Abstract
Home mouse allergen exposure is associated with asthma morbidity, but little is known about the shape of the dose-response relationship or the relevance of location of exposure within the home.
Asthma outcome and allergen exposure data were collected every three months for 1 year in 150 urban children with asthma. Participants were stratified by mouse sensitization and relationships between continuous measures of mouse allergen exposure and outcomes of interest were analyzed.
Every ten-fold increase in the bed mouse allergen level was associated with an 87% increase in the odds of any asthma-related health care use among mouse sensitized (OR (95% CI): 1.87 (1.21–2.88)), but not non-mouse sensitized participants. Similar relationships were observed for emergency department visit and unscheduled doctor visit among mouse sensitized participants. Kitchen floor and bedroom air mouse allergen concentrations were also associated with greater odds of asthma-related healthcare utilization; however, the magnitude of the association was less than that observed for bed mouse allergen concentrations.
In this population of urban children with asthma, there is a linear dose-response relationship between mouse allergen concentrations and asthma morbidity among mouse-sensitized asthmatics. Bed and bedroom air mouse allergen exposure compartments may have a greater impact on asthma morbidity than other compartments.
Keywords: Mouse allergen, inner-city asthma, childhood asthma, exposure compartments, indoor allergens, asthma morbidity
INTRODUCTION
Pest allergens, such as cockroach and mouse allergens, are the predominant urban allergens and are important causes of asthma morbidity in urban populations. (Rosenstreich 1997; Gruchalla et al. 2005; Phipatanakul et al. 2005; Donohue 2008 et al.; Matsui et al. 2010) Although there has been more than a decade of research investigating the role of cockroach allergen in urban asthma, mouse allergen has only recently emerged as an important contributor to asthma morbidity in this population. (Matsui 2006 et al.; Pongracic et al. 2008).
Previous studies have assessed mouse allergen exposure in settled dust samples from the child’s bedroom or kitchen and reported associations between certain threshold concentrations of settled dust mouse allergen and asthma morbidity (Matsui et al. 2006; Pongracic et al. 2008), but several questions remain unanswered. First, there has been little examination of the dose-response relationship between mouse allergen exposure and asthma morbidity among mouse-sensitized children. Understanding the shape of the dose-response relationship has important implications for the design of home-based environmental interventions targeting mouse allergen. For example, a linear relationship would suggest that a decrease in mouse allergen concentrations would be associated with an incremental decrease in risk of morbidity, whereas a threshold relationship would suggest that allergen concentrations must be lowered below a particular threshold in order to expect a clinical effect.
A second question that has implications for the design of home-based environmental interventions but remains unanswered is whether a particular site or source of mouse allergen exposure is more strongly associated with morbidity among mouse-sensitized children with asthma. For example, children may have greater exposure to allergen in bedding than the bedroom floor due to the duration of time spent sleeping as well as the close proximity of the nose and mouth to bedding. On the other hand, a bedroom airborne dust sample may be a better reflection of actual exposure since this type of sample more closely reflects the material that is being inhaled with each breath. Should one compartment be the major driver of asthma morbidity, strategies to augment integrated pest management could be implemented to target specifically this compartment. These might include air purifiers for the airborne allergen compartment or small pore allergen-proof mattress/pillow encasements, which can block animal allergens, (Vaughan et al. 1999) and washing of bedding for the bed dust compartment. Therefore, we sought to examine relationships between continuous measures of bed, bedroom floor, kitchen and airborne mouse allergen exposure and asthma morbidity among urban children with asthma.
METHODS
Study Population
One hundred fifty Baltimore City children (5–17y) with persistent asthma were enrolled and followed for one year. Participants had to be diagnosed with asthma by a physician at least one year prior to the baseline study visit. Participants had to be on a controller medication or meet criteria for persistent asthma defined by the National Asthma Education and Prevention Program guidelines. (National Heart, Lung, and Blood Institute National Asthma Education and Prevention Program 2007) All participants had to have had an exacerbation in the previous year and reside in Baltimore City. Smokers were excluded. Participants also had to sleep in the same home for at least 4 nights per week to be enrolled in the study. All screened participants who met the inclusion criteria were enrolled. Clinical assessments were performed at baseline and every three months. Exposure assessments were also performed at baseline and every three months, within ±2 weeks of the clinical assessments. Written informed consent was obtained and the study was approved by the Johns Hopkins Institutional Review Board.
Clinical Assessments
Allergy skin testing was performed at the baseline clinic visit using the MultiTest II device (Lincoln Diagnostics, Decatur, IL). The allergens tested were dog, cat, Dermatophagoides pteryonyssinus, Dermatophagoides farinae, rat epithelia, German cockroach, American cockroach, mouse epithelia, oak, grass mix, Alternaria tenius, Aspergillus fumigatus, common ragweed, and Cladosporium herbarum (Greer Laboratories, Lenoir, NC). A skin test was considered positive if the net orthogonal wheal diameter was ≥ 3mm. Blood was collected at baseline and serum total IgE levels quantified using the ImmunoCAP system (Thermo-Fisher, Uppsala, Sweden).
Spirometry was performed at each clinic visit using a KoKo spirometer (nSpire Health, Inc. Longmont, Colorado) and percent predicted values were determined using Hankinson equations. (Hankinson et al. 1999) Fractional exhaled nitric oxide (FENO) was measured at each clinic visit using the NIOX Mino (Aerocrine, Uppsala, Sweden) according to manufacturer’s instructions.
Questionnaires that captured medication use, asthma symptoms, and health care utilization were administered at all clinic visits. The questionnaires captured symptoms and medication use over a two week period of time and asthma-related health care utilization was captured for the preceding three month period. (Morgan et al. 2004) Every 3 months, the study participants returned to the research clinic and were asked if they had any asthma related hospitalizations, ED visits or unscheduled doctor visits. An acute visit was defined as any one of these three types of unscheduled asthma-related health care visit.
Exposure Assessments
Settled dust samples from the bed, bedroom floor and kitchen floor were collected using a handheld vacuum cleaner and a Mitest dust collector (Indoor Biotechnologies, Charlottesville, VA). Airborne particulate matter was sampled by placing an air monitor off the floor in the child’s bedroom. Airborne particulate matter ≤10 microns was collected on 37mm Teflon filters over 5–7 days at a flow rate of 4 liters per minute. (Matsui et al. 2007) Protein was extracted from the settled dust samples and air filters and Mus m 1 content was quantified by ELISA. The limit of detection for the assay was 2.2ng/g (0.0022 μg/g).
Statistical Analyses
All analyses were performed with StataSE 11.0 (StataCorp, College Station, TX). Descriptive statistics for the variables of interest were generated using tabulations and displaying summaries of continuous variables that included measures of central tendency. Participants were stratified by mouse sensitization status and relationships between outcomes of interest and mouse allergen exposure were inspected using exploratory graphs to examine the shape of the exposure-asthma morbidity relationships. Participants were considered mouse sensitized if the net mouse epithelia wheal was ≥3mm. Final analyses used generalized estimating equations to account for repeated outcome measures. The statistical models estimated the relative odds of the outcomes of interest (e.g. acute visit) for every 10-fold change in mouse allergen exposure. Final models adjusted for age, gender, total IgE and type of health insurance. The variables that were included in the final models were selected because of general knowledge about their relationships to asthma morbidity, exposure, and/or degree of allergic sensitization. Type of health insurance was coded dichotomously as public or private insurance. To assess linearity of the relationship between mouse allergen exposure and asthma morbidity, a quadratic term was added and did not improve the fit of the linear model. Interactions between exposure and age, sex, and public health insurance could not be formally examined because of the modest sample size of the population. The criterion for statistical significance was a p value < 0.05.
RESULTS
Study Population Characteristics
One hundred and fifty participants were enrolled in the study. Fifty-seven percent were male and the median age was 11 years. Participants were predominantly African American (91%) with an annual household income of less than $25,999. (Table 1) Participants had frequent symptoms and rescue medication use and lung function and inflammation parameters were typical of a pediatric population with persistent, atopic asthma. The mean FEV1/FVC% was 80.9% and the median FENO was 33ppb. (Table 2) Ninety-one percent of the participants were atopic and 52% sensitized to mouse. Sensitization to other indoor allergens was also common (Table 3)
Table 1.
Sociodemographic Characteristics (n=150)
| Age (y), median (25th%–75th%) | 11 (8–15) |
|
| |
| Male, n (%) | 85 (57) |
|
| |
| Annual Household Income ($), n (%) | |
| <25,000 | 75 (50) |
| 25,000–49,999 | 40 (27) |
| ≥50,000 | 12 (8) |
| Don’t know or refused | 23 (15) |
|
| |
| Black/African American, n (%) | 137 (91) |
|
| |
| Insurance, n (%) | |
| Public | 128 (86) |
| Private | 18 (12) |
| Self Pay | 2 (1) |
| Don’t know | 2 (1) |
Table 2.
Asthma Characteristics at Baseline
| mean±SD | |
|---|---|
| FEV1, % predicted* | 93.5±18.5 |
| FVC, % predicted* | 99.7±15.8 |
| FEV1/FVC %* | 80.9±9.9 |
| Days of symptoms/2 weeks | 4.9±4.8 |
| Days of short-acting beta agonist use/2 weeks | 4.2±5.0 |
| Controller medication, n (%) | 108 (72) |
| FENO (ppb), median (25th%–75th%) | 33 (16–62) |
n=140
Table 3.
Atopic Characteristics
| Total Serum IgE* (kU/L), median (25th%–75th%) | 189 (48–458) |
|
| |
| Skin Test Sensitivity¥ | n (%) |
| Atopic (≥1 +SPT) | 135 (91) |
| Cat | 97 (65) |
| Cockroach | 92 (62) |
| Dust mite | 85 (57) |
| Mouse | 78 (52) |
n=147
n=149
At the baseline home visit, mouse allergen was detectable in 100% of bed dust samples, 99% of bedroom floor dust samples, 100% of kitchen samples and 91% of air samples. Mouse allergen concentrations were as follows (median (25th–75th)): bed: 1.2 μg/g (0.3–3.9); bedroom floor: 2.3 μg/g (0.6–12.7); kitchen: 8.0 μg/g (0.3–34.1); and air: 0.03ng/m3 (0.01–0.08) (Table 4).
Table 4.
Baseline Mouse Allergen Concentrations
| median (25th–75th) | |
|---|---|
| Settled Dust (μg/g) | |
| Kitchen* | 8.0 ( 0.3–34.1) |
| Bedroom£ | 2.3 (0.6–12.7 ) |
| Bed¥ | 1.2 (0.3–3.9) |
| Air (ng/m3) | |
| Bedroom€ | 0.03 (0.01–0.08) |
n=110;
n=127;
n=118;
n=124
Mouse Allergen Exposure and Asthma Morbidity Dose-Response Relationships
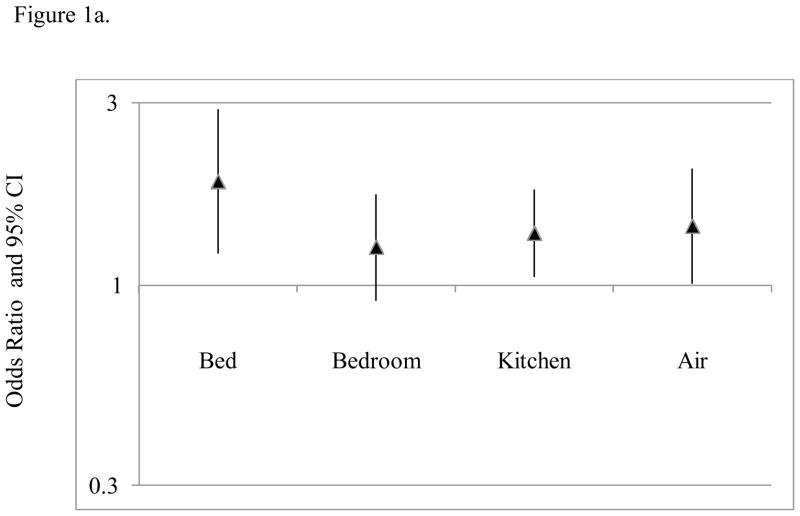
Increasing bed mouse allergen concentrations were associated with an increasing odds of asthma-related health care use among mouse sensitized, but not non-mouse sensitized participants in both unadjusted and adjusted analyses. For example, every 10-fold increase in bed dust mouse allergen concentration was associated with an 87% increase in the odds of having an acute visit for asthma among mouse-sensitized participants in the final adjusted model; similar relationships were observed for emergency department visit and unscheduled doctor visit (OR (95% CI): 1.66 (1.07–2.56) and 2.10 (1.12–3.93), respectively). (Table 5 and Figure 1) Bed mouse allergen concentrations were not associated with hospitalization among either mouse-sensitized or non-mouse sensitized participants.
Table 5.
Relationships Between Mouse Allergen Exposure Compartment and Acute Asthma Visit, by Mouse Skin Test Sensitization Status§
| Acute Visit OR (95%CI) | ED Visit OR (95%CI) | Unscheduled Doctor Visit OR (95%CI) | |
|---|---|---|---|
| mSPT(+)* | |||
| Bed | 1.87 (1.21–2.88) | 1.66 (1.07–2.56) | 2.10 (1.12–3.93) |
| Bedroom | 1.26 (0.91–1.73) | 1.36 (0.96–1.91) | 1.49 (0.93–2.38) |
| Kitchen | 1.37 (1.05–1.78) | 1.43 (1.07–1.90) | 1.41 (0.96–2.06) |
| Air | 1.43 (1.01–2.02) | 1.45 (01.00–2.11) | 1.77 (1.06–2.94) |
| mSPT(−) ¥ | |||
| Bed | 1.08 (0.71–1.64) | 1.24 (0.78–2.00) | 0.95 (0.56–1.60) |
| Bedroom | 1.07 (0.80–1.45) | 1.27 (0.89–1.82) | 0.98 (0.68–1.42) |
| Kitchen | 1.11 (0.85–1.46) | 1.07 (0.78–1.49) | 1.18 (0.84–1.66) |
| Air | 1.20 (0.84–1.73) | 1.19 (0.78–1.81) | 1.27 (0.82–1.97) |
Results presented are derived from models adjusted for age, gender, total IgE, and health insurance. Relationships between mouse allergen exposure and the outcomes are modeled for every 10-fold increase in mouse allergen exposure.
mSPT(+): positive mouse skin test
mSPT(−): negative mouse skin test
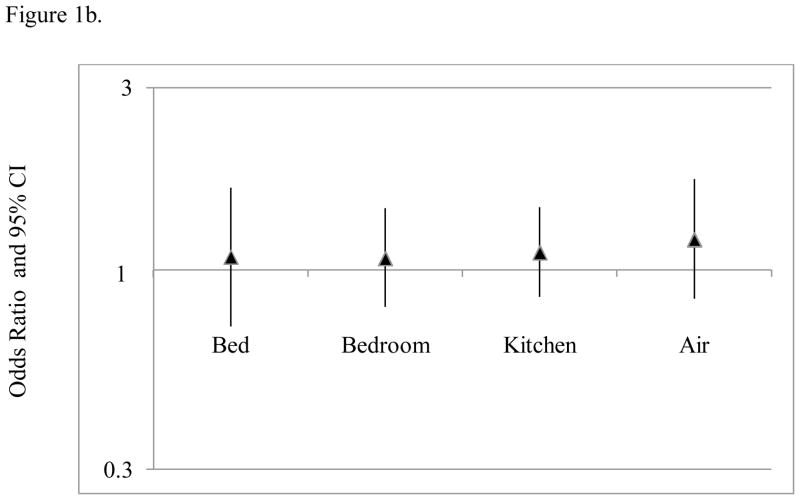
Figure 1.
Relationships between mouse allergen exposure and asthma-related health care use among (a) mouse sensitized and (b) non-mouse sensitized participants. Estimates and 95% CIs were generated using multivariate regression modeling and GEE to account for repeated outcome measures. Mus m 1 was log10 transformed. All statistical models adjusted for age, gender, total IgE and type of health insurance.
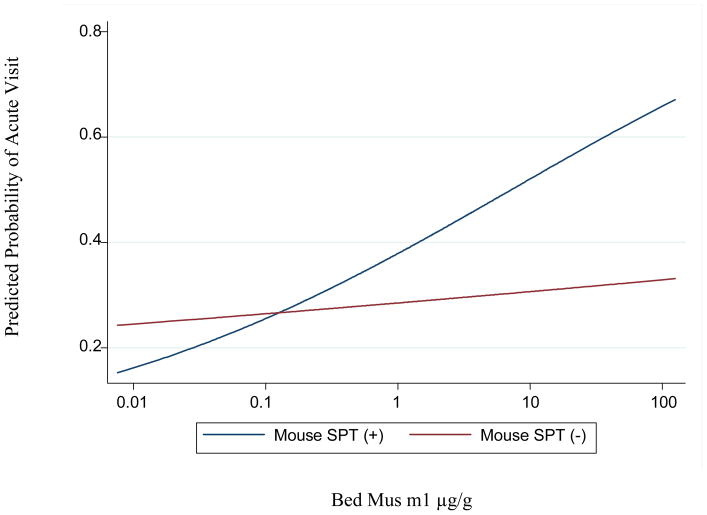
Figure 2 is a plot of the predicted probability of an acute visit derived from the regression models for mouse sensitized and non-mouse sensitized participants. There is a linear relationship between bed mouse allergen levels and the probability of an acute visit among mouse sensitized individuals, while there is little evidence of a relationship between exposure and the probability of an acute visit for non-mouse sensitized participants. A quadratic term was also added to the linear model and did not provide a better fit of the data than the linear model (data not shown).
Figure 2.
Predicted probabilities of health care utilization and bed mouse allergen levels among mouse SPT (+) and SPT (−) participants. The predicted probabilities were generated from unadjusted models using GEE to account for repeated outcome measures.
Mouse Allergen Exposure Compartments and Asthma Morbidity
Kitchen and air mouse allergen concentrations were also associated with an increased risk asthma-related healthcare utilization in models adjusted for age, gender, total IgE and insurance; however, the magnitude of the association was less than that observed for bed mouse allergen concentrations. (Table 5, Figure 1) For example, for every 10 fold increase in kitchen mouse allergen, there was a 37% increase in the odds of having an acute visit for asthma among mouse sensitized participants in the final adjusted model, which was less than half the estimated effect that was observed for bed mouse allergen concentrations. Bedroom floor mouse allergen exposure was not significantly associated with any asthma-related health care use. (Table 5, Figure 1)
Multivariate models of relationships between bed mouse allergen concentrations and acute asthma visit were also adjusted for the other exposure compartments (bedroom floor, kitchen, and air). When these other exposure compartments were included in the final model, the point estimates for the relationships between bed mouse allergen exposure and asthma morbidity changed to some extent, but, by and large, bed exposure remained the compartment with the strongest association with morbidity. (Online Supplement)
Mouse Allergen Exposure and Pulmonary Function and Inflammation
Relationships between the various measures of mouse allergen exposure and pulmonary inflammation and lung function were less robust or absent. Increasing airborne mouse allergen exposure was associated with increasing FENO (coefficient (95% CI): 0.04 (−0.01 – 0.08)), but this relationship did not meet statistical significance. There were no relationships observed between any measure of mouse allergen exposure and lung function (data not shown).
DISCUSSION
This is the first study, to our knowledge, to find a linear dose response relationship between mouse allergen concentrations and asthma morbidity among mouse-sensitized asthmatics. Previous studies have reported threshold concentrations of mouse allergen associated with increased morbidity risk (Matsui et al. 2006; Pongracic et al. 2008), suggesting that a reduction in mouse allergen concentrations to a level below a threshold is necessary to reduce asthma morbidity, but our findings suggest that progressive decrements in mouse allergen concentrations should be associated with an incremental reduction in risk of asthma morbidity. Our findings also support the concept, previously identified for dust mite (Raja et al. 2010, Tovey and Marks 2011), that specific exposure compartments may have particular clinical relevance. Specifically, we found that bed and airborne mouse allergen exposure may have a greater impact on asthma morbidity than bedroom floor or kitchen exposures. These findings suggest that augmenting an integrated pest management (IPM) intervention with environmental control practices focused on reducing bed mouse allergen concentrations could improve the efficacy of an IPM intervention.
The relationship between mouse allergen exposure and asthma morbidity in sensitized asthmatics has been reported in several studies, including one of Baltimore City pre-school children (Matsui et al. 2006) and one multi-center study of urban school age asthmatics (Pongracic et al. 2008). However, these studies reported threshold mouse allergen concentrations that were associated with asthma morbidity and did not directly address hypotheses related to the shape of the dose-response relationship. Although Salo and colleagues evaluated continuous mouse allergen levels, their outcome of interest was current asthma and not asthma morbidity among a population of asthmatics. In addition, the analyses were not stratified by mouse allergen sensitization status, but atopy. (Salo et al.2009) Beyond confirming the association between mouse allergen exposure and asthma morbidity reported in these previous studies, our study extends these previous observations by addressing explicitly the shape of the dose-response curve and the relative clinical importance of different mouse allergen exposure compartments.
Threshold exposure values associated with health risks are appealing because thresholds are straightforward to apply in clinical decision-making and are easily understood by both scientists and laypeople. However, there are some important limitations to the use of thresholds for allergen exposure-biologic response relationships. For example, thresholds are often chosen, arbitrarily, by necessity, and are likely to be, at least in part, a function of the underlying distribution of allergen concentrations in the population under investigation. In fact, three previous studies of mouse allergen exposure and asthma morbidity utilized three different threshold values of mouse allergen exposure: 1.6μg/g in the kitchen, 0.5μg/g, and approximately 0.01μg/g in the bedroom. (Phipatanakul et al. 2000, Matsui et al. 2006, Pongracic et al. 2008) Because of these limitations, thresholds may not be relevant for setting goals for allergen reduction outside of the populations in which they were observed. As such, we examined the shape of the dose response relationship and found a strong linear relationship between mouse allergen concentrations and asthma morbidity and no evidence of clinical tolerance at higher mouse allergen concentrations. This finding suggests that a reduction in mouse allergen concentrations would be expected to be associated with a concomitant reduction in risk of morbidity, even among those with the highest concentrations of mouse allergen.
While our study population resides in Baltimore City so that our results may not be generalizable to other populations, similar concentrations of mouse allergen and prevalences of mouse sensitization are found in asthmatic populations in other major cities in the Midwest and Northeast US, suggesting that our findings are likely to have relevance to pediatric asthma populations in other major US cities.(Pongracic et al. 2008) Although we found no relationship between mouse allergen exposure and lung function among mouse sensitized participants, the lack of an association with lung function but strong association with symptoms and morbidity is common in pediatric asthma populations. (Morgan et al. 2004) Our study design had the advantage of being a prospective cohort study with analysis of continuous mouse allergen exposures. Although this study was an observational study, it lays the groundwork for environmental intervention trials to evaluate the effect of mouse allergen reduction on asthma morbidity in mouse-sensitized asthmatics.
Because our primary outcome was derived from a questionnaire, there could have been misclassification of the outcome variable. If this did, indeed, occur, it is likely this would have resulted in non-differential misclassification, which would have attenuated estimates of the associations between exposure and morbidity. It is also possible, that with a larger sample size, some of the other exposure compartments, such as airborne mouse allergen exposure, could have emerged as independent predictors of morbidity. However, the point estimates for the other exposure compartments were much lower than those observed for bed exposure, so that the contribution of these other exposure compartments to asthma morbidity is still likely to be less than bed exposure in studies with larger sample sizes.
In summary, this study provides strong evidence of a linear dose response relationship between mouse allergen concentrations and asthma morbidity among mouse-sensitized urban children and adolescents, suggesting that a decrease in mouse allergen concentrations would be associated with a concomitant decrease in morbidity. Although sustained eradication of mouse infestation may be difficult, or even impossible, to achieve in some homes, these findings support intervening to reduce allergen concentrations even when complete eradication of infestation is not feasible. While we await more definitive studies, augmenting IPM strategies with air purifiers, frequent laundering of bed linens and allergen-proof mattress and pillow encasements may result in greater reduction of mouse allergen in the most clinically relevant sites of exposure.
Supplementary Material
Practical Implications.
The linear dose response relationship between mouse allergen concentrations and asthma morbidity among mouse-sensitized urban children and adolescents, suggests that a decrease in mouse allergen concentrations would be associated with a concomitant decrease in morbidity. For mouse sensitized children and adolescents with asthma, it may be beneficial to augment integrative pest management (IPM) strategies with air purifiers, frequent laundering of bed linens and allergen-proof mattress and pillow encasements since the bed and air exposure compartments are most strongly associated with morbidity.
Acknowledgments
Funding: This work was supported by the National Institute of Environmental Health Sciences (P50ES015903, P01ES018176), the Environmental Protection Agency (R832139), the National Institute of Allergy and Infectious Diseases (R01AI070630), and by the Johns Hopkins University School of Medicine General Clinical Research Center grant number M01-RR00052, from the National Center for Research Resources/NIH.
ABBREVIATIONS
- ED
Emergency Department
- FENO
Fractional Exhaled Nitric Oxide
- FEV1
Forced Expiratory Volume in 1 second
- FVC
Forced Vital Capacity
- OR
Odds Ratio
- ELISA
Enzyme-Linked Immunosorbent Assay
References
- Chew GL, Perzanowski MS, Miller RL, Correa JC, Hoepner LA, Jusino CM, et al. Distribution and determinants of mouse allergen exposure in low-income New York City apartments. Environ Health Perspect. 2003 Aug;111(10):1348–51. doi: 10.1289/ehp.6124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohue KM, Al-alem U, Perzanowski MS, Chew GL, Johnson A, Divjan A, et al. Anti-cockroach and anti-mouse IgE are associated with early wheeze and atopy in an inner-city birth cohort. J Allergy Clin Immunol. 2008 Nov;122(5):914–20. doi: 10.1016/j.jaci.2008.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruchalla RS, Pongracic J, Plaut M, Evans R, 3rd, Visness CM, Walter M, et al. Inner City Asthma Study: relationships among sensitivity, allergen exposure, and asthma morbidity. J Allergy Clin Immunol. 2005 Mar;115(3):478–85. doi: 10.1016/j.jaci.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159(1):179–87. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- Matsui EC, Sampson HA, Bahnson HT, Gruchalla RS, Pongracic JA, Teach SJ, et al. Allergen-specific IgE as a biomarker of exposure plus sensitization in inner-city adolescents with asthma. Allergy. 2010 Nov;65(11):1414–22. doi: 10.1111/j.1398-9995.2010.02412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui EC, Breysse P, Diette GB. Mouse allergen levels vary over time in inner-city homes. J Allergy Clin Immunol. 2007;120(4):956–959. doi: 10.1016/j.jaci.2007.07.053. [DOI] [PubMed] [Google Scholar]
- Matsui EC, Buckley TJ, Krishnan JA, Breysse PN, Rand CS, Diette GB. Household mouse allergen exposure and asthma morbidity in inner-city preschool children. Ann Allergy Asthma Immunol. 2006;97(4):514–20. doi: 10.1016/S1081-1206(10)60943-X. [DOI] [PubMed] [Google Scholar]
- Morgan WJ, Crain EF, Gruchalla RS, O’Connor GT, Kattan M, Evans R, 3rd, Stout J, Malindzak G, Smartt E, Plaut M, Walter M, Vaughn B, Mitchell H. Results of a home-based environmental intervention among urban children with asthma. N Engl J Med. 2004;9;351(11):1068–80. doi: 10.1056/NEJMoa032097. [DOI] [PubMed] [Google Scholar]
- Guidelines for the Diagnosis and Management of Asthma Full Report. 2007. National Heart, Lung, and Blood Institute National Asthma Education and Prevention Program Expert Panel Report 3. [Google Scholar]
- Phipatanakul W, Eggleston P, Wright E, Wood R. Mouse allergen. II. The relationship of mouse allergen exposure to mouse sensitization and asthma morbidity in inner-city children with asthma. J Allergy Clin Immunol. 2000;106(6) doi: 10.1067/mai.2000.110795. [DOI] [PubMed] [Google Scholar]
- Phipatanakul W, Celedón JC, Sredl DL, Weiss ST, Gold DR. Mouse exposure and wheeze in the first year of life. Ann Allergy Asthma Immunol. 2005 May;94(5):593–9. doi: 10.1016/S1081-1206(10)61139-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pongracic JA, Gruchalla RS, Evans R, 3rd, Mitchell HE. Effect of mouse allergen and rodent environmental intervention on asthma in inner-city children. Ann Allergy Asthma Immunol. 2008;101(1):35–41. doi: 10.1016/S1081-1206(10)60832-0. [DOI] [PubMed] [Google Scholar]
- Raja S, Xu Y, Ferro AR, Jaques PA, Hopke PK. Resuspension of indoor aeroallergens and relationship to lung inflammation in asthmatic children. Environ Int. 2010;36:8–14. doi: 10.1016/j.envint.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Rosenstreich D, Eggleston P, Kattan M. The role of cockroach allergy and exposure to cockroach allergen in causing morbidity among inner-city children with asthma. NEJM. 1997;336(19) doi: 10.1056/NEJM199705083361904. [DOI] [PubMed] [Google Scholar]
- Salo PM, Cohn RD, London SJ, Zeldin DC. Exposure to mouse allergen in U.S. homes associated with asthma symptoms. Environ Health Perspect. 2009;117(3):387–391. doi: 10.1289/ehp.11847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelmach I, Jerzynska J, Stelmach W, Majak P, Chew G, Kuna P. The prevalence of mouse allergen in inner-city homes. Pediatr Allergy Immunol. 2002 Aug;13(4):299–302. doi: 10.1034/j.1399-3038.2002.01079.x. [DOI] [PubMed] [Google Scholar]
- Tovey ER, Marks GB. It’s time to rethink mite allergen avoidance. J Allergy Clin Immunol. 2011 Oct;128(4):723–727. doi: 10.1016/j.jaci.2011.07.009. [DOI] [PubMed] [Google Scholar]
- Vaughan JW, McLaughlin TE, Perzanowski MS, Platts-Mills TA. Evaluation of materials used for bedding encasement: effect of pore size in blocking cat and dust mite allergen. J Allergy Clin Immunol. 1999 Feb;103(2 Pt 1):227–31. doi: 10.1016/s0091-6749(99)70495-1. [DOI] [PubMed] [Google Scholar]
- Wilson J, Dixon SL, Breysse P, Jacobs D, Adamkiewicz G, Chew GL, et al. Housing and allergens: a pooled analysis of nine US studies. Environ Res. 2010 Feb;110(2):189–98. doi: 10.1016/j.envres.2009.10.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.