Abstract
Adjuvants modulate protective CD8+ T cell responses generated by cancer vaccines. We have previously shown that immunostimulatory CpG ODN significantly augments tumor protection in mice given adenovirus cancer vaccines. Here, we examined the impact of chitosan, another candidate vaccine adjuvant, on protection conferred by adenovirus cancer vaccines. Unexpectedly, immunization of mice with adenovirus cancer vaccines in combination with chitosan provided little protection against tumor challenge. This directly correlated with reduced detection of Ag-specific CD8+ T cells, IFN-γ production and cytotoxic T cell activity. We ruled out immunosuppressive regulatory T cells since frequency did not change regardless of whether chitosan was delivered. In mammalian cell lines, chitosan did not interfere with adenovirus transgene expression. However, infection of primary murine bone marrow-derived dendritic cells with adenovirus complexed with chitosan significantly reduced viability, transgene expression and upregulation of MHC class I and CD86. Our in vitro observations indicate that chitosan dramatically inhibits adenovirus-mediated transgene expression and antigen presenting cell activation, without which CD8+ T cell activation cannot occur in vivo. These surprising data demonstrate for the first time that chitosan vaccine formulations can negatively impact the induction of CD8+ T cell responses via its effect on dendritic cells, which is clinically important since consideration of chitosan as an adjuvant for vaccine formulations is growing.
Keywords: Chitosan, CpG ODN, adenovirus, cancer vaccine, dendritic cell, CD8+ T cell
INTRODUCTION
The development of cancer vaccines is an area of intense interest since they have the potential to offer a safe and effective alternative to conventional modalities of cancer treatment, i.e. surgery, radiation therapy and chemotherapy. Among the choices for vaccine platforms capable of delivering tumor antigens (Ag) are recombinant, replication-deficient adenoviruses, which have been effectively used in several mouse tumor models1–9 and in Phase I clinical trials for prostate cancer therapy10. Their success is due to their ability to infect dendritic cells (DC), resulting in upregulation of MHC I and II and costimulatory molecules along with enhanced presentation of tumor Ag epitopes8, 11, 12. This ultimately results in the induction of protective Ag-specific cytotoxic T lymphocyte (CTL) responses that have been shown to be important for carrying out immune-mediated tumor rejection13. Recent studies using adenovirus-based tumor Ag vaccines have aimed to further improve their efficacy by the addition of adjuvants.
Bacterial DNA and certain oligonucleotides containing unmethylated CpG motifs can stimulate murine and human lymphocytes, whereas eukaryote DNA and methylated oligonucleotides cannot. Synthetic CpG ODN are known to directly stimulate B cells, macrophages and DCs, causing an increase in cytokine secretion, especially TH1- like cytokines such as IL-12 and IL-18, costimulatory molecule expression and antigen presentation14. As such, CpG ODN, a TLR9 agonist, has become increasingly popular as an adjuvant and has been shown to augment anti-tumor responses elicited by vaccines5, 6, 15–20.
Chitosan, a biocompatible and mucoadhesive polysaccharide, has been widely used in vaccination formulations because of its ability to enhance immunogenicity (21–26 and reviewed in27). This is in part due to its intrinsic adjuvant activity that has been reported to augment expression of the activation marker CD69 on murine B and T cells and to stimulate pro-inflammatory cytokine production from human PBMCs28. Furthermore, chitosan-based nanoparticle vaccines have been explored as a means to encapsulate protein Ags, thus providing protection from degradation during delivery and resulting in better uptake by antigen presenting cells (APC)29–33. Although chitosan has been used successfully in a range of vaccination strategies, the primary measure of its efficacy has been the development of Ag-specific antibodies. This effect has been reported in, but is not limited to, mouse models of vaccination with sheep red blood cells34, tetanus toxoid30, ovalbumin25, 32, 35, Hepatitis B surface Ag29, 33, 36, 37, inactivated influenza22, 23, 31, 38, influenza M1/M2 proteins26, 39 and pneumococcal surface antigen40. Studies into the effect of chitosan-formulated vaccines on protective CD8+ T cell responses are limited. Those that directly measured Ag-specific CD8+ T cell activity after vaccination have used chitosan not as an adjuvant, but as a delivery vehicle for immunostimulatory cytokines, i.e. GM-CSF41, IL-1242, and IL-1543 or chemotherapeutic drugs, i.e. doxorubicin44, 45. While these studies suggest that chitosan can be indirectly beneficial for CD8+ T cell responses when used to deliver secondary vaccination components, the effect of combining chitosan directly with promising adenovirus cancer vaccines remains unknown.
Here, we present data in which mice were given prophylactic adenovirus-based cancer vaccines, formulated with two distinct classes of adjuvants: chitosan and/or immunostimulatory CpG ODN. For these studies, OVA and prostate specific antigen (PSA) served as the model tumor Ags. Ovalbumin was utilized as a model protein antigen in our vaccine formulations, while PSA provided a model with specific clinical relevance. We confirmed that vaccines formulated with CpG ODN significantly increased protective tumor Ag-specific CD8+ T cell responses. In contrast, chitosan negatively modulated protective CD8+ T cell responses in both the OVA and PSA tumor models. The reduced responses in vivo corresponded directly to the negative impact of chitosan on viability, transduction and activation of primary murine bone marrow-derived dendritic cells (BMDC) in vitro. Our findings give further indication of the strong potential CpG ODN has as an adjuvant in adenovirus based cancer vaccines and describe for the first time some unexpected limitations of the use of chitosan in adenovirus vaccine formulations where the primary goal is induction of protective CD8+ T cell responses.
EXPERIMENTAL SECTION
Mice and tumor cell lines
All studies involving mice were approved by and performed according to guidelines established by the University of Iowa Institutional Animal Care and Use Committee. Inbred 6–8 week old male C57BL/6 and Balb/c were obtained from Jackson Laboratories and maintained in filtered cages. E.G7-OVA and EL4 tumor cell lines were obtained from American Type Culture Collection (Manassas, VA) and grown in RPMI-1640 (GIBCO, Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (Atlanta Biologicals, Lawrenceville, GA), 1mM sodium pyruvate (GIBCO), 10 mM HEPES (GIBCO), 0.05 mM 2-mercaptoethanol, and 50 µg/mL gentimicin sulfate (Mediatech, Inc., Manassas, VA). E.G7-OVA cell culture was also maintained with 0.4 mg/mL G418 (GIBCO). E5 (PSA expressers) and RM11 (PSA negative) mouse prostate tumor cell lines were generated as previously described3. These cells were maintained in DMEM (GIBCO) supplemented exactly as described for RPMI-1640.
Generation of adenovirus-CpG ODN-chitosan vaccine formulations
Replication deficient adenovirus encoding OVA, PSA, LacZ or GFP (Ad5-OVA, Ad5-PSA, Ad5-LacZ, or Ad5-GFP) was obtained from the University of Iowa Gene Transfer Vector Core (Iowa City, IA), as previously described3, 6. Endotoxin-free, nonmethylated CpG ODN ODN 1826 (5’-TCCATGACGTTCCTGACGTT-3’) with a phosphorothioate-modified backbone for nuclease resistance was obtained from Coley Pharmaceutical Group (Wellesley, MA). Low molecular weight chitosan (Sigma Aldrich, St. Louis, MO) was purified, characterized for degree of deacetylation, and resuspended in 1% glacial acetic acid at a final concentration of 10 mM. For vaccine formulations, 108 pfu of adenovirus, 50 µg CpG ODN, and a final chitosan concentration of 3.75 mM was used, unless otherwise noted. The concentration of chitosan used corresponds to a final nitrogen:phosphate ratio (N:P) of 10:1, which was determined to be optimal when complexing chitosan with DNA. Vaccine components were pre-mixed by vortexing and incubated for 30 min at room temperature to allow for nanoparticle complexation. All mice were vaccinated by subcutaneous injection in the right flank.
Particle size and zeta potential analysis
After mixing of adenovirus/CpG ODN/chitosan formulations, spontaneous nanoparticles form based on electrostatic interactions. Nanoparticle size measurements were conducted using the Zetasizer Nano ZS (Malvern, Southborough, MA), as previously described46. Briefly, the nanoparticles were suspended in deionized water at a concentration of 1 mg/mL. The size measurements were performed at 25°C at a 173° scattering angle. The mean hydrodynamic diameter was determined by cumulative analysis. The zeta potential determinations were based on electrophoretic, mobility of the nanoparticles in the aqueous medium, which were performed using folded capillary cells in automatic mode.
In vivo tumor challenge
For tumor challenge, C57BL/6 mice were anesthetized by intraperitoneal injection of a ketamine/xylazine mix, at a final concentration of 87.5 mg/kg ketamine and 2.5 mg/kg xylazine. E.G7-OVA and EL4 cells tumor cells were harvested from cell culture, washed and resuspended immediately prior to injection in room temperature sterile 1X PBS (GIBCO). Each mouse was challenged subcutaneously in the right flank with 107 tumor cells. Tumor outgrowth, determined by tumor size as a function of time, was measured twice a week and tumor volume was calculated by the equation for determining the volume of an ellipsoid: [(Diameter 1 × Diameter 2 × Height) × (π/6)], as previously described6.
Intracellular cytokine staining
Fourteen days after viral vaccination, splenocytes were prepared and restimulated with 5 µg of peptide Ag (OVA peptide=SIINFEKL258–265). After 4hrs, cells were stained with anti-CD8 and anti-CD3 mAbs (eBioscience, San Diego, CA). Following surface marker staining, cells were fixed, permeabilized and stained with IFN-γ-PE mAb, according to manufacturer’s protocol (BD Cytofix/Cytoperm™ Fixation/Permeabilization Kit, BD Biosciences, San Diego, CA). Samples were acquired on a FACScan flow cytometer (Becton Dickinson, Franklin Lakes, NJ) and data analyzed with FlowJo software (TreeStar, Ashland, OR).
Tetramer staining
Fourteen days after Ad5-OVA vaccination, splenocytes were prepared and the frequency of OVA-specific CD8+ T cells was determined by tetramer staining, as previously described6. The tetramer used was the H2-Kb SIINFEKL Class I iTAg™ MHC Tetramer (Kb-OVA257) (Beckman Coulter, Fullerton, CA). Surface molecules were stained with anti-CD8 and anti-CD3 mAbs (eBioscience). Samples were acquired and analyzed as described in ‘Intracellular cytokine staining’ section.
Measurement of cytotoxic T cell activity
To expand and detect OVA- or PSA-specific cytotoxic T cells, spleens were harvested 14 days after viral vaccination. Spleens were homogenized and RBCs removed by treatment with ACK lysis buffer, followed by washing and resuspension of splenocytes in complete RPMI-1640. OVA- or PSA-expressing stimulator cells were prepared by treatment with mitomycin C (50µg/mL) for 45 min at 37°C. Splenocytes and stimulator cells were then plated and incubated at 37°C in 6-well plates at a ratio of 50:1 with IL-2 added to a final concentration of 10 Units/mL. After 5 days, live splenocytes were harvested by Fico/Lite-LM (Atlanta Biologicals) and used as effectors in a 4hr Na51CrO4 release assay. 5×103 Na51CrO4-labeled targets were used per well. Supernatants were harvested after incubation and measured in a gamma counter (Beckman, Palo Alto, CA). Percent lysis was calculated by the following formula:
[(sample cpm-spontaneous release cpm) ÷ (max release cpm-spontaneous release cpm)]×100
Detection of CD4+Foxp3+ Treg cells
CD4+Foxp3+ splenocytes were detected using the mouse/rat-specific Foxp3 Staining Set (eBioscience), according to the manufacturer’s protocol. Briefly, spleens were harvested 14 days after immunization and splenocyte suspensions prepared as described before. Cells were first stained with anti-CD4 and anti-CD3 mAbs (eBioscience), fixed and permeabilized, and then stained with anti-Foxp3 mAb (eBioscience). Samples were acquired and analyzed as described in ‘Intracellular cytokine staining’ section.
In vitro infectivity assay
RM11 cells were seeded at 106/well in culture medium and infected with Ad5-GFP +/− chitosan at an MOI of 100 for 12, 24 or 36 hours. Cells were then harvested and flow cytometric analysis performed for GFP expression. Permissive HEK293 cells were incubated with Ad5-PSA +/− chitosan at an MOI of 100 for 36 hours, followed by detection of PSA in the culture supernatant by PSA-specific immunoassay (IMx, Abbott Laboratories, Chicago, IL). Primary murine bone marrow-derived DCs (BMDC) were generated as previously described47, and seeded at 106/well in DMEM culture medium supplemented with 20 ng/mL of recombinant murine GM-CSF (PeproTech, Rocky Hill, NJ). Cells were infected with Ad5-GFP or Ad5-OVA at MOIs of 100, 50, 25, 10 and 1 +/− chitosan at final concentrations of 500, 250, 125, 50, and 5 µM for 24 hours. Cells were stained with either anti-CD86 or anti-H-2Kb (MHC class I) mAb (eBioscience), or propidium iodide (PI) (Invitrogen, Eugene, OR). Samples were acquired and analyzed as described in ‘Intracellular cytokine staining’ section.
Statistical analysis
Tumor growth was analyzed by linear mixed effect models to estimate and compare group-specific tumor growth curves. Pairwise comparisons were performed to identify group differences (Table 1, Supporting Information). Survival differences between groups were compared using the log-rank test (Table 2, Supporting Information). All tests were two-sided and carried out at 5% level of significance. Tumor growth and survival curve analyses were performed using the SAS 9.2 software package by the Department of Biostatistics, College of Public Health at the University of Iowa. Tetramer and intracellular cytokine staining frequencies were compared using one-way ANOVA; PI staining frequencies and GFP, H-2Kb, and CD86 expression levels were compared using two-way ANOVA (GraphPad Prism version 5.00 for Windows, GraphPad Software, San Diego, CA, www.graphpad.com).
RESULTS
Chitosan abrogates tumor protection generated by adenovirus cancer vaccines
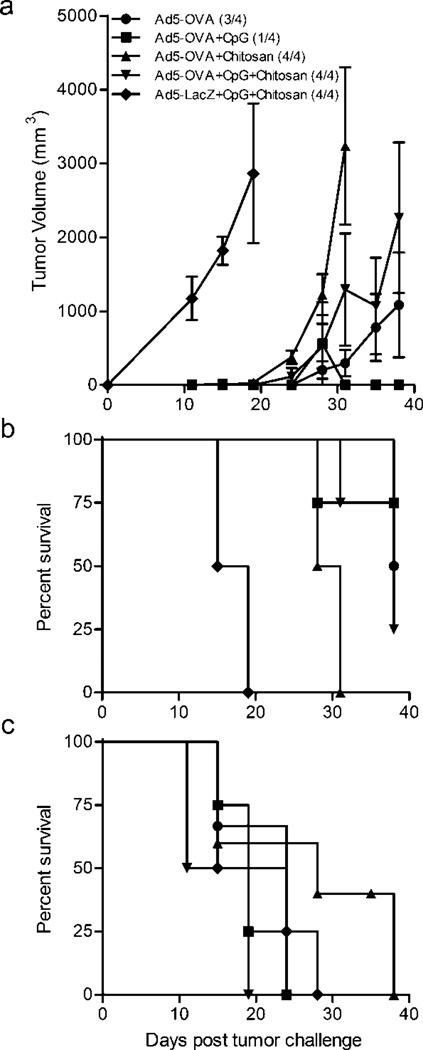
In this study, we tested the ability of adenovirus cancer vaccines complexed with chitosan to stimulated protective CD8+ anti-tumor responses. When chitosan is combined with DNA or adenoviruses, nanoparticles form (200–300 nm) through electrostatic interactions (Table 1). Mice immunized prophylactically with Ad5-OVA/chitosan nanoparticles had a significant reduction in tumor protection upon challenge with the OVA-expressing E.G7 tumor cells, as compared to Ad5-OVA alone or the positive control Ad5-OVA+CpG ODN immunized mice (Figure 1). Tumor incidence in mice immunized with chitosan-formulated Ad5-OVA or the negative control Ad5-LacZ was 100%, as compared to 75% and 25% for those immunized with Ad5-OVA and Ad5-OVA+CpG ODN, respectively (Figure 1a). Furthermore, mean tumor volumes were higher in mice whose immunizations were formulated with chitosan (Table 2) and tumor burdens in these mice resulted in reduced survival times (Figure 1b and Table 3). The reduction in tumor protection observed in the mice given Ad5-OVA+chitosan was tumor antigen (OVA) specific since none of the mice were protected when challenged with EL4 parental tumor cells (Figure 1c).
Table 1.
Size and zeta potential determinations for chitosan particle formulations
| Formulation | Diameter (nm) | Zeta Potential |
|---|---|---|
| Chitosan+CpG ODN | 256 ± 4.5 | 19.79 ± 2.24 |
| Chitosan+Adenovirus+CpG ODN | 291 ± 2.4 | 18.49 ± 5.45 |
Values are means ± SD from cumulative analysis of the particles formed.
N:P ratio used was 10:1, and is defined as the ratio of primary amino groups in chitosan to phosphate groups in DNA (CpG ODN).
Figure 1.
CpG ODN enhances and chitosan reduces in vivo tumor protection and survival generated by adenovirus cancer vaccines. C57BL/6 mice were challenged subcutaneously with E.G7-OVA (upper two panels) or parental EL4 (bottom panel) tumor cells 14 days after adenovirus immunization. (a) Tumor volume and (b,c) percent survival were determined over time. Means ± SEM are shown and numbers in parentheses (upper panel) indicate tumor incidence.
Table 2.
Summary statistics for tumor volume measurements
| Treatment Group | N | Mean (Tumor Volume) |
|---|---|---|
| AdOVA | 4 | 1585.75 |
| AdOVA+Chitosan | 4 | 2226.00 |
| AdOVA+CpG | 4 | 560.75 |
| AdOVA+CpG+Chitosan | 4 | 2819.75 |
| AdLacZ+CpG+Chitosan | 4 | 2484.75 |
Values were calculated from the mixed linear regression analysis of tumor growth.
Table 3.
Summary statistics for survival in days
| Treatment Group | Mean (Days) | Median (Days) |
|---|---|---|
| AdOVA | 48 | 48 |
| AdOVA+Chitosan | 29.5 | 29.5 |
| AdOVA+CpG | 52 | 60 |
| AdOVA+CpG+Chitosan | 40.75 | 38 |
| AdLacZ+CpG+Chitosan | 17 | 17 |
Generation of Ag-specific CD8+ T cell responses by adenovirus cancer vaccines is inhibited by chitosan
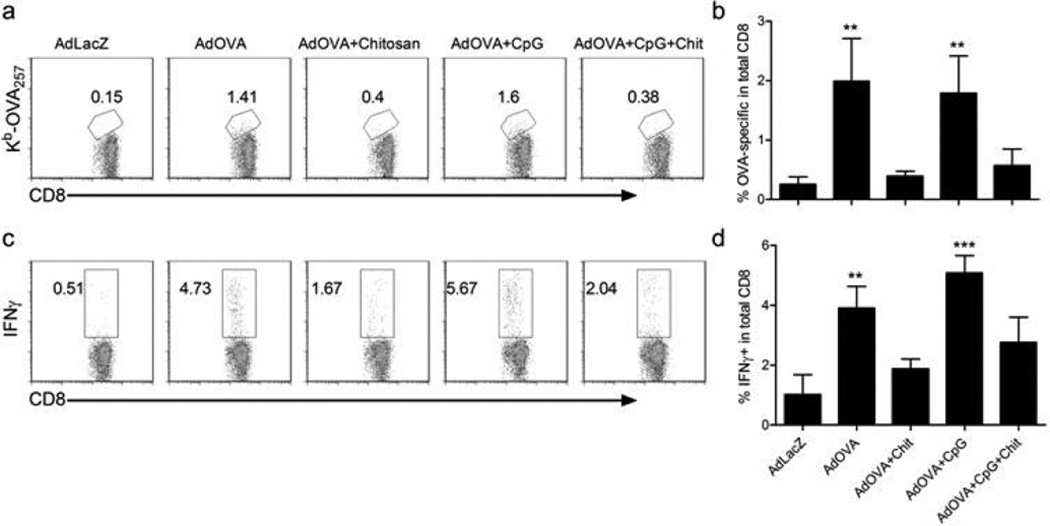
Since it has been previously shown that tumor protection conferred by adenovirus cancer vaccines is primarily mediated by CD8+ T cells3, 6, we wanted to determine the impact that the chitosan vaccination formulations were having on production of tumor Ag-specific CD8+ T cells, as compared to CpG ODN. Fourteen days after immunization, OVA-specific CD8+ T cells were not detected by Kb-OVA257 tetramer staining in mice immunized with Ad5-OVA +/− CpG formulated with chitosan, whereas excluding chitosan from the Ad5-OVA formulation (+/− CpG ODN) induced significantly higher frequencies of these cells, as compared to Ad5-LacZ controls (Figure 2a,b). These trends were mirrored in the frequencies of IFNγ+ CD8+ T cells detected by intracellular cytokine staining (Figure 2c,d). Of note was the apparent inability of CpG ODN to counteract the prohibitive effect of chitosan on Ad5-OVA-induced tumor protection and CD8+ T cell anti-tumor responses.
Figure 2.
Production of OVA-specific CD8+ T cells is inhibited by the addition of chitosan to adenovirus vaccines. (a,b) Detection by Kb-OVA257 tetramer staining and statistical analysis of the frequency of OVA-specific CD8+ splenocytes. (c,d) Intracellular cytokine staining for IFNγ and statistical analysis of the frequency of IFNγ+ CD8+ splenocytes. Analysis was performed 14 days after immunization of C57BL/6 mice. Means + SD from 3 independent experiments are shown. Statistical analysis was performed by comparing Ad5-OVA groups to the Ad5-LacZ control group. *, p<0.05, **, p<0.01, ***, p<0.001.
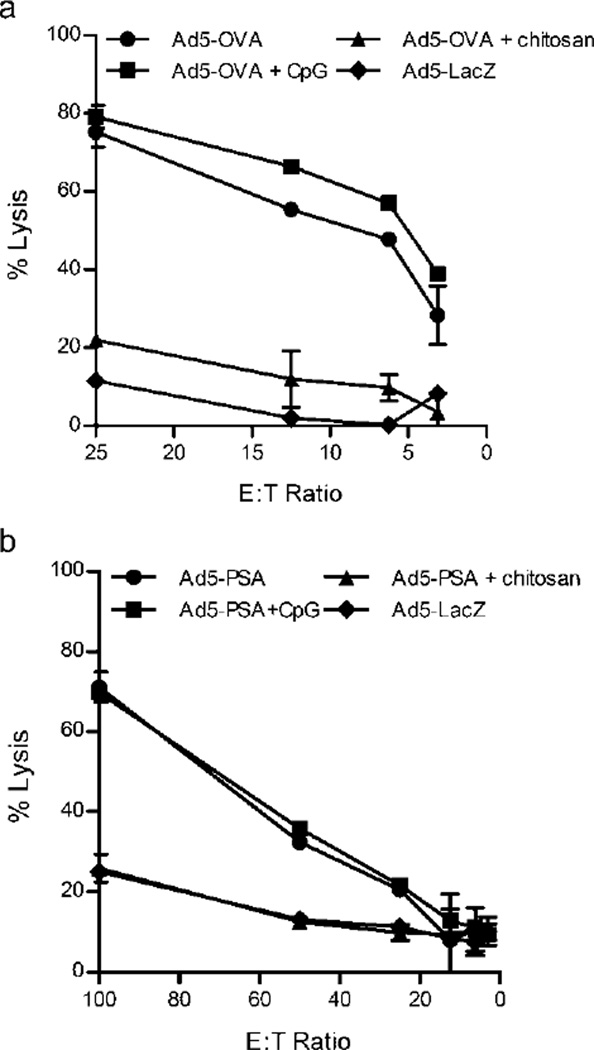
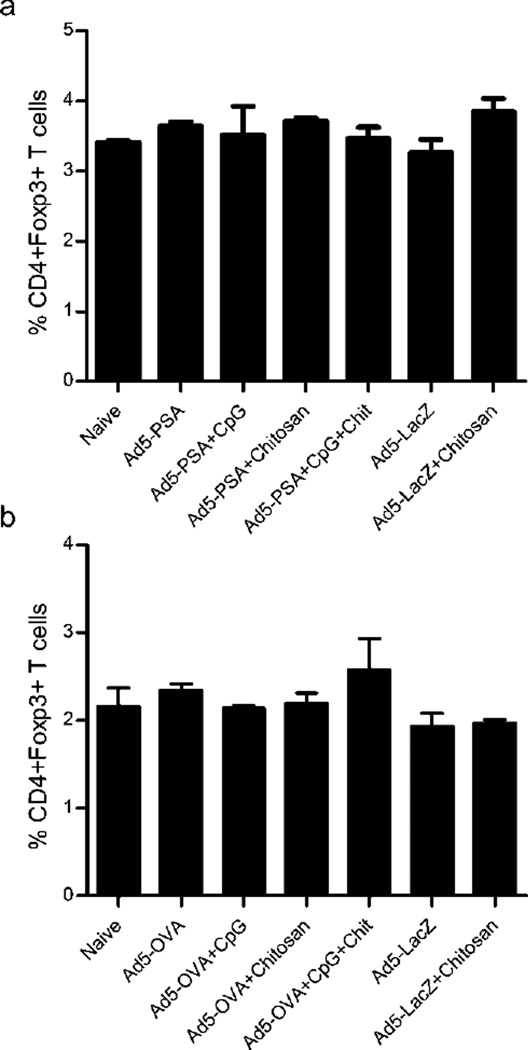
Next we wanted to corroborate our findings in the artificial OVA tumor antigen model with a more clinically relevant mouse tumor model of prostate cancer. To do this we compared the generation of tumor antigen-specific CTL activity in mice immunized with either Ad5-OVA or Ad5-PSA (prostate specific antigen) formulated with chitosan, or CpG ODN as a positive control (Figure 3). Immunization with either Ad5-OVA (Figure 3a) or Ad5-PSA (Figure 3b) +/− CpG ODN induced high tumor antigen-specific CTL activity. However we again observed a negative effect of chitosan with CTL activity completely eliminated, regardless of the tumor antigen model. The suppressed tumor-specific CTL activity did not appear to be related to an augmentation in the Treg population, since the frequency of CD4+Foxp3+ T cells was equivalent after immunization of mice with Ad5-PSA (Figure 4a) or Ad5-OVA (Figure 4b) +/− chitosan.
Figure 3.
Cytotoxic T cell activity is reduced in mice immunized with adenovirus/chitosan formulations. (a) Balb/c mice were immunized with Ad5-PSA and (b) C57BL/6 with Ad5-OVA +/− CpG +/− chitosan. Spleens were harvested 14 days later for determination of cytotoxic T cell activity against PSA-expressing (E5) or OVA-expressing (EG.7) target cells. Ad5-LacZ immunization served as a negative control. Means ± SD of samples performed in duplicate are shown.
Figure 4.
Immunization with adenovirus formulations containing chitosan does not augment Treg populations. (a) Balb/c mice were immunized with Ad5-PSA and (b) C57BL/6 with Ad5-OVA formulated with CpG and/or chitosan. CD4+Foxp3+ Treg cells were detected 14 days later by flow cytometry. Means ± SD from 2 mice/immunization group are shown.
Chitosan must complex with the adenovirus to reduce CD8+ T cell response induction
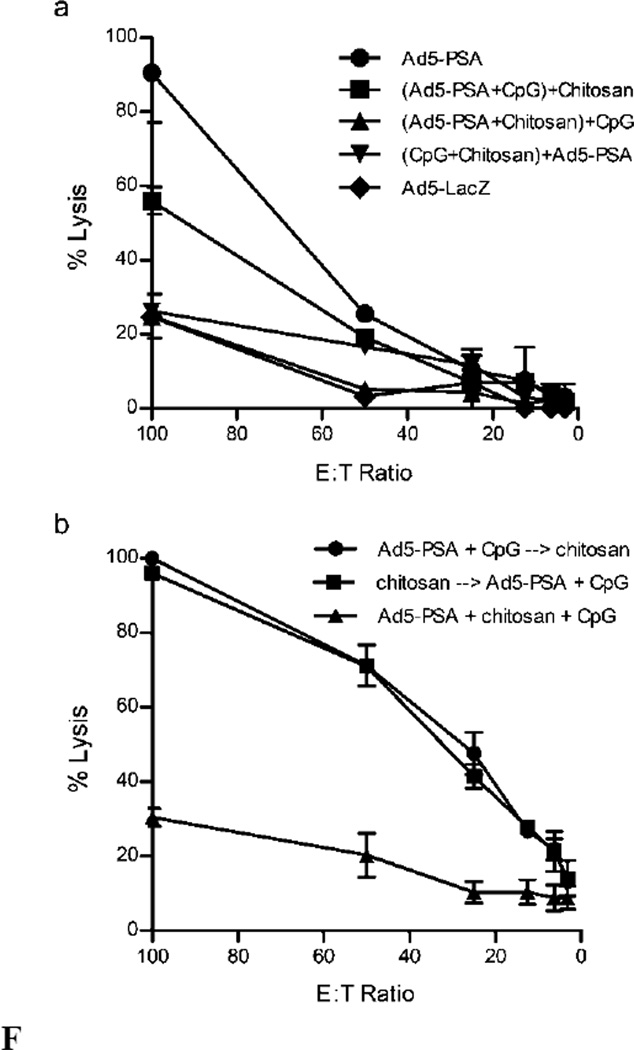
Because chitosan and the adenovirus interact through electrostatic interactions and complex to form nanoparticles, we next carried out experiments that determined if the degree of complexation was directly responsible for the reduced CD8+ T cell response. To test this, we first modified the way in which the formulations were mixed prior to immunization. Originally, all of the components were mixed and left to complex spontaneously for 30 minutes, followed by immunization of mice. Here, we altered the mixing order of the components to see if reducing the amount of time chitosan was allowed to complex with the virus would result in augmented CD8+ T cell responses. The formulations were mixed as follows: 1st component+2nd component, followed by 30 minute incubation and then the 3rd component added and mixed immediately prior to immunization. We found that any formulation containing chitosan still resulted in lower CTL activity, regardless of mixing order (Figure 5a). However, when chitosan was added as the 3rd component and therefore was not allowed to fully complex with the virus ((Ad5-PSA+CpG)+chitosan), CTL activity was not completely eliminated. The most inhibitory to PSA-specific CTL induction was the formulation where Ad5-PSA and chitosan were mixed first and incubated, with CpG ODN added last ((Ad5-PSA+chitosan)+CpG ODN). This data suggests that full complexation between these two components is most likely reducing CD8+ T cell responses normally generated by the adenovirus immunization.
Figure 5.
Chitosan-mediated reduction in CTL activity requires direct mixing with the adenovirus. (a) Ad5-PSA, CpG and chitosan were mixed in different orders ((1st + 2nd) + 3rd) prior to immunization of Balb/c mice, followed by determination of cytotoxic T cell activity 14 days later. (b) Balb/c mice were injected with chitosan 24 hrs before or after Ad5-PSA+CpG immunization, and 14 days later cytotoxic T cell activity was compared to mice immunized with a formulation containing all three components. Means ± SD of samples performed in duplicate are shown.
We also tested whether chitosan was capable of inhibiting CD8+ T cell responses when delivered separately from Ad5-PSA. To do this, we injected chitosan on its own either 24hrs before or after immunization with Ad5-PSA+CpG ODN and compared the CTL activity to that induced by immunization with all three components complexed together (Ad5-PSA+CpG ODN+chitosan) (Figure 5b). Administration of chitosan before or after adenovirus immunization did not inhibit induction of a CTL response, as compared to the complete lack of activity elicited by the fully complexed formulation. This demonstrates that chitosan inhibits CD8+ T cell responses when administered at the same time as the adenovirus vaccine.
Chitosan does not impair adenovirus infection of mammalian cell lines
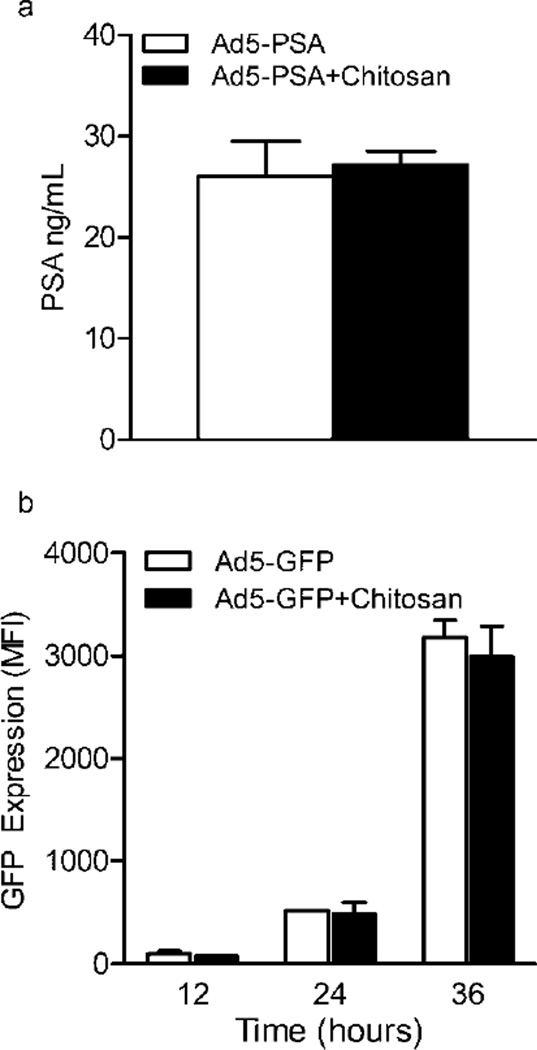
Since optimal transgene expression by our adenovirus vaccines is needed to induce T cell responses we tested whether chitosan complexation was inhibiting viral infectivity and/or expression of the model antigenic proteins encoded by the virus. We addressed this possibility with two separate assays based on infectivity of mammalian cell lines. First, HEK293 were infected with Ad5-PSA +/− chitosan for 36hrs, followed by detection of PSA in culture supernatants. Both treatments (+/− chitosan) resulted in similar levels of secreted PSA being detected in the culture supernatant (Figure 6a). Second, Rm11 cells were infected at an MOI of 100 with adenovirus encoding GFP (Ad5-GFP) +/− chitosan for 12, 24 or 36 hrs, followed by flow cytometric analysis of GFP expression levels (Figure 6b). At no timepoints did we observe an inhibitory effect of chitosan on GFP expression levels after infection of this cell line. As was previously reported48, these infectivity assays confirm that adenoviruses complexed with chitosan are able to infect mammalian cell lines, resulting in successful expression and secretion of their transgenes.
Figure 6.
Complexation of adenovirus with chitosan does not inhibit infectivity, production or secretion of the encoded transgene in mammalian cell lines. (a) HEK293 cells were incubated with Ad5-PSA at an MOI of 100 +/− chitosan for 36 hours, followed by detection of PSA in the culture supernatant. (b) Rm11 tumor cells were incubated with Ad5-GFP at an MOI of 100 +/− chitosan for 12, 24 and 36 hours, followed by flow cytometric analysis of GFP expression (mean fluorescence intensity, MFI). Means ± SD from samples performed in duplicate are shown.
Chitosan complexation with adenovirus reduces viability, infectivity and activation of primary murine BMDCs
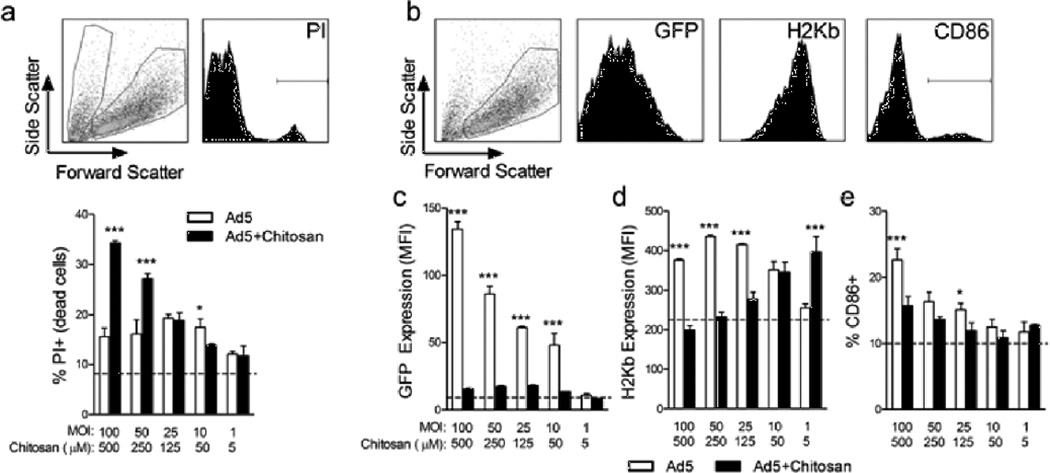
The use of mammalian cell lines to test adenovirus infectivity is commonly employed3, 48, but might not accurately reflect what occurs in vivo. The primary cellular targets of our adenovirus cancer vaccines in vivo are DCs, which act as potent APCs for CD8+ T cells. Thus we examined how infection with adenovirus complexed with chitosan impacted DC viability, transgene expression and maturation (Figure 7). Cells were incubated for 24 hrs with adenovirus +/− chitosan at one of five different viral MOIs/chitosan concentrations. The highest viral MOI/chitosan concentration used (MOI 100/500µM) corresponds to the amount delivered when immunizing mice. First, we observed that the formulations containing the two highest concentrations of chitosan resulted in significantly higher cell death, as compared to treatment with virus alone (Figure 7a). A similar reduction in viability was also observed when BMDCs were incubated with chitosan alone (data not shown), thus indicating an innate characteristic of this polymer. Next, expression of the GFP transgene, MHC class I (H-2Kb), and CD86 was analyzed, gating on live cells only (Figure 7b). Chitosan complexation with Ad5-GFP nearly eliminated GFP expression in BMDCs at all concentrations tested (Figure 7c), and at the highest concentrations significantly reduced H-2Kb and CD86 expression (Figure 7d,e). Comprehensively, these in vitro data show that chitosan dramatically affects DCs in a way that likely diminishes their function as APCs in vivo.
Figure 7.
Complexation of adenovirus with chitosan significantly reduces viability and activation of primary BMDCs. C57BL/6 BMDCs were infected in vitro for 24 hrs with Ad5-OVA or Ad5–GFP +/− chitosan at final viral MOIs of 100, 50, 25, 10 or 1 and final chitosan concentrations of 500, 250, 125, 50 or 5 µM. (a) Representative dot and histogram plots of BMDCs stained with PI to detect dead cells (scatter dot plot: left gate contains PI+ dead cells; right gate contains PI− live cells); statistical analysis of %PI+ dead cells/treatment group. (b) Representative dot and histogram plots (gated on live cells) of GFP, H-2Kb and CD86 expression. (c–e) Statistical analysis of GFP MFI, H-2Kb MFI and %CD86+ cells/treatment group. Dotted line represents levels observed in uninfected control cells. Means ± SD from treatment groups performed in triplicate are shown. *, p<0.05, **, ***, p<0.001.
DISCUSSION
Here we present surprising and novel data on the negative effect that chitosan has on anti-tumor CD8+ T cell responses elicited by adenovirus cancer vaccines. This was shown both quantitatively, i.e. enumeration by tetramer staining, and qualitatively, i.e. intracellular staining for IFN-γ, CTL activity, and tumor protection. The diminished development of CD8+ T cell responses was directly related to complexation between chitosan and the adenovirus, and ultimately, the negative impact of chitosan on DCs. Chitosan’s “three-pronged assault” on DCs, which resulted in reduced viability, transgene expression and activation spell out major hurdles for the successful use of this polymer as an adjuvant for induction of protective CD8+ T cell responses.
Given the implications of our novel findings, it is important to understand the difference between our study and common ways chitosan has been used in other vaccine studies. First, chitosan solutions have been applied to vaccine preparations where both its adjuvant activity and depot forming ability are desired21, 22, 24. The inherent viscosity of 0.5–1% chitosan solutions provides a gelatinous matrix that protects antigenic proteins from degradation after administration. While also acting as a potent adjuvant, chitosan solutions have been shown to enhance both the stability and immunogenicity of vaccines. Applications of chitosan solutions have revealed significant augmentation of Ag-specific antibody responses21–23, 26, 38, 39, but no evidence of benefiting CD8+ T cell responses. We based our vaccination strategy on another popular approach involving the administration of Ag-loaded chitosan nanoparticles25, 29–33. This strategy has also shown to result in significant antibody production25, 29–33, but again there is little data regarding how chitosan nanoparticle vaccinations affect CD8+ T cells. The polycationic nature of chitosan allows it to complex with antigenic peptides/proteins, or in our case CpG ODN and adenovirus, thus forming nanoparticulated vaccine components. Since we were testing a novel delivery of chitosan-adenovirus complexes we first confirmed the formation of nanoparticles. Upon mixing of chitosan and adenovirus we observed nanoparticles that were roughly 300 nm in diameter (Table 1), which is an optimal size for uptake by DCs49. Additionally, the nanoparticles exhibited a net positive surface charge, which should further enhance phagocytosis by APCs. Despite the promise of chitosan-Ag nanoparticle vaccines seen in other studies, we found that complexation with chitosan abrogates the Ag-specific CD8+ T-cell response stimulated by adenovirus cancer vaccines.
The mechanism by which CpG ODN enhances vaccine efficacy has been described previously5, 6, 18. Because this is the first report to look at the effect that chitosan complexation has on CD8+ T cell responses generated by adenovirus vaccines, we evaluated the mechanism by which inhibition was occurring. First, we examined the possibility that chitosan-containing vaccines were immunosuppressive in vivo. Treg populations are known to be a barrier to successful cancer vaccine and immunotherapy strategies that are reliant on strong induction of effector T cell responses50. So we quantified CD4+Foxp3+ Treg cells in spleens of vaccinated mice in order to determine if chitosan affected this population. Regardless of whether the vaccines contained chitosan or not, Treg frequencies were unaffected and equivalent to untreated mice (Fig. 4). This strongly suggests that this cell population is unaffected by chitosan and that they do not play a role in the lack of CD8+ T cell responses and tumor protection we observed.
The second explanation that we considered was that the physical complexation of chitosan with adenovirus was directly responsible for the effect observed in vivo. Indeed, we found that allowing for full complexation between chitosan and adenovirus resulted in the most dramatic inhibition of CD8+ T cell responses (Figure 5a) and that delivering chitosan separately from the adenovirus vaccine restored CTL activity (Figure 5b). Interestingly, we have found that complexation of our adenovirus cancer vaccine with another cationic polymer, polyethylenimine (PEI), also resulted in a significant reduction of CD8+ T cell responses in vivo; whereas, a non-cationic co-block polymer, pluronics F-127, that does not complex with the adenovirus, did not inhibit induction of T cell responses upon vaccination (unpublished data). Because these data implicated that complexation of chitosan with adenovirus might be inhibiting infectivity in vivo, we next tested this possibility using an in vitro infectivity assay. Consistent with previous findings48, we did not observe any reduction in transfection and transgene expression when mammalian cell lines were infected with adenovirus complexed with chitosan (Figure 6). However, when infectivity was tested in primary murine DCs, which are themain targets of adenovirus vaccine in vivo, we saw a significant reduction in transgene expression (Figure 7c). Additionally, adenovirus complexed with chitosan resulted in reduced surface MHC class I (H-2Kb) and CD86 (Figure 7d,e), and importantly caused dramatic cell death (Figure 7a). In support of these findings we observed in vivo that immunization with adenovirus complexed with chitosan resulted in modest, yet reproducible decreases in the frequency of CD11c+ dendritic cells found in the draining lymph nodes and in their surface expression of both CD86 and MHC class I (data not shown). Taken together these effects on DCs both in vitro and in vivo strongly suggest that direct complexation between chitosan and the adenovirus is responsible for reducing DC activation, which ultimately eliminates induction of CD8+ T cell responses.
This study highlights the differential modulation that different classes of adjuvants can have on the CD8+ T cell response stimulated by cancer vaccines. In this study, we confirmed that CpG ODN enhances the effectiveness of the adenovirus cancer vaccines5, 6, 18, while we show for the first time that chitosan reduces Ag-specific CD8+ T cell responses. This novel finding can be directly related to chitosan’s negative impact on the viability of DCs and its ability to inhibit viral vaccine infectivity upon complexation. We feel that our data provides an important delineation between studies that have demonstrated advantages of chitosan as an adjuvant for inducing protective humoral responses, and here where the goal was induction of protective CD8+ T cell responses. These data have serious implications for future chitosan vaccine formulations and should be carefully considered when determining the desired outcome of a vaccine approach.
Supplementary Material
ACKNOWLEDGEMENTS
We gratefully acknowledge support from the American Cancer Society (RSG-09-015-01-CDD), the National Cancer Institute at the National Institutes of Health (1R21CA13345-01/UI Mayo Clinic Lymphoma SPORE), and the Pharmaceutical Research and Manufacturers of America (PhRMA) Foundation. C. Lemke acknowledges support from the PhRMA foundation for a post-doctoral fellowship.
REFERENCES
- 1.Brossart P, Goldrath AW, Butz EA, Martin S, Bevan MJ. Virus-mediated delivery of antigenic epitopes into dendritic cells as a means to induce CTL. J Immunol. 1997;158(7):3270–3276. [PubMed] [Google Scholar]
- 2.Rosenberg SA, Zhai Y, Yang JC, Schwartzentruber DJ, Hwu P, Marincola FM, Topalian SL, Restifo NP, Seipp CA, Einhorn JH, Roberts B, White DE. Immunizing patients with metastatic melanoma using recombinant adenoviruses encoding MART-1 or gp100 melanoma antigens. J Natl Cancer Inst. 1998;90(24):1894–1900. doi: 10.1093/jnci/90.24.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elzey BD, Siemens DR, Ratliff TL, Lubaroff DM. Immunization with type 5 adenovirus recombinant for a tumor antigen in combination with recombinant canarypox virus (ALVAC) cytokine gene delivery induces destruction of established prostate tumors. Int J Cancer. 2001;94(6):842–849. doi: 10.1002/ijc.1556. [DOI] [PubMed] [Google Scholar]
- 4.Siemens DR, Elzey BD, Lubaroff DM, Bohlken C, Jensen RJ, Swanson AK, Ratliff TL. Cutting edge: restoration of the ability to generate CTL in mice immune to adenovirus by delivery of virus in a collagen-based matrix. J Immunol. 2001;166(2):731–735. doi: 10.4049/jimmunol.166.2.731. [DOI] [PubMed] [Google Scholar]
- 5.Lubaroff DM, Karan D, Andrews MP, Acosta A, Abouassaly C, Sharma M, Krieg AM. Decreased cytotoxic T cell activity generated by co-administration of PSA vaccine and CpG ODN is associated with increased tumor protection in a mouse model of prostate cancer. Vaccine. 2006;24(35–36):6155–6162. doi: 10.1016/j.vaccine.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 6.Karan D, Krieg AM, Lubaroff DM. Paradoxical enhancement of CD8 T cell-dependent anti-tumor protection despite reduced CD8 T cell responses with addition of a TLR9 agonist to a tumor vaccine. Int J Cancer. 2007;121(7):1520–1528. doi: 10.1002/ijc.22873. [DOI] [PubMed] [Google Scholar]
- 7.Xia D, Moyana T, Xiang J. Combinational adenovirus-mediated gene therapy and dendritic cell vaccine in combating well-established tumors. Cell Res. 2006;16(3):241–259. doi: 10.1038/sj.cr.7310032. [DOI] [PubMed] [Google Scholar]
- 8.Xie J, Xiong L, Tao X, Li X, Su Y, Hou X, Shi H. Antitumor effects of murine bone marrow-derived dendritic cells infected with xenogeneic livin alpha recombinant adenoviral vectors against Lewis lung carcinoma. Lung Cancer. 2009 doi: 10.1016/j.lungcan.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 9.Sorensen MR, Holst PJ, Pircher H, Christensen JP, Thomsen AR. Vaccination with an adenoviral vector encoding the tumor antigen directly linked to invariant chain induces potent CD4(+) T-cell-independent CD8(+) T-cell-mediated tumor control. Eur J Immunol. 2009;39(10):2725–2736. doi: 10.1002/eji.200939543. [DOI] [PubMed] [Google Scholar]
- 10.Lubaroff DM, Konety BR, Link B, Gerstbrein J, Madsen T, Shannon M, Howard J, Paisley J, Boeglin D, Ratliff TL, Williams RD. Phase I clinical trial of an adenovirus/prostate-specific antigen vaccine for prostate cancer: safety and immunologic results. Clin Cancer Res. 2009;15(23):7375–7380. doi: 10.1158/1078-0432.CCR-09-1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morelli AE, Larregina AT, Ganster RW, Zahorchak AF, Plowey JM, Takayama T, Logar AJ, Robbins PD, Falo LD, Thomson AW. Recombinant adenovirus induces maturation of dendritic cells via an NF-kappaB-dependent pathway. J Virol. 2000;74(20):9617–9628. doi: 10.1128/jvi.74.20.9617-9628.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller G, Lahrs S, Pillarisetty VG, Shah AB, DeMatteo RP. Adenovirus infection enhances dendritic cell immunostimulatory properties and induces natural killer and T-cell-mediated tumor protection. Cancer Res. 2002;62(18):5260–5266. [PubMed] [Google Scholar]
- 13.Russell JH, Ley TJ. Lymphocyte-mediated cytotoxicity. Annu Rev Immunol. 2002;20:323–370. doi: 10.1146/annurev.immunol.20.100201.131730. [DOI] [PubMed] [Google Scholar]
- 14.Wagner H. Interactions between bacterial CpG-DNA and TLR9 bridge innate and adaptive immunity. Curr Opin Microbiol. 2002;5(1):62–69. doi: 10.1016/s1369-5274(02)00287-4. [DOI] [PubMed] [Google Scholar]
- 15.Rieger R, Kipps TJ. CpG oligodeoxynucleotides enhance the capacity of adenovirus-mediated CD154 gene transfer to generate effective B-cell lymphoma vaccines. Cancer Res. 2003;63(14):4128–4135. [PubMed] [Google Scholar]
- 16.Tormo D, Ferrer A, Bosch P, Gaffal E, Basner-Tschakarjan E, Wenzel J, Tuting T. Therapeutic efficacy of antigen-specific vaccination and toll-like receptor stimulation against established transplanted and autochthonous melanoma in mice. Cancer Res. 2006;66(10):5427–5435. doi: 10.1158/0008-5472.CAN-06-0399. [DOI] [PubMed] [Google Scholar]
- 17.Salucci V, Mennuni C, Calvaruso F, Cerino R, Neuner P, Ciliberto G, La Monica N, Scarselli E. CD8+ T-cell tolerance can be broken by an adenoviral vaccine while CD4+ T-cell tolerance is broken by additional co-administration of a Toll-like receptor ligand. Scand J Immunol. 2006;63(1):35–41. doi: 10.1111/j.1365-3083.2006.01706.x. [DOI] [PubMed] [Google Scholar]
- 18.Lubaroff DM, Karan D. CpG oligonucleotide as an adjuvant for the treatment of prostate cancer. Adv Drug Deliv Rev. 2009;61(3):268–274. doi: 10.1016/j.addr.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 19.Aurisicchio L, Peruzzi D, Conforti A, Dharmapuri S, Biondo A, Giampaoli S, Fridman A, Bagchi A, Winkelmann CT, Gibson R, Kandimalla ER, Agrawal S, Ciliberto G, La Monica N. Treatment of mammary carcinomas in HER-2 transgenic mice through combination of genetic vaccine and an agonist of Toll-like receptor 9. Clin Cancer Res. 2009;15(5):1575–1584. doi: 10.1158/1078-0432.CCR-08-2628. [DOI] [PubMed] [Google Scholar]
- 20.Dharmapuri S, Peruzzi D, Mennuni C, Calvaruso F, Giampaoli S, Barbato G, Kandimalla ER, Agrawal S, Scarselli E, Mesiti G, Ciliberto G, La Monica N, Aurisicchio L. Coadministration of telomerase genetic vaccine and a novel TLR9 agonist in nonhuman primates. Mol Ther. 2009;17(10):1804–1813. doi: 10.1038/mt.2009.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zaharoff DA, Rogers CJ, Hance KW, Schlom J, Greiner JW. Chitosan solution enhances both humoral and cell-mediated immune responses to subcutaneous vaccination. Vaccine. 2007;25(11):2085–2094. doi: 10.1016/j.vaccine.2006.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghendon Y, Markushin S, Krivtsov G, Akopova I. Chitosan as an adjuvant for parenterally administered inactivated influenza vaccines. Arch Virol. 2008;153(5):831–837. doi: 10.1007/s00705-008-0047-4. [DOI] [PubMed] [Google Scholar]
- 23.Ghendon Y, Markushin S, Vasiliev Y, Akopova I, Koptiaeva I, Krivtsov G, Borisova O, Ahmatova N, Kurbatova E, Mazurina S, Gervazieva V. Evaluation of properties of chitosan as an adjuvant for inactivated influenza vaccines administered parenterally. J Med Virol. 2009;81(3):494–506. doi: 10.1002/jmv.21415. [DOI] [PubMed] [Google Scholar]
- 24.Saenz L, Neira-Carrillo A, Paredes R, Cortes M, Bucarey S, Arias JL. Chitosan formulations improve the immunogenicity of a GnRH-I peptide-based vaccine. Int J Pharm. 2009;369(1–2):64–71. doi: 10.1016/j.ijpharm.2008.10.033. [DOI] [PubMed] [Google Scholar]
- 25.Slutter B, Soema PC, Ding Z, Verheul R, Hennink W, Jiskoot W. Conjugation of ovalbumin to trimethyl chitosan improves immunogenicity of the antigen. J Control Release. 2010 doi: 10.1016/j.jconrel.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 26.Sui Z, Chen Q, Wu R, Zhang H, Zheng M, Wang H, Chen Z. Cross-protection against influenza virus infection by intranasal administration of M2-based vaccine with chitosan as an adjuvant. Arch Virol. 2010 doi: 10.1007/s00705-010-0621-4. [DOI] [PubMed] [Google Scholar]
- 27.Arca HC, Gunbeyaz M, Senel S. Chitosan-based systems for the delivery of vaccine antigens. Expert Rev Vaccines. 2009;8(7):937–953. doi: 10.1586/erv.09.47. [DOI] [PubMed] [Google Scholar]
- 28.Li H, Willingham SB, Ting JP, Re F. Cutting edge: inflammasome activation by alum and alum's adjuvant effect are mediated by NLRP3. J Immunol. 2008;181(1):17–21. doi: 10.4049/jimmunol.181.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Borges O, Cordeiro-da-Silva A, Tavares J, Santarem N, de Sousa A, Borchard G, Junginger HE. Immune response by nasal delivery of hepatitis B surface antigen and codelivery of a CpG ODN in alginate coated chitosan nanoparticles. Eur J Pharm Biopharm. 2008;69(2):405–416. doi: 10.1016/j.ejpb.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 30.Sayin B, Somavarapu S, Li XW, Sesardic D, Senel S, Alpar OH. TMC-MCC (N-trimethyl chitosan-mono-N-carboxymethyl chitosan) nanocomplexes for mucosal delivery of vaccines. Eur J Pharm Sci. 2009;38(4):362–329. doi: 10.1016/j.ejps.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 31.Hagenaars N, Verheul RJ, Mooren I, de Jong PH, Mastrobattista E, Glansbeek HL, Heldens JG, van den Bosch H, Hennink WE, Jiskoot W. Relationship between structure and adjuvanticity of N,N,N-trimethyl chitosan (TMC) structural variants in a nasal influenza vaccine. J Control Release. 2009;140(2):126–133. doi: 10.1016/j.jconrel.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 32.Slutter B, Plapied L, Fievez V, Sande MA, des Rieux A, Schneider YJ, Van Riet E, Jiskoot W, Preat V. Mechanistic study of the adjuvant effect of biodegradable nanoparticles in mucosal vaccination. J Control Release. 2009;138(2):113–121. doi: 10.1016/j.jconrel.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 33.Prego C, Paolicelli P, Diaz B, Vicente S, Sanchez A, Gonzalez-Fernandez A, Alonso MJ. Chitosan-based nanoparticles for improving immunization against hepatitis B infection. Vaccine. 2010 doi: 10.1016/j.vaccine.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 34.Marcinkiewicz J, Polewska A, Knapczyk J. Immunoadjuvant properties of chitosan. Arch Immunol Ther Exp (Warsz) 1991;39(1–2):127–132. [PubMed] [Google Scholar]
- 35.Bal SM, Slutter B, van Riet E, Kruithof AC, Ding Z, Kersten GF, Jiskoot W, Bouwstra JA. Efficient induction of immune responses through intradermal vaccination with N-trimethyl chitosan containing antigen formulations. J Control Release. 2010;142(3):374–383. doi: 10.1016/j.jconrel.2009.11.018. [DOI] [PubMed] [Google Scholar]
- 36.Borges O, Silva M, de Sousa A, Borchard G, Junginger HE, Cordeiro-da-Silva A. Alginate coated chitosan nanoparticles are an effective subcutaneous adjuvant for hepatitis B surface antigen. Int Immunopharmacol. 2008;8(13–14):1773–1780. doi: 10.1016/j.intimp.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 37.Sivakumar SM, Sukumaran N, Nirmala L, Swarnalakshmi R, Anilbabu B, Siva L, Anbu J, Shanmugarajan TS, Ravichandran V. Immunopotentiation of hepatitis B vaccine using biodegradable polymers as an adjuvant. J Microbiol Immunol Infect. 2010;43(4):265–270. doi: 10.1016/S1684-1182(10)60042-4. [DOI] [PubMed] [Google Scholar]
- 38.Chang H, Li X, Teng Y, Liang Y, Peng B, Fang F, Chen Z. Comparison of adjuvant efficacy of chitosan and aluminum hydroxide for intraperitoneally administered inactivated influenza H5N1 vaccine. DNA Cell Biol. 2010;29(9):563–568. doi: 10.1089/dna.2009.0977. [DOI] [PubMed] [Google Scholar]
- 39.Sui Z, Chen Q, Fang F, Zheng M, Chen Z. Cross-protection against influenza virus infection by intranasal administration of M1-based vaccine with chitosan as an adjuvant. Vaccine. 2010 doi: 10.1016/j.vaccine.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 40.Xu J, Dai W, Wang Z, Chen B, Li Z, Fan X. Intranasal vaccination with chitosan-DNA nanoparticles expressing pneumococcal surface antigen A (PsaA) protects mice against nasopharyngeal colonization by Streptocoocus pneumoniae. Clin Vaccine Immunol. 2010 doi: 10.1128/CVI.00263-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zaharoff DA, Rogers CJ, Hance KW, Schlom J, Greiner JW. Chitosan solution enhances the immunoadjuvant properties of GM-CSF. Vaccine. 2007;25(52):8673–8686. doi: 10.1016/j.vaccine.2007.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heffernan MJ, Zaharoff DA, Fallon JK, Schlom J, Greiner JW. In vivo efficacy of a chitosan/IL-12 adjuvant system for protein-based vaccines. Biomaterials. 2010 doi: 10.1016/j.biomaterials.2010.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang X, Zhang X, Kang Y, Jin H, Du X, Zhao G, Yu Y, Li J, Su B, Huang C, Wang B. Interleukin-15 enhance DNA vaccine elicited mucosal and systemic immunity against foot and mouth disease virus. Vaccine. 2008;26(40):5135–5144. doi: 10.1016/j.vaccine.2008.03.088. [DOI] [PubMed] [Google Scholar]
- 44.Han HD, Song CK, Park YS, Noh KH, Kim JH, Hwang T, Kim TW, Shin BC. A chitosan hydrogel-based cancer drug delivery system exhibits synergistic antitumor effects by combining with a vaccinia viral vaccine. Int J Pharm. 2008;350(1–2):27–34. doi: 10.1016/j.ijpharm.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 45.Seo SH, Han HD, Noh KH, Kim TW, Son SW. Chitosan hydrogel containing GMCSF and a cancer drug exerts synergistic anti-tumor effects via the induction of CD8+ T cell-mediated anti-tumor immunity. Clin Exp Metastasis. 2009;26(3):179–187. doi: 10.1007/s10585-008-9228-5. [DOI] [PubMed] [Google Scholar]
- 46.Intra J, Salem AK. Characterization of the transgene expression generated by branched and linear polyethylenimine-plasmid DNA nanoparticles in vitro and after intraperitoneal injection in vivo. J Control Release. 2008;130(2):129–138. doi: 10.1016/j.jconrel.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lutz MB, Kukutsch N, Ogilvie AL, Rossner S, Koch F, Romani N, Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223(1):77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- 48.Kawamata Y, Nagayama Y, Nakao K, Mizuguchi H, Hayakawa T, Sato T, Ishii N. Receptor-independent augmentation of adenovirus-mediated gene transfer with chitosan in vitro. Biomaterials. 2002;23(23):4573–4579. doi: 10.1016/s0142-9612(02)00203-x. [DOI] [PubMed] [Google Scholar]
- 49.Foged C, Brodin B, Frokjaer S, Sundblad A. Particle size and surface charge affect particle uptake by human dendritic cells in an in vitro model. Int J Pharm. 2005;298(2):315–322. doi: 10.1016/j.ijpharm.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 50.Mittendorf EA, Sharma P. Mechanisms of T-cell inhibition: implications for cancer immunotherapy. Expert Rev Vaccines. 2010;9(1):89–105. doi: 10.1586/erv.09.144. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.