Abstract
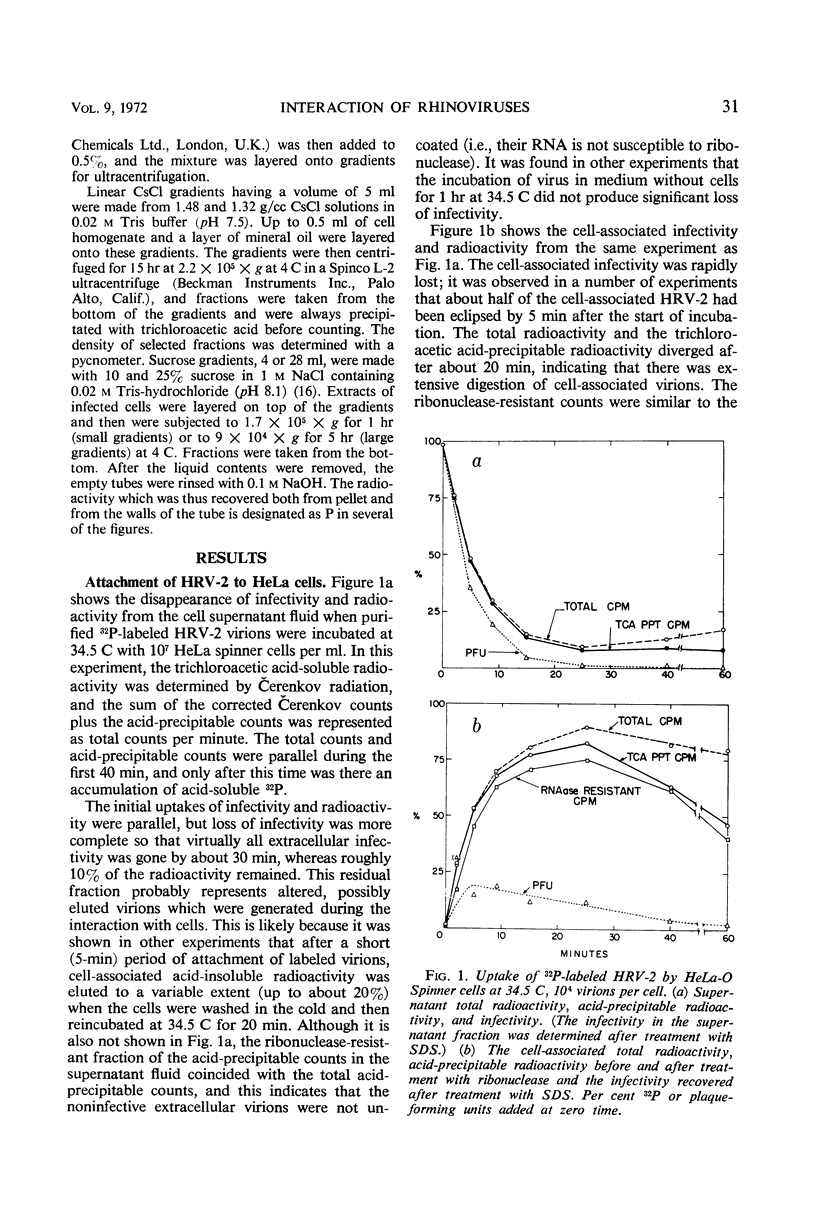
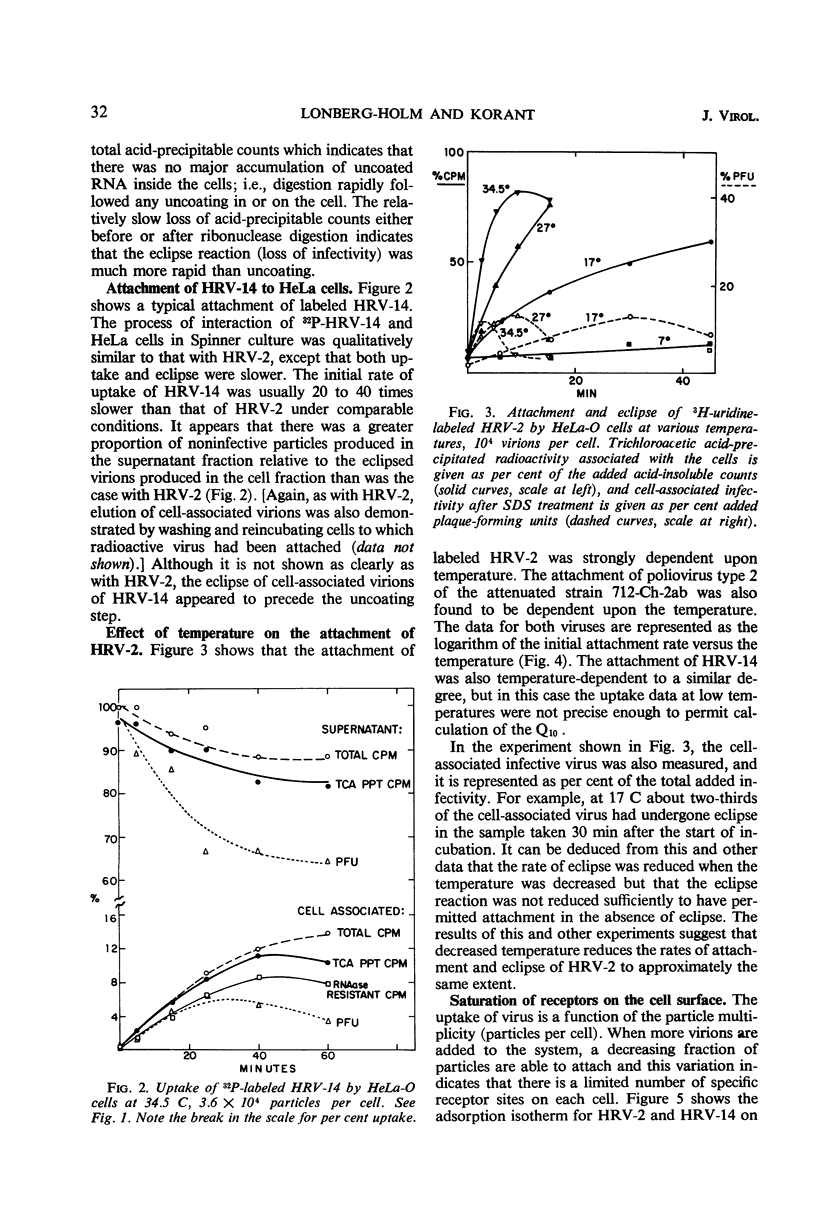
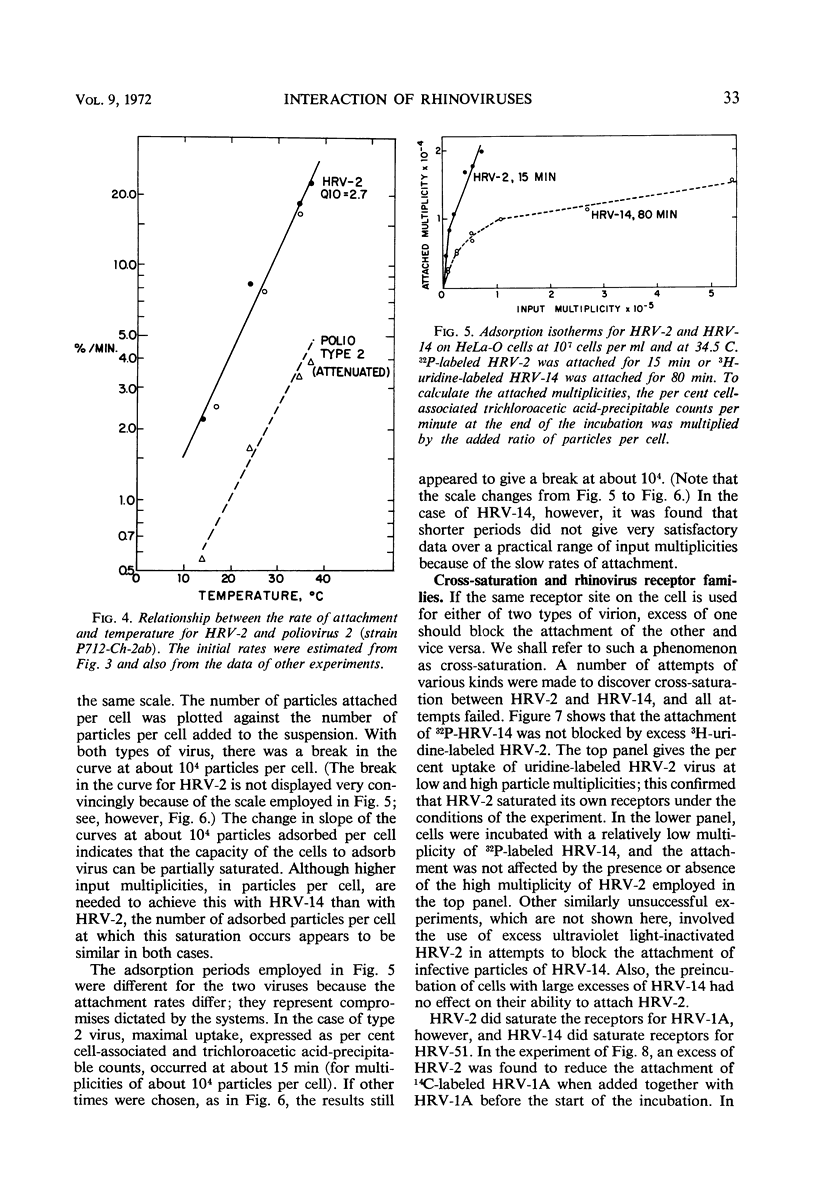
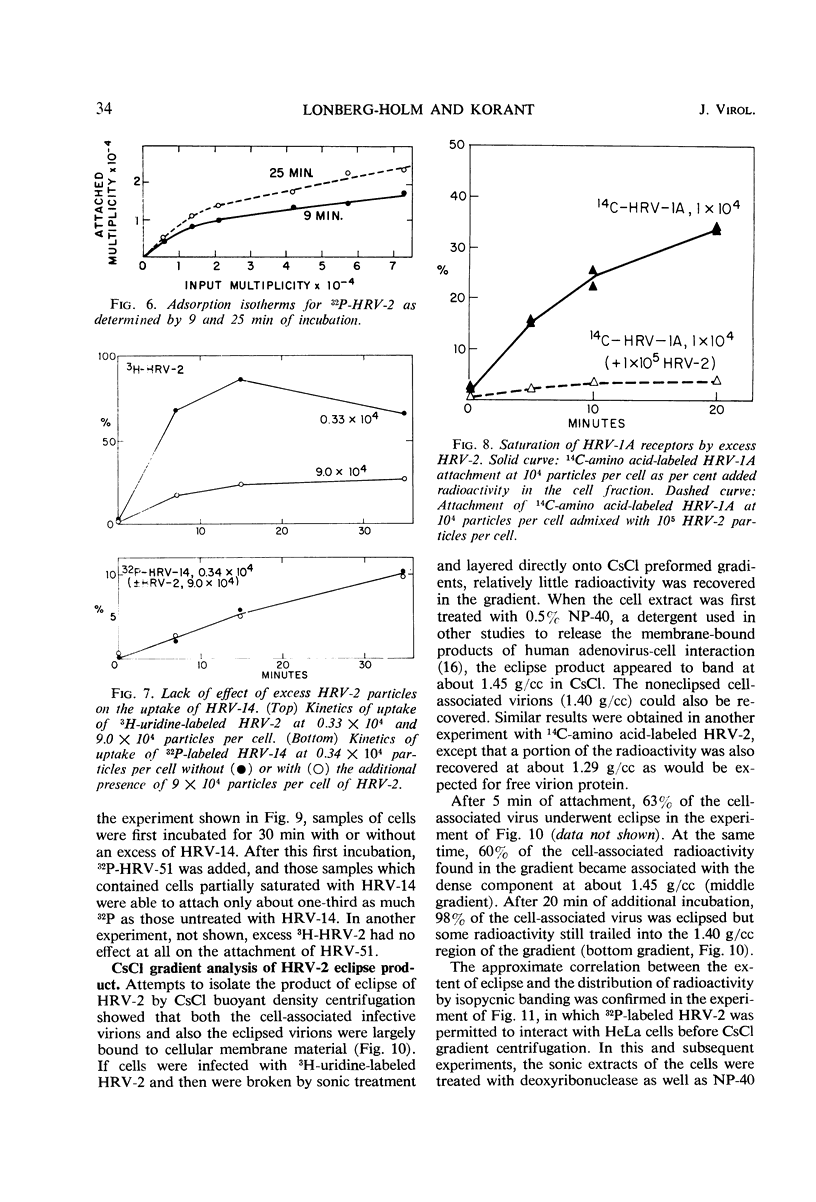
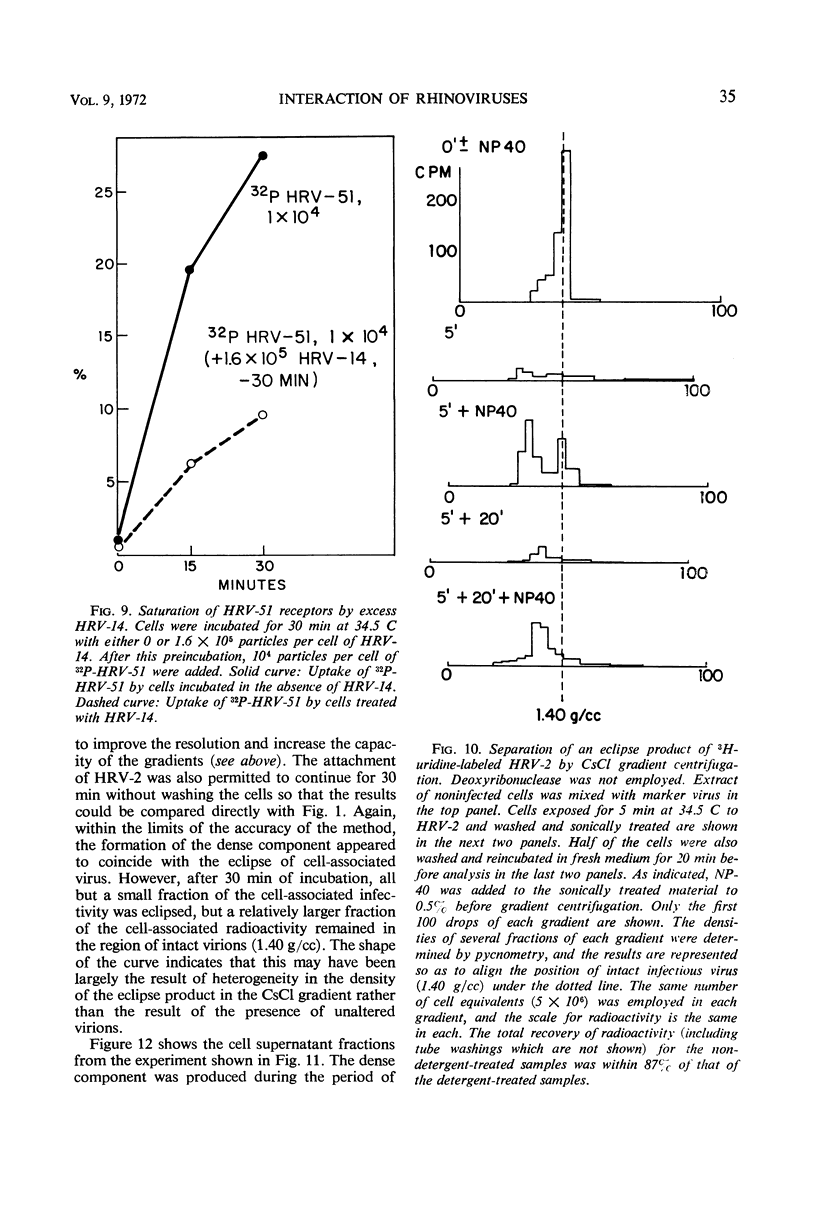
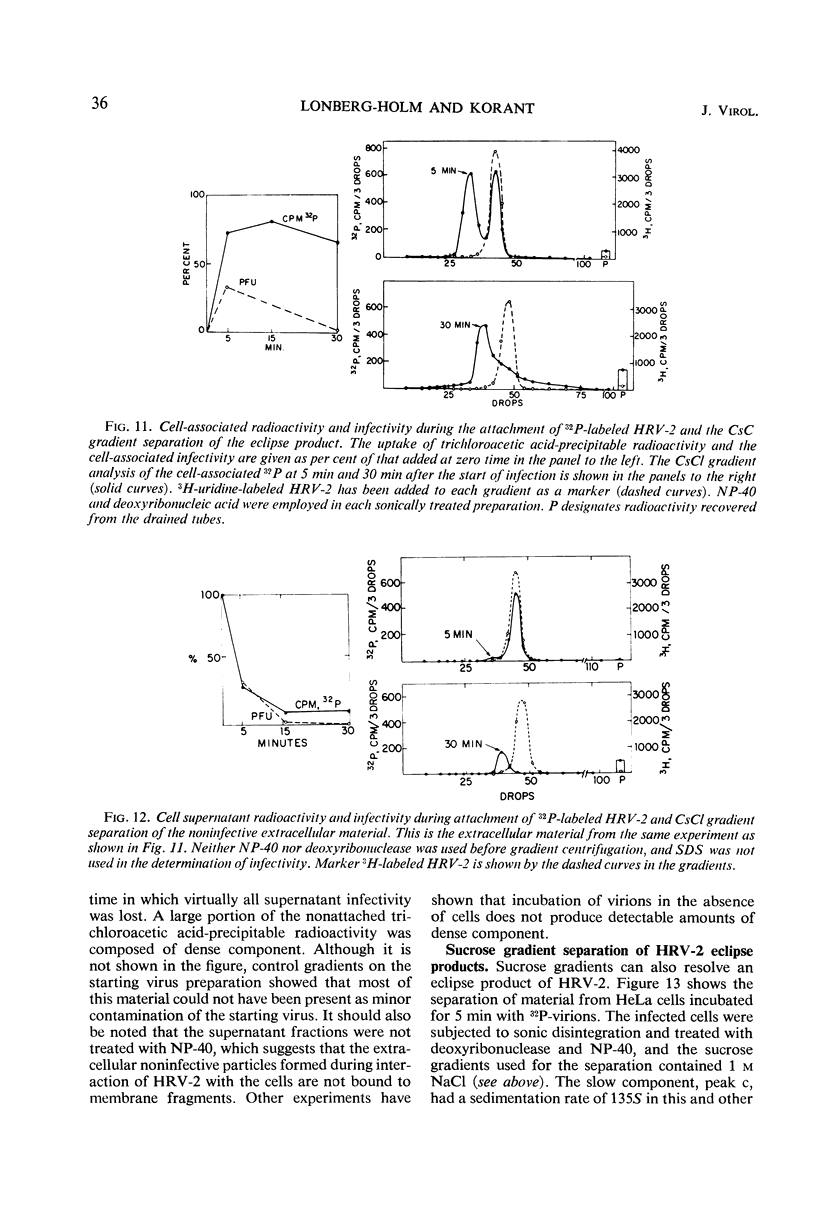
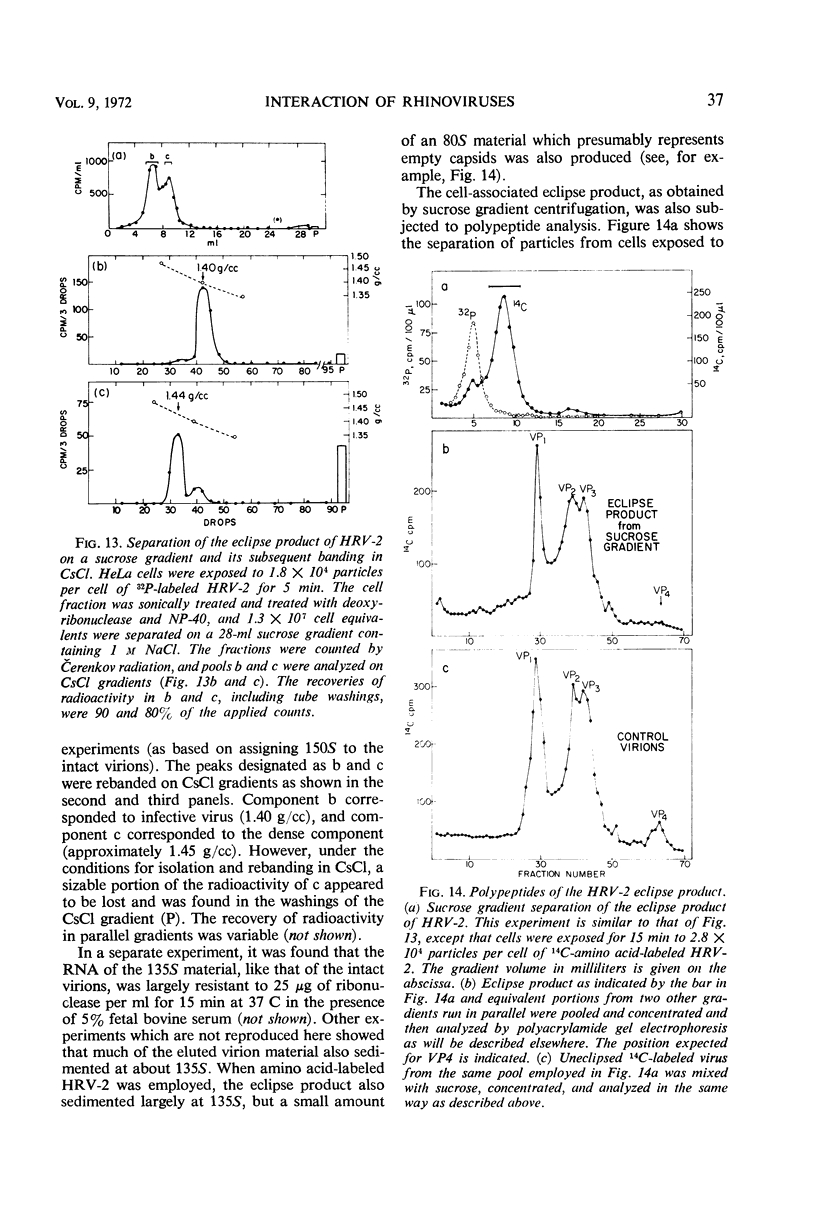
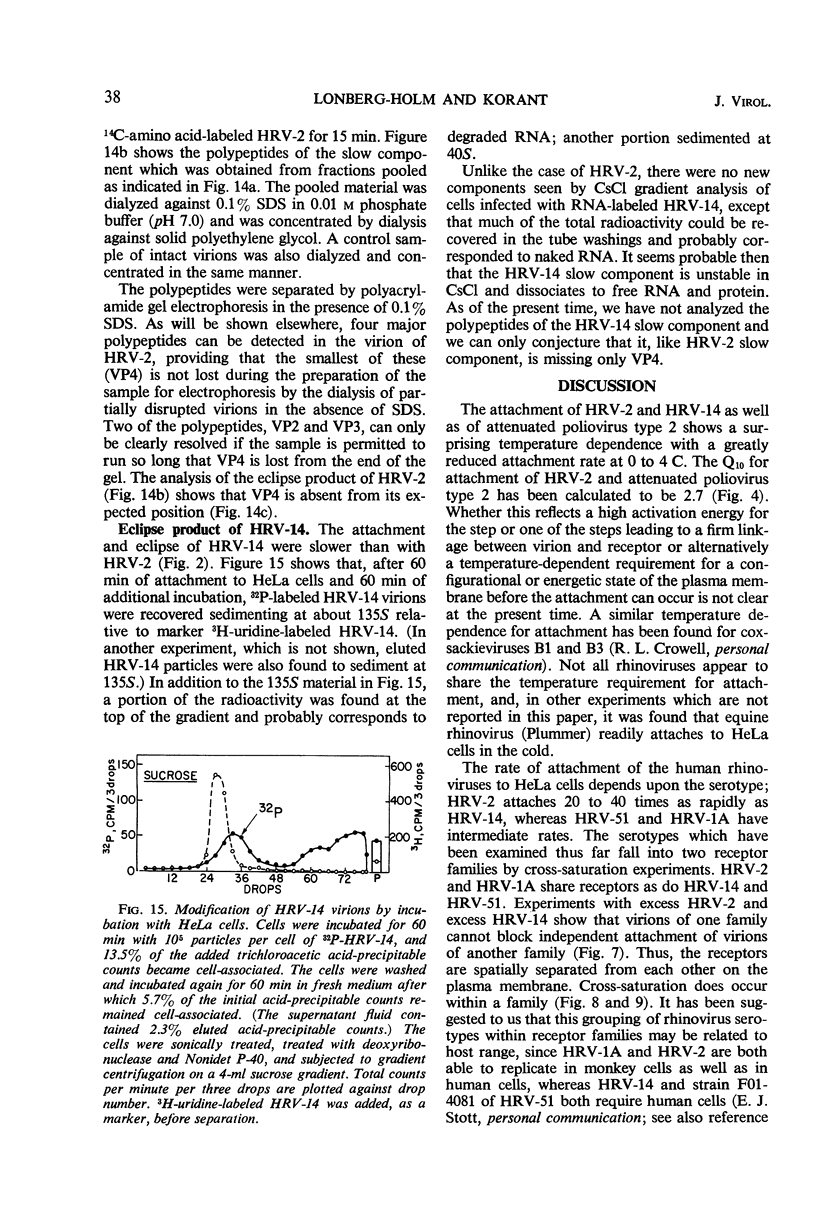
The rate of attachment of type 2 virions to suspensions of HeLa cells is much greater than that of type 14, but the number of receptor sites per cell is similar for each type. The receptor sites may be partly saturated with excess virions; attachment is greatly reduced after about 104 particles have been taken up per cell. A lack of saturation of type 14 receptors by excess type 2 indicates that their receptor sites are separate on the cell surface. Excess of type 2 blocks attachment of type 1A, however, and excess of type 14 blocks type 51. Attachment of the human rhinoviruses is temperature-dependent with a Q10 of 2.7. The eclipse reaction is also temperature-dependent. At 34.5 C, the irreversible eclipse of cell-associated rhinovirus type 2 requires only a few minutes, whereas the rate of eclipse of cell-associated type 14 is considerably slower. The eclipse product of type 2 rhinovirus has been recovered from infected cells. It sediments at about 90% of the rate of the infective virions and is missing virus polypeptide 4 (the smallest of the capsid polypeptides). Upon being subjected to CsCl gradient centrifugation, virus polypeptide 2 is also lost but the product still contains ribonucleic acid and bands at about 1.45 g/cc.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BACHTOLD J. G., BUBEL H. C., GEBHARDT L. P. The primary interaction of poliomyelitis virus with host cells of tissue culture origin. Virology. 1957 Dec;4(3):582–589. doi: 10.1016/0042-6822(57)90087-9. [DOI] [PubMed] [Google Scholar]
- BROWN F., CARTWRIGHT B., STEWART D. L. Further studies on the infection of pig-kidney cells by foot-and-mouth disease virus. Biochim Biophys Acta. 1962 May 14;55:768–774. doi: 10.1016/0006-3002(62)90855-7. [DOI] [PubMed] [Google Scholar]
- Breindl M. The structure of heated poliovirus particles. J Gen Virol. 1971 Jun;11(3):147–156. doi: 10.1099/0022-1317-11-3-147. [DOI] [PubMed] [Google Scholar]
- Buck C. A., Granger G. A., Taylor M. W., Holland J. J. Efficient, inefficient, and abortive infection of different mammalian cells by small RNA viruses. Virology. 1967 Sep;33(1):36–46. doi: 10.1016/0042-6822(67)90091-8. [DOI] [PubMed] [Google Scholar]
- Crowell R. L., Philipson L. Specific alterations of coxsackievirus B3 eluted from HeLa cells. J Virol. 1971 Oct;8(4):509–515. doi: 10.1128/jvi.8.4.509-515.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- FENWICK M. L., COOPER P. D. Early interactions between poliovirus and ERK cells: some observations on the nature and significance of the rejected particles. Virology. 1962 Oct;18:212–223. doi: 10.1016/0042-6822(62)90007-7. [DOI] [PubMed] [Google Scholar]
- HOLLAND J. J. Irreversible eclipse of poliovirus by HeLa cells. Virology. 1962 Feb;16:163–176. doi: 10.1016/0042-6822(62)90292-1. [DOI] [PubMed] [Google Scholar]
- HOLLAND J. J., McLAREN L. C. The mammalian cell-virus relationship. II. Adsorption, reception, and eclipse of poliovirus by HeLa cells. J Exp Med. 1959 May 1;109(5):487–504. doi: 10.1084/jem.109.5.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haff R. F., Wohlsen B., Force E. E., Stewart R. C. Growth characteristics of two rhinovirus strains in WI-26 and monkey kidney cells. J Bacteriol. 1966 Jun;91(6):2339–2342. doi: 10.1128/jb.91.6.2339-2342.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall L., Rueckert R. R. Infection of mouse fibroblasts by cardioviruses: premature uncoating and its prevention by elevated pH and magnesium chloride. Virology. 1971 Jan;43(1):152–165. doi: 10.1016/0042-6822(71)90233-9. [DOI] [PubMed] [Google Scholar]
- JOKLIK W. K., DARNELL J. E., Jr The adsorption and early fate of purified poliovirus in HeLa cells. Virology. 1961 Apr;13:439–447. doi: 10.1016/0042-6822(61)90275-6. [DOI] [PubMed] [Google Scholar]
- Lonberg-Holm K., Philipson L. Early events of virus-cell interaction in an adenovirus system. J Virol. 1969 Oct;4(4):323–338. doi: 10.1128/jvi.4.4.323-338.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak T. W., O'Callaghan D. J., Colter J. S. Studies of the early events of the replicative cycle of three variants of Mengo encephalomyelitis virus in mouse fibroblast cells. Virology. 1970 Dec;42(4):1087–1096. doi: 10.1016/0042-6822(70)90356-9. [DOI] [PubMed] [Google Scholar]
- McGregor S., Mayor H. D. Internal components released from rhinovirus and poliovirus by heat. J Gen Virol. 1971 Feb;10(2):203–207. doi: 10.1099/0022-1317-10-2-203. [DOI] [PubMed] [Google Scholar]
- McLAREN L. C., HOLLAND J. J., SYVERTON J. T. The mammalian cell-virus relationship. I. Attachment of poliovirus to cultivated cells of primate and non-primate origin. J Exp Med. 1959 May 1;109(5):475–485. doi: 10.1084/jem.109.5.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PHILIPSON L. THE EARLY INTERACTION OF ANIMAL VIRUSES AND CELLS. Prog Med Virol. 1963;5:43–78. [PubMed] [Google Scholar]
- Philipson L., Lonberg-Holm K., Pettersson U. Virus-receptor interaction in an adenovirus system. J Virol. 1968 Oct;2(10):1064–1075. doi: 10.1128/jvi.2.10.1064-1075.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stott E. J., Heath G. F. Factors affecting the growth of Rhinovirus 2 in suspension cultures of L132 cells. J Gen Virol. 1970 Jan;6(1):15–24. doi: 10.1099/0022-1317-6-1-15. [DOI] [PubMed] [Google Scholar]
- Thomas D. C., Conant R. M., Hamparian V. V. Rhinovirus replication in suspension cultures of HeLa cells. Proc Soc Exp Biol Med. 1970 Jan;133(1):62–65. doi: 10.3181/00379727-133-34407. [DOI] [PubMed] [Google Scholar]
- VOGT M., DULBECCO R. Properties of a HeLa cell culture with increased resistance to poliomyelitis virus. Virology. 1958 Jun;5(3):425–434. doi: 10.1016/0042-6822(58)90037-0. [DOI] [PubMed] [Google Scholar]
- Wall R., Taylor M. W. Host-dependent restriction of mengovirus replication. J Virol. 1969 Nov;4(5):681–687. doi: 10.1128/jvi.4.5.681-687.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall R., Taylor M. W. Mengovirus RNA synthesis in productive and restrictive cell lines. Virology. 1970 Sep;42(1):78–86. doi: 10.1016/0042-6822(70)90240-0. [DOI] [PubMed] [Google Scholar]
- ZAJAC I., CROWELL R. L. EFFECT OF ENZYMES ON THE INTERACTION OF ENTEROVIRUSES WITH LIVING HELA CELLS. J Bacteriol. 1965 Mar;89:574–582. doi: 10.1128/jb.89.3.574-582.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]



