Abstract
We have recently reported that selective cannabinoid 2 (CB2) receptor agonists upregulate 5-HT2A receptors by enhancing ERK1/2 signaling in prefrontal cortex (PFCx). Increased activity of cortical 5-HT2A receptors has been associated with several neuropsychiatric disorders such as anxiety and schizophrenia. Here we examine the mechanisms involved in this enhanced ERK1/2 activation in rat PFCx and in a neuronal cell model. Sprague-Dawley rats treated with a non-selective cannabinoid agonist (CP55940, 50 μg/kg, 7 days, i.p.) showed enhanced co-immunoprecipitation of β-Arrestin 2 and ERK1/2, enhanced pERK protein levels, and enhanced expression of β-Arrestin 2 mRNA and protein levels in PFCx. In a neuronal cell line, we found that selective CB2 receptor agonists upregulate β-Arrestin 2, an effect that was prevented by selective CB2 receptor antagonist JTE-907 and CB2 shRNA lentiviral particles. Additionally, inhibition of clathrin-mediated endocytosis, ERK1/2, and the AP-1 transcription factor also prevented the cannabinoid receptor-induced upregulation of β-Arrestin 2. Our results suggest that sustained activation of CB2 receptors would enhance β-Arrestin 2 expression possibly contributing to its increased interaction with ERK1/2 thereby driving the upregulation of 5-HT2A receptors. The CB2 receptor-mediated upregulation of β-Arrestin 2 would be mediated, at least in part, by an ERK1/2-dependent activation of AP-1. These data could provide the rationale for some of the adverse effects associated with repeated cannabinoid exposure and shed light on some CB2 receptor agonists that could represent an alternative therapeutic because of their minimal effect on serotonergic neurotransmission.
Keywords: Cannabinoid receptors, 5-HT2A receptor, ERK1/2, β-Arrestin 2, prefrontal cortex
1. Introduction
Cannabinoid receptor agonists are being shown to have wide therapeutic applications in the treatment of conditions such as stroke, neuropathic pain, neurodegenerative diseases, and cocaine addiction [1–4]. However, recent and independent clinical studies provide strong evidence indicating that sustained use of nonselective cannabinoid agonists may precipitate the onset of mental disorders associated with dysfunction of serotonin 2A (5-HT2A) receptor neurotransmission in prefrontal cortex (PFCx), such as schizophrenia, psychosis and anxiety [5–8]. Although the precise mechanism by which repeated cannabinoid exposure may precipitate these disorders is unknown, we have recently provided evidence that cannabinoid agonists induce a strong upregulation and increase activity of 5-HT2A receptors in vivo and in vitro [9;10].
Cannabinoid agonists can produce their physiological effects through the activation of two G-protein coupled cannabinoid receptors in the brain, CB1 and CB2 receptors [11;12]. CB1 and CB2 receptors bind endocannabinoids, synthetic cannabinoids, and cannabinoids found in nature (such as Cannabis sativa) with high affinity [11;12]. Although only CB1 receptors were initially identified in the brain [13], later studies have identified CB2 receptors in several brain areas such as PFCx, hippocampus, amygdala, substantia nigra, and cerebellum [14;15], triggering a reevaluation of the possible roles that CB2 receptors may play in the brain. These cannabinoid receptors couple to Gi/o class of G-proteins and can activate ERK1/2 signaling in a either G-protein or β-Arrestin dependent pathway [12;16]. While G-protein-mediated activation of ERK1/2 is transient and peaks within 2–5 minutes [17;18], β-Arrestins can form a scaffolding complex with Raf-1, MEK, and ERK1/2 which can regulate the long-term activation of ERK1/2 after β-Arrestin mediated internalization of the G-protein coupled receptor or GPCR [17–19].
We recently reported that 5-HT2A receptors are upregulated by repeated exposures to cannabinoid agonists through a mechanism that would involve CB2 receptor-mediated activation of ERK1/2 signaling, and that is independent of CB1 receptor activation [9;10]. Moreover, we presented experimental evidence that sustained treatment with a non-selective cannabinoid agonist (CP55940) or selective CB2 receptor agonists (JWH-133 or GP1a) upregulate 5-HT2A receptors in a neuronal cell line [10], an effect that was not replicated by selective CB1 agonists [10]. The CB2 receptor is a class A GPCR which means it would preferentially interact with β-Arrestin 2 to form a scaffolding complex with ERK1/2 [20]. Accordingly, we also reported that the cannabinoid receptor agonist-induced upregulation of 5-HT2A receptors was prevented in cells stably transfected with either CB2 or β-Arrestin 2 shRNA lentiviral particles [10].
Here we examined mechanisms which could contribute to the CB2 receptor- and ERK1/2- mediated enhanced activation of 5-HT2A receptors. We studied the involvement of selective CB1 and CB2 receptor agonists on the regulation of β-Arrestin 2 expression and the formation of a β-Arrestin 2 and ERK1/2 protein complex. Our results indicate that repeated exposure to cannabinoids enhance the protein interaction between β-Arrestin 2 and ERK1/2. Furthermore, cannabinoid agonists upregulated β-Arrestin 2 by a mechanism that would require internalization of CB2 receptors, activation of ERK1/2, and activation of the transcription factor AP-1. We also detected a strong CB2, but not CB1, receptor activation upregulation of β-Arrestin 2 in a neuronal cell line. We hypothesize that the data presented here could provide, at least in part, a molecular mechanism by which repeated exposure to cannabinoids might be relevant to some cognitive and mood disorders by upregulating and enhancing the activity of 5-HT2A receptors.
2. Materials and Methods
2.1 Drugs
(−)-cis-3-[2-Hydroxy-4-(1,1-dimethylheptyl)phenyl]-trans-4-(3-hydroxypropyl)cyclohexanol (CP55940), a CB1 and CB2 receptor agonist; (6aR,10aR)-3-(1,1-Dimethylbutyl)-6a,7,10,10a-tetrahydro-6,6,9-trimethyl-6H-dibenzo[b,d]pyran (JWH-133) a selective CB2 agonist; N-(Piperidin-1-yl)-1-(2,4-dichlorophenyl)-1,4-dihydro-6-methylindeno[1,2-c]pyrazole-3-carboxamide (GP1a) a highly selective CB2 receptor agonist; N-(2-Chloroethyl)-5Z,8Z,11Z,14Z-eicosatetraenamide (ACEA) a highly selective CB1 receptor agonist; 2-(2-Chlorophenyl)-3-(4-chlorophenyl)-7-(2,2-difluoropropyl)-6,7-dihydro-2H-pyrazolo[3,4-f][1,4]oxazepin-8(5H)-one (PF-514273), a selective CB1 receptor antagonist; N-(1,3-Benzodioxol-5-ylmethyl)-1,2-dihydro-7-methoxy-2-oxo-8-(pentyloxy)-3-quinolinecarboxamide (JTE-907) a selective CB2 receptor antagonist/inverse agonist; N-(Cyclopropylmethoxy)-3,4,5-trifluoro-2-[(4-iodo-2-methylphenyl)amino]-benzamide (PD198306) a potent and selective ERK1/2 inhibitor; and (E,E,Z,E)-3-Methyl-7-(4-methylphenyl)-9-(2,6,6-trimethyl-1-cyclohexen-1-yl)-2,4,6,8-nonatetraenoic acid (SR11302), an inhibitor of activating protein-1 transcription factor activity were purchased from Tocris (Ellisville, MO). Naphthol AS-E phosphate, a CREB inhibitor, was purchased from Sigma-Aldrich Inc. (St. Louis, MO). Naphthalen-1-yl-(1-butylindol-3-yl)methanone (JWH-073), a CB1/CB2 receptor agonist, was synthesized in the laboratory of Dr. Thomas Prisinzano as described previously [21].
2.2 Animal Experimental Protocol
Male Sprague-Dawley rats (225–275 g; Harlan Laboratories, Indianapolis, IN) were housed two per cage in a temperature-, humidity-, and light-controlled room (12 hr light/dark cycle, lights on 7:00 AM–7:00 PM). Food and water were available ad libitum. All procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals as approved by the University of Kansas Institutional Animal Care and Use Committee (IACUC).
After arrival, the rats were allowed to acclimate to their environment for at least 4 days prior to the start of the treatment period. Eight rats were randomly assigned to each group, cage mates were assigned to the same treatment group. The body weight of each rat was recorded every other day. All solutions were made fresh before administration and rats were injected with either vehicle (Tween-80/ethanol/saline (1:1:18); 1mL/kg, i.p.) or CP55940 (0.05 mg/kg, i.p.) once a day for 7 days. Rats were sacrificed by decapitation 48 h after the last CP55940 injection. The dose and time course for CP55940 were chosen based upon the literature that reported that similar doses induced increased anxiety-like behaviors [22;23] and upregulation of 5-HT2A receptors in rat PFCx [9]. In our preliminary experiments we also noticed that doses higher than 0.2 mg/kg prevent weight gain in rats after 2 days of CP55940 exposure. After sacrifice, brains were immediately removed and PFCx was dissected and frozen in dry ice.
2.3 Co-Immunoprecipitation
Co-immunoprecipitation (co-IP) was conducted with the Thermo Scientific Pierce co-IP kit following manufacturer’s protocol and as previously described [9]. The β-Arrestin 2 and ERK1/2 antibody was purchased from Santa Cruz, CA. Briefly, β-Arrestin 2 antibody was incubated with AminoLink Plus coupling resin for 2 hrs. This resin was incubated with pre-cleared PFCx lysate (300 μg) from either vehicle or CP55940 treated rats overnight. A negative control included a non-reactive resin that was incubated with β-Arrestin 2 antibody for 2 hrs and then pre-cleared PFCx lysate from either CP55940 or vehicle-treated rats overnight. After the overnight incubation, the resins were washed (3x) and the protein eluted using elution buffer. Samples were analyzed by Western blot using ERK1/2 antibody. The specificity of the β-Arrestin 2 or ERK1/2 antibody has been verified in the literature [10;24;25].
2.4 Western Blot
Membrane-associated fractions were isolated using the ProteoExtract™ Native Membrane Protein Extraction kit (Calbiochem, La Jolla, CA) and Nuclear-associated fractions were isolated using NE-PER ® Nuclear and Cytoplasmic Extraction Reagents (Thermo Scientific, IL). Expression of β-Arrestin 2, pERK, and ERK1/2 were determined by western blot analysis as previous described [26]. pERK antibody was purchased from Santa Cruz, CA. The specificity of the antibody has been verified in the literature [27]. Protein loading for each lane was verified using an anti-actin antibody (Santa Cruz Biotechnology, Inc.). Negative controls included either the omission of primary antibody or addition of preimmune rabbit immunoglobulins. Films were analyzed densitometrically with values calculated from the integrated optical density (IOD) of each band using Scion Image software (Scion Corporation, Frederick, MD, USA), as previously described [26;28]. All samples were standardized to controls and normalized to their respective actin levels.
2.5 CP55940-induced ERK1/2 activation in PFCx tissue
This assay was based upon a PLCβ assay that has been previously described [26]. PFCx tissue from vehicle or CP55940 treated rats was homogenized in homogenization buffer (1 mL/0.1 g of tissue) with protease inhibitor for 3 seconds. Samples were vortexed and centrifuged at 13,000 rpm at 4°C for 10 minutes. The supernatant was removed, 500 μL of homogenate buffer was added to the pellet, sampes were vortexed and centrifuged at 13,000 rpm at 4°C for 10 minutes. This wash process was repeated three times. Protein levels were measured and equalized. Vehicle and CP55940 samples were treated with 1 nM CP55940 and incubated for 15 minutes. Western blot analysis was utilized to measure pERK levels as previously described above.
2.6 Quantitative Real-Time PCR
These reactions were prepared using QuantiFast SYBR Green PCR Kit (Qiagen, Valencia, CA) and the ABI 7500 fast real time PCR system (Applied Biosystems, Foster City, CA) and then data was analyzed using the comparative cycle threshold (Ct) method as previously described [9]. The primers used in this manuscript were: 5-HT2A (F:5′-AACGGTCCATCCACAGAG-3′ and R:5′-AACAGGAAGAACACGATGC-3′), β-Arrestin 2 (F:5′-AGCACCGCGCAGTACAAGT-3′, 5′-R:CACGCTTCTCTCGGTTGTCA -3′) and GAPDH (F: 5′-TGGAGTCTACTGGCGTCTTCAC-3′ and R:5′-GGCATGGACTGTGGTCATGA-3′). These primers have been previously validated in the literature [9;10;29;30].
2.7 Cell Culture Protocol
CLU213 cells, a rat neuronal cell line that co-expresses 5-HT2A, D2, CB1 and CB2 receptors, were purchased from Cedarlane Laboratories (Burlington, NC). CLU213 were grown on 100-mm2 plates treated with polystyrene (Corning Incorporated, Corning, NY) and maintained in 5% CO2 at 37°C, in Dulbecco’s modified eagle medium (DMEM; Mediatech Inc, Manassas, VA) containing 10% fetal bovine serum (FBS; Thermo Scientific, Logan, UT).
2.7.1 Effect of Non-Selective and Selective CB1 and CB2 Receptor Agonists on β-Arrestin 2 mRNA and protein levels
CLU213 cells were incubated with either vehicle (ethanol 0.01% final concentration), CP55940 1 nM (CB1 and CB2 agonist, Ki: 0.58 nM and 0.68 nM for CB1 and CB2 receptors, respectively) [31]; JWH-133 30 nM (selective CB2 agonist, Ki: 3.4 nM and 677 nM for CB2 and CB1 receptors, respectively) [32], GP1a 1 nM (highly selective CB2 agonist, Ki: 0.037 nM and 353 nM for CB2 and CB1 receptors, respectively) [33], ACEA 15 nM (selective CB1 agonist, Ki: 1.4 nM and 3.1 μM for CB1 and CB2 receptors, respectively) [34] or JWH-073 40 nM (CB1 and CB2 agonist, Ki: 8.9 nM and 38 nM for CB1 and CB2 receptors, respectively) [21;35] for 24 hours. mRNA was isolated and qRT-PCR for β-Arrestin 2 mRNA were performed as described above.
In a separate experiment, cells were treated with vehicle (ethanol 0.01% final concentration), CP55940 (1 nM), JWH-133 (30 nM) or GP1a (1 nM) for 72 hours. Cells were washed (3x) with PBS every 24 hours and fresh vehicle, CP55940, JWH-133 or GP1a were added. Expression of membrane-associated β-Arrestin 2 was determined by Western blot as previously described.
2.7.2 Effect of selective cannabinoid receptor antagonists on the CP55940-induced upregulation of β-Arrestin 2
CLU213 cells were pretreated with either vehicle (ethanol 0.01% final concentration); PF-514273 20 nM (CB1 antagonist, Ki: 1 nM and >10,000 nM for CB1 and CB2 receptor, respectively) [36]; or JTE-907 10 nM (CB2 antagonist, Ki: 0.38 and 1,050 nM for CB2 and CB1 receptors, respectively) [37]. Twenty minutes later cells were incubated with either vehicle (ethanol 0.01% final concentration) or CP55940 1 nM for 24 hours. mRNA was isolated and qRT-PCR for β-Arrestin 2 mRNA was performed.
2.7.3 Effect of selective CB2 receptor antagonist on GP1a-induced upregulation of β-Arrestin 2
CLU213 cells were pretreated with either vehicle (ethanol 0.01% final concentration) or JTE-907 (10 nM, a CB2 receptor antagonist) [37]. Twenty min later cells were incubated with either vehicle (ethanol 0.01% final concentration) or GP1a (1nM) for 24 hours. mRNA was isolated and qRT-PCR for β-Arrestin 2 mRNA was determined.
2.7.4 Lentivirus and stable transduction of shRNAs in CLU213 cells
β-Arrestin 2 shRNA (r), CB2 shRNA (r), CB1 shRNA (r), copGFP control, control shRNA lentiviral particles, polybrene, and puromyocin were purchased from Santa Cruz, CA. Optimal transduction conditions were elucidated utilizing copGFP control lentiviral particles prior to transduction with β-Arrestin 2 shRNA or CB2 shRNA lentiviral particles and then transduction was conducted as previously described [10]. Cells were analyzed for β-Arrestin 2, CB2, or CB1 knockdown one week after initiation of puromyocin selection.
2.7.5 Effect of CB2 or CB1 shRNA lentivirus transfection on cannabinoid-induced upregulation of β-Arrestin 2
After confirming that treatment with the CB2 or CB1 shRNA lentivirus reduced CB2 or CB1 mRNA 80% and 70%, respectively, control, CB2, or CB1 shRNA treated cells were treated with either vehicle (ethanol 0.01% final concentration), CP55940 1 nM, JWH-133 30 nM, or GP1a 1 nM for 24 hours. mRNA was isolated from cells and qRT PCR was performed for β-Arrestin 2 mRNA levels as previously described above.
2.7.6 Effect of Concanavalin A (ConA) treatment on cannabinoid-induced increases in β-Arrestin 2 mRNA
Cells were pretreated with either vehicle or 250 μg/mL ConA for 20 minutes [10;38]. Twenty minutes later cells were incubated with either vehicle (ethanol 0.01% final concentration), CP55940 (1 nM), or GP1a (1 nM). mRNA was isolated and qRT-PCR was performed for β-Arrestin 2 mRNA levels.
2.7.7 Effect of a selective ERK1/2 inhibitor on GP1a-Induced Increases in β-Arrestin 2 mRNA
CLU213 cells were treated with either vehicle (ethanol 0.01% final concentration) or PD198306 (200 nM) [10]. Twenty minutes later cells were incubated with either vehicle (ethanol 0.01% final concentration) or GP1a (1 nM). mRNA was isolated and qRT-PCR was used to measure β-Arrestin 2 mRNA levels.
2.7.8 Effect of β-Arrestin 2 shRNA lentivirus transfection on cannabinoid-induced ERK1/2 activation
After confirming that treatment with the β-Arrestin 2 lentivirus particles reduced β-Arrestin 2 mRNA and protein levels 85%, control or β-Arrestin 2 treated cells were treated with either vehicle (ethanol 0.01% final concentration), CP55940 1 nM or GP1a 1 nM for 15 minutes. Nuclear fractions were isolated and western blot analysis was utilized to measure nuclear-associated pERK levels.
2.7.9 Effect of Transcription Factor Inhibitors on GP1a-Induced Upregulation of β-Arrestin 2 mRNA
CLU213 cells were treated with either vehicle (ethanol 0.01%), Naphthol AS-E phosphate (10 μM) [39] or SR11302 (1 μM) [40] for 20 minutes. Cells were then treated with either vehicle (ethanol 0.01%) or GP1a (1 nM) for 24 hours. mRNA was isolated and qRT-PCR for β-Arrestin 2 mRNA was performed as previously described.
2.7.10 Effect of β-Arrestin 2 shRNA lentivirus treatment on GP1a-induced upregulation of 5-HT2A mRNA
Control or β-Arrestin 2 shRNA lentivirus treated cells were treated with either vehicle (ethanol 0.01% final concentration), GP1a 1 nM, ACEA 15 nM or JWH-073 40 nM for 24 hours. mRNA was isolated and qRT-PCR for β-Arrestin 2 mRNA was performed.
2.8 Statistics
All data are expressed as the mean ± S.E.M., where n indicates the number of rats or cell culture plates per group. Data was analyzed by an unpaired Student’s t-test or ANOVA (Newman-Keuls post-hoc test). GB-STAT software (Dynamic Microsystems, Inc., Silver Spring, MD, USA) was used for all statistical analyses.
3. Results
3.1 Chronic CP55940 treatment induces enhanced β-Arrestin 2 and ERK1/2 interaction in PFCx
Our previous work has shown that some cannabinoid agonists can enhance 5-HT2A receptor expression by means of a mechanism that involves CB2 receptor regulation of ERK1/2 activation. [9;10]. Cannabinoid receptors could produce a long-term ERK1/2 activation by a mechanism that may involve a β-Arrestin-ERK1/2 scaffolding complex [17–19]. Specifically, CB2 receptors that are a class A GPCR would preferentially interact with β-Arrestin 2, which may facilitate and enhance the interaction between β-Arrestin and ERK1/2 resulting in long-term ERK1/2 activation [20]. Here, we used co-immunoprecipitation protocols to study the effect of CP55940 treatment on the physical interaction between β-Arrestin 2 and ERK1/2 in rat PFCx (Fig. 1. A). We used β-Arrestin 2 antibody as bait and ERK1/2 antibody as prey. Inactive columns which are unable to bind β-Arrestin 2 antibody were used as a control as described in methods. We found that ERK1/2 co-precipitates with β-Arrestin 2 when we used β-Arrestin 2 as bait (Fig. 1. A, lanes 3 & 4). Interestingly, we detected a significant (p<0.05) two-fold increase in the interaction between β-Arrestin 2 and ERK1/2 in PFCx of CP55940-treated rats compared to vehicle treated controls (Fig. 1. A, lane 3 and 4, vehicle- and CP55940-treated animals, respectively). No co-precipitation of β-Arrestin 2 and ERK1/2 was detected using the inactive columns (Fig. 1. A, lanes 5 & 6).
Figure 1. CP55940-induced enhanced co-immunoprecipitation of β-Arrestin 2 and ERK1/2 and increased β-Arrestin 2 protein expression in rat PFCx.
(A) Enhanced immunoprecipitation of the ERK1/2 (Lane 4) compared to vehicle-treated controls (Lane 3). Negative controls (Lanes 5 and 6) received the same concentration of β-Arrestin 2 antibody except that the coupling resin was replaced with control agarose resin that is not amine reactive. All columns were incubated with prefrontal cortex lysate (300 μg) from vehicle (Lanes 3 and 5 ) or CP55940 (Lanes 4 and 6) treated rats. Prefrontal cortex lysate (30 μg of protein) was used as an input control (Lane 1 and 2). (B) Increased pERK protein levels in CP55940 treated rats compared to vehicle treated rats. **p<0.01, significant effect of CP55940 treatment compared to vehicle-treated controls. (C) Increased membrane associated β-Arrestin 2 protein levels in PFCx of CP55940 treated rats. **p<0.01 significant effect of CP55940 treatment compared to vehicle-treated controls. (D) CP55940 treatment does not affect total ERK1/2 expression in the PFCx. (E) Increased β-Arrestin 2 mRNA levels in PFCx of CP55940 treated rats. *p<0.01 significant effect of CP55940 treatment compared to vehicle treated controls. (F) β-Arr2 shRNA lentivurs transfection prevents GP1a-induced increases in 5-HT2A receptor mRNA. **p<0.01, significant effect of GP1a treatment on 5-HT receptor mRNA levels in control shRNA tranfected cells compared 2A to vehicle-treated controls. ##p<0.01, significant effect of β-Arr2 shRNA transfection on the GP1a-induced upregulation of 5-HT2A receptors. Representative Western blots are shown in this figure and IOD was calculated as described in Experimental Procedures. The data represent mean ± SEM (n=6–8).
3.2 Chronic CP55940 treatment enhances ERK1/2 activation in PFCx homogenates after an acute challenge with CP55940
The increased interaction between β-Arrestin 2 and ERK1/2 proteins could lead to an enhanced ERK1/2 signaling pathway activity. We then designed an in vitro experiment to measure acute CP55940-induced ERK phosphorylation in PFCx homogenates of vehicle and CP55940-treated rats. ERK activation (phosphorylation) was induced by a short (15min) incubation of the homogenates with 1nM CP55940. We found that this CP55940 challenge induced a significantly (p<0.01) greater ERK1/2 phosphorylation in PFCx homogenates of CP55940 treated rats compared to vehicle controls (78 ± 5% increase in CP55940 compared to controls, Fig. 1. B). No significant differences (p>0.05) in total ERK1/2 protein levels were detected between both experimental groups.
3.3 Chronic CP55940 treatment upregulates β-Arrestin 2 expression but not ERK1/2 in rat PFCx
We also studied the effect of repeated exposure of CP55940 on β-Arrestin 2 and ERK1/2 protein expression in rat PFCx since changes in the levels of these proteins could explain the enhanced: (1) β-Arrestin 2 and ERK1/2 interaction (Fig. 1.A); and (2) ERK1/2 signaling pathway (Fig. 1.B) in PFCx of CP55940-treated rats compared to vehicle controls. Here, we examined the effect of repeated CP55940 (50μg/kg for 7 days) exposure on the expression of membrane-associated β-Arrestin 2 and total ERK1/2 protein levels in rat PFCx. CP55940 treatment produced a significant (p<0.01) increase in β-Arrestin 2 membrane-associated protein levels compared to vehicle treated controls (108 ± 13% increase compared to controls, Fig. 1.C). Interestingly, CP55940 treatment did not significantly alter (p>0.05) total ERK1/2 protein levels in rat PFCx (Fig. 1.D). Actin was used as a protein loading controls in the Western blots as described in methods. Cannabinoid-induced upregulation of β-Arrestin 2 mRNA synthesis could contribute to the changes in membrane-associated β-Arrestin 2 protein levels so we also determined the effect of CP55940 treatment on β-Arrestin 2 mRNA levels. β-Arrestin 2 mRNA was significantly (p<0.05) increased (two-fold) in PFCx of CP55940-treated rats compared to vehicle-treated controls (107 ± 15% increase compared to controls, Fig. 1.E).
3.4 β-Arrestin 2 is involved in the cannabinoid-induced upregulation of 5-HT2A receptors
We used neuronal cells stably transfected with either β-Arrestin 2 or control shRNA lentiviral particles to study the contribution of β-Arrestin 2 on the cannabinoid-induced upregulation of 5-HT2A receptors. We have previous shown that GP1a (a selective CB2 receptor agonist) upregulates 5-HT2A receptors while treatment with ACEA (a selective CB1 receptor agonist) does not significantly alter 5-HT2A receptor mRNA levels [9]. Treatment with β-Arrestin 2 shRNA lentiviral particles significantly (p<0.01) reduced β-Arrestin 2 mRNA and protein levels by approximately 85% [10]. Here, cells were treated with either vehicle, GP1a (1 nM), or ACEA (15 nM) for 24 hours. We found that GP1a upregulated 5-HT2A mRNA in control shRNA treated cells by 109 ± 2% (Fig. 1.F). ACEA did not significantly modify 5-HT2A receptor mRNA levels in control shRNA treated cells compared to vehicle treated controls. Noteworthy, the GP1a-induced upregulation of 5-HT2A mRNA levels was significantly (p<0.05) reduced in cells stably transfected with β-Arrestin 2 shRNA lentiviral particles. GP1a-mediated increases in 5-HT2A mRNA levels was prevented in β-Arrestin 2 shRNA treated cells (Fig. 1.F). The two-way ANOVA showed main effects of transfection (F1,63.37 =0.39, p<0.0001), CB2 agonist (F3,71.59 =0.44, p<0.0001), and a main interaction between these two factors (F3,61.22 =0.38, p<0.0001) on 5-HT2A mRNA levels. In summary, these data suggest that CP55940 treatment might heighten the β-Arrestin 2 and ERK1/2 interaction that would enhance ERK1/2 signaling in rat PFCx through upregulation of β-Arrestin 2. This later protein is necessary for the cannabinoid-induced upregulation of 5-HT2A receptors as indicated in studies conducted in our neuronal cell model.
3.5 CP55940 and selective CB2 receptor agonists, JWH-133 and GP1a, upregulate β-Arrestin 2 in CLU213 cells
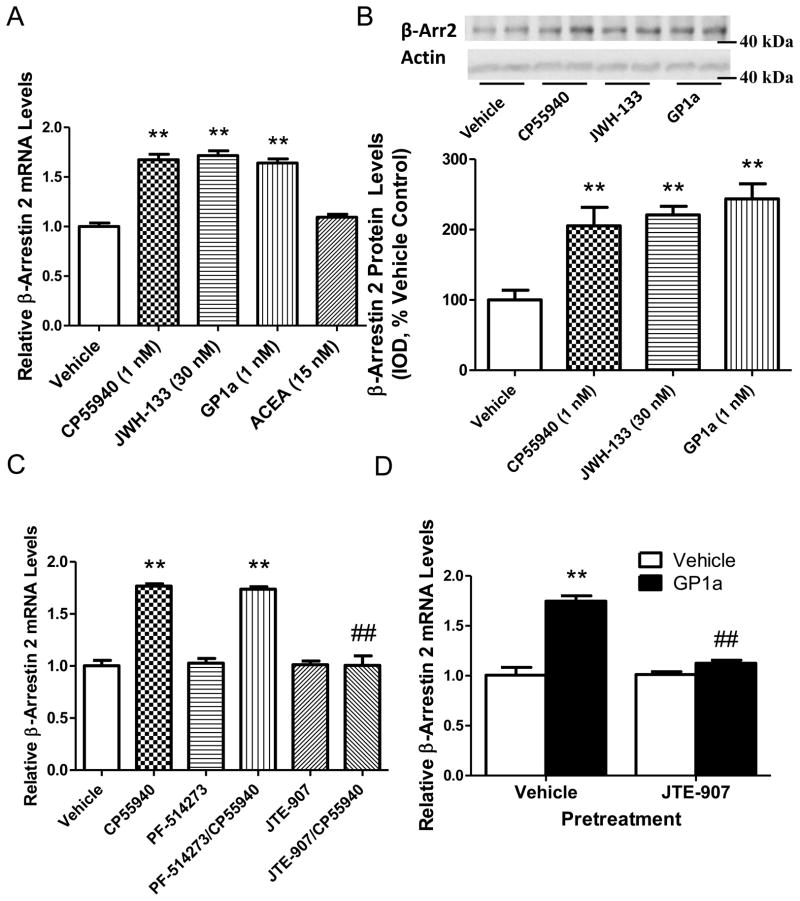
Next, we used a neuronal cell culture model to elucidate the mechanisms underlying cannabinoid-induced upregulation of β-Arrestin 2. Here, cells were incubated with either vehicle, CP55940 (CB1/CB2 receptor agonist, 1nM), selective CB2 receptor agonists JWH-133 (30nM) or GP1a (1nM), or a selective CB1 agonist ACEA (15nM) for 24 hours. We found that sustained CP55940 exposure significantly (p<0.01) increased β-Arrestin 2 mRNA levels compared to vehicle treated cells (67 ± 5% increase, Fig. 2.A). Noteworthy, sustained treatment with either JWH-133 or GP1a, but not ACEA, significantly increased (p<0.01) β-Arrestin 2 mRNA levels (72 ± 5% and 64 ± 4% increase compared to controls, respectively)(Fig. 2.A). We also determined what effect CP55940, JWH-133, and GP1a treatment had on β-Arrestin 2 protein levels. We found that treatment with CP55940 (1nM), JWH-133 (30nM), and GP1a (1nM) also significantly increased (p<0.01) β-Arrestin 2 protein levels compared to vehicle treated controls (105 ± 26%, 120 ± 12%, and 143 ± 21% increase compared to vehicle controls, respectively)(Fig. 2B).
Figure 2. Selective CB2 receptor agonists, GP1a and JWH-133, upregulate β-Arrestin 2 and selective CB2 receptor antagonist, JTE-907, prevent cannabinoid-induced upregulation of β-Arrestin 2 in CLU213 cells.
(A) Increased β-Arrestin 2 mRNA levels in CP55940, JWH-133, and GP1a treated cells. ACEA treatment did not significantly alter β-Arrestin 2 mRNA levels. **p<0.01 significant effect of CP55940, JWH-133, or GP1a treatment compared to vehicle-treated controls. (B) Increased β-Arrestin 2 protein levels in CP55940, JWH-133, and GP1a treated cells. **p<0.01 significant effect of CP55940, JWH-133, or GP1a compared to vehicle-treated controls (C) Selective CB2 receptor antagonists pretreatment JTE-907 prevents CP55940-induced increases in β-Arrestin 2 mRNA. **p<0.01, significant effect of CP55940 treatment on β-Arrestin 2 mRNA levels compared to vehicle-treated controls. ##p<0.01, significant effect of JTE-907 pretreatment on the CP55940-induced upregulation of β-Arrestin 2 mRNA levels (D) JTE-907 prevents GP1a-induced increases in β-Arrestin 2 mRNA levels. **p<0.01, significant effect of GP1a treatment on β-Arrestin 2 mRNA levels compared to vehicle treated controls. ##p<0.01, significant effect of pretreatment on GP1a-induced upregulation of β-Arrestin 2 mRNA levels. Representative Western blots are shown in this figure and IOD was calculated as described in Experimental Procedures. The data represent mean ± SEM (n=3).
3.6 A selective CB2 receptor antagonist, JTE-907, prevents CP55940- and the GP1a-induced increases in β-Arrestin 2 mRNA levels
The evidence presented above suggested that the cannabinoid-induced upregulation of β-Arrestin 2 might be mediated by the CB2 receptors. In order to examine the relative roles of CB1 and CB2 receptors in the upregulation of β-Arrestin 2, cells were pretreated with either vehicle, a selective CB1 receptor antagonist PF-514273 (20nM), or a selective CB2 receptor antagonist JTE-907 (10nM) and then treated with either vehicle or CP55940 (1nM) for 24 hours. CP55940 treatment significantly (p<0.01) increased β-Arrestin 2 mRNA levels compared to vehicle treated controls (75 ± 2% increase compared to controls, Fig. 2.C). We found that PF-514273 pretreatment did not inhibit or significantly (p>0.05) modify the CP55940-induced increases in β-Arrestin 2 mRNA levels. Noteworthy, pretreatment with a selective CB2 receptor antagonist, JTE-907, prevented the CP55940-induced increases in β-Arrestin 2 mRNA levels (p<0.01, Fig. 2.C). No significant (p>0.05) effect of PF-514273 or JTE-907 pretreatment was found on basal β-Arrestin 2 mRNA levels. The two-way ANOVA showed main effects of cannabinoid antagonists (F2,37.3 =0.28, p<0.0001), cannabinoid agonists (F1,143.3 =1.08, p<0.0001), and a main interaction between these two factors (F2,36.9 =0.28, p<0.0001) on β-Arrestin 2 mRNA levels.
In order to more thoroughly investigate the role of CB2 receptors in the cannabinoid-induced upregulation of β-Arrestin 2 mRNA levels, we pretreated cells with the either vehicle or selective CB2 antagonist JTE-907 and then treated cells with vehicle or a highly selective CB2 receptor agonist GP1a. As expected, GP1a treatment significantly (p<0.01) increased β-Arrestin 2 mRNA levels (74 ± 5% increase compared to controls, Fig. 2.D). This GP1a-induced upregulation of β-Arrestin 2 was prevented (p<0.01) in cells pretreated with JTE-907 (Fig. 2.D). The two-way ANOVA showed main effects of JTE-907 pretreatment (F1,35.4 =0.28, p<0.0003), GP1a treatment (F1,68.3 = 0.54, p<0.0001), and a main interaction between these two factors (F1,37.1 =0.29, p<0.0003) on β-Arrestin 2 mRNA levels.
3.7 CB2, but not CB1, shRNA lentiviral particle treatment prevents cannabinoid-induced increases in β-Arrestin 2 mRNA level
We also used cells stably transfected with control, CB2, or CB1 shRNA lentiviral particles to study whether the effect of cannabinoid agonists on β-Arrestin 2 mRNA levels. CB1 and CB2 mRNA levels were significantly reduced by approximately 75–80% in neuronal cells treated with either CB1 or CB2 shRNA lentiviral particles, respectively (Fig. 3.A). We have previously reported similar reductions (75–80%) in protein levels of these receptors after transfection with these shRNA lentiviral particles [10].
Figure 3. CB2 receptors are necessary for CP55940, JWH-133, and GP1a-induced upregulation of β-Arrestin 2 in CLU213 cells.
(A) Reduced CB2 or CB1 mRNA levels in cells treated with CB2 or CB1 shRNA lentiviral particles, respectively. **p<0.01 significant effect of CB2 or CB1 shRNA compared to control shRNA treated cells. (B) CB2 shRNA lentivitrarus transfection prevents CP55940, JWH-133 and GP1a-induced increases in β-Arrestin 2 mRNA. **p<0.01, significant effect of CP55940, JWH-133, and GP1a treatment on β-Arrestin 2 mRNA levels in control shRNA lentivirus tranfected cells compared to vehicle-treated controls. ##p<0.01, significant effect of CB2 shRNA lentivirus transfection on the CP55940, JWH-133, and GP1a-induced upregulation of β-Arrestin 2. (C) CB1 shRNA lentivirus transfection does not prevent CP55940, JWH-133 or GP1a-induced increases β-Arrestin 2. **p<0.01, significant effect of CP55940, JWH-133 or GP1a treatment on β-Arrestin 2 mRNA levels in control or CB1 shRNA lentvirus treated cells compared to vehicle-treated controls. The data represent mean ± SEM (n=3).
Control or CB2 shRNA lentiviral transfected cells were treated with either vehicle, CP55940 (1nM), JWH-133 (30nM) or GP1a (1nM). We found that treatment with CP55940, JWH-133, or GP1a significantly (p<0.01) increased β-Arrestin 2 mRNA levels in control shRNA treated cells compared to vehicle-treated controls (76 ± 3%, 65 ± 2%, and 72 ± 5% increase, respectively)(Fig 3B). Noteworthy, CB2 shRNA treatment prevented (p<0.01) the CP55940, JWH-133, and GP1a induced increases in β-Arrestin 2 mRNA levels. CB2 shRNA lentivirus treatment did not significantly (p>0.01) alter basal β-Arrestin 2 mRNA levels. The two-way ANOVA for β-Arrestin 2 mRNA showed significant main effects of transfection (F3,97.9 = 0.83, p<0.001) and cannabinoid agonist treatment (F1,33.2 =0.28, p<0.0001). There was a significant interaction between transfection and cannabinoid agonist treatment (F3,23.1 =0.19, p<0.0001).
We also determined what effect CP55940, JWH-133 and GP1a treatment had on β-Arrestin 2 upregulation in control or CB1 shRNA lentivirus transfected cells. In control shRNA treated cells, CP55940, JWH-133, and GP1a treatment significantly (p<0.01) increased β-Arrestin 2 mRNA levels compared to vehicle treated controls (68 ± 3%, 74 ± 5%, and 71 ± 5% increase, respectively)(Fig. 3.C). Noteworthy, treatment with CB1 shRNA lentiviral particles did not prevent or significantly modify the CP55940, JWH-133, or GP1a-induced increases in β-Arrestin 2 mRNA levels compared to control shRNA treated cells (Fig. 3. C). The two-way ANOVA for β-Arrestin 2 mRNA showed significant main effects of transfection (F1,7.05 = 0.03, p<0.0173) and cannabinoid agonist treatment (F3,180.67 =0.75, p<0.0001). There was no significant interaction between transfection and cannabinoid agonist treatment (F3,0.11=0.0005, p>0.9526). This evidence indicates that CB2, but not CB1, receptors, would mediate the cannabinoid-induced upregulation of β-Arrestin 2.
3.8 Effect of cannabinoid receptor internalization on the CB2-mediated upregulation of β-Arrestin 2 mRNA
Next, we investigated whether CB2 receptor internalization may be involved in the cannabinoid-induced upregulation of β-Arrestin 2. Internalization of membrane-associated receptors could be a very important step in the signaling of CB2 receptors [41]. Specifically, CB2-induced ERK1/2 activation may require the internalization of membrane-associated CB2 receptors [41]. Here, we used two different approaches to study the role of internalization of CB2 receptors on β-Arrestin 2 upregulation: (1) ConA treatment to prevent CB2 receptor internalization [10]; and (2) different cannabinoid agonists that are either good or poor internalizers of CB2 receptors [41].
Currently, there are several protocols that are commonly used to prevent clathrin-mediated internalization of GPCR which include: ConA, hypertonic sucrose, or depletion of intercellular potassium [38]. Here we pretreated cells with vehicle or ConA and then treated cells with either vehicle, CP55940 (1nM) or GP1a (1nM) for 24h. Our previous studies suggested that this ConA pretreatment decreased the cannabinoid-induced translocation of CB2 receptors from the membrane to the cytosol and prevented the cannabinoid-induced ERK1/2 activation [10]. CP55940 and GP1a significantly (p<0.01) increased β-Arrestin 2 mRNA levels compared to vehicle treated cells (76 ± 3%, and 77 ± 3% increase, respectively) (Fig. 4. A). ConA pretreatment significantly (p<0.01) blocked the effect of CP55940 and GP1a on β-Arrestin 2 mRNA levels compared to controls (Fig. 4. A). The two-way ANOVA showed a main effect of ConA (F1,107 =0.93, p<0.0001), cannabinoid agonists (F2,35 =0.31, p<0.0001), and a main interaction between these two factors (F2,32 =0.276, p<0.0001) on β-Arrestin 2 mRNA levels.
Figure 4. CP55940 and GP1a may upregulate β-Arrestin 2 via CB2 receptor internalization in CLU213 cells.
(A) Increased β-Arrestin 2 mRNA levels in cells treated with either CP55940 or GP1a. significant effect of CP55940 and GP1a treatment on β-Arrestin 2 mRNA levels compared to vehicle-treated controls. ##p<0.01, significant effect of ConA pretreatment on the CP55940 and GP1a-induced upregulation of β-Arrestin 2. The data represent mean ± SEM (n=3).
Interestingly, a recent study shows that some cannabinoid agonists are more efficacious CB2 receptor internalizers than other cannabinoid agonists [41]. Indeed, CP55940 and GP1a would be efficacious CB2 receptor internalizers while aminoakyindoles, such as JWH-073, were classified as poor CB2 receptor internalizers [41]. We then tested the effect of either vehicle, JWH-073, GP1a, or CP55940 on β-Arrestin 2 mRNA levels in neuronal cells. We found that either GP1a or CP55940 treatment produced significant (p<0.01) increases in the β-Arrestin 2 mRNA levels while JWH-073 treatment did not significantly alter β-Arrestin 2 mRNA levels (Fig 4B). These results obtained seems to indicate that CB2 internalization of CB2 receptors is a critical step in the upregulation of β-Arrestin 2 mRNA.
3.9 Agonists of CB2 receptors would mediate the upregulation of β-Arrestin 2 via ERK1/2 in CLU213 cells
We also aimed to investigate the mechanism by which CB2 receptor induces the upregulation of β-Arrestin 2. CB2 receptors are positively coupled to the ERK1/2 signaling pathway [16]. We used PD198306, a selective ERK1/2 inhibitor [42], to study the effect of GP1a-induced ERK1/2 activation on β-Arrestin 2 upregulation. GP1a exhibits higher selectivity for CB2 receptors than JWH-133 therefore we utilized GP1a in these experiments (approx. 9,000- and 200-fold selectivity between CB2/CB1 receptors for GP1a and JWH-133, respectively) [32;33]. Here, cells were pretreated with either vehicle or PD198306 (200nM) for 20 min and then treated with either vehicle or GP1a (1nM) for 24h. GP1a treatment significantly (p<0.01) increased β-Arrestin 2 mRNA levels compared to vehicle treated controls (104 ± 10% increase, Fig 5A). This GP1a-induced upregulation of β-Arrestin 2 was prevented (p<0.01) in cells pretreated with PD198306 (Fig. 5.A). The two-way ANOVA for β-Arrestin 2 mRNA showed significant main effects of PD198306 pretreatment (F1,30.2 =0.54, p<0.0006) and GP1a treatment (F1,48.8 =0.87, p<0.0001). There was a significant interaction between PD198306 and GP1a treatment (F1,41.6 =0.74, p<0.0002).
Figure 5. GP1a, a selective CB2 receptor agonist, upregulate β-Arrestin 2 via ERK1/2 signaling CLU213 cells.
(A) PD198306, potent ERK1/2 inhibitor, pretreatment prevents GP1a-induced increases β-Arrestin 2 mRNA levels. **p<0.01, significant effect of GP1a treatment compared to vehicle-treated controls. ##p<0.01, significant effect of PD198306 pretreatment on GP1a-induced increases β-Arrestin 2 mRNA levels compared to vehicle-treated controls (B) β-Arrestin 2 shRNA lentivirus treatment significantly reduced CP55940 induced increased in nuclear-associated pERK levels. **p<0.01, significant effect of CP55940 treatment on nuclear-associated pERK levels in control or β-Arrestin 2 shRNA treated cells compared to vehicle-treated. ##p<0.01, significant effect of β-Arrestin 2 shRNA treatment in CP55940 treated cells compared to control shRNA transfect/CP55940 treated cells. (C) β-Arrestin shRNA lentivirus treatment significantly reduced GP1a-induced increases in nuclear-associated pERK levels. **p<0.01, significant effect of GP1a treatment on nuclear-associated pERK levels in control or β-Arrestin 2 shRNA treated cells compared to vehicle-treated controls. ##p<0.01, significant effect of β-Arrestin 2 shRNA treatment in CP55940 treated cells compared to control shRNA transfected/CP55940 treated cells. The data represent mean ± SEM (n=3).
We then used cells stably transfected with either β-Arrestin 2 or control shRNA lentiviral particles to study the contribution of β-Arrestin 2 to the cannabinoid-induced increases in nuclear-associated pERK. ERK1/2 is activated through phosphorylation and once phosphorylated this protein can translocate from the cytoplasm to the nucleus [43]. Cells transfected with either control or β-Arrestin 2 shRNA lentiviral particles were treated with vehicle or CP55940 (1nM) for 15 min. We found that treatment with CP55940 significantly (p<0.01) increased nuclear-associated pERK levels in control shRNA treated cells compared to vehicle treated controls (Fig. 5.B). The CP55940 induced increases in nuclear-associated pERK levels were significantly (p<0.01) reduced in cells stably transfected with β-Arrestin 2 shRNA lentiviral particles (Fig. 5.B). Additionally, CP55940 treatment significantly (p<0.01) increased nuclear-associated pERK levels in β-Arrestin 2 shRNA lentiviral treated cells compared to vehicle treated controls. The two-way ANOVA for nuclear-associated pERK showed significant main effects of transfection (F1,429084 =39.41, p<0.0001) and CP55940 treatment (F1,444167 = 40.79, p<0.0001). There was a significant interaction between transfection and CP55940 treatment (F1,191213 =175.7, p<0.0001).
Cells transfected with either control or β-Arrestin 2 shRNA lentiviral particles were also treated with either vehicle or GP1a for 15 minutes. We found that treatment with GP1a significantly (p<0.01) increased nuclear-associated pERK levels in control shRNA treated cells compared to vehicle treated controls (Fig 5C). β-Arrestin 2 shRNA lentivirus transfection significantly (p<0.01) reduced GP1a-induced increases in pERK levels (Fig 5C). Treatment with GP1a significantly (p<0.01) increased nuclear-associated pERK levels in β-Arrestin 2 shRNA treated cells compared to vehicle treated controls. The two-way ANOVA for nuclear-associated pERK showed significant main effects of transfection (F1,131794 =18.3, p<0.0001) and GP1a treatment (F1,142369 = 19.8, p<0.0008). There was a significant interaction between transfection and GP1a treatment (F1,252455 =350.5, p<0.0001).
3.10 Transcription factor AP-1, but not CREB, would be involved in GP1a induced upregulation of β-Arrestin 2 in CLU213 cells
Here we wanted to identify possible transcription factor(s) that would contribute to GP1a-induced increases in β-Arrestin 2 mRNA. The results presented above seem to indicate that ERK1/2 activation is a mechanism involved in the CB2 upregulation of β-Arrestin 2 mRNA. In the nucleus, phosphorylated ERK (pERK) can activate several transcription factors including CREB, c-Fos, SP-1, and EGR-1 [43]. The transcription factors CREB and AP-1 have consensus sequences within the promoter region of the rat β-Arrestin 2 gene [44]. Therefore, we decided to test the effects of inhibitors of these transcription factors on the GP1a-induced upregulation of β-Arrestin 2 mRNA.
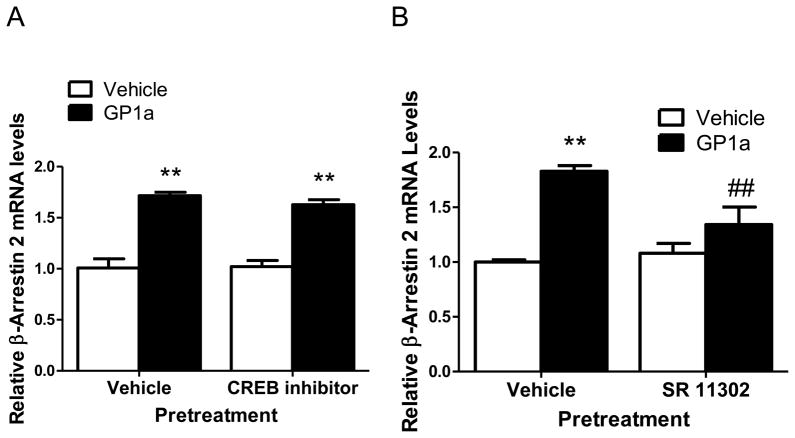
CREB is a transcription factor which binds the cAMP response element (CRE) to regulate the transcription of genes [45]. c-Fos belongs to the immediate early gene family of transcription factors and this family can dimerize with c-jun to form the AP-1 transcription factors to upregulate transcription of various genes [46]. In our first experiment, we studied the effect of CREB inhibitor pretreatment on the GP1a-induced upregulation of β-Arrestin 2. Here, CLU213 cells were treated with either vehicle or Naphthol AS-E phosphate (10μM) for 20 min and then treated with vehicle or GP1a (1nM). Naphthol AS-E phosphate blocks cAMP-induction of CREB-dependent gene transcription (Ki=10μM) [39]. We found that Naphthol AS-E phosphate did not inhibit or significantly decrease GP1a-induced increases in β-Arrestin 2 mRNA (Fig. 6.A). No significant (p>0.05) effect of Naphthol AS-E phosphate was found on basal β-Arrestin 2 mRNA levels either. The two-way ANOVA for β-Arrestin 2 mRNA showed no significant main effect of Naphthol AS-E phosphate pretreatment (F1,0.004 =0.038, p>0.0557) and a significant main effect of GP1a treatment (F1,1.29 =116.6, p<0.0001). There was no significant interaction between Naphthol AS-E pretreatment and GP1a treatment (F1,0.007 =0.683, p>0.683) on.
Figure 6. CB2 receptor-induced upregulation of β-Arrestin 2 involves AP-1, but not CREB, activation.
(A) Inhibition of CREB activation did not prevent or significantly reduce GP1a-induced increases in β-Arrestin 2 mRNA. **p<0.01, significant effect of GP1a and CREB/GP1a treatment on β-Arrestin 2 mRNA levels compared to vehicle-treated controls. (B) CB2 receptor-mediated upregulation of β-Arrestin 2 involves AP-1 transcription factor activity. **p<0.01, significant effect of GP1a treatment compared to vehicle-treated controls. ##p<0.01, significant effect of AP-1 transcription factor inhibitor pretreatment on GP1a-induced increases in β-Arrestin 2 mRNA. The data represent mean ± SEM (n=3).
We also studied the effect of AP-1 inhibition on GP1a-induced increases in β-Arrestin 2 mRNA. Here neuronal cells were treated with either vehicle or SR11302 (1μM) for 20 min then vehicle or GP1a (1nM) was added to the incubation media. SR11302 is a retinoid which transrepresses AP-1 without transactivating the retinoic acid response element (Emax= 1 μM) [47]. As expected, GP1a induced a significant (p<0.01) increase in β-Arrestin 2 mRNA levels (Fig. 6.B). SR11302 pretreatment significantly reduced (approximately 49% decrease, p<0.01) the GP1a-induced upregulation of the β-Arrestin 2 mRNA (Fig. 6B). No significant (p>0.05) effect of SR11302 was found in basal β-Arrestin 2 mRNA levels. The two-way ANOVA for β-Arrestin 2 mRNA did not show a significant main effect of SR11302 pretreatment (F1,0.12 =4.46, p>0.0676) but did show a significant effect of GP1a treatment (F1,0.88 p<0.0005). There was a significant interaction between SR11302 pretreatment and GP1a treatment (F 1,0.24 =8.71, p<0.0184). These results suggest that AP-1, but not CREB, would mediate at least in part the GP1a-induced upregulation of β-Arrestin 2.
4. Discussion
β-Arrestin 1 and 2 were initially found to hinder G protein coupling of agonist-activated G-protein coupled receptors (GPCR) resulting in GPCR desensitization; however, recent evidence shows that β-Arrestins can also function to activate signaling cascades independent of G protein activation and can function in receptor internalization [17–19]. The classical paradigm of agonist-induced GPCR mediated signal transduction involves agonist-induced dissociation of the G proteins from the GPCR and subsequent G protein regulation of secondary messengers [48;49]. It was found that G protein-coupled receptor kinases (GRKs) could terminate this agonist-induced signaling response through phosphorylation of the receptor [17]. GRK mediated phosphorylation of the receptor triggers the binding of β-Arrestins to the receptor preventing further G-protein mediated activation of the secondary messengers [17]. However, recent evidence has identified that β-Arrestins can recruit proteins such as ERK1/2 to the GPCR to form scaffolding complexes that can regulate the activation of signaling cascades [48;49].
Interestingly, previous reports showed that chronic exposure to THC (tetrahydrocannabinol, CB1/CB2 receptor agonist) upregulates β-Arrestin1 in striatum and β-Arrestin 2 in cerebellum and hippocampus [50] but did not upregulate total ERK1/2 levels in hippocampus [51]. Currently, the consequences of this upregulation of β-Arrestins on scaffolding mediated regulation of signaling cascades are unknown. Other research has identified that β-Arrestins can bind proteins involved in receptor internalization and can bring activated receptors along with scaffolding proteins to clathrin-coated pits for endocytosis [48;49]. After internalization of the GPCR, evidence indicates that the scaffolding complex can continue to regulate signaling cascades [19].
Our results indicate that exposure to CP55940, a CB1/CB2 receptor agonist, increases the interaction between β-Arrestin 2 and ERK1/2 in rat PFCx (Fig. 1.A). Co-immunoprecipitation has been successfully used by some groups to demonstrate an interaction between β-Arrestin 2 and its scaffolding proteins [52–54]. Interestingly, this enhanced β-Arrestin 2 and ERK1/2 interaction in the PFCx of CP55940 treated rats was associated with enhanced CP55940-induced ERK1/2 activation (Fig. 1.B). In previous work we have found cannabinoid-induced ERK1/2 activation is needed for the upregulation of 5-HT2A receptors hence cannabinoid regulation of the β-Arrestin and ERK1/2 interaction could play an essential role in the upregulation of 5-HT2A receptors through regulation of ERK1/2 signaling. Changes in β-Arrestin 2 and/or ERK1/2 expression could contribute to this cannabinoid agonist-induced enhanced β-Arrestin 2 and ERK1/2 interaction. Interestingly, we found that CP55940 treatment increased β-Arrestin 2 protein levels while ERK1/2 protein levels were not significantly altered in the PFCx of rats (Fig. 1.C and 1D). Therefore changes in β-Arrestin 2 protein expression but not ERK1/2 protein expression may be contributing to this enhanced β-Arrestin 2 and ERK1/2 interaction in the PFCx. Moreover, we found increased β-Arrestin 2 mRNA in PFCx of CP55940 treated rats compared to controls (Fig. 1.E) suggesting that increases in β-Arrestin 2 expression most likely occurs through cannabinoid-mediated enhanced transcription of the β-Arrestin 2 gene. Finally, treatment with β-Arrestin 2 lentiviral particles prevented the cannabinoid-induced upregulation of 5-HT2A receptors (Fig. 1.F). This evidence indicates that β-Arrestin 2 is necessary for the cannabinoid-induced upregulation of 5-HT2A receptors and suggests that regulation of 5-HT2A receptor expression may be mediated by changes in β-Arrestin 2 expression and formation of the scaffolding complex.
We used a neuronal cell line, selective CB1 and CB2 receptor agonists or antagonists, and CB1 and CB2 shRNA lentiviral particles to determine the contribution of CB1 and CB2 receptors to the regulation of β-Arrestin 2 mRNA levels. In our neuronal cell line which expresses CB1 and CB2 receptors, we found that treatment with the non-selective CB1/CB2 agonist CP55940 and treatment with the selective CB2 receptors agonists, JWH-133 and GP1a, increased β-Arrestin 2 mRNA and protein levels (Fig. 2.A and 2B). This cannabinoid–induced upregulation of β-Arrestin 2 was not mimicked by a selective CB1 agonist (ACEA) (Fig. 2.A) suggesting that CB2 receptors may be mediating this response. Additionally, we found that a selective CB2 receptor antagonist JTE-907, but not a selective CB1 receptor antagonist PF-514273, prevented the CP55940-induced increases in β-Arrestin 2 mRNA levels (Fig. 2.C). Moreover, treatment with CB2 shRNA lentiviral particles, but not CB1 shRNA lentiviral particles, prevented the CP55940, JWH-133, and GP1a-induced increases β-Arrestin 2 mRNA levels (Fig. 3.B and 3C). This evidence indicates the CB2 receptors are required for the cannabinoid-induced upregulation of β-Arrestin 2.
Recent evidence indicates that CB2 receptor ligands can distinctly regulate the signal transduction mechanisms associated with the CB2 receptors, a phenomenon known as functional selectivity [19;41;55]. Atwood et al. have identified different classes of cannabinoid receptor agonists which differ in their ability induce internalization of CB2 receptors [41]. They generalize that bicyclic cannabinoid such as CP55940 would be efficacious CB2 receptor internalizers while aminoakylindoles would be poor CB2 receptor internalizers. We found that CP55940, but not JWH-073, induced β-Arrestin 2 upregulation (Fig. 4.B), suggesting that upregulation of β-Arrestin 2 is dependent on the ability of the agonists to promote internalization of CB2 receptors. To further explore this possibility, we pretreated cells with ConA which is commonly used to prevent clathrin mediated endocytosis of GPCRs [38;56] and then treated cells with CP55940 or GP1a. Interestingly, ConA pretreatment prevented the CP55940- and GP1a-induced increases in β-Arrestin 2 mRNA levels in cultured cells (Fig. 4.A). This evidence further suggests that internalization of the CB2 receptor is needed for the cannabinoid-induced upregulation of β-Arrestin 2.
We tested the effect of PD198306, a potent ERK1/2 inhibitor, on the CB2-induced upregulation of β-Arrestin 2 [12;42]. Previous studies have identified that 200 nM of PD198306 can prevent ERK1/2 activation in breast cancer cells [42]. We found the PD198306 pretreatment prevented the selective CB2 receptor agonist induced increases in β-Arrestin 2 mRNA levels (Fig. 5.A). This evidence suggests that CB2 receptors can induce the upregulation of β-Arrestin 2 through a mechanism that involves ERK activation. Currently, the mechanism by which CB2 receptors mediate the activation of ERK1/2 has not been well defined. It is known that β-Arrestins can form scaffolding complexes with ERK1/2 which can mediate its activation [19]. We found that β-Arrestin 2 shRNA lentiviral particle treatment significantly reduced the CP55940 and GP1a-induced increases in nuclear-associated pERK compared to vehicle treated controls (Fig. 5.B and 5C). Yet treatment with CP55940 and GP1a significantly increased nuclear pERK levels in β-Arrestin 2 shRNA lentivirus treated cells compared vehicle treated controls. This evidence suggests that CB2 receptors can mediate ERK1/2 activation, at least in part, through β-Arrestin 2. Here the CP55940 and GP1a induced increased in pERK levels in β-Arrestin 2 shRNA lentivirus treated cells could be attributed to: (1) residual β-Arrestin 2 left over after β-Arrestin 2 shRNA lentivirus treatment (approximately 15%) and/or (2) β-Arrestin 1 mediated activation of ERK1/2 signaling. It has been found that β-Arrestins can functionally substitute for the other isoform to some degree [49]. However, internalization of GPCRs is typically mediated primarily by one isoform of β-Arrestin [49].
Our results suggest that inhibition of AP-1, but not CREB, significantly decreased the GP1a-induced upregulation of 5-HT2A receptors (Fig. 6.A and 6B). The partial inhibition of the GP1a-induced increases in β-Arrestin 2 mRNA levels by SR11302 suggest that other transcription factors yet to be identified could also contribute to this upregulation.
Chronic cannabinoid receptor agonist exposure have been associated with several neuropsychiatric disorders such as schizophrenia, anxiety, and depression [6–8;57]. Interestingly, dysregulation of 5-HT2A and D2 receptor signaling has been associated with schizophrenia, stress response, anxiety, and depression [58–61]. While a causal link between chronic cannabinoid agonist exposure and these neuropsychiatric disorders has not been found, it has been suggested that long-term cannabinoid agonist exposure may precipitate these neuropsychiatric disorders [6–8;57]. Interestingly, repeated exposure to a cannabinoid agonist, CP55940, leads to increased anxiety and long-term memory impairments that irrespective of the age at which drug exposure occurs [62]. More importantly, recent evidence indicates that repeated exposure to a selective CB2 receptor agonist, JWH-133, induces anxiety-like behaviors in rodents that are blocked by a selective CB2 receptor antagonist [63]. This evidence highlights the need to identify the mechanisms by which sustained cannabinoid exposure induces and/or contributes to neuropsychiatric disorders. Noteworthy, in our previous reports we found that CB2 receptor–mediated upregulation of 5-HT2A receptors [10] could contribute to the cannabinoid-induced enhanced interaction between 5-HT2A and D2 receptors in PFCx [9]. Here, we aimed to identify the molecular mechanisms contributing to this upregulation of 5-HT2A receptors and specifically we focused on the role of β-Arrestin 2 in this phenomenon. In summary, our results suggest that sustained activation of CB2 receptors would enhance β-Arrestin 2 expression possibly contributing to its increased interaction with ERK1/2 thereby driving the upregulation of 5-HT2A receptors. The CB2 receptor-mediated upregulation of β-Arrestin 2 would be mediated, at least in part, by an ERK1/2-dependent activation of AP-1.
5. Conclusion
Emerging studies are identifying that selective CB2 receptor agonists such as JWH-133 have wide therapeutic application in the treatment of conditions such as stroke, neurogenerative diseases, and neuropathic pain [1–4]. However, the mechanism that we are currently defining could represent a potential adverse effect of the long-term use of selective CB2 receptor agonists such as GP1a and JWH-133. Repeated exposure to these agonists, that are efficacious CB2 receptor internalizers, could be associated with potential adverse effects as mentioned above. However, our evidence suggest that CB2 receptor agonists such as JWH-073, that are categorized as poor CB2 receptor internalizers, could represent an alternative therapeutic approach that may have minimal effect on serotonergic neurotransmission in brain thereby reducing the adverse effects that may be associated with enhanced 5-HT2A receptor function.
Acknowledgments
Funding Acknowledgements:
This work was supported by National Institute of Health/National Institute on Drug Abuse DA024329 and DA034315 and University of Kansas Startup Funds.
Non-standards abbreviations
- 5-HT
5-hydroxytryptamine
- 5-HT2A
Serotonin 2A
- Δ9-THC
Δ9-Tetrahydrocannabinol
- ERK
extracellular regulated kinase
- PFCx
prefrontal cortex
- GPCR
G-protein coupled receptor
- MAP
Mitogen Activated Protein kinase
- pERK
phosphorylated extracellular regulated kinase
Footnotes
Conflict of Interest Statement:
The Authors declare that there is no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Zarruk JG, Fernandez-Lopez D, Garcia-Yebenes I, Garcia-Gutierrez MS, Vivancos J, Nombela F, Torres M, Burguete MC, Manzanares J, Lizasoain I, Moro MA. Cannabinoid type 2 receptor activation downregulates stroke-induced classic and alternative brain macrophage/microglial activation concomitant to neuroprotection. Stroke. 2012;43:211–9. doi: 10.1161/STROKEAHA.111.631044. [DOI] [PubMed] [Google Scholar]
- 2.Guindon J, Hohmann AG. Cannabinoid CB2 receptors: a therapeutic target for the treatment of inflammatory and neuropathic pain. Br J Pharmacol. 2008;153:319–34. doi: 10.1038/sj.bjp.0707531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sagredo O, Gonzalez S, Aroyo I, Pazos MR, Benito C, Lastres-Becker I, Romero JP, Tolon RM, Mechoulam R, Brouillet E, Romero J, Fernandez-Ruiz J. Cannabinoid CB2 receptor agonists protect the striatum against malonate toxicity: relevance for Huntington’s disease. Glia. 2009;57:1154–67. doi: 10.1002/glia.20838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morales M, Bonci A. Getting to the core of addiction: Hooking CB2 receptor into drug abuse? Nat Med. 2012;18:504–5. doi: 10.1038/nm.2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patton GC, Coffey C, Carlin JB, Degenhardt L, Lynskey M, Hall W. Cannabis use and mental health in young people: cohort study. BMJ. 2002;325:1195–8. doi: 10.1136/bmj.325.7374.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Henquet C, Murray R, Linszen D, van OJ. The environment and schizophrenia: the role of cannabis use. Schizophr Bull. 2005;31:608–12. doi: 10.1093/schbul/sbi027. [DOI] [PubMed] [Google Scholar]
- 7.Kuepper R, van OJ, Lieb R, Wittchen HU, Hofler M, Henquet C. Continued cannabis use and risk of incidence and persistence of psychotic symptoms: 10 year follow-up cohort study. BMJ. 2011;342:d738. doi: 10.1136/bmj.d738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crippa JA, Zuardi AW, Martin-Santos R, Bhattacharyya S, Atakan Z, McGuire P, Fusar-Poli P. Cannabis and anxiety: a critical review of the evidence. Hum Psychopharmacol. 2009;24:515–23. doi: 10.1002/hup.1048. [DOI] [PubMed] [Google Scholar]
- 9.Franklin JM, Carrasco GA. Cannabinoid-induced enhanced interaction and protein levels of serotonin 5-HT2A and dopamine D2 receptors in rat prefrontal cortex. J Psychopharmacol. 2012;26:915–29. doi: 10.1177/0269881112450786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franklin JM, Vasiljevik T, Prisinzano TE, Carrasco GA. Cannabinoid 2 Receptor- and Beta Arrestin 2-Dependent Upregulation of Serotonin 2A Receptors. Eur Neuropsychopharmacol. 2012 doi: 10.1016/j.euroneuro.2012.06.012. in press: http://dx.doi.org/10.1016/j.euroneuro.2012.06.012. [DOI] [PMC free article] [PubMed]
- 11.Howlett AC. Cannabinoid receptor signaling. Handb Exp Pharmacol. 2005:53–79. doi: 10.1007/3-540-26573-2_2. [DOI] [PubMed] [Google Scholar]
- 12.Felder CC, ckason-Chesterfield AK, Moore SA. Cannabinoids biology: the search for new therapeutic targets. Mol Interv. 2006;6:149–61. doi: 10.1124/mi.6.3.6. [DOI] [PubMed] [Google Scholar]
- 13.Abood ME, Martin BR. Molecular neurobiology of the cannabinoid receptor. Int Rev Neurobiol. 1996;39:197–221. doi: 10.1016/s0074-7742(08)60667-4. [DOI] [PubMed] [Google Scholar]
- 14.Gong JP, Onaivi ES, Ishiguro H, Liu QR, Tagliaferro PA, Brusco A, Uhl GR. Cannabinoid CB2 receptors: immunohistochemical localization in rat brain. Brain Res. 2006;1071:10–23. doi: 10.1016/j.brainres.2005.11.035. [DOI] [PubMed] [Google Scholar]
- 15.den Boon FS, Chameau P, Schaafsma-Zhao Q, van AW, Bari M, Oddi S, Kruse CG, Maccarrone M, Wadman WJ, Werkman TR. Excitability of prefrontal cortical pyramidal neurons is modulated by activation of intracellular type-2 cannabinoid receptors. Proc Natl Acad Sci U S A. 2012;109:3534–9. doi: 10.1073/pnas.1118167109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bouaboula M, Poinot-Chazel C, Marchand J, Canat X, Bourrie B, Rinaldi-Carmona M, Calandra B, Le FG, Casellas P. Signaling pathway associated with stimulation of CB2 peripheral cannabinoid receptor. Involvement of both mitogen-activated protein kinase and induction of Krox-24 expression. Eur J Biochem. 1996;237:704–11. doi: 10.1111/j.1432-1033.1996.0704p.x. [DOI] [PubMed] [Google Scholar]
- 17.Reiter E, Lefkowitz RJ. GRKs and beta-arrestins: roles in receptor silencing, trafficking and signaling. Trends Endocrinol Metab. 2006;17:159–65. doi: 10.1016/j.tem.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 18.Shenoy SK, Drake MT, Nelson CD, Houtz DA, Xiao K, Madabushi S, Reiter E, Premont RT, Lichtarge O, Lefkowitz RJ. beta-arrestin-dependent, G protein-independent ERK1/2 activation by the beta2 adrenergic receptor. J Biol Chem. 2006;281:1261–73. doi: 10.1074/jbc.M506576200. [DOI] [PubMed] [Google Scholar]
- 19.Lefkowitz RJ, Shenoy SK. Transduction of receptor signals by beta-arrestins. Science. 2005;308:512–7. doi: 10.1126/science.1109237. [DOI] [PubMed] [Google Scholar]
- 20.Oakley RH, Laporte SA, Holt JA, Caron MG, Barak LS. Differential affinities of visual arrestin, beta arrestin1, and beta arrestin2 for G protein-coupled receptors delineate two major classes of receptors. J Biol Chem. 2000;275:17201–10. doi: 10.1074/jbc.M910348199. [DOI] [PubMed] [Google Scholar]
- 21.Huffman JW, Zengin G, Wu MJ, Lu J, Hynd G, Bushell K, Thompson AL, Bushell S, Tartal C, Hurst DP, Reggio PH, Selley DE, Cassidy MP, Wiley JL, Martin BR. Structure-activity relationships for 1-alkyl-3-(1-naphthoyl)indoles at the cannabinoid CB(1) and CB(2) receptors: steric and electronic effects of naphthoyl substituents. New highly selective CB(2) receptor agonists. Bioorg Med Chem. 2005;13:89–112. doi: 10.1016/j.bmc.2004.09.050. [DOI] [PubMed] [Google Scholar]
- 22.Rubino T, Vigano D, Massi P, Parolaro D. Changes in the cannabinoid receptor binding, G protein coupling, and cyclic AMP cascade in the CNS of rats tolerant to and dependent on the synthetic cannabinoid compound CP55,940. J Neurochem. 2000;75:2080–6. doi: 10.1046/j.1471-4159.2000.0752080.x. [DOI] [PubMed] [Google Scholar]
- 23.Marco EM, Perez-Alvarez L, Borcel E, Rubio M, Guaza C, Ambrosio E, File SE, Viveros MP. Involvement of 5-HT1A receptors in behavioural effects of the cannabinoid receptor agonist CP 55,940 in male rats. Behav Pharmacol. 2004;15:21–7. doi: 10.1097/00008877-200402000-00003. [DOI] [PubMed] [Google Scholar]
- 24.Yaniv SP, Lucki A, Klein E, Ben-Shachar D. Dexamethasone enhances the norepinephrine-induced ERK/MAPK intracellular pathway possibly via dysregulation of the alpha2-adrenergic receptor: implications for antidepressant drug mechanism of action. Eur J Cell Biol. 2010;89:712–22. doi: 10.1016/j.ejcb.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 25.Odemis V, Lipfert J, Kraft R, Hajek P, Abraham G, Hattermann K, Mentlein R, Engele J. The presumed atypical chemokine receptor CXCR7 signals through G(i/o) proteins in primary rodent astrocytes and human glioma cells. Glia. 2012;60:372–81. doi: 10.1002/glia.22271. [DOI] [PubMed] [Google Scholar]
- 26.Carrasco GA, Battaglia G. Withdrawal from a single exposure to cocaine increases 5-HT2A receptor and G protein function. Neuroreport. 2007;18:51–5. doi: 10.1097/01.wnr.0000246324.43567.55. [DOI] [PubMed] [Google Scholar]
- 27.Wang HY, Crupi D, Liu J, Stucky A, Cruciata G, Di RA, Friedman E, Quartarone A, Ghilardi MF. Repetitive transcranial magnetic stimulation enhances BDNF-TrkB signaling in both brain and lymphocyte. J Neurosci. 2011;31:11044–54. doi: 10.1523/JNEUROSCI.2125-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carrasco GA, Van de Kar LD, Sullivan NR, Landry M, Garcia F, Muma NA, Battaglia G. Cocaine-mediated supersensitivity of 5-HT2A receptors in hypothalamic paraventricular nucleus is a withdrawal-induced phenomenon. Neuroscience. 2006;143:7–13. doi: 10.1016/j.neuroscience.2006.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh RK, Shi J, Zemaitaitis BW, Muma NA. Olanzapine increases RGS7 protein expression via stimulation of the Janus tyrosine kinase-signal transducer and activator of transcription signaling cascade. J Pharmacol Exp Ther. 2007;322:133–40. doi: 10.1124/jpet.107.120386. [DOI] [PubMed] [Google Scholar]
- 30.Yang CH, Huang HW, Chen KH, Chen YS, Sheen-Chen SM, Lin CR. Antinociceptive potentiation and attenuation of tolerance by intrathecal beta-arrestin 2 small interfering RNA in rats. Br J Anaesth. 2011;107:774–81. doi: 10.1093/bja/aer291. [DOI] [PubMed] [Google Scholar]
- 31.Showalter VM, Compton DR, Martin BR, Abood ME. Evaluation of binding in a transfected cell line expressing a peripheral cannabinoid receptor (CB2): identification of cannabinoid receptor subtype selective ligands. J Pharmacol Exp Ther. 1996;278:989–99. [PubMed] [Google Scholar]
- 32.Huffman JW, Liddle J, Yu S, Aung MM, Abood ME, Wiley JL, Martin BR. 3-(1′,1′-Dimethylbutyl)-1-deoxy-delta8-THC and related compounds: synthesis of selective ligands for the CB2 receptor. Bioorg Med Chem. 1999;7:2905–14. doi: 10.1016/s0968-0896(99)00219-9. [DOI] [PubMed] [Google Scholar]
- 33.Gorantla S, Makarov E, Roy D, Finke-Dwyer J, Murrin LC, Gendelman HE, Poluektova L. Immunoregulation of a CB2 receptor agonist in a murine model of neuroAIDS. J Neuroimmune Pharmacol. 2010;5:456–68. doi: 10.1007/s11481-010-9225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hillard CJ, Manna S, Greenberg MJ, DiCamelli R, Ross RA, Stevenson LA, Murphy V, Pertwee RG, Campbell WB. Synthesis and characterization of potent and selective agonists of the neuronal cannabinoid receptor (CB1) J Pharmacol Exp Ther. 1999;289:1427–33. [PubMed] [Google Scholar]
- 35.Aung MM, Griffin G, Huffman JW, Wu M, Keel C, Yang B, Showalter VM, Abood ME, Martin BR. Influence of the N-1 alkyl chain length of cannabimimetic indoles upon CB(1) and CB(2) receptor binding. Drug Alcohol Depend. 2000;60:133–40. doi: 10.1016/s0376-8716(99)00152-0. [DOI] [PubMed] [Google Scholar]
- 36.Dow RL, Carpino PA, Hadcock JR, Black SC, Iredale PA, Silva-Jardine P, Schneider SR, Paight ES, Griffith DA, Scott DO, O’Connor RE, Nduaka CI. Discovery of 2-(2-chlorophenyl)-3-(4-chlorophenyl)-7-(2,2-difluoropropyl)-6,7-dihydro-2 H-pyrazolo[3,4-f][1,4]oxazepin-8(5H)-one (PF-514273), a novel, bicyclic lactam-based cannabinoid-1 receptor antagonist for the treatment of obesity. J Med Chem. 2009;52:2652–5. doi: 10.1021/jm900255t. [DOI] [PubMed] [Google Scholar]
- 37.Iwamura H, Suzuki H, Ueda Y, Kaya T, Inaba T. In vitro and in vivo pharmacological characterization of JTE-907, a novel selective ligand for cannabinoid CB2 receptor. J Pharmacol Exp Ther. 2001;296:420–5. [PubMed] [Google Scholar]
- 38.Trincavelli ML, Tuscano D, Cecchetti P, Falleni A, Benzi L, Klotz KN, Gremigni V, Cattabeni F, Lucacchini A, Martini C. Agonist-induced internalization and recycling of the human A(3) adenosine receptors: role in receptor desensitization and resensitization. J Neurochem. 2000;75:1493–501. doi: 10.1046/j.1471-4159.2000.0751493.x. [DOI] [PubMed] [Google Scholar]
- 39.Best JL, Amezcua CA, Mayr B, Flechner L, Murawsky CM, Emerson B, Zor T, Gardner KH, Montminy M. Identification of small-molecule antagonists that inhibit an activator: coactivator interaction. Proc Natl Acad Sci U S A. 2004;101:17622–7. doi: 10.1073/pnas.0406374101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang C, Ma WY, Dawson MI, Rincon M, Flavell RA, Dong Z. Blocking activator protein-1 activity, but not activating retinoic acid response element, is required for the antitumor promotion effect of retinoic acid. Proc Natl Acad Sci U S A. 1997;94:5826–30. doi: 10.1073/pnas.94.11.5826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Atwood BK, Wager-Miller J, Haskins C, Straiker A, Mackie K. Functional selectivity in CB(2) cannabinoid receptor signaling and regulation: implications for the therapeutic potential of CB(2) ligands. Mol Pharmacol. 2012;81:250–63. doi: 10.1124/mol.111.074013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aksamitiene E, Kholodenko BN, Kolch W, Hoek JB, Kiyatkin A. PI3K/Akt-sensitive MEK-independent compensatory circuit of ERK activation in ER-positive PI3K-mutant T47D breast cancer cells. Cell Signal. 2010;22:1369–78. doi: 10.1016/j.cellsig.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chang F, Steelman LS, Lee JT, Shelton JG, Navolanic PM, Blalock WL, Franklin RA, McCubrey JA. Signal transduction mediated by the Ras/Raf/MEK/ERK pathway from cytokine receptors to transcription factors: potential targeting for therapeutic intervention. Leukemia. 2003;17:1263–93. doi: 10.1038/sj.leu.2402945. [DOI] [PubMed] [Google Scholar]
- 44.Bjork K, Hansson AC, Sommer WH. Genetic variation and brain gene expression in rodent models of alcoholism implications for medication development. Int Rev Neurobiol. 2010;91:129–71. doi: 10.1016/S0074-7742(10)91005-2. [DOI] [PubMed] [Google Scholar]
- 45.Chrivia JC, Kwok RP, Lamb N, Hagiwara M, Montminy MR, Goodman RH. Phosphorylated CREB binds specifically to the nuclear protein CBP. Nature. 1993;365:855–9. doi: 10.1038/365855a0. [DOI] [PubMed] [Google Scholar]
- 46.Karin M. The regulation of AP-1 activity by mitogen-activated protein kinases. J Biol Chem. 1995;270:16483–6. doi: 10.1074/jbc.270.28.16483. [DOI] [PubMed] [Google Scholar]
- 47.Fanjul A, Dawson MI, Hobbs PD, Jong L, Cameron JF, Harlev E, Graupner G, Lu XP, Pfahl M. A new class of retinoids with selective inhibition of AP-1 inhibits proliferation. Nature. 1994;372:107–11. doi: 10.1038/372107a0. [DOI] [PubMed] [Google Scholar]
- 48.Lefkowitz RJ, Whalen EJ. beta-arrestins: traffic cops of cell signaling. Curr Opin Cell Biol. 2004;16:162–8. doi: 10.1016/j.ceb.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 49.DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK. Beta-arrestins and cell signaling. Annu Rev Physiol. 2007;69:483–510. doi: 10.1146/annurev.physiol.69.022405.154749. [DOI] [PubMed] [Google Scholar]
- 50.Rubino T, Vigano D, Premoli F, Castiglioni C, Bianchessi S, Zippel R, Parolaro D. Changes in the expression of G protein-coupled receptor kinases and beta-arrestins in mouse brain during cannabinoid tolerance: a role for RAS-ERK cascade. Mol Neurobiol. 2006;33:199–213. doi: 10.1385/MN:33:3:199. [DOI] [PubMed] [Google Scholar]
- 51.Derkinderen P, Valjent E, Toutant M, Corvol JC, Enslen H, Ledent C, Trzaskos J, Caboche J, Girault JA. Regulation of extracellular signal-regulated kinase by cannabinoids in hippocampus. J Neurosci. 2003;23:2371–82. doi: 10.1523/JNEUROSCI.23-06-02371.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Beaulieu JM, Sotnikova TD, Marion S, Lefkowitz RJ, Gainetdinov RR, Caron MG. An Akt/beta-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell. 2005;122:261–73. doi: 10.1016/j.cell.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 53.Terrillon S, Bouvier M. Receptor activity-independent recruitment of betaarrestin2 reveals specific signalling modes. EMBO J. 2004;23:3950–61. doi: 10.1038/sj.emboj.7600387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Urs NM, Daigle TL, Caron MG. A dopamine D1 receptor-dependent beta-arrestin signaling complex potentially regulates morphine-induced psychomotor activation but not reward in mice. Neuropsychopharmacol. 2011;36:551–8. doi: 10.1038/npp.2010.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shoemaker JL, Ruckle MB, Mayeux PR, Prather PL. Agonist-directed trafficking of response by endocannabinoids acting at CB2 receptors. J Pharmacol Exp Ther. 2005;315:828–38. doi: 10.1124/jpet.105.089474. [DOI] [PubMed] [Google Scholar]
- 56.Macey TA, Ingram SL, Bobeck EN, Hegarty DM, Aicher SA, Arttamangkul S, Morgan MM. Opioid receptor internalization contributes to dermorphin-mediated antinociception. Neuroscience. 2010;168:543–50. doi: 10.1016/j.neuroscience.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Large M, Sharma S, Compton MT, Slade T, Nielssen O. Cannabis Use and Earlier Onset of Psychosis: A Systematic Meta-analysis. Arch Gen Psychiatry. 2011;68:555–61. doi: 10.1001/archgenpsychiatry.2011.5. [DOI] [PubMed] [Google Scholar]
- 58.Zhang Y, Damjanoska KJ, Carrasco GA, Dudas B, D’Souza DN, Tetzlaff J, Garcia F, Hanley NR, Scripathirathan K, Petersen BR, Gray TS, Battaglia G, Muma NA, Van de Kar LD. Evidence that 5-HT2A receptors in the hypothalamic paraventricular nucleus mediate neuroendocrine responses to (−)DOI. J Neurosci. 2002;22:9635–42. doi: 10.1523/JNEUROSCI.22-21-09635.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weisstaub NV, Lira A, Zhou M, Bradley-Moore M, Merker R, Gingrich JA. Altered anxiety-related behaviors in 5-HT2A receptor knock out mice. Neuroscience Abst. 2004;468(10) [Google Scholar]
- 60.Ichikawa J, Dai J, Meltzer HY. DOI, a 5-HT2A/2C receptor agonist, attenuates clozapine-induced cortical dopamine release. Brain Res. 2001;907:151–5. doi: 10.1016/s0006-8993(01)02596-3. [DOI] [PubMed] [Google Scholar]
- 61.Shelton RC, Sanders-Bush E, Manier DH, Lewis DA. Elevated 5-HT 2A receptors in postmortem prefrontal cortex in major depression is associated with reduced activity of protein kinase A. Neuroscience. 2009;158:1406–15. doi: 10.1016/j.neuroscience.2008.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.O’Shea M, McGregor IS, Mallet PE. Repeated cannabinoid exposure during perinatal, adolescent or early adult ages produces similar longlasting deficits in object recognition and reduced social interaction in rats. J Psychopharmacol. 2006;20:611–21. doi: 10.1177/0269881106065188. [DOI] [PubMed] [Google Scholar]
- 63.Garcia-Gutierrez MS, Garcia-Bueno B, Zoppi S, Leza JC, Manzanares J. Chronic blockade of cannabinoid CB2 receptors induces anxiolytic-like actions associated with alterations in GABA(A) receptors. Br J Pharmacol. 2012;165:951–64. doi: 10.1111/j.1476-5381.2011.01625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]