The fetal circulation uses a series of adaptive mechanisms to maximize delivery of oxygen to the most metabolically active tissues. Blood returning from the placenta through the umbilical vein has the highest oxygen content, and is directed from the right atrium through the foramen ovale into the left atrium, where it is ejected from the left ventricle into the ascending aorta, thus becoming available to the heart and brain. Blood returning from the brain and heart has the lowest oxygen content, and is directed from the right atrium into the right ventricle. High pressure is maintained in the fetal pulmonary vasculature, which serves to help direct most of the right ventricular output through the ductus arteriosus into the descending aorta and back to the placenta through the umbilical arteries.
The fetal circulatory system must also prepare for a rapid and dramatic switch to allow oxygen uptake by the lungs at birth. In preparation for this perinatal redirection of blood flow, anatomic development and growth of the lungs must occur, but separate regulation of pulmonary vascular function is required to maintain the metabolic efficiency of the fetal circulation. As a result, despite the rapid increase in the number of small pulmonary arteries,1 high pulmonary vascular pressure is maintained by active vasoconstriction of fetal vessels.
After birth, the pulmonary vascular resistance (PVR) decreases and pulmonary vascular flow increases in response to lung expansion, increased oxygen tension, and increased pH caused by more efficient clearance of carbon dioxide. The normal perinatal physiologic changes start to occur within the first few breaths, and full pulmonary perfusion is normally achieved within minutes after birth. Pulmonary artery pressure and vascular resistance decrease more slowly, with pulmonary arterial pressure reaching its nadir approximately 2 to 3 weeks after birth.
PULMONARY HYPERTENSION
Persistent pulmonary hypertension of the newborn (PPHN) is the result of an abnormal early adaptation to the perinatal circulatory transition. PPHN is a syndrome characterized by common pathophysiologic features including sustained elevation of PVR; decreased perfusion of the lungs; and continued right-to-left shunting of blood through the fetal channels (foramen ovale and ductus arteriosus). When PVR remains high after birth, right (and sometimes left) ventricular function and cardiac output are depressed. Moderate or severe PPHN is believed to affect up to 2 to 6 per 1000 live births, and complicates the course of 10% of all infants admitted to neonatal intensive care.2 These circulatory abnormalities are also responsible for an 8% to 10% risk of death and a 25% risk of long-term neurodevelopmental morbidity.
PPHN occurs in association with a diverse group of neonatal respiratory illnesses, but generally presents as one of three patterns: (1) a structurally normal but abnormally constricted pulmonary vasculature, which is the most common type and includes such diagnoses as meconium aspiration syndrome, respiratory distress syndrome, and sepsis; (2) a structurally abnormal vasculature that arises from antenatal remodeling, and is often termed “idiopathic PPHN”; or (3) a hypoplastic vasculature, such as is seen in congenital diaphragmatic hernia (CDH) or alveolar capillary dysplasia, a rare malformation of lung development. Although idiopathic pulmonary hypertension occurs in the minority (~20%–25%) of infants with PPHN, severe cases of meconium aspiration syndrome are almost certainly affected by parenchymal and vascular disease.
Significant pulmonary hypertension may also develop in neonates and young infants as a result of bronchopulmonary dysplasia (BPD) or cardiac disease. Pulmonary hypertension affects roughly one-third of infants with moderate-to-severe BPD,3,4 and results in greater morbidity and mortality, poor growth and neurodevelopmental outcome, long-term mechanical ventilation support, and death caused by right heart dysfunction and multiorgan failure.5,6 There is an increasing awareness that this association occurs frequently in high-risk populations. For instance, in a recent epidemio-logic study, Slaughter and colleagues3 reported a 37% incidence of pulmonary hypertension at 1 month of life among chronically ventilated BPD infants.
ENDOGENOUS REGULATORS OF PULMONARY VASCULAR TONE
Pulmonary vascular tone is mediated by competing vasodilatory and vasoconstrictive factors, with interrelated signaling pathways. The balance of expression and activity of intermediates in these signaling pathways determines the net pulmonary vascular tone (Figs. 1 and 2). As gestation progresses, mediators of pulmonary vasodilation become more dominant.
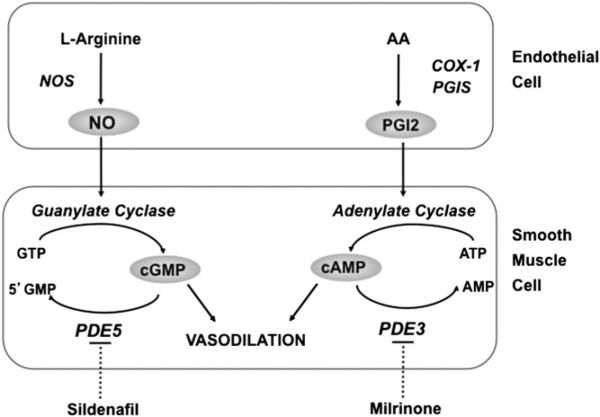
Fig. 1.
Nitric oxide (NO) and prostacyclin (PGI2) signaling pathways in the regulation of pulmonary vascular tone. NO is synthesized by NO synthase (NOS) from the terminal nitrogen of l-arginine. NO stimulates soluble guanylate cyclase (sGC) to increase intracellular cGMP. PGI2 is an arachidonic acid (AA) metabolite formed by cyclooxygenase (COX-1) and prostacyclin synthase (PGIS) in the vascular endothelium. PGI2 stimulates adenylate cyclase in vascular smooth muscle cells, which increases intracellular cAMP. Both cGMP and cAMP indirectly decrease free cytosolic calcium, resulting in smooth muscle relaxation. Specific phosphodiesterases (PDE) hydrolyze cGMP and cAMP, thus regulating the intensity and duration of their vascular effects. Inhibition of these PDE with such agents as sildenafil and milrinone may enhance pulmonary vasodilation. (From Steinhorn RH. Lamb models of pulmonary hypertension. Drug Discov Today Dis Models 2010;7:99–105; with permission.)
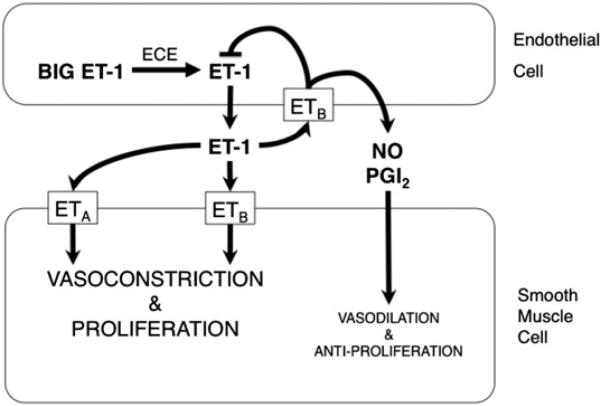
Fig. 2.
Endothelin (ET)-1 signaling pathway in the regulation of pulmonary vascular tone. Big ET-1 is cleaved to ET-1 by endothelin converting enzyme (ECE) in endothelial cells. ET-1 binds its specific receptors ETA and ETB with differential effects. Binding of ET-1 to ETA or ETB on smooth muscle cells leads to vasoconstrictive and proliferative effects. ETB are transiently expressed on endothelial cells after birth; binding of ET-1 to ETB on endothelial cells leads to downregulation of ECE activity and increased production of nitric oxide (NO) and prostacyclin (PGI2), which led to vasodilation and antiproliferation.
The postnatal stimuli that lead to pulmonary vasodilation (lung inflation with a gas, increase in oxygen tension, and decrease in carbon dioxide tension) each independently decrease PVR and increase pulmonary blood flow, but they also interact with each other synergistically. For instance, oxygen directly and indirectly stimulates the activity of endothelial nitric oxide synthase (eNOS) and cyclooxygenase (COX)-1 immediately after birth, leading to increased levels of the vasodilators, NO, and prostacyclin (PGI2). Shear stress is also known to regulate the synthesis of NO in the fetal circulation. During the perinatal transition, the initial increase in pulmonary blood flow in response to ventilation or oxygenation likely leads to increased shear stress in the vasculature, which further potentiates NO production. In contrast, type-5 phosphodiesterase (PDE5) expression and activity normally fall after birth in the pulmonary vasculature,7,8 further accentuating upstream effects leading to increased cyclic guanosine monophosphate (cGMP) and vasodilation.
Understanding the mechanisms that lead to cardiopulmonary dysfunction in PPHN is essential to selecting appropriate pharmacotherapy. Because of pathophysiologic differences among the patterns of PPHN, not all therapeutic interventions have equal effectiveness. Successful approaches in infants with lung injury may not be as successful in infants with developmental lung disorders. Understandably, many advances in PPHN therapy are derived from results of laboratory-based research. A newborn lamb created by antenatal ductal ligation is frequently used for PPHN investigations because the lambs display a phenotype that is strikingly similar to severe PPHN in human infants.9 They have pulmonary vascular smooth muscle cell hypertrophy and require aggressive care after birth because of high mortality, severe hypoxemia and respiratory failure, and circulatory instability. Models of pulmonary hypertension caused by BPD are even more difficult to generate, but have been developed in mice, rats, and baboons exposed to hyperoxia or mechanical ventilation.
PHARMACOTHERAPY FOR PULMONARY HYPERTENSION
The primary aim of PPHN therapy is selective pulmonary vasodilatation. In all cases, treatment of pulmonary hypertension includes optimization of lung function and oxygen delivery, and support of cardiac function. Optimal lung inflation is essential because PVR is increased when the lungs are underexpanded or overexpanded, independent of lung disease. The use of lung recruitment strategies, such as high-frequency ventilation and exogenous surfactant administration, is particularly important in infants with PPHN associated with parenchymal disease, but has limited impact in infants with primary vascular disease. Correction of severe acidosis and avoidance of hypoxemia are important because both stimuli promote pulmonary vasoconstriction. Maintaining a normal hematocrit is also important to ensure adequate oxygen carrying capacity while avoiding polycythemia, because hyperviscosity can increase PVR. The focus of this article is the pharmacotherapies that specifically target pulmonary vascular tone.
Nitric Oxide
NO is the gas molecule produced endogenously by the conversion of arginine to citrulline by the enzyme NOS. Three isoforms of NOS exist, although eNOS is regarded as the most important regulator of NO production in the perinatal lung vasculature. NO generated in the endothelium readily diffuses into adjacent vascular smooth muscle cells where it stimulates soluble guanylate cyclase activity and increases cGMP, the critically important second messenger that mediates the vasodilatory pathway.
Lung eNOS mRNA and protein are present in the early fetus, but both increase toward the end of gestation, so that the lung is poised to adapt to the postnatal need for pulmonary vasodilation. This increase in lung eNOS content also explains the growing capacity of the fetal pulmonary vasculature to respond to endothelium-dependent vasodilators, such as oxygen and acetylcholine.10 Many factors associated with pulmonary hypertension have the capacity to perturb eNOS function, even if protein levels are sufficient. Presumably, this is because the normal catalytic function of eNOS depends on numerous posttranslational modifications, including association with the chaperone protein Hsp90 and availability of essential substrates and cofactors including l-arginine, tetrahydrobiopterin (BH4), the reduced form of nicotinamide adenine dinucleotide phosphate, and calcium–calmodulin. Depletion of Hsp90 or biopterin reduces production or bioavailability of NO, but also “uncouples” eNOS, resulting in incomplete reduction of molecular oxygen with subsequent formation of superoxide, essentially turning the enzyme into a source of oxidant stress.11,12
Inhaled NO (iNO) has many features of an ideal pulmonary vasodilator, including a particularly rapid onset of action, typically within minutes. Because eNOS is decreased or dysfunctional in PPHN, iNO could provide specific replacement therapy that is delivered by inhalation directly to airspaces approximating the pulmonary vascular bed. Although it is most commonly administered with mechanical ventilation, iNO can also be provided by continuous positive airway pressure or nasal cannula devices, although the concentration may need to be increased to account for the entrainment of room air.13 It has been assumed that because NO is a small lipophilic molecule, iNO simply diffuses from alveoli through epithelial cells to gain direct access to the vasculature. However, it is now understood that NO is a free radical that can be inactivated through interaction with reactive oxygen species found in the alveolar space or alveolar lining. Others propose that NO gas gains entry into alveolar epithelium in part by forming a nitrosothiol derivative of cysteine that enters by an amino acid transporter.14 Once in the bloodstream, NO binds avidly to hemoglobin, which is subsequently reduced by methemoglobin reductase.
The safety and efficacy of iNO for PPHN have been particularly well studied through large placebo-controlled trials. In most of these, the use of extracorporeal life support (ECMO) served as a primary endpoint in combination with mortality (Table 1). iNO significantly decreases the need for ECMO support in newborns with PPHN. However, it should be noted that up to 40% of infants did not improve oxygenation or maintain a response to iNO, and iNO did not reduce mortality or length of hospitalization in any study. By encompassing a range of disease severity, these studies also highlight that starting iNO for respiratory failure that is in earlier stages of evolution (for an oxygenation index of 15–25) did not decrease the incidence of ECMO or death or improve other patient outcomes (see Table 1), including the incidence of neurodevelopmental impairment.15,16 However, delaying iNO initiation until respiratory failure is advanced (oxygenation index of >40) may increase length of time on oxygen.17 In longer-term follow-up, iNO did not significantly alter the incidence of chronic lung disease or neurodevelopmental impairment relative to placebo. This last observation may indicate that the underlying disease is associated with early neurologic injury that cannot be reversed by NO.
Table 1.
Summary of the large, multicenter, randomized trials of iNO in term newborns with hypoxemic respiratory failure or PPHN, showing the effect of iNO on ECMO use, mortality, and neurodevelopmental impairment
| Initial Oxygenation Index |
% ECMO |
% Mortality |
% Neurodevelopmental Impairment |
|||||
|---|---|---|---|---|---|---|---|---|
| Study | N | Control | iNO | Control | iNO | Control | iNO | |
| Neonatal Inhaled | 235 | 44 | 55 | 39* | 17 | 14 | 30 | 35 |
| Nitric Oxide | ||||||||
| Study Group19 | ||||||||
| Roberts et al75 | 58 | 44.4 | 71 | 40* | 7 | 7 | – | – |
| Clark et al76,77 | 248 | 39 | 65 | 48* | 8 | 7 | 14 | 19 |
| Davidson et al22 | 155 | 24.7 | 34 | 22 | 2 | 8 | 20 | 18 |
| Konduri et al15,16 | 299 | 19.2 | 12 | 10 | 9 | 7 | 25 | 28 |
P<.05; significant reduction.
After the introduction of high-frequency ventilation, surfactant, and iNO in the early 1990s, the patient demographic of neonatal support with ECMO changed. Data from the large registry maintained by the Extracorporeal Life Support Organization indicate that the use of these therapies has increased steadily since they have become clinically available, accompanied by a greater than 40% reduction in the number of neonates cannulated for ECMO. However, because overall ECMO survival has diminished over the same time period, some physicians have speculated that these new treatment modalities may delay ECMO cannulation and negatively affect mortality and morbidity in those infants that require ECMO. In an analysis of data from the Extracorporeal Life Support Organization registry between 1996 and 2003, use of iNO, high-frequency ventilation, and surfactant was not associated with any adverse outcomes during ECMO, including increased hours on ECMO or increased time to extubation.18 Furthermore, surfactant and iNO use were associated with lower ECMO mortality, and iNO use was associated with a decreased risk of cardiac arrest before cannulation. Because ECMO has been proved to reduce mortality for severe respiratory failure, it is reassuring that these new therapies have not had a negative impact on the most severely affected infants. More likely, the increase in ECMO mortality represents increased use in sicker infants who have failed to respond to advanced medical therapies.
The most important criterion for starting iNO is evidence for pulmonary hypertension. Clinical manifestations of PPHN include differential saturation (higher Spo2 in the right upper extremity compared with a lower extremity in most cases), labile oxygenation, or profound hypoxemia despite optimal mechanical strategies to expand the lungs. Unfortunately, these findings are not specific for PPHN, and the accurate diagnosis of PPHN requires echocardiography to rule out congenital heart disease and rule in extrapulmonary shunting. Echocardiography also rules out left ventricular insufficiency, which could trigger pulmonary venous hypertension that would only be aggravated by a pulmonary vasodilator.
Based on the available data, iNO should be initiated at a dose of 20 ppm when there is evidence of PPHN and significant hypoxemia. Studies that adjusted the iNO dosing based on oxygenation response showed that failure to respond to 20 ppm was only rarely followed by improvement at higher doses.19 Other studies used lower starting doses, but produced concern that inadequate early treatment could diminish the oxygenation response if escalation of the iNO dose became necessary.20 Fineman and colleagues21 directly measured the pulmonary artery pressure by catheterization to study the effect of delivery of iNO at 5, 20, and 40 ppm in random order. Oxygenation was improved with all three iNO doses, but a significant improvement in the ratio of pulmonary to systemic artery pressure was only seen when the iNO dose was at least 20 ppm. However, increased methemoglobinemia and nitrogen dioxide levels were reported with doses greater than 20 ppm for as little as 24 hours.22
Given its delivery by inhalation, the areas of the lung that are preferentially ventilated will receive more exogenous NO, resulting in improvement in ventilation–perfusion matching. The greatest improvement in oxygenation after iNO would be expected in infants with extrapulmonary right-to-left shunting but without significant pulmonary parenchymal disease. Even if the total PVR does not decrease, iNO can improve oxygenation through a “microselective” effect that reduces intrapulmonary shunting. For this reason, neonates with significant parenchymal pulmonary disease are less likely to respond to iNO unless lung expansion is optimized with high-frequency ventilation or surfactant administration.23
Data addressing optimal weaning procedures for iNO are more limited. After oxygenation improves, iNO usually can be weaned relatively rapidly to 5 ppm without difficulty. In the large randomized studies, most infants were treated with iNO for less than 5 days. Infants who remain hypoxemic with evidence of PPHN beyond that time are more likely to have an underlying cause of dysregulated pulmonary vascular tone, such as developmental lung abnormalities like alveolar capillary dysplasia,24 severe pulmonary hypoplasia, or progressive lung injury. When iNO is stopped abruptly, “rebound” pulmonary hypertension sometimes develops.25 This interesting phenomenon can occur even if no improvement in oxygenation was observed, can be life-threatening, and has raised questions about whether vascular cells respond to NO by upregulating vasoconstrictor pathways.26,27 However, from a practical standpoint, the clinical problem can usually be overcome by weaning iNO to 1 ppm before discontinuation.
The same mechanisms that contribute to rebound pulmonary hypertension can interfere with the response to iNO. The reason some patients do not respond to iNO may be related to the severe antenatal lung vascular injury with impaired vasodilation caused by altered downstream signaling pathways, including soluble guanylate cyclase and cGMP-PDEs. For example, studies in newborn lambs indicate that iNO responsiveness is significantly blunted after even brief (30 minute) periods of ventilation with 100% O2,28,29 and that oxidant stress alters NO responsiveness in part through increasing expression and activity of cGMP-specific PDEs.30 NO can also react with other radicals, such as superoxide to form peroxynitrite (ONOO–), an exceptionally reactive oxidant that can aggravate lung injury; produce nonspecific nitration of proteins, such as eNOS; and cause further dysregulation of vasoactive signaling pathways.31,32
The use of iNO for infants with pulmonary hypertension associated with CDH is less clearly defined. The lungs of infants with CDH are characterized by parenchymal and vascular hypoplasia, and typically do not respond to lung recruitment measures. Randomized studies have not demonstrated a consistent improvement in oxygenation after early use of iNO in these infants.33 However, the benefits of stabilizing infants and preventing cardiac arrest before ECMO suggest a potential role for iNO in some infants with CDH.18 Worsening pulmonary hypertension after surgical repair of the diaphragmatic defect may also benefit from iNO treatment, and noninvasive delivery techniques may allow for long-term therapy.13
Although less well studied in humans, iNO may also have a role in the management of infants with pulmonary hypertension caused by chronic lung disease. Up to one-third of premature infants with moderate or severe BPD develop some degree of pulmonary hypertension or cor pulmonale.3,4 Although the alterations in pulmonary vascular tone signaling are not identical to those seen in PPHN, alterations in eNOS and NO signaling seem to play a role in the vascular and lung injury.34 Preclinical studies also indicated that prolonged NO inhalation in preterm baboons or rats with hyperoxia-induced lung injury improved lung compliance and lung growth and architecture.35,36 Clinical trials have shown a modest benefit when iNO is used to prevent BPD,37,38 although one large trial found that a more prolonged period of inhalation in high-risk infants led to improvements in lung function that persisted through 12 months of age.39,40 A recent case series also indicates that iNO may reduce established BPD-associated pulmonary hypertension to a greater degree than oxygen alone.41 However, these studies certainly highlight the complex, multifactorial nature of BPD, and further investigation is needed to understand the role of endogenous and inhaled NO for BPD prevention and therapy.
Sildenafil
cGMP is the second messenger that regulates contractility of smooth muscle through activation of cGMP-dependent kinases, PDEs, and ion channels. In vascular smooth muscle cells, NO-mediated activation of soluble guanylate cyclase is a major source of cGMP production. Because cGMP is such a central mediator of vascular contractility, it is not surprising that its concentrations are regulated within a relatively narrow range to allow fine-tuning of vascular responses to oxygen, NO, and other stimuli.
PDEs are a large family of enzymes that hydrolyze and inactivate cGMP and cAMP, thus regulating their concentrations and effects, and facilitating “cross-talk” between the two cyclic nucleotides. PDE5 is especially highly expressed in the lung, and not only uses cGMP as a substrate but also contains a specific cGMP binding domain that serves to activate its catabolic activity. As the primary enzyme responsible for regulating cGMP, PDE5 may well represent the most important regulator of NO-mediated vascular relaxation in the normal pulmonary vascular transition after birth.42
Fetal and neonatal lung development, along with commonly used neonatal intensive care unit therapies, seems to regulate PDE expression and activity. In developing lambs and rats, PDE5 is expressed according to specific developmental trajectories that result in a peak of expression during late fetal life, followed by an acute fall around the time of birth.8,43 This drop in PDE5 activity would be expected to amplify the effects of NO produced by birth-related stimuli, such as oxygen and shear stress. In contrast, when pulmonary vessels of fetal lambs undergo remodeling by chronic intrauterine pulmonary hypertension, PDE5 activity increases relative to controls.44,45 Even more striking is that after birth, PDE5 activity does not fall in PPHN lambs, but rather increases dramatically above fetal levels, and above levels observed in spontaneously breathing or ventilated control lambs.8,45 This abnormal increase in activity would be expected to diminish responses to endogenous and exogenous NO, and could explain the incomplete clinical efficacy of iNO in some patients.
Infants with pulmonary hypertension are commonly treated with high concentrations of oxygen to reverse hypoxemia. However, even brief exposures to hyperoxia diminish pulmonary vascular responses to endogenous and exogenous NO in healthy late-gestation lambs and lambs with pulmonary hypertension (who remain hypoxemic despite ventilation with 100% O2). These findings suggest that lung oxidant stress exerts rapid and powerful effects on the signaling pathways that mediate vascular responses to NO. In pulmonary artery smooth muscle cells from healthy lambs, exposure to 24 hours of hyperoxia increased intracellular oxidant stress, increased PDE5 mRNA and protein expression and activity, and reduced cGMP generation in response to NO.30 A dose-dependent effect of hyperoxia was observed, as indicated by a stepwise increase in PDE5 activity as oxygen concentration was increased from 21%, to 50%, and 95% O2. Treatment of cells with sildenafil partially restored the cGMP response to exogenous NO, further highlighting that inhibition of PDE5 activity can counterregulate abnormal vascular cGMP responses after hyperoxia exposure.
In lambs with experimental PPHN, enteral and aerosolized sildenafil dilate the pulmonary vasculature and augment the pulmonary vascular response to iNO.46,47 In a piglet model of meconium aspiration syndrome, intravenous sildenafil produced selective pulmonary vasodilation with efficacy equivalent to iNO, although hypotension and worsening oxygenation resulted when it was used in combination with iNO.48 In a rat model of hyperoxia-induced BPD, chronic use of sildenafil decreased medial wall thickness and RVH and improved alveolarization.49 Another recent study showed that antenatal administration of sildenafil to the dam reduced PDE5 activity and increased cGMP, and produced striking reductions in the vascular findings of persistent pulmonary hypertension in rat pups with experimental CDH.50 This is the first indication that pulmonary hypertension can be treated before birth, and will likely open up a productive line of investigation in antenatal diagnosis and treatment.
The first clinical report of sildenafil use in infants was to facilitate weaning from iNO after corrective surgery for congenital heart disease.51 In this initial case series, enteral sildenafil increased circulating cGMP and allowed two of three infants to wean from iNO without rebound pulmonary hypertension. Subsequent case series expanded these initial observations to show that enteral sildenafil may facilitate iNO discontinuation in infants with critical illness,52 and may also reduce duration of mechanical ventilation and intensive care unit length of stay.53 The clinical use of enteral sildenafil has also been reported in infants with PPHN, including one small, randomized controlled trial with oral sildenafil that showed a dramatic improvement in oxygenation and survival.54
Enteral administration of sildenafil could raise concerns about gastrointestinal absorption, particularly in conditions that may have an element of compromised intestinal perfusion. A recent open-label pilot trial demonstrated that intravenous sildenafil, delivered as a continuous infusion, improved oxygenation in a series of 36 infants with PPHN.55 This study also showed that sildenafil clearance in neonates increases rapidly through the first week of life, likely reflecting the relative immaturity of the hepatic CYP system in the early neonatal period.56 Hypotension was the most commonly reported adverse effect, although it was not observed when the loading dose was delivered over 3 hours or eliminated. Although most infants were treated while receiving iNO, seven infants received sildenafil without prior use of iNO, and all experienced a significant improvement in oxygenation within 4 hours after sildenafil administration (Fig. 3). Only one of these seven infants required iNO, and the other six infants improved and survived to hospital discharge without requiring either iNO or ECMO.55 A randomized controlled trial is currently underway to evaluate the efficacy of intravenous sildenafil in infants with moderate PPHN (NCT01409031).
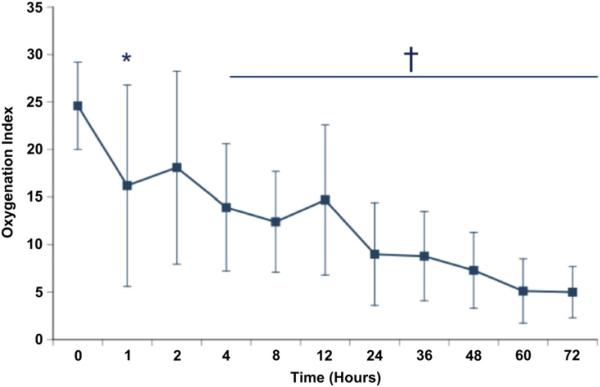
Fig. 3.
Response to intravenous sildenafil. Seven infants were treated with open-label intravenous sildenafil before initiation of iNO. Oxygenation index was improved by 1 hour (24.6 ± 4.6 to 16.1 ± 9.9; *P = .0502), with significant and sustained improvement by 4 hours after initiation of sildenafil (14.7 ± 6.4; †P = .0088). Only one infant went on to require therapy with iNO. (From Steinhorn RH, Kinsella JP, Butrous G, et al. Open-label, multicentre, pharmacokinetic study of iv sildenafil in the treatment of neonates with persistent pulmonary hypertension of the newborn (PPHN). Circulation 2007;116:II-614; with permission.)
Sildenafil is also an attractive therapeutic option for infants with chronic pulmonary hypertension caused by CDH or BPD because it can be given orally, and over longer periods of time with apparent low toxicity. A recent case series examined the effect of oral sildenafil in 25 infants and children (<2 years of age) with pulmonary hypertension caused by chronic lung disease (mostly BPD). Most patients showed some improvement after a median treatment interval of 40 days, and most infants were able to wean off iNO.57 Five patients died after initiation of sildenafil treatment, but none died from refractory pulmonary hypertension or right heart failure. A similar approach might benefit some infants with chronic pulmonary hypertension associated with lung hypoplasia.58 These important pilot studies suggest that sildenafil is well tolerated in infants with pulmonary hypertension caused by chronic lung disease, and paves the way to further studies in this especially challenging population.
Prostacyclin
Another important vasodilatory pathway in the fetal lung is mediated by PGI2 and cAMP (see Fig. 1). COX is the rate-limiting enzyme that generates prostacyclin from arachadonic acid. COX-1 is upregulated as lung development progresses in utero, leading to an increase in prostacyclin production in late gestation and early postnatal life.59,60 Prostacyclin produced by endothelial cells initiates its signaling pathway by binding to the smooth muscle cell membrane bound receptor (IP), stimulating the associated adenylate cyclase to increase conversion of adenosine triphosphate to cAMP. Like cGMP, cAMP leads to decreased intracellular calcium ion resulting in vasodilation. In addition, PGI2 inhibits pulmonary artery smooth muscle cell proliferation in vitro.
Systemic administration of PGI2 is considered the most effective therapy for severe pulmonary hypertension in adults. However, rapid dosage escalation of infusions of PGI2 may be necessary to promote acute pulmonary vasodilation. In infants, this can produce systemic hypotension, which can further compromise circulatory function. In addition, a dedicated central venous catheter is necessary for the delivery of intravenous PGI2, with associated risks of infection and other line complications. Other systemic side effects (especially pain) also limit the use of systemic PGI2 in the acute setting. When given as an aerosol by inhalation, PGI2 has been shown to have vasodilator effects limited to the pulmonary circulation, making this strategy appealing when acute pulmonary vasodilation is desirable. Reports in children have been positive, but to date, there are few studies reporting the use of inhaled PGI2 in neonates with PPHN.61–63
Experience suggests that inhaled PGI2 is well tolerated and may assist in recovery without ECMO in infants with severe PPHN and inadequate response to iNO.64 The optimal dosing of inhaled PGI2 in critically ill mechanically ventilated infants is not known. In short-term studies of newborn lambs with pulmonary hypertension, increasing the dose of inhaled PGI2 up to 500 ng/kg/min produced progressive improvements in pulmonary artery pressure, PVR, and pulmonary blood flow.65 In clinical experience, the authors have used 50 to 100 ng/kg/min and have observed rapid improvement in oxygenation and limited progression to ECMO in infants with inadequate response to iNO. Concerns regarding the use of inhaled PGI2 in critically ill infants include airway irritation from the alkaline solution needed to maintain drug stability, rebound pulmonary hypertension if the drug is abruptly discontinued, loss of medication into the circuit because of condensation, and alteration of characteristics of mechanical ventilation from the added gas flow needed for the nebulization. Prolonged use of continuous inhaled PGI2 may also lead to damage of mechanical ventilator valves.
Further investigations will likely focus on preparations specifically designed for inhalation, such as iloprost or treprostinil. Because these medications are more stable than PGI2 they do not need to be dissolved in an alkaline solution, which could decrease the risk of lung injury. In addition, their longer half-lives allow for intermittent dosing using ultrasonic nebulizers, which would allow for interruption of mechanical ventilation for airway suctioning and probably interfere less with the flow patterns of mechanical ventilation. In critically ill mechanically ventilated patients, the effective dosing of inhaled prostanoids is likely to be higher and more frequent than in spontaneously breathing patients because of loss of the medication into the humidified ventilator circuit.
Although systemic hypotension may limit the use of intravenous prostanoids in the acute setting, long-term systemic prostanoids may be useful to protect the right ventricle from failing in infants with chronically progressive or severe pulmonary hypertension. This more chronic setting allows for a slower escalation of dose, which decreases the risk of side effects. However, the care of infants with severe pulmonary hypertension in the neonatal intensive care unit is often complicated by limited vascular access in older, chronically ill patients. This problem has prompted use of subcutaneous treprostinil in a small, heterogeneous cohort of infants with severe pulmonary hypertension. In three extremely premature infants with severe pulmonary hypertension associated with BPD, right ventricle size and function improved over time, and local site pain (typically a significant problem in adults) was not evident. However, the use of systemic prostanoids should be approached with caution. In particular, care must be taken to avoid excessive prostanoid therapy, which may produce severe discomfort, and could also lead to generalized vasodilation and high-output heart failure.
Milrinone
Prostacyclin–cAMP signaling operates in parallel to the NO–cGMP pathway for peri-natal pulmonary vasodilation and is regulated in part by cAMP-hydrolyzing PDE isoforms, such as PDE3 and PDE4. The authors recently reported that PDE3A expression and activity in the resistance pulmonary arteries increase dramatically by 24 hours after birth.66 These results were surprising and unexpected, because the authors would have predicted that similar to PDE5, PDE3 activity would decrease after birth to facilitate increased cAMP levels. This increase may be acting to establish cAMP-containing regulatory regions within the pulmonary vascular smooth muscle cell after birth, although it is unclear what role PDE3 has in the normal pulmonary vascular transition after birth. They also observed that addition of NO dramatically increased PDE3 levels, which suggests that milrinone might enhance the vasodilatory effects of iNO-cGMP signaling in addition to its expected effects on the cAMP pathways.67
The PGI2 receptor (IP) is decreased in adult and pediatric patients with pulmonary hypertension, and animal studies point to its contribution to altered vasodilation in PPHN.67 Bypassing these abnormalities in PGI2 signaling by enhancing availability of cAMP may represent a useful therapeutic approach. Inhibition of PDE3 activity would be expected to increase cAMP concentration, and thus promote vasodilation. Milrinone, an inhibitor of PDE3, is frequently used in intensive care units to improve myocardial contractility and reverse diastolic dysfunction. In animal studies, milrinone has been shown to decrease pulmonary artery pressure and resistance and to act additively with iNO.65,68 Clinical reports indicate that milrinone may decrease rebound pulmonary hypertension after iNO is stopped,27 and may enhance pulmonary vasodilation of infants with PPHN refractory to iNO.69 A study to better define the pharmacokinetic profile of milrinone in infants with PPHN is ongoing (NCT01088997), and should lead to clinical trials designed to test its efficacy.
Bosentan
Endothelin-1 (ET-1) is a 21–amino acid protein formed by serial enzymatic cleavage of a larger prepropeptide to the vasoactive form, and is one of the most potent vasoconstrictors described in the pulmonary vasculature (see Fig. 2). ET-1 is principally produced in endothelial cells in response to hypoxia, and is known to promote endothelial cell dysfunction, smooth muscle cell proliferation and remodeling, and inflammation and fibrosis.70 ET-1 binds to two receptor subtypes, ET receptors A and B, and the binding of ET-1 to the ETA receptor on smooth muscle cells produces vasoconstriction. Increased ET-1 production and altered ET receptor activity have been consistently reported in neonatal and adult animal models of pulmonary hypertension, and lung ET-1 expression and plasma ET levels were elevated in severe pulmonary arterial hypertension in adults.71 The use of ET-1 receptor antagonists, such as bosentan, has been shown to improve outcomes in adults with pulmonary hypertension. In PPHN, ET-1 is believed to play a role in the pathogenesis of PPHN, and ET blockade augments pulmonary vasodilation.70 A recent prospective examination of 40 newborns with CDH and poor outcome also indicated that plasma ET-1 levels were highly correlated with the severity of pulmonary hypertension.72 Recent case reports suggest that bosentan, an ET blocking agent, can improve oxygenation in neonates with PPHN.73,74 A randomized controlled trial is currently underway to investigate the efficacy of bosentan in infants with severe PPHN (NCT01389856).
SUMMARY
No single therapeutic approach has been shown to be universally effective in the treatment of PPHN, and because of the complexity of the signaling pathways, this is not surprising. Although each of the therapies described in this article likely plays a role, using combinations of therapies is especially intriguing. Because the signaling pathways that regulate pulmonary vascular tone are highly interrelated, correcting only one pathway may not fully correct the vascular abnormality, and can even perturb the balance of vasodilator and vasoconstrictor production. The combination of strategies that increase cGMP and cAMP together may be more effective than either treatment alone. A thoughtful, multipronged approach may allow for more effective and efficient therapy, perhaps minimizing side effects and lung injury, especially in the most severe cases.
Acknowledgments
The preparation of this manuscript was supported in part by R01HL54705 (NHLBI) and U01HL102235 (NHLBI), both to RHS.
REFERENCES
- 1.Levin DL, Rudolph AM, Heymann MA, et al. Morphological development of the pulmonary vascular bed in fetal lambs. Circulation. 1976;53:144–51. doi: 10.1161/01.cir.53.1.144. [DOI] [PubMed] [Google Scholar]
- 2.Walsh-Sukys MC, Tyson JE, Wright LL, et al. Persistent pulmonary hypertension of the newborn in the era before nitric oxide: practice variation and outcomes. Pediatrics. 2000;105:14–20. doi: 10.1542/peds.105.1.14. [DOI] [PubMed] [Google Scholar]
- 3.Slaughter JL, Pakrashi T, Jones DE, et al. Echocardiographic detection of pulmonary hypertension in extremely low birth weight infants with bronchopulmonary dysplasia requiring prolonged positive pressure ventilation. J Perinatol. 2011;31:635–40. doi: 10.1038/jp.2010.213. [DOI] [PubMed] [Google Scholar]
- 4.An HS, Bae EJ, Kim GB, et al. Pulmonary hypertension in preterm infants with bronchopulmonary dysplasia. Korean Circ J. 2010;40:131–6. doi: 10.4070/kcj.2010.40.3.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farquhar M, Fitzgerald DA. Pulmonary hypertension in chronic neonatal lung disease. Paediatr Respir Rev. 2010;11:149–53. doi: 10.1016/j.prrv.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Khemani E, McElhinney DB, Rhein L, et al. Pulmonary artery hypertension in formerly premature infants with bronchopulmonary dysplasia: clinical features and outcomes in the surfactant era. Pediatrics. 2007;120:1260–9. doi: 10.1542/peds.2007-0971. [DOI] [PubMed] [Google Scholar]
- 7.Sanchez LS, Del La Monte SM, Filippov G, et al. Cyclic-GMP-binding, cyclic-GMP-specific phosphodiesterase gene expression is regulated during rat pulmonary development. Pediatr Res. 1998;43:163–8. doi: 10.1203/00006450-199802000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Farrow KN, Lakshminrusimha S, Czech L, et al. Superoxide dismutase and inhaled nitric oxide normalize phosphodiesterase 5 expression and activity in neonatal lambs with persistent pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2010;299:L109–16. doi: 10.1152/ajplung.00309.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steinhorn RH. Lamb models of pulmonary hypertension. Drug Discov Today Dis Models. 2010;7:99–105. [Google Scholar]
- 10.Tiktinsky MH, Morin FC., III Increasing oxygen tension dilates fetal pulmonary circulation via endothelium-derived relaxing factor. Am J Physiol Heart Circ Physiol. 1993;265:H376–80. doi: 10.1152/ajpheart.1993.265.1.H376. [DOI] [PubMed] [Google Scholar]
- 11.Mata-Greenwood E, Jenkins C, Farrow KN, et al. eNOS function is developmentally regulated: uncoupling of eNOS occurs postnatally. Am J Physiol Lung Cell Mol Physiol. 2006;290:L232–41. doi: 10.1152/ajplung.00393.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farrow KN, Lakshminrusimha S, Reda WJ, et al. Superoxide dismutase restores eNOS expression and function in resistance pulmonary arteries from neonatal lambs with persistent pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2008;295:L979–87. doi: 10.1152/ajplung.90238.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kinsella JP, Parker TA, Ivy DD, et al. Noninvasive delivery of inhaled nitric oxide therapy for late pulmonary hypertension in newborn infants with congenital diaphragmatic hernia. J Pediatr. 2003;142:397–401. doi: 10.1067/mpd.2003.140. [DOI] [PubMed] [Google Scholar]
- 14.Brahmajothi MV, Mason SN, Whorton AR, et al. Transport rather than diffusion-dependent route for nitric oxide gas activity in alveolar epithelium. Free Radic Biol Med. 2010;49:294–300. doi: 10.1016/j.freeradbiomed.2010.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Konduri GG, Solimani A, Sokol GM, et al. A randomized trial of early versus standard inhaled nitric oxide therapy in term and near-term newborn infants with hypoxic respiratory failure. Pediatrics. 2004;113:559–64. doi: 10.1542/peds.113.3.559. [DOI] [PubMed] [Google Scholar]
- 16.Konduri GG, Vohr B, Robertson C, et al. Early inhaled nitric oxide therapy for term and near-term newborn infants with hypoxic respiratory failure: neurodevelopmental follow-up. J Pediatr. 2007;150:235–40. 240.e1. doi: 10.1016/j.jpeds.2006.11.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gonzalez A, Fabres J, D'Apremont I, et al. Randomized controlled trial of early compared with delayed use of inhaled nitric oxide in newborns with a moderate respiratory failure and pulmonary hypertension. J Perinatol. 2010;30:420–4. doi: 10.1038/jp.2009.171. [DOI] [PubMed] [Google Scholar]
- 18.Fliman PJ, deRegnier RA, Kinsella JP, et al. Neonatal extracorporeal life support: impact of new therapies on survival. J Pediatr. 2006;148:595–9. doi: 10.1016/j.jpeds.2005.12.024. [DOI] [PubMed] [Google Scholar]
- 19.Neonatal Inhaled Nitric Oxide Study Group Inhaled nitric oxide in full-term and nearly full-term infants with hypoxic respiratory failure. N Engl J Med. 1997;336:597–604. doi: 10.1056/NEJM199702273360901. [DOI] [PubMed] [Google Scholar]
- 20.Cornfield DN, Maynard RC, deRegnier RO, et al. Randomized, controlled trial of low-dose inhaled nitric oxide in the treatment of term and near-term infants with respiratory failure and pulmonary hypertension. Pediatrics. 1999;104:1089–94. doi: 10.1542/peds.104.5.1089. [DOI] [PubMed] [Google Scholar]
- 21.Tworetzky W, Bristow J, Moore P, et al. Inhaled nitric oxide in neonates with persistent pulmonary hypertension. Lancet. 2001;357:118–20. doi: 10.1016/S0140-6736(00)03548-0. [DOI] [PubMed] [Google Scholar]
- 22.Davidson D, Barefield ES, Kattwinkel J, et al. Inhaled nitric oxide for the early treatment of persistent pulmonary hypertension of the term newborn: a randomized, double-masked, placebo-controlled, dose-response, multicenter study. Pediatrics. 1998;101:325–34. doi: 10.1542/peds.101.3.325. [DOI] [PubMed] [Google Scholar]
- 23.Kinsella JP, Truog WE, Walsh WF, et al. Randomized, multicenter trial of inhaled nitric oxide and high-frequency oscillatory ventilation in severe, persistent pulmonary hypertension of the newborn. J Pediatr. 1997;131:55–62. doi: 10.1016/s0022-3476(97)70124-0. [DOI] [PubMed] [Google Scholar]
- 24.Bishop NB, Stankiewicz P, Steinhorn RH. Alveolar capillary dysplasia. Am J Respir Crit Care Med. 2011;184:172–9. doi: 10.1164/rccm.201010-1697CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davidson D, Barefield ES, Kattwinkel J, et al. Safety of withdrawing inhaled nitric oxide therapy in persistent pulmonary hypertension of the newborn. Pediatrics. 1999;104:231–6. doi: 10.1542/peds.104.2.231. [DOI] [PubMed] [Google Scholar]
- 26.Black SM, Heidersbach RS, McMullan DM, et al. Inhaled nitric oxide inhibits NOS activity in lambs: potential mechanism for rebound pulmonary hypertension. Am J Physiol Heart Circ Physiol. 1999;277:H1849–56. doi: 10.1152/ajpheart.1999.277.5.H1849. [DOI] [PubMed] [Google Scholar]
- 27.Thelitz S, Oishi P, Sanchez LS, et al. Phosphodiesterase-3 inhibition prevents the increase in pulmonary vascular resistance following inhaled nitric oxide withdrawal in lambs. Pediatr Crit Care Med. 2004;5:234–9. doi: 10.1097/01.pcc.0000124021.25393.2d. [DOI] [PubMed] [Google Scholar]
- 28.Lakshminrusimha S, Russell JA, Steinhorn RH, et al. Pulmonary hemodynamics in neonatal lambs resuscitated with 21%, 50%, and 100% oxygen. Pediatr Res. 2007;62:313–8. doi: 10.1203/PDR.0b013e3180db29fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lakshminrusimha S, Swartz DD, Gugino SF, et al. Oxygen concentration and pulmonary hemodynamics in newborn lambs with pulmonary hypertension. Pediatr Res. 2009;66:539–44. doi: 10.1203/PDR.0b013e3181bab0c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Farrow KN, Groh BS, Schumacker PT, et al. Hyperoxia increases phosphodiesterase 5 expression and activity in ovine fetal pulmonary artery smooth muscle cells. Circ Res. 2008;102:226–33. doi: 10.1161/CIRCRESAHA.107.161463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lassegue B, Griendling KK. Reactive oxygen species in hypertension: an update. Am J Hypertens. 2004;17:852–60. doi: 10.1016/j.amjhyper.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 32.Lakshminrusimha S, Russell J, Wedgwood S, et al. Superoxide dismutase improves oxygenation and reduces oxidation in neonatal pulmonary hypertension. Am J Respir Crit Care Med. 2006;174:1370–7. doi: 10.1164/rccm.200605-676OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neonatal Inhaled Nitric Oxide Study Group Inhaled nitric oxide and hypoxic respiratory failure in infants with congenital diaphragmatic hernia. Pediatrics. 1997;99:838–45. doi: 10.1542/peds.99.6.838. [DOI] [PubMed] [Google Scholar]
- 34.Afshar S, Gibson LL, Yuhanna IS, et al. Pulmonary NO synthase expression is attenuated in a fetal baboon model of chronic lung disease. Am J Physiol Lung Cell Mol Physiol. 2003;284:L749–58. doi: 10.1152/ajplung.00334.2002. [DOI] [PubMed] [Google Scholar]
- 35.McCurnin DC, Pierce RA, Chang LY, et al. Inhaled NO improves early pulmonary function and modifies lung growth and elastin deposition in a baboon model of neonatal chronic lung disease. Am J Physiol Lung Cell Mol Physiol. 2005;288:L450–9. doi: 10.1152/ajplung.00347.2004. [DOI] [PubMed] [Google Scholar]
- 36.Lin YJ, Markham NE, Balasubramaniam V, et al. Inhaled nitric oxide enhances distal lung growth after exposure to hyperoxia in neonatal rats. Pediatr Res. 2005;58:22–9. doi: 10.1203/01.PDR.0000163378.94837.3E. [DOI] [PubMed] [Google Scholar]
- 37.Donohue PK, Gilmore MM, Cristofalo E, et al. Inhaled nitric oxide in preterm infants: a systematic review. Pediatrics. 2011;127:e414–22. doi: 10.1542/peds.2010-3428. [DOI] [PubMed] [Google Scholar]
- 38.Steinhorn RH, Shaul PW, deRegnier RA, et al. Inhaled nitric oxide and bronchopulmonary dysplasia. Pediatrics. 2011;128:e255–6. doi: 10.1542/peds.2011-1270A. [author reply: e256–7] [DOI] [PubMed] [Google Scholar]
- 39.Ballard RA, Truog WE, Cnaan A, et al. Inhaled nitric oxide in preterm infants undergoing mechanical ventilation. N Engl J Med. 2006;355:343–53. doi: 10.1056/NEJMoa061088. [DOI] [PubMed] [Google Scholar]
- 40.Hibbs AM, Walsh MC, Martin RJ, et al. One-year respiratory outcomes of preterm infants enrolled in the Nitric Oxide (to prevent) Chronic Lung Disease trial. J Pediatr. 2008;153:525–9. doi: 10.1016/j.jpeds.2008.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mourani PM, Ivy DD, Gao D, et al. Pulmonary vascular effects of inhaled nitric oxide and oxygen tension in bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2004;170:1006–13. doi: 10.1164/rccm.200310-1483OC. [DOI] [PubMed] [Google Scholar]
- 42.Farrow KN, Steinhorn RH. Phosphodiesterases: emerging therapeutic targets for neonatal pulmonary hypertension. Handb Exp Pharmacol. 2011;204:251–77. doi: 10.1007/978-3-642-17969-3_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sanchez LS, Filippov G, Zapol WM, et al. cGMP-binding, cGMP-specific phosphodiesterase gene expression is regulated during lung development. Pediatr Res. 1995;37:348A. doi: 10.1203/00006450-199802000-00002. [DOI] [PubMed] [Google Scholar]
- 44.Hanson KA, Abman SH, Clarke WR. Elevation of pulmonary PDE5-specific activity in an experimental fetal ovine perinatal pulmonary hypertension model. Pediatr Res. 1996;39:334A. [Google Scholar]
- 45.Farrow KN, Wedgwood S, Lee KJ, et al. Mitochondrial oxidant stress increases PDE5 activity in persistent pulmonary hypertension of the newborn. Respir Physiol Neurobiol. 2010;174:272–81. doi: 10.1016/j.resp.2010.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weimann J, Ullrich R, Hromi J, et al. Sildenafil is a pulmonary vasodilator in awake lambs with acute pulmonary hypertension. Anesthesiology. 2000;92:1702–12. doi: 10.1097/00000542-200006000-00030. [DOI] [PubMed] [Google Scholar]
- 47.Ichinose F, Erana-Garcia J, Hromi J, et al. Nebulized sildenafil is a selective pulmonary vasodilator in lambs with acute pulmonary hypertension. Crit Care Med. 2001;29:1000–5. doi: 10.1097/00003246-200105000-00024. [DOI] [PubMed] [Google Scholar]
- 48.Shekerdemian L, Ravn H, Penny D. Intravenous sildenafil lowers pulmonary vascular resistance in a model of neonatal pulmonary hypertension. Am J Respir Crit Care Med. 2002;165:1098–102. doi: 10.1164/ajrccm.165.8.2107097. [DOI] [PubMed] [Google Scholar]
- 49.Ladha F, Bonnet S, Eaton F, et al. Sildenafil improves alveolar growth and pulmonary hypertension in hyperoxia-induced lung injury. Am J Respir Crit Care Med. 2005;172:750–6. doi: 10.1164/rccm.200503-510OC. [DOI] [PubMed] [Google Scholar]
- 50.Luong C, Rey-Perra J, Vadivel A, et al. Antenatal sildenafil treatment attenuates pulmonary hypertension in experimental congenital diaphragmatic hernia. Circulation. 2011;123:2120–31. doi: 10.1161/CIRCULATIONAHA.108.845909. [DOI] [PubMed] [Google Scholar]
- 51.Atz AM, Wessel DL. Sildenafil ameliorates effects of inhaled nitric oxide withdrawal. Anesthesiology. 1999;91:307–10. doi: 10.1097/00000542-199907000-00041. [DOI] [PubMed] [Google Scholar]
- 52.Lee JE, Hillier SC, Knoderer CA. Use of sildenafil to facilitate weaning from inhaled nitric oxide in children with pulmonary hypertension following surgery for congenital heart disease. J Intensive Care Med. 2008;23:329–34. doi: 10.1177/0885066608321389. [DOI] [PubMed] [Google Scholar]
- 53.Namachivayam P, Theilen U, Butt WW, et al. Sildenafil prevents rebound pulmonary hypertension after withdrawal of nitric oxide in children. Am J Respir Crit Care Med. 2006;174:1042–7. doi: 10.1164/rccm.200605-694OC. [DOI] [PubMed] [Google Scholar]
- 54.Baquero H, Soliz A, Neira F, et al. Oral sildenafil in infants with persistent pulmonary hypertension of the newborn: a pilot randomized blinded study. Pediatrics. 2006;117:1077–83. doi: 10.1542/peds.2005-0523. [DOI] [PubMed] [Google Scholar]
- 55.Steinhorn RH, Kinsella JP, Pierce C, et al. Intravenous sildenafil in the treatment of neonates with persistent pulmonary hypertension. J Pediatr. 2009;155:841–7. doi: 10.1016/j.jpeds.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 56.Mukherjee A, Dombi T, Wittke B, et al. Population pharmacokinetics of sildenafil in term neonates: evidence of rapid maturation of metabolic clearance in the early postnatal period. Clin Pharmacol Ther. 2009;85:56–63. doi: 10.1038/clpt.2008.177. [DOI] [PubMed] [Google Scholar]
- 57.Mourani PM, Sontag MK, Ivy DD, et al. Effects of long-term sildenafil treatment for pulmonary hypertension in infants with chronic lung disease. J Pediatr. 2009;154:379–84. 384, e1–2. doi: 10.1016/j.jpeds.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Keller RL, Moore P, Teitel D, et al. Abnormal vascular tone in infants and children with lung hypoplasia: findings from cardiac catheterization and the response to chronic therapy. Pediatr Crit Care Med. 2006;7:589–94. doi: 10.1097/01.PCC.0000244401.53189.CB. [DOI] [PubMed] [Google Scholar]
- 59.Brannon TS, MacRitchie AN, Jaramillo MA, et al. Ontogeny of cyclooxygenase-1 and cyclooxygenase-2 gene expression in ovine lung. Am J Physiol. 1998;274:L66–71. doi: 10.1152/ajplung.1998.274.1.L66. [DOI] [PubMed] [Google Scholar]
- 60.Brannon TS, North AJ, Wells LB, et al. Prostacyclin synthesis in ovine pulmonary artery is developmentally regulated by changes in cyclooxygenase-1 gene expression. J Clin Invest. 1994;93:2230–5. doi: 10.1172/JCI117220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bindl L, Fahnenstich H, Peukert U. Aerosolised prostacyclin for pulmonary hypertension in neonates. Arch Dis Child Fetal Neonatal Ed. 1994;71:F214–6. doi: 10.1136/fn.71.3.f214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Soditt V, Aring C, Groneck P. Improvement of oxygenation induced by aerosolized prostacyclin in a preterm infant with persistent pulmonary hypertension of the newborn. Intensive Care Med. 1997;23:1275–8. doi: 10.1007/s001340050498. [DOI] [PubMed] [Google Scholar]
- 63.Olmsted K, Oluola O, Parthiban A, et al. Can inhaled prostacyclin stimulate surfactant in ELBW infants? J Perinatol. 2007;27:724–6. doi: 10.1038/sj.jp.7211811. [DOI] [PubMed] [Google Scholar]
- 64.Kelly LK, Porta NF, Goodman DM, et al. Inhaled prostacyclin for term infants with persistent pulmonary hypertension refractory to inhaled nitric oxide. J Pediatr. 2002;141:830–2. doi: 10.1067/mpd.2002.129849. [DOI] [PubMed] [Google Scholar]
- 65.Kumar VH, Swartz DD, Rashid N, et al. Prostacyclin and milrinone by aerosolization improve pulmonary hemodynamics in newborn lambs with experimental pulmonary hypertension. J Appl Physiol. 2010;109:677–84. doi: 10.1152/japplphysiol.01082.2009. [DOI] [PubMed] [Google Scholar]
- 66.Chen B, Lakshminrusimha S, Czech L, et al. Regulation of phosphodiesterase 3 in the pulmonary arteries during the perinatal period in sheep. Pediatr Res. 2009;66:682–7. doi: 10.1203/PDR.0b013e3181bce574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lakshminrusimha S, Porta NF, Farrow KN, et al. Milrinone enhances relaxation to prostacyclin and iloprost in pulmonary arteries isolated from lambs with persistent pulmonary hypertension of the newborn. Pediatr Crit Care Med. 2009;10:106–12. doi: 10.1097/PCC.0b013e3181936aee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Deb B, Bradford K, Pearl RG. Additive effects of inhaled nitric oxide and intravenous milrinone in experimental pulmonary hypertension. Crit Care Med. 2000;28:795–9. doi: 10.1097/00003246-200003000-00031. [DOI] [PubMed] [Google Scholar]
- 69.McNamara PJ, Laique F, Muang- S, et al. Milrinone improves oxygenation in neonates with severe persistent pulmonary hypertension of the newborn. J Crit Care. 2006;21:217–22. doi: 10.1016/j.jcrc.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 70.Abman SH. Role of endothelin receptor antagonists in the treatment of pulmonary arterial hypertension. Annu Rev Med. 2009;60:13–23. doi: 10.1146/annurev.med.59.110106.212434. [DOI] [PubMed] [Google Scholar]
- 71.Giaid A, Yanagisawa M, Lagleben D, et al. Expression of endothelin-1 in the lungs of patients with pulmonary hypertension. N Engl J Med. 1993;328:1732–9. doi: 10.1056/NEJM199306173282402. [DOI] [PubMed] [Google Scholar]
- 72.Keller RL, Tacy TA, Hendricks-Munoz K, et al. Congenital diaphragmatic hernia: endothelin-1, pulmonary hypertension, and disease severity. Am J Respir Crit Care Med. 2010;182:555–61. doi: 10.1164/rccm.200907-1126OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nakwan N, Choksuchat D, Saksawad R, et al. Successful treatment of persistent pulmonary hypertension of the newborn with bosentan. Acta Paediatr. 2009;98:1683–5. doi: 10.1111/j.1651-2227.2009.01386.x. [DOI] [PubMed] [Google Scholar]
- 74.Goissen C, Ghyselen L, Tourneux P, et al. Persistent pulmonary hypertension of the newborn with transposition of the great arteries: successful treatment with bosentan. Eur J Pediatr. 2008;167:437–40. doi: 10.1007/s00431-007-0531-y. [DOI] [PubMed] [Google Scholar]
- 75.Roberts JD, Fineman J, Morin FC, III, et al. Inhaled nitric oxide and persistent pulmonary hypertension of the newborn. N Engl J Med. 1997;336:605–10. doi: 10.1056/NEJM199702273360902. [DOI] [PubMed] [Google Scholar]
- 76.Clark RH, Huckaby JL, Kueser TJ, et al. Low-dose nitric oxide therapy for persistent pulmonary hypertension: 1-year follow-up. J Perinatol. 2003;23:300–3. doi: 10.1038/sj.jp.7210908. [DOI] [PubMed] [Google Scholar]
- 77.Clark RH, Kueser TJ, Walker MW, et al. Low dose nitric oxide therapy for persistent pulmonary hypertension of the newborn. N Engl J Med. 2000;342:469–74. doi: 10.1056/NEJM200002173420704. [DOI] [PubMed] [Google Scholar]