Abstract
BACKGROUND
Although concerns surrounding high-dose dextromethorphan (DXM) abuse have recently increased, few studies have examined the acute cognitive effects of high doses of DXM. The aim of this study was to compare the cognitive effects of DXM with those of triazolam and placebo.
METHODS
Single, acute, oral doses of DXM (100, 200, 300, 400, 500, 600, 700, 800 mg/70 kg), triazolam (0.25, 0.5 mg /70 kg), and placebo were administered p.o. to twelve healthy volunteers with histories of hallucinogen use, under double-blind conditions, using an ascending dose run-up design. Effects on cognitive performance were examined at baseline and after drug administration for up to 6 hours.
RESULTS
Both triazolam and DXM produced acute impairments in attention, working memory, episodic memory, and metacognition. Impairments observed following doses of 100-300 mg/70 kg DXM were generally smaller in magnitude than those observed after 0.5 mg/70 kg triazolam. Doses of DXM that impaired performance to the same extent as triazolam were in excess of 10-30 times the therapeutic dose of DXM.
CONCLUSION
The magnitude of the doses required for these effects and the absence of effects on some tasks within the 100-300 mg/70 kg dose range of DXM, speak to the relatively broad therapeutic window of over-the-counter DXM preparations when used appropriately. However, the administration of supratherapeutic doses of DXM resulted in acute cognitive impairments on all tasks that were examined. These findings are likely relevant to cases of high-dose DXM abuse.
Keywords: Dextromethorphan, triazolam, cognitive, memory, Robitussin, Coricidin
1. INTRODUCTION
Dextromethorphan (DXM) is a drug that was approved by the US Food and Drug Administration (FDA) in 1958 as a non-prescription cough medication. Today, DXM is available in the US in over 125 different over-the-counter (OTC) products and formulations for the treatment of unproductive cough (FDA Briefing Information, 2010). Current common trade names of DXM-containing products include Robitussin (liquids and capsules) and Coricidin (capsules; Bem and Peck 1992). In addition, DXM can be legally purchased in the US currently as an unfinished drug product (i.e., as a bulk powder); however, such formulation is not approved for medical use (H.R. 1259 (111th): Dextromethorphan Distribution Act of 2009). Although DXM is available OTC and is not scheduled under Federal Law, DXM has liability for abuse (Banken and Foster, 2008; Reissig et al., 2012; Romanelli and Smith, 2009; Schutz and Soyka, 2000; Soyka et al., 2000; Zawertailo et al., 1998; Ziaee et al. 2005;) and the sale of DXM has already been regulated or restricted by several States in the US (Erowid, 2012). Epidemiological studies have documented cases of DXM abuse that have been reported to the National Poison Data System in the US (Wilson et al., 2011). Recently, there has been an increasing concern surrounding DXM abuse as studies have reported an increase in the number of cases of DXM abuse from 2000 to 2006, although the frequency of these cases appears to have been stable from 2006 to 2010 (Wilson et al., 2011).
The therapeutic dose range for cough suppression for DXM is 10-30 mg. In contrast, doses of DXM that are used recreationally often exceed several hundred mg (e.g., 600-900 mg/70 kg, Boyer, 2004; 75-2700 mg/70 kg, Ziaee et al., 2005). At high concentrations, DXM binds to n-methyl-D-aspartate (NMDA) receptors as an antagonist (Church et al., 1994) in a manner similar to that of phencyclidine (PCP; Morris et al., 2005; Newell et al., 2007) and ketamine (Sinner and Graf, 2008). In addition, the primary active metabolite of DXM, dextrorphan, binds to NMDA receptors with greater affinity than DXM and functions as an antagonist (Franklin and Murray 1992; Parsons et al. 1995; Werling et al. 2007). It is likely that most of the cases of DXM abuse, sometimes referred to as “Dexing” or “Robotripping,” are the result of a user seeking effects similar to those produced by classic hallucinogens, which occur at very large doses of DXM (Reissig et al., 2012). Cognitive impairments associated with these high doses of DXM might contribute to the reports of adverse events and toxicity that have been reported in recent years.
Part of the rationale for this investigation is that few studies have examined the effects of DXM in human participants within the range of doses that are frequently abused (i.e., 400-1,000 mg/70 kg; Steinberg et al., 1996; Zawertailo et al., 1998). In studies that have examined the effects of supratherapeutic doses of DXM, effects on cognitive functions such as attention and memory processing were not evaluated (Schutz and Soyka, 2000; Soyka et al., 2000; Steinberg et al., 1996; Zawertailo et al., 1998). Given the increasing concern surrounding high-dose DXM abuse, it is important to know if and how high-dose DXM abuse might affect cognitive functioning. Thus, one of the aims of this study was to examine the acute dose effects of high doses of DXM on a variety of different cognitive measures. A second aim was to extend previous findings from our laboratory on the comparative pharmacology and the differential profiles of the cognitive effects of benzodiazepines such as triazolam (Carter et al., 2006, 2007, 2009; Mintzer and Griffiths 2002, 2005) and NMDA antagonists such as ketamine (Carter et al., submitted; Lofwall et al., 2006). On the basis of the results from these previous studies, we hypothesized that the effects of DXM on participants’ subjective ratings of drug effects and of their own cognitive performance impairment would be greater than those of triazolam, whereas the cognitive impairments observed after administration of triazolam would be greater than those observed after DXM.
2. MATERIALS AND METHODS
A brief description of the general methods is provided below. This report describing the cognitive effects of DXM and triazolam comes from a larger study in which additional measures of physiological effects, psychomotor performance, subjective effects, and hallucinogen-like effects were examined. A more detailed description of those measures and the complete study methods can be found in a prior publication (Reissig et al., 2012).
2.1 Participants
Twelve adult volunteers (9 males) completed this study. Participants ranged in age from 20 to 40 years (mean 27.5 years), were medically and psychologically healthy, and had a history of hallucinogen use. Ten participants were Caucasian (83%), one was African-American, and one was Asian-American. All volunteers reported past use of LSD (range: 2-500 lifetime uses, mean: 58.2 uses) and psilocybin (range: 4-60 lifetime uses, mean: 21 uses). Seven of the twelve volunteers had used DXM previously for recreational purposes (range: 1-10 lifetime uses, mean of these seven volunteers: 4.8 uses), and three of the twelve had experience with either PCP or ketamine. Individuals were excluded from participation if they had a history of substance dependence according to DSM-IV-TR criteria (excluding nicotine or caffeine), were pregnant or nursing, had a current significant medical condition or had a contraindication to receiving sedatives or anesthetics. A detailed psychiatric history was taken during the screening interview to exclude individuals with a personal or immediate family history of schizophrenia, bipolar affective disorder, delusional disorder, paranoid disorder, or schizoaffective disorder. The Johns Hopkins University School of Medicine Institutional Review Board approved this study and it was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and all applicable U.S. Laws. Participants gave their written informed consent before beginning the study and were paid for their participation.
2.2 General Procedures
During the study, participants could receive a maximum of 11 different conditions over 11 experimental sessions (placebo, 0.25 and 0.5 mg/70 kg triazolam, and 100, 200, 300, 400, 500, 600, 700 and 800 mg/70 kg DXM). Consecutive sessions for each participant were separated by a minimum of 48 hours and the median number of days between sessions for all participants was 6. The order of the three types of conditions (triazolam, DXM, and placebo) was counterbalanced across participants. Within each drug condition, the sequence of the doses was ascending, and dosing with triazolam or DXM was completed before proceeding to another type (e.g., both doses of triazolam were administered before proceeding to DXM dosing and vice versa). The ascending sequence was used to determine the maximum dose of DXM that could be safely tolerated by individual participants and to avoid adverse events that might result if an individual was particularly sensitive to DXM. As described elsewhere (Reissig et al., 2012), all participants received placebo, both doses of triazolam (0.25 and 0.5 mg/70 kg), and at least four doses of DXM (100, 200, 300, and 400 mg/70 kg). The highest dose of DXM administered varied across participants (400 mg/70 kg, n=2; 500 mg/70 kg n=2; 600 mg/70 kg, n=4; 700 mg/70 kg, n = 2; 800 mg/70 kg, n=2). Seven volunteers reached their stop point because of significant behavioral impairment. Two volunteers reached their stop point because the investigators judged administration of higher doses to be inadvisable. One volunteer reached a stop point because the participant stated that he/she did not want to receive that dose of drug again.
Prior to the first experimental session, participants practiced the experimental tasks to achieve a stable level of performance. Participants were asked to refrain from using any drugs other than non-prescription pain relievers, tobacco, and caffeinated products while enrolled in the study. They were asked to consume their usual amounts of tobacco or caffeine and a low fat breakfast before arriving for each session. At the beginning of each experimental session participants’ urine was tested for the presence of cocaine, benzodiazepines, and opioids using an EMIT system (Syva Co., Palo Alto, CA, USA) and expired air was tested for the presence of alcohol using a breathalyzer test (Alco-Sensor IV, Intoximeters, Inc., St, Louis, MO); urine was not screened for the presence of THC or THC metabolites. Female participants were asked to take a pregnancy test at the beginning of each session and were only allowed to continue in the study with the provision of a negative result.
2.3 Drugs
Drugs and placebo were orally administered in size 0 aqua colored opaque capsules with approximately 200 ml of water. Four identical capsules were administered during each experimental session. Capsules were filled with lactose monohydrate (placebo; Ruger Chemical Company, Linden NJ, USA); powdered dextromethorphan hydrobromide (Spectrum Chemical, Gardena CA, USA) and lactose; or commercially available crushed triazolam tablets (Halcion; The Upjohn Company, Kalamazoo, MI, USA) and lactose. All doses were adjusted for participant body weight. Doses of dextromethorphan are expressed as the salt.
2.4 Cognitive Measures
Participants completed the Digit-Symbol-Substitution Task, Divided Attention task, and Working Memory task before capsule administration (baseline or pre-drug) and at 120, 240, and 360 min after capsule administration. Tasks assessing episodic memory and metacognition were administered as described below.
2.4.1 Digit-Symbol-Substitution Task (DSST)
This task was a computer version of the digit-symbol-substitution task (McLeod et al., 1982), which is a measure of focused attention and pattern recognition. Dependent measures were the number of trials attempted and the proportion of trials completed correctly within 90 sec.
2.4.2 Divided attention
In this task, which has been previously described in detail (Kleykamp et al., 2010), participants were presented with a diamond stimulus that moved back-and-forth horizontally in the center of the computer screen, in addition to five non-moving single digit integers with one digit presented in each of the four corners of the screen in white font and one digit presented in the center of the bottom of the screen in green font. Participants were instructed to concurrently track the moving diamond stimulus using the computer mouse, which controlled a crosshair depicted on the screen (tracking), and to click the computer mouse whenever one of the four digits in the corners of the screen matched the green digit at the center of the bottom of the screen (monitoring). The primary dependent measure associated with the tracking component was tracking deviation (distance in pixels between the diamond stimulus and cross hair). The primary dependent measure associated with the digit monitoring component was proportion correct (number of times a mouse press was made when the target digit was presented in the corner of the screen out of a total possible of 24).
2.4.3 Working memory
The working memory task that was used is a variant of the classic Sternberg task (Sternberg 1969) and was administered using procedures similar to those described by Mintzer and Griffiths (2007). During each experimental session, standardized instructions were read to the participants before the task and practice trials were presented before the experimental trials to ensure that the participants understood and performed the task correctly. A memory set consisting of 7 randomly selected and randomly ordered consonant letters (e.g., ZHFKDXW) was presented on the screen followed by a probe consisting of a lower case letter–digit pair (e.g., f–4), and participants were asked to decide whether the probed letter had appeared in the memory set in the ordinal position represented by the digit (e.g., 4 = 4th position in the memory set). Participants completed 36 trials consisting of 12 trials in each of the three conditions: non-memory control (i.e., the memory set remained on the screen during probe presentation), 0-sec delay (between memory set and probe presentation), and 12-sec delay. The order of presentation of trials from the three conditions was random and the probed digit represented the correct position of the probed letter on half of the trials. In addition to the accuracy of the response (i.e., yes or no), participants’ reaction time (RT) from the onset of probe presentation to their response was recorded and is presented for correct trials only.
2.4.4 Episodic memory
Stimuli for these tasks were sets of 36 words. The words were presented sequentially and the participants were asked to remember them. During each session, participants studied a list of 36 words at a time point that corresponded to 120 min after capsule administration, which was anticipated to be times of peak drug effects for each drug (Carter et al., 2009; Reissig et al., 2012). During the study phase of the task, participants categorized the concrete noun represented by each word as “artificial” (i.e., man-made) or “natural” to encourage them to attend to and think about each word that was presented as has been described previously (Carter et al., 2009).
2.4.5 Free recall
Participants’ memory for the words (i.e., free recall) was tested 200 min after they had studied the list of words, a time point that corresponded to 320 min after capsule administration. Free recall was assessed by giving participants 5 min to write down all the words they could remember on a sheet of lined paper. The dependent measure was the number of correct words recalled (written down) within 5 min.
2.4.6 Word recognition
Recognition memory was tested immediately following the test of free recall. In the word recognition task, 72 words were randomly presented one at a time on the computer screen, and participants selected one of six options using a 6-point confidence scale (definitely old, probably old, maybe old, maybe new, probably new, definitely new). The 72 words presented during the word recognition task were comprised of the 36 words from the list that was presented in the study phase of the task (“old” words) and 36 words from a list that had not been previously presented (“new” words). The dependent measures were the proportion of old words correctly identified as old (collapsed across definitely old, probably old, and maybe old; this is the hit rate), the proportion of new words incorrectly identified as old (collapsed across definitely old, probably old, and maybe old; this is the false alarm rate), and signal detection measures of sensitivity in distinguishing between old and new words (d’) and response bias (C) (Snodgrass and Corwin 1988).
2.4.7 Metacognition
Metacognition is the knowledge or self-awareness that one has regarding their cognitive state. For the DSST and working memory task, participants estimated how well they expected to perform (before completing the task) or just performed (after completing the task) on these tasks using a 100 mm visual analog scale (VAS) scale. Participant estimates of performance were compared to the actual task scores by calculating the difference between an individual’s performance estimate and his/her actual performance (each expressed as a percentage of baseline or pre-drug responding). Thus, a positive score represents an under-estimation of performance impairment, while a negative score represents an over-estimation of performance impairment. Metamemory was assessed by calculating the Goodman-Kruskal gamma correlation (a correlation between confidence and correctness in recognition; Goodman and Kruskal, 1954) for the word recognition memory task. It is presumed that greater awareness of the state of one’s memory results in greater confidence in correct responses and lower confidence in incorrect responses. Gamma values can range from -1 (complete discordance between confidence ratings and recognition memory accuracy) to 1 (complete concordance between confidence ratings and recognition memory accuracy). The dependent measure is the gamma correlation from the word recognition task.
2.5 Statistical analyses
The maximum tolerable dose of DXM varied across the 12 volunteers and ranged from 400-800 mg/70kg (see General Procedures). As a result, peak effect (maximum or minimum values observed across the time course of effects) and one-time measures (e.g., word recall and recognition) were analyzed two different ways, consistent with previous analyses of data from similar ascending dose run-up designs (Carter et al., 2006; Reissig et al., 2012). One analysis included placebo, 0.25 and 0.5 mg/70kg triazolam, and 100, 200, and 300 mg/70kg DXM (low dose analysis). Higher doses were not included in this analysis because some participants either did not receive some of the higher doses or did not complete assessments at higher doses. A second analysis included placebo, 0.25 and 0.5 mg/70kg triazolam, and the penultimate DXM dose that was administered to each participant (i.e., the dose that preceded the maximum dose received; high dose analysis). Penultimate doses ranged from 300-700 mg/kg. An analysis of the maximum dose that each participant received was not performed for these measures because some participants did not complete all assessments at those doses.
Data were analyzed using repeated measures regression models in SAS Proc Mixed (SAS Institute Inc., Cary, NC, USA). These models allow for the specification of the covariance structure of the repeated measures and have better mechanisms for handling missing data and thus are preferable to traditional ANOVA models (Wolfinger and Chang 1995). The implementation in PROC MIXED is preferable to GEE models for datasets with small to moderate numbers of subjects. We report Type III tests of fixed effects. Data for measures that were assessed repeatedly as part of the battery of assessments are presented as a percentage of pre-drug (baseline) scores. The non-memory control condition of the working memory task was used as a covariate in the model to control for any drug effects on the non-memory components of this task. For all the peak effect and one-time measures, Fisher’s Least Significant Difference post hoc tests were used to compare placebo with the active doses when the F statistic of the ANOVA was significant. The mean ± standard error of the mean (SEM) is presented throughout. Statistical tests were considered significant at p ≤ 0.05.
3. RESULTS
3.1 Digit-Symbol Substitution Test
Significant main effects of dose were observed on the number of DSST trials attempted in both the low dose [F(5, 55)=18.63, p<0.0001] and high doses analyses [F(3, 33)=16.27, p<0.0001; Figure 1]. There was a significant main effect on the proportion of trials completed correctly in the high dose analysis [F(3, 33)=3.44, p<0.05; Figure 1], but not in the low dose analysis [F(5, 55)=2.25, p=0.062]. The largest doses of both drugs (0.5 mg/70 kg triazolam and 300 mg/70 kg DXM) that were studied in all individuals significantly decreased the number of trials attempted (Figure 1).
Figure 1.
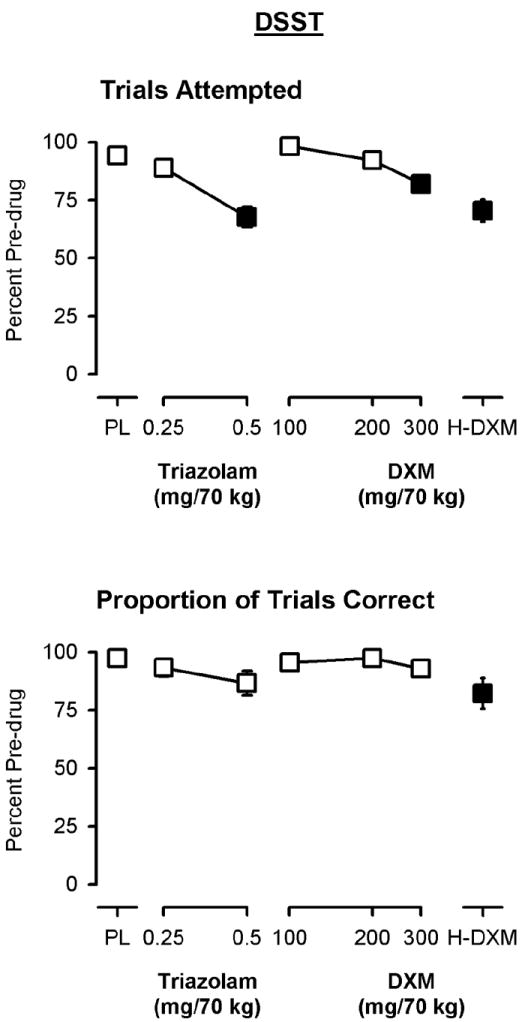
Peak effects of triazolam and dextromethorphan (DXM) on the number of trials attempted (top panel) and the number of trials correct (bottom panel) on the DSST. Y-axes show the number of trials, expressed as a percent of pre-drug (baseline) responding. X-axes show dose in milligrams per 70 kilograms (log scale). PL designates placebo and H-DXM designates the penultimate dose of DXM that was received. Data points show means, brackets show ±1 SEM, and the absence of brackets indicates that 1 SEM fell within the area of the data symbol. Filled symbols indicate a significant difference from placebo.
3.2 Divided attention
Significant main effects of dose were observed on the monitoring component of the divided attention task (the proportion of targets correctly identified) in both the low dose [F(5, 54)=18.49, p<0.0001] and high dose [F(3, 32)=17.33, p<0.0001] analyses (Figure 2, top panel). A significant main effect of dose on the tracking component (tracking; deviation; distance in pixels between the diamond stimulus and cross hair), however, was only observed in the high dose analysis [F(3, 32)=4.24, p<0.05; Figure 2, bottom panel]. The effects of 100-300 mg/70 kg of DXM were not significantly different from placebo on the tracking component, and the magnitude of effects were less than 0.5 mg/70 kg triazolam on both components of the task (Figure 2). However, the penultimate dose of DXM did significantly decrease performance on both components of the task and did so to a similar extent as 0.5 mg/70 kg triazolam (Figure 2).
Figure 2.
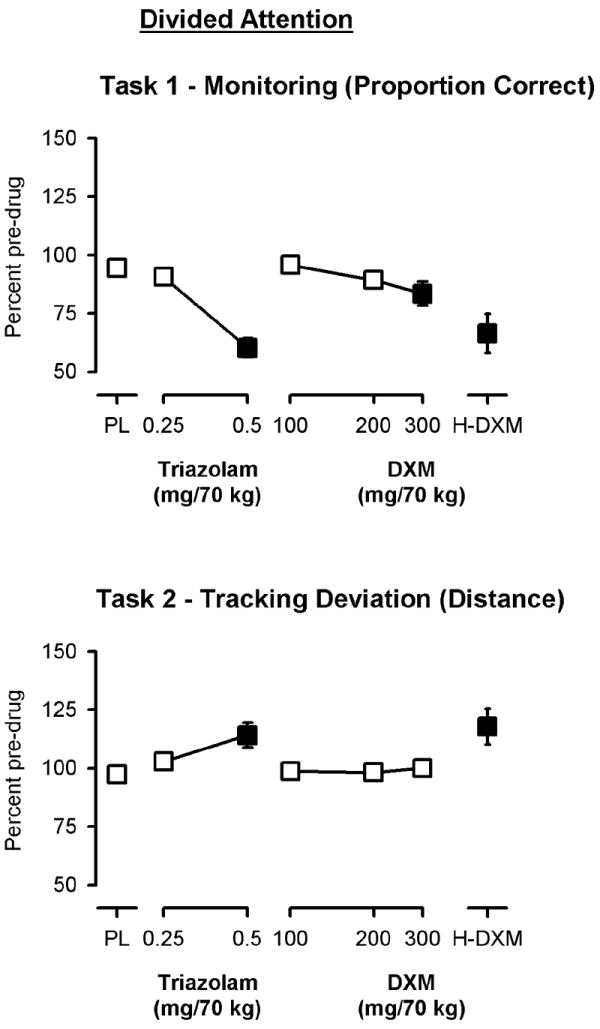
Peak effects of triazolam and dextromethorphan (DXM) on the monitoring (top panel) and tracking (bottom panel) components of the Divided Attention task. Y-axes show the proportion of items correct and the mean distance from the target, respectively, expressed as a percent of pre-drug (baseline) responding. X-axes show dose in milligrams per 70 kilograms (log scale). PL designates placebo and H-DXM designates the penultimate dose of DXM that was received. Data points show means, brackets show ±1 SEM, and the absence of brackets indicates that 1 SEM fell within the area of the data symbol. Filled symbols indicate a significant difference from placebo.
3.3 Working Memory
Significant main effects of dose, but not delay, were observed for accuracy (proportion of trials correct) and response time on the working memory task (Figure 3, top left and center panels). In the absence of a main effect of delay, data from the 0 and 12 sec delay conditions were collapsed and analyzed together (the non-memory control condition in which the prompt remained on screen was entered as a covariate). As shown in Figure 3 (top left panel), only the penultimate dose of DXM resulted in a significant decrease in accuracy as compared to placebo on this working memory task [high dose analysis, F(3, 32)=4.27, p<0.05]. In contrast, both doses of triazolam, 300 mg/70 kg DXM, and the penultimate dose of DXM significantly increased median response times on the task [low dose analysis, F(5, 54)=8.71, p<0.0001; high dose analysis, F(3, 32)=7.40, p=0.001; Figure 3, top center panel].
Figure 3.
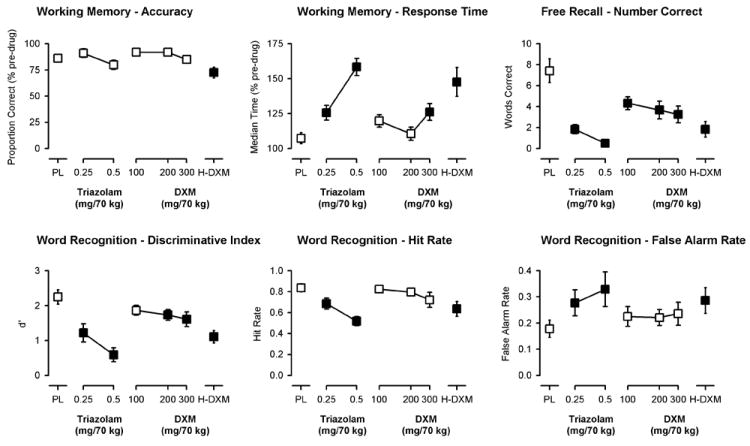
Peak effects of triazolam and dextromethorphan on working memory and episodic memory measures (word recall and word recognition). Y-axes show the proportion of trials correct and the median reaction time expressed as a percent of pre-drug (baseline) responding, respectively, on the working memory task (top left and center panels); the number of correct words recalled on the Free Recall task (top right panel); and the ability to discriminate (discriminative index, d’) between old and new words (bottom left panel), the hit rate (“old words” correctly identified as “old”; bottom center panel), and the false alarm rate (“new words” incorrectly identified as “old”; bottom right panel) on the Word Recognition task. X-axes show dose in milligrams per 70 kilograms (log scale). PL designates placebo and H-DXM designates the penultimate dose of DXM that was received. Data points show means, brackets show ±1 SEM, and the absence of brackets indicates that 1 SEM fell within the area of the data symbol. Filled symbols indicate a significant difference from placebo.
3.4 Episodic memory
Significant main effects of dose were observed on the number of words correctly recalled in the free recall task in both analyses [low dose analysis, F(5, 55)=15.71, p<0.0001; high dose analysis, F(3, 33)=19.15, p<0.0001]. The number of words correctly recalled was significantly decreased by both doses of triazolam and by 100, 200, and 300 mg/70 kg DXM and by the penultimate DXM dose; however, the doses of triazolam appeared to have a greater amnesic effect than those doses of DXM (Figure 3, top right panel). The mean (± 1 SEM) number of words recalled following the penultimate dose of DXM (1.8±0.7) was similar to that of 0.25 mg/70 kg triazolam (1.8±0.4).
Significant main effects of dose were also observed on participants’ ability to discriminate between words that had and had not been previously studied (i.e., “old” and “new” words) in the word recognition task [low dose analysis, F(5, 55)=15.27, p<0.0001; high dose analysis, F(3, 33)=21.47, p<0.0001]. On this measure, all dose conditions except for the lowest dose of DXM (100 mg/70 kg) significantly decreased the discriminative index (d’) and, as with the free recall data, the doses of triazolam studied appeared to have greater amnesic effects than those of DXM (Figure 3, bottom left panel). Differences between triazolam and DXM on recognition memory are more apparent when one examines the individual components of the discriminative index separately. The discriminative index is calculated from the hit rate (proportion of old words correctly identified as old) and the false alarm rate (the proportion of new words incorrectly identified as old) on the task. The bottom center and right panels of Figure 3 show that both doses of triazolam and the penultimate dose of DXM, but not the lower doses of 100, 200, or 300 mg/70 kg DXM significantly decreased the hit rate (Figure 3, top right panel) and increased the false alarm rate (Figure 3, bottom right panel). There was no significant main effect on response bias (C) (data not shown).
3.5 Metacognition
Participants’ estimate of their performance on the DSST (collapsed across before and after ratings) was not significantly affected (i.e., significantly different from placebo) after any dose of triazolam, DXM (100-300 mg/70 kg), or the penultimate dose of DXM (Figure 4, top panel). Although there was a trend for an over-estimation of performance impairment (negative values) at 300 mg/70 kg DXM, the main effect of dose was not statistically significant for either analysis. In contrast, participants’ estimate of their performance on the working memory task was significantly affected by triazolam and DXM [low dose analysis, F(5, 54)=4.04, p<0.005; high dose analysis, F(3, 32)=4.31, p<0.05]. Performance estimates were significantly negative (indicating an over-estimation of impaired performance) after administration of 0.5 mg/70 kg triazolam and doses ≥200 mg/70 kg of DXM (Figure 4, center panel). There were also significant main effects for the Goodman-Kruskal gamma correlations between the relative confidence in responses and the accuracy of those responses on the word recognition task [low dose analysis, F(5,55)=2.43, p<0.05; high dose analysis, F(3, 33)=3.30, p<0.05]. Gamma correlations were positive (indicating a positive relationship between confidence and correctness) and were significantly decreased by both doses of triazolam, 300 mg/70 kg DXM, and the penultimate dose of DXM (Figure 4, bottom panel), indicating impairments in metacognition.
Figure 4.
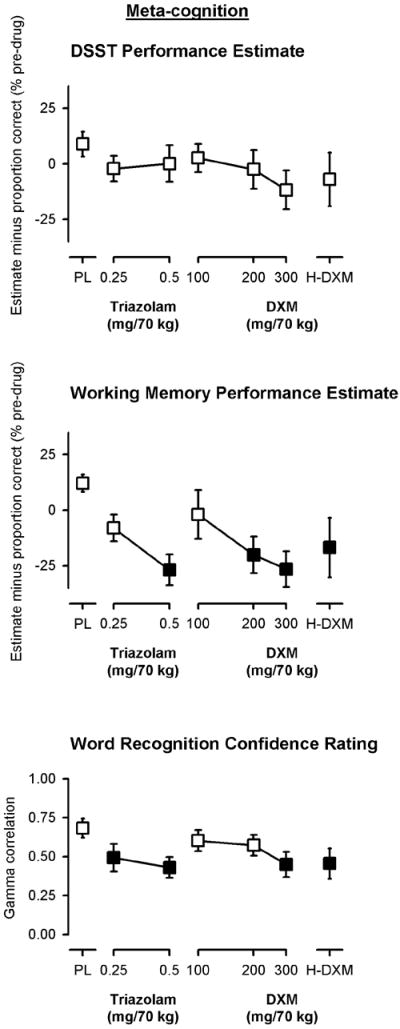
Peak effects of triazolam and dextromethorphan on measures of metacognition. Y-axes show ratings of performance (estimates) minus actual task performance, each expressed as a percent of pre-drug (baseline) responding (top and center panels) or the gamma correlation between confidence and correctness (bottom panel). X-axes show dose in milligrams per 70 kilograms (log scale). PL designates placebo and H-DXM designates the penultimate dose of DXM that was received. Data points show means, brackets show ±1 SEM, and the absence of brackets indicates that 1 SEM fell within the area of the data symbol. Filled symbols indicate a significant difference from placebo. Estimates from the DSST and working memory task are collapsed across pre- and post-performance estimates.
4. DISCUSSION
This study was designed to examine the effects of supratherapeutic doses of DXM as compared to those of triazolam and placebo. Reported elsewhere, we have described the effects of DXM and triazolam on physiological measures, psychomotor performance, subjective measures, observer-rated measures, and assessments of hallucinogen-like effects, in addition to the ascending dose run-up design that led to seven of the 12 volunteers reaching a stop point because of significant behavioral impairment, two volunteers reaching a stop point because an investigator judged administration of higher doses to be inadvisable, and one volunteer reaching a stop point because the participant did not want to receive that drug dose again. Two participants received all planned doses of DXM (Reissig et al., 2012). In this report, we describe the cognitive effects of DXM using a variety of different measures of attention, working memory, episodic memory, and metacognition in healthy adults.
There are several important findings from this study. First, acute supratherapeutic doses of DXM produced orderly, dose-related, and temporary impairments in focused attention, divided attention, working memory, episodic memory, and metacognition. In most cases, impairments in performance and changes in subjective effects resolved by the end of the experimental session (Reissig et al., 2012); however, in all cases, performance and subjective effects had returned to baseline measures before participants were allowed to leave the laboratory (note: participants were not allowed to drive home after sessions). The time course of DXM effects on several psychomotor and subjective measures are presented elsewhere (Reissig et al., 2012) and generally peaked within 2-4 hours after administration and resolved within 6-8 hours after administration. Second, the acute and time-limited impairments in cognitive functioning observed following doses of 100-300 mg/70 kg DXM were generally smaller in magnitude than those observed after administration of a large dose (0.5 mg/70 kg) of triazolam. Third, analysis of the penultimate dose of DXM revealed that the doses of DXM required to impair cognitive performance to the same extent as a large dose of triazolam (0.5 mg/70 kg) were in excess of 10-30 times the therapeutic dose. This is in contrast to some of the other effects in which greater relative drug effect was observed for DXM as compared to triazolam. Specifically, the penultimate dose of DXM resulted in greater psychomotor impairment of balance and hand-eye coordination (i.e., circular lights performance) and higher subjective ratings of drug effect (97.4 ± 1.1 on a 0-100 VAS) as compared to that of 0.5 mg/70 kg triazolam (64.4 ± 6.5 on a 0-100 VAS) (Reissig et al., 2012). Nonetheless, the magnitude of the doses required to observe acute impairments in cognitive performance speak to the relatively broad therapeutic window of OTC DXM preparations when used appropriately, and is consistent with DXM’s long track record of safety when used therapeutically as an antitussive.
As far as we are aware, this is the first study to examine the cognitive effects of large, supratherapeutic doses of DXM (ranging from 100-800 mg/70 kg) in a controlled setting (see also, Reissig et al., 2012). Large doses of DXM resulted in impairments in cognitive performance. These effects were consistent with previous studies of the NMDA antagonist ketamine. In previous studies, ketamine significantly decreased episodic word recall, word recognition, and working memory accuracy (Lofwall et al., 2006; Carter et al., submitted). Likewise, the profile of triazolam effects was also consistent with previous studies in that triazolam produced significant impairments in psychomotor and cognitive performance (Carter et al., 2009; Kleykamp et al., 2010; Mintzer and Griffiths, 2002; Reissig et al., 2012). However, in this study we did not observe an impairment in working memory accuracy as a result of the administration of triazolam, which is somewhat puzzling, but might have been due to the timing of the first cognitive assessment battery (120 min post-administration) as compared to previous studies (e.g., 30-90 min; Carter et al., 2009; Carter et al., submitted). Consistent with previous studies is the observation that impairments in reaction time tend to be more sensitive to the effects of triazolam as compared to impairments in accuracy on this working memory task (Carter et al., submitted).
Also consistent with previous studies of ketamine and triazolam are data from the word recall and word recognition tasks in which the magnitude of the impairment of episodic memory produced by DXM (including that observed after the second-highest dose of DXM that each participant received, i.e., in the high dose analyses), was numerically less than that observed after administration of 0.5 mg/70 kg triazolam across measures (Figure 3). Thus, the profile of cognitive effects and metacognitive effects (awareness of one’s cognitive state) that has been observed for NMDA antagonists such as DXM and ketamine appears to be different from the profile of effects that has been observed with triazolam. That is, participants tend to underestimate the cognitive impairments associated with triazolam (Carter et al., 2009; Kleykamp et al., 2010; Mintzer and Griffiths, 2002), whereas participants tend to overestimate the cognitive impairments associated with ketamine (Carter et al., 2009; Lofwall et al., 2006) or DXM (cf., Figures 3-4 and Reissig et al., 2012).
There are several potential limitations of this study. First, the participants in this outpatient study had a history of recreational drug use, including hallucinogen use, and it is possible that the use of drugs outside of the laboratory for which we did not test (e.g., hallucinogens, cannabis) might have influenced the results. In addition, these participants might not be representative of the general population, which could limit the generalizability of these results. Second, these data can speak to the acute effects of DXM and triazolam, but not to any risks related to the chronic use of these drugs. Third, although this study design used the fairly rigorous approach of an ascending dose run-up for both drugs, DXM was studied up to a maximum dose that could be safely tolerated by individual participants, whereas the same approach was not employed for triazolam, which might limit some of the conclusions drawn from these data. Fourth, one of the advantages of this study is that it was conducted under controlled laboratory conditions; however, it is important to note that recreational drug use is often characterized by the concomitant use of multiple drugs and environmental contexts that are quite different from a controlled laboratory setting, which can have dramatic effects on the relative safety or risk associated with the administration of a given dose of a drug.
Acknowledgments
The authors thank Mary Cosimano, M.S.W., Lilian Salinas, and Jenna Cohen, for serving as assistant session monitors (see Reissig et al., 2012 for further detail). We also thank John Yingling for technical assistance, Barine Duman for data analysis assistance, and Linda Felch and Paul Nuzzo for statistical assistance.
Role of Funding Source
Funding for this study was provided by NIDA grants R01 DA03889 and T32 DA07209; NIDA had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Contributors
Authors Carter, Reissig, Johnson, Griffiths, and Mintzer designed the study and wrote the protocol. Authors Carter and Reissig managed the literature searches and summaries of previous related work. Author Klinedinst prepared the data for statistical analyses, and author Carter wrote the first draft of the manuscript. All authors contributed to and have approved the final manuscript.
Conflict of Interest
In the past three years, Lawrence Carter has served as a Special Government Employee for FDA and as a consultant for Dan’s Plan, LLC, Jazz Pharmaceuticals, Inc., KemPharm, Inc., and UCB, SA. He is currently employed by Jazz Pharmaceuticals plc and has been granted stock options in the company. During the past 3 years, on issues related to drug abuse liability, Matthew Johnson has been a consultant to Eli Lilly and Co. During the past 3 years, on issues related to drug abuse liability, Roland Griffiths has been a consultant to or has received contracts or grants from: Abbott Laboratories, Alexza Pharmaceuticals, Bristol-Myers Squibb, Hoffman-La Roche Inc., Jazz Pharmaceuticals, Merck & Co, Neurocrine Biosciences, Novartis, Pharmacia Corporation, Pfizer, Sanofi-Aventis, Somaxon Pharmaceuticals, Transcept Pharmaceuticals Inc., Vanda Pharmaceuticals, and Wyeth Pharmaceuticals. All other authors have no conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Lawrence P. Carter, University of Arkansas for Medical Sciences, Department of Pharmacology & Toxicology, 4301 W. Markham Street, Little Rock, AR 72205
Chad J. Reissig, Department of Psychiatry and Behavioral Sciences, Johns Hopkins University School of Medicine, 5510 Nathan Shock Drive, Baltimore, MD 21224-6823, USA
Matthew W. Johnson, Department of Psychiatry and Behavioral Sciences, Johns Hopkins University School of Medicine, 5510 Nathan Shock Drive, Baltimore, MD 21224-6823, USA
Margaret A. Klinedinst, Department of Psychiatry and Behavioral Sciences, Johns Hopkins University School of Medicine, 5510 Nathan Shock Drive, Baltimore, MD 21224-6823, USA
Roland R. Griffiths, Department of Psychiatry and Behavioral Sciences, Johns Hopkins University School of Medicine, 5510 Nathan Shock Drive, Baltimore, MD 21224-6823, USA Department of Neuroscience, Johns Hopkins University School of Medicine, 5510 Nathan Shock Drive, Baltimore, MD 21224-6823, USA.
Miriam Z. Mintzer, Johns Hopkins University School of Medicine, Department of Psychiatry and Behavioral Sciences, 5510 Nathan Shock Drive, Baltimore, MD 21224, mmintzer@jhmi.edu, telephone: 410-550-0529, fax: 410-550-0030
References
- Banken JA, Foster H. Dextromethorphan. Ann N Y Acad Sci. 2008;1139:402–411. doi: 10.1196/annals.1432.003. [DOI] [PubMed] [Google Scholar]
- Bem JL, Peck R. Dextromethorphan. An overview of safety issues Drug Saf. 1992;7:190–199. doi: 10.2165/00002018-199207030-00004. [DOI] [PubMed] [Google Scholar]
- Boyer EW. Dextromethorphan abuse. Pediatr Emerg Care. 2004;20:858–863. doi: 10.1097/01.pec.0000148039.14588.d0. [DOI] [PubMed] [Google Scholar]
- Carter LP, Griffiths RR, Mintzer MZ. Cognitive, psychomotor, and subjective effects of sodium oxybate and triazolam in healthy volunteers. Psychopharmacology (Berl) 2009;206:141–54. doi: 10.1007/s00213-009-1589-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter LP, Griffiths RR, Suess PE, Casada JH, Wallace CL, Roache JD. Relative abuse liability of indiplon and triazolam in humans: a comparison of psychomotor, subjective, and cognitive effects. J Pharmacol Exp Ther. 2007;322:749–759. doi: 10.1124/jpet.107.119693. [DOI] [PubMed] [Google Scholar]
- Carter LP, Kleykamp BA, Griffiths RR, Mintzer MZ. Cognitive effects of intramuscular ketamine and oral triazolam in healthy volunteers. Psychopharmacology (Berl) doi: 10.1007/s00213-012-2883-x. (under review) submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter LP, Richards BD, Mintzer MZ, Griffiths RR. Relative abuse liability of GHB in humans: a comparison of psychomotor, subjective, and cognitive effects of supratherapeutic doses of triazolam, pentobarbital, and GHB. Neuropsychopharmacol. 2006;31:2537–2551. doi: 10.1038/sj.npp.1301146. [DOI] [PubMed] [Google Scholar]
- Church J, Sawyer D, McLarnon JG. Interactions of dextromethorphan with the N-methyl-D-aspartate receptor-channel complex: single channel recordings. Brain Res. 1994;666:189–194. doi: 10.1016/0006-8993(94)90771-4. [DOI] [PubMed] [Google Scholar]
- [June 24, 2012];Erowid Dextromethorphan Legal Status Page. http://www.erowid.org/chemicals/dxm/dxm_law.shtml.
- FDA Briefing Information, Dextromethorphan (DXM); September 14, 2010; Meeting of the Drug Safety and Risk Management Advisory Committee; [February 26, 2012]. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/DrugSafetyandRiskManagementAdvisoryCommittee/UCM224446.pdf. [Google Scholar]
- Franklin PH, Murray TF. High affinity [3H]dextrorphan binding in rat brain is localized to a noncompetitive antagonist site of the activated N-methyl-D-aspartate receptor-cation channel. Mol Pharmacol. 1992;41:134–146. [PubMed] [Google Scholar]
- Goodman LA, Kruskal WH. Measures of association for cross-classifications. J Am Stat Assoc. 1954;49:732–764. [Google Scholar]
- [June 24, 2012];H R 1259 (111th): Dextromethorphan Distribution Act of 2009. http://www.govtrack.us/congress/bills/111/hr1259.
- Kleykamp BA, Griffiths RR, Mintzer MZ. Dose effects of triazolam and alcohol on cognitive performance in healthy volunteers. Exp Clin Psychopharmacol. 2010;18:1–16. doi: 10.1037/a0018407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lofwall MR, Griffiths RR, Mintzer MZ. Cognitive and subjective acute dose effects of intramuscular ketamine in healthy adults. Exp Clin Psychopharmacol. 2006;14:439–49. doi: 10.1037/1064-1297.14.4.439. [DOI] [PubMed] [Google Scholar]
- McLeod DR, Griffiths RR, Bigelow GE, Yingling J. An automated version of the digit symbol substitution test (DSST) Behav Res Methods Instrument. 1982;14:463–466. [Google Scholar]
- Mintzer MZ, Griffiths RR. Alcohol and triazolam: differential effects on memory, psychomotor performance and subjective ratings of effects. Behav Pharmacol. 2002;13:653–658. doi: 10.1097/00008877-200212000-00007. [DOI] [PubMed] [Google Scholar]
- Mintzer MZ, Griffiths RR. An abuse liability comparison of flunitrazepam and triazolam in sedative drug abusers. Behav Pharmacol. 2005;16:579–584. doi: 10.1097/01.fbp.0000172736.11994.3c. [DOI] [PubMed] [Google Scholar]
- Mintzer MZ, Griffiths RR. Differential effects of scopolamine and lorazepam on working memory maintenance versus manipulation processes. Cogn Affect Behav Neurosci. 2007;7:120–129. doi: 10.3758/cabn.7.2.120. [DOI] [PubMed] [Google Scholar]
- Morris BJ, Cochran SM, Pratt JA. PCP: from pharmacology to modeling schizophrenia. Curr Opin Pharmacol. 2005;5:101–106. doi: 10.1016/j.coph.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Newell KA, Zavitsanou K, Huang XF. Short and long term changes in NMDA receptor binding in mouse brain following chronic phencyclidine treatment. J Neural Transm. 2007;114:995–1001. doi: 10.1007/s00702-007-0668-x. [DOI] [PubMed] [Google Scholar]
- Parsons CG, Quack G, Bresink I, Baran L, Przegalinski E, Kostowski W, Krzascik P, Hartmann S, Danysz W. Comparison of the potency, kinetics and voltage-dependency of a series of uncompetitive NMDA receptor antagonists in vitro with anticonvulsive and motor impairment activity in vivo. Neuropharmacology. 1995;34:1239–1258. doi: 10.1016/0028-3908(95)00092-k. [DOI] [PubMed] [Google Scholar]
- Reissig CJ, Carter LP, Johnson MW, Mintzer MZ, Klinedinst MA, Griffiths RR. High doses of dextromethorphan, an NMDA antagonist, produce effects similar to classic hallucinogens. Psychopharmacology (Berl) in press. 2012 doi: 10.1007/s00213-012-2680-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanelli F, Smith KM. Dextromethorphan abuse: clinical effects and management. J Am Pharm Assoc. 2009;49:e20–e25. doi: 10.1331/JAPhA.2009.08091. [DOI] [PubMed] [Google Scholar]
- Schutz CG, Soyka M. Dextromethorphan challenge in alcohol-dependent patients and controls. Arch Gen Psychiatry. 2000;57:291–292. doi: 10.1001/archpsyc.57.3.291. [DOI] [PubMed] [Google Scholar]
- Sinner B, Graf BM. Ketamine. Handb Exp Pharmacol. 2008:313–333. doi: 10.1007/978-3-540-74806-9_15. [DOI] [PubMed] [Google Scholar]
- Soyka M, Bondy B, Eisenburg B, Schutz CG. NMDA receptor challenge with dextromethorphan - subjective response, neuroendocrinological findings and possible clinical implications. J Neural Transm. 2000;107:701–714. doi: 10.1007/s007020070071. [DOI] [PubMed] [Google Scholar]
- Snodgrass JG, Corwin J. Pragmatics of measuring recognition memory: applications to dementia and amnesia. J Exp Psychol Gen. 1988;117:34–50. doi: 10.1037//0096-3445.117.1.34. [DOI] [PubMed] [Google Scholar]
- Steinberg GK, Bell TE, Yenari MA. Dose escalation safety and tolerance study of the N-methyl-D-aspartate antagonist dextromethorphan in neurosurgery patients. J Neurosurg. 1996;84:860–866. doi: 10.3171/jns.1996.84.5.0860. [DOI] [PubMed] [Google Scholar]
- Sternberg S. The discovery of processing stages: Extensions of Donder’s method. Acta Psychologica. 1969;30:276–315. [Google Scholar]
- Werling LL, Keller A, Frank JG, Nuwayhid SJ. A comparison of the binding profiles of dextromethorphan, memantine, fluoxetine and amitriptyline: treatment of involuntary emotional expression disorder. Exp Neurol. 2007;207:248–257. doi: 10.1016/j.expneurol.2007.06.013. [DOI] [PubMed] [Google Scholar]
- Wilson MD, Ferguson RW, Mazer ME, Litovitz TL. Monitoring trends in dextromethorphan abuse using the National Poison Data System: 2000-2010. Clin Toxicol (Phila) 2011;49:409–415. doi: 10.3109/15563650.2011.585429. [DOI] [PubMed] [Google Scholar]
- Zawertailo LA, Kaplan HL, Busto UE, Tyndale RF, Sellers EM. Psychotropic effects of dextromethorphan are altered by the CYP2D6 polymorphism: a pilot study. J Clin Psychopharmacol. 1998;18:332–337. doi: 10.1097/00004714-199808000-00014. [DOI] [PubMed] [Google Scholar]
- Ziaee V, Akbari HE, Hoshmand A, Amini H, Kebriaeizadeh A, Saman K. Side effects of dextromethorphan abuse, a case series. Addict Behav. 2005;30:1607–1613. doi: 10.1016/j.addbeh.2005.02.005. [DOI] [PubMed] [Google Scholar]


