Abstract
Helicobacter pylori (H. pylori) is considered the second most prevalent infection in man. A precise diagnosis is important for treating patients with the indicative gastrointestinal symptoms. The present study analyzes the effectiveness of a molecular biology method (PCR) comparing the results obtained with the histology and with the rapid urease tests. PCR was used in the detection and genotyping of the H. pylori urease-C gene and the patterns which were obtained from the patients studied. 141 biopsy samples from 131 patients were evaluated. 59 paraffin biopsies samples were positive for H. pylori according to the histological examination. Of those, 59/12 (20.3%) were amplified using PCR. Of the 82 samples from the fresh biopsies, 64 were positive for H. pylori according to the rapid urease test (78%); there was an agreement of 100% with PCR. Sixty positive H. pylori samples were genotyped (58 samples of fresh biopsies and 2 samples of paraffin biopsies) using two restriction enzymes. The patterns observed were analyzed with the computational program BIO 1D; 11 patterns with the enzyme HhaI and 12 patterns with the enzyme MboI were found. However, it was not possible to find a statistically significant correlation between the specific genotypes and digestive pathologies. Accordingly, future research should be performed to confirm a statistically significant relationship between genotyping and gastrointestinal symptoms.
1. Introduction
The Helicobacter pylori (H. pylori) infection is currently endemic worldwide with high prevalence (up to 60%) in developing regions such as South America. The infection causes chronic Gastritis, gastric and duodenal ulcers, gastric adenocarcinoma, and mucosa-associated lymphoid tissue [1–6]. H. pylori is associated with several autoimmune diseases, including idiopathic thrombocytopenic purpura (ITP), Sjögren syndrome, systemic sclerosis [7], Graves' disease [8], and autoimmune pancreatitis [9]. As a result of this association with autoimmune diseases, we hypothesized that H. pylori might induce systemic immunological changes. Although the seroprevalence of H. pylori may be high in the normal population, a minority develops peptic ulcers [10, 11]. Some possibilities could justify this data: genetic differences in the host's environmental factors and bacterial strains.
A variety of tests are now available to diagnose H. pylori infection. Histological examination of gastric tissue, bacterial cultures, rapid urease test, use of DNA probes, and PCR analysis, when used to test gastric tissue, all require endoscopy. In contrast, breath tests, serology, gastric juice PCR, and urinary excretion of N15 ammonia are noninvasive tests that do not require endoscopy. PCR offers high sensitivity and specificity as a technique for the detection of H. pylori although the accuracy of such techniques varies widely [12]. The aim of this work is to analyze the effectiveness of the molecular biology method PCR in the detection of H. pylori in patients with gastrointestinal symptoms, comparing the results with the histology and the rapid urease test and using the PCR-RFLP technique to detect H. pylori subtypes in endoscopic biopsy samples obtained from Brazilian patients.
2. Material and Methods
2.1. Patients
141 samples were collected from 131 patients with several diagnoses of gastrointestinal pathologies. Among them, 99 patients who were involved in this study had Gastritis, 29 had ulcers, 5 had Gastritis and ulcers, 3 had esophagitis, and 5 had other gastrointestinal diseases. The patients were 48 years old on average; their ages varied from 4 to 90 years old. 81 were males and 50 females (Table 1). All patients were submitted to an endoscopy at the Gastrocentro (Center of Digestive Tract Studies), University Hospital, State University of Campinas, SP, Brazil, after informed consent was obtained and protocol approved by the Hospital's Ethics Committee.
Table 1.
Patient characteristics.
| Total (n = 141) | |
|---|---|
| Patients | |
| Sex (male/female) | 81/50 |
| Age years (median) | 48 years (range 4–90) |
| Disease | |
| Gastritis | 99 (70.2%) |
| Ulcers | 29 (20.6%) |
| Gastritis + ulcers | 5 (3.5%) |
| Esophagitis | 3 (2%) |
| Other* | 5 (3.5%) |
*Inflammation, duodenitis, and splenomegaly.
2.2. Methods
The methods used for the detection of H. pylori were polymerase chain reaction (PCR) and PCR-RFLP for genotyping. They were chosen in order to detect the bacterium and its subtypes in endoscopic biopsies of fresh tissues and paraffin tissues. The fresh biopsy samples were conserved in physiologic serum 0.9% until the DNA was extracted. At least two, 5 to 10 mm, ribbons of paraffin were collected from the paraffin tissue. In the fresh biopsies, at least two fragments were collected.
2.2.1. DNA Extraction—Gastric Paraffin Biopsy
DNA extraction from the endoscopic biopsies fastened in paraffin followed the method described by Davis et al., 1995 [13], with some modifications.
At least two, 5 to 10 mm, ribbons of paraffin were placed in a 1.5 mL Eppendorf tube. One mL of xylene was added to the samples. They were shaken, allowed to rest for 3 to 5 minutes, and then centrifuged for 5 minutes, discarding the xylene afterwards. After three washes in 100%, 95%, and 70% ethanol, respectively, the samples were dried at room temperature. Next, the material was resuspended in a solution of Proteinase K, 50 mM Tris, 0.5% SDS, and sterile water. 430 μL of phenol were added to the sample, which was homogenized and centrifuged for another 30 minutes at 14,000 RPM. The supernatant containing DNA was transferred to a new tube and 430 μL of phenol/chloroform (1 : 1) were added and centrifuged again for 5 minutes at 14,000 RPM. Chloroform/isoamyl ethanol was added (24 : 1) to the supernatant, which was homogenized and centrifuged for another 30 minutes at 14,000 RPM. After the addition of 75 μL of ammonium acetate and 750 μL of 100%, ethanol samples were inverted several times and incubated overnight to −20°C. After centrifugation for 30 minutes at 12,000 RPM to −4°C, the supernatant was discarded. The precipitate was carefully washed with 500 μL of chilled 70% ethanol, which was immediately discarded. The material was dried at room temperature and resuspended in a solution containing 50 mL of sterile water, 10 M of Tris (pH 8.0), and 1 mL of EDTA and stored at −20°C until its use.
2.2.2. DNA Extraction: Fresh Biopsy
Firstly, a fresh 3 to 7 mm biopsy section was placed in a 1.5 mL sterile tube with 190 μL of a solution that contained 0.1 M of Tris HCl (pH 7.5) and 1% of SDS. Secondly, 10 μL of proteinase K were added (10 mg/mL) to the solution. The sample was macerated and incubated overnight at 55°C. After that, 200 μL of phenol and 200 μL of both chloroform and isoamyl alcohol (24 : 1) were added. The solution was then homogenized and centrifuged for one minute. Next, the supernatant was removed, and 200 μL of chloroform/isoamyl alcohol were added, homogenized, and centrifuged for 1 minute. Next, the supernatant was removed again, and 25 μL of sodium acetate 3 M and 900 μL of 100% ethanol at −20°C were added; after vortexing the mixture, it was incubated for 30 minutes at −70°C. The samples were centrifuged for 15 minutes at 15,000 RPM. The supernatant was discarded. Lastly, the DNA was resuspended in 25 μL of distilled and sterile water. [14].
2.2.3. PCR Amplification of the H. pylori
The polymerase chain reaction followed the method described by Saiki and col. [15], with some modifications.
For each amplification reaction, 0.5 to 0.7 μL of the DNA under investigation were used, for a total reaction volume of 20.0 μL. The reaction buffer contained 50 mM KCL, 10 mM Tris-HCL pH 8.4, 2.5 mM MgCL2, 2.0 pmol of each “primer,” 200 μM of each deoxynucleotide triphosphate (dATP, dCTP, dGTP, and dTTP), 2.5 units of Taq polymerase (Gibco-BRL), and sufficient water to give the total volume of 20.0 μL. The reaction mixture was covered with 100 μL of mineral oil and the tubes were placed in a DNA Thermal Cycler (Perkin-Elmer).
The reactions that followed were found to be optimal. The samples were heated to 94°C for 60 s to denature the DNA, cooled to 57°C for 90 s to allow the primers and the DNA to reanneal, and then heated to 72°C for 120 s for primer extension. By the final cycle, the extension period was 7 min. A total of 40 cycles were performed. The amplified product was detected by direct gel analysis. 5 μL of the reaction mixture were subjected to electrophoresis with 2% agarose minigel, and the DNA was visualized using UV fluorescence after staining with ethidium bromide. Molecular weight markers were included in each gel. An 820 base-pair band was seen when samples were amplified using primers P1 and P2 to detect H. pylori (Table 1 and Figure 1).
Figure 1.
Automated sequencing (Abi Prism 377) for the ureC region of H. pylori. Positive samples for H. pylori infection obtained from PCR in order to prove that the sequences being amplified did belong to the genetic sequence of a DNA segment of the urease-C area of H. pylori (enzymes HhaI and MboI). All DNA samples taken from the patients presented genetic sequences similar to the one of H. pylori, as described for the urease-C area of the Gene Bank (I Square 1).
2.2.4. PCR-Based Restriction Fragment Length Polymorphism Typing of Helicobacter pylori (RFLP)
After the amplification was confirmed, the PCR product was submitted to digestion with the restriction enzymes HhaI and MboI for fragmentation of Urease-C [16].
The fragments which were produced were submitted to electrophoresis in a 2% gel agarose 1000 (Gibco-BRL), stained with ethidium bromide, visualized under ultraviolet light and photographed in Polaroid System. The patterns which were found were compared and analyzed with the computational program Bio 1D (Analysis of Restriction—PCR-RFLP—Restriction Fragment Length Polymorphism), version 99 (Vilber Loumart) (Figures 2 and 3).
Figure 2.
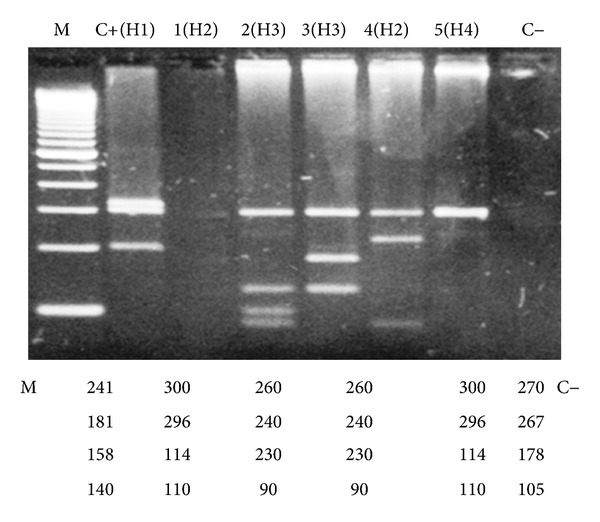
Digestion patterns with the enzyme HhaI found in some analyzed samples. Electrophoresis in 2% agarose gel 1000, stained with bromide ethidium. M marker of molecular weight, C+ (1), 1(H2), 2(H3), 3(H4), 4(H4), 5(H2), C-negative control. Note: the most frequent pattern found was H4, with 14 patients (25.8%).
Figure 3.
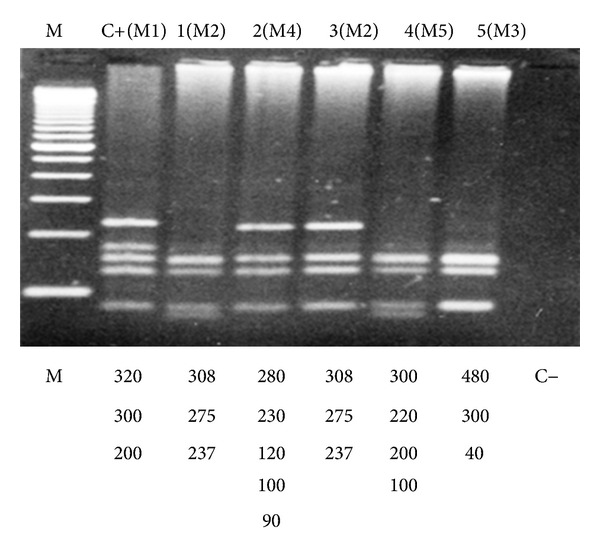
Digestion patterns with the enzyme MboI found in some analyzed samples. Electrophoresis in 2% agarose gel 1000, stained with ethidium bromide. M marker of molecular weight; C+(M1); 1(M2); 2(M4); 3(M2); 4(M5); 5(M3); C-negative control. Note 1: Pattern M4 was the most frequent, with 15 patients (25.8%). Note 2: patient 1(M2) presented very clear bands in this analysis, but in a posterior analysis it was possible to classify this patient in group M2.
Approximately 10 μL of the amplified product were used for the digestion process which also contained 2.0 μL of the corresponding enzyme. Water was added to fill 20.0 μL and the mixture was placed in a 37°C bath overnight.
2.2.5. Automatic Sequencing
Automatic sequencing was performed using the program Abi Prism, model 377, version 3.4, and Abi 100, version 3.2. Sequencing allowed for the identification of the studied DNA region (Primers P1 and P2). Figure 1 shows the automatic sequencing, proving that the sequence is Helicobacter pylori.
3. Results
A total of 141 endoscopic biopsy samples from 131 patients were studied for H. pylori infections with PCR and the results were compared with Urease and Histology tests. 82/64 (78%) fresh samples had a positive Urease test for H. pylori. A PCR test detected all of the 64 positive samples identified by the Urease test (100%) (Table 2).
Table 2.
Comparison between PCR and urease test in fresh biopsy samples.
| Urease test | PCR | |
|---|---|---|
| Positive | 64 | 64 |
| Negative | 18 | 18 |
| Total | 82 | 82 |
100% agreement.
Fifty-nine paraffin biopsies, all found to be positive through a histological examination, were submitted to the DNA extraction procedure and Beta-Globin PCR to prove the quality and the presence of DNA in the extracted samples. Only 14/59 (23.7%) samples were positive for the Beta Globin gene, but in two of them H. pylori was not amplified by PCR, even though they had a positive Histology test (Table 3). In the other 45 samples, it was impossible to detect Beta Globin in the DNA using PCR, primarily because of the low amount of paraffin samples and/or because the reaction was inhibited due to paraffin and xilol in the extraction procedures. No contamination occurred and the samples were tested two times.
Table 3.
Comparison between PCR and histology test in paraffin biopsy samples.
| Histology | PCR | |
|---|---|---|
| Positive | 59 | 12 |
| Negative | 0 | 47 |
|
| ||
| Total | 59 | 59 |
|
| ||
| Positive Beta Globin (DNA detection) | — | 14 |
| Negative Beta Globin (no DNA detection) | — | 45 |
|
| ||
| Total | 0 | 59 |
*Two paraffin samples were positive for the Beta Globin gene, but negative for the H. pylori gene.
Among the 141 fresh endoscopic biopsy samples, 58 were tested using the RFLP technique to detect the different H. pylori strainswith the restriction enzymes HhaI and MboI. All 58 samples showed positive PCR for Beta Globin and H. pylori genes. The product obtained from the H. pylori amplification gene by direct PCR was 820 base pairs. Eleven digestion patterns for HhaI and twelve for MboI were found (Table 4). The most frequent patterns were HhaI–3 with 12.58 (18.3%), HhaI-4 with 14.58 (23.3%), MboI-2 with 13.58 (21.7%), and MboI-4, with 15.58 (23.3%). The median age was 45, 39, 39, and 57, respectively, in each of the detected patterns. The most frequent diseases in the patients of this study were Gastritis and ulcers.
Table 4.
Use of the Restriction Fragment Length Products (RFLP) technique for genotyping the positive H. pylori PCR products using restriction enzymes (HhaI and MboI).
| Restriction enzyme | Frequency (%) | Disease | Median age (years) |
|---|---|---|---|
| HhaI-1 | 1/58 ( 1.7) | Gastritis + ulcers | 41 |
| HhaI-2 | 5/58 (8.6) | 3 Gastritis; 2 ulcers | 29 |
| HhaI-3 | 12/58 (20.7) | 9 Gastritis; 3 ulcers; | 45 |
| HhaI-4 | 14/58 (24.1) | 8 Gastritis; 3 ulcers; 1 esophagitis; 1 Inflamation | 39 |
| HhaI-5 | 3/58 (5.2) | 1 Gastritis; 2 ulcers | 54 |
| HhaI-6 | 6/58 (10.3) | 3 Gastritis; 2 ulcers; 1 Gastritis + ulcers | 39 |
| HhaI-7 | 1/58 (1.7) | 1 ulcers | 19 |
| HhaI-8 | 4/58 (6.9) | 1 Gastritis; 2 ulcers; 1 esophagitis | 48 |
| HhaI-9 | 2/58 (3.4) | 1 Gastritis; 1 ulcers | 37 |
| HhaI-10 | 2/58 (3.4) | 2 ulcers | 72 |
| HhaI-11 | 8/58 (13.8) | 6 Gastritis; 1 inflamation; 1 ulcers | 54 |
| MboI-1 | 2/58 (3.4) | 2 Gastritis | 72 |
| MboI-2 | 13/58 (22.4) | 7 Gastritis; 5 ulcers, 1 esophagitis | 39 |
| MboI-3 | 8/58 (13.8) | 3 Gastritis; 3 ulcers; 1 inflamation | 49 |
| MboI-4 | 15/58 (25.8) | 10 Gastritis; 5 ulcers | 57 |
| MboI-5 | 4/58 (6.9) | 1 Gastritis; 1 ulcers; 1 esophagitis; 1 Gastritis ulcers | 27 |
| MboI-6 | 2/58 (3.4) | 1 Gastritis; 1 ulcers | 37 |
| MboI-7 | 4/58 (6.9) | 1 Gastritis, 1 splenomegaly; 2 ulcers | 50 |
| MboI-8 | 1/58 (1.7) | 1 Gastritis | 45 |
| MboI-9 | 5/58 (8.6) | 2 Gastritis; 2 ulcers; 1 Gastritis + ulcers | 47 |
| MboI-10 | 2/58 (3.4) | 2 Gastritis | 28 |
| MboI-11 | 1/58 (1.7) | 1 Gastritis | 31 |
| MboI-12 | 1/58 (1.7) | 1 Gastritis | 38 |
4. Discussion
Gastric cancer is one of neoplasms that cause the majority of deaths not only in Brazil but all over the world. The type of cancer caused by H. pylori could be linked to gastric chronic. Differences in the degree of virulence between strains have lead to an increased risk of developing gastric diseases [17].
The H. pylori infection is distributed in a cosmopolitan way, reaching mainly the adult population of low socioeconomic levels in developing countries. The discharge infection rate is correlated with bacterial virulence and inherent factors of the particular host, mainly with respect to the immune system [18].
It should be noticed that the route of fecal-oral transmission appears to be the biggest problem in the prevalence of infection, making H. pylori a serious public health problem in both developed and developing countries [19].
The present study analyzes the effectiveness of the molecular biology method PCR in the detection of H. pylori in patients with gastrointestinal symptoms, comparing it with the histology and rapid urease test.
The polymerase chain reaction (PCR) for the diagnosis of H. pylori is a very sensitive and specific method [20], providing fast and safe diagnosis. Many results indicate that PCR sensitivity is close to that of culture tests [21], but for verifying the eradication of H. pylori the effectiveness of PCR can be markedly superior [22, 23]. The methodology used in other studies to distinguish the different H. pylori subtypes has been PCR-RFLP [24] that through analyzis of the PCR product with restriction endonucleases that resulting fragments of different sizes and the digestion profile is decisive to define the strains. The restriction enzymes HhaI and MboI were used for the Urease-C area [16]. The extreme degree of variability observed among the strains of H. pylori became an important focus of scientific attention, as the investigators recognized the significant impact that this phenomenon can have on several research areas, such as the development of vaccines, the development of resistance to antimicrobial agents, and the study of the pathogen-host interaction [25, 26]. Considering that 10 to 20% of people infected with H. pylori develop obvious diseases, the reliable identification of the lineages could actually be very beneficial [27]. Previous studies that have used several techniques characterized H. pylori as a highly variable species that presents countless lineages, each one with its own and different genotype [28–31].
The genotyping of H. pylori is important for characterizing the most pathogenic genotype and the most frequent strain. This information can be used for clinical and epidemic studies. Even if many infections are clinically silent, the organism infected with H. pylori presents increased morbidity and mortality [5, 32, 33].
In the present study we standardized PCR with material obtained from the fresh endoscopic biopsies samples of patients attending Gastrocentro (the Center of Digestive Tract Studies), Medical School, UNICAMP. Some of the gastric biopsy samples were collected in paraffin and some were not. With regard to the standardization of the DNA extraction technique from the paraffin biopsy samples, several difficulties were found, because the samples contained a small amount of tissue fragments and many of the paraffin samples did not amplify the β-Globin gene, demonstrating degraded DNA of poor or inhibited quality.
PCR was used because it is more specific and faster when compared to other methods; the product of PCR can be processed with restriction enzymes to verify H. Pylori strains. Besides, starting with the PCR, DNA sequencing can be made to verify mutations, which no other technique is capable of doing.
As an internal control of the reaction was used in all samples (human β-Globin gene), in the fresh-air biopsy samples positive for H. pylori, we had 100% PCR amplification. However, in several paraffin samples, the β-Globin did not amplify, indicating an inefficient DNA extraction of the samples.
The efficiency of H. pylori detection PCR in fresh samples was superior to that in the paraffin samples. We suspect that PCR inhibition may have occurred due to the method used in DNA extraction from paraffin or the fact that that the samples were insufficient.
The extraction of DNA from fresh samples had excellent results. Among the 82 analyzed samples, 64 were positive and 18 negative, with 100% in agreement with PCR.
In the present study, we used the PCR-RFLP method for the differentiation of H. pylori strains from specimens obtained from gastric biopsies taken from Brazilian patients. Using this methodology we observed that 12 and 11 patterns were produced, respectively, by the two restriction enzymes MboI and HhaI from 58 specimens obtained from gastric biopsies. Two were samples of biopsies in paraffin and 58 were samples of nonfastened gastric biopsies (Table 1).
This data suggests that genotyping using PCR-RFLP can be useful as a fast procedure for the specific identification of H. pylori lineages in gastric biopsies specimens [16]. Several protocols of genotyping analysis were proposed for distinguishing the lineages of clinically isolated H. pylori [34–38]. Several primer pairs were described for detection and the typing of H. pylori was based on the amplification of the ureA [34], ureA plus ureB [35], and ureC genes [36, 38]. These results demonstrate great diversity in the urease genes in clinical H. pylori samples. Li et al. [16] found 3, 11, and 6 different patterns which were produced by 19 clinically isolated samples, respectively, digested by the restriction enzymes HhaI, MboI and AluI. Foxall et al. [35] found 10 different patterns which were produced by 22 clinically isolated samples, when the restriction enzyme HaeIII digested the PCR product of 2.4 Kb which had been amplified by the ureA and ureB genes. Lopez et al. [37] found that the patterns generated by the digestion of PCR products with the HaeIII enzyme, starting from ureA and ureB, were almost as different as the standard HaeIII. Akopyanz et al. [28] found 18 MboI and 27 HaeIII RFLP patterns, PCR products amplified by ureA and ureB genes of 2.4 Kb of 60 H. pylori lineages, and that the patterns distinguished 44 separate groups. Each isolated group did not differ from the other ones in the RFLP analyses of ureA and ureB products, but differed in MboI digestion of the 1.7 Kb ureC and ureD segments. Such a fact indicates that PCR-RFLP analyses of ureC genes can produce a great number of standard RFLPs.
Several studies have confirmed that PCR-RFLP analysis of the ureC gene can differentiate clinically isolated H. pylori. Using restriction endonucleases, Moore et al. [38] analyzed the 1.1 Kb portion of the ureC gene amplified by the “PCR” of 21 clinically isolated H. pylori. The samples were divided into four groups after digestion with the enzyme HindIII, while the lineages were divided into 15 groups after they were digested with the enzymes AluI and PvuI. Fujimoto et al. [36] demonstrated that the digestion of 820 bp of the H. pylori ureC gene with the restriction enzymes HhaI, MboI, and MseI resulted in 10, 10, and 11 different patterns, respectively. Dooley et al. [3] used three types of enzymes in the PCR product of a 1.179 bp portion of the H. pylori ureC gene. Eleven, 10, and 6 digestion patterns were produced by the HhaI, MboI, and AluI enzymes, respectively.
In our study we used two types of restriction enzymes in the amplified products of 820 pb of the H. pylori ureC gene. We obtained 11 and 12 different patterns, respectively, from the 58 clinically isolated samples which were studied. Our results suggest that the PCR-RFLP analysis of this portion of the H. pylori ureC gene is a reliable method, and that genotyping of PCR in this area can be used for epidemic studies and for the differentiation of isolated H. pylori strands.
This study also revealed a high level of genetic diversity isolated in the different H. pylori positive patients studied in Brazil.
The obtained genotyping patterns were compared using the computational program Bio 1D. We found 11 patterns with the HhaI enzyme and 12 patterns with the MboI enzyme. The reason for the small number of studied samples was due to the fact that it was not possible to establish a significant statistical correlation between specific digestive pathologies and standard genotypes.
We believe that the genotyping of H. pylori can contribute to the study of the microorganism's characteristics, facilitating the detection of pathogenic or nonpathogenic strains and, in turn, providing a better understanding of the several virulence factors that the bacterium uses to cause diseases.
4.1. Statistics
Percentage agreement was calculated to compare H. pylori genotypes obtained from PCR performed directly on gastric biopsies, with the genotypes obtained from the PCR of DNA extracted from paraffin and fresh samples, as well as histology and urease tests.
Conflict of Interests
Authors have no conflict to declare.
Acknowledgment
The authors thanks the patients who agreed to participate in this study.
References
- 1.Graham DY, Malaty HM, Evans DG, Evans DJ, Klein PD, Adam E. Epidemiology of Helicobacter pylori in an asymptomatic population in the United States: effect of age, race, and socioeconomic status. Gastroenterology. 1991;100(6):1495–1501. doi: 10.1016/0016-5085(91)90644-z. [DOI] [PubMed] [Google Scholar]
- 2.Scheiman JM, Cutler AF. Helicobacter pylori and gastric cancer. American Journal of Medicine. 1999;106(2):222–226. doi: 10.1016/s0002-9343(98)00393-3. [DOI] [PubMed] [Google Scholar]
- 3.Dooley CP, Cohen H, Fitzgibbons PL, et al. Prevalence of Helicobacter pylori infection and histologic gastritis in asymptomatic persons. The New England Journal of Medicine. 1989;321(23):1562–1566. doi: 10.1056/NEJM198912073212302. [DOI] [PubMed] [Google Scholar]
- 4.Taylor NS, Fox JG, Akopyants NS, et al. Long-term colonization with single and multiple strains of Helicobacter pylori assessed by DNA fingerprinting. Journal of Clinical Microbiology. 1995;33(4):918–923. doi: 10.1128/jcm.33.4.918-923.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Megraud F. Epidemiology of Helicobacter pylori infection. Gastroenterology Clinics of North America. 1993;22(1):73–88. [PubMed] [Google Scholar]
- 6.Atherton JC. The clinical relevance of strain types of Helicobacter pylori . Gut. 1997;40(6):701–703. doi: 10.1136/gut.40.6.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Radić M, Kaliterna DM, Radić J. Helicobacter pylori infection and systemic sclerosis-is there a link? Joint Bone Spine. 2011;78(4):337–340. doi: 10.1016/j.jbspin.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 8.Bassi V, Santinelli C, Iengo A, Romano C. Identification of a Correlation between Helicobacter pylori Infection and Graves’ Disease. Helicobacter. 2010;15(6):558–562. doi: 10.1111/j.1523-5378.2010.00802.x. [DOI] [PubMed] [Google Scholar]
- 9.Jesnowski R, Isaksson B, Möhrcke C, et al. Helicobacter pylori in autoimmune pancreatitis and pancreatic carcinoma. Pancreatology. 2010;10(4):462–466. doi: 10.1159/000264677. [DOI] [PubMed] [Google Scholar]
- 10.Go MF, Graham DY. How does Helicobacter pylori cause duodenal ulcer disease: the bug, the host, or both? Journal of Gastroenterology and Hepatology. 1994;9(supplement 1):S8–S10. doi: 10.1111/j.1440-1746.1994.tb01294.x. [DOI] [PubMed] [Google Scholar]
- 11.Labigne A, De Reuse H. Determinants of Helicobacter pylori pathogenicity. Infectious Agents and Disease. 1996;5(4):191–202. [PubMed] [Google Scholar]
- 12.Dunn BE, Cohen H, Blaser MJ. Helicobacter pylori . Clinical Microbiology Reviews. 1997;10(4):720–741. doi: 10.1128/cmr.10.4.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis LG, Dibner MD, Battey JF. Basic Metthods in Molecular Biology. New York, NY, USA: Elsevier; 1995. [Google Scholar]
- 14.Latchman DS. PCR Applications in Pathology. Principles and Practice. Oxford University Press; 1995. [Google Scholar]
- 15.Saiki RK, Scharf S, Faloona F. Enzymatic amplification of β-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- 16.Li C, Ha T, Chi DS, et al. Differentiation of Helicobacter pylori strains directly from gastric biopsy specimens by PCR-based restriction fragment length polymorphism analysis without culture. Journal of Clinical Microbiology. 1997;35(12):3021–3025. doi: 10.1128/jcm.35.12.3021-3025.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kague E, Thomazini CM, Pardini MIDCM, De Carvalho F, Leite CV, Pinheiro NA. Methylation status of CDH1 gene in samples of gastric mucous from Brazilian patients with chronic gastritis infected by Helicobacter pylori . Arquivos de Gastroenterologia. 2010;47(1):7–12. doi: 10.1590/s0004-28032010000100002. [DOI] [PubMed] [Google Scholar]
- 18.Dorrell N, Crabtree JE, Wren BW. Host-bacterial interactions and the pathogenesis of Helicobacter pylori infection. Trends in Microbiology. 1998;6(10):379–380. doi: 10.1016/s0966-842x(98)01367-5. [DOI] [PubMed] [Google Scholar]
- 19.Quaglia NC, Dambrosio A, Normanno G, Celano GV. Evaluation of a Nested-PCR assay based on the phosphoglucosamine mutase gene (glmM) for the detection of Helicobacter pylori from raw milk. Food Control. 2009;20(2):119–123. [Google Scholar]
- 20.Van Doorn L-J, Figueiredo C, Sanna R, et al. Expanding allelic diversity of Helicobacter pylori vacA. Journal of Clinical Microbiology. 1998;36(9):2597–2603. doi: 10.1128/jcm.36.9.2597-2603.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Zwet AA, Thijs JC, Kooistra-Smid AMD, Schirm J, Snijder JAM. Sensitivity of culture compared with that of polymerase chain reaction for detection of Helicobacter pylori from antral biopsy samples. Journal of Clinical Microbiology. 1993;31(7):1918–1920. doi: 10.1128/jcm.31.7.1918-1920.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Engstrand L, Pahlson C, Gustavsson S, Schwan A. Monoclonal antibodies for rapid identification of Campylobacter pyloridis . The Lancet. 1986;2(8520):1402–1403. doi: 10.1016/s0140-6736(86)92049-0. [DOI] [PubMed] [Google Scholar]
- 23.Lamouliatte H, Hua J, Birac C, Caular R, Mégraud F. Posttreatment follow-up of anti-Helicobacter pylori regimen: standard bacteriology or PCR? Acta Gastro-Enterologica Belgica. 1993;56(supplement):104–119. [Google Scholar]
- 24.Mégraud F. Advantages and disadvantages of current diagnostic tests for the detection of Helicobacter pylori . Scandinavian Journal of Gastroenterology, Supplement. 1996;31(215):57–62. doi: 10.3109/00365529609094536. [DOI] [PubMed] [Google Scholar]
- 25.Logan RPH, Berg DE. Genetic diversity of Helicobacter pylori . The Lancet. 1996;348(9040):1462–1463. doi: 10.1016/s0140-6736(05)65885-0. [DOI] [PubMed] [Google Scholar]
- 26.Cover TL. The vacuolating cytotoxin of Helicobacter pylori . Molecular Microbiology. 1996;20(2):241–246. doi: 10.1111/j.1365-2958.1996.tb02612.x. [DOI] [PubMed] [Google Scholar]
- 27.Forbes KJ, Fang Z, Pennington TH. Allelic variation in the Helicobacter pyloriflagellin genes flaA and flaB: its consequences for strain typing schemes and population structure. Epidemiology and Infection. 1995;114(2):257–266. doi: 10.1017/s0950268800057927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Akopyanz N, Bukanov NO, Westblom TU, Berg DE. PCR-based RFLP analysis of DNA sequence diversity in the gastric pathogen Helicobacter pylori . Nucleic Acids Research. 1992;20(23):6221–6225. doi: 10.1093/nar/20.23.6221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taylor DE, Eaton M, Chang N, Salama SM. Construction of a Helicobacter pylori genome map and demonstration of diversity at the genome level. Journal of Bacteriology. 1992;174(21):6800–6806. doi: 10.1128/jb.174.21.6800-6806.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akopyanz N, Bukanov NO, Westblom TU, Kresovich S, Berg DE. DNA diversity among clinical isolates of Helicobacter pylori detected by PCR-based RAPD fingerprinting. Nucleic Acids Research. 1992;20(19):5137–5142. doi: 10.1093/nar/20.19.5137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Desai M, Linton D, Owen RJ, Stanley J. Molecular typing of Helicobacter pylori isolates from asymptomatic, ulcer and gastritis patients by urease gene polymorphism. Epidemiology and Infection. 1994;112(1):151–160. doi: 10.1017/s0950268800057514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blaser MJ. Intrastrain differences in Helicobacter pylori: a key question in mucosal damage? Annals of Medicine. 1995;27(5):559–563. doi: 10.3109/07853899509002469. [DOI] [PubMed] [Google Scholar]
- 33.NIH Consensus Conference. Helicobacter pylori in peptic ulcer disease. NIH Consensus Development Panel on Helicobacter pylori in Peptic Ulcer Disease. JAMA. 1994;272(1):65–69. [PubMed] [Google Scholar]
- 34.Clayton CL, Kleanthous H, Morgan DD, Puckey L, Tabaqchali S. Rapid fingerprinting of Helicobacter pylori by polymerase chain reaction and restriction fragment length polymorphism analysis. Journal of Clinical Microbiology. 1993;31(6):1420–1425. doi: 10.1128/jcm.31.6.1420-1425.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Foxall PA, Hu LT, Mobley HLT. Use of polymerase chain reaction-amplified Helicobacter pylori urease structural genes for differentiation of isolates. Journal of Clinical Microbiology. 1992;30(3):739–741. doi: 10.1128/jcm.30.3.739-741.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fujimoto S, Marshall B, Blaser MJ. PCR-based restriction fragment length polymorphism typing of Helicobacter pylori . Journal of Clinical Microbiology. 1994;32(2):331–334. doi: 10.1128/jcm.32.2.331-334.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lopez CR, Owen RJ, Desai M. Differentiation between isolates of Helicobacter pylori by PCR-RFLP analysis of urease A and B genes and comparison with ribosomal RNA gene patterns. FEMS Microbiology Letters. 1993;110(1):37–44. doi: 10.1111/j.1574-6968.1993.tb06292.x. [DOI] [PubMed] [Google Scholar]
- 38.Moore RA, Kureishi A, Wong S, Bryan LE. Categorization of clinical isolates of Helicobacter pylori on the basis of restriction digest analyses of polymerase chain reaction-amplified ureC genes. Journal of Clinical Microbiology. 1993;31(5):1334–1335. doi: 10.1128/jcm.31.5.1334-1335.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]


