Abstract
Primary effusion lymphoma (PEL) is a rare extranodal lymphoma that typically presents in a body cavity in the absence of a detectable tumor mass and that occurs predominantly in immunosuppressed individuals. The neoplastic lymphoid cells are frequently infected with human herpes virus 8 (HHV8), also known as Kaposi sarcoma herpes virus (KSHV). We describe two HIV-negative patients who presented with primary effusion lymphoma of B-cell lineage involving the pleural cavity, but whose tumor cells lacked infection by HHV8. We review the English language literature of HHV8-negative PEL of B-cell lineage and compare these lymphomas to HHV8-associated PEL with regard to clinical and pathological characteristics, therapy, and outcome.
1. Introduction
Primary effusion lymphoma (PEL) is a rare extranodal lymphoma of large B cells with characteristic clinicopathologic features including: initial presentation as a body cavity lymphomatous effusion in the absence of a detectable tumor mass; occurrence mostly in human immunodeficiency virus (HIV)-positive individuals; and expression of antigens associated with a late stage of B-cell differentiation, such as CD138 and MUM1/IRF4, without pan-B-cell antigen expression [1]. Human herpes virus-8 (HHV8), also known as Kaposi's sarcoma herpes virus (KSHV), is strongly causally related to PEL and its presence has been incorporated as a diagnostic criterion for PEL [2].
Diffuse large B-cell lymphoma (DLBCL) constitutes approximately 30–40% of all non-Hodgkin's lymphoma (NHL) and typically presents with a rapidly enlarging symptomatic mass, usually due to nodal enlargement. Extranodal disease with involvement of tissue other than lymph node, spleen, Waldeyer's ring or thymus is quite common in DLBCL, as is secondary involvement of a body cavity by DLBCL [3]. However, primary presentation of DLBCL as a body cavity lymphomatous effusion without any detectable solid mass, similar to HHV8-associated PEL, is extremely rare. Reports of such cases of HHV8-negative PEL of B-cell lineage are limited to isolated case reports and small series. We report two additional cases of this aggressive extranodal lymphoma that presented as a solitary pleural effusion without other sites of disease at the time of diagnosis. In addition, we perform a comprehensive literature review of similar cases with the aim of further characterizing this unusual lymphoma subtype.
Case 1 —
An 87-year-old HIV-negative Portuguese female with a past medical history of heart failure with preserved ejection fraction (EF = 60%), hypertension, atrial fibrillation, dyslipidemia, and degenerative joint disease was admitted with progressive shortness of breath of two weeks' duration. Complete blood count on admission revealed WBC count of 9600/μL, hemoglobin of 13.7 g/dL, hematocrit of 42.0%, and platelet count of 160,000/μL. Serum total protein and LDH were 6.4 g/dL and 184 IU, respectively. The chest X-ray showed an enlarged cardiac silhouette with bilateral pleural effusions. Thoracocentesis revealed the pleural fluid to be exudative with glucose of 3 mg/dL, protein of 3.5 g/dL, LDH of 1341 U/L and 9600 nucleated cells/μL, of which 5100 were normal-appearing white blood cells (6% neutrophils, 91% lymphocytes, 3% monocytes) and 4500 were malignant-appearing cells.
Cytocentrifuge preparation showed the malignant cells to be large lymphoid cells with irregular nuclei and deeply basophilic cytoplasm with prominent vacuoles (Figures 1(a) and 1(b)). Flow cytometry of the pleural fluid showed that the large cells were positive for CD45, CD19, CD20, CD22, CD79a, CD38, HLA-DR, and surface IgM, with aberrant expression of the T-cell antigen, CD8, and the myeloid antigen, CD13. They were negative for surface and cytoplasmic light chains, MPO, TdT and other T-cell antigens (CD2, CD3, CD4, CD7). Immunoperoxidase stains showed that neoplastic cells were positive for CD45 (Figure 1(c)), CD20 (Figure 1(d)), CD79a (Figure 1(e)), bcl-2, bcl-6 (>50%), Ki-67 (>90%), epithelial membrane antigen (<50%) and negative for CD10, CD30 and CD138 (Figure 1(f)). Immunohistochemical staining for HHV8 latency associated nuclear antigen (LANA)-1 and in-situ hybridization (ISH) for Epstein-Barr virus (EBV) were negative. The patient was diagnosed with DLBCL. Further staging to exclude a primary extra-cavitary site of involvement was performed; however, no mass, organomegaly or lymphadenopathy was detected on computed tomography (CT) scans of the chest, abdomen or pelvis. Ultimately, it was felt that a diagnosis of HHV8-negative PEL was most appropriate. The patient was treated only with talc pleurodesis as she declined chemotherapy and radiotherapy. She is alive approximately 24 months after the procedure and a total of 29 months after her initial presentation of bilateral pleural effusions.
Figure 1.
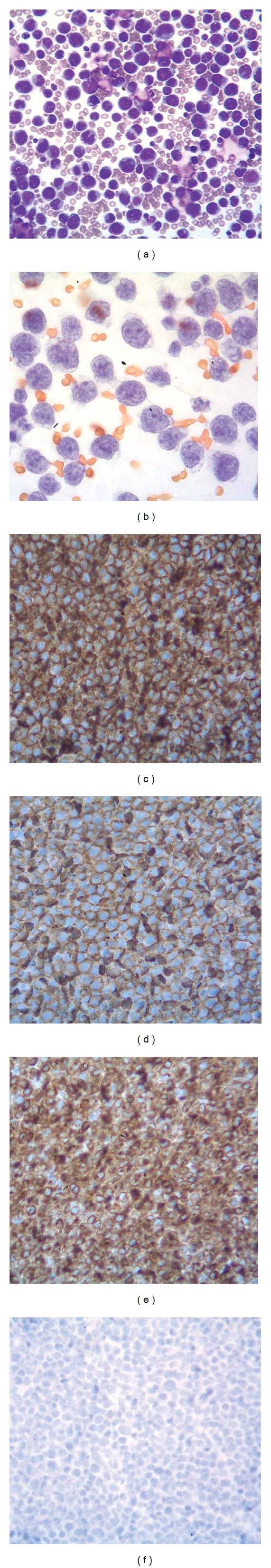
Cytological analysis revealed large atypical lymphoid cells with irregular nuclei, prominent nucleoli and basophilic vacuolated cytoplasm ((a), May-Grünwald-Giemsa; (b), Papanicolaou). By immunohistochemistry of a corresponding cell block specimen, the large cells were strongly positive for CD45 (c), CD20 (d), and CD79a (e), and were negative for CD138 (f), indicative of a mature B-cell immunophenotype.
Case 2 —
An 82-year-old HIV-negative Caucasian female with a past medical history of hypertension, sick sinus syndrome, abdominal aortic aneurysm and chronic obstructive pulmonary disease was admitted with dyspnea. Ten years earlier, she was diagnosed with non-small cell lung cancer that was treated with concurrent neoadjuvant chemotherapy and radiation followed by lobectomy. She had no interval clinical or imaging evidence of recurrence of her thoracic malignancy. Chest radiograph during the admission showed a right-sided pleural effusion. Thoracocentesis revealed malignant cells in the pleural fluid that were large lymphoid cells with irregular nuclear contours, basophilic cytoplasm and multiple nucleoli (Figures 2(a) and 2(b)). Immunohistochemical stains showed the neoplastic cells to be positive for CD20 (Figure 2(c)), PAX5/BSAP, bcl-6, MUM1/IRF4 (subset) and kappa light chain (Figure 2(d)), weakly positive for bcl-2, and negative for CD5, CD10, CD15, CD30, CD138, cyclin D1, lambda light chain (Figure 2(e)) and HHV8 LANA-1. ISH for EBV-encoded RNA (EBER) was negative. Immunoglobulin heavy chain (IGH@) gene rearrangement studies showed a clonal pattern [4]. F-Fluorodeoxyglucose (FDG) positron emission tomography (PET)/CT whole body scan for staging did not demonstrate additional sites of disease. The expression of mature B-cell markers and absence of HHV8, EBV, CD30 and CD138 expression excluded the diagnosis of HHV8-associated PEL and the patient was given the diagnosis of HHV8-negative PEL.
Figure 2.
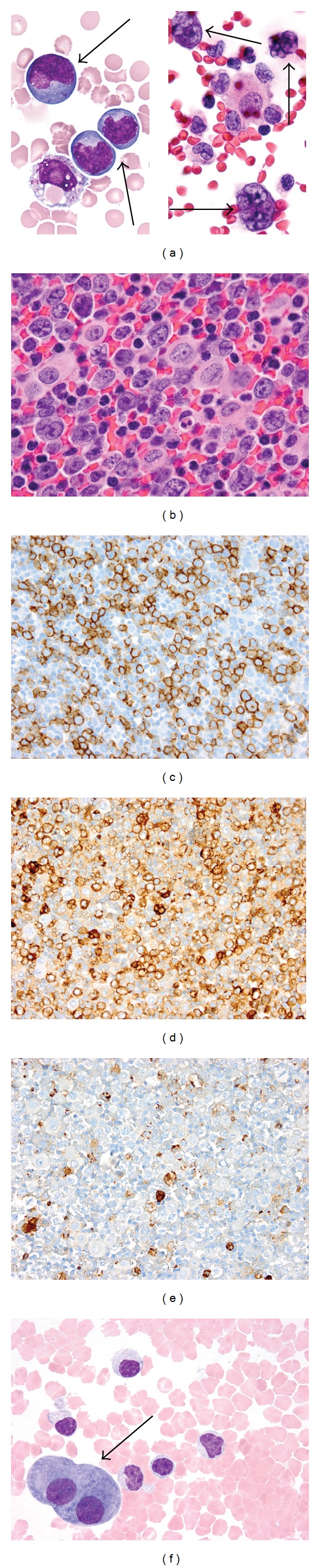
Examination of the initial thoracentesis fluid demonstrated scattered large atypical lymphoid cells with multilobated nuclei, vesicular chromatin and multiple prominent nucleoli (arrows), in a background of benign mesothelial cells, histiocytes, small lymphocytes and neutrophils ((a), left, May-Grünwald-Giemsa, and right, hematoxylin and eosin). The corresponding cell block specimen showed similar findings (hematoxylin and eosin). Immunohistochemical stains showed the scattered large cells to be positive for CD20 (c) and kappa light chain-restricted (d), with few lambda-positive cells in the background (e). A repeat thoracentesis specimen taken 1 month later showed no evidence of malignancy, with only benign mesothelial cells (arrow) and hematopoietic elements (Wright-Giemsa).
The patient became symptomatic with dyspnea a month later and chest X-ray showed recurrent pleural effusion. Thoracocentesis was repeated and examination of the pleural fluid by cytology and flow cytometry revealed only reactive mesothelial cells and histiocytes, without evidence of malignant-appearing cells (Figure 2(f)). No clonal B-cell population was detected by concurrent flow cytometry. Spontaneous regression of lymphoma was re-confirmed with repeat thoracocentesis a week later yielding no malignant cells. However, follow-up FDG PET/CT whole body scans done 4 months later showed a new FDG-avid pleural-based small nodule and various nodularities in the omentum. Tissue biopsy of these nodules was not attempted, but they were believed to be consistent with metastatic progression of the lymphoma. The patient refused any chemotherapy and died 11 months after her diagnosis of lymphoma.
2. Design and Methods
“Primary effusion lymphoma” and “body cavity based lymphoma” were used as search terms to identify English-language articles from PubMed published in the past 15 years (January 1997 to June 2012). Primary effusion lymphoma was defined by the presence of malignant lymphoma cells exclusively in a body cavity or cavities without any contiguous or non-contiguous tumor mass or lymph node enlargement at the time of presentation. The review was restricted to reports of primary effusion lymphomas that were negative for HHV8 and that showed expression of mature pan-B-cell antigens. Editorials, reviews without additional cases, and non-published abstracts were excluded.
Clinical information abstracted for each case included: age at presentation; sex; HIV status by enzyme-linked immunosorbent assay (ELISA) or Western blot studies; detection of hepatitis C virus (HCV) by serologic studies or polymerase chain reaction (PCR); detection of EBV by PCR; site(s) of disease; therapy; and outcome. Pathological data collected for each case included: lymphoma cell morphology and immunophenotype; HHV8 LANA-1 expression by immunohistochemistry or detection of HHV8 by PCR or ISH; detection of EBV by EBV latent membrane protein-1 (LMP1) expression or EBER ISH; and results of IGH@ gene rearrangement and cytogenetic studies.
3. Results
The preliminary search for reports using the above mentioned terms yielded 1187 articles. After excluding reports of HHV8-associated PEL and cases of T-cell or null immunophenotype, we identified 34 articles describing 46 unique cases [4–37]. Our review includes these 46 cases and our 2 cases for a total of 48 reported cases of HHV8-negative PEL.
Clinical characteristics are summarized in Table 1 and detailed clinical and pathological findings in each case are listed in Table 2. The 48 patients had a median age at diagnosis of 74 years (range: 14–99 years) with a male-to-female ratio of 3 : 2. Information regarding HIV status was available in 41 patients, and none were reported to be HIV-positive. The association with HCV and EBV infection was found to be 22.2% and 21.3%, respectively. For the 41 patients with information available regarding site of disease, the frequencies of various sites of involvement were as follows: pleura: 65.9%, peritoneum: 39.0%, and pericardium: 36.6%. A single case (case 48) involved the scrotum.
Table 1.
Summary of clinical characteristics of 48 patients with HHV8-negative effusion lymphomas of B-cell lineage.
| Characteristics | Number of patients (%) |
|---|---|
| Age (n = 48) | |
| Age > 60 | 10 (20.8) |
| Age < 60 | 38 (79.2) |
| Sex (n = 48) | |
| Male | 29 (60.4) |
| Female | 19 (39.6) |
| EBV status (n = 47) | |
| Positive | 10 (21.3) |
| Negative | 37 (78.7) |
| HCV status (n = 36) | |
| Positive | 8 (22.2) |
| Negative | 28 (77.8) |
| Site(s) involved (n = 41) | |
| Pleura | 27 (65.9) |
| Peritoneum | 16 (39.0) |
| Pericardium | 15 (36.6) |
| Treatment (n = 48) | |
| No chemotherapy | 17 (35.42) |
| CHOP | 11 (22.92) |
| CHOP + R | 3 (6.25) |
| THP-CVP | 6 (12.5) |
| THP-CVP + R | 4 (8.3) |
| Other regimens | 6 (12.5) |
| Unknown | 1 (2.0) |
| Outcome | |
| At 6 months (n = 45) | |
| Dead | (10/45) 22.2% |
| Alive | (35/45) 77.8% |
| At 1 year (n = 36) | |
| Dead | (14/36) 38.9% |
| Alive | (22/36) 61.1% |
Abbreviations: CHOP: cyclophosphamide, doxorubicin, vincristine, prednisone; R: Rituximab; THP-CVP-pirarubicin, cyclophosphamide, vincristine, prednisone; EBV: Epstein-Barr virus, HCV: hepatitis C virus.
Table 2.
Detailed clinical characteristics of 48 cases of HHV8-negative effusion lymphomas of B-cell lineage.
| Case | Ref no. | Age/sex | Other disease | HIV | EBV | HCV | Sites involved | Morphology | Immunophenotype# | Molecular genetics/cytogenetics | Therapy | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Case 1 | 87/F | CHF, Afib | — | — | — | Pleura | Large | CD19, CD45, CD20, CD79a | * | Pleurodesis | Alive 21 mo |
| 2 | Case 2 | 82/F | HTN, sick sinus syndrome, COPD | — | — | — | Pleura | Large | CD20, bcl-6, MUM1/IRF4, PAX5 | Clonal IGH@ | Pleural effusion drainage | Died 13 mo |
| 3 | [5] | 99/F | * | — | — | — | Pleura, pericardium | Medium to large | CD19, CD20, CD5, CD25, IgM, IgD | MYC amplification but no rearrangement, Clonal IGH@ | Pleural drainage | Alive 16 mo |
| 4 | [5] | 85/M | HTN, Afib | — | — | — | Pleura, pericardium | Medium to large | CD20 | Clonal IGH@, no MYC rearrangement | No treatment | Alive 11 mo |
| 5 | [6] | 79/M | HTN, CHF | — | — | — | Pleura | Large pleomorphic | CD45, CD20, CD79a, bcl-2, bcl-6, MUM1 | Clonal IGH@ | Pleurodesis with doxycycline | Alive 55 mo |
| 6 | [7] | 67/F | RA | — | — | — | Pericardium | Medium to large | CD20, CD79a | Clonal IGH@ | CHOP and then followed by MEPP, DEVIC | Died 16 mo |
| 7 | [8] | 74/M | * | — | — | — | Pericardium | Medium to large | CD20 | * | Rituximab + CHOP | Died 7 mo |
| 8 | [9] | 63/M | DM | — | + | — | Peritoneum, pleura | Large pleomorphic | CD19, CD20, CD22, CD45, HLA-DR, bcl-2, kappa | Clonal IGH@ | CHOP | Died 5 mo |
| 9 | [10] | 82/M | * | — | + | — | Pleura. pericardium |
Medium to large | CD20, CD79a, Ig light chain restriction | * | CHOP | Alive 18 mo |
| 10 | [10] | 73/M | * | — | — | — | Pleura. Pericardium, peritoneum |
Large | CD20 | * | CHOP | Alive 12 mo |
| 11 | [11] | 77/M | Prostate ca, MI, idiopathic CD4+ T-cell lymphopenia | — | + | — | Pleura | Large | CD45, CD19, CD20, CD79a, CD38, CD71, CD30, lambda | Trisomy 18. No rearrangements involving MYC, BCL2, BCL6, ALK and IGH | CHOP | lost to follow up |
| 12 | [12] | 68/M | * | — | — |
— |
Pleura | Large | CD20, CD79a | Clonal IGH@. No MYC rearrangement | R − CHOP | Alive 22 mo |
| 13 | [13] | 78/M | Idiopathic CD4+ T-cell lymphopenia | — | + | — |
Pleura, pericardium | Large | CD19, CD20, CD22, HLA-DR, IgM, bcl-6 | Additional unknown material at 3q27 (BCL6). No MYC rearrangement | R + THP-COP | Alive 30 mo |
| 14 | [4] | 88/M | CAD | — | — |
— |
Pleura | Large | CD20, CD30, CD79a, CD45 | * | R + CHOP | Alive 11 mo |
| 15 | [14] | 69/M | None | — | — | — | Pericardium, pleura | Large pleomorphic | CD19, CD20, CD5, kappa, bcl2, cyclin D1 | t(8; 14) (q24; q32); MYC-IGH rearrangement. Clonal IGH@ | THP-COP | Died 5 mo |
| 16 | [15] | 52/F | * | — | — | * | Pleura, pericardium | Large pleomorphic | CD19, CD20, CD22, CD45, HLA-DR | Clonal IGH@ | * | * |
| 17 | [16] | 59/F | Hep C cirrhosis | — | − | + | Peritoneum | Small to medium-sized | CD20, CD10, IgG | 48,XX,t(8;22)(q24;q11), +16, +21; MYC-IGL rearrangement. Clonal IGH@ | None | Died 2 mo |
| 18 | [17] | 57/M | * | — | + | * | Peritoneum | Monomorphic, small to medium-sized | CD19, CD22, CD79a, CD10, CD23, CD38, IgM | 46,XY,t(8;22)(q24; q11); MYC-IGL rearrangement | None | Died 1 w |
| 19 | [18] | 63/M | Hep C cirrhosis, HCC | — | − | + | Peritoneum | Medium to large size | CD19, CD20, CD22, IgG lambda | Complex karyotype with t(9;14). No MYC rearrangement. Clonal IGH@ | None | Died 22 mo |
| 20 | [19] | 60/F | Cholesteatoma | — | + | − | Peritoneum | Large | CD19, CD20, CD22, HLA-DR | Complex karyotype including der(8) t(2;8) (q31;q24), but no MYC rearrangement identified by Southern blot | None | Alive 24 mo |
| 21 | [20] | 65/M | Hep C cirrhosis | — | − | + | Peritoneum | Large | CD19, CD20, CD22, IgH@ | Clonal IGH@. No MYC rearrangement | Prednisolone, etoposide | Alive 8 mo |
| 22 | [21] | 65/M | Alcoholic cirrhosis | — | + | − | Peritoneum | Large Immunoblastic | CD19, lambda | Clonal IGH@ | CHOP | Died 12 mo |
| 23 | [22] | 75/M | * | — | − | − | Pleura | Large | CD19, CD20, HLA-DR, kappa | Complex karyotype including MYC amplification. Clonal IGH@ | CHOP | Died 15 mo |
| 24 | [22] | 76/M | * | — | − | * | Pleura | Large | CD19, CD20, CD10, HLA-DR | Complex karyotype with t(8; 22)(q24,q11); Clonal IGH@ | None | Alive 6 mo |
| 25 | [22] | 32/F | Congenital protein-losing enteropathy | — | − | * | Peritoneum | Large | CD19, CD20, CD10, HLA-DR | Complex karyotype including MYC amplification. Clonal IGH@ | CHOP, PBSCT | Alive 13 mo |
| 26 | [22] | 81/M | * | — | — | * | Pleura | Large | CD19, CD20, CD10, HLA-DR, CD5 | Complex karyotype including MYC amplification. Clonal IGH@ | None | Alive 2 mo |
| 27 | [23] | 58/F | DM, Hep C, hypothyroidism | — | − | + | Peritoneum | Large | CD19, CD20, CD4, CD5 | Hyperdiploid karyotype including MYC rearrangement. Clonal IGH@ | None | Died 7 mo |
| 28 | [24] | 58/F | CVID | — | + | − | Pleura, pericardium | Large | CD19, CD20, CD22, HLA-DR, kappa | No MYC rearrangement | Prednisolone | Died 18day |
| 29 | [25] | 58/M | Hep C cirrhosis | — | − | + | Peritoneum | Large | CD45, CD19, CD20, CD22, CD10, FMC7, HLA-DR | Clonal IGH@ | CVP | Died 5 mo |
| 30 | [26] | 90/F | Afib | — | − | − | Pleura peritoneum, pericardium | Large | CD20, CD79a, bcl-2 | MYC rearrangement. Clonal IGH@ | None | Died 5 mo |
| 31 | [27] | 70/F | None | — | − | − | Pleura, pericardium | Large | CD19, CD20, CD22, CD24, CD8, CD10, HLA-DR, CD38 | Complex karyotype. No MYC rearrangement. Clonal IGH@ | CHOP, Sobuzoxane | Alive 30 mo |
| 32 | [28] | 32/F | Lymphangioma, protein-losing enteropathy, chylothorax, Hep C | — | − | + | Pleura, peritoneum | Large | CD19, CD20, CD10, HLA-DR | Complex karyotype including MYC amplification | THP-COP, PBSCT | Died 18 mo |
| 33 | [29] | 74/F | Hep C cirrhosis, allergic granulo-matous angiitis | — | − | + | Pleura, pericardium, peritoneum | Large | CD45, CD19, CD20, CD25, HLA-DR, kappa | No MYC rearrangement. Clonal IGH@ | Rituximab + THP-COP | Alive 26 mo |
| 34 | [30] | 75/F | − | — | − | − | Pericardium | Large | CD20, CD79a | t(1;22)(q21;q11), t(14;17)(q32;q23). No MYC rearrangement | CHOP | Alive 36 mo |
| 35 | [31] | 90/M | History of TB | — | − | − | Pleura | Large | CD19, CD20, CD30 | Complex karyotype including add(8)(q24). Clonal IGH@ | Rituximab + THP-COP | Alive 38 mo |
| 36 | [31] | 87/F | * | — | − | − | Pleura | Large | CD20, CD30, kappa | * | Rituximab | Alive 32 mo |
| 37 | [32] | 74/M | CKD, pulmonary infarction, DM | * | — | * | * | Large | CD19, CD20, MUM1, BLIMP1 | Clonal IGH@ | Pleural effusion drainage | Died 80 mo |
| 38 | [32] | 87/M | DM | * | — | * | * | Large immunoblasts type | CD19, CD20, MUM1 | Clonal IGH@ | THP-CVP | Died 16 mo |
| 39 | [32] | 66/M | DM, HTN, MI | * | — | * | * | Large | CD19, CD20, MUM1 | Clonal IGH@ | THP CVP + rituximab | Alive 9 mo |
| 40 | [32] | 94/F | Afib | * | — | * | * | Large | CD20 | * | THP-CVP | Died 1 mo |
| 41 | [32] | 92/M | CRF | * | — | — | * | Medium to large sized | CD19, CD20, bcl-6, MUM1 | Clonal IGH@ | No chemotherapy initially; THP-CVP 4 mo later | Died 9 mo |
| 42 | [32] | 79/M | DM, CRF | * | — | * | * | Large | CD19, CD20, MUM1 | Clonal IGH@ | None | Alive 7 mo |
| 43 | [33] | 76/F | Hypothyroidism, pulmonary emphysema | * | * | * | Pleura, pericardium | Medium-sized monomorphic cells | CD19, CD20, CD21, surface Ig, HLA-DR | X,Xq-,2q-,5q+,-6, +7p,+9p,+15,+r. Clonal IGH@ | Prednisolone | Died 15 mo |
| 44 | [34] | 55/M | Autoimmune hemolytic anemia | — | + | — | Peritoneum | Large | CD45, CD20, CD79a, CD38, IgM | 49,XY,add(3)(q11), der(8)t(1;8)(q12;p11),+r, +2mar. Clonal IGH@ | CHOP | Died 3 mo |
| 45 | [20] | 65/F | Liver cirrhosis, Hep C | — | — | + | Peritoneum | Large | CD19, CD20, CD22 | No MYC rearrangement | Prednisone, etoposide | Alive 8 mo |
| 46 | [35] | 92/F | HTN, DM, ESRD | — | — | * | Pleura | Large | CD20, CD45, bcl-2 | * | None | Died 2 mo |
| 47 | [36] | 70/M | Hep B, liver transplant | — | + | — | Pleura | Large | CD19, CD20 | * | None | Alive 8 mo |
| 48 | [37] | 51/M | None | — | — | — | Scrotum | Medium to large size | CD45, CD19, CD20, CD79a | Clonal IGH@ | Carboplatin, etoposide, mitoxantrone, prednisone + radiotherapy | Alive 8 mo |
#Immunophenotype includes only positively expressed antigens. *Information not available or mentioned. Abbreviations: Afib: atrial fibrillation; ca: carcinoma; CAD: coronary artery disease; CHF: congestive heart failure; CHOP: cyclophosphamide, daunorubicin, oncovin, prednisolone; CKD: chronic kidney disease; COPD: chronic obstructive pulmonary disease; CRF: chronic renal failure; CVID: common variable immune deficiency; DEVIC: dexamethasone, etoposide, ifosfamide and carboplatin; DM: diabetes mellitus; ESRD: end-stage renal disease; HCC: hepatocellular carcinoma; Hep: hepatitis; HTN: hypertension; IGH@: immunoglobulin heavy chain gene rearrangement study; MEPP: mitoxantrone hydrochloride, etoposide, cisplatin and prednisolone; MI: myocardial infarction; mo: months; PBSCT: peripheral blood stem cell transplantation; R: rituximab; RA: rheumatoid arthritis; TB: tuberculosis; THP-COP: pirarubicin, cyclophosphamide, oncovin, prednisolone
Most cases consisted of medium-sized to large or large-sized cells that were occasionally described as pleomorphic. All cases expressed one or more pan-B-cell antigens (CD19, CD20 and/or CD79a) and several cases expressed surface and/or cytoplasmic immunoglobulin, antigens typically absent in HHV8-associated PEL [16]. The immunophenotype was variable with regard to germinal center (CD10, bcl-6) and post-germinal center (MUM1/IRF4) markers, but no case was reported to express CD138, a plasmacytic antigen typically seen in HHV8-associated PEL. Expression of T-cell antigens, a feature reported in occasional cases of HHV8-associated PEL, was seen in only rare cases (two cases with CD5 co-expression [22, 23] and case 1 reported above with CD8 co-expression). At least some cytogenetic information (FISH and/or karyotype) was available in 26 cases. Of these, 12 cases showed a rearrangement or amplification involving MYC at 8q24 and 13 were reported to harbor a complex karyotype, although full karyotypic information was available in only a small number of cases.
Thirty patients (62.5%) received chemotherapy with a variety of regimens, including cyclophosphamide, doxorubicin, vincristine, prednisolone (CHOP) in 11 patients; CHOP with rituximab (R) in 3 patients; THP-CVP (pirarubicin, cyclophosphamide, vincristine, prednisolone) in 6 patients; THP-CVP + R in 4 patients; and other chemotherapy regimens in 6 patients. Treatment was unknown in 1 patient. The remaining 17 patients (35.4%) received no chemotherapy and were treated with fluid drainage alone or fluid drainage and pleurodesis. The median overall survival (OS) was 11 months. The number of patients alive at 6 months and 1 year following symptomatic presentation was 77.8% and 61.1% respectively. Patients who received no chemotherapy had a median OS of 8 months (range: 1 week to 80 months) versus 12 months (range: 18 days to 38 months) in patients who received any kind of chemotherapy. The rate of death with any kind of chemotherapy at 6 months was 20% and at 1 year was 33%, compared to 25% and 42% without any chemotherapy. Among the 22 patients who died of their lymphoma, the median OS was 7 months (range: 1 week to 80 months). The median follow-up period in 24 living patients was approximately 14.5 months (range: 2 months to 55 months).
4. Discussion
Body cavity-based lymphomas are a heterogeneous group of rare non-Hodgkin's lymphomas that arise primarily in the serous body cavities and that result in recurrent effusions. This group includes pyothorax-associated lymphoma and PEL. Pyothorax-associated lymphoma presents as a solid mass localized in the thoracic cavity that is contiguous with the effusion; it is EBV-associated and arises in the setting of long-standing pyothorax resulting from iatrogenic pneumothorax used to treat tuberculosis [38]. In contrast, PEL is typically confined to a body cavity and grows in a liquid phase, without any detectable nodal or extranodal mass elsewhere in the body. As illustrated in Figure 3, PEL can be broadly divided into two categories: HHV8-associated PEL, a subtype of DLBCL and a distinct category in the 2008 WHO Classification of Neoplasms of Haematopoietic and Lymphoid Tissues [2], and HHV8-negative PEL, of which the majority (~80% of cases) express mature pan-B-cell antigens [39]. As only rare cases of HHV8-negative PEL with neoplastic cells of T-cell derivation [40–44] or null/indeterminate immunophenotype [22, 45, 46] have been reported, we restricted our review to HHV8-negative PEL of B-cell lineage. Our report of two cases and review of the literature demonstrates that these neoplasms are heterogenous, but share common features that distinguish them from HHV8-associated PEL with regard to pathogenesis, clinical characteristics, immunophenotype and prognosis.
Figure 3.
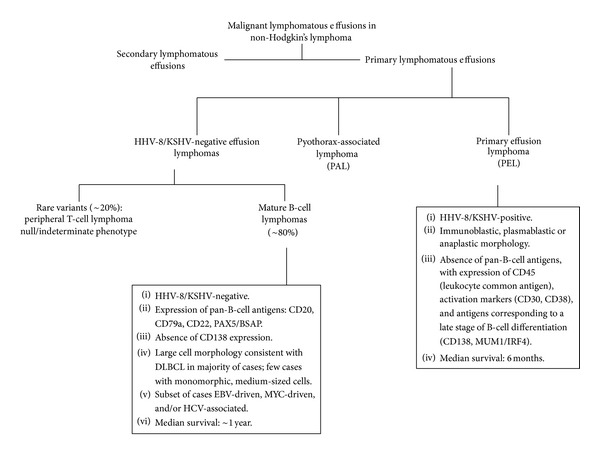
A schema of malignant lymphomatous effusions in non-Hodgkin's lymphoma highlighting the differences between HHV8-associated PEL and HHV8-negative PEL.
While none of the 48 patients with HHV8-negative PEL were HIV-positive, EBV infection was seen in 21.3% of cases, suggesting that altered immunosurveillance may play a pathogenetic role in some cases. Among the 10 EBV-positive cases, all patients were >50 years old, 2 patients had idiopathic CD4+ T-cell lymphopenia and 1 patient had a history of common variable immunodeficiency, supporting this hypothesis [47]. Similarly, 22.2% of patients had underlying HCV infection. HCV has been shown to be lymphotropic and has been implicated as an etiological factor in lymphomagenesis for various NHL subtypes [48, 49]. Ascoli et al. detected HCV RNA in the ascitic fluid of a patient with HHV8-negative PEL and proposed that HCV may play a causative role in these lymphomas by chronic stimulation of B cells followed by clonal expansion [16].
In our review, a number of cases were negative for both EBV and HCV (19/48; 39.6%), suggesting that other etiologies also play a role in lymphomagenesis. Among cases with available cytogenetic information, 50% (12/26) harbored amplification or rearrangement of the MYC oncogene, a likely driver of neoplasia in these cases. In terms of B-cell NHL subclassification, most MYC-rearranged cases had large cell morphology or a complex karyotype, consistent with DLBCL [14, 19, 22, 23, 26, 31, 50] Two cases (cases 17 and 18) reportedly had monomorphic medium-sized cells, a germinal center immunophenotype (expression of CD10), and a relatively simple background karyotype, suggesting that these cases may represent an unusual extranodal presentation of Burkitt's lymphoma [16, 17].
Unlike HHV8-associated PEL, HHV8-negative PEL of B-cell lineage shares several clinical features with nodal or extranodal DLBCL presenting with a mass lesion, and differentiating HHV8-negative PEL from conventional DLBCL complicated by secondary lymphomatous effusion requires a thorough staging evaluation to exclude the presence of a mass lesion at the time of diagnosis. Based on our literature review, HHV8-negative PEL presents at an older median age (74 years) compared to that reported for HHV8-associated PEL (44 years) and exhibits a lower male-to-female ratio, similar to DLBCL [2, 44, 51, 52]. The overall favorable prognosis compared to HHV8-associated PEL was underscored by a survival rate of approximately 60% at 1 year and a median OS of 11 months. While this compares favorably to HHV8-associated PEL with a reported median OS of 4–6 months [2, 44], the range was quite wide (1 week to 80 months). This heterogeneity in clinical behavior is further highlighted by our finding of a small number of patients who presented with involvement limited to a single body cavity site, but who developed mass lesions outside of the body cavity at the time of disease progression, similar to our case 2 [32]. At the other extreme, our case 1 showed regression of the malignancy after pleurodesis and drainage of the pleural fluid without any chemotherapy, and in our literature review we identified 7 other patients in whom the lymphoma similarly regressed following drainage of the effusion [5–7, 12, 18, 23].
There is no clear consensus on the appropriate treatment of HHV8-negative PEL due to the limited number of cases reported. Our findings suggest that chemotherapy benefits most patients, as those treated with any type of chemotherapy overall had a lower rate of death compared to patients who received no chemotherapy. Remarkably, the addition of rituximab to chemotherapy regimens induced remission in all 8 patients, 7 of whom were alive at the time of last follow-up [4, 8, 12, 13, 29, 31, 32]. The single patient who died following a rituximab-containing regimen died prematurely of a cause unrelated to lymphoma [8]. Therefore, treatment with drainage of the effusion followed by chemoimmunotherapy with rituximab and CHOP, particularly in CD20-positive cases, appears to offer the possibility of prolonged survival in a subset of patients. Further study of rare patients who undergo spontaneous regression of their lymphoma following drainage alone may help to identify clinical or pathological features that predict for a good outcome following only minimal therapy.
Acknowledgments
All authors acknowledge no financial interests or motives in contribution of the manuscript.
Authors' Contributions
Each author has participated sufficiently in the work to take public responsibility for the content.
References
- 1.Carbone A, Gloghini A. PEL and HHV8-unrelated effusion lymphomas: classification and diagnosis. Cancer. 2008;114(4):225–227. doi: 10.1002/cncr.23597. [DOI] [PubMed] [Google Scholar]
- 2.Said J, Cesarman E, Harris NL. Primary effusion lymphoma. In: Swerdlow SH, editor. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th edition. Lyon, France: IARC Press; 2008. pp. 260–261. [Google Scholar]
- 3.Møller MB, Pedersen NT, Christensen BE. Diffuse large B-cell lymphoma: clinical implications of extranodal versus nodal presentation—a population-based study of 1575 cases. British Journal of Haematology. 2004;124(2):151–159. doi: 10.1046/j.1365-2141.2003.04749.x. [DOI] [PubMed] [Google Scholar]
- 4.Youngster I, Vaisben E, Cohen H, Nassar F. An unusual cause of pleural effusion. Age and Ageing. 2006;35(1):94–96. doi: 10.1093/ageing/afj009. [DOI] [PubMed] [Google Scholar]
- 5.Terasaki Y, Yamamoto H, Kiyokawa H, et al. Disappearance of malignant cells by effusion drainage alone in two patients with HHV-8-unrelated HIV-negative primary effusion lymphoma-like lymphoma. International Journal of Hematology. 2011;94:279–284. doi: 10.1007/s12185-011-0906-8. [DOI] [PubMed] [Google Scholar]
- 6.Wang T, Nava VE, Schechter GP, Lichy JH. Human herpes virus 8-unrelated primary effusion lymphoma-like lymphoma: a patient successfully treated with pleurodesis. Journal of Clinical Oncology. 2011;29:e747–e750. doi: 10.1200/JCO.2011.35.7509. [DOI] [PubMed] [Google Scholar]
- 7.Inoue S, Miyamoto T, Yoshino T, Yamadori I, Hagari Y, Yamamoto O. Primary effusion lymphoma with skin involvement. Journal of Clinical Pathology. 2006;59(11):1221–1222. doi: 10.1136/jcp.2005.031807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kagoya Y, Takahashi T, Yoshimoto T, et al. Recurrent pericardial effusion after treatment for primary effusion lymphoma-like lymphoma: an autopsied case. Annals of Hematology. 2011;90(2):219–220. doi: 10.1007/s00277-010-0975-4. [DOI] [PubMed] [Google Scholar]
- 9.Ceran F, Aydin Y, Özçakar L, Han U, Yildiz M. Primary effusion lymphoma: an untrivial differential diagnosis for ascites. Yonsei Medical Journal. 2009;50(6):862–864. doi: 10.3349/ymj.2009.50.6.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takahashi T, Hangaishi A, Yamamoto G, Ichikawa M, Imai Y, Kurokawa M. HIV-negative, HHV-8-unrelated primary effusion lymphoma-like lymphoma: report of two cases. American Journal of Hematology. 2010;85(1):85–87. doi: 10.1002/ajh.21568. [DOI] [PubMed] [Google Scholar]
- 11.Tsagarakis NJ, Argyrou A, Gortzolidis G, et al. Report of an HIV and HHV-8 negative case of primary effusion lymphoma with idiopathic T4 lymphocytopenia. International Journal of Hematology. 2009;90(1):94–98. doi: 10.1007/s12185-009-0343-0. [DOI] [PubMed] [Google Scholar]
- 12.Terasaki Y, Okumura H, Saito K, et al. HHV-8/KSHV-negative and CD20-positive primary effusion lymphoma successfully treated by pleural drainage followed by chemotherapy containing rituximab. Internal Medicine. 2008;47(24):2175–2178. doi: 10.2169/internalmedicine.47.1565. [DOI] [PubMed] [Google Scholar]
- 13.Niino D, Tsukasaki K, Torii K, et al. Human herpes virus 8-negative primary effusion lymphoma with BCL6 rearrangement in a patient with idiopathic CD4 positive T-lymphocytopenia. Haematologica. 2008;93(1):e21–e23. doi: 10.3324/haematol.12085. [DOI] [PubMed] [Google Scholar]
- 14.Fujisawa S, Tanioka F, Matsuoka T, Ozawa T. CD5+ diffuse large B-cell lymphoma with c-myc/IgH rearrangement presenting as primary effusion lymphoma. International Journal of Hematology. 2005;81(4):315–318. doi: 10.1532/IJH97.04155. [DOI] [PubMed] [Google Scholar]
- 15.Hermine O, Michel M, Buzyn-Veil A, et al. Body-cavity-based lymphoma in an HIV-seronegative patient without Kaposi’s sarcoma-associated herpesvirus-like DNA sequences. New England Journal of Medicine. 1996;334(4):272–273. doi: 10.1056/NEJM199601253340417. [DOI] [PubMed] [Google Scholar]
- 16.Ascoli V, Lo Coco F, Artini M, Levrero M, Fruscalzo A, Mecucci C. Primary effusion Burkitt’s lymphoma with t(8;22) in a patient with hepatitis C virus related cirrhosis. Human Pathology. 1997;28(1):101–104. doi: 10.1016/s0046-8177(97)90287-2. [DOI] [PubMed] [Google Scholar]
- 17.Carbone A. Establishment of HHV-8-positive and HHV-8-negative lymphoma cell lines from primary lymphomatous effusions. International Journal of Cancer. 1997;73:562–569. doi: 10.1002/(sici)1097-0215(19971114)73:4<562::aid-ijc18>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 18.Ichinohasama R, Miura I, Kobayashi N, et al. Herpes virus type 8-negative primary effusion lymphoma associated with PAX-5 gene rearrangement and hepatitis C virus. American Journal of Surgical Pathology. 1998;22(12):1528–1537. doi: 10.1097/00000478-199812000-00010. [DOI] [PubMed] [Google Scholar]
- 19.Ashihara E, Shimazaki C, Hirai H, et al. Human herpes virus 8-negative primary effusion lymphoma in a patient with a ventriculoperitoneal shunt tube. International Journal of Hematology. 2001;74(3):327–332. doi: 10.1007/BF02982069. [DOI] [PubMed] [Google Scholar]
- 20.Hara T, Nishi S, Horimoto A, Takenaka S, Ibata Y, Akamatsu H. Primary effusion lymphoma in a patient with hepatitis C virus-related liver cirrhosis. Journal of Gastroenterology and Hepatology. 2001;16(8):948–949. [PubMed] [Google Scholar]
- 21.Rodriguez J, Romaguera JE, Katz RL, Said J, Cabanillas F. Primary effusion lymphoma in an Hiv-negative patient with no with no serologic evidence of Kaposi’s sarcoma virus. Leukemia and Lymphoma. 2001;41(1-2):185–189. doi: 10.3109/10428190109057969. [DOI] [PubMed] [Google Scholar]
- 22.Ohshima K, Ishiguro M, Yamasaki S, et al. Chromosomal and comparative genomic analyses of HHV-8-negative primary effusion lymphoma in five HIV-negative Japanese patients. Leukemia and Lymphoma. 2002;43(3):595–601. doi: 10.1080/10428190290012100. [DOI] [PubMed] [Google Scholar]
- 23.Saiki M, Saitoh T, Inoue M, et al. Human herpesvirus-8 negative primary effusion lymphoma with complete clinical remission after removal of ascites. The Japanese Journal of Clinical Hematology. 2002;43(7):548–553. [PubMed] [Google Scholar]
- 24.Hisamoto A, Yamane H, Hiraki A, et al. Human herpes virus-8-negative primary effusion lymphoma in a patient with common variable immunodeficiency. Leukemia and Lymphoma. 2003;44(11):2019–2022. doi: 10.1080/1042819031000110955. [DOI] [PubMed] [Google Scholar]
- 25.Paner GP, Jensen J, Foreman KE, Reyes CV. HIV and HHV-8 negative primary effusion lymphoma in a patient with hepatitis C virus-related liver cirrhosis. Leukemia and Lymphoma. 2003;44(10):1811–1814. doi: 10.1080/1042819031000104015. [DOI] [PubMed] [Google Scholar]
- 26.Shimazaki M, Fujita M, Tsukamoto K, et al. An unusual case of primary effusion lymphoma in a HIV-negative patient not pathogenetically associated with HHV8. European Journal of Haematology. 2003;71(1):62–67. doi: 10.1034/j.1600-0609.2003.00083.x. [DOI] [PubMed] [Google Scholar]
- 27.Inoue Y, Tsukasaki K, Nagai K, Soda H, Tomonaga M. Durable remission by sobuzoxane in an HIV-seronegative patient with human herpesvirus 8-negative primary effusion lymphoma. International Journal of Hematology. 2004;79(3):271–275. doi: 10.1532/ijh97.03107. [DOI] [PubMed] [Google Scholar]
- 28.Nonami A, Yokoyama T, Takeshita M, Ohshima K, Kubota A, Okamura S. Human herpes virus 8-negative primary effusion lymphoma (PEL) in a patient after repeated chylous ascites and chylothorax. Internal Medicine. 2004;43(3):236–242. doi: 10.2169/internalmedicine.43.236. [DOI] [PubMed] [Google Scholar]
- 29.Takao T, Kobayashi Y, Kuroda J, et al. Rituximab is effective for human herpesvirus-8-negative primary effusion lymphoma with CD20 phenotype associated hepatitis C virus-related liver cirrhosis. American Journal of Hematology. 2004;77(4):419–420. doi: 10.1002/ajh.20227. [DOI] [PubMed] [Google Scholar]
- 30.Fujiwara T, Ichinohasama R, Miura I, et al. Primary effusion lymphoma of the pericardial cavity carrying t(1;22)(q21;q11) and t(14;17)(q32;q23) Cancer Genetics and Cytogenetics. 2005;156(1):49–53. doi: 10.1016/j.cancergencyto.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 31.Matsumoto Y, Nomura K, Ueda K, et al. Human herpesvirus 8-negative malignant effusion lymphoma: a distinct clinical entity and successful treatment with rituximab. Leukemia and Lymphoma. 2005;46(3):415–419. doi: 10.1080/10428190400018364. [DOI] [PubMed] [Google Scholar]
- 32.Kishimoto K, Kitamura T, Hirayama Y, Tate G, Mitsuya T. Cytologic and immunocytochemical features of EBV negative primary effusion lymphoma: report on seven Japanese cases. Diagnostic Cytopathology. 2009;37(4):293–298. doi: 10.1002/dc.21022. [DOI] [PubMed] [Google Scholar]
- 33.Iwahashi M, Iida S, Sako S, et al. Primary effusion lymphoma with B-cell phenotype. American Journal of Hematology. 2000;64:317–318. doi: 10.1002/1096-8652(200008)64:4<317::aid-ajh15>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 34.Chiba H, Matsunaga T, Kuribayashi K, et al. Autoimmune hemolytic anemia as a first manifestation of primary effusion lymphoma. Annals of Hematology. 2003;82(12):773–776. doi: 10.1007/s00277-003-0734-x. [DOI] [PubMed] [Google Scholar]
- 35.Nemr S, Mayor-Modesto MH, Schwartz S, Summerhill EM. A 92-year-old woman with recurrent pleural effusions. Chest. 2008;134(1):196–199. doi: 10.1378/chest.07-2529. [DOI] [PubMed] [Google Scholar]
- 36.Ohori NP, Whisnant RE, Nalesnik MA, Swerdlow SH. Primary pleural effusion posttransplant lymphoproliferative disorder: distinction from secondary involvement and effusion lymphoma. Diagnostic Cytopathology. 2001;25(1):50–53. doi: 10.1002/dc.2001. [DOI] [PubMed] [Google Scholar]
- 37.Nakamura Y, Tajima F, Omura H, et al. Primary effusion lymphoma of the left scrotum. Internal Medicine. 2003;42:351–353. doi: 10.2169/internalmedicine.42.351. [DOI] [PubMed] [Google Scholar]
- 38.Nakatsuka S, Yao M, Hoshida Y, et al. Pyothorax-associated lymphoma: a review of 106 cases. Journal of Clinical Oncology. 2002;20:4260–4255. doi: 10.1200/JCO.2002.09.021. [DOI] [PubMed] [Google Scholar]
- 39.Adiguzel C, Bozkurt SU, Kaygusuz I, et al. Human herpes virus 8-unrelated primary effusion lymphoma-like lymphoma: report of a rare case and review of the literature. Acta Pathologica, Microbiologica, et Immunologica Scandinavica. 2009;117:222–29. doi: 10.1111/j.1600-0463.2008.00005.x. [DOI] [PubMed] [Google Scholar]
- 40.Venizelos I, Tamiolakis D, Lambropoulou M, et al. An unusual case of posttransplant peritoneal primary effusion lymphoma with T-cell phenotype in a HIV-negative female, not associated with HHV-8. Pathology and Oncology Research. 2005;11(3):178–181. doi: 10.1007/BF02893396. [DOI] [PubMed] [Google Scholar]
- 41.Chan ACL, Chan JKC, Yan KW, Kwong YL. Anaplastic large cell lymphoma presenting as a pleural effusion and mimicking primary effusion lymphoma: a report of 2 cases. Acta Cytologica. 2003;47(5):809–816. doi: 10.1159/000326611. [DOI] [PubMed] [Google Scholar]
- 42.Yamamoto Y, Kitajima H, Sakihana H, Shigeki T, Fukuhara S. T-cell lymphoma with clinical features of primary effusion lymphoma: an autopsy case. International Journal of Hematology. 2001;74(4):442–446. doi: 10.1007/BF02982089. [DOI] [PubMed] [Google Scholar]
- 43.Yasuda H, Nakao M, Kanemasa H, et al. T-cell lymphoma presenting with pericardial and pleural effusion as the initial and primary lesion: cytogenetic and molecular evidence. Internal Medicine. 1996;35(2):150–154. doi: 10.2169/internalmedicine.35.150. [DOI] [PubMed] [Google Scholar]
- 44.Kobayashi Y, Kamitsuji Y, Kuroda J, et al. Comparison of human herpes virus 8 related primary effusion lymphoma with human herpes virus 8 unrelated primary effusion lymphoma-like lymphoma on the basis of HIV: report of 2 cases and review of 212 cases in the literature. Acta Haematologica. 2007;117(3):132–144. doi: 10.1159/000097460. [DOI] [PubMed] [Google Scholar]
- 45.Tanaka S, Katano H, Tsukamoto K, et al. HHV8-negative primary effusion lymphoma of the peritoneal cavity presenting with a distinct immunohistochemical phenotype. Pathology International. 2001;51(4):293–300. doi: 10.1046/j.1440-1827.2001.01189.x. [DOI] [PubMed] [Google Scholar]
- 46.Carbone A, Gloghini A, Vaccher E, et al. Kaposi’s sarcoma-associated herpesvirus DNA sequences in AIDS-related and AIDS-unrelated lymphomatous effusions. British Journal of Haematology. 1996;94(3):533–543. doi: 10.1046/j.1365-2141.1996.d01-1826.x. [DOI] [PubMed] [Google Scholar]
- 47.Nakamura S, Jaffe E, Swerdlow S. EBV positive diffuse large B-cell of the elderly. In: Swerdlow S, editor. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC Press; 2008. pp. 243–244. [Google Scholar]
- 48.De Vita S, Sansonno D, Dolcetti R, et al. Hepatitis C virus within a malignant lymphoma lesion in the course of type II mixed cryoglobulinemia. Blood. 1995;86(5):1887–1892. [PubMed] [Google Scholar]
- 49.Franzin F, Efremov DG, Pozzato G, Tulissi P, Batista F, Burrone OR. Clonal B-cell expansions in peripheral blood of HCV-infected patients. British Journal of Haematology. 1995;90(3):548–552. doi: 10.1111/j.1365-2141.1995.tb05582.x. [DOI] [PubMed] [Google Scholar]
- 50.Sohani AR, Hasserjian RP. Diagnosis of Burkitt lymphoma and related high-grade B-cell neoplasms. Surgical Pathology Clinics. 2010;3(4):1035–1059. doi: 10.1016/j.path.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 51.Ferry JA. Lymphomas of the thorax. In: Ferry JA, editor. Extranodal Lymphomas. 2nd edition. Philadelphia, Pa, USA: Elsevier; 2011. pp. 93–96. [Google Scholar]
- 52.Stein H, Warnke RA, Chan WC, et al. Diffuse large B-cell lymphoma, not otherwise specified. In: Swerdlow SH, Campo E, Harris NL, et al., editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC Press; 2008. pp. 233–237. [Google Scholar]


