Abstract
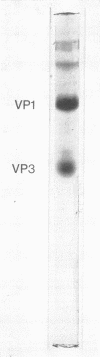
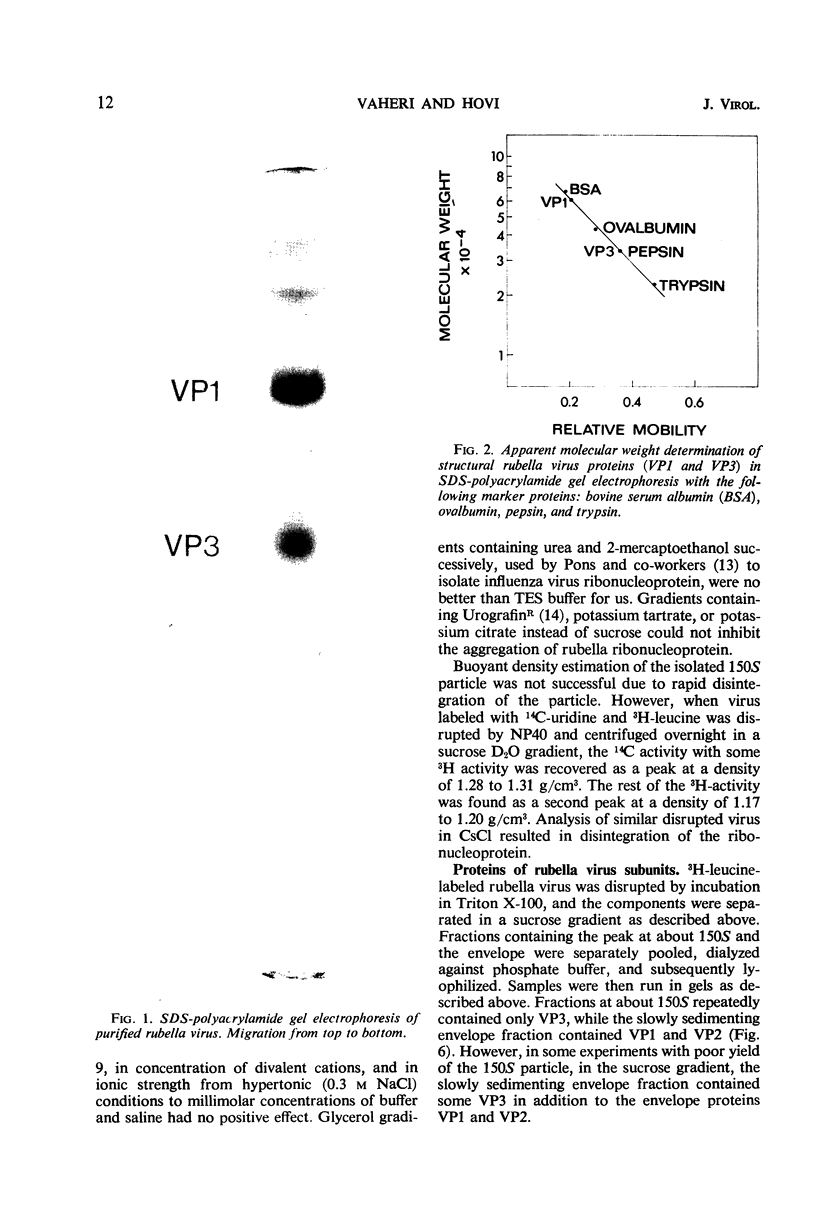
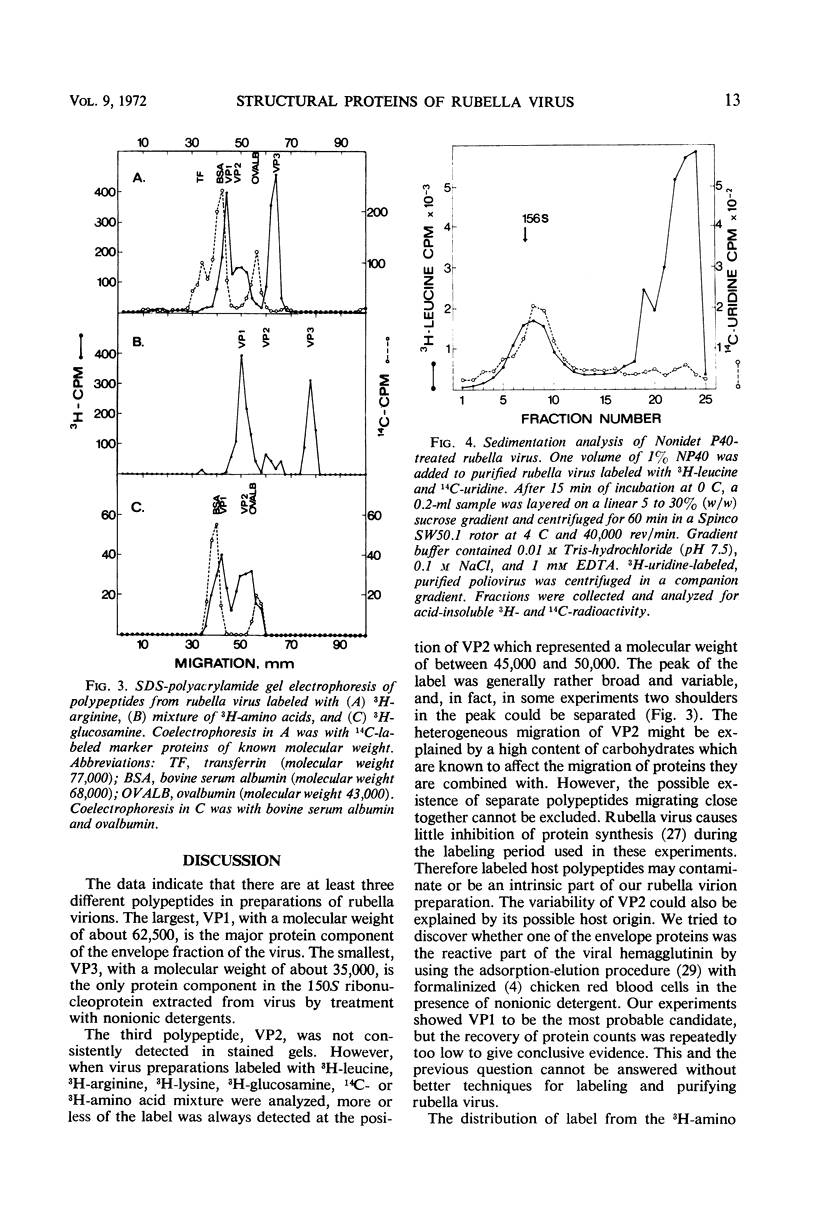
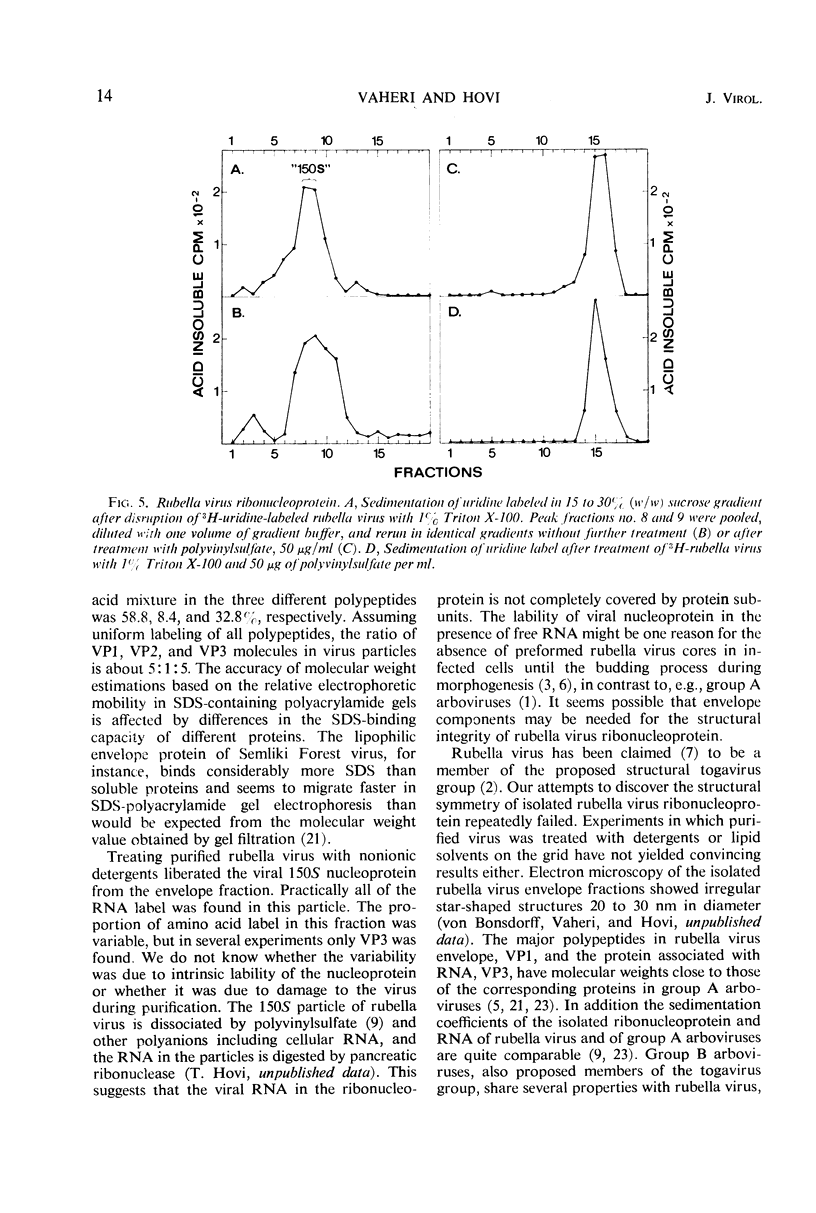
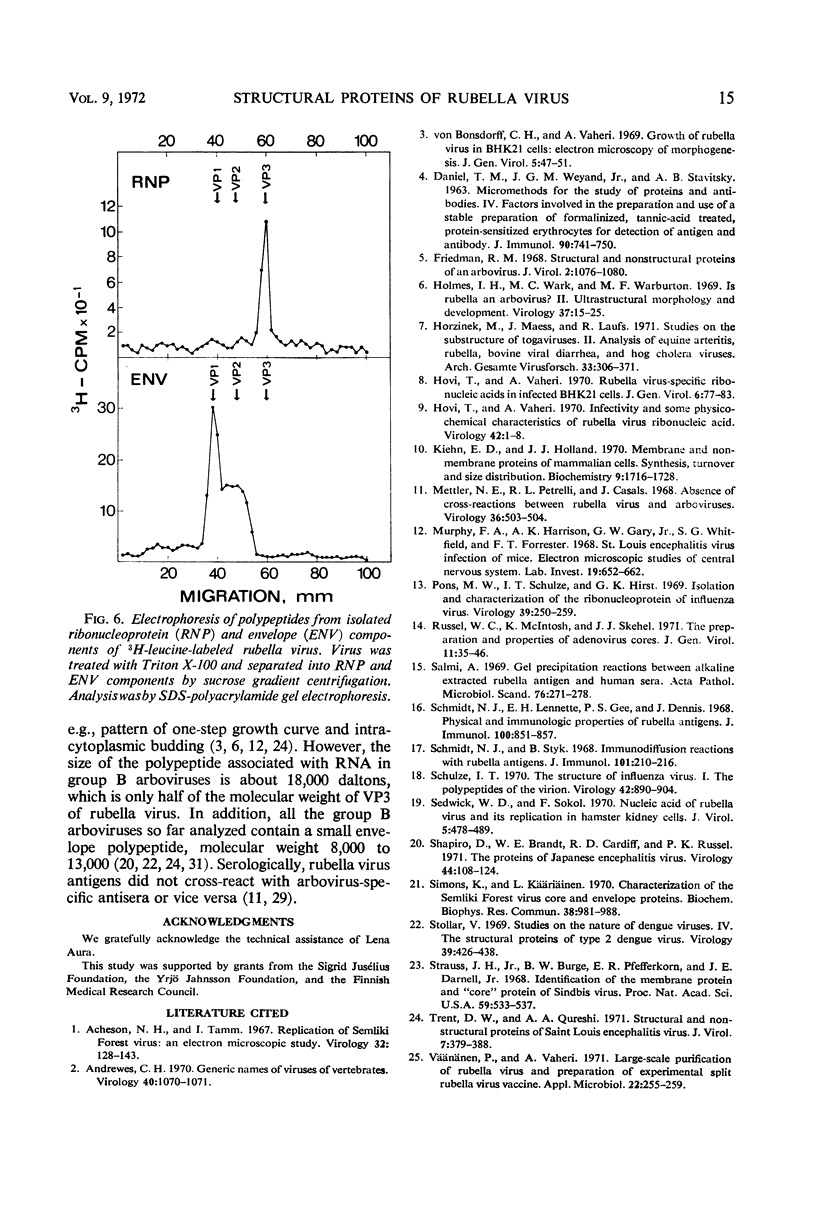
Polyacrylamide gel electrophoresis of purified rubella virus revealed two distinct structural proteins VP1 and VP3, which had molecular weights of 62,500 and 35,000, respectively. In addition, a broad variable peak, designated VP2, with a molecular weight of about 47,500, was seen. Sucrose gradient analysis of virus disrupted by neutral detergents separated a labile 150S ribonucleoprotein, containing 40S ribonucleic acid and VP3, from the envelope fraction containing VP1 and VP2. VP1 and particularly VP2 were labeled with glucosamine and are thus glycoproteins. Labeling the polypeptides with different amino acids indicated that VP3, the “core” protein, is relatively rich in arginine but not in lysine. The size of the two main polypeptides, VP1 and VP3, corresponds to those of group A arboviruses.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acheson N. H., Tamm I. Replication of Semliki Forest virus: an electron microscopic study. Virology. 1967 May;32(1):128–143. doi: 10.1016/0042-6822(67)90261-9. [DOI] [PubMed] [Google Scholar]
- DANIEL T. M., WEYAND J. G., Jr, STAVITSKY A. B. MICROMETHODS FOR THE STUDY OF PROTEINS AND ANTIBODIES. IV. FACTORS INVOLVED IN THE PREPARATION AND USE OF A STABLE PREPARATION OF FORMALINIZED, TANNIC ACID-TREATED, PROTEIN-SENSITIZED ERYTHROCYTES FOR DETECTION OF ANTIGEN AND ANTIBODY. J Immunol. 1963 May;90:741–750. [PubMed] [Google Scholar]
- Friedman R. M. Structural and nonstructural proteins of an arbovirus. J Virol. 1968 Oct;2(10):1076–1080. doi: 10.1128/jvi.2.10.1076-1080.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes I. H., Wark M. C., Warburton M. F. Is rubella an arbovirus? II. Ultrastructural morphology and development. Virology. 1969 Jan;37(1):15–25. doi: 10.1016/0042-6822(69)90301-8. [DOI] [PubMed] [Google Scholar]
- Horzinek M., Maess J., Laufs R. Studies on the substructure of togaviruses. II. Analysis of equine arteritis, rubella, bovine viral diarrhea, and hog cholera viruses. Arch Gesamte Virusforsch. 1971;33(3):306–318. [PubMed] [Google Scholar]
- Hovi T., Vaheri A. Infectivity and some physicochemical characteristics of rubella virus ribonucleic acid. Virology. 1970 Sep;42(1):1–8. doi: 10.1016/0042-6822(70)90232-1. [DOI] [PubMed] [Google Scholar]
- Hovi T., Vaheri A. Rubella virus-specific ribonucleic acids in infected BHK21 cells. J Gen Virol. 1970 Jan;6(1):77–83. doi: 10.1099/0022-1317-6-1-77. [DOI] [PubMed] [Google Scholar]
- Kiehn E. D., Holland J. J. Membrane and nonmembrane proteins of mammalian cells. Synthesis, turnover, and size distribution. Biochemistry. 1970 Apr 14;9(8):1716–1728. doi: 10.1021/bi00810a010. [DOI] [PubMed] [Google Scholar]
- Mettler N. E., Petrelli R. L., Casals J. Absence of antigenic cross-reactions between rubella virus and arbouviruses. Virology. 1968 Nov;36(3):503–504. doi: 10.1016/0042-6822(68)90175-x. [DOI] [PubMed] [Google Scholar]
- Murphy F. A., Harrison A. K., Gary G. W., Jr, Whitfield S. G., Forrester F. T. St. Louis encephalitis virus infection in mice. Electron microscopic studies of central nervous system. Lab Invest. 1968 Dec;19(6):652–662. [PubMed] [Google Scholar]
- Pons M. W., Schulze I. T., Hirst G. K., Hauser R. Isolation and characterization of the ribonucleoprotein of influenza virus. Virology. 1969 Oct;39(2):250–259. doi: 10.1016/0042-6822(69)90045-2. [DOI] [PubMed] [Google Scholar]
- Russell W. C., McIntosh K., Skehel J. J. The preparation and properties of adenovirus cores. J Gen Virol. 1971 Apr;11(1):35–46. doi: 10.1099/0022-1317-11-1-35. [DOI] [PubMed] [Google Scholar]
- Salmi A. A. Gel precipitation reactions between alkaline extracted rubella antigens and human sera. Acta Pathol Microbiol Scand. 1969;76(2):271–278. doi: 10.1111/j.1699-0463.1969.tb03257.x. [DOI] [PubMed] [Google Scholar]
- Schmidt N. J., Lennette E. H., Gee P. S., Dennis J. Physical and immunologic properties of rubella antigens. J Immunol. 1968 Apr;100(4):851–857. [PubMed] [Google Scholar]
- Schmidt N. J., Styk B. Immunodiffusion reactions with rubella antigens. J Immunol. 1968 Aug;101(2):210–216. [PubMed] [Google Scholar]
- Schulze I. T. The structure of influenza virus. I. The polypeptides of the virion. Virology. 1970 Dec;42(4):890–904. doi: 10.1016/0042-6822(70)90338-7. [DOI] [PubMed] [Google Scholar]
- Sedwick W. D., Sokol F. Nucleic acid of rubella virus and its replication in hamster kidney cells. J Virol. 1970 Apr;5(4):478–489. doi: 10.1128/jvi.5.4.478-489.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro D., Brandt W. E., Cardiff R. D., Russell P. K. The proteins of Japanese encephalitis virus. Virology. 1971 Apr;44(1):108–124. doi: 10.1016/0042-6822(71)90158-9. [DOI] [PubMed] [Google Scholar]
- Simons K., Käriäinen L. Characterization of the Semliki Forest virus core and envelope protein. Biochem Biophys Res Commun. 1970 Mar 12;38(5):981–988. doi: 10.1016/0006-291x(70)90818-1. [DOI] [PubMed] [Google Scholar]
- Stollar V. Studies on the nature of dengue viruses. IV. The structural proteins of type 2 dengue virus. Virology. 1969 Nov;39(3):426–438. doi: 10.1016/0042-6822(69)90091-9. [DOI] [PubMed] [Google Scholar]
- Strauss J. H., Jr, Burge B. W., Pfefferkorn E. R., Darnell J. E., Jr Identification of the membrane protein and "core" protein of Sindbis virus. Proc Natl Acad Sci U S A. 1968 Feb;59(2):533–537. doi: 10.1073/pnas.59.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trent D. W., Qureshi A. A. Structural and nonstructural proteins of Saint Louis encephalitis virus. J Virol. 1971 Mar;7(3):379–388. doi: 10.1128/jvi.7.3.379-388.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaheri A., Cristofalo V. J. Metabolism of rubella virus-infected BHK 21 cells. Enhanced glycolysis and late cellular inhibition. Arch Gesamte Virusforsch. 1967;21(3):425–436. doi: 10.1007/BF01241741. [DOI] [PubMed] [Google Scholar]
- Vaheri A., Sedwick W. D., Plotkin S. A., Maes R. Cytopathic effect of rubella virus in RHK21 cells and growth to high titers in suspension culture. Virology. 1965 Oct;27(2):239–241. doi: 10.1016/0042-6822(65)90170-4. [DOI] [PubMed] [Google Scholar]
- Vaheri A., Vesikari T. Small size rubella virus antigens and soluble immune complexes: analysis by the platelet aggregation technique. Arch Gesamte Virusforsch. 1971;35(1):10–24. doi: 10.1007/BF01249748. [DOI] [PubMed] [Google Scholar]
- Vänänen P., Vaheri A. Large-scale purification of rubella virus and preparation of an experimental split rubella virus vaccine. Appl Microbiol. 1971 Sep;22(3):255–259. doi: 10.1128/am.22.3.255-259.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Westaway E. G., Reedman B. M. Proteins of the group B arbovirus Kunjin. J Virol. 1969 Nov;4(5):688–693. doi: 10.1128/jvi.4.5.688-693.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bonsdorff C. H., Vaheri A. Growth of rubella virus in BHK21 cells: electron microscopy of morphogenesis. J Gen Virol. 1969 Jul;5(1):47–51. doi: 10.1099/0022-1317-5-1-47. [DOI] [PubMed] [Google Scholar]
- von Bonsdorff C. H., Vaheri A. Growth of rubella virus in BHK21 cells: electron microscopy of morphogenesis. J Gen Virol. 1969 Jul;5(1):47–51. doi: 10.1099/0022-1317-5-1-47. [DOI] [PubMed] [Google Scholar]