Abstract
Purpose
Asymptomatic Clostridium difficile carriage has a prevalence reported as high as 51% to 85%; with up to 84% of incident hospital-acquired infections linked to carriers. Accurately identifying carriers may limit the spread of Clostridium difficile.
Methods
Since new technology adoption depends heavily on its economic value, we developed a analytic simulation model to determine the cost-effectiveness screening hospital admissions for Clostridium difficile from the hospital and third party payer perspectives. Isolation precautions were applied to patients testing positive, preventing transmission. Sensitivity analyses varied Clostridium difficile colonization rate, infection probability among secondary cases, contact isolation compliance, and screening cost.
Results
Screening was cost-effective [i.e., incremental cost-effectiveness ratio (ICER) ≤$50,000/QALY] for every scenario tested; all ICER values ≤$256/QALY. Screening was economically dominant (i.e., saved costs and provided health benefits) with a ≥10.3% colonization rate and ≥5.88% infection probability when contact isolation compliance was ≥25% (hospital perspective). Under some conditions screening led to cost-savings per case averted (range: $53 to $272).
Conclusion
Clostridium difficile screening, coupled with isolation precautions, may be a cost-effective intervention to hospitals and third party payers, based on prevalence. Limiting Clostridium difficile transmission can reduce the number of infections, thereby reducing its economic burden to the healthcare system.
Keywords: Clostridium difficile, Screening, Economics, Cost-Effectiveness, Modeling
INTRODUCTION
Clostridium difficile (C. difficile) can cause a wide range of clinical disease in hospitalized patients[1-2] and result in substantial healthcare costs.[3] It is the leading cause of infectious diarrhea in hospitalized patients and especially affects elderly and frail patients.[4-5] Studies have found asymptomatic C. difficile carriage rates to be as high as 51% to 85% in nursing facilities and in selected inpatient populations with a prolonged length of stay.[6-8] Previous typing studies have suggested that as many as 84% of incident hospital acquired infections are linked to asymptomatic carriers.[8] Accurately identifying these carriers and taking appropriate precautions may limit the spread of C. difficile to other hospitalized patients.
Health care facilities do not routinely screen for this hospital-acquired pathogen. Detecting asymptomatic C. difficile carriers was previously limited to research laboratories equipped to perform anaerobic culture for C. difficile. Because non-toxigenic strains of C. difficile incapable of causing human infections exist, each isolate recovered by culture required confirmation of toxin production; the entire process of detecting asymptomatic carriers was thus impractical as a surveillance test, as the average turn-around time of 5-10 days exceeded the median length of stay for most hospitals. Existing C. difficile tests for toxin production could not be used as effective screening tools because of low sensitivity compared to the gold standard of culture, as even PCR-based assays have been shown to have sensitivities of 77-86% compared to toxigenic culture (i.e., the gold standard [9]), which is too low to serve as an effective screening test for carriage.[10-12] In addition, existing clinical tests for C. difficile toxin require use of a stool sample, which makes the sampling process inefficient for screening purposes. However, a novel screening method has recently been developed.[13] This method utilizes peri-rectal surveillance specimens that are pre-amplified in a C. difficile selective medium followed by toxin detection using a polyermerase chain reaction (PCR) assay, combining the sensitivity of anaerobic culture with the specificity and rapid turn-around of PCR-based testing without needing stool samples. The broth amplification process results in a 1.25 to 3.25 day turnaround time, which is comparable to other healthcare associated (HAI) surveillance tests.[13]
Since adoption of this technology depends heavily on its economic value, we developed a computational analytic simulation model to determine the cost-effectiveness of this novel C. difficile screening method. Sensitivity analyses varied the key parameters of C. difficile colonization rate among admitted patients, the probability of infection, contact isolation compliance, and the cost of screening. Decisions makers such as hospital administrators and third party payers can use the result of our study to make decisions about implementing C. difficile screening programs and reimbursement rates.
METHODS
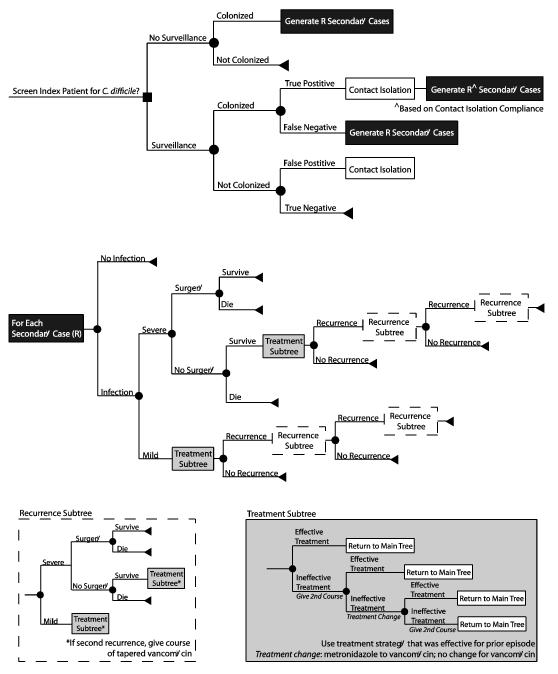
We further adapted our previously published C. difficile outcomes model[3] developed in TreeAge Pro 2009 (Williamstown, MA) to determine the cost-effectiveness of screening all hospital admissions for C. difficile from the hospital and third-party payer perspectives. Figure 1 outlines the general structure of our model and Table 1 provides the input parameters with values and sources. Upon admission, all patients (≥1 year old) were either screened (via peri-rectal swabbing) or not screened for C. difficile. Patients with a positive screening test were placed under contact isolation precautions (i.e., the use of gloves and gowns for each patient contact), regardless of true colonization status (i.e., true and false positive tests). Staff compliance with the contact isolation precautions reduced transmission of C. difficile to other patients (i.e., a reduction in R based on compliance rates). Patient age and length of stay (LOS) for all admissions was based on statistics for all hospital stays from the Healthcare Utilization Project (HCUP)[14] (Table 1).
Figure 1.
Model structure
Table 1.
Model input parameters and values
| Mean* | Standard Deviation^ or Range |
Source | |
|---|---|---|---|
| Costs ($US 2011) | |||
| Screening | 7.66 | 3.32 – 15.88 | [13] |
| Gloves (per pair) | 0.0861 | [45] | |
| Gown | 0.922 | [45] | |
| Technician Wage (median hourly) | 17.96 | 14.34 – 22.63 | [46] |
| Nurse Wage (median hourly) | 31.10 | 21.24 – 45.74 | [46] |
| Hospitalization | |||
| 1 to 17 years old | 7,695.72 | 1157^ | [14] |
| 18 to 44 years old | 8,557.51 | 318^ | [14] |
| 45 to 64 years old | 10,833.81 | 250.5^ | [14] |
| 65 to 85 years | 11,476.37 | 246.90^ | [14] |
| 85 years and older | 10,324.00 | 215.6^ | [14] |
| Hospital Bed Day | 1,560 | 33.55^ | [14] |
| Peripheral Intravenous Line Insertion |
97.63 | [47] | |
| CT Scan | 284.38 | 30.50 | [47] |
| Colectomy | |||
| 1 to 17 years old | 34,417.94 | 5731.92^ | [14] |
| 18 to 44 years old | 32,982.51 | 3608.08^ | [14] |
| 45 to 64 years old | 38,472.33 | 19626.24^ | [14] |
| 65 to 85 years | 46,566.65 | 1,594.21^ | [14] |
| 85 years and older | 45,913.49 | 3316.95^ | [14] |
| Antibiotics (Full Course): | |||
| Metronidazole (IV) | 116.36 | 10.88 | [48] |
| Metronidazole (Oral) | 57.41 | 38.59 | [48] |
| Vancomycin (Oral) | 1,347.39 | 77.52 | [48] |
| Vancomycin (Tapered) | 2,069.21 | 119.04 | [48] |
| Utility Weights | |||
| Age 1 to 17 years | 1.0 | [49] | |
| Age 18 to 64 years | 0.96 | [49] | |
| Age 65 years and older | 0.84 | [49] | |
| Mild CDI | 0.88 | [16-17] | |
| Severe CDI | 0.817 | [16-17] | |
| Colectomy | 0.536 | [18] | |
| Probabilities | |||
| PCR Sensitivity | 99 | 94.9 – 100 | [13] |
| PCR Specificity | 99.1 | 97.6 – 99.7 | [13] |
| Given Infection: | |||
| Severe Disease | 15.8 | 5.46 | [50,6,51-52] |
| Colectomy | 0.27 | [53] | |
| Colectomy Mortality | 41.7 | 37.2 – 46.3 | [54-55] |
| Mortality if No Colectomy | 58.3 | [56] | |
| Recurrence | 18.9 | 6.77 | [52,57-58] |
| Treatment Efficacies: | |||
| Metronidazole | 87 | 85.4 – 88.2 | [59] |
| Vancomycin | 90.2 | 87.9 – 92.3 | [59] |
| Vancomycin (Tapered) | 72.2 | 55 – 86 | [60] |
| Durations | |||
| Reproductive Rate | 1.04 | 0.52 – 1.99 | [32] |
| Attributable CDI Length of Stay | 3.6 | 1.5 – 6.2 | [24] |
| Attributable CDI Length of Stay † | 7.14 | 2.18 | [25-29] |
| Turnaround Time | 2.25 | 1.25 – 3.25 | [13] |
| Technician Time (minutes) | 10 – 12 | [13] | |
| Patient Contacts per Day | 25 – 50 | [61] | |
| Time Don/Doff (minutes) | 1 | [45] | |
| Patient Characteristics | |||
| Age | |||
| 1 to 17 years old | 4.64 | [14] | |
| 18 to 44 years old | 28.56 | [14] | |
| 45 to 64 years old | 27.7 | [14] | |
| 65 to 85 years | 30.2 | [14] | |
| 85 years and older | 8.9 | [14] | |
| Length of Stay for Index Patient | |||
| 1 to 17 years old | 3.6 | 0.1 | [14] |
| 18 to 44 years old | 3.6 | [14] | |
| 45 to 64 years old | 5.0 | [14] | |
| 65 to 85 years | 5.4 | [14] | |
| 85 years and older | 5.5 | 0.1 | [14] |
Mean value unless otherwise noted
Denotes value is a standard error
Longer CDI attributable LOS used in additional analysis
C. difficile screening consisted of peri-rectal swabbing, pre-amplification in a selective medium, and the use of real time PCR assay.[13] The test assumed a mean sensitivity of 99% and specificity of 99.1% (Table 1). Testing also had a 1.25 to 3.25 day turnaround time[13], during which colonized patients could freely transmit C. difficile to other patients, based on its reproductive rate (R), which is the average number of secondary colonizations generated by one colonized patient, regardless of the mode of transmission (e.g., person-to-person, environmental).
All secondary colonizations generated could develop a C. difficile infection (CDI) or remain colonized. Those developing CDI could have mild/moderate or severe CDI. We used standard treatments for CDI therapy [9,5,15]. Severe CDI patients could undergo surgery (i.e., a total colectomy), based on the results of a computed tomography (CT) scan. Patients undergoing surgery received enteral (PO) vancomycin and IV metronidazole (500 mg, every 8 hours for 10-14 days[5,9,15]), requiring the use of additional peripheral intravenous central catheter (PICC) line. Those patients not undergoing surgery were treated with oral vancomycin (125 mg, 4 times daily for 10-14 days[5,9,15]), which had a probability of being effective; if ineffective, a second course was given. Severe CDI episodes, regardless of surgery, were associated with a probability of mortality. Patients with mild/moderate CDI received oral metronidazole (500 mg, 3 times daily for 10-14 days[5,15]). If treatment was ineffective, a second course was given, if this failed again, the patient was switch to vancomycin. Upon the second recurrence, all patients were given tapered vancomycin (4 times daily for 14 days, 2 times daily for 7 days, once daily for 7 days, once every 2 days for 8 days, once every 3 days for 15 days[5,15]), regardless of disease severity. All secondary cases could experience up to 2 recurrences, for a total of ≤3 episodes of CDI. CDI’s severity was independent of the previous episode’s severity (i.e., a patient with mild CDI could have a recurrence with severe CDI and a severe CDI could recur as a mild CDI).
Each simulation sent 1,000 patient admissions (1st order trial or microsimulation) through the model 1,000 times (2nd order trial), for a total of one million trials with unique outcomes. The incremental cost-effectiveness ratio (ICER) was calculated for each simulation as:
where effectiveness was measured in quality-adjusted life years (QALYs). CDI patients received a decrement to their age dependent healthy QALY value by the CDI’s utility weight based on disease severity for the duration of each episode. QALY decrements for non-infectious diarrhea[16-18] were used as a proxy for C. difficile diarrhea, as more specific estimates are not yet published. Those who do not develop CDI received the full healthy QALY value for the duration of the simulation. For example, a 65 year old patient who has one episode of severe CDI would receive 0.69 QALYs for 10 days (0.84*0.817; 65 year old healthy QALY value*severe CDI’s utility weight) and would receive 0.84 QALYs for the remaining time in the model. The age of secondary cases was also determined by the statistics for all stays from HCUP. ICER values ≤$50,000/QALY were considered to be cost-effective[19] and screening was considered economically dominant when it saved both costs and QALYs. We also calculated the cost per case averted when screening is implemented (i.e., the difference in cost between screening and no screening divided by the number of cases that screening would prevent).
The hospital perspective measured illness costs in lost bed days (i.e., additional length of stay attributable to CDI) by a methods described by Graves[20]. The third party payer prospective included the direct costs of illness, such as hospitalization, diagnostic tests, and treatment. All costs, where applicable, were age-dependent. Other costs, such as screening (i.e., materials and technician time) and contact isolation of those testing positive were incurred by the each perspective being modeled. The cost of contact isolation included the cost of gloves and gowns for each patient contact per day for the duration of their hospitalization. Contact isolation was considered standard treatment for all secondary cases and its cost was included only from the hospital perspective.
Sensitivity Analyses
Monte Carlo probabilistic sensitivity analyses simultaneously varied the parameters in Table 1 throughout the ranges listed. Sensitivity analysis varied the probability of colonization (0.5% to 20%[8,21-22]) of the admitted patient, to represent differences in location and the risks associated with antibiotic exposure and hospitalization. The probability of infection given colonization for secondary cases was varied from 5.88%[8] to 18.6%[23]. Contact isolation compliance was ranged from 25% to 75% (efficacy was assumed to be 100% if implemented correctly). The sensitivity of the screening test was varied from the baseline in Table 1 to 75%. Initial scenarios assumed a CDI attributable LOS based on Kyne et al. (mean: 3.6; 95% confidence interval: 1.5 - 6.2) [24]; additional runs explored the effects of an increased CDI attributable LOS (mean: 7.14; standard deviation: 2.18) [25-29].
RESULTS
Table 2 shows the ICER values for C. difficile screening of hospital admissions from both the hospital and third party payer perspectives by varying rates of colonization, infection, and contact isolation compliance. Screening was cost-effective (i.e., ≤$50,000/QALY) for every scenario tested, with all ICER values ≤$256/QALY from both perspectives. C. difficile screening was economically dominant (i.e., saved costs and QALYs) under several scenarios (Table 2). When the colonization rate was ≥10.3% and probability of infection after C. difficile spore acquisition was ≥5.88%, C. difficile screening dominated no screening when contact isolation compliance was a least 25% from the hospital perspective. For a 5% colonization rate, screening was economically dominant when the probability of C. difficile infection after spore acquisition rate was 18.6% and contact isolation compliance was ≥25% (hospital perspective). C. difficile screening remained cost-effective when costing $50 (ICERs ≤$930/QALY) or $75 (ICERs ≤$1,376/QALY) from both perspectives.
Table 2.
Incremental cost effectiveness ratio (ICER, $/QALY) for C. difficile screening compared to no screening
|
C. difficile Colonization on Admission (%) |
Contact Isolation Compliance (%) |
||
|---|---|---|---|
| 25 | 50 | 75 | |
| Hospital Perspective | |||
| Probability of Infection after Colonization = 5.88% | |||
| 0.5 | 256 | 241 | 208 |
| 1 | 122 | 105 | 94 |
| 5 | 5 | 3 | 1 |
| 10.3 | Screen | Screen | Screen |
| 15 | Screen | Screen | Screen |
| 20 | Screen | Screen | Screen |
| Probability of Infection after Colonization = 18.6% | |||
| 0.5 | 207 | 186 | 157 |
| 1 | 64 | 42 | 40 |
| 5 | Screen | Screen | Screen |
| 10.3 | Screen | Screen | Screen |
| 15 | Screen | Screen | Screen |
| 20 | Screen | Screen | Screen |
| Third Party Payer Perspective | |||
| Probability of Infection after Colonization = 5.88% | |||
| 0.5 | 235 | 212 | 187 |
| 1 | 97 | 85 | 73 |
| 5 | Screen | Screen | Screen |
| 10.3 | Screen | Screen | Screen |
| 15 | Screen | Screen | Screen |
| 20 | Screen | Screen | Screen |
| Probability of Infection after Colonization = 18.6% | |||
| 0.5 | 131 | 100 | 76 |
| 1 | Screen | Screen | Screen |
| 5 | Screen | Screen | Screen |
| 10.3 | Screen | Screen | Screen |
| 15 | Screen | Screen | Screen |
| 20 | Screen | Screen | Screen |
Screen = screening was dominant (less costly and more effective) than no screening
The cost of one secondary case having up to 3 CDI episodes was a median $7,178 (mean: $7,177; range: $6,817 to $7,562) from the hospital perspective and a median $12,979 (mean: $12,979; range: $12,403 to $13,629) from the third party payer perspective. Table 3 reports the cost per case averted from the hospital perspective. In some scenarios, the costs of C. difficile screening exceeded the cost-savings in CDI cases averted, with $12 to $4,072 spent per case averted (Table 3). In some scenarios (when the population entering the model had a C. difficile prevalence ≥10.3%), C. difficile screening led to cost savings to avert a case (i.e., negative values). Cost savings ranged from $53 to $272 per case averted in these scenarios. Screening always provided savings in scenarios where the population entering the model had a C. difficile prevalence ≥7.5% when contact isolation compliance was ≥25% (5.88% probability of C. difficile infection after spore acquisition). The savings provided by screening were even higher with an 18.6% infection rate and ranged from $250 (5% admission colonization rate, contact isolation compliance 25%) to $2,249 (20% colonization rate on admission, contact isolation compliance 75%).
Table 3.
Cost per case averted utilizing screening with a 5.88% probability of C. difficile infection from the hospital perspective
|
C. difficile Colonization on Admission (%) |
Contact Isolation Compliance (%) |
||
|---|---|---|---|
| 25 | 50 | 75 | |
| 0.5 | 4,072 | 3,787 | 3,238 |
| 1 | 1,936 | 1,655 | 1,482 |
| 5 | 77 | 47 | 12 |
| 7.5 | −53 | −89 | −136 |
| 10.3 | −146 | −157 | −189 |
| 12 | −163 | −195 | −227 |
| 15 | −190 | −214 | −241 |
| 20 | −235 | −242 | −271 |
Note: Negative values imply cost savings
Increasing the attributable LOS to an average 7.14 days, decreased the ICER values from the hospital perspective (all ICERs ≤$226/QALY) and screening become the dominant strategy at a 5% admission colonization prevalence when contact isolation compliance was ≥25% (5.88% probability of infection after acquisition). For third party payers, the increased LOS did not have an effect on the cost-effectiveness of screening (all ICERs ≤$235/QALY). A test with 75% sensitivity still yielded screening to be cost-effective with all ICER values ≤$344/QALY from both perspectives.
Annual Hospital Savings
Assuming a 10.3% colonization rate on admission[8], a hospital with 1,000 annual admissions would experience cost savings of $10,256, $12,278, and $16,071 when implementing universal screening plus contact isolation with 25%, 50%, and 75% compliance, respectively. The costs or savings increased with increasing annual admissions. For 5,000 and 10,000 annual admissions, hospitals could save $51,280 to $80,356 and $102,560 to -$160,712, respectively with contact isolation compliance rates ≥25%. Extrapolating to the entire US, with 34,705,583 annual discharges in 2009[14], cost savings could range from $152 million (7.5% admission prevalence, 25% contact isolation compliance) to $1.6 billion (20% admission prevalence, 75% contact isolation compliance).
DISCUSSION
C. difficile has become an increasing healthcare concern and can be costly pathogen.[3] Reducing the number of healthcare associated CDI cases can in turn reduce CDI’s costs. The cost of one secondary case in our model (median: $5,953 and $10,547 for 1 episode and a median: $7,178 and $12,979 for up to 3 episodes, from the hospital and third party payer perspectives, respectively) is consistent with previously published range of CDI costs ($2,000 to $72,000). [4,30-31] While the number of secondary cases an index case will generate remains unclear, an extensive mathematical model has simulated a basic reproductive rate, suggesting that transmission within a ward is insufficient to account for sustained, endemic CDI within hospital facilities. This model, as well as other studies, has suggested that admission colonized patients play a key role in sustaining C. difficile transmission.[32,8] Our results show that screening coupled with contact isolation precautions may be a cost-effective way to reduce the number of secondary CDI cases, leading to cost-savings by averting cases. Economically dominant results strongly support the implementation of screening, as the intervention not only saves costs, but also provides health benefits.
Screening for other HAIs (i.e., Staphylococcus aureus and Acinetobacter baumannii) has shown cost-effectiveness in various populations.[33-38] Our goal was to inform various decision makers (e.g., infection control specialists, hospital administrators, insurance companies) about the potential cost-effectiveness of C. difficile screening, not to make the decision about implementation. Decision makers can use the results of our study to make decisions based on their own local circumstances. Our results suggest that C. difficile screening, even just at admission, is cost-effective over a range of colonization and contact isolation compliance rates. For community hospitals, where the population served might have prevalence of C. difficile colonization closer to that observed in healthy adults (0-15%) based on prior studies,[39-42] screening can be cost-effective and even cost saving if implemented with contact isolation compliance rates ≥25%. For a community hospital with a 5% prevalence of C. difficile carriers entering the facility, increasing contact isolation compliance can reduce the cost per case averted generated by screening (Table 3). It should be noted that the implementation of a new screening method may require additional start up costs (e.g., new equipment) and ongoing microbiology laboratory personnel costs which were not included in the model.
Although we did not explicitly model other inpatient populations, our results could be particularly important for tertiary referral centers, long-term acute care hospitals, and some nursing facilities which have been associated with high prevalence of C. difficile carriage in previous studies.[7] However, they may not be applicable to those long-term acute care facilities and nursing homes, which have ongoing transmission from long-term residents with C. difficile colonization, in which repeated screening may be necessary. They also may not be applicable to hospitals that primarily serve pediatric patients (patients <1 year old were not included in this study), as neonates are known to have very high rates of asymptomatic C. difficile carriage.
Our model attempted to be conservative about the benefits of C. difficile screening. We limited the number of unique CDI episodes to 3 per secondary case; some persons may experience more. The costs evaluated were only for the duration of hospitalization (with continuing treatment after discharge to complete the full course of antibiotics), additional costs may be associated with a longer time frame. Additionally, we used only the standard treatment regimens for CDI, other drug therapies may be used such as reconstituted IV vancomycin and fidaxomicin. We excluded rare CDI complications and co-morbid conditions (e.g., irritable bowel syndrome or immunosuppressed patients), which may lead to additional costs. The health impact of CDI may be underestimated in our model; the QALY decrements used in our study are for non-infectious diarrhea, while diarrhea caused by C. difficile may be more severe, resulting in a larger decrement. Furthermore, our model only focused on identifying C. difficile carriers and how this can reduce its spread to other patients, but not how it may lead to the implementation of appropriate antibiotic treatment for those who may progress to infection or reduction in transmission of other epidemiologically significant organisms within hospitals. Although not explicitly modeled, screening may have additional benefits in increased environmental cleaning in rooms for those who test positive, further reducing transmission. However, it should be noted that a positive screening test should not prompt treatment in patients with minimal or no symptoms[5]; patients may acquire CDI as a result of the misguided treatment. Additional data on the colonization rate on admission in different inpatient and long-term care populations are needed to get a more accurate picture about the potential benefits C. difficile screening. The probability of infection given C. difficile colonization may vary as most studies do not report this since a patient’s colonization status is not known.
By definition, all models are simplifications of real life[43] and therefore cannot account for every possible CDI event or outcome. Nor can the full spectrum of socio-demographic and clinical heterogeneity among admitted patients being screened or among secondary cases be represented. Our model inputs were derived from studies of varying quality. While adverse events attributable to contact isolation precautions have been reported[44], there are no published cost or utility estimates to quantify these effects and where therefore not included. In addition, contact precautions did not include the use of an isolation/private room, which may incur additional costs (e.g., patient transfer and cleaning. However, this arrangement is becoming more obsolete as new hospital construction in the US now provides for 100% single occupancy rooms. As constructed, our model does not account for potential transmission events from patients with negative admission screens who may go on to acquire C. difficile on a hospital ward.
Conclusion
Our model showed that C. difficile screening, coupled with contact isolation precautions, may be a cost-effective intervention (≤$256/QALY) to hospitals and third party payers. Reducing the transmission of C. difficile can reduce the number of CDI cases and episodes, therefore reducing its large economic burden to the healthcare system. Under some conditions, screening was economically dominant and could save costs if implemented.
ACKNOWLEDGMENTS
This study was supported by the Pennsylvania Department of Health (grant #4100047864) and the National Institute of General Medical Sciences Models of Infectious Disease Agent Study (MIDAS) grant 1U54GM088491-0109. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
CONFLICT OF INTEREST The authors are not aware of any substantial conflict of interest.
REFERENCES
- 1.Heinlen L, Ballard JD. Clostridium difficile infection. American Journal of the Medical Sciences. 2010;340(3):247–252. doi: 10.1097/MAJ.0b013e3181e939d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Missaghi B, Valentia AJ, Owens RCJ. Clostridium difficile infection: a critical overview. Current Infectious Disease Reports. 2008;10:165–173. doi: 10.1007/s11908-008-0028-5. [DOI] [PubMed] [Google Scholar]
- 3.McGlone SM, Bailey RR, Zimmer SM, Popovich MJ, Tian Y, Ufberg PJ, Muder RR, Lee BY. The economic burden of Clostridium difficile. Clinical Microbiology and Infection. 2011 doi: 10.1111/j.1469-0691.2011.03571.x. doi: 10.1111/j.1469-0691.2011.03571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dubberke ER, Wertheimer AI. Review of current literature on the economic burden of Clostridium difficile infection. Infection Control and Hospital Epidemiology. 2009;30(1):57–66. doi: 10.1086/592981. [DOI] [PubMed] [Google Scholar]
- 5.Kelly CP, LaMont JT. Clostridium difficile - more difficult than ever. New England Journal of Medicine. 2008;359:1932–1940. doi: 10.1056/NEJMra0707500. [DOI] [PubMed] [Google Scholar]
- 6.McFarland LV, Mulligan ME, Kowk RYY, Stamm WE. Nosocomial acquisition of Clostridium difficile infection. New England Journal of Medicine. 1989;320(4):204–210. doi: 10.1056/NEJM198901263200402. [DOI] [PubMed] [Google Scholar]
- 7.Riggs MM, Sethi AK, Zabarsky TF, Eskstein Ec, Jump RLP, Donskey CJ. Asymptomatic carriers are a potential source for transmission of epidemic and nonepidemic Clostridium difficile strains among long-term care facility residents. Clinical Infectious Diseases. 2007;45(992-998) doi: 10.1086/521854. [DOI] [PubMed] [Google Scholar]
- 8.Clabots CR, Johnson S, Olson MM, Peterson LR, Gerding DN. Acquisition of Clostridium difficile by hospitalized patients: evidence for colonized new admissions as a source of infection. Journal of Infectious Diseases. 1992;166:561–567. doi: 10.1093/infdis/166.3.561. [DOI] [PubMed] [Google Scholar]
- 9.Cohen SH, Gerding DN, Johnson S, Kelly CP, Loo VG, McDonald LC, Pepin J, Wilcox MH. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infections Diseases Society of America (IDSA) Infection Control and Hospital Epidemiology. 2010;31(5):431–455. doi: 10.1086/651706. [DOI] [PubMed] [Google Scholar]
- 10.Sloan LM, Duresko BJ, Gustafson DR, Rosenblatt JE. Comparison of real-time PCR for detection of the tcdC gene with four toxin immunoassays and culture in diagnosis of Clostridium difficile infection. Journal of Clinical Microbiology. 2008;46(6):1996–2001. doi: 10.1128/JCM.00032-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stamper PD, Alcabasa R, Aird D, Babiker W, Wehrlin J, Ikpeama I, Carroll KC. Comparison of a commercial real-time PCR assay for tcdB detection to a cell culture cytotoxicity assay and toxigenic culture for direct detection of toxin-producing Clostridium difficile in clinical samples. Journal of Clinical Microbiology. 2009;47(2):373. doi: 10.1128/JCM.01613-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stamper PD, Babiker W, Alcabasa R, Aird D, Wehrlin J, Ikpeama I, Gluck L, Carroll KC. Evaluation of a new commercial TaqMan PCR assay for direct detection of the Clostridium difficile toxin B gene in clinical stool samples. Journal of Clinical Microbiology. 2009;47(12):3846. doi: 10.1128/JCM.01490-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Curry SR, Gee JL, Hamilton TM, Henderson TK, Brown NT, Marsh JW, Shutt KA, Brooks MM, Pasculle AW, Muto CA, Harrison LH. Peri-rectal swab surveillance for Clostridium difficile using selective broth pre-amplification and real-time PCR detection of tcdB. Journal of Clinical Microbiology. 2011 doi: 10.1128/JCM.00679-11. Epub Ahead of Print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.United States Department of Health & Human Services HCUP facts and figures: statistics on hospital-based care in the United States [Accessed Feb 2 2012];AHRQ: Agency for Healthcare Research and Quality. 2009 http://www.hcup-us.ahrq.gov/reports.jsp. [PubMed]
- 15.Leffler DA, LaMont JT. Treatment of Clostridium difficile-associated disease. Gastroenterology. 2009;136:1899–1912. doi: 10.1053/j.gastro.2008.12.070. [DOI] [PubMed] [Google Scholar]
- 16.Ramsey S, Veenstra D, Clarke L, Gandhi S, Hirsch M, Penson D. Is combined androgen blockade with bicalutamide cost-effective compared with combined androgen blockade with flutamide? Urology. 2005;66(4):835–839. doi: 10.1016/j.urology.2005.04.028. [DOI] [PubMed] [Google Scholar]
- 17.Penson D, Ramsey S, Veenstra D, Clarke L, Gandhi S, Hirsch M. The cost-effectiveness of combined androgen blockade with bicalutamide and luteinizing hormone releasing agonist in men with metastatic prostate cancer. Journal of Urology. 2005;174(2):547–552. doi: 10.1097/01.ju.0000165569.48372.4c. [DOI] [PubMed] [Google Scholar]
- 18.Hayes JL, Hansen P. Is laparoscopic colectomy for cancer cost-effective relative to open colectomy? ANZ Journal of Surgery. 2007;77(9):782–786. doi: 10.1111/j.1445-2197.2007.04226.x. [DOI] [PubMed] [Google Scholar]
- 19.Neumann PJ, Sandberg EA, Bell CM, Stone PW, Chapman RH. Are pharmaceuticals cost-effective? A review of the evidence. Health Affairs. 2000;19(2):92–109. doi: 10.1377/hlthaff.19.2.92. [DOI] [PubMed] [Google Scholar]
- 20.Graves N. Economics and preventing hospital-acquired infection. Emerging Infectious Diseases. 2004;10(4):561–566. doi: 10.3201/eid1004.020754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marciniak C, Chen D, Stein AC, Semik PE. Prevalence of Clostridium difficile colonization at admission to rehabilitation. Archives of Physical Medicine and Rehabiliation. 2006;87(8):1086–1890. doi: 10.1016/j.apmr.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 22.Loo VG, Bourgault A-M, Poirier L, Lamothe F, Michaud S, Turgeon N, Toye B, Beaudoin A, Frost EH, Gilca R, Brassard P, Dendukuri N, Beliveau C, Oughton M, Brukner I, Dascal A. Host and pathogen factors for Clostridium difficile infection and colonization. New England Journal of Medicine. 2011;365:1693–1703. doi: 10.1056/NEJMoa1012413. [DOI] [PubMed] [Google Scholar]
- 23.Shim JK, Johnson S, Samore MH, Bliss DZ, Gerding DN. Primary symptomless colonisation by Clostridium difficile and decreased risk of subsequent diarrhoea. Lancet. 1998;351:633–636. doi: 10.1016/S0140-6736(97)08062-8. [DOI] [PubMed] [Google Scholar]
- 24.Kyne L, Hamel MB, Polavaram R, Kelly CP. Health care costs and mortality associated with nosocomial diarrhea due to Clostridium difficile. Clinical Infectious Diseases. 2002;34:346–353. doi: 10.1086/338260. [DOI] [PubMed] [Google Scholar]
- 25.Pepin J, Valiquette L, Cossette B. Mortality attributable to nosocomial Clostridium difficile-associated disease during an epidemic caused by a hypervirulent strain in Quebec. Canadian Medical Association Journal. 2005;173(9):1037. doi: 10.1503/cmaj.050978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Song X, Bartlett JG, Speck K, Naegeli A, Carroll KC, Perl TM. Rising economic impact of Clostridium difficile - associated diesease in adult hopsitalized patient population. Infection Control and Hospital Epidemiology. 2008;29(9):823–828. doi: 10.1086/588756. [DOI] [PubMed] [Google Scholar]
- 27.Forster AJ, Taljarrd M, Oake N, Wilson K, Roth V, van Walraven C. The effect of hospital-acquired infection with Clostridium difficile on length of stay in hospital. Canadian Medical Association Journal. 2012;184(1):37–42. doi: 10.1503/cmaj.110543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pakyz A, Carroll NV, Harpe SE, Oinonen M, Polk RE. Economic impact of Clostridium difficile infection in a mulithospital cohort of academic health centers. Pharmacotherapy. 2011;31(6):546–551. doi: 10.1592/phco.31.6.546. [DOI] [PubMed] [Google Scholar]
- 29.Vonberg R-P, Reichardt C, Behnke M, Schwab F, Zindler S, Gastmeier P. Costs of nosocomial Clostridium difficile-associated diarrhoea. Journal of Hospital Infection. 2008;70(1):15–20. doi: 10.1016/j.jhin.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 30.Ghantoji SS, Sail K, Lairson D, DuPont HL, Garey KW. Economic healthcare costs of Clostridium difficile infection: a systematic review. Journal of Hospital Infection. 2010;74:309–318. doi: 10.1016/j.jhin.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 31.McGlone SM, Bailey RR, Zimmer SM, Popovich MJ, Tian Y, Ufberg PJ, Muder RR, Lee BY. The economic burden of Clostridium difficile. Clinical Microbiology and Infection. 2012;18(3):282–289. doi: 10.1111/j.1469-0691.2011.03571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lanzas C, Dubberke ER, Lu Z, Reske KA, Grohn YT. Epidemiological model for Clostridium difficile transmission in healthcare settings. Infection Control and Hospital Epidemiology. 2011;32(6):553–561. doi: 10.1086/660013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee BY, Bailey RR, Smith KJ, Muder RR, Strotmeyer ES, Lewis GJ, Ufberg PJ, Song Y, Harrison LH. Universal methicillin-resistant Staphylococcus aureus (MRSA) surveillance for adults at hospital admission: an economic model and analysis. Infection Control and Hospital Epidemiology. 2010;31(6):598–606. doi: 10.1086/652524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee BY, McGlone SM, Doi Y, Bailey RR, Harrison LH. Economic value of Acinetobacter baumannii screening in the intensive care unit (ICU) Clinical Microbiology and Infection. 2011;17(11):1691–1697. doi: 10.1111/j.1469-0691.2011.03491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee BY, Song Y, McGlone SM, Bailey RR, Feura J, Tai JHY, Lewis GJ, Wiringa AE, Smith KJ, Muder RR, Harrison LH, Piraino B. The economic value of screening haemodialysis patients for methicillin-resistant Staphylococcus aureus in the USA. Clinical Microbiology and Infection. 2011;17(11):1717–1726. doi: 10.1111/j.1469-0691.2011.03525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee BY, Tsui B, Bailey RR, Smith KJ, Muder RR, Lewis GJ, Harrison LH. Should vascular surgery patients be screened preoperatively for methicillin-resistant Staphylococcus aureus? Infection Control and Hospital Epidemiology. 2009;30(12):1158–1165. doi: 10.1086/648087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee BY, Wiringa AE, Bailey RR, Goyal V, Lewis GJ, Tsui B, Smith KJ, Muder RR. Screening cardiac surgery patients for MRSA: an economic computer model. American Journal of Managed Care. 2010;16(7):e163–e173. [PMC free article] [PubMed] [Google Scholar]
- 38.Lee BY, Wiringa AE, Bailey RR, Goyal V, Tsui B, Lewis GJ, Muder RR, Harrison LH. The economic effect of screening orthopedic surgery patients preoperatively for methicillin-resistant Staphylococcus aureus. Infection Control and Hospital Epidemiology. 2010;31(11):1130–1138. doi: 10.1086/656591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kato H, Kita H, Karasawa T, Maegawa T, Koino Y, Takakuwa H, Saikai T, Kobayashi K, Yamagishi T, Nakamura S. Colonisation and transmission of Clostridium difficile in healthy individuals examined by PCR ribotyping and pulsed-field gel electrophoresis. J Med Microbiol. 2001;50(8):720–727. doi: 10.1099/0022-1317-50-8-720. [DOI] [PubMed] [Google Scholar]
- 40.Nakamura S, Mikawa M, Nakashio S, Takabatake M, Okado I, Yamakawa K, Serikawa T, Okumura S, Nishida S. Isolation of Clostridium difficile from the feces and the antibody in sera of young and elderly adults. Microbiol Immunol. 1981;25(4):345–351. doi: 10.1111/j.1348-0421.1981.tb00036.x. [DOI] [PubMed] [Google Scholar]
- 41.Ozaki E, Kato H, Kita H, Karasawa T, Maegawa T, Koino Y, Matsumoto K, Takada T, Nomoto K, Tanaka R, Nakamura S. Clostridium difficile colonization in healthy adults: transient colonization and correlation with enterococcal colonization. J Med Microbiol. 2004;53(Pt 2):167–172. doi: 10.1099/jmm.0.05376-0. [DOI] [PubMed] [Google Scholar]
- 42.Viscidi R, Willey S, Bartlett JG. Isolation rates and toxigenic potential of Clostridium difficile isolates from various patient populations. Gastroenterology. 1981;81(1):5–9. [PubMed] [Google Scholar]
- 43.Lee BY. Digital decision making: computer models and antibiotic prescribing in the twenty-first century. Clinical Infectious Diseases. 2008;46(8):1139–1141. doi: 10.1086/529441. [DOI] [PubMed] [Google Scholar]
- 44.Abad C, Fearday A, Safdar N. Adverse events of isolation in hospitalised patients: a systematic review. Journal of Hospital Infection. 2010;76:97–102. doi: 10.1016/j.jhin.2010.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Puzniak LA, Gillespie KN, Leet T, Kollef M, Mundy LM. A cost-benefit analysis of gown use in controlling vancomycin-resistant Enterococcus transmission: is it worth the price? Infection Control and Hospital Epidemiology. 2004;25:418–424. doi: 10.1086/502416. [DOI] [PubMed] [Google Scholar]
- 46.Bureau of Labor Statistics [Accessed November 2011];Occupational employment statistics: May 2009 national occupational employment and wage estimates, United States. U.S. Bureau of Labor Statistics Division of Occupational Employment Statistics. 2010 http://stat.bls.gov/oes/2008/may/oes_nat.htm#b00-0000.
- 47.American Medical Association [Accessed April 27 2011];CPT Code/Relative Value Search. 2011 https://ocm.ama-assn.org/OCM/CPTRelativeValueSearch.do.
- 48.PDR . Red Book Pharmacy’s Fundamental Reference. Thompson Reuters (Healthcare), Inc.; Montvale, NJ: 2010. [Google Scholar]
- 49.Gold MR, Franks P, McCoy KI, Fryback DG. Toward consistency in cost-utility analyses: using national measures to create condition-specific values. Medical Care. 1998;36(6):778–792. doi: 10.1097/00005650-199806000-00002. [DOI] [PubMed] [Google Scholar]
- 50.Fujitani S, George WL, Murthy AR. Comparison of clinical severity score indices for Clostridium difficile infection. Infection Control and Hospital Epidemiology. 2011;32(3):220–228. doi: 10.1086/658336. [DOI] [PubMed] [Google Scholar]
- 51.Henrich TJ, Krakower D, Bitton A, Yokoe DS. Clinical risk factors for severe Clostridium difficile-associated disease. Emerging Infectious Diseases. 2009;15(13):415–422. doi: 10.3201/eid1503.080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barbut F, Gariazzo B, Bonne L, Lalande V, Burghoffer B, Luiuz R, Petit H-C. Clinical features of Clostridium difficile-associated infections and molecular characterization of strains: results of a retrospective study, 2000-2004. Infection Control and Hospital Epidemiology. 2007;28(2):131–139. doi: 10.1086/511794. [DOI] [PubMed] [Google Scholar]
- 53.Ricciardi R, Rothenberger DA, Madoff RD, Baxter NN. Increasing prevalence and severity of Clostridium difficile colitis in hospitalized patients in the United States. Archives of Surgery. 2007;142(7):624–631. doi: 10.1001/archsurg.142.7.624. [DOI] [PubMed] [Google Scholar]
- 54.Jaber MR, Olfsson S, Fung WL, Reeves ME. Clinical review of the management of fulminant Clostridium difficile infection. American Journal of Gastroenterology. 2008;103:3195–3203. doi: 10.1111/j.1572-0241.2008.02198.x. [DOI] [PubMed] [Google Scholar]
- 55.Pepin J, Vo TT, Boutros M, Marcotte E, Dial S, Bube S, Vasilevsky C-A, McFadden N, Patino C, Labbe A-C. Risk factors for mortality following emergency colectomy for fulminant Clostridium difficile infection. Diseases of the Colon and Rectum. 2009;52(3):400–405. doi: 10.1007/DCR.0b013e31819a69aa. [DOI] [PubMed] [Google Scholar]
- 56.Lamontagne F, Labbe A-C, Haeck O, Lesur O, Lalancette M, Patino C, Leblanc M, Laverdiere M, Pepin J. Impact of emergency colectomy on survival of patients with fulminant Clostridium difficile colitis during an epidemic caused by a hypervirulent strain. Annals of Surgery. 2007;245(2):267–272. doi: 10.1097/01.sla.0000236628.79550.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Barbut F, Richard A, Hamadi K, Chomette V, Burghoffer B, Petit J-C. Epidemiology of recurrences or reinfections of Clostridium difficile-associated diarrhea. Journal of Clinical Microbiology. 2000;38(6):2386–2388. doi: 10.1093/gao/9781884446054.article.t031141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fekety R, McFarland LV, Surawicz CM, Greenberg RN, Elmer GW, Mulligan ME. Recurrent Clostridium difficile diarrhea: characteristics of and risk factors for patients enrolled in a prospective, randomized, double-blinded trial. Clinical Infectious Diseases. 1997;24(3):324–333. doi: 10.1093/clinids/24.3.324. [DOI] [PubMed] [Google Scholar]
- 59.Bauer MP, Kuijper EJ, van Dissel JT. European Society of Clinical Microbiology and Infectious Diseases (ESCMID): treatment guidance document for Clostridium difficile infection (CDI) Clinical Microbiology and Infection. 2009;15:1067–1079. doi: 10.1111/j.1469-0691.2009.03099.x. [DOI] [PubMed] [Google Scholar]
- 60.McFarland LV, Elmer GW, Surawicz CM. Breaking the cycle: treatment strategies for 163 cases of recurrent Clostridium difficile disease. Am J Gastroenterol. 2002;97(7):1769–1775. doi: 10.1111/j.1572-0241.2002.05839.x. doi:10.1111/j.1572-0241.2002.05839.x. [DOI] [PubMed] [Google Scholar]
- 61.Jernigan JA, Clemence MA, Stott GA, Titus MG, Alexander CH, Palumbo CM, Farr BM. Control of methicillin-resistant Staphylococcus aureus at a university hospital: one decade later. Infection Control and Hospital Epidemiology. 1995;16(12):686–696. doi: 10.1086/647042. [DOI] [PubMed] [Google Scholar]