Abstract
The liver is a common site for metastatic malignancies, particularly from the gastrointestinal tract. It also may be involved by primary neoplasms, both benign and malignant. Cytopathologic examination of mass lesions of the liver with pertinent use of ancillary studies is a useful method of establishing a correct diagnosis for patient management. The authors reviewed the literature for articles pertaining to cytologic characteristics of specific tumor types, utility of immunohistochemical markers and pertinent molecular studies, differential diagnoses and pitfalls.
Key Words: Cytology, liver, brush cytology, endoscopic ultrasound, fine needle aspiration
Introduction
The main indication for fine needle aspiration (FNA) of the liver is in the diagnosis of focal mass lesions. This includes both primary as well as metastatic neoplasms. Blind percutaneous biopsy with a large core needle is the preferred method for evaluating diffuse liver diseases (hepatitis, cirrhosis) where architectural details are important. FNA is not useful in identifying diffuse liver disorders such as hepatitis or cirrhosis, but may be employed to rule out neoplasms from the differential diagnosis when inflammatory or diffuse liver diseases appear to be non-homogenous or mimic mass-like lesions on radiology.
The definition of FNA is arbitrarily defined as that performed with needles of 1mm diameter or less, so practically any needles smaller than 19 G. FNA is usually performed percutaneously via abdominal/lower thoracic wall with a 22-23 G needle, 80-150 mm in length, under CT or ultrasound guidance, or EUS for smaller masses. Blind needle aspirates may be performed for large palpable masses (1).
FNA is a rapid, safe and extremely cost effective method for diagnosis. Studies have shown FNA to be more sensitive (81-93.5%) and specific technique for diagnosing malignancy than conventional biopsy in experienced hands (2,3). The smaller diameter of the needle allows more extensive and multiple samples to be obtained; any region of the liver, including lesions in the left lobe and the porta hepatis may be sampled (which is too risky to perform using large bore needles).
Multiple samples are easy to obtain, allowing for greater sampling. All portions of the liver can be safely sampled. Sensitivity and specificity of malignant diagnoses are high. False positive diagnoses are uncommon (4). Combined cytology and histology are often complimentary and increase diagnostic sensitivity (washings and imprints from needle biopsy specimens, cell block preparations) and is the ideal approach to obtain maximal diagnostic information. Often the radiologists will first perform FNA with on-site evaluation of adequacy by the cytologist and end the procedure with a final core biopsy specimen. There are instances where the FNA contains abundant diagnostic material but the tissue biopsy is non-diagnostic and vice-versa. For hilar lesions of the liver, brushings obtained at the time of ERCP and endoscopically guided FNA are the preferred methods to obtain diagnostic material.
Complications of FNA are rare, hemorrhage, hematoma formation, bile peritonitis, pneumothorax, Gram-negative sepsis and tumor seeding has been reported. These complications are less than those reported for wider bore biopsies (5-6).
Contraindications include refractory bleeding diathesis, uncooperative patient, massive ascites, severe emphysema, suspected hydatid cyst (as rupture may precipitate an anaphylactic reaction). Cytology is less useful in diagnosing specific localized non neoplastic and benign liver lesions, but is nevertheless helpful in excluding a malignant process.
Sample preparation
Aspirate smears should be made rapidly to avoid clotting artifacts, which will seriously compromise the cytologist’s rendering of a complete and accurate diagnosis. Diff-Qiuk stain on air dried smears or Toluidin Blue on alcohol fixed smears may be used to immediately assess the quality of the biopsy. Cell blocks prepared from rinsing the FNA needle after smear preparation as well as harvesting the entire contents of one or more FNA passes are extremely helpful. Several reasonably sized (0.5 to 1 mm) fragments of tissue should be obtained for cell block. If concomitant core biopsy specimens are obtained they should be processed separately.
Normal liver
Needle aspirates of normal liver consist predominantly of hepatocytes, with admixed biliary epithelial cells, Kupffer cells and endothelial cells. The hepatocytes are present as single cells, or monolayered small cell groups and sheets. The cells are round, polygonal, have well defined cell borders, and granular dense cytoplasm. Hepatocytes frequently contain cytoplasmic pigments (lipofuscin, hemosiderin, bile pigment, copper). The hepatocyte nucleus is round/oval, with smooth nuclear contour, fine evenly dispersed chromatin, and conspicuous nucleolus. There may be mild anisonucleosis (in extreme uniformity a well-differentiated neoplasm needs to be excluded). Scattered binucleation may be present. Numerous intranuclear inclusions are seen in Diabetes Mellitus and Wilson’s disease.
Bile duct cells are sparse in normal liver aspirates. They appear as monolayer sheets of uniform columnar to cuboidal cells with evenly spaced nuclei (“honeycomb appearance”). Bile ductal cells have less cytoplasm than hepatocytes, and contain round to oval nuclei with indistinct nucleoli (Figure 1).
Figure 1.
Normal polygonal hepatocytes with granular eosinophilic cytoplasm and distinct cell borders; a small cluster of tiny bile ductal cells in a honeycomb pattern is present at the lower right corner (Pap stain, 400×)
Reactive hepatocytes show nuclear size variation, the nuclear volume between cells can exceed a 4:1 ratio. There may be macronucleoli. However the nucleus to cytoplasmic ratio is normal and the nuclear membranes are smooth, and mitotic activity is rare. There may be a background of necrotic debris, histiocytes and acute inflammatory cells. If exceedingly large polygonal cells with bizarre shaped nuclei, hyperchromasia and multinucleation are seen, liver cell dysplasia is suggested (nuclear volume ratios are in the 8:1 range) as seen in cirrhosis, hepatitis B or hepatocellular carcinoma.
Hepatocellular carcinoma
Hepatocellular carcinoma (HCC) accounts for 90% of all primary cancers of the liver. It is far more common in Africa and Asia, where it constitutes 20-40% of all malignancies, often in the 3rd-4th decade. In the Western world HCC accounts for 2% of all malignancies, and usually presents in the 6th-7th decade. Serum alpha-fetoprotein (AFP) levels >1,000 ng/mL are virtually diagnostic, but are not always elevated. HCC is associated with cirrhosis, Hepatitis B and C, and congenital metabolic diseases.
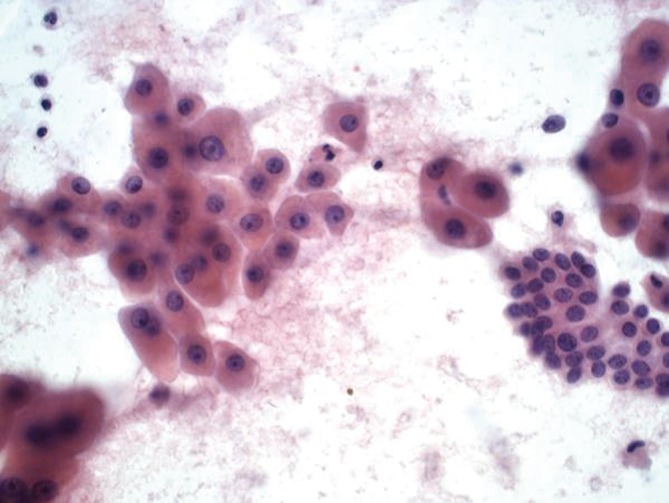
Aspirates of HCC show increased cellularity and discohesiveness, with crowding and piling within cell groupings. A variable number of single cells may be present. The polygonal neoplastic hepatocytes are present in abnormally thick trabecular cords (Figure 2). Endothelial cells surround the abnormal trabecular cords, which are greater than three cell layers thick. Solid sheets, tubular or pseudoglandular structures may be present, resembling metastatic carcinoma. Tumor cells have granular cytoplasm, and there may be evidence of bile production. Atypical stripped hepatocyte nuclei may be seen (Figure 3). The hepatocyte nuclei are large round with prominent nucleoli. Nucleus to cytoplasmic ratio is increased. Intranuclear inclusions may be present. 10% of HCC may have clear cytoplasm (resembling clear cell carcinomas from the kidney and adrenal cortex) (7). Spindle, pleomorphic and multinucleated tumor giant cells may be present.
Figure 2.
Hepatocellular carcinoma with abnormally thick trabecular cords (DQ stain, 200×)
Figure 3.
Hepatocellular carcinoma with cells displaying prominent nucleoli, increased nuclear:cytoplasmic ratios, and atypical stripped nuclei (Pap stain, 400×)
Immunohistochemical stains are particularly helpful in differentiating HCC from benign lesions, other primary and metastatic malignancies. Polyclonal CEA (Figure 4), CD10, and villin stain normal and neoplastic hepatocytes in a canalicular pattern. HCC is AFP, alpha-1-antitrypsin, low molecular weight keratin, CAM 5.2 and Hep Par 1 positive. CD 34 immunohistochemical stain shows positivity in the lining sinusoidal cells. TTF-1 may show cytoplasmic positivity. Cytokeratin 7, high molecular weight keratin and keratin AE1-AE3 are negative (8-11).
Figure 4.
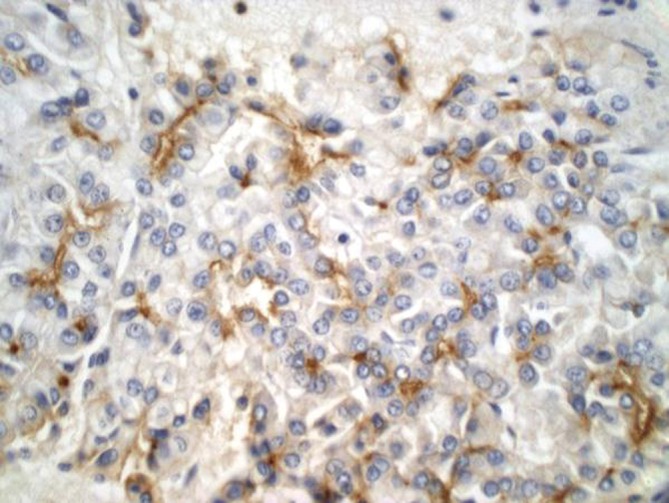
Hepatocellular carcinoma showing a characteristic canalicular polyclonal CEA staining pattern (pCEA stain, 400×)
Fibrolamellar carcinoma
This variant of HCC is seen predominantly in young adults (average age, mid 20s), and is not associated with cirrhosis. Radiologically the liver mass shows a central stellate scar. Serum AFP levels are normal. Recognition of this variant is important as the prognosis is more favorable with a 50% five year survival rate.
Aspirates are cellular with scattered mostly single large polygonal tumor cells with abundant eosinophilic granular cytoplasm. Cells are three to four times larger than hepatocytes, which they resemble (Figure 5). Some cells may show hyaline intracytoplasmic (fibrinogen) globules. Parallel bands of fibrous connective tissue (lamellar fragments) surround tumor cells (12,13).
Figure 5.
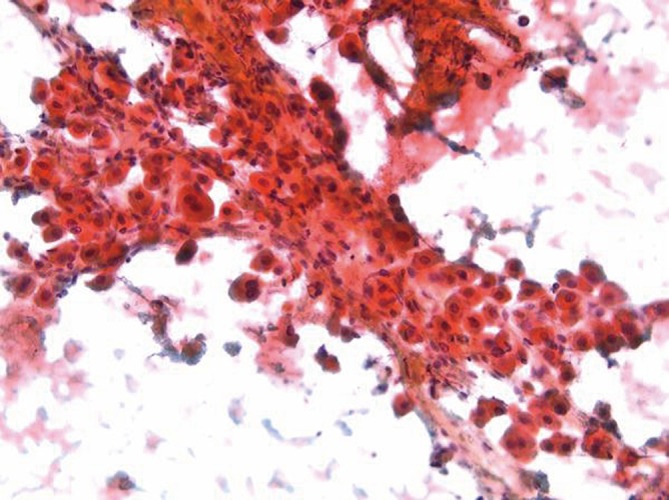
Fibrolamellar carcinoma showing scattered large eosinophilic cells surrounded by parallel bands of fibrosis (Pap stain, 200×)
Hepatoblastoma
Hepatoblastoma is a rare tumor seen in infancy and childhood. It is the most common primary hepatic tumor of childhood, usually seen in children less than three years of age. Serum AFP levels are markedly elevated. Epithelial (fetal, embryonal) and mixed epithelial/mesenchymal types are recognized.
These tumors are seldom aspirated. Cytologic smears may resemble HCC with larger more pleomorphic cells, or those from small, blue, round cell tumors of childhood. Mesenchymal elements and hematopoetic cells are rarely seen in aspirates, but may be seen in cell block preparations. Mitotic figures are frequent (Figure 6).
Figure 6.
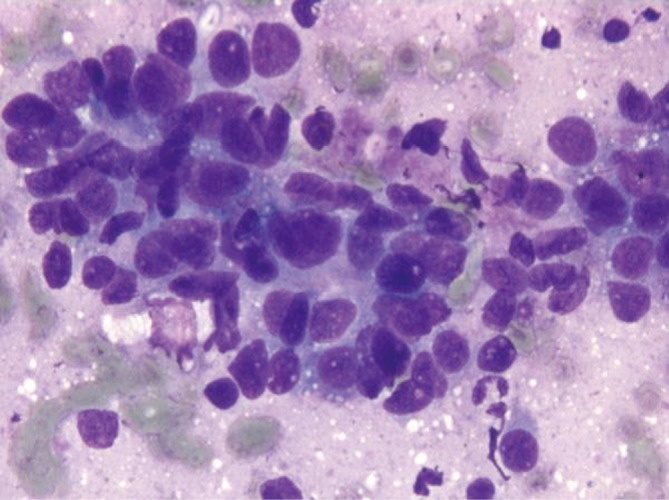
Hepatoblastoma showing small round cells with a small amount of cytoplasm (DQ stain, 400×)
Tumor cells are low molecular weight cytokeratin and AFP positive. The differential diagnosis includes HCC, other small cell neoplasms of childhood (neuroblastoma, rhabdomyosarcoma, Wilm tumor, lymphoma).
Undifferentiated embryonal sarcoma
Undifferentiated embryonal sarcoma (UES) is a rare primary hepatic pediatric neoplasm, accounting for 13% of pediatric hepatic malignancies (14). Rare cases have been reported in adults. Prognosis is poor, but survival has been reported after complete surgical resection. It is a neoplasm with primitive mesenchymal phenotype. Spindle, oval or stellate cells are admixed with myxoid stroma (Figure 7). Tumor is positive for desmin, keratin, alpha-1-trypsin and alpha-1-chymotrypsin.
Figure 7.
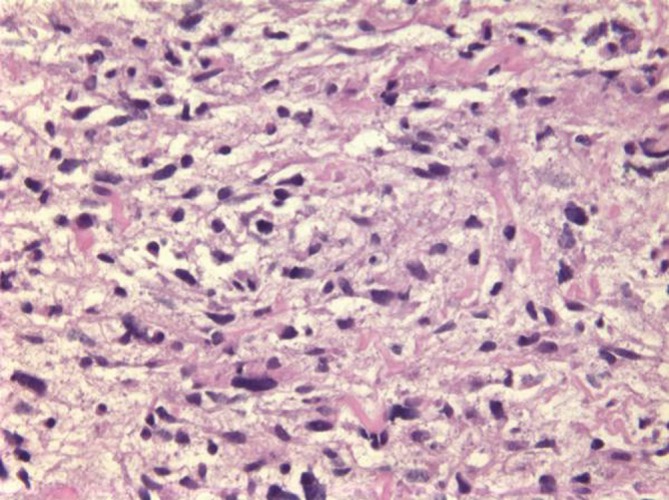
Undifferentiated embryonal sarcoma, with spindled and stellate cells in a background of myxoid stroma (H&E, 200×)
Cholangiocarcinoma
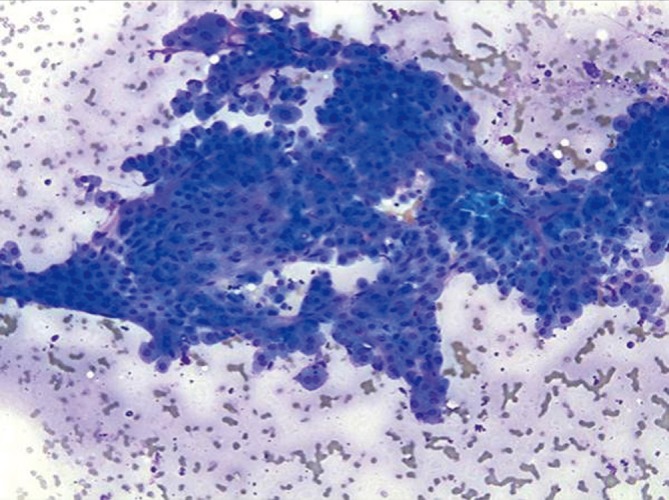
Cholangiocarcinoma is less common than HCC. It is not associated with cirrhosis. Ca 19-9 and CEA levels are significantly elevated. Patients are usually in 6th-7th decade of life. Parasites (chlonorchis sinensis), intrahepatic lithiasis, and sclerosing cholangitis are predisposing factors. The diagnosis can be made in the majority of patients using bile cytology, brush cytology or percutaneous FNA (15).
Ductal components are abundant and in broader sheets admixed with varying numbers of non-neoplastic hepatocytes (Figures 8,9). Glandular differentiation may be better appreciated on cell block preparations. Tumor cells resemble other well differentiated adenocarcinomas (mucin secreting, signet ring cell), adenosquamous and squamous cell carcinoma. The differential diagnosis includes HCC and metastatic adenocarcinoma. Tumor cells are high and low molecular weight keratins, CK7, CK19, and polyclonal CEA (cytoplasmic) positive. AFP stain is negative. Mucicarmine stain is positive in tumor cells, unlike HCC.
Figure 8.
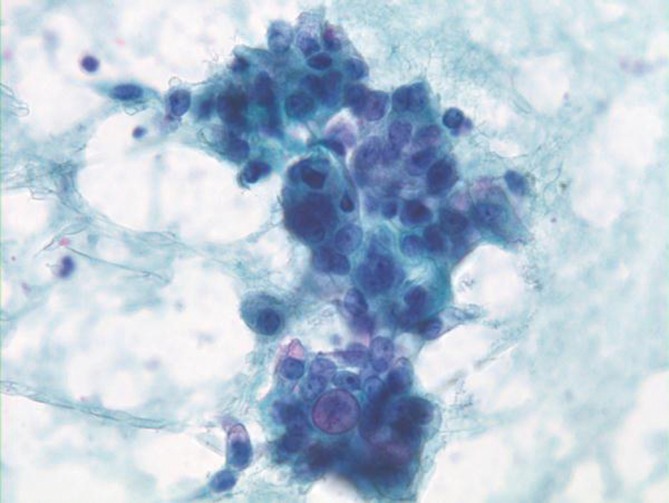
Cholangiocarcinoma with pleomorphic, enlarged hypochromatic nuclei and occasional nucleoli (Pap stain, 400×)
Figure 9.
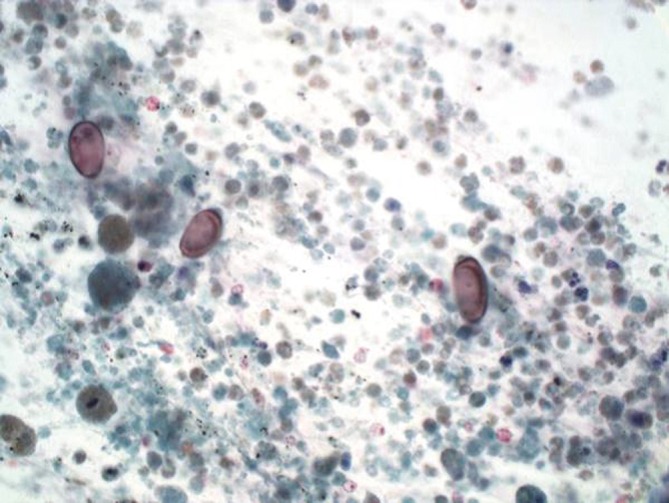
Cholangiocarcinoma with tumor diathesis and brown oval chlonorchis eggs (Pap stain, 400×)
The distinction between cholangiocarcinoma and metastatic carcinoma is challenging, and is best made on clinical grounds by ruling out other primary sites.
Angiosarcoma
Angiosarcoma is an uncommon highly malignant tumor. It represents less than 1% of primary hepatic malignancies. A third of these tumors arise in a setting of cirrhosis. It is seen with increased frequency in patients who have been exposed to arsenic compounds, polyvinylchloride or thorotrast radiographic contrast agent. Tumors may be well or poorly differentiated. Massive bleeding is a potential complication of FNA (5).
Aspirates are often very bloody. Suspect angiosarcoma when unexpected cellularity is obtained despite significant blood. Well differentiated tumors show spindle cells singly and in tight clusters. Less well differentiated tumors show larger, pleomorphic bizarre cells, frequently with ingested cytoplasmic material (Figure 10) Factor 8 and CD 34 staining is helpful in confirming the diagnosis and differentiating the tumor cells from metastatic sarcomas (most commonly leiomyosarcoma).
Figure 10.
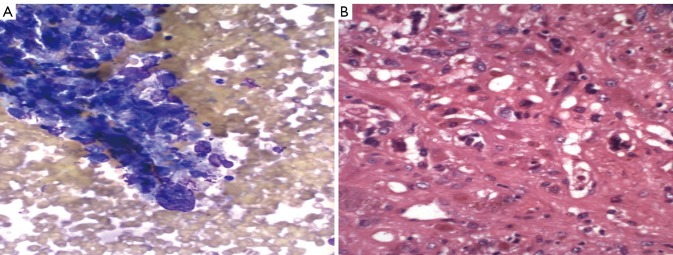
A. angiosarcoma showing tight clusters of pleomorphic bizarre cells in a bloody background (DQ stain, 400×); B. angiosarcoma with bizarre cells and numerous mitotic figures (H&E, 400×)
Epitheliod hemangioendothelioma
Epitheliod hemangioendothelioma is an uncommon tumor that may arise in the liver. It is also a tumor of endothelial cells but behaves in a less malignant fashion than angiosarcoma. Tumor cells are positive for endothelial markers. Cytologic findings may be non-diagnostic, inconclusive or at most suggestive of the diagnosis.
Metastatic tumors
The liver is a common site for metastases, especially from malignant epithelial tumors in sites drained by the portal venous system (GI tract, pancreas). Other common primary sites include the lung, breast, kidney and melanoma. Sarcomas, sarcomatoid carcinomas and lymphomas may also involve the liver. FNA plays an important role in the diagnosis of metastatic disease in the liver. Most aspirated masses prove to be metastatic malignancies (16).
Clinical, laboratory and radiologic findings are important. Multiple nodules of various sizes distributed randomly suggest metastases. The primary site may already be known during aspiration. Occasionally the primary tumor is not known and the pathologist is expected to help identify the cell or origin. This is of vital importance as frequently therapy is based on tumor type. Cytomorphology of the tumor cells, comparison with available cytology or histology of the original tumor and pertinent ancillary studies are helpful in suggesting a specific diagnosis.
Tumors with suspected metastatic disease can be divided into three main groups. Tumors in which likely tumor and primary site can be predicted with a high level of confidence based on cytologic appearances, tumors having characteristic cytologic pattern, but without specific clues to primary site, and undifferentiated neoplasms. Small cell carcinoma of lung, renal clear cell carcinoma, colon carcinoma and breast carcinoma fall into the first category. Most adenocarcinomas, squamous cell carcinomas and lymphomas fall into the second category.
Colonic adenocarcinoma
There is often necrotic debris, often dominating the aspirate (Figures 11,12). Cells are columnar or cuboidal with nuclear polarity. Glandular, palisading arrangement of tumor cells may be seen. Nuclei are characteristically cigar-shaped (enlarged, elongated), hyperchromatic with clumped chromatin. Tumor cells are CK 20, CEA, villin and CDX2 positive.
Figure 11.
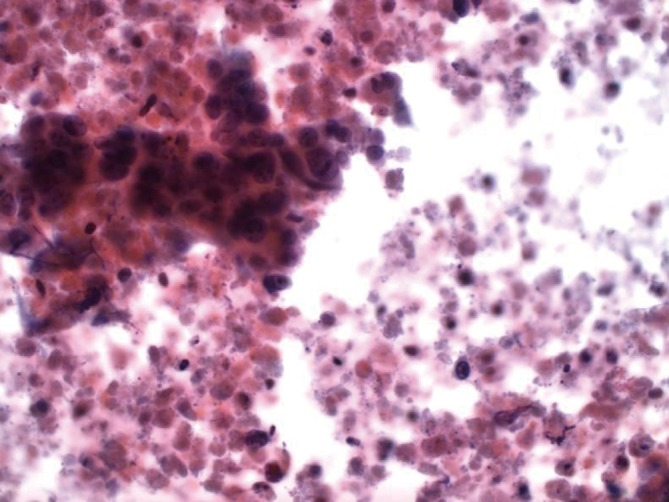
Colon carcinoma metastatic to the liver, showing glandular arrangement of cells with background of substantial necrosis (Pap stain, 400×)
Figure 12.
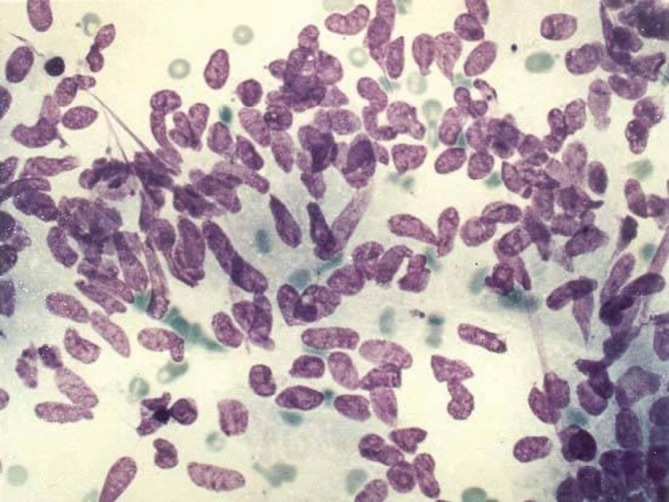
Metastatic colon carcinoma with cigar-shaped nuclei (DQ stain, 400×)
Breast carcinoma
There is usually a known history of breast cancer. Tumor cells with intracytoplasmic lumen formation and intracytoplasmic mucin (targetoid bodies) may be seen. These features are best seen in lobular carcinoma of breast. Tumor cells are CK7, GCDFP-15 positive.
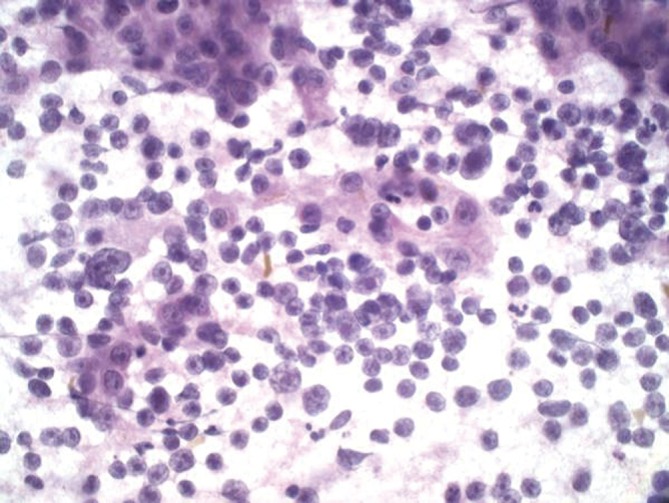
Small cell carcinoma
Hypercellular aspirates with tumor cells four to six times the size of red blood cells. Small cell clusters and single cells present. Nuclear/cytoplasmic ratios are extremely high. There may be nuclear molding. Crushed nuclei are common. Chromatin is granular with inconspicuous nucleoli (Figures 13,14). Mitotic figures are present. Widespread and single cell necrosis is seen. The differential diagnosis includes lymphoma. Small cell carcinoma is positive for CK7, TTF-1 and neuroendocrine markers.
Figure 13.
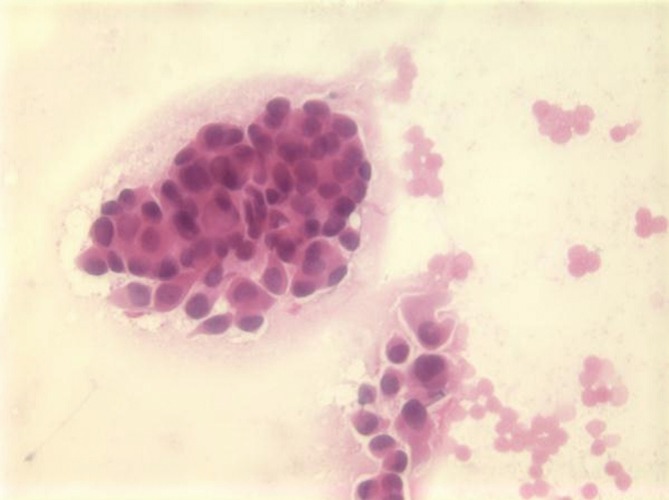
Metastatic ductal breast carcinoma with plasmacytoid nuclei forming ball-like clusters (Pap stain, 400×)
Figure 14.
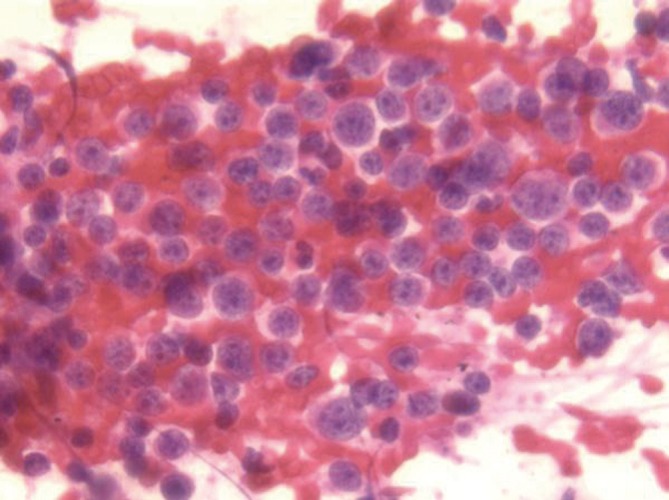
Small cell carcinoma metastatic to the liver, displaying numerous cells with minimal cytoplasm and granular “salt and pepper” chromatin (Pap stain, 400×)
Other neuroendocrine tumors
History or serum detection of single or multiple hormones supports and may predict the diagnosis. Cells are uniform in size and may be round/oval, polygonal or fusiform in shape. Mitoses and necrosis are usually not evident. Tumor cells have coarse “salt and pepper” chromatin and plasmacytoid/eccentric nuclei. The cells may be arranged in small rosette-like aggregates (Figure 15). Neuroendocrine markers are positive. The differential includes lymphoma.
Figure 15.
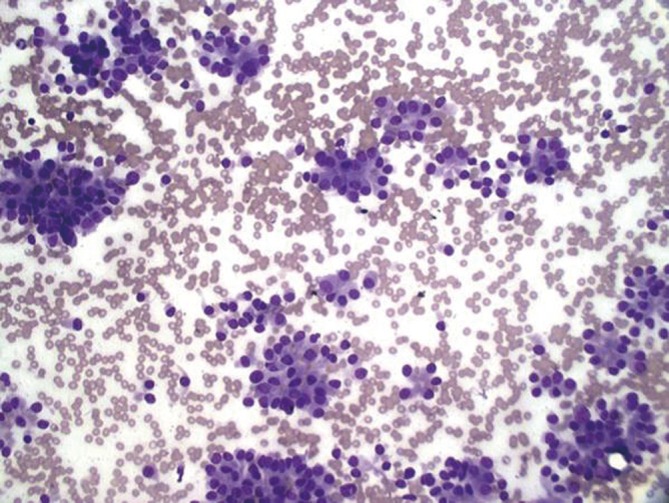
Low grade neuroendocrine carcinoma, showing rosette-like aggregates and eccentric nuclei. No necrosis or mitoses present (DQ, 200×).
Lymphoma
Aspirates are cellular and consist of dispersed single cells with high nuclear/cytoplasmic ratios. Smear background shows lymphoglandular bodies (“blue blobs”-rounded cytoplasmic fragments), these are best appreciated on Diff-Quik stains. The scant cytoplasm frequently stains intensely blue on Diff-Quik stains (Figure 16). The differential diagnosis includes small cell carcinoma and other neuroendocrine neoplasms. Flow cytometry and immunostains for various lymphoid markers (CD45) are positive for exact classification (17).
Figure 16.
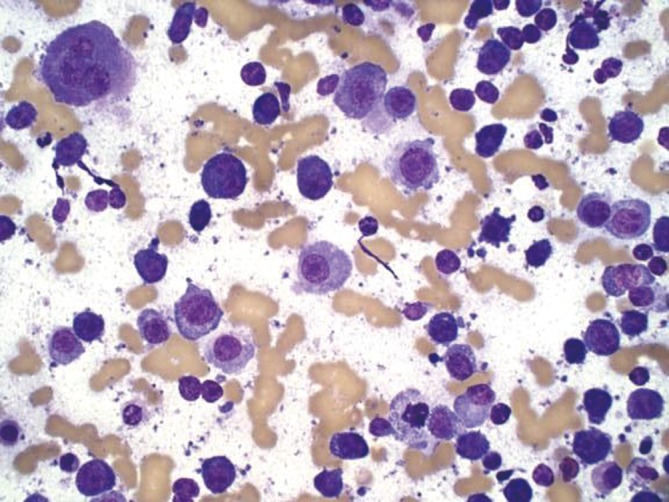
Lymphoma involving the liver with lymphoglandular bodies and a monotonous population of small cells with scant cytoplasm; the larger cells are reactive hepatocytes (DQ stain, 400×)
Melanoma
Aspirates are cellular consisting of dispersed single cells or pseudo cohesive when there is marked cellularity. Cells are usually polygonal, but may be spindle, small cell or anaplastic in morphology. Nuclei may be plasmacytoid/eccentric with single large nucleoli (Figure 17). Mirror-image binucleation is common. Melanin pigment if present will be dark blue on Diff-Qiuik stain, and yellow-brown, non refractile on Papanicolaou stain. Tumor cells are S-100, HMB-45, CD117 Melan-A positive (18).
Figure 17.
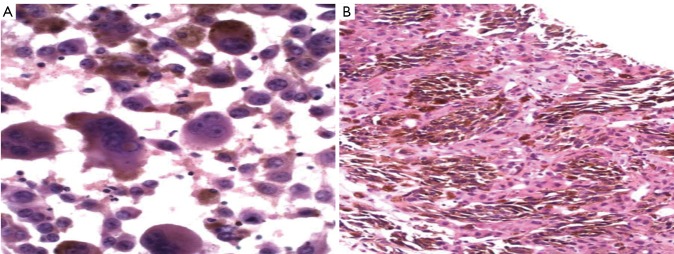
A. melanoma of an obvious nature, with pleomorphic multinucleated cells, plentiful brown melanin pigment present (Pap stain, 400×); B. melanoma metastatic to liver with copious melanin pigment (H&E, 200×)
Metastatic melanoma is particularly challenging on liver aspiration as the tumor cells have several features in common with both normal and neoplastic hepatocytes - polygonal cells with granular cytoplasm, and intra-nuclear cytoplasmic inclusions (Figure 18). The diagnosis may be missed if appropriate stains are not ordered in cases where the primary tumor is unknown or the information has not been relayed to the pathologist. Ocular melanomas have a peculiar tendency to metastasize to the liver, often many years after the initial diagnosis. Therefore beware the glass-eyed patient with liver enlargement!
Figure 18.
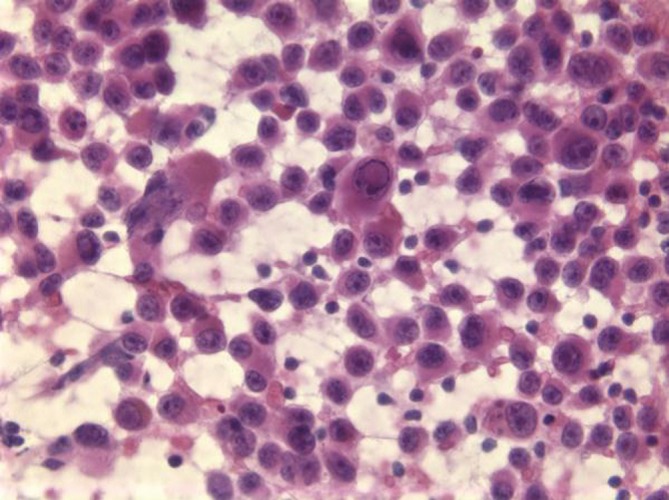
Amelanotic melanoma with single cells and occasional intranuclear pseudo-inclusions mimicking poorly differentiated HCC (Pap stain, 400×)
Clear cell renal cell carcinoma
Tumor cells have abundant clear cytoplasm and central round nuclei. The nuclear/cytoplasmic ratios do not appear to be elevated. There is often a prominent capillary vascular network (Figure 19). Tumor cells are vimentin and CD10 positive. The differential includes clear cell variant of HCC.
Figure 19.
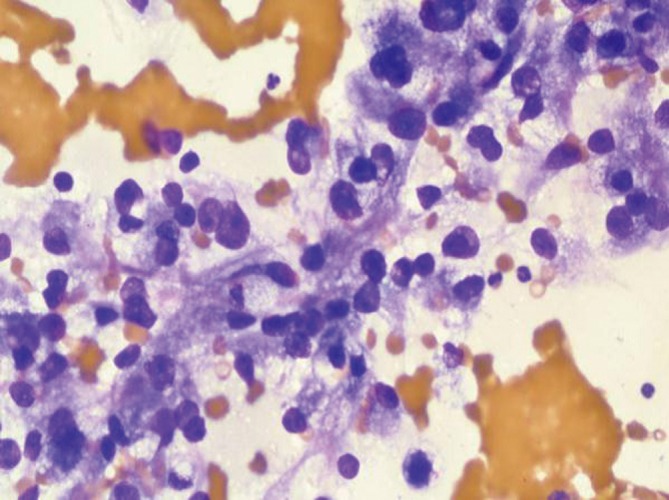
Clear cell renal cell carcinoma, showing abundant wispy cytoplasm and round central nuclei with prominent nucleoli (DQ stain, 400×)
Diagnostic pitfalls of liver cytology
Failure to recognize non hepatocyte tissue sampling can lead to errors in diagnosis. Sheets of mesothelial cells in trans-abdominal aspirations, or sampling of adjacent viscera (kidney, adrenal cortex and lung, particularly in right sided aspirates need to be recognized as such and not misinterpreted.
Normal and reactive hepatocytes may also have quite prominent nucleoli, but this should not be a uniform feature. Regenerative hepatocytes in cirrhotic livers may also show various degrees of dysplastic change. Benign bile ductal epithelial sheets may be diagnosed as metastatic adenocarcinoma if attention is not paid to the cohesive, uniform honeycomb appearance of the cells and two-dimensional sheets, rather than a haphazard three-dimensional grouping of tumor cells.
Malignant melanoma may resemble hepatocellular carcinoma. Clear cell HCC resembles metastatic clear cell renal cell carcinoma.
Summary
Cytology of the liver is a safe and sensitive technique for the diagnosis of mass forming lesions of the liver. Adequate, well preserved and prepared cytologic sampling is essential. The vast majority of primary or metastatic neoplasms can be identified morphologically and particularly with the help of confirmatory ancillary studies. Occasionally however well differentiated primary neoplasms (both benign and malignant) and rare lesions may be difficult to diagnose. Complete history, clinical, serologic and radiologic findings are essential. Thorough sampling, adequate well preserved and well prepared specimens (preferably in conjunction with cell blocks and even core biopsy) and expert interpretation are necessary for optimal results. The new trends in personalized molecular targeted therapy require better characterization and prediction of tumor behavior. Cytologic sampling is ideally suited for the procurement of tumor for these molecular studies.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- 1.Edoute Y, Osamah H, Malberger E, et al. Diagnostic accuracy of direct fine needle aspiration of liver lesions: a prospective study of 107 patients in a peripheral community center with limited technological capability. Arch Gastroenterohepatol 2000;2:1-2 [Google Scholar]
- 2.Sbolli G, Fornari F, Civardi G, et al. Role of ultrasound guided fine needle aspiration biopsy in the diagnosis of hepatocellular carcinoma. Gut 1990;31:1303-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nggada H, Ahidjo A, Ajayi N, et al. Correlation between ultrasound findings and ultrasound-guided fine needle aspiration cytology in the diagnosis of hepatic lesions: a Nigeriian tertiary hospital experience. Int J Gastroenterd 2007;5:1 [Google Scholar]
- 4.Wee A. Fine-needle aspiration biopsy of hepatocellular carcinoma and related hepatocellular nodular lesions in cirrhosis: controversies, challenges, and expectations. Patholog Res Int 2011;2011:587936. [DOI] [PMC free article] [PubMed]
- 5.Hertzanu Y, Peiser J, Zirkin H.Massive bleeding after fine needle aspiration of liver angiosarcoma. Gastrointest Radiol 1990;15:43-6 [DOI] [PubMed] [Google Scholar]
- 6.Tung WC, Huang YJ, Leung SW, et al. Incidence of needle tract seeding and responses of soft tissue metastasis by hepatocellular carcinoma postradiotherapy. Liver Int 2007;27:192-200 [DOI] [PubMed] [Google Scholar]
- 7.Singh HK, Silverman JF, Geisinger KR. Fine-needle aspiration cytomorphology of clear-cell hepatocellular carcinoma. Diagn Cytopathol 1997;17:306-10 [DOI] [PubMed] [Google Scholar]
- 8.Lau SK, Prakash S, Geller SA, et al. Comparative immunohistochemical profile of hepatocellular carcinoma, cholangiocarcinoma, and metastatic adenocarcinoma. Hum Pathol 2002;33:1175-81 [DOI] [PubMed] [Google Scholar]
- 9.Saad RS, Luckasevic TM, Noga CM, et al. Diagnostic value of HepPar1, pCEA, CD10, and CD34 expression in separating hepatocellular carcinoma from metastatic carcinoma in fine-needle aspiration cytology. Diagn Cytopathol 2004;30:1-6 [DOI] [PubMed] [Google Scholar]
- 10.Saleh HA, Aulicino M, Zaidi SY, et al. Discriminating hepatocellular carcinoma from metastatic carcinoma on fine-needle aspiration biopsy of the liver: the utility of immunocytochemical panel. Diagn Cytopathol 2009;37:184-90 [DOI] [PubMed] [Google Scholar]
- 11.Wang L, Vuolo M, Suhrland MJ, et al. HepPar1, MOC-31, pCEA, mCEA and CD10 for distinguishing hepatocellular carcinoma vs. metastatic adenocarcinoma in liver fine needle aspirates. Acta Cytol 2006;50:257-62 [DOI] [PubMed] [Google Scholar]
- 12.Kunz G, Jr, Chung J, Ali SZ. Hepatocellular carcinoma-fibrolamellar variant: cytopathology of an unusual case. Diagn Cytopathol 2002;26:257-61 [DOI] [PubMed] [Google Scholar]
- 13.Pérez-Guillermo M, Masgrau NA, García-Solano J, et al. Cytologic aspect of fibrolamellar hepatocellular carcinoma in fine-needle aspirates. Diagn Cytopathol 1999;21:180-7 [DOI] [PubMed] [Google Scholar]
- 14.Sakellaridis T, Panagiotou I, Georgantas T, et al. Undifferentiated embryonal sarcoma of the liver mimicking acute appendicitis. Case report and review of the literature. World J Surg Oncol 2006;4:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aljiffry M, Walsh MJ, Molinari M. Advances in diagnosis, treatment and palliation of cholangiocarcinoma: 1990-2009. World J Gastroenterol 2009;15:4240-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Centeno BA. Pathology of liver metastases. Cancer Control 2006;13:13-26 [DOI] [PubMed] [Google Scholar]
- 17.Shetuni B, Lakey M, Kulesza P.Optimal specimen processing of fine needle aspirates of non-hodgkin lymphoma. Diagn Cytopathol 2012;40:984-6 [DOI] [PubMed] [Google Scholar]
- 18.Hookim K, Roh MH, Willman J, et al. Application of immunocytochemistry and BRAF mutational analysis to direct smears of metastatic melanoma. Cancer Cytopathol 2012;120:52-61 [DOI] [PubMed] [Google Scholar]


