Abstract
Various findings concerning the clinical significance of quantitative changes in hepatitis B surface antigen (HBsAg) during the acute and chronic phase of hepatitis B virus (HBV) infection have been reported. In addition to being a biomarker of HBV-replication activity, it has been reported that HBsAg could contribute to the immunopathogenesis of HBV persistent infection. Moreover, HBsAg could become an attractive target for immune therapy, since the cellular and humeral immune response against HBsAg might be able to control the HBV replication and life cycle. However, several reports have described the immune suppressive function of HBsAg. HBsAg might suppress monocytes, dendritic cells (DCs), natural killer (NK), and natural killer T (NK-T) cells by direct interaction. On the other hand, cytotoxic T lymphocytes (CTLs) and helper T (Th) cells were exhausted by high amounts of HBsAg. In this paper, we focused on the immunological aspects of HBsAg, since better understanding of the interaction between HBsAg and immune cells could contribute to the development of an immune therapy as well as a biomarker of the state of HBV persistent infection.
1. Introduction
Hepatitis B virus (HBV) is basically a noncytopathic DNA virus that causes chronic hepatitis and hepatocellular carcinoma (HCC) as well as acute hepatitis and fulminant hepatitis [1]. HBV now affects more than 400 million people worldwide and, in approximately 5% of adults and 95% of neonates who become infected with HBV, persistent infection develops [2]. HBV contains a small (3.2 kb), circular, partially double-strand DNA organized into four open-reading frames. The longest open-reading frame encodes the viral polymerase. The envelope open-reading frame is located within the polymerase open-reading frame in a frame-shift manner. The core and X open-reading frames partially overlap with the envelope open-reading frame [3, 4]. The covalently closed circular DNA (ccc DNA) is the template that is transcribed to generate four major RNA species: the 3.5 kb, 2.4 kb, 2.1 kb, and 0.7 kb viral RNA transcripts [5]. HBV produces Hepatitis B core antigen (HBcAg), Hepatitis B envelope antigen (HBeAg), Hepatitis B X antigen (HBxAg), and Hepatitis B surface antigen (HBsAg) that could contribute to the HBV life cycle. HBsAg was found by Blumberg et al. in 1965 and regarded as an HBV-related antigen in 1968 [6, 7]. The clinical significance of quantitative changes in HBsAg during the acute and chronic phase of HBV infection has been reported [8–10]. The amount of HBsAg has been found to be closely related to the activity of HBV replication in hepatocytes [8]. In addition to serving as a biomarker of HBV-replication activity, it has been reported that HBsAg could contribute to the immunopathogenesis of HBV persistent infection (Table 1) [11–17].
Table 1.
Functions and the effect of HBsAg among the various kinds of lymphoid cells.
| Lymphoid cells | Function | The effect of HBsAg for immune response | Reference |
|---|---|---|---|
| Innate immune response | |||
| NK/NK-T cells | Independent of epitope cytotoxic function | Suppression of cytotoxic activity of Intrahepatic NK cells | [14] |
| Cytokine secreation (IFN-gamma etc.) | |||
| Monocyte | cytokine, chemokine expression | Suppression of monocyte activation | |
| Suppression of LPS and IL-2 induced cytokines production | [13, 15] | ||
| Intrahepatocyte reaction (TLR signaling) |
Detection of pathogen-associated molecular pattern | Unclear | |
| Adaptive immune response | |||
| CD8+ CTL | HBV-specific cytotoxic function | CTL exhaustion/peripheral tolerance | [18–22] |
| (Perforin, IFN-gamma, etc.) | |||
| CD4+ Th cells | HBV-specific IFN-gamma secretion | Th2 commitment | [23] |
| Tregs | Immune suppresion via IL10 and/or cells to cell contact | Enhancing Tregs activity via stress-related proteins | [21, 23, 24] |
| Dendritice cells | HBV antigen presentation and secretion of cytokines | Inhibit the upregulation of costimulatory molecules on mDCs | [12, 25–27] |
| Suppression of pDC function |
It has been shown that cellular immune responses including those of cytotoxic T lymphocytes (CTLs), Type 1 helper CD4+ T lymphocytes (Th1), FoxP3+ regulatory T lymphocytes (Tregs), and dendritic cells (DCs) play a central role in the control of virus infection [18–20, 28–34]. Moreover, Type 2 helper CD4+ T lymphocytes (Th2), B cells, and plasma cells could contribute to the production of HBV neutralizing and/or nonneutralizing antibody. Not only adaptive immune responses but also innate immune responses, including the intrahepatocyte innate immune response, and those of natural killer cells (NK), natural killer T cells (NK-T) and monocytes, might be involved in the coordination of all immune responses [15, 35–37]. Several reports have described that HBsAg could be involved in the orchestration of the immune responses, especially in disturbances of the appropriate immune responses [11, 14, 15, 38].
Recently, nucleos(t)ide analogs, such as lamivudine, adefovir, entecavir and tenofovir, and interferon-based therapy have been employed to control HBV [39, 40]. Unfortunately, the efficacy of nucleos(t)ide analogs is limited by viral reactivation through the emergence of escaped mutants in cases of prolonged treatment [39, 40]. Therefore, immunotherapy including interferon-based therapy is one of the significant options to eradicate or control HBV replication [41]. The aim of immunotherapy is to control the activity of HBV replication and to eradicate infected hepatocytes [42]. For this reason, the quantification of HBsAg and stimulation of HBsAg-specific immune responses might be important. It is necessary for the development of new strategies to understand the immuno-pathogenesis of HBV infection [43]. In this review, we focus on the immunological aspect of HBsAg based on various reports regarding HBsAg, lymphoid cells, and HBsAg-related immunotherapy.
2. HBsAg, T (CTLs, Th, Tregs, etc.) and B Lymphocytes
In the resolution of HBV infection, efficient recognition of the intracellular HBV antigens by the host immune cells is essential [32, 33, 44, 45]. It has been shown that the cellular immune system, including CTL, Th1, and Tregs, plays a central role in the control of virus infection. The hyporesponsiveness of HBV-specific CTL, Th1 cells, and excessive regulatory function of Tregs in peripheral blood have been reported in chronic hepatitis B (CHB) patients [2, 23, 24, 28, 29, 33, 46, 47]. Many groups including ours indicated that the treatment with nucleos(t)ide analogs in CHB could restore both CD4+ T cells and CTL hyporesponsiveness following the decline of serum levels of HBV-DNA and HBV-derived antigens (Figure 1) [20, 24, 46].
Figure 1.
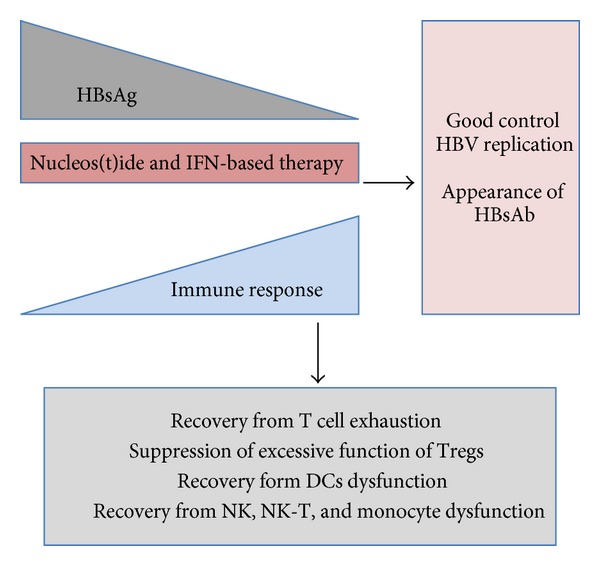
Scheme of recovery from immune suppression. The reduction of HBsAg could result in recovery from various kinds of immune suppression and possibly achieve resolution of HBV persistent infection or good control of HBV replication.
Although there are few reports indicating a direct suppression of the T cell immune response by HBsAg, we cannot exclude the possibility of a direct suppression of the T cell immune responses. It has been reported that T cell hyporesponsiveness in CHB might be induced by peripheral tolerance such as exhaustion [18, 20, 21, 30]. Recently, much attention has been devoted to the relevance of inhibitory receptor expression on T cells during chronic infections, including programmed death-1 receptor (PD-1) [22]. An HBV transgenic mouse model revealed the effect of PD-1 mediated T cell exhaustion [18].
CTLs recognize viral antigens synthesized within infected cells in the form of oligopeptides that are presented on HLA class I molecules [48]. Although the responses of HBV-specific CTLs are observed in a strong, polyclonal, and multi-specific way in patients with self-limited infection [47], hyporesponsiveness of HBV specific CTLs in the peripheral blood has been demonstrated in patients with HBV persistent infection. Various kinds of epitopes in HBV-core, surface, and polymerase were reported by many groups [48]. We also identified new, HLA-A24 restricted CTL epitopes in core and surface antigens [46]. Therefore, the amount of HBsAg in peripheral blood might influence the HBV-specific CTL response.
Previously, we examined the mechanisms of hyporesponsiveness of HBV-specific CD4+ T cells by evaluating the Th1/Th2 commitment and activity of Tregs [23]. In CHB patients, HBsAg stimulation induced upregulation of GATA-3 mRNA compared with that in healthy volunteers, while the expression level of Th1-related mRNA remained unchanged. However, the suppression of either direction, to Th1 or Th2, by HBcAg stimulation was observed. HBcAg-specific Tregs produced IL10 and suppressed the immune response, while HBsAg stimulation favored Th2 deviation in CHB [23]. Negative regulation of CD8+ T cell responses during chronic HBV infection can also be mediated by immuno-suppressive cytokines such as IL10 and TGF-beta [29]. Recently, there has been increasing evidence indicating a role of Tregs in maintaining HBV infection [28, 29]. However, the relation between HBsAg and Tregs has not been clarified yet.
The extent to which the humoral immune response contributes to the control of chronic HBV infection is less clear. HBV-specific antibodies are indicators of certain stages of these diseases. HBsAg specific antibodies (HBsAb), detectable in patients who have recovered from acute HBV infection and in HBV-vaccinated individuals, serve as neutralizing antibodies that can inhibit viral attachment and entry [49]. The induction of HBsAb is sufficient to prevent infection. In CHB patients, seroconversion to HBsAb is considered to be a marker of disease resolution [50, 51]. Although B cells and plasma cells are important for producing HBsAb, there are few reports indicating direct suppression of the B cell functions by HBsAg in CHB patients. We need to consider the possibility of B cell dysfunction in CHB since the appearance of HBsAb during CHB treatment represents a favorable condition of the immune response, as mentioned above.
3. HBsAg, NK, and NK-T Cells
NK cells play a role in controlling the innate immune response during viral pathogen invasion in the early stage of infection, while regulating the adaptive immune responses in a persistently infected host. They are especially enriched in the liver where they comprise about 50% of the intrahepatic lymphocyte portion, as compared to the peripheral blood where they represent about 10% of the total lymphocyte population [52, 53]. NK cells can be detected by flow cytometry by the expression of CD56 and lack of CD3. Additionally, they can be separated into CD56dim and CD56bright cells. CD56dim cells are considered to be mature NK cells that constitute majority of the population, whereas CD56bright cells are the minority and thought to be at an early stage of maturation [35, 36]. They mediate the recognition and lysis of viral-infected cells and the production of immunoregulatory cytokines. Through the release of cytokines such as IFN-gamma GM-CSF, TNF-alpha TGF-beta IL-2, and IL-10, they may influence the adaptive immune system [54–56].
NK-T cells are included in a lymphocyte population identified with the expression of surface markers of NK cells together with T cell antigen receptor [57]. NK-T cells as well as NK cells are found markedly in the liver, with fewer found in the spleen or bone marrow [37]. NK-T cells produce large numbers of cytokines more quickly than NK cells. Therefore, NK-T cells have been considered to largely influence the innate and the adaptive immune response [58].
Little is known about the specific immune suppression of NK and NK-T cells by HBV-encoded antigens such as HBsAg. In 2005, Chen et al., showed that the number of hepatic NK cells was decreased with the expression of HBsAg and their cytotoxicity was attenuated in transgenic mice. In addition, the response of hepatic NK cells to specific stimulation by Poly (I:C) was changed and the increase in the antitumor cytotoxic activity of intrahepatic activated NK cells was markedly impaired [14]. However, in responders to vaccination with HBV, Alabarran et al. reported that NK and NK-T cells are effectively activated against HBsAg, as reflected in the elevated ratio of CD56bright cells, increased NK-T cells, specific high levels of IL-2 and IFN-gamma intracellular cytokines in NK-T, and increase of IFN-gamma in NK cells. However, in nonresponders to vaccination, NK and NK-T cells showed an inactivation of capability and a diminished regulatory cytokine production [59].
4. HBsAg and Monocytes
Monocytes play roles in immune function and migrate from the bloodstream to other tissues to differentiate into tissue-resident macrophages or dendritic cells. They are characterized by the high expression of the CD14. Some groups have reported about the association between monocytes and HBV infection. Vanlandschoot et al., described that HBsAg suppressed the activity of monocytes and confirmed that recombinant HBsAg particles could bind almost exclusively to monocytes and that the binding to monocytes was enhanced by a heat-labile serum protein that was inhibited by Ca2M/Mg2M, low pH, and an HBsAg-specific monoclonal antibody. Moreover, the LPS and IL-2-induced production of cytokines was suppressed [15]. A study of the association between the human immune function and HBsAg using THP-1 cells, a human monocyte cell-line, indicated that HBsAg inhibits LPS-induced COX-2 expression and suggested that hepatitis B virus may regulate IFN-gamma production by inhibiting IL-18 and IL-12 production [13].
5. HBs-Ag and Dendritic Cells
Dendritic cells are professional APC that initiate and mediate immune responses against pathogens and tumors. Typically, immature DCs capture and process antigens to peptides which are then presented in the context of MHC class II or class I molecules. They migrate to lymphoid tissues and present antigenic peptides to naive T cells. Previous studies demonstrated that the myeloid dendritic cells (mDCs) of patients with chronic HBV are indeed impaired in their capacity to mature compared to mDC of healthy controls, as shown by their decreased capacity to upregulate costimulatory molecules, produce proinflammatory cytokines, and stimulate T cells [12]. It has been also reported that monocyte-derived DCs and plasmacytoid DCs(pDCs) are functionally impaired by the presence of HBV [12, 25]. Concerning the inhibitory mechanism, whether HBV directly interferes with the DC function is not known, as only HBV DNA could be detected but no evidence was found for viral replication in circulating mDCs and pDCs [12, 26], nor in monocyte-derived DCs [27]. Thus, although HBV may be incapable of replicating in DCs, the binding and uptake of viral particles by these cells may be responsible for the impaired function of DCs in HBV-infected patients. Op den Brouw et al. reported that HBsAg is internalized by mDCs and inhibits the upregulation of costimulatory molecules on mDCs [11], and Woltman et al. reported that analyzing different HBV proteins revealed that HBeAg and especially HBsAg are involved in the suppression of pDCs function, and HBsAg abrogated the CpG-induced mTOR-mediated phosphorylation of S6, the subsequent phosphorylation of IRF7 and the transcription of IFN-α genes [60]. However, the direct immune regulatory effect of HBV and circulating HBsAg particles on the function of DCs can be considered as part of the mechanism by which HBV escapes immunity (Figure 2).
Figure 2.
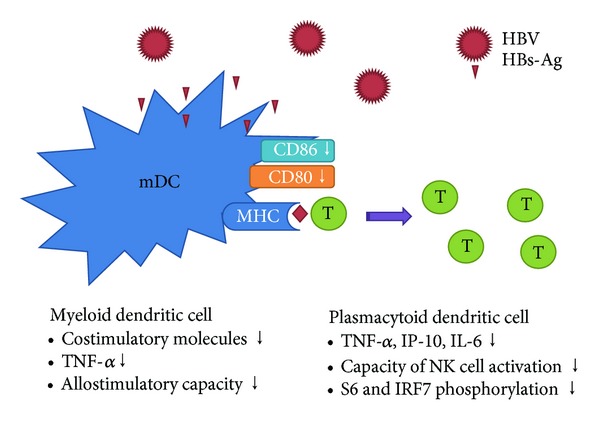
A schematic diagram of DC dysfunction in patients with HBV.
6. HBsAg Contributing to Carcinogenesis and Immune-Suppression of HBV-Related HCC
Up to now, carcinogenesis of the HCC by HBV has been studied. HBV-associated carcinogenesis can be seen as a multifactorial process that includes a direct mechanism involving viral protein, indirect mechanisms through the chronic inflammation, and the integration of HBV DNA [61]. As for the direct mechanism of the viral protein, it has been reported that the HBx gene [62–65] and PreS2 gene [66] act as promoters of carcinogenesis, based on a transgenic mouse model and force expression model of cell lines. The Pre S2 protein is encoded by HBsAg. It activates mitogen-activated protein kinase (MAPK), which is a signal molecule that is involved in cell proliferation [67]. Moreover, PreS2 protein accumulates in the endoplasmic reticulum (ER) of hepatocytes, and DNA injury is caused in the cell by ER stress [68, 69]. These mechanisms are considered to be a cause of carcinogenesis.
However, it is also thought that evasion from self-immunity is necessary for the growth of cancer. In recent studies, it was revealed that HBsAg carriers have 25–37 times increased risk of developing HCC as compared to noninfected people [70, 71]. Accordingly, it is thought that HBsAg functions in immune evasion, not only in promoting carcinogenesis. Actually, the accumulation of ER stress in hepatocytes causes the degeneration of protein, and evasion from self-immunity [72]. Moreover, it was reported that Pre S2 mutants increased hepatocellular carcinoma. These mutants reveal shorter forms of large, HBV surface antigens (LHBs), proteins with internal deletion. The deletion site (nucleotides 4–57) of Pre S2 has been recognized to correlate with an epitope of the CD8 T-cell response and B cell neutralization [33]. Therefore, Pre S2 mutants are involved in immune evasion. The immune evasion mechanism of HBV oncogenesis has not been fully elucidated, but further clarification is expected in the future.
It has been reported that the immune response could be suppressed by various kinds of mechanisms in HCC [73–81]. One of the important roles of immune suppression is induced by Tregs, as seen in HBV persistent infection [75–79, 82]. The frequency of Tregs infiltrating HCC was significantly higher than in non-HCC regions of the liver [83–86]. Moreover, HBV itself could induce excessive Tregs function [23, 24]. Therefore, the interaction between HBV and immune suppressive factors of HCC might strongly suppress cellular immune responses, including DCs, CTL, and Th1, and so forth, that are important for controlling HCC proliferation and HBV replication [87]. However, the relation between HBsAg expression and immune suppression in HCC is still unclear. In our ongoing study, the expression patterns of chemokines produced from several HCC cell lines with HBV replication were clearly different from those without HBV replication. Therefore, it is urgent to analyze the mechanisms of immune-suppression observed in HBV-related HCC.
7. HBsAg and Immunotherapy
In addition to HBcAg, HBsAg is one of the most important HBV-antigens that could induce HBV-specific cellular immune responses. HBsAg stimulation might easily induce Th2 immune responses. However, one group reported that HBsAg and CpG motif-containing oligodeoxynucleotides could induce Th1 immune responses [17]. Various kinds of HBsAg delivery systems were examined since the induction of a favorable immune response that could control HBV used to be difficult [17, 88–94]. Many groups have described that various kinds of recombinant hepatitis B vaccines could have a specific but transient effect on viral replication in HBsAg-positive CHB [95]. However, vaccines consisting of recombinant HBsAg and anti-HBs immunoglobulins could induce HBs-specific T cells efficiently since the formation of Ag-Ab immune complexes could be easily captured and taken up by DCs [96]. Although HBsAg might suppress the functions of DCs [11], HBsAg-pulsed DCs might enhance HBV-specific immune response in CHB patients [97].
8. Conclusion
HBsAg is not only a useful biomarker but also a protein that might suppress various kinds of immune cells contributing to innate and adaptive immune systems. Moreover, HBsAg could become an attractive target of immune therapy, since the cellular and humeral immune response against HBsAg might be able to control HBV replication and life cycles. Better understating of the interaction between HBsAg and immune cells could contribute to the development of immune therapy and a biomarker of the clinical state for HBV persistent infection.
References
- 1.Tiollais P, Pourcel C, Dejean A. The hepatitis B virus. Nature. 1985;317(6037):489–495. doi: 10.1038/317489a0. [DOI] [PubMed] [Google Scholar]
- 2.Lai CL, Ratziu V, Yuen MF, Poynard T. Viral hepatitis B. The Lancet. 2003;362(9401):2089–2094. doi: 10.1016/S0140-6736(03)15108-2. [DOI] [PubMed] [Google Scholar]
- 3.Summers J, Mason WS. Replication of the genome of a hepatitis B-like virus by reverse transcription of an RNA intermediate. Cell. 1982;29(2):403–415. doi: 10.1016/0092-8674(82)90157-x. [DOI] [PubMed] [Google Scholar]
- 4.Mason WS, Aldrich C, Summers J, Taylor JM. Asymmetric replication of duck hepatitis B virus DNA in liver cells: free minus-strand DNA. Proceedings of the National Academy of Sciences of the United States of America. 1982;79(13):3977–4001. doi: 10.1073/pnas.79.13.3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ganem D. The X files—one step closer to closure. Science. 2001;294(5550):2299–2300. doi: 10.1126/science.1067850. [DOI] [PubMed] [Google Scholar]
- 6.Prince AM. An antigen detected in the blood during the incubation period of serum hepatitis. Proceedings of the National Academy of Sciences of the United States of America. 1968;60(3):814–821. doi: 10.1073/pnas.60.3.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blumberg BS, Alter HJ, Visnich S. A “New” Antigen in Leukemia Sera. The Journal of the American Medical Association. 1965;191:541–546. doi: 10.1001/jama.1965.03080070025007. [DOI] [PubMed] [Google Scholar]
- 8.Thompson AJV, Nguyen T, Iser D, et al. Serum hepatitis B surface antigen and hepatitis B e antigen titers: disease phase influences correlation with viral load and intrahepatic hepatitis B virus markers. Hepatology. 2010;51(6):1933–1944. doi: 10.1002/hep.23571. [DOI] [PubMed] [Google Scholar]
- 9.Gramenzi A, Loggi E, Micco L, et al. Serum hepatitis B surface antigen monitoring in long-term lamivudine-treated hepatitis B virus patients. Journal of Viral Hepatitis. 2011;18(10):e468–e474. doi: 10.1111/j.1365-2893.2011.01473.x. [DOI] [PubMed] [Google Scholar]
- 10.Chan HL-Y, Thompson A, Martinot-Peignoux M, et al. Hepatitis B surface antigen quantification: why and how to use it in 2011—a core group report. Journal of Hepatology. 2011;55(5):1121–1131. doi: 10.1016/j.jhep.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 11.Op den Brouw ML, Binda RS, van Roosmalen MH, et al. Hepatitis B virus surface antigen impairs myeloid dendritic cell function: a possible immune escape mechanism of hepatitis B virus. Immunology. 2009;126(2):280–289. doi: 10.1111/j.1365-2567.2008.02896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van der Molen RG, Sprengers D, Binda RS, et al. Functional impairment of myeloid and plasmacytoid dendritic cells of patients with chronic hepatitis B. Hepatology. 2004;40(3):738–746. doi: 10.1002/hep.20366. [DOI] [PubMed] [Google Scholar]
- 13.Cheng J, Imanishi H, Morisaki H, et al. Recombinant HBsAg inhibits LPS-induced COX-2 expression and IL-18 production by interfering with the NFκB pathway in a human monocytic cell line, THP-1. Journal of Hepatology. 2005;43(3):465–471. doi: 10.1016/j.jhep.2005.02.033. [DOI] [PubMed] [Google Scholar]
- 14.Chen Y, Wei H, Sun R, Tian Z. Impaired function of hepatic natural killer cells from murine chronic HBsAg carriers. International Immunopharmacology. 2005;5(13-14):1839–1852. doi: 10.1016/j.intimp.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 15.Vanlandschoot P, van Houtte F, Roobrouck A, Farhoudi A, Leroux-Roels G. Hepatitis B virus surface antigen suppresses the activation of monocytes through interaction with a serum protein and a monocyte-specific receptor. Journal of General Virology. 2002;83, part 6:1281–1289. doi: 10.1099/0022-1317-83-6-1281. [DOI] [PubMed] [Google Scholar]
- 16.Vanlandschoot P, Roobrouck A, van Houtte F, Leroux-Roels G. Recombinant HBsAg, an apoptotic-like lipoprotein, interferes with the LPS-induced activation of ERK-1/2 and JNK-1/2 in monocytes. Biochemical and Biophysical Research Communications. 2002;297(3):486–491. doi: 10.1016/s0006-291x(02)02243-x. [DOI] [PubMed] [Google Scholar]
- 17.Zhou X, Zheng L, Liu L, Xiang L, Yuan Z. T helper 2 immunity to hepatitis B surface antigen primed by gene-gun-mediated DNA vaccination can be shifted towards T helper 1 immunity by codelivery of CpG motif-containing oligodeoxynucleotides. Scandinavian Journal of Immunology. 2003;58(3):350–357. doi: 10.1046/j.1365-3083.2003.01310.x. [DOI] [PubMed] [Google Scholar]
- 18.Maier H, Isogawa M, Freeman GJ, Chisari FV. PD-1:PD-L1 interactions contribute to the functional suppression of virus-specific CD8+ T lymphocytes in the liver. Journal of Immunology. 2007;178(5):2714–2720. doi: 10.4049/jimmunol.178.5.2714. [DOI] [PubMed] [Google Scholar]
- 19.Kanto T. Virus associated innate immunity in liver. Frontiers in Bioscience. 2008;13:6183–6192. doi: 10.2741/3146. [DOI] [PubMed] [Google Scholar]
- 20.Reignat S, Webster GJM, Brown D, et al. Escaping high viral load exhaustion: CD8 cells with altered tetramer binding in chronic hepatitis B virus infection. Journal of Experimental Medicine. 2002;195(9):1089–1101. doi: 10.1084/jem.20011723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roh S, Kim K. Overcoming tolerance in hepatitis B virus transgenic mice: a possible involvement of regulatory T cells. Microbiology and Immunology. 2003;47(6):453–460. doi: 10.1111/j.1348-0421.2003.tb03370.x. [DOI] [PubMed] [Google Scholar]
- 22.Frebel H, Richter K, Oxenius A. How chronic viral infections impact on antigen-specific T-cell responses. European Journal of Immunology. 2010;40(3):654–663. doi: 10.1002/eji.200940102. [DOI] [PubMed] [Google Scholar]
- 23.Kondo Y, Kobayashi K, Ueno Y, et al. Mechanism of T cell hyporesponsiveness to HBcAg is associated with regulatory T cells in chronic hepatitis B. World Journal of Gastroenterology. 2006;12(27):4310–4317. doi: 10.3748/wjg.v12.i27.4310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kondo Y, Ueno Y, Kobayashi K, et al. Hepatitis B virus replication could enhance regulatory T cell activity by producing soluble heat shock protein 60 from hepatocytes. Journal of Infectious Diseases. 2010;202(2):202–213. doi: 10.1086/653496. [DOI] [PubMed] [Google Scholar]
- 25.Möröy T, Etiemble J, Trépo C, Tiollais P, Buendia MA. Transcription of woodchuck hepatitis virus in the chronically infected liver. The EMBO Journal. 1985;4(6):1507–1514. doi: 10.1002/j.1460-2075.1985.tb03810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tavakoli S, Mederacke I, Herzog-Hauff S, et al. Peripheral blood dendritic cells are phenotypically and functionally intact in chronic hepatitis B virus (HBV) infection. Clinical and Experimental Immunology. 2008;151(1):61–70. doi: 10.1111/j.1365-2249.2007.03547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Untergasser A, Zedler U, Langenkamp A, et al. Dendritic cells take up viral antigens but do not support the early steps of hepatitis B virus infection. Hepatology. 2006;43(3):539–547. doi: 10.1002/hep.21048. [DOI] [PubMed] [Google Scholar]
- 28.Manigold T, Racanelli V. T-cell regulation by CD4 regulatory T cells during hepatitis B and C virus infections: facts and controversies. Lancet Infectious Diseases. 2007;7(12):804–813. doi: 10.1016/S1473-3099(07)70289-X. [DOI] [PubMed] [Google Scholar]
- 29.Billerbeck E, Böttler T, Thimme R. Regulatory T cells in viral hepatitis. World Journal of Gastroenterology. 2007;13(36):4858–4864. doi: 10.3748/wjg.v13.i36.4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sette AD, Oseroff C, Sidney J, et al. Overcoming T cell tolerance to the hepatitis B virus surface antigen in hepatitis B virus-transgenic mice. Journal of Immunology. 2001;166(2):1389–1397. doi: 10.4049/jimmunol.166.2.1389. [DOI] [PubMed] [Google Scholar]
- 31.Böcher WO, Galun E, Marcus H, et al. Reduced hepatitis B virus surface antigen-specific Th1 helper cell frequency of chronic HBV carriers is associated with a failure to produce antigen-specific antibodies in the trimera mouse. Hepatology. 2000;31(2):480–487. doi: 10.1002/hep.510310231. [DOI] [PubMed] [Google Scholar]
- 32.Guidotti LG, Ishikawa T, Hobbs MV, Matzke B, Schreiber R, Chisari FV. Intracellular inactivation of the hepatitis B virus by cytotoxic T lymphocytes. Immunity. 1996;4(1):25–36. doi: 10.1016/s1074-7613(00)80295-2. [DOI] [PubMed] [Google Scholar]
- 33.Chisari FV, Ferrari C. Hepatitis B virus immunopathogenesis. Annual Review of Immunology. 1995;13:29–60. doi: 10.1146/annurev.iy.13.040195.000333. [DOI] [PubMed] [Google Scholar]
- 34.Kennedy PTF, Gehring AJ, Nowbath A, et al. The expression and function of NKG2D molecule on intrahepatic CD8+ T cells in chronic viral hepatitis. Journal of Viral Hepatitis. 2008;15(12):901–909. doi: 10.1111/j.1365-2893.2008.01049.x. [DOI] [PubMed] [Google Scholar]
- 35.Romagnani C, Juelke K, Falco M, et al. CD56brightCD16-killer Ig-like receptor-NK cells display longer telomeres and acquire features of CD56dim NK cells upon activation. Journal of Immunology. 2007;178(8):4947–4955. doi: 10.4049/jimmunol.178.8.4947. [DOI] [PubMed] [Google Scholar]
- 36.Chan A, Hong DL, Atzberger A, et al. CD56bright human NK cells differentiate into CD56dim cells: role of contact with peripheral fibroblasts. Journal of Immunology. 2007;179(1):89–94. doi: 10.4049/jimmunol.179.1.89. [DOI] [PubMed] [Google Scholar]
- 37.Seaman WE. Natural killer cells and natural killer T cells. Arthritis & Rheumatism. 2000;43(6):1204–1217. doi: 10.1002/1529-0131(200006)43:6<1204::AID-ANR3>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 38.Carey I, D’Antiga L, Bansal S, et al. Immune and viral profile from tolerance to hepatitis B surface antigen clearance: a longitudinal study of vertically hepatitis B virus-infected children on combined therapy. Journal of Virology. 2011;85(5):2416–2428. doi: 10.1128/JVI.01449-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Langley DR, Walsh AW, Baldick CJ, et al. Inhibition of hepatitis B virus polymerase by entecavir. Journal of Virology. 2007;81(8):3992–4001. doi: 10.1128/JVI.02395-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tong MJ, Tu SS. Treatment of patients with chronic hepatitis B with adefovir dipivoxil. Seminars in Liver Disease. 2004;24(supplement 1):37–44. doi: 10.1055/s-2004-828677. [DOI] [PubMed] [Google Scholar]
- 41.Guan R. Interferon monotherapy in chronic hepatitis B. Journal of Gastroenterology and Hepatology. 2000;15:E34–E40. doi: 10.1046/j.1440-1746.2000.02101.x. [DOI] [PubMed] [Google Scholar]
- 42.Pol S, Michel ML. Therapeutic vaccination in chronic hepatitis B virus carriers. Expert Review of Vaccines. 2006;5(5):707–716. doi: 10.1586/14760584.5.5.707. [DOI] [PubMed] [Google Scholar]
- 43.Kondo Y, Ueno Y, Shimosegawa T. Immunopathogenesis of hepatitis B persistent infection: Implications for immunotherapeutic strategies. Clinical Journal of Gastroenterology. 2009;2(2):71–79. doi: 10.1007/s12328-009-0074-z. [DOI] [PubMed] [Google Scholar]
- 44.Rahman F, Dahmen A, Herzog-Hauff S, Böcher WO, Galle PR, Löhr HE. Cellular and humoral immune responses induced by intradermal or intramuscular vaccination with the major hepatitis B surface antigen. Hepatology. 2000;31(2):521–527. doi: 10.1002/hep.510310237. [DOI] [PubMed] [Google Scholar]
- 45.Hsu HY, Chang MH, Hsieh RP, Ni YH, Chi WK. Humoral and cellular immune responses to hepatitis B vaccination in hepatitis B surface antigen-carrier children who cleared serum-hepatitis B surface antigen. Hepatology. 1996;24(6):1355–1360. doi: 10.1002/hep.510240607. [DOI] [PubMed] [Google Scholar]
- 46.Kondo Y, Asabe S, Kobayashi K, et al. Recovery of functional cytotoxic T lymphocytes during lamivudine therapy by acquiring multi-specificity. Journal of Medical Virology. 2004;74(3):425–433. doi: 10.1002/jmv.20194. [DOI] [PubMed] [Google Scholar]
- 47.Kondo Y, Kobayashi K, Asabe S, et al. Vigorous response of cytotoxic T lymphocytes associated with systemic activation of CD8+ T lymphocytes in fulminant hepatitis B. Liver International. 2004;24(6):561–567. doi: 10.1111/j.1478-3231.2004.0982.x. [DOI] [PubMed] [Google Scholar]
- 48.Nayersina R, Fowler P, Guilhot S, et al. HLA A2 restricted cytotoxic T lymphocyte responses to multiple hepatitis B surface antigen epitopes during hepatitis B virus infection. Journal of Immunology. 1993;150(10):4659–4671. [PubMed] [Google Scholar]
- 49.Rehermann B, Nascimbeni M. Immunology of hepatitis B virus and hepatitis C virus infection. Nature Reviews Immunology. 2005;5(3):215–229. doi: 10.1038/nri1573. [DOI] [PubMed] [Google Scholar]
- 50.McMahon BJ. The natural history of chronic hepatitis B virus infection. Hepatology. 2009;49(supplement 5):S45–S55. doi: 10.1002/hep.22898. [DOI] [PubMed] [Google Scholar]
- 51.McMahon BJ. The influence of hepatitis B virus genotype and subgenotype on the natural history of chronic hepatitis B. Hepatology International. 2009;3(2):334–342. doi: 10.1007/s12072-008-9112-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grégoire C, Chasson L, Luci C, et al. The trafficking of natural killer cells. Immunological Reviews. 2007;220(1):169–182. doi: 10.1111/j.1600-065X.2007.00563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Doherty DG, O’Farrelly C. Innate and adaptive lymphoid cells in the human liver. Immunological Reviews. 2000;174:5–20. doi: 10.1034/j.1600-0528.2002.017416.x. [DOI] [PubMed] [Google Scholar]
- 54.Loza MJ, Zamai L, Azzoni L, Rosati E, Perussia B. Expression of type 1 (interferon gamma) and type 2 (interleukin-13, interleukin-5) cytokines at distinct stages of natural killer cell differentiation from progenitor cells. Blood. 2002;99(4):1273–1281. doi: 10.1182/blood.v99.4.1273. [DOI] [PubMed] [Google Scholar]
- 55.Cooper MA, Fehniger TA, Turner SC, et al. Human natural killer cells: a unique innate immunoregulatory role for the CD56bright subset. Blood. 2001;97(10):3146–3151. doi: 10.1182/blood.v97.10.3146. [DOI] [PubMed] [Google Scholar]
- 56.Gray JD, Hirokawa M, Ohtsuka K, Horwitz DA. Generation of an inhibitory circuit involving CD8+ T cells, IL-2, and NK cell-derived TGF-β: Contrasting effects of anti-CD2 and anti-CD3. Journal of Immunology. 1998;160(5):2248–2254. [PubMed] [Google Scholar]
- 57.Porcelli S, Yockey CE, Brenner MB, Balk SP. Analysis of T cell antigen receptor (TCR) expression by human peripheral blood CD4-8- α/β T cells demonstrates preferential use of several Vβ genes and an invariant TCR α chain. Journal of Experimental Medicine. 1993;178(1):1–16. doi: 10.1084/jem.178.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim CH, Johnston B, Butcher EC. Trafficking machinery of NKT cells: shared and differential chemokine receptor expression among Vα24+Vβ11+ NKT cell subsets with distinct cytokine-producing capacity. Blood. 2002;100(1):11–16. doi: 10.1182/blood-2001-12-0196. [DOI] [PubMed] [Google Scholar]
- 59.Albarran B, Goncalves L, Salmen S, et al. Profiles of NK, NKT cell activation and cytokine production following vaccination against hepatitis B. APMIS. 2005;113(7-8):526–535. doi: 10.1111/j.1600-0463.2005.apm_191.x. [DOI] [PubMed] [Google Scholar]
- 60.Woltman mail AM, Op den Brouw ML, Biesta PJ, Shi CC. Hepatitis B virus lacks immune activating capacity, but actively inhibits plasmacytoid dendritic cell function. PLOS One. 2011;6(1) doi: 10.1371/journal.pone.0015324.e15324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Imazeki F, Omata M, Yokosuka O, Okuda K. Integration of hepatitis B virus DNA in hepatocellular carcinoma. Cancer. 1986;58(5):1055–1060. doi: 10.1002/1097-0142(19860901)58:5<1055::aid-cncr2820580513>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 62.Koike K, Tsutsumi T, Fujie H, Shintani Y, Moriya K. Molecular mechanism of viral hepatocarcinogenesis. Oncology. 2002;62(supplement 1):29–37. doi: 10.1159/000048273. [DOI] [PubMed] [Google Scholar]
- 63.Staib F, Hussain SP, Hofseth LJ, Wang XW, Harris CC. TP53 and liver carcinogenesis. Human Mutation. 2003;21(3):201–216. doi: 10.1002/humu.10176. [DOI] [PubMed] [Google Scholar]
- 64.Cougot D, Neuveut C, Buendia MA. HBV-induced carcinogenesis. Journal of Clinical Virology. 2005;34(supplement 1):S75–S78. doi: 10.1016/s1386-6532(05)80014-9. [DOI] [PubMed] [Google Scholar]
- 65.Chan HLY, Sung JJY. Hepatocellular carcinoma and hepatitis B virus. Seminars in Liver Disease. 2006;26(2):153–161. doi: 10.1055/s-2006-939753. [DOI] [PubMed] [Google Scholar]
- 66.Kekule AS, Lauer U, Meyer M, Caselmann WH, Hofschneider PH, Koshy R. The preS2/S region of integrated hepatitis B virus DNA encodes a transcriptional transactivator. Nature. 1990;343(6257):457–461. doi: 10.1038/343457a0. [DOI] [PubMed] [Google Scholar]
- 67.Hung JH, Su IJ, Lei HY, et al. Endoplasmic reticulum stress stimulates the expression of cyclooxygenase-2 through activation of NF-κB and pp38 mitogen-activated protein kinase. Journal of Biological Chemistry. 2004;279(45):46384–46392. doi: 10.1074/jbc.M403568200. [DOI] [PubMed] [Google Scholar]
- 68.Wang HC, Wu HC, Chen CF, Fausto N, Lei HY, Su IJ. Different types of ground glass hepatocytes in chronic hepatitis B virus infection contain specific pre-S mutants that may induce endoplasmic reticulum stress. American Journal of Pathology. 2003;163(6):2441–2449. doi: 10.1016/S0002-9440(10)63599-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kadowaki H, Nishitoh H, Ichijo H. Survival and apoptosis signals in ER stress: the role of protein kinases. Journal of Chemical Neuroanatomy. 2004;28(1-2):93–100. doi: 10.1016/j.jchemneu.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 70.Hassan MM, Hwang LY, Hatten CJ, et al. Risk factors for hepatocellular carcinoma: synergism of alcohol with viral hepatitis and diabetes mellitus. Hepatology. 2002;36(5):1206–1213. doi: 10.1053/jhep.2002.36780. [DOI] [PubMed] [Google Scholar]
- 71.Yang HI, Lu SN, Liaw YF, et al .Hepatitis B e antigen and the risk of hepatocellular carcinoma. The New England Journal of Medicine. 2002;347(3):168–174. doi: 10.1056/NEJMoa013215. [DOI] [PubMed] [Google Scholar]
- 72.Wang HC, Huang W, Lai MD, Su IJ. Hepatitis B Virus pre-S mutants, endoplasmic reticulum stress and hepatocarcinogenesis. Cancer Science. 2006;97(8):683–688. doi: 10.1111/j.1349-7006.2006.00235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ke W, Kryczek I, Chen L, Zou W, Welling TH. Kupffer cell suppression of CD8+ T cells in human hepatocellular carcinoma is mediated by B7-H1/programmed death-1 interactions. Cancer Research. 2009;69(20):8067–8075. doi: 10.1158/0008-5472.CAN-09-0901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Matsuzaki K, Date M, Furukawa F, et al. Regulatory mechanisms for transforming growth factor β as an autocrine inhibitor in human hepatocellular carcinoma: implications for roles of smads in its growth. Hepatology. 2000;32(2):218–227. doi: 10.1053/jhep.2000.9145. [DOI] [PubMed] [Google Scholar]
- 75.Chen KJ, Lin SZ, Zhou L, et al. Selective recruitment of regulatory T cell through CCR6-CCL20 in hepatocellular carcinoma fosters tumor progression and predicts poor prognosis. PLoS One. 2011;6(9) doi: 10.1371/journal.pone.0024671.e24671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lee WC, Wu TJ, Chou HS, et al. The impact of CD4+CD25+ T cells in the tumor microenvironment of hepatocellular carcinoma. Surgery. 2012;151(2):213–222. doi: 10.1016/j.surg.2011.07.029. [DOI] [PubMed] [Google Scholar]
- 77.Chen KJ, Zhou L, Xie HY, Ahmed TE, Feng XW, Zheng SS. Intratumoral regulatory T cells alone or in combination with cytotoxic T cells predict prognosis of hepatocellular carcinoma after resection. Medical Oncology. 2012;29(3):1817–1826. doi: 10.1007/s12032-011-0006-x. [DOI] [PubMed] [Google Scholar]
- 78.Takata Y, Nakamoto Y, Nakada A, et al. Frequency of CD45RO+ subset in CD4+CD25high regulatory T cells associated with progression of hepatocellular carcinoma. Cancer Letters. 2011;307(2):165–173. doi: 10.1016/j.canlet.2011.03.029. [DOI] [PubMed] [Google Scholar]
- 79.Cabrera R, Ararat M, Eksioglu EA, et al. Influence of serum and soluble CD25 (sCD25) on regulatory and effector T-cell function in hepatocellular carcinoma. Scandinavian Journal of Immunology. 2010;72(4):293–301. doi: 10.1111/j.1365-3083.2010.02427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gao Q, Wang XY, Qiu SJ, et al. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clinical Cancer Research. 2009;15(3):971–979. doi: 10.1158/1078-0432.CCR-08-1608. [DOI] [PubMed] [Google Scholar]
- 81.Hoechst B, Ormandy LA, Ballmaier M, et al. A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4+CD25+Foxp3+ T cells. Gastroenterology. 2008;135(1):234–243. doi: 10.1053/j.gastro.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 82.Miroux C, Vausselin T, Delhem N. Regulatory T cells in HBV and HCV liver diseases: implication of regulatory T lymphocytes in the control of immune response. Expert Opinion on Biological Therapy. 2010;10(11):1563–1572. doi: 10.1517/14712598.2010.529125. [DOI] [PubMed] [Google Scholar]
- 83.Shirabe K, Motomura T, Muto J, et al. Tumor-infiltrating lymphocytes and hepatocellular carcinoma: pathology and clinical management. International Journal of Clinical Oncology. 2010;15(6):552–558. doi: 10.1007/s10147-010-0131-0. [DOI] [PubMed] [Google Scholar]
- 84.Yang ZQ, Yang ZY, Zhang LD, et al. Increased liver-infiltrating CD8+FoxP3+ regulatory T cells are associated with tumor stage in hepatocellular carcinoma patients. Human Immunology. 2010;71(12):1180–1186. doi: 10.1016/j.humimm.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 85.Shen X, Li N, Li H, Zhang T, Wang F, Li Q. Increased prevalence of regulatory T cells in the tumor microenvironment and its correlation with TNM stage of hepatocellular carcinoma. Journal of Cancer Research and Clinical Oncology. 2010;136(11):1745–1754. doi: 10.1007/s00432-010-0833-8. [DOI] [PubMed] [Google Scholar]
- 86.Fu J, Xu D, Liu Z, et al. Increased regulatory T cells correlate with CD8 T-cell impairment and poor survival in hepatocellular carcinoma patients. Gastroenterology. 2007;132(7):2328–2339. doi: 10.1053/j.gastro.2007.03.102. [DOI] [PubMed] [Google Scholar]
- 87.Yamamoto M, Tatsumi T, Miyagi T, et al. α-Fetoprotein impairs activation of natural killer cells by inhibiting the function of dendritic cells. Clinical and Experimental Immunology. 2011;165(2):211–219. doi: 10.1111/j.1365-2249.2011.04421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhou C, Peng G, Jin X, Tang J, Chen Z. Vaccination with a fusion DNA vaccine encoding hepatitis B surface antigen fused to the extracellular domain of CTLA4 enhances HBV-specific immune responses in mice: implication of its potential use as a therapeutic vaccine. Clinical Immunology. 2010;137(2):190–198. doi: 10.1016/j.clim.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 89.Memarnejadian A, Roohvand F. Fusion of HBsAg and prime/boosting augment Th1 and CTL responses to HCV polytope DNA vaccine. Cellular Immunology. 2010;261(2):93–98. doi: 10.1016/j.cellimm.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 90.Yang K, Whalen BJ, Tirabassi RS, et al. A DNA vaccine prime followed by a liposome-encapsulated protein boost confers enhanced mucosal immune responses and protection. Journal of Immunology. 2008;180(9):6159–6167. doi: 10.4049/jimmunol.180.9.6159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Menne S, Tennant BC, Gerin JL, Cote PJ. Chemoimmunotherapy of chronic hepatitis B virus infection in the woodchuck model overcomes immunologic tolerance and restores T-cell responses to pre-S and S regions of the viral envelope protein. Journal of Virology. 2007;81(19):10614–10624. doi: 10.1128/JVI.00691-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Iglesias E, Thompson R, Carrazana Y, et al. Coinoculation with hepatitis B surface and core antigen promotes a Th1 immune response to a multiepitopic protein of HIV-1. Immunology and Cell Biology. 2006;84(2):174–183. doi: 10.1111/j.1440-1711.2005.01408.x. [DOI] [PubMed] [Google Scholar]
- 93.Qiu SJ, Lu L, Qiao C, et al. Induction of tumor immunity and cytotoxic t lymphocyte responses using dendritic cells transduced by adenoviral vectors encoding HBsAg: comparison to protein immunization. Journal of Cancer Research and Clinical Oncology. 2005;131(7):429–438. doi: 10.1007/s00432-004-0616-1. [DOI] [PubMed] [Google Scholar]
- 94.Zhang L, Widera G, Bleecher S, Zaharoff DA, Mossop B, Rabussay D. Accelerated immune response to DNA vaccines. DNA and Cell Biology. 2003;22(12):815–822. doi: 10.1089/104454903322625028. [DOI] [PubMed] [Google Scholar]
- 95.Michel ML, Mancini-Bourgine M. Therapeutic vaccination against chronic hepatitis B virus infection. Journal of Clinical Virology. 2005;34(supplement 1):S108–S114. doi: 10.1016/s1386-6532(05)80019-8. [DOI] [PubMed] [Google Scholar]
- 96.Wen YM, Wu XH, Hu DC, Zhang QP, Guo SQ. Hepatitis B vaccine and anti-HBs complex as approach for vaccine therapy. The Lancet. 1995;345(8964):1575–1576. doi: 10.1016/s0140-6736(95)91126-x. [DOI] [PubMed] [Google Scholar]
- 97.Akbar SMF, Horiike N, Chen S, et al. Mechanism of restoration of immune responses of patients with chronic hepatitis B during lamivudine therapy: increased antigen processing and presentation by dendritic cells. Journal of Viral Hepatitis. 2011;18(3):200–205. doi: 10.1111/j.1365-2893.2010.01300.x. [DOI] [PubMed] [Google Scholar]


