Abstract
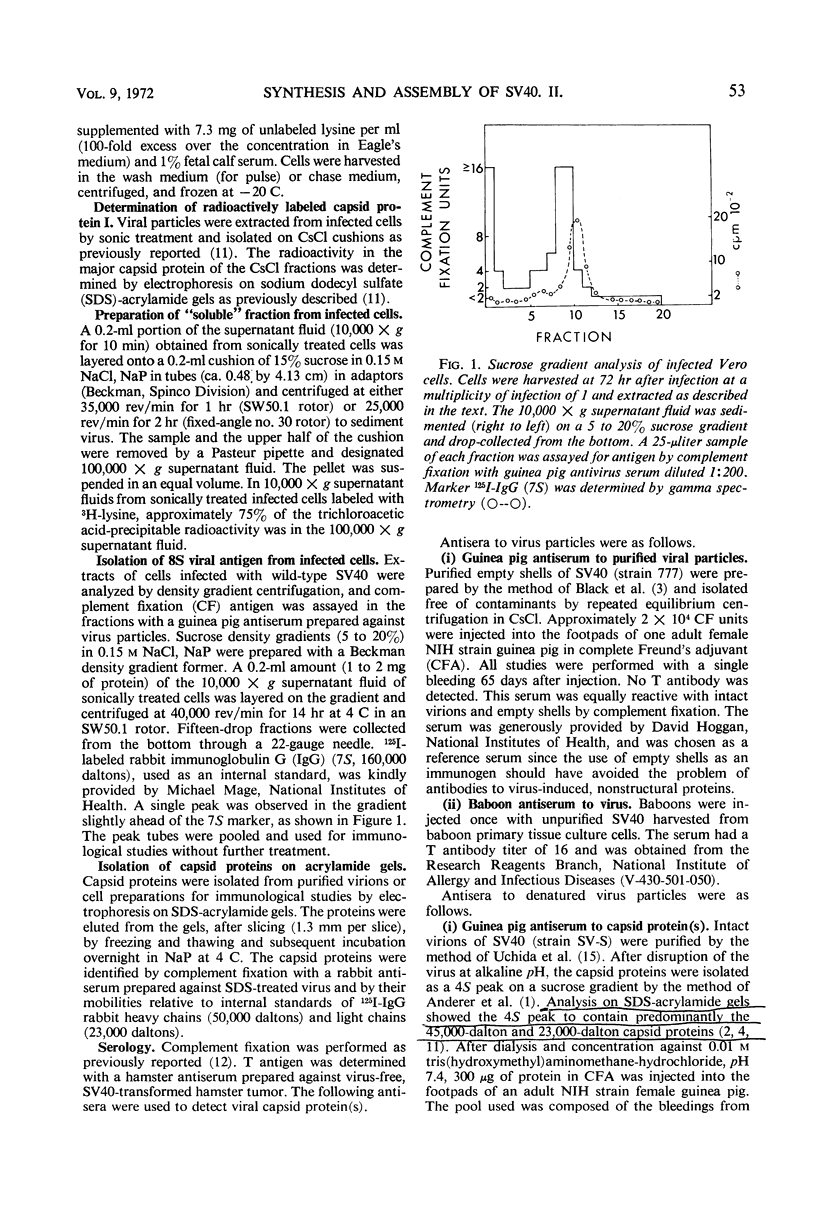
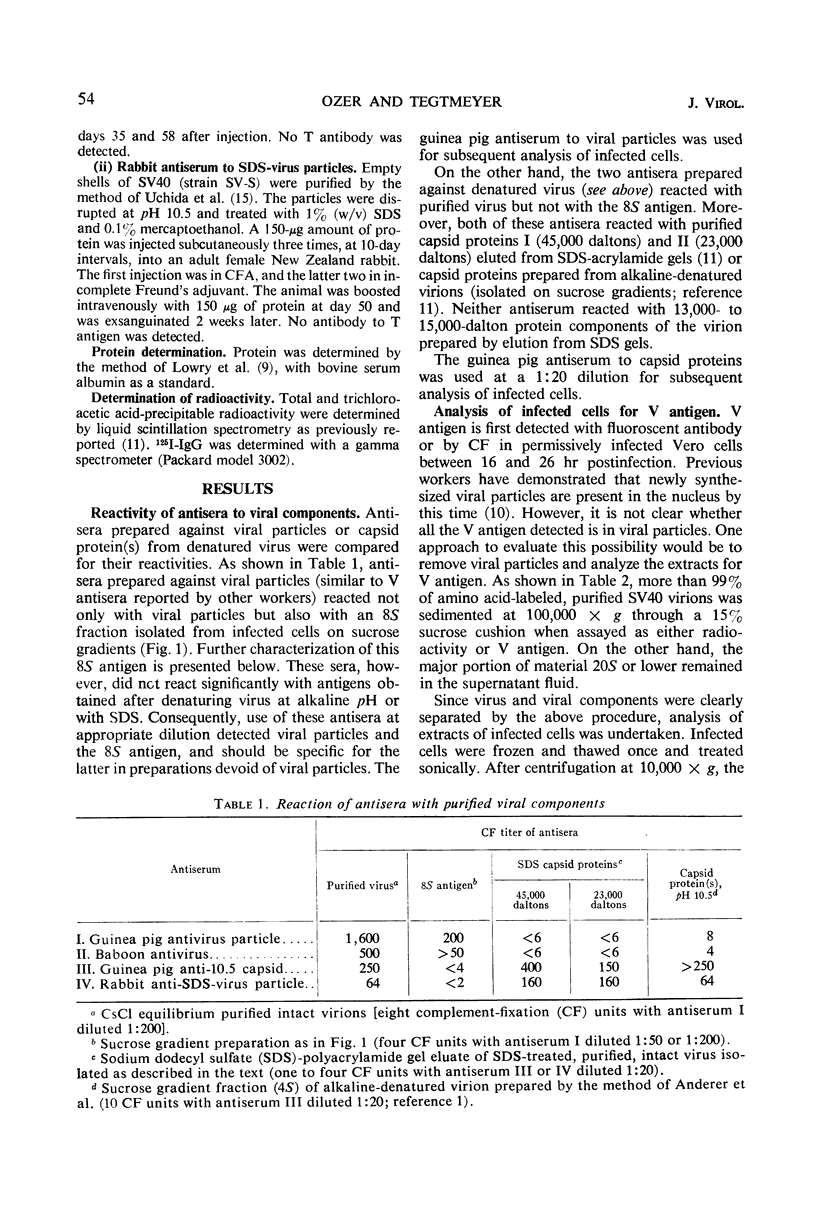
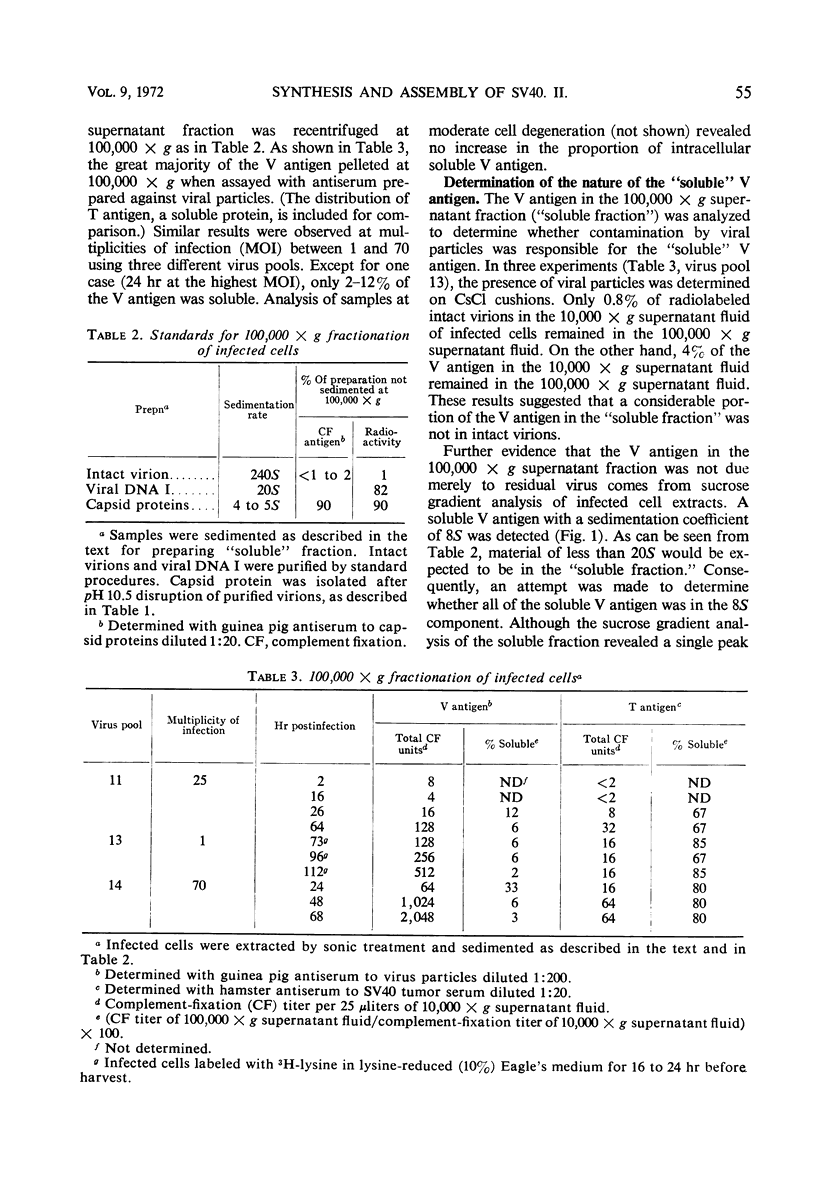
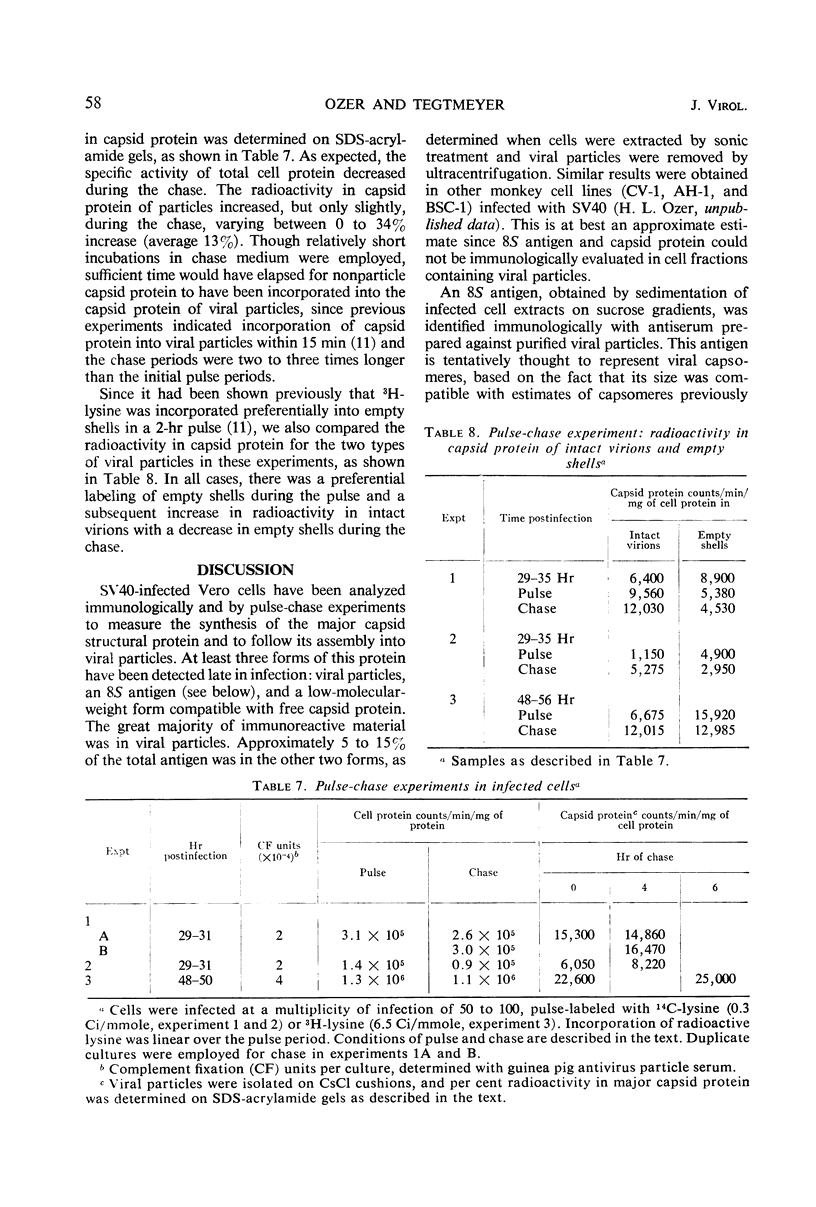
African green monkey kidney cells infected by simian virus 40 were analyzed for the presence of the major capsid protein (capsid protein I) by immunological and radiolabeling techniques. Antisera with different specificities were prepared by immunization with intact or denatured viral particles. Antisera prepared against intact virus reacted by complement fixation with viral particles and with an 8S subunit containing the capsid protein I. Antisera prepared against denatured viral particles reacted with unassembled capsid protein(s) as well as with viral particles. These antisera were used to detect 8S viral subunits or unassembled viral capsid protein in soluble extracts of infected cells after centrifugation at 100,000 × g to remove viral particles. The soluble antigen pool was found to be small during infection with wild-type virus or a temperature-sensitive mutant deficient in the synthesis of viral particles. Pulse-chase experiments, performed at a high multiplicity of infection, also indicated a small pool of nonparticle capsid protein I. Radioactive lysine was incorporated into capsid protein I of virus particles during a 2-hr pulse. A subsequent chase with excess unlabeled lysine resulted in only a slight increase in the radio-activity found in capsid protein I of viral particles. Furthermore, in the same experiments, capsid protein I was incorporated preferentially into empty shells during the pulse with a shift in radioactivity to intact virions during the chase period, indicating a possible precursor relationship between the two types of virus particles.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderer F. A., Koch M. A., Schlumberger H. D. Structure of simian virus 40. 3. Alkaline degradation of the virus particle. Virology. 1968 Mar;34(3):452–458. doi: 10.1016/0042-6822(68)90065-2. [DOI] [PubMed] [Google Scholar]
- BLACK P. H., CRAWFORD E. M., CRAWFORD L. V. THE PURIFICATION OF SIMIAN VIRUS 40. Virology. 1964 Nov;24:381–387. doi: 10.1016/0042-6822(64)90175-8. [DOI] [PubMed] [Google Scholar]
- Barban S., Goor R. S. Structural proteins of simian virus 40. J Virol. 1971 Feb;7(2):198–203. doi: 10.1128/jvi.7.2.198-203.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes M. K., Huang E. S., Pagano J. S. Structural polypeptides of simian virus 40. J Virol. 1971 May;7(5):635–641. doi: 10.1128/jvi.7.5.635-641.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer H., Munk K. The pattern of protein synthesis in SV40-infected CV-1 cells. Int J Cancer. 1970 Jan 15;5(1):21–27. doi: 10.1002/ijc.2910050104. [DOI] [PubMed] [Google Scholar]
- Friedmann T. In vitro reassembly of shell-like particles from disrupted polyoma virus. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2574–2578. doi: 10.1073/pnas.68.10.2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson M. F., Baltimore D. Morphogenesis of poliovirus. I. Association of the viral RNA with coat protein. J Mol Biol. 1968 Apr 28;33(2):369–378. doi: 10.1016/0022-2836(68)90195-2. [DOI] [PubMed] [Google Scholar]
- Koch M. A., Eggers H. J., Anderer F. A., Schlumberger H. D., Frank H. Structure of simian virus 40. I. Purification and physical characterization of the virus particle. Virology. 1967 Jul;32(3):503–510. doi: 10.1016/0042-6822(67)90302-9. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MAYOR H. D., STINEBAUGH S. E., JAMISON R. M., JORDAN L. E., MELNICK J. L. Immunofluorescent, cytochemical, and microcytological studies on the growth of the simian vacuolating virus (SV-40) in tissue culture. Exp Mol Pathol. 1962 Oct;1:397–416. doi: 10.1016/0014-4800(62)90033-3. [DOI] [PubMed] [Google Scholar]
- Ozer H. L. Synthesis and assembly of simian virus 40. I. Differential synthesis of intact virions and empty shells. J Virol. 1972 Jan;9(1):41–51. doi: 10.1128/jvi.9.1.41-51.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozer H. L., Takemoto K. K. Site of host restriction of simian virus 40 mutants in an established African green monkey kidney cell line. J Virol. 1969 Oct;4(4):408–415. doi: 10.1128/jvi.4.4.408-415.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAPP F., BUTEL J. S., FELDMAN L. A., KITAHARA T., MELNICK J. L. DIFFERENTIAL EFFECTS OF INHIBITORS ON THE STEPS LEADING TO THE FORMATION OF SV40 TUMOR AND VIRUS ANTIGENS. J Exp Med. 1965 Jun 1;121:935–944. doi: 10.1084/jem.121.6.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P., Ozer H. L. Temperature-sensitive mutants of simian virus 40: infection of permissive cells. J Virol. 1971 Oct;8(4):516–524. doi: 10.1128/jvi.8.4.516-524.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]



