Abstract
Objective
While neuropathy is common in the elderly, nerve conduction (NC) reproducibility in older adults is not well-established. We sought to evaluate intraobserver reproducibility of peroneal motor NC measures in a diverse sample of older adults.
Methods
We measured peroneal motor NC amplitude and velocity in a subset of participants (mean age=82.9 ± 2.7, n=62, 50% female, 51.6% black, 35.5% DM) in the Health, Aging, and Body Composition Study. Using coefficients of variation (CVs), intraclass correlation coefficients (ICCs), and Bland Altman Plots, we compared two sets of measurements taken by the same examiner hours apart on the same day.
Results
Low CVs (2.15–4.24%) and moderate to high ICCs (0.75–0.99) were observed. No systematic variation was found across measures. Despite small numbers in some subgroups, we found no differences in reproducibility by diabetes, race, or study site.
Conclusion
NC measures have moderate to high intraobsever reproducibility in older adults and are not affected by diabetes, race, or gender.
Significance
These data provide evidence to support use of these measures in aging research.
Keywords: Motor nerve conduction, aging, peripheral nerve function, reproducibility, diabetes
Introduction
Peroneal nerve conduction (NC) studies objectively measure impairments in motor nerve function, a common complication of diabetes mellitus (DM) and an important risk factor for musculoskeletal impairments (Cauley et al., 2010, Lauretani et al., 2006) and mobility limitations in old age (Resnick et al., 2002, Strotmeyer et al., 2008). NC studies are the most sensitive and specific method to detect diabetic peripheral neuropathy (Perkins et al., 2001) and can quantify change over time in nerve function (Brown et al., 2004, Dyck et al., 1997a, Dyck et al., 1997b, Partanen et al., 1995, Sosenko et al., 1993, Sosenko et al., 1992). In clinical settings, NC studies are typically used to confirm diagnosis of neuropathy in patients with symptoms; however, the Diabetic Neuropathy Study Group of the European Association for the Study of Diabetes (NEURODIAB) and the International Symposium on Diabetic Neuropathy endorse the use of NC studies as a measure of early nerve decline in pre-symptomatic individuals (Tesfaye et al., 2010). By identifying abnormalities prior to onset of neuropathic symptoms (DCCT Research Group, 2002) these studies permit earlier and potentially more successful intervention for modifiable risk factors (Vinik et al., 2008). In addition, there is evidence that the natural history of diabetic neuropathy may be changing with less deterioration in NC over time (Tesfaye et al., 2007). Therefore, it has become necessary to detect small changes in NC, dictating a need for greater accuracy and precision.
Whether assessing the degree of abnormality or quantifying change, it is essential to use an accurate method of measurement. Test-retest reliability, otherwise known as reproducibility, is a necessary component of accuracy. Moderate to high reproducibility has been found for peroneal motor NC in healthy (Herrera et al., 2009, Loseth et al., 2007) and diabetic populations (Bird et al., 2006, Dyck et al., 2003, Dyck et al., 2007); however, these studies included few older adults and lacked racial diversity.
Potential lack of reproducibility in NC studies in older adults is particularly problematic because peripheral nerve function decline is common in this age group. Both incidence (Baldereschi et al., 2007) and prevalence of poor peripheral nerve function increase with age (Baldereschi, Inzitari, 2007, Buschbacher, 1999, Gregg et al., 2004, Resnick et al., 2001, Rivner et al., 2001), among persons with and without DM (Baldereschi, Inzitari, 2007, Gregg, Sorlie, 2004). The 1999–2000 National Health and Nutrition Examination Survey (NHANES) found that 28% of adults ages 70–79 and 35% of adults 80 years and older had impaired nerve function based on a simple screen for reduced sensation at the foot. However, this is likely an underestimate of nerve dysfunction due to use of less sensitive measures such as self-reported symptoms and monofilament testing (Gregg, Sorlie, 2004). While DM is the most common cause of peripheral neuropathy in the elderly (George and Twomey, 1986, Huang, 1981, Verghese et al., 2001), age itself is also an independent predictor of peripheral nerve impairments (Bouche et al., 1993).
Regardless of etiology, decreased nerve function in old age has been independently associated with physical function limitations and impairments (Resnick, Stansberry, 2002, Resnick et al., 2000, Strotmeyer, de Rekeneire, 2008) and increased risk of falls (Cavanagh et al., 1992, Ferrucci et al., 2004, Richardson et al., 1992). Additionally, neuromuscular impairments and denervation of motor units that occur with age have become particularly important within gerontological research given their potential effects on conditions salient to older adults such as sarcopenia (Lauretani, Bandinelli, 2006), osteoporosis (Cauley, Blackwell, 2010, Strotmeyer et al., 2006), frailty (Runge and Hunter, 2006), and disability (Resnick, Stansberry, 2002, Resnick, Vinik, 2000). Therefore, it is crucial to evaluate these methods of nerve function assessment in older adults since they are essential for investigating the pathophysiology of neuromuscular dysfunction in late-life and its relationship to key outcomes.
While it has been established that values for NC change with aging, less is known about pathophysiology and electrodiagnostic testing changes in older adults (Stetson et al., 1992, Verghese, Bieri, 2001). We investigated reproducibility of conduction velocities and amplitudes of peroneal motor responses (CMAPs) recorded at the extensor digitorum brevis muscle with stimulation at the ankle, popliteal fossa, and fibular head in a diverse sample of older adults from the Health, Aging, and Body Composition (Health ABC) Study to determine if the technique is sufficiently robust to establish the natural history of peripheral nerve decline in aging.
Materials and Methods
Health ABC is an ongoing prospective cohort study of well functioning older adults (n = 3,075; 48.4% male; 41.6% black; 70–79 years of age at baseline) that was established in 1997–1998 to investigate changes in body composition and disability in old age. Study participants were recruited through mailings to a random sample of white Medicare beneficiaries and all black community residents eligible by age. Eligibility was determined by phone interview, and included having no difficulty walking a quarter of a mile or walking up 10 steps, and no difficulty performing activities of mobility-related daily living, as well as having no life-threatening cancers with active treatment within the past 3 years, and planning to remain in the study area for at least 3 years. Informed consent was provided prior to examination and approved by the institutional review boards at the University of Pittsburgh and the University of Tennessee Health Science Center. In the present study, a subset of 66 Health ABC participants were recruited from the 2007–2008 clinic visit at both sites to obtain approximately equal numbers of black and white men and women (black women: n = 16, black men: n = 17; white women: n = 17; white men: n = 16).
Measures included in the 2007–2008 clinic visits were designed to assess long term change in peripheral nerve function, with specific regard to musculoskeletal and mobility outcomes. Participants were excluded from peroneal NC testing if they had bilateral lower limb amputation or bilateral knee replacement. NC was measured using surface electrodes on the right leg, unless the left leg was tested at the prior visit or testing on the right leg was contraindicated. Contraindications included lower limb amputation, knee replacement, surgery, trauma, or ulcers. If testing on the right leg was contraindicated, measures were performed on the left leg unless its use was also contraindicated.
NC was measured using the NeuroMax 8 electrodiagnostic device (XLTEK, Oakville, ON, Canada) while the participant lay supine on the examining table with their leg exposed. Amplifier filter settings were set at 2.0 Hz and 2 kHz, without notch filter. Supramaximal pulse stimulation lasting 0.20 ms was gradually increased as needed up to 100 mA. Prior to testing, the surface temperature on the dorsum of the foot was measured using a surface thermometer. If the temperature of the foot was below 30°C, the foot was warmed with a heating pad until 30°C was reached. If the foot did not reach 30°C after 5 minutes of warming, the achieved temperature was recorded and the examiner proceeded with testing. This only occurred once, in which case the participant’s final foot temperature was recorded as 29.6°C. Surface electrodes with conducting gel were placed over the anterior ankle, over the fifth metatarsophalangeal joint (lateral to long extensor tendons), and over the base of the extensor digitorum brevis muscle (1 cm distal to calcaneous bone). The peroneal nerve was stimulated at: 1) the ankle, 8.5 cm from the electrode placed at the base of the extensor digitorum brevis muscle, approximately 5 cm proximal of the malleoli; 2) the fibular head, immediately below the fibula; and 3) the popliteal fossa, approximately 10 cm proximal to the fibular head. Motor response (CMAP, compound action potential) was recorded at the extensor digitorum brevis muscle. The nerve conduction velocities (NCV) with stimulation of the peroneal nerve at the fibular head and popliteal fossa were calculated by the dividing the distance between the stimulation sites by the latency difference. Two NC exams were performed for each participant by the same examiner, on the same day 1 to 3 hours apart, during which time the participants were allowed to get up from the table and move around freely. All electrodes and skin marking indicating electrode placement were removed after the first measurement and examiners were instructed not to refer to the first measurements when performing the second.
The two study sites (Memphis and Pittsburgh) each had clinic staff examiners trained by an expert technician with extensive experience in clinical trials using NC measures as outcomes and a board certified neurologist with additional certifications in electrodiagnostic medicine and neuromuscular medicine, qualifications in clinical neurophysiology, and specialization in neuromuscular disorders. Data and waveforms collected during 2007–2008 clinic visits of the Health ABC Study were reviewed by the neurologist for the first 200 participants to ensure that the protocol for collecting data was producing the highest quality waveforms. After the first 200 participants, the neurologist only reviewed data flagged for quality control. CMAP amplitude values of less than 1 millivolt (mV) and NCV values of less than 20 m/s or greater than 70 m/s were flagged for quality control and reviewed. Tests were immediately repeated if there was a difference greater than 10 m/s between the fibular head and the popliteal fossa NCV measures. NC measures with abnormal waveform morphology were identified by the neurologist during quality control after testing. Final CMAP amplitudes ranged from 0.1 to 8.0 mV and NCV ranged from 32.6 to 57.8 m/s.
Participant characteristics were stratified by DM status due to its strong relationship with peripheral nerve function in older adults (Baldereschi, Inzitari, 2007), and analyzed using Pearson chi-squared, Fisher’s exact, and t-tests. DM was defined as self-reported physician diagnosis, hypoglycemic medication use, or fasting glucose greater than 126 mg/dL (47.0 mmol/L) after an 8-hour or longer fast. We estimated possible prevalent peripheral neuropathy using a composite of clinic measures. These included reporting pain or numbness in the lower extremity and having a peroneal NCV of <40 m/s or being unable to detect a 10g monofilament. However, this was not a clinical diagnosis of PN. Signed rank and paired t-tests were used to compare the two sets of measures. We calculated coefficients of variation (CVs) and interclass correlation coefficients (ICCs). Two-sided t-test approximations for the Wilcoxon rank sum test were used to compare CVs between study site, race, gender, DM, and obesity groups defined by body mass index (normal weight = BMI<25, overweight = 25≤BMI<30, obese = BMI≥30). Bland Altman plots were graphed for each measure and the correlation coefficient was calculated for each plot. Values for NC measures with abnormal waveform morphology could not be included in the analyses using these methods. However, we performed an additional analysis using Kappa statistics to compare agreement between primary and reproduced measures based on whether normal waveform morphology did or did not occur, which allowed full use of all data collected.
Out of the 66 participants sampled, four were excluded due to missing fasting glucose. We were unable to report numeric values for NC parameters for ankle stimulation on four participants and fibular head and popliteal fossa stimulation on six participants due to abnormal waveform morphology.
Results
Participants with and without DM did not differ by age, sex, body composition, or lifestyle characteristics (Table 1). A significantly higher proportion of diabetic participants self-identified as black (72.7% vs. 40.0%, p = 0.01). Approximately 36% of participants with DM had it for 10 or more years and 40% had hemoglobin A1C levels ≥ 7%, as an indicator of poor diabetes control (American Diabetest Association, 2012). Participants with DM had a higher percentage of glaucoma (45.0% vs. 17.5%, p = 0.02) but did not differ from those without DM by any other chronic health condition.
Table 1.
Participant characteristics by diabetes status
| Characteristic | DM (n = 22) | NonDM (n = 40) | p-value |
|---|---|---|---|
| Age (years) | 82.8 ± 2.7 | 83.0 ± 2.7 | 0.78 |
| Female (%) | 40.9% | 55.0% | 0.29 |
| Black race (%)* | 72.7% | 40.0% | 0.01 |
| Body composition | |||
| Height (m) | 1.642 ± 0.104 | 1.648 ± 0.097 | 0.82 |
| BMI (kg/m2) | 29.8 ± 5.4 | 27.0± 5.5 | 0.05 |
| Obese (%) | 36.4% | 25.0% | 0.35 |
| Lifestyle characteristics | |||
| Current smoker (%) | 0% | 5.0% | 0.55 |
| Drinking frequency > 1/week (%) | 45.0% | 47.5 % | 0.85 |
| Physical activity (kcal/kg/week) | 6.8 ± 16.2 | 7.7 ± 11.8 | 0.79 |
| Monofilament detection | |||
| Able 10g (%) | 81.8% | 84.6% | 1.00 |
| Able 1.4g (%) | 59.1% | 59.0% | 0.99 |
| PN Symptoms | |||
| Numbness/tingling (%) | 45.0% | 30.0% | 0.25 |
| Stabbing/burning pain (%) | 20.0% | 17.5% | 1.00 |
| Persistent sore/gangrene (%) | 5.0% | 0% | 0.33 |
| Chronic health conditions | |||
| DM duration | |||
| ≥ 10 years (%) | 36.4% | -- | -- |
| ge; 5 to < 10 years (%) | 36.4% | -- | -- |
| A1C (%)† | 7.0 ± 1.2 | 5.8 ± 0.4 | 0.0001 |
| A1C ≥ 7% (%)‡ | 40.0% | 0% | <0.0001 |
| Insulin & oral hypoglycemic medication (%) | 4.6% | -- | -- |
| Insulin only (%) | 9.1% | -- | -- |
| Oral hypoglycemic medication only (%) | 54.6% | -- | -- |
| Possible peripheral neuropathy (%) | 31.8% | 20.5% | 0.32 |
| Ankle-arm index < 0.9 (%) | 66.7% | 76.5% | 0.43 |
| Hypertension (%) | 100.0% | 90.0% | 0.12 |
| Hypertension medication (%) | 90.9% | 80.0% | 0.47 |
| Systolic blood pressure (mmHg) | 136.5 ± 23.4 | 130.8 ± 21.0 | 0.33 |
| Diastolic blood pressure (mmHg) | 68.9 ± 11.1 | 68.1 ± 11.3 | 0.77 |
| Cholesterol (mg/dl) | 188.7 ± 42.4 | 195.7 ± 42.0 | 0.54 |
| Retinal disease/retinopathy (%) | 5.0% | 2.5% | 1.00 |
| Glaucoma (%)* | 45.0% | 17.5% | 0.02 |
| Cataracts (%) | 80.0% | 77.5% | 1.00 |
| Cystatin-C (mg/l) | 0.9 ± 0.3 | 1.0 ± 0.3 | 0.28 |
| Creatinine ≥ 1.5 men/1.3 women (%) | 15.0% | 8.6% | 0.66 |
p<0.05;
p<0.001;
p<0.0001; Data are means ± SD unless otherwise specified; DM = diabetes mellitus; SD = standard deviation; PN = peripheral nerve
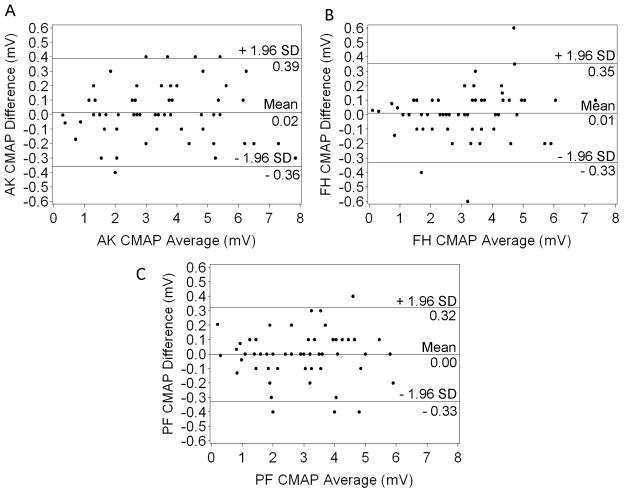
Table 2 shows no significant differences between mean primary and reproduced measures of ankle, popliteal fossa, and fibular head CMAP amplitude, or popliteal fossa and fibular head NCV for the total study population. No mean CV exceeded 5%. ICCs for the total study population were all above 0.9, except for popliteal fossa NCV, which was 0.75 (95% CI: 0.60–0.84). Mean differences between primary and reproduced measures, reported in the Bland Altman plots (Figure 1 and Figure 2), were not significantly different than 0 (all p > 0.05). These plots show the vast majority of data points fall within the range of 1.96 standard deviations above and below the mean differences. Correlation coefficients for all Bland Altman plots were not significantly different than 0 (ankle amplitude: r = −0.002, fibular head amplitude: r = 0.16, popliteal fossa amplitude: r = 0.004, fibular head NCV: r = 0.03, popliteal fossa NCV: r = 0.06; all p > 0.05).
Table 2.
Primary and reproduced peroneal motor nerve conduction measures (mean ± SD), correlation coefficients, mean coefficients of variation, and intraclass correlation coefficients
| Parameter | Primary Measure | Reproduced Measure | Mean CV % | ICC | ICC 95% CI |
|---|---|---|---|---|---|
| CMAP amplitude (mV) | |||||
| Ankle (n=58) | 3.46 ± 1.8 | 3.48 ± 1.8 | 3.66 | 0.99 | 0.99–1.00 |
| Popliteal Fossa (n=56) | 2.94 ± 1.4 | 2.93 ± 1.4 | 4.24 | 0.99 | 0.99–1.00 |
| Fibular Head (n=57) | 3.09 ± 1.5 | 3.10 ± 1.6 | 3.26 | 0.99 | 0.99–1.00 |
| NCV (m/s) | |||||
| Popliteal Fossa (n=56) | 42.0± 3.3 | 41.9 ± 3.4 | 2.15 | 0.90 | 0.83–0.94 |
| Fibular Head (n=57) | 42.3 ± 4.1 | 42.2 ± 4.2 | 2.70 | 0.75 | 0.60–0.84 |
NC = nerve conduction; SD = standard deviation; CV = coefficient of variation; ICC = intraclass correlation coefficient; CMAP = compound muscle action potential; NCV = nerve conduction velocity; CI = confidence interval
Figure 1.
Bland Altman Plots for Ankle (A), Fibular Head (B), and Popliteal Fossa (C) CMAP amplitudes.
AK = ankle; FH = fibular head; PF = popliteal fossa; CMAP = compound muscle action potential; SD = standard deviation.
Figure 2.
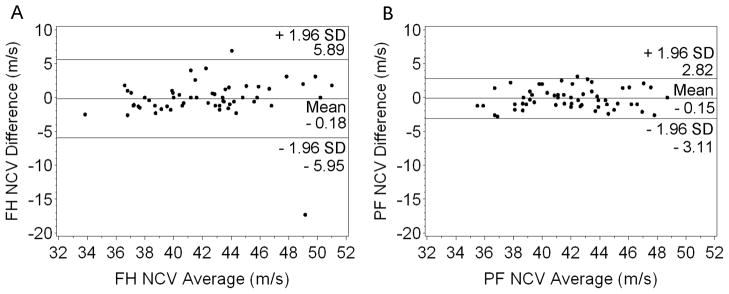
Bland Altman Plots for Fibular Head (A) and Popliteal Fossa (B) NCV.
FH = fibular head; PF = popliteal fossa; NCV = nerve conduction velocity; SD = standard deviation.
When stratified by DM, all CVs were below 5% and were not significantly different by group (results not shown). All CMAP amplitude ICCs were above 0.9 and all NCV ICCs exceeded 0.8, except for the fibular head NCV for the nondiabetic group (ICC = 0.687; 95% CI: 0.469–0.826). Stratification by obesity status (Table 3) showed a slight (but nonsignificant) increasing trend for all CMAP CVs. Only the CV for popliteal fossa CMAP amplitude in obese participants exceeded 5% (6.88%). Women had higher fibular head NCV CV than men; however, neither CV was above 5% (men = 1.77%, women = 3.54%, p = 0.03). No differences in reproducibility were found by race or study site for any measure (results not shown). All NC measures had high Kappa coefficients (0.78–0.88; all p < 0.05) between primary and reproduced measures by waveform morphology status (Table 4), indicating good to very good agreement (Altman, 1991).
Table 3.
Nerve conduction measures (mean ± SD) and coefficients of variation by obesity status
| Obese (n = 16) | Overweight (n = 23) | Normal weight (n = 19) | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Parameter | Mean ± SD | CV | Mean ± SD | CV | Mean ± SD | CV | p-value† |
| CMAP amplitude (mV) | |||||||
| Ankle | 3.20 ± 1.7 | 4.77% | 3.42 ± 2.0 | 3.74% | 3.74 ± 1.6 | 2.62% | 0.78 |
| Popliteal Fossa | 2.97 ± 1.3 | 6.88% | 2.88 ± 1.6 | 3.33% | 3.15 ± 1.4 | 3.20% | 0.49 |
| Fibular Head | 2.98 ± 1.3 | 4.56% | 3.04 ± 1.7 | 3.01% | 3.24 ± 1.4 | 2.54% | 0.67 |
| NCV (m/s) | |||||||
| Popliteal Fossa | 43.3 ± 2.6 | 1.81% | 42.2 ± 3.8 | 2.02% | 40.7 ± 2.6 | 2.55% | 0.29 |
| Fibular Head | 42.5 ± 2.7 | 2.60% | 42.8 ± 5.4 | 3.28% | 41.6 ± 3.2 | 2.08% | 0.90 |
NC = nerve conduction; SD = standard deviation; CV = coefficient of variation; CMAP = compound muscle action potential; NCV = nerve conduction velocity;
p-value for CV trend; no significant pairwise comparisons for CV
Table 4.
Kappa statistics for agreement of primary and reproduced NC measures by waveform morphology status
| Parameter | Kappa Coefficient | 95% CI* | |
|---|---|---|---|
| CMAP amplitude (mV) | |||
| Ankle | 0.85 | 0.56 – 1.00 | |
| Popliteal Fossa | 0.78 | 0.49 – 1.00 | |
| Fibular Head | 0.88 | 0.65 – 1.00 | |
| NCV (m/s) | |||
| Popliteal Fossa | 0.78 | 0.49 – 1.00 | |
| Fibular Head | 0.88 | 0.65 – 1.00 | |
p<0.05; NC = nerve conduction; SD = standard deviation; CV = coefficient of variation; ICC = intraclass correlation coefficient; CMAP = compound muscle action potential; NCV = nerve conduction velocity; CI = confidence interval
Discussion
The present study provides evidence that NC measures are reproducible in a diverse population of oldest-old, community dwelling adults with and without DM, and across BMI values. The reproducibility of NC study measures has not been demonstrated in older populations despite the critical impact on clinical testing and accurate diagnosis of peripheral neuropathy in the elderly. Important additional considerations specific to this age group exist that could affect reproducibility, such as increased variability due, in part, to comorbid conditions and recovery from illness or injury. Reproducibility for evaluating nerve function impairment in older adults is absolutely crucial for studies aimed at assessing risk factors and interventions for nerve function decline and the role that it plays in late-life conditions. The reproducibility of these measures is also critical for clinical trials that need to detect longitudinal change, such as those assessing medications or other interventions to prevent or treat peripheral neuropathy in the elderly. Poor peripheral nerve function is common in older adults with multiple comorbidities such as DM and obesity (Baldereschi, Inzitari, 2007, Buschbacher, 1999, Gregg, Sorlie, 2004, Rivner, Swift, 2001) and can greatly impact physical function (Cavanagh, Derr, 1992, De Rekeneire et al., 2003, Resnick, Vinik, 2000, Ryerson et al., 2003). Reproducible NC measures in this population are essential for the validity of their assessment in epidemiologic and clinical research settings.
Because our sample was selected with respect to race and gender and included older adults with and without DM across a broad range of BMI, we compared reproducibility within and between these characteristics. Reproducibility did not significantly differ by DM, obesity status, race or study site, although we had small numbers in each subgroup, which limited statistical power for these analyses. While women had significantly lower reproducibility of NCV than men with stimulation at the fibular head, CV values were still in the range of high reproducibility (under 5%).
Few studies have examined the effects of increased adipose tissue on NC; however, the thickness of subcutaneous tissue has been noted to influence the amplitude and latency of surface EMG measures (Buschbacher, 1998, Farina et al., 2004). Buschbacher found that participants with a higher BMI had shorter peroneal and ulnar motor latencies and lower sensory and mixed nerve amplitudes (Buschbacher, 1998) and hypothesized that increased adipose tissue may provide better insulation for the axon, resulting in a faster impulse, while a thicker subcutaneous layer may diminish amplitude measures. It is reasonable to hypothesize that increased adipose tissue and risk factors for poor nerve function, such as advanced age and DM could affect reproducibility of NC measures. However, to our knowledge, no studies have compared the reproducibility of NC measures by these characteristics. In our study, we found a nonsignificant increasing trend between obesity categories and CMAP CVs, with the overweight and obese groups having higher CV values and lower reproducibility. No significant differences were found for peroneal motor NC reproducibility by DM or obesity status and these measures were moderately to highly reproducible in older adults with or without DM whether they were normal weight, overweight, or obese.
Our results are comparable to reproducibility studies on younger, less diverse study populations, which found ICCs for peroneal motor NCV between 0.52 and 0.89 (Bird, Brown, 2006, Dyck, Norell, 2007, Herrera, Camargo, 2009). Our sample had overall ICCs for NCV ranging from 0.75–0.90. This study supports that clinical research staff can be trained to measure NC with high test-retest reliability. These findings have positive implications for the accurate assessment of peripheral nerve function in the elderly using peroneal motor NC testing in future epidemiologic studies. We provide evidence that these measures are sufficiently reproducible and sensitive for detecting subclinical decline over short durations for clinical trials in the elderly. We used multiple statistical methods to thoroughly test various components of intraobserver test-retest reliability. Low CVs illustrated small dispersion between the two measures while accounting for the magnitude of the measures’ values. Moderate to high ICC values showed that the majority of variability could be attributed to differences between individuals rather than measurement variability within individuals. Bland Altman plots showed no systematic variation between the repeated tests.
It is very likely that some of the abnormal waveform morphology were due to poor or absent nerve responses common with increasing age (Rivner, Swift, 2001). Rivner and colleagues found that an absent nerve response occurred 6.67% of the time in participants from 70–79 years of age and 25% of the time in those from 80–89 years, although the number of participants in this age group was small. We were not able to classify participants with abnormal waveform morphology as having absent nerve responses, since waveforms were reviewed by a neurologist after the testing. Approximately 8% of measures in this study had abnormal waveform morphology. This percentage is reasonable, given the advanced age of this study population. Moreover, using Kappa coefficients, we found good to very good agreement between primary and reproduced NC measures based on waveform morphology status. Future NC studies in older adults should take into account the likelihood of absent responses in a population of this advanced age.
Our study also had several limitations. To duplicate test conditions and isolate participant variation, the same examiner performed both examinations, so we were not able to assess interexaminer variation. The effects of interexaminer variability on reproducibility of NC measures in older adults should be investigated in future studies. Measures included in the 2007–2008 clinic visits were designed to assess long term change in peripheral nerves and only motor nerve conduction had been previously measured. Due to time limitations within the study visit, our NC measures were limited to the peroneal motor nerve because of its superficial location, easy accessibility, and association with lower extremity physical function limitations and impairments (Resnick, Stansberry, 2002, Resnick, Vinik, 2000, Strotmeyer, de Rekeneire, 2008). Additionally, due to known issues in obtaining sural nerve responses at advanced age, we did not include sural NC measures in our initial study since we anticipated an unacceptable level of missing data. Future studies should similarly examine the reproducibility of NC measures on other nerves in older adults. Participants with DM in our study may have been healthier than those with DM in the general population. However, the rates of peripheral neuropathy in both the NonDM and DM group were similar to (Gregg et al., 2004) or exceeded rates found in other studies of older adults (Baldereschi, Inzitari, 2007). It is possible that participants in this reproducibility study were healthier than those in the general Health ABC study, causing selection bias. To assess this, we compared DM status, DM duration, hemoglobin A1C, possible peripheral neuropathy, and all NC measures in those that participated in the reproducibility study with participants from the general study (results not shown). Participants in the reproducibility study had higher CMAP amplitudes (ankle: 3.5 vs. 2.9 mV; popliteal fossa: 3.0 vs. 2.5 mV; fibular head: 3.1 vs. 2.6 mV; all p<0.05), but did not differ by any other characteristic, suggesting that this group was only minimally different with respect to measures of peripheral nerve function and disease status.
Peroneal motor NC testing is a reproducible technique for measuring nerve function in diverse older adults with and without DM, across a wide range of BMIs. Reproducible NC measurements are critical for clinical diagnoses of peripheral nerve dysfunction, examining longitudinal change in motor NC, and evaluating interventions to prevent or treat peripheral neuropathy. Given the current diabetes epidemic combined with the high incidence and prevalence (Baldereschi, Inzitari, 2007, Gregg, Sorlie, 2004) of poor peripheral nerve function in the elderly and their potential implications, these findings are particularly important for assessing motor nerve conduction during the course of potential preventive and treatment strategies.
Highlights.
Motor nerve conduction reproducibility is poorly established in very old adults, despite a high burden of sensorimotor decline and overt neuropathy.
Moderate to high intraobserver reliability exists for peroneal motor nerve conduction in very old, racially diverse men and women.
The results have critical implications for diagnosing motor nerve dysfunction, measuring age-related change, and evaluating interventions to prevent or treat neuropathy.
Acknowledgments
This research was supported in part by the Intramural Research Program of the NIH, National Institute on Aging; Ward RE, NIA Aging Training Grant: T32-AG-000181; Strotmeyer ES, NIH/NIA R01-AG 028050-01; Health ABC, NIH/NIA N01-AG-6-2101, N01-AG-6-2103, and N01-AG-6-2106; Ward RE, K. Leroy Irvis Fellowship, University of Pittsburgh; University of Pittsburgh Claude D. Pepper Center Older Americans Independence Center, P30-AG024827. We would like to acknowledge Chris Taylor, PhD and Mei Yang, MS for their work in data management, Amy Schorr for data collection (CT, MY and AS from the Department of Epidemiology, University of Pittsburgh), and Health ABC participants, staff and investigators.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altman DG. Practical statistics for medical research. London: Chapman and Hall; 1991. [Google Scholar]
- American Diabetes Association. Standards of medical care in diabetes--2012. Diabetes Care. 2012;35 (Suppl 1):S11–63. doi: 10.2337/dc12-s011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldereschi M, Inzitari M, Di Carlo A, Farchi G, Scafato E, Inzitari D. Epidemiology of distal symmetrical neuropathies in the Italian elderly. Neurology. 2007;68:1460–7. doi: 10.1212/01.wnl.0000260606.36443.29. [DOI] [PubMed] [Google Scholar]
- Bird SJ, Brown MJ, Spino C, Watling S, Foyt HL. Value of repeated measures of nerve conduction and quantitative sensory testing in a diabetic neuropathy trial. Muscle Nerve. 2006;34:214–24. doi: 10.1002/mus.20577. [DOI] [PubMed] [Google Scholar]
- Bouche P, Cattelin F, Saint-Jean O, Leger JM, Queslati S, Guez D, et al. Clinical and electrophysiological study of the peripheral nervous system in the elderly. J Neurol. 1993;240:263–8. doi: 10.1007/BF00838158. [DOI] [PubMed] [Google Scholar]
- Brown MJ, Bird SJ, Watling S, Kaleta H, Hayes L, Eckert S, et al. Natural progression of diabetic peripheral neuropathy in the Zenarestat study population. Diabetes Care. 2004;27:1153–9. doi: 10.2337/diacare.27.5.1153. [DOI] [PubMed] [Google Scholar]
- Buschbacher RM. Body mass index effect on common nerve conduction study measurements. Muscle Nerve. 1998;21:1398–404. doi: 10.1002/(sici)1097-4598(199811)21:11<1398::aid-mus6>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Buschbacher RM. Peroneal nerve motor conduction to the extensor digitorum brevis. Am J Phys Med Rehabil. 1999;78:S26–31. doi: 10.1097/00002060-199911001-00006. [DOI] [PubMed] [Google Scholar]
- Cauley JA, Blackwell T, Zmuda JM, Fullman RL, Ensrud KE, Stone KL, et al. Correlates of trabecular and cortical volumetric bone mineral density at the femoral neck and lumbar spine: the osteoporotic fractures in men study (MrOS) J Bone Miner Res. 2010;25:1958–71. doi: 10.1002/jbmr.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh PR, Derr JA, Ulbrecht JS, Maser RE, Orchard TJ. Problems with gait and posture in neuropathic patients with insulin-dependent diabetes mellitus. Diabet Med. 1992;9:469–74. doi: 10.1111/j.1464-5491.1992.tb01819.x. [DOI] [PubMed] [Google Scholar]
- De Rekeneire N, Resnick HE, Schwartz AV, Shorr RI, Kuller LH, Simonsick EM, et al. Diabetes is associated with subclinical functional limitation in nondisabled older individuals: the Health, Aging, and Body Composition study. Diabetes Care. 2003;26:3257–63. doi: 10.2337/diacare.26.12.3257. [DOI] [PubMed] [Google Scholar]
- Dyck PJ, Davies JL, Litchy WJ, O’Brien PC. Longitudinal assessment of diabetic polyneuropathy using a composite score in the Rochester Diabetic Neuropathy Study cohort. Neurology. 1997a;49:229–39. doi: 10.1212/wnl.49.1.229. [DOI] [PubMed] [Google Scholar]
- Dyck PJ, Litchy WJ, Daube JR, Harper CM, Davies J, O’Brien PC. Individual attributes versus composite scores of nerve conduction abnormality: sensitivity, reproducibility, and concordance with impairment. Muscle Nerve. 2003;27:202–10. doi: 10.1002/mus.10320. [DOI] [PubMed] [Google Scholar]
- Dyck PJ, Melton LJ, 3rd, O’Brien PC, Service FJ. Approaches to improve epidemiological studies of diabetic neuropathy: insights from the Rochester Diabetic Neuropathy Study. Diabetes. 1997b;46 (Suppl 2):S5–8. doi: 10.2337/diab.46.2.s5. [DOI] [PubMed] [Google Scholar]
- Dyck PJ, Norell JE, Tritschler H, Schuette K, Samigullin R, Ziegler D, et al. Challenges in design of multicenter trials: end points assessed longitudinally for change and monotonicity. Diabetes Care. 2007;30:2619–25. doi: 10.2337/dc06-2479. [DOI] [PubMed] [Google Scholar]
- Farina D, Merletti R, Enoka RM. The extraction of neural strategies from the surface EMG. J Appl Physiol. 2004;96:1486–95. doi: 10.1152/japplphysiol.01070.2003. [DOI] [PubMed] [Google Scholar]
- Ferrucci L, Bandinelli S, Cavazzini C, Lauretani F, Corsi A, Bartali B, et al. Neurological examination findings to predict limitations in mobility and falls in older persons without a history of neurological disease. Am J Med. 2004;116:807–15. doi: 10.1016/j.amjmed.2004.01.010. [DOI] [PubMed] [Google Scholar]
- George J, Twomey JA. Causes of polyneuropathy in the elderly. Age Ageing. 1986;15:247–9. doi: 10.1093/ageing/15.4.247. [DOI] [PubMed] [Google Scholar]
- Gregg EW, Sorlie P, Paulose-Ram R, Gu Q, Eberhardt MS, Wolz M, et al. Prevalence of lower-extremity disease in the US adult population >=40 years of age with and without diabetes: 1999–2000 national health and nutrition examination survey. Diabetes Care. 2004;27:1591–7. doi: 10.2337/diacare.27.7.1591. [DOI] [PubMed] [Google Scholar]
- Herrera E, Camargo DM, Delgado DC, Salvini TF. Reliability of superficial peroneal, sural, and medial plantar nerve conduction studies: analysis of statistical methods. J Clin Neurophysiol. 2009;26:372–9. doi: 10.1097/WNP.0b013e3181baaaea. [DOI] [PubMed] [Google Scholar]
- Huang CY. Peripheral neuropathy in the elderly: a clinical and electrophysiologic study. J Am Geriatr Soc. 1981;29:49–54. doi: 10.1111/j.1532-5415.1981.tb01226.x. [DOI] [PubMed] [Google Scholar]
- Lauretani F, Bandinelli S, Bartali B, Di Iorio A, Giacomini V, Corsi AM, et al. Axonal degeneration affects muscle density in older men and women. Neurobiol Aging. 2006;27:1145–54. doi: 10.1016/j.neurobiolaging.2005.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loseth S, Nebuchennykh M, Stalberg E, Mellgren SI. Medial plantar nerve conduction studies in healthy controls and diabetics. Clin Neurophysiol. 2007;118:1155–61. doi: 10.1016/j.clinph.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Partanen J, Niskanen L, Lehtinen J, Mervaala E, Siitonen O, Uusitupa M. Natural history of peripheral neuropathy in patients with non-insulin-dependent diabetes mellitus. N Engl J Med. 1995;333:89–94. doi: 10.1056/NEJM199507133330203. [DOI] [PubMed] [Google Scholar]
- Perkins BA, Olaleye D, Zinman B, Bril V. Simple screening tests for peripheral neuropathy in the diabetes clinic. Diabetes Care. 2001;24:250–6. doi: 10.2337/diacare.24.2.250. [DOI] [PubMed] [Google Scholar]
- Resnick HE, Stansberry KB, Harris TB, Tirivedi M, Smith K, Morgan P, et al. Diabetes, peripheral neuropathy, and old age disability. Muscle Nerve. 2002;25:43–50. doi: 10.1002/mus.1217. [DOI] [PubMed] [Google Scholar]
- Resnick HE, Vinik AI, Heimovitz HK, Brancati FL, Guralnik JM. Age 85+ years accelerates large-fiber peripheral nerve dysfunction and diabetes contributes even in the oldest-old: the Women’s Health and Aging Study. J Gerontol A Biol Sci Med Sci. 2001;56:M25–31. doi: 10.1093/gerona/56.1.m25. [DOI] [PubMed] [Google Scholar]
- Resnick HE, Vinik AI, Schwartz AV, Leveille SG, Brancati FL, Balfour J, et al. Independent effects of peripheral nerve dysfunction on lower-extremity physical function in old age: the Women’s Health and Aging Study. Diabetes Care. 2000;23:1642–7. doi: 10.2337/diacare.23.11.1642. [DOI] [PubMed] [Google Scholar]
- Richardson JK, Ching C, Hurvitz EA. The relationship between electromyographically documented peripheral neuropathy and falls. J Am Geriatr Soc. 1992;40:1008–12. doi: 10.1111/j.1532-5415.1992.tb04477.x. [DOI] [PubMed] [Google Scholar]
- Rivner MH, Swift TR, Malik K. Influence of age and height on nerve conduction. Muscle Nerve. 2001;24:1134–41. doi: 10.1002/mus.1124. [DOI] [PubMed] [Google Scholar]
- Runge M, Hunter G. Determinants of musculoskeletal frailty and the risk of falls in old age. J Musculoskelet Neuronal Interact. 2006;6:167–73. [PubMed] [Google Scholar]
- Ryerson B, Tierney EF, Thompson TJ, Engelgau MM, Wang J, Gregg EW, et al. Excess physical limitations among adults with diabetes in the U.S. population, 1997–1999. Diabetes Care. 2003;26:206–10. doi: 10.2337/diacare.26.1.206. [DOI] [PubMed] [Google Scholar]
- Sosenko JM, Kato M, Soto R, Bild DE. A prospective study of sensory function in patients with type 2 diabetes. Diabet Med. 1993;10:110–4. doi: 10.1111/j.1464-5491.1993.tb00026.x. [DOI] [PubMed] [Google Scholar]
- Sosenko JM, Kato M, Soto R, Goldberg RB. Sensory function at diagnosis and in early stages of NIDDM in patients detected through screening. Diabetes Care. 1992;15:847–52. doi: 10.2337/diacare.15.7.847. [DOI] [PubMed] [Google Scholar]
- Stetson DS, Albers JW, Silverstein BA, Wolfe RA. Effects of age, sex, and anthropometric factors on nerve conduction measures. Muscle Nerve. 1992;15:1095–104. doi: 10.1002/mus.880151007. [DOI] [PubMed] [Google Scholar]
- Strotmeyer ES, Cauley JA, Schwartz AV, de Rekeneire N, Resnick HE, Zmuda JM, et al. Reduced peripheral nerve function is related to lower hip BMD and calcaneal QUS in older white and black adults: the Health, Aging, and Body Composition Study. J Bone Miner Res. 2006;21:1803–10. doi: 10.1359/jbmr.060725. [DOI] [PubMed] [Google Scholar]
- Strotmeyer ES, de Rekeneire N, Schwartz AV, Faulkner KA, Resnick HE, Goodpaster BH, et al. The relationship of reduced peripheral nerve function and diabetes with physical performance in older white and black adults: the Health, Aging, and Body Composition (Health ABC) study. Diabetes Care. 2008;31:1767–72. doi: 10.2337/dc08-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesfaye S, Boulton AJ, Dyck PJ, Freeman R, Horowitz M, Kempler P, et al. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010;33:2285–93. doi: 10.2337/dc10-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesfaye S, Tandan R, Bastyr EJ, 3rd, Kles KA, Skljarevski V, Price KL. Factors that impact symptomatic diabetic peripheral neuropathy in placebo-administered patients from two 1-year clinical trials. Diabetes Care. 2007;30:2626–32. doi: 10.2337/dc07-0608. [DOI] [PubMed] [Google Scholar]
- Verghese J, Bieri PL, Gellido C, Schaumburg HH, Herskovitz S. Peripheral neuropathy in young-old and old-old patients. Muscle Nerve. 2001;24:1476–81. doi: 10.1002/mus.1171. [DOI] [PubMed] [Google Scholar]
- Vinik AI, Strotmeyer ES, Nakave AA, Patel CV. Diabetic neuropathy in older adults. Clin Geriatr Med. 2008;24:407–35. v. doi: 10.1016/j.cger.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Writing Team for Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. Effect of intensive therapy on the microvascular complications of type 1 diabetes mellitus. JAMA. 2002;287:2563–9. doi: 10.1001/jama.287.19.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]