, 8-12 year old children with SCD18 reported night awakenings, difficulty falling asleep, and daytime sleepiness on more than 30% of daily diary days. On the CSHQ, parents of children aged 4-10 years with SCD reported significantly more night awakenings and SDB symptoms in their children than parents of healthy controls.33 PSG studies in SCD largely focus on SDB, a significant concern in this population, and often fail to report other sleep characteristics. In PSG studies, 36% of children with SCD have SDB,34 and up to 40% experience nocturnal desaturation,35 which is associated with short sleep duration, high sleep fragmentation and movement, high latency to REM sleep, and short REM sleep duration.36 However, findings from one study suggest that other sleep characteristics do not differ between children with SCD with and without SDB.37 In another PSG study of 64 children with SCD aged 2-1838, 23% were diagnosed with periodic limb movement disorder (PLM), identifying an important area for future research and clinical assessment in this population. In summary, physiological sleep research in pediatric SCD has largely focused on SDB, but findings suggest other sleep disruptions in SCD warrant further investigation.
Headache
Approximately 70-90% of youth suffer from headaches39-40 with 5% of children and adolescents aged 10-18 years reporting daily headaches.41 The majority (15) of identified sleep studies in clinical pediatric headache samples employed behavioral measures (Table 1); 2 used actigraphy42 and 1 PSG (without a healthy comparison group).
Behavioral measures identify significantly more sleep problems in children and adolescents with headache versus healthy controls.43-49 In a study of 69 adolescents with headache,45 insufficient sleep, daytime sleepiness, difficulty falling asleep, and night awakenings were identified as frequent problems on the School Sleep Habits Questionnaire.46 Using the CSHQ,50 parents rated 6-18 year old children with migraine as having longer sleep durations, more daytime sleepiness, and longer sleep onset delays than healthy siblings. Findings from studies using actigraphy, wrist-watch size computers that monitor movement to identify sleep and wake patterns,42 were mixed. One study found no group differences in sleep patterns of 8-12 year olds with and without headaches,51 while another study found that children with headache rose earlier than healthy children.52 The only PSG study in children with headache is a chart review53 of 90 headache patients aged 5-19 years who screened positive for behavioral signs of a sleep disorder. Children with chronic migraine had significantly less total sleep time, longer sleep latencies, and greater arousal indexes than children with less frequent migraines and other types of headaches. SDB was detected in 49% of the total sample, identifying an important aspect of sleep for research and clinical assessment in children with headache. Overall, behavioral and PSG findings indicate that children with headaches experience significant sleep problems; however, actigraphy findings are equivocal.
Mixed Pediatric Pain Samples
Twenty-two studies were identified that included mixed samples of children with musculoskeletal pain, complex regional pain syndrome, functional abdominal pain, juvenile fibromyalgia syndrome, or at least two different pediatric pain conditions, (e.g., JIA, SCD, headache). The majority of studies (19) utilized behavioral measures, six used actigraphy, and 2 used PSG (Table 1).
Behavioral assessments consistently found that children with persistent pain experience poor sleep,54-56 particularly in comparison to healthy controls.57-60 In a study of 59 adolescents with persistent pain and 56 healthy controls59 using the Adolescent Sleep Wake Scale,61 sleep hygiene, and pre-sleep arousal questionnaires there was an association between persistent pain and insomnia; adolescents with chronic pain also reported higher levels of pre-sleep arousal. In a study of 100 children with persistent pain (headache, JIA, or SCD),60 53% of the pain sample scored at or above the clinical cutoff for total sleep problems versus 23% of the normative community sample for the CSHQ. A few studies with mixed samples used actigraphy, but with inconclusive findings.62-65 In an age and sex matched sample, adolescents with persistent pain experienced lower sleep efficiency, more night awakenings, and shorter total sleep times on actigraphy than healthy peers.63 However, two larger studies conducted by the same investigative team64-65 found that adolescents with persistent pain reported poorer subjective sleep quality on daily diaries, but there were no between-group differences on actigraphic variables. Two studies that used PSG in children and adolescents with juvenile fibromyalgia syndrome 66-67 found that the sleep architecture of children and adolescents with juvenile fibromyalgia syndrome differed significantly from healthy controls.
Overall, behavioral assessments and PSG consistently identified more sleep disturbances in children and adolescents with persistent pain compared to healthy children; actigraphy results were equivocal. Also, consistent with studies using a single pediatric pain population, the majority of studies were cross-sectional and did not address whether differences in sleep disturbances change over time.
A Model of the Pain-Sleep Relationship in Pediatric Persistent Pain Populations
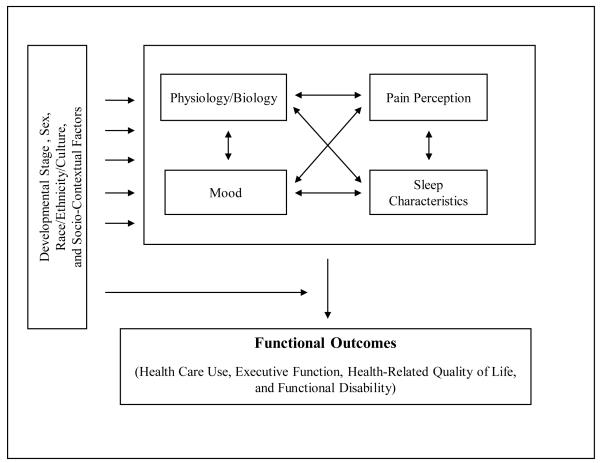
Understanding the pain-sleep relationship in pediatric persistent pain populations within a broader biopsychosocial context is needed to develop appropriate research and clinical recommendations. Thus, the following model (see Figure 2) was developed to reflect complex bidirectional relationships between sleep, pain, physiology/biology, mood, developmental stage, sex, race/ethnicity/culture, socio-contextual factors, and functional outcomes in pediatric pain populations. In the proposed model, pain perception and sleep quality have a bidirectional relationship and interact with physiology/biology and mood to influence functional outcomes, including health-related quality of life (HRQoL), health care use, and functional disability. In addition, though not fully explored in current research, developmental stage, sex, race/ethnicity/culture, and socio-contextual factors are proposed to influence the interaction between pain, sleep, physiology, and mood, and to modify their influence on functional outcomes. The following sections review supporting evidence for the relationships proposed in the model.
Figure 2.
A Model of the Pain-Sleep Relationship in Pediatric Persistent Pain Populations
Evidence for a Bi-directional Relationship between Sleep and Pain Intensity
Studies using behavioral assessments26,45,50,68-70, and PSG16,53,66 in pediatric pain samples generally detected a relationship between high pain and disrupted sleep. In addition, most reviewed studies indicate that even after controlling for other factors, high pain is predictive of disrupted sleep patterns. In a study of children with SCD that controlled for age, sex, and SCD genotype,18 high pain predicted poor self-reported sleep quality. A study of 118 children with headache71 found that longer headache duration predicted higher sleep anxiety and more bedtime resistance on the CSHQ after controlling for age, sex, and race/ethnicity. Also, more frequent headaches predicted symptoms of parasomnia, sleep walking, and bruxism (e.g., clenching or grinding teeth during sleep). In a study of 86 adolescents with headache, JIA, or SCD,10 higher pain intensity and frequency predicted more sleep problems after controlling for socioeconomic status and illness group.
Mounting evidence shows that poorer sleep is predictive of higher pain in pediatric pain populations. A diary study of 20 children with SCD aged 8-12 years19 found that high pain intensity predicted poor sleep quality and poor sleep quality predicted high pain the following day. Similarly, diary studies of children with JIA aged 8-1615 and adolescents with persistent pain65 found that though pain intensity did not predict sleep quality, poor sleep quality predicted high pain intensity the following day. Findings from studies of headache,72-73 musculoskeletal pain,74 and mixed pain samples75 also suggest that children with persistent pain may use sleep for pain coping. However, a prospective diary study of 25 children with headache aged 8-17 years76 found that although 68% of the sample endorsed lack of sleep as a headache trigger, shorter sleep duration, increased number of night awakenings, and feeling unrested in the morning did not predict subsequent headache occurrence. These findings support complex, bidirectional associations between pain characteristics and sleep quality in children with persistent pain.
Lastly, a few small uncontrolled intervention studies targeting pain appeared to impact sleep outcomes and vice versa.77-79 In a small, uncontrolled trial of cognitive behavioral therapy for youth with juvenile fibromyalgia syndrome78, incorporating pain and sleep treatment components, youth displayed significant improvement in pain, sleep, fatigue, and psychological and functional ability. In addition, Bruni and colleagues79 randomly assigned 70 children with headache to either a parent implemented sleep hygiene intervention or maintenance of current sleep routines. The intervention group reported significant reductions in headache frequency compared to the control group at three and six months. Clearly, additional intervention studies are needed in pediatric pain populations to better assess the impact of pain and sleep management techniques on improving health outcomes.
Factors Influencing and Mediating the Pain-Sleep Relationship
Mood
Research has consistently linked mood to both pain and sleep. Studies of children with persistent pain report that increased depressive symptoms and poorer negative mood regulation are predictive of high pain intensity.80-81 In addition, higher pain predicted more depressive symptoms and greater negative mood in children with persistent pain.20,82 There is also a body of literature linking mood fluctuations to poor sleep.83 A study of adolescents with persistent pain and healthy controls65 found that more depressive symptoms predicted poorer sleep quality. In a daily diary study of children with SCD20, negative mood partially mediated the influence of poor sleep quality on high pain intensity the following day and of high pain intensity and poor sleep quality that night. This is consistent with a recent review exploring the pain-emotion relationship in adult persistent pain populations indicating that poor sleep may strengthen the association between high pain and emotional distress.84 Additionally, there is evidence that positive emotions act as a protective factor in the pain-sleep relationship. Studies in SCD20 and JIA15 found that the influence of poor sleep on high pain the following day was weakened at increasing levels of positive mood. Unfortunately, most studies do not utilize mood assessments that separately assess negative and positive mood.
Developmental Stage
Studies indicate that adolescents are more prone to sleep problems than school age children,85 that these differences are probably linked to pubertal and psychosocial changes across development,86 and that persistent pain prevalence steadily increases, peaking during adolescence.1,87-88 However, the majority of studies included in this review report broad age ranges and fail to investigate the possible influence of developmental stage, or the role of pubertal status, which may also contribute to age and sex-specific differences. The few studies using child or adolescent only samples consistently indicate that both children and adolescents with persistent pain experience sleep problems.
Sex
Findings concerning the role of sex on sleep in pediatric pain populations are limited and equivocal, likely due to the higher prevalence of females in pain samples, making examination of sex differences more difficult.18,71,89 Results from daily diaries and actigraphy in 28 children with headache52 indicated that sex may moderate the relationship between headache and sleep. However, these findings should be interpreted cautiously as they are based upon a small sample of children with headache further subdivided by sex.
Race/Ethnicity/Culture and Socio-Contextual Factors
Race, ethnicity, culture, and socio-contextual factors (e.g., socio-economic status, neighborhood disadvantage, and family environment) are seen as interconnected factors often studied as a cluster. A recent review90 highlights how cultural and socio-contextual factors can predispose chronically ill children to poor sleep patterns, but also underscores vast limitations in the evidence base. This is similar in the literature on pediatric persistent pain as only one study on the pain-sleep association examined these factors,91 finding that minority children with persistent pain reported sleeping less than Caucasian children with persistent pain. Of note, while SCD is seen primarily in minority populations, most other pediatric pain conditions are more common in Caucasians, complicating research on the influence of race/ethnicity/culture and socio-contextual factors in pediatric pain.
The Impact of Pain and Sleep on Functional Outcomes
Studies have linked sleep problems in pediatric pain populations to a range of functional outcomes. A series of investigations27,92 support the relationship between sleep and executive functioning in children with JIA aged 6-11 years. Higher mean number of night awakenings predicted poorer rapid visual processing, higher apnea severity scores predicted longer reaction times, and higher total sleep disturbances on the CSHQ predicted slower reaction times for all children; however, group membership (JIA versus comparison) did not predict slower reaction times. Studies of children with persistent pain60,68,89 also related behavioral reports of poorer sleep to poorer HRQoL. Lower sleep efficiency and less sleep per night were related to a high number of daytime naps in adolescent girls with persistent pain93 and higher pain frequency and lower sleep efficiency predicted activity limitations in 40 adolescents with and without persistent pain.94
DISCUSSION
Overall, research supports that children and adolescents with persistent pain suffer from sleep impairment. Behavioral sleep assessments indicate that children, adolescents, and their parents report poorer subjective sleep quality as evidenced by more night awakenings and daytime sleepiness versus healthy controls. Findings from PSG studies in pediatric rheumatology populations also indicate that children and adolescents with persistent pain experience more disrupted sleep, including lower sleep efficiency, more alpha/delta activity, and the presence of more physiological sleep disorders (e.g., PLM and SDB), compared to healthy peers. Additional investigation is needed into the epidemiology of sleep disorders in pediatric pain populations.
Sleep actigraphy, on the other hand, has been less frequently used with pediatric pain populations than behavioral questionnaires or PSG. When used, sleep actigraphy has indicated less sleep pattern differences between children with persistent pain and healthy controls. Given high concordance rates previously observed between sleep actigraphy and PSG,42 these inconsistencies warrant further investigation. A lack of standardized sleep actigraphy scoring may impact researchers’ ability to detect between-group differences and small sample sizes may also contribute to equivocal findings. In addition, specific aspects of sleep assessed via actigraphy versus those assessed using behavioral reports or PSG may have different relationships with pain and related factors. Lastly, the reviewed studies demonstrate that sleep difficulties in children with persistent pain are strongly predictive of function, including impaired executive dysfunction and poorer HRQoL. These findings highlight the critical need to consider assessment and management of sleep problems in the care of pediatric pain populations.
A limitation of the current literature is the cross sectional design of most studies describing sleep patterns in children with persistent pain. The lack of longitudinal study designs does not allow researchers to elucidate the nature of sleep disturbances in this population over time, to discern how changes over time compare to normal developmental sleep changes, or to detect associations with variations in disease activity. Another key limitation of the literature is the lack of focus on mechanisms by which children with persistent pain experience disrupted sleep. It has been proposed that sleep disturbances in children with persistent pain are associated with underlying disease-related mechanisms, treatment regimens, or hospitalizations.2-5 Additionally, the influence of mood, developmental stage, sex, race/ethnicity/culture, and socio-contextual factors may be important, but the influence of these factors on sleep in pediatric pain populations has not been extensively researched. There is also a critical need for further investigation into the functional consequences of poor sleep in pediatric pain populations. For example, the lack of information on the possible influences of pain and sleep on pain management behaviors, and on the interactive influences of pain and sleep on functional outcomes, hinder the ability of clinicians to develop standardized approaches to treatment.
Clinical Implications
Findings from this systematic review underline the need to assess and manage sleep problems in children with persistent pain. Clinical assessment of the nature of a child’s sleep disruptions is critical for individualizing interventions. One useful screening tool available to pediatricians is the BEARS,95 a brief interview designed for pediatric clinical settings, assessing Bedtime problems, Excessive sleepiness, Awakenings, Regularity of sleeping, and Sleep-disordered breathing. Though not a comprehensive research assessment, the BEARS is a developmentally appropriate screening tool.
Children with evidence of excessive daytime sleepiness, poor quality sleep, and heavy snoring, gasping, or long pauses in breathing, may require formal evaluation by a sleep medicine specialist for SDB. However, other sleep problems necessitate thorough assessment of sleep patterns and behaviors (e.g., sleep schedule, routines, habits, behaviors during the night) via clinical interview, diaries, and/or standardized pediatric sleep measures (reviewed by Lewandowski, et al.96). Following assessment, behavioral interventions may target sleep habits or sleep hygiene; improving habits that interfere with sleep onset or maintenance (e.g., restricting evening caffeine intake, eliminating naps, creating a sleep routine/schedule, restricted use of electronic devices at night). A thorough sleep assessment for youth with persistent pain includes a detailed review of medications, including doses and timing. Many commonly used medications for persistent pain effect sleep staging.96 Onen and colleagues3 have recently reviewed the medical management of sleep problems in adult pain populations.
Following assessment by the pediatrician, children with persistent sleep problems should be referred to a sleep medicine specialist for additional evaluation, including a 1 to 2 night sleep study (overnight PSG) and the multiple sleep latency test,97 an indicator of excessive daytime sleepiness. Some sleep disorders, such as SDB and PLM, may require medical interventions (e.g., continuous positive airway pressure), and sleep medicine management, overseen by a sleep medicine specialist. Children with inadequate sleep, insomnia, or delayed sleep phase, may benefit from cognitive behavioral therapy with a behavioral sleep specialist. Cognitive behavioral therapy has been recommended by the American Academy of Sleep Medicine for the treatment of adult insomnia based on a large evidence base in diverse adult patient populations.98 There is evidence that cognitive behavioral therapy leads to reduction of insomnia symptoms and pain in older adults with persistent pain.99
In conclusion, children with persistent pain commonly suffer from disturbed sleep, which puts them at risk for poor functional outcomes. Pediatricians who treat children with persistent pain can optimize care by integrating the assessment and management of sleep problems into routine practice, focusing on the interactions between pain, sleep, and moderating biopsychosocial factors. Embedding medical and behavioral interventions to enhance sleep into existing pain management interventions for children with persistent pain will improve health outcomes. However, more systematic research investigating the mechanisms associating sleep and pediatric persistent pain is warranted.
Acknowledgments
This investigation was supported by NIHK01HL103155 (C.R.V) from the National Heart, Lung, and Blood Institute, NIHK24HD060068 (T.P.) from the National Institute of Child Health and Human Development, and NIHR01AR53845 (M.H.B., L.E.S.) from the National Institute for Arthritis, Musculoskeletal, and Skin Diseases.
Footnotes
Disclosure: The authors declare no conflicts of interest.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stanford EA, Chambers CT, Biesanz JC, et al. The frequency, trajectories and predictors of adolescents recurrent pain: A population-based approach. Pain. 2008;138:11–21. doi: 10.1016/j.pain.2007.10.032. [DOI] [PubMed] [Google Scholar]
- 2.Menefee LA, Cohen MJ, Anderson WR, et al. Sleep disturbance and nonmalignant chronic pain: A comprehensive review of the literature. Pain Med. 2000;1:156–172. doi: 10.1046/j.1526-4637.2000.00022.x. [DOI] [PubMed] [Google Scholar]
- 3.Onen SH, Onen F, Courpron P, et al. How pain and analgesics disturb sleep. Clin J Pain. 2005;21:422–431. doi: 10.1097/01.ajp.0000129757.31856.f7. [DOI] [PubMed] [Google Scholar]
- 4.Lewin DS, Dahl RE. Importance of sleep in the management of pediatric pain. J Dev Behav Pediatr. 1999;20:244–252. doi: 10.1097/00004703-199908000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Lewandowski AS, Ward TM, Palermo TM. Sleep problems in children and adolescents with common medical conditions. Pediatr Clin North Am. 2011;58:699–713. doi: 10.1016/j.pcl.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Sleep Foundation [Accessed September 20, 2006];Adolescent Sleep Needs and Patterns: Research Reports and Resource Guide. 2000 Available at: http://www.sleepfoundation.org/_content/hottopics/sleep_and_teens_report1.pdf.
- 7.O’Brien LM, Gozal D. Neurocognitive dysfunction and sleep in children: From human to rodent. Pediatr Clin North Am. 2004;51:187–202. doi: 10.1016/s0031-3955(03)00184-6. [DOI] [PubMed] [Google Scholar]
- 8.Owens JA. Neurocognitive and behavioral impact of sleep disordered breathing in children. Pediatr Pulmonol. 2009;44:417–422. doi: 10.1002/ppul.20981. [DOI] [PubMed] [Google Scholar]
- 9.Taras H, Potts-Datema W. Sleep and student performance at school. J Sch Health. 2005;75:248–254. doi: 10.1111/j.1746-1561.2005.00033.x. [DOI] [PubMed] [Google Scholar]
- 10.Palermo TM, Kiska R. Subjective sleep disturbances in adolescents with chronic pain: Relationship to daily functioning and quality of life. J Pain. 2005;6:201–207. doi: 10.1016/j.jpain.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 11.Motivala SJ, Irwin MR. Sleep and immunity: Cytokine pathways linking sleep and health outcomes. Curr Dir Psychol Sci. 2007;16:21–25. [Google Scholar]
- 12.Lautenbacher S, Kundermann B, Krieg JC. Sleep deprivation and pain perception. Sleep Med Rev. 2006;10:357–369. doi: 10.1016/j.smrv.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 13.Smith M, Edwards R, McCann U. The effects of sleep deprivation on pain inhibition and spontaneous pain in women. Sleep. 2007;30:494–505. doi: 10.1093/sleep/30.4.494. [DOI] [PubMed] [Google Scholar]
- 14.National Heart, Lung, and Blood Institute [Accessed September 15, 2012];What is Insomnia? 2011 Dec 13; Available at: http://www.nhlbi.nih.gov/health/health-topics/topics/inso/
- 15.Bromberg MH, Gil KM, Schanberg LE. Daily sleep quality and mood as predictors of pain in children with juvenile polyarticular arthritis. Health Psychol. 2011;31:202–209. doi: 10.1037/a0025075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Passarelli CM, Roizenblatt S, Len CA, et al. A case-controlled sleep study in children with polyarticular juvenile rheumatoid arthritis. J Rheumatol. 2006;33:796–802. [PubMed] [Google Scholar]
- 17.Ward TM, Brandt P, Archbold K, et al. Introduction to the special issue: Polysomnography and self-reported sleep, pain, fatigue, and anxiety in children with active and inactive juvenile rheumatoid arthritis. J Pediatr Psychol. 2008;33:232–241. doi: 10.1093/jpepsy/jsm121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Valrie CR, Gil KM, Redding-Lallinger R, et al. The influence of pain and stress on sleep in children with sickle cell disease. Child Health Care. 2007;36:335–353. [Google Scholar]
- 19.Valrie CR, Gil KM, Redding-Lallinger R, et al. Sleep in children with sickle cell disease: An analysis of daily diaries utilizing multilevel models. J Pediatr Psychol. 2007;32:857–861. doi: 10.1093/jpepsy/jsm016. [DOI] [PubMed] [Google Scholar]
- 20.Valrie CR, Gil KM, Redding-Lallinger R, et al. Daily mood as a mediator or moderator of the pain-sleep relationship in children with sickle cell disease. J Pediatr Psychol. 2008;33:317–322. doi: 10.1093/jpepsy/jsm058. [DOI] [PubMed] [Google Scholar]
- 21.De la Vega R, Miró J. [Accessed September 15, 2012];The assessment of sleep in pediatric chronic pain sufferers [published online ahead of print June 29 2012] Sleep Med Rev. 2012 doi: 10.1016/j.smrv.2012.04.002. http://www.journals.elsevier.com/sleep-medicine-reviews/ [DOI] [PubMed]
- 22.Sacks JJ, Helmick CG, Luo YH, et al. Prevalence of and annual ambulatory health care visits for pediatric arthritis and other rheumatologic conditions in the United States in 2001-2004. Arthritis Rheum. 2007;57:1439–1445. doi: 10.1002/art.23087. [DOI] [PubMed] [Google Scholar]
- 23.Schanberg LE, Anthony KK, Gil KM, et al. Daily pain and symptoms in children with polyarticular arthritis. Arthritis Rheum. 2003;48:1390–1397. doi: 10.1002/art.10986. [DOI] [PubMed] [Google Scholar]
- 24.American Academy of Sleep Medicine Task Force Sleep-related breathing disorders in adults: Recommendation for syndrome definition and measurement techniques in clinical research. Sleep. 1999;22:667–689. [PubMed] [Google Scholar]
- 25.Owens JA, Spirito A, McGuinn M. The Children’s Sleep Habits Questionnaire (CSHQ): Psychometric properties of a survey instrument for school-aged children. Sleep. 2000;23:1043–1051. [PubMed] [Google Scholar]
- 26.Bloom BJ, Owens JA, McGuinn M, et al. Sleep and its relationship to pain, dysfunction, and disease activity in juvenile rheumatoid arthritis. J Rheumatol. 2002;29:169–173. [PubMed] [Google Scholar]
- 27.Ward TM, Ringold S, Metz J, et al. Sleep disturbances and neurobehavioral functioning in children with and without juvenile idiopathic arthritis. Arthritis Care & Research. 2011;63:1006–1012. doi: 10.1002/acr.20469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zamir G, Press J, Asher T, et al. Sleep fragmentation in children with juvenile rheumatoid arthritis. J Rheumatol. 1998;25:1191–1197. [PubMed] [Google Scholar]
- 29.Lopes MC, Guilleminault C, Rosa A, et al. Delta sleep instability in children with chronic arthritis. Brazilian Journal of Medical and Biological Research. 2008;41:938–943. doi: 10.1590/s0100-879x2008001000018. [DOI] [PubMed] [Google Scholar]
- 30.Steinberg MH. Management of sickle cell disease. N Engl J Med. 1999;340:1021–1030. doi: 10.1056/NEJM199904013401307. [DOI] [PubMed] [Google Scholar]
- 31.Dampier C, Setty BNY, Eggleston B, et al. Vaso-occlusion in children with sickle cell disease: Clinical characteristics and biologic correlates. J Pediatr Hematol Oncol. 2004;26:785–790. [PubMed] [Google Scholar]
- 32.Walco GA, Dampier CD. Pain in children and adolescents with sickle cell disease: A descriptive study. J Pediatr Psychol. 1990;15:643–658. doi: 10.1093/jpepsy/15.5.643. [DOI] [PubMed] [Google Scholar]
- 33.Daniel LC, Grant M, Kothare SV, et al. Sleep patterns in pediatric sickle cell disease. Pediatr Blood Cancer. 2010;55:501–507. doi: 10.1002/pbc.22564. [DOI] [PubMed] [Google Scholar]
- 34.Samuels MP, Stebbens VA, Davies SC, et al. Sleep related upper airway obstruction and hypoxaemia in sickle cell disease. Arch Dis Child. 1992;67:925–929. doi: 10.1136/adc.67.7.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Caboot JB, Allen JL. Pulmonary complications of sickle cell disease in children. Curr Opin Pediatr. 2008;20:279–287. doi: 10.1097/MOP.0b013e3282ff62c4. [DOI] [PubMed] [Google Scholar]
- 36.Souza LC, Viegas CA. Quality of sleep and pulmonary function in clinically stable adolescents with sickle cell anemia. J Bras Pneumol. 2007;33:275–281. doi: 10.1590/s1806-37132007000300008. [DOI] [PubMed] [Google Scholar]
- 37.Salles C, Ramos RT, Daltro C, et al. Prevalence of obstructive sleep apnea in children and adolescents with sickle cell anemia. J Bras Pneumol. 2009;35:1075–1083. doi: 10.1590/s1806-37132009001100004. [DOI] [PubMed] [Google Scholar]
- 38.Rogers VE, Marcus CL, Jawad AF, et al. Periodic limb movements and disrupted sleep in children with sickle cell disease. Sleep. 2011;34:899–908. doi: 10.5665/SLEEP.1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bille B. Migraine in childhood and its prognosis. Cephalalgia. 1981;1:71–75. doi: 10.1111/j.1468-2982.1981.tb00012.x. [DOI] [PubMed] [Google Scholar]
- 40.Rhee H. Prevalence and predictors of headaches in US adolescents. Headache. 2000;40:528–538. doi: 10.1046/j.1526-4610.2000.00084.x. [DOI] [PubMed] [Google Scholar]
- 41.King S, Chambers CT, Huguet A, et al. The epidemiology of chronic pain in children and adolescents revisited: A systematic review. Pain. 2011;152:2729–2738. doi: 10.1016/j.pain.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 42.Sadeh A, Alster J, Urbach D, et al. Actigraphically based automatic bedtime sleep-wake scoring: Validity and clinical applications. J Ambul Monitoring. 1989;2:209–216. [Google Scholar]
- 43.Luc ME, Gupta A, Birnberg JM, et al. Characterization of symptoms of sleep disorders in children with headache. Pediatr Neurol. 2006;34:7–12. doi: 10.1016/j.pediatrneurol.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 44.Zarowski M, Mlodzikowska-Albrecht J, Steinborn B. The sleep habits and sleep disorders in children with headache. Adv Med Sci. 2007;52:194–196. [PubMed] [Google Scholar]
- 45.Gilman DK, Palermo TM, Kabbouche MA, et al. Primary headache and sleep disturbances in adolescents. Headache. 2007;47:1198–1194. doi: 10.1111/j.1526-4610.2007.00885.x. [DOI] [PubMed] [Google Scholar]
- 46.Wolfson AR, Carskadon MA. Sleep schedules and daytime functioning in adolescents. Child Dev. 1998;69:875–887. [PubMed] [Google Scholar]
- 47.Pakalnis A, Spaingard M, Splaingard D, et al. Serotonin effects on sleep and emotional disorders in adolescent migraine. Headache. 2009;49:1486–1492. doi: 10.1111/j.1526-4610.2009.01392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bruni O, Fabrizi P, Ottaviano S, et al. Prevalence of sleep disorders in childhood and adolescence with headache: A case-controlled study. Cephalalgia. 1997;17:492–498. doi: 10.1046/j.1468-2982.1997.1704492.x. [DOI] [PubMed] [Google Scholar]
- 49.Carotenuto M, Giudetti V, Ruju F, et al. Headache disorders as risk factors for sleep disturbances in school aged children. J Headache Pain. 2005;6:268–270. doi: 10.1007/s10194-005-0204-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Heng K, Wirrell E. Sleep disturbances in children with migraine. J Child Neurol. 2006;21:761–766. doi: 10.1177/08830738060210092201. [DOI] [PubMed] [Google Scholar]
- 51.Bruni O, Russo PM, Violani C, et al. Sleep and migraine: An actigraphic study. Cephalalgia. 2004;24:134–139. doi: 10.1111/j.1468-2982.2004.00657.x. [DOI] [PubMed] [Google Scholar]
- 52.Bursztein C, Steinberg T, Sadeh A. Sleep, sleepiness, and behavior problems in children with headache. J Child Neurol. 2006;21:1012–1019. doi: 10.1177/7010.2006.00239. [DOI] [PubMed] [Google Scholar]
- 53.Vendrame M, Kaleyias J, Valencia I, et al. Polysomnographic findings in children with headaches. Pediatr Neurol. 2008;39:6–11. doi: 10.1016/j.pediatrneurol.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 54.Siegel DM, Janeway D, Baum J. Fibromyalgia syndrome in children and adolescents: Clinical features at presentation and status at follow-up. Pediatrics. 1998;101:377–382. doi: 10.1542/peds.101.3.377. [DOI] [PubMed] [Google Scholar]
- 55.Gedalia A, Garcia CO, Molina JF, et al. Fibromyalgia syndrome: Experience in a pediatric rheumatology clinic. Clin Exp Rheumatol. 2000;18:415–419. [PubMed] [Google Scholar]
- 56.Konijnenberg AY, Uuterwaal CS, Kimpen JL, et al. Children with unexplained chronic pain: Substantial impairment in everyday life. Arch Dis Child. 2005;90:680–686. doi: 10.1136/adc.2004.056820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huntley ED, Campo JV, Dahl RE, et al. Sleep characteristics of youth with functional abdominal pain and a healthy comparison group. J Pediatr Psychol. 2007;32:938–49. doi: 10.1093/jpepsy/jsm032. [DOI] [PubMed] [Google Scholar]
- 58.Yunus MB, Masi AT. Juvenile primary fibromyalgia syndrome. A clinical study of thirty-three patients and matched normal controls. Arthritis Rheum. 1985;28:138–145. doi: 10.1002/art.1780280205. [DOI] [PubMed] [Google Scholar]
- 59.Palermo TM, Wilson AC, Lewandowski AS, et al. Behavioral and psychosocial factors associated with insomnia in adolescents with chronic pain. Pain. 2011;152:89–94. doi: 10.1016/j.pain.2010.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Long AC, Krishnamurthy V, Palermo TM. Sleep disturbances in school-age children with chronic pain. J Pediatr Psychol. 2008;33:258–268. doi: 10.1093/jpepsy/jsm129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.LeBourgeois MK, Giannotti F, Cortesi F, et al. The relationship between reported sleep quality and sleep hygiene in Italian and American adolescents. Pediatrics. 2005;115:257–265. doi: 10.1542/peds.2004-0815H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Haim A, Pillar G, Pecht A, et al. Sleep patterns in children and adolescents with functional recurrent abdominal pain: objective versus subjective assessment. Acta Paediatr. 2004;93:677–80. [PubMed] [Google Scholar]
- 63.Palermo TM, Toliver-Sokol M, Fonareva I, et al. Objective and subjective assessment of sleep in adolescents with chronic pain compared to healthy adolescents. Clin J Pain. 2007;23:812–820. doi: 10.1097/AJP.0b013e318156ca63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Law EF, Dufton L, Palermo TM. Daytime and nighttime sleep patterns in adolescents with and without chronic pain. Health Psychol. 2011 doi: 10.1037/a0026485. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lewandowski AS, Palermo TM, De l Motte S, et al. Temporal daily associated between pain and sleep in adolescents with chronic pain versus healthy adolescents. Pain. 2010;151:220–225. doi: 10.1016/j.pain.2010.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Roizenblatt S, Tufik S, Goldenberg J, et al. Juvenile fibromyalgia: clinical and polysomnographic aspects. J Rheumatol. 1997;24:579–585. [PubMed] [Google Scholar]
- 67.Tayag-Kier CE, Keenan GF, Scalzi LV, et al. Sleep and periodic limb movement in sleep in juvenile fibromyalgia. Pediatrics. 2000;106:E70. doi: 10.1542/peds.106.5.e70. [DOI] [PubMed] [Google Scholar]
- 68.Aviel YB, Stremler R, Benseler SM, et al. Sleep and fatigue and the relationship to pain, disease activity and quality of life in juvenile idiopathic arthritis and juvenile dermatomyositis. Rheumatology. 2011;50:2051–2060. doi: 10.1093/rheumatology/ker256. [DOI] [PubMed] [Google Scholar]
- 69.Shapiro BS, Dinges DF, Orne EC, et al. Home management of sickle cell-related pain in children and adolescents: Natural history and impact on school attendance. Pain. 1995;61:139–144. doi: 10.1016/0304-3959(94)00164-A. [DOI] [PubMed] [Google Scholar]
- 70.Jacob E, Miashowski C, Savendra M, et al. Changes in sleep, food intake, and activity levels during acute painful episodes in children with sickle cell disease. J Pediatr Nurs. 2006;21:23–34. doi: 10.1016/j.pedn.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 71.Miller VA, Palermo TM, Powers SW, et al. Migraine headaches and sleep disturbances in children. Headache. 2003;43:362–368. doi: 10.1046/j.1526-4610.2003.03071.x. [DOI] [PubMed] [Google Scholar]
- 72.Rossi LN, Cortinovis I, Menegazzo L, et al. Classification criteria and distinction between migraine and tension-type headache in children. Dev Med Child Neurol. 2001;43:45–51. doi: 10.1017/s001216220100007x. [DOI] [PubMed] [Google Scholar]
- 73.Aaltonen K, Hämäläinen ML, Hoppu K. Migraine attacks and sleep in children. Cephalalgia. 2000;20:580–584. doi: 10.1046/j.1468-2982.2000.00089.x. [DOI] [PubMed] [Google Scholar]
- 74.Meltzer LJ, Logan DE, Mindell JA. Sleep patterns in female adolescents with chronic musculoskeletal pain. Behav Sleep Med. 2005;3:193–208. doi: 10.1207/s15402010bsm0304_2. [DOI] [PubMed] [Google Scholar]
- 75.Claar RL, Scharff L. Parent and child perceptions of chronic pain treatments. Child Health Care. 2007;36:285–301. [Google Scholar]
- 76.Connelly M, Bickel J. An electronic daily diary process study of stress and health behavior triggers of primary headaches in children. J Pediatr Psychol. 2011;36:852–862. doi: 10.1093/jpepsy/jsr017. [DOI] [PubMed] [Google Scholar]
- 77.Chalkiadis GA. Management of chronic pain in children. Med J Aust. 2001;175:476–479. doi: 10.5694/j.1326-5377.2001.tb143680.x. [DOI] [PubMed] [Google Scholar]
- 78.Degotardi PJ, Klass ES, Rosenberg BS, et al. Development and evaluation of a cognitive-behavioral intervention for juvenile fibromyalgia. J Pediatr Psychol. 2006;31:714–723. doi: 10.1093/jpepsy/jsj064. [DOI] [PubMed] [Google Scholar]
- 79.Bruni O, Galli F, Guidetti V. Sleep hygiene and migraine headache in children and adolescents. Cephalalgia. 1999;19:25–57. doi: 10.1177/0333102499019s2516. [DOI] [PubMed] [Google Scholar]
- 80.Connelly M, Bromberg MH, Anthony KK, et al. Emotion regulation predicts pain and functioning in children with juvenile idiopathic arthritis: An electronic diary study. J Pediatr Psychol. 2012;37:43–52. doi: 10.1093/jpepsy/jsr088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hoff AL, Palermo TM, Schluchter M, et al. Longitudinal relationships of depressive symptoms to pain intensity and functional disability among children with disease-related pain. J Pediatr Psychol. 2006;31:1046–1056. doi: 10.1093/jpepsy/jsj076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sandstrom MJ, Schanberg LE. Peer rejection, social behavior, and psychosocial adjustment in children with juvenile rheumatic disease. J Pediatr Psychol. 2004;29:29–34. doi: 10.1093/jpepsy/jsh004. [DOI] [PubMed] [Google Scholar]
- 83.Buysse DJ, Germain A, Nofzinger EA, et al. Mood disorders and sleep. In: Stein DJ, Kupfer DJ, Schatzberg AF, editors. The American Psychiatric Publishing Textbook of Mood Disorders. American Psychiatric Publishing, Inc; Washington, DC: 2006. pp. 717–737. [Google Scholar]
- 84.Dima AL, Gillanders DT, Power MJ. Dynamic pain-emotion relations in chronic pain: A theoretical review of moderation studies. Health Psychol Rev. 2011 online:1-68. [Google Scholar]
- 85.Carskadon MA. Adolescents Sleep Patterns: Biological, Social, and Psychological Influences. Cambridge University Press; New York: 2002. [Google Scholar]
- 86.Dahl RE, Lewin DS. Pathways to adolescent health sleep regulation and behavior. J Adolesc Health. 2002;31:175–184. doi: 10.1016/s1054-139x(02)00506-2. [DOI] [PubMed] [Google Scholar]
- 87.Lateef TM, Merikangas KR, He J, et al. Headache in a national sample of American children: Prevalence and comorbidity. J Child Neurol. 2009;24:536–43. doi: 10.1177/0883073808327831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Perquin CW, Hazebroek-Kampschreur AA, Hunfeld JA, et al. Pain in children and adolescents: A common experience. Pain. 2000;87:51–58. doi: 10.1016/S0304-3959(00)00269-4. [DOI] [PubMed] [Google Scholar]
- 89.LaPlant MM, Adams BS, Haftel HM, et al. Insomnia and quality of life in children referred for limb pain. J Rheumatol. 2007;34:2486–90. [PubMed] [Google Scholar]
- 90.Boergers J, Koinis-Mitchell D. Sleep and culture in children with medical conditions. J Pediatr Psychol. 2010;35:915–926. doi: 10.1093/jpepsy/jsq016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Evans S, Taub R, Tsao JC, et al. Sociodemographic factors in a pediatric chronic pain clinic: The roles of age, sex, and minority status in pain and health characteristics. J Pain Manag. 2010;3:273–281. [PMC free article] [PubMed] [Google Scholar]
- 92.Ward TM, Archbold K, Lentz M, et al. Sleep disturbance, daytime sleepiness, and neurocognitive performance in children with juvenile idiopathic arthritis. Sleep. 2010;33:252–259. doi: 10.1093/sleep/33.2.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tsai SY, Labyak SE, Richardson LP, et al. Actigraphic sleep and daytime naps in adolescent girls with chronic musculoskeletal pain. J Pediatr Psychol. 2008;33:307–311. doi: 10.1093/jpepsy/jsm117. [DOI] [PubMed] [Google Scholar]
- 94.Palermo TM, Fonareva I, Janosy NR. Sleep quality and efficiency in adolescents with chronic pain: Relationship with activity limitations and health-related quality of life. Behav Sleep Med. 2008;6:234–250. doi: 10.1080/15402000802371353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Owens JA, Dalzell V. Use of the ‘BEARS’ sleep screening tool in a pediatric residents’ continuity clinic: A pilot study. Sleep Med. 2005;6:63–69. doi: 10.1016/j.sleep.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 96.Lewandowski AS, Toliver-Sokol M, Palermo TM. Evidence-based review of subjective pediatric sleep measures. J Pediatr Psychol. 2011;36:780–793. doi: 10.1093/jpepsy/jsq119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Carskadon MA. Evaluation of excessive daytime sleepiness. Clin Neurophysiol. 1993;23:91–97. doi: 10.1016/s0987-7053(05)80287-4. [DOI] [PubMed] [Google Scholar]
- 98.Perlis ML, Jungquist C, Smith M, et al. Cognitive Behavioral Treatment of Insomnia: A Session-By-Session Guide. Springer; New York: 2005. [Google Scholar]
- 99.Vitiello MV, Rybarczyk B, Von Korff M, et al. Cognitive behavioral therapy for insomnia improves sleep and decreases pain in older adults with co-morbid insomnia and osteoarthritis. J Clin Sleep Med. 2009;5:355–362. [PMC free article] [PubMed] [Google Scholar]