Abstract
Some have claimed that the medial prefrontal cortex (mPFC) mediates decision making. Others suggest mPFC is selectively involved in the retrieval of remote long-term memory. Yet others suggests mPFC supports memory and consolidation on time-scales ranging from seconds to days. How can all these roles be reconciled? We propose that the function of the mPFC is to learn associations between context, locations, events, and corresponding adaptive responses, particularly emotional responses. Thus, the ubiquitous involvement of mPFC in both memory and decision making may be due to the fact that almost all such tasks entail the ability to recall the best action or emotional response to specific events in a particular place and time. An interaction between multiple memory systems may explain the changing importance of mPFC to different types of memories over time. In particular, mPFC likely relies on the hippocampus to support rapid learning and memory consolidation.
INTRODUCTION
The empirical literature on the medial prefrontal cortex (mPFC) is dominated by studies of its role in decision making, including conflict monitoring (Botvinick et al., 2004), error detection (Holroyd et al., 2002), executive control (Posner et al., 2007; Ridderinkhof et al., 2004), reward-guided learning (Rushworth et al., 2011), and decision making about risk and reward (Bechara and Damasio, 2005). However, the mPFC also plays a key role in memory, as highlighted by its selective involvement in the retrieval of “remote” memories (i.e., items learned several weeks earlier) (Bontempi et al., 1999; Frankland et al., 2004; Takashima et al., 2006b). Other studies implicate mPFC in “recent” memory, learned 1–2 days earlier. For example, inactivation of mPFC impairs the recall of fear memory learned the previous day (Corcoran and Quirk, 2007). Hence, the mPFC plays a role in both recent and remote memory. Other studies have emphasized the role of mPFC in the consolidation of memories, in that interfering with mPFC immediately after learning disrupts subsequent recall in many tasks (e.g., Tronel and Sara, 2003). All of these studies implicate mPFC in what might be defined as “long-term” memory (i.e., memory spanning several hours or longer). There is also evidence that mPFC is important for “short-term” memory, spanning seconds to minutes. For example, rats with mPFC lesions have difficulty recalling place-reward associations over a 30 minute delay (Seamans et al., 1995) or waiting for a response cue over a 30 second delay (Narayanan et al., 2006). In summary, there is evidence that the mPFC plays a critical role in remote, recent and short-term memories over a broad range of tasks.
Theories of medial prefrontal function have emphasized its role in adaptive decision making. Earl Miller and colleagues have suggested that the entire prefrontal cortex receives a broad range of sensory and limbic inputs which can activate contextually appropriate representations of goals or task rules (Miller, 2000; Miller and Cohen, 2001). Active maintenance of these goals provide a “top-down” bias signal which can influence stimulus-response mappings in other areas of the brain. They also suggest that outcome feedback drives synaptic plasticity in prefrontal cortex, ensuring that the appropriate goal state is enabled in the appropriate context (Miller and Cohen, 2001). Other theories, focused more specifically on mPFC, have suggested it guides decisions by anticipating emotional outcomes and enacting them as bodily states (Bechara and Damasio, 2005; Fellows, 2007).
This review represents an attempt to explain the mnemonic functions of mPFC as an aspect of the mPFC's more general role in guiding adaptive behavior. Our proposal builds upon the aforementioned theories, but seeks to extend them to accommodate the burgeoning evidence implicating mPFC in different types of memory. Based on anatomical and electrophysiological evidence, we propose that mPFC takes as inputs the current context and events and predicts the most adaptive response based on past experience. Hence, what differentiates mPFC from other areas of the cortex is not its mnemonic capabilities, which we believe are shared with other cortical areas, but rather its specific involvement in guiding adaptive behavior. We further suggest that rapidly acquired input-output mappings in mPFC are initially supported by the hippocampus, but later become independent. This framework unifies the known representational capabilities of mPFC with its role in a broad range of memory studies.
THE MPFC PREDICTS ADAPTIVE RESPONSES
One of the most consistent findings regarding mPFC is that it is strongly modulated by motivationally salient events, both positive and negative. As exemplified in Figure 1, a large number of studies in humans, monkeys, and rodents have shown activity in ventral mPFC and medial orbitofrontal cortex (OFC) tied to the subjective value of anticipated or actual outcomes (Rushworth et al., 2011). In rodent mPFC, usually about one third of cells show firing rate changes tied to reward and reward expectancy (Burton et al., 2009; Gruber et al., 2010; Pratt and Mizumori, 2001). Neural activity in mPFC is also strongly modulated by negative outcomes (Figure 2). Specifically, the primate rostral cingulate zone has been repeatedly found to be activated by the subjective experience of pain (reviewed in Shackman et al., 2011). The rodent anterior cingulate plays a similar role in the experience of pain (Johansen et al., 2001). Further, a subset of rodent mPFC cells respond selectively to the expectation of aversive events (Baeg et al., 2001; Gilmartin and McEchron, 2005). In primate anterior cingulate, partially overlapping groups of cells respond to both reward and lack of expected reward (Quilodran et al., 2008).
Figure 1.
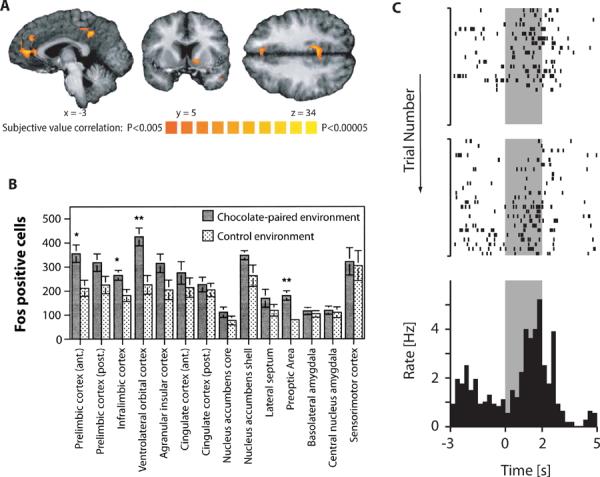
mPFC Encodes Expectation of Positive Outcomes. (A) Brain areas correlated with subjective value in humans. Subjects were asked to accept or reject offers of different amounts of money to be delivered after a specific delay. The mPFC, together with ventral striatum and posterior cingulate cortex, showed activity during the decision process related specifically to the subjective value of the current offer. The color scale represents the t-value of the contrast testing for a significant effect of subjective value (Kable and Glimcher, 2007). (B) Rat brain areas related to expectation of reward. Bars indicate relative numbers of Fos-positive cells in different brain areas after exposure to an environment where rats had previously been given chocolate chips compared to a neutral environment. Among cortical areas, there is selective activation of prelimbic and infralimbic cortex as well as lateral orbital cortex (Schroeder et al., 2001). (C) Single neuron encoding of reward expectation in rat mPFC. Rats had to enter a specific location and stay there until a food pellet was dropped at a separate location. Top two plots show spikes from one cell over two sessions. The bottom panel shows the binned peri-event time histogram for both sessions. This cell shows ramping activity during the delay between zone entry at time 0 and pellet release at time 2 seconds (delay indicated in gray) (Burton et al., 2009).
Figure 2.
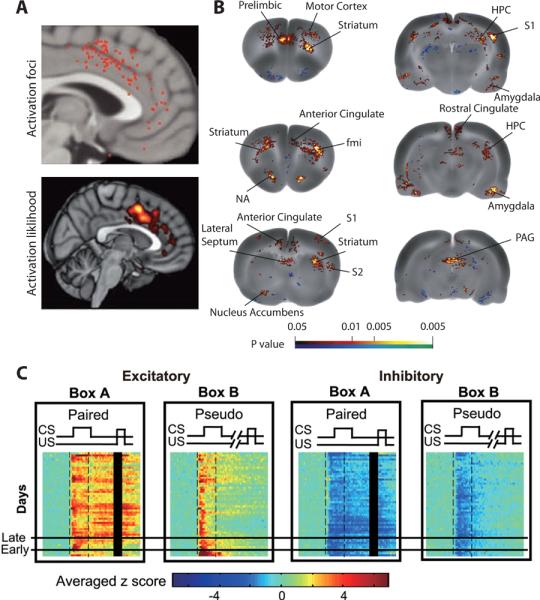
mPFC Encodes Expectation of Negative Outcomes. (A) Pain-associated foci in human mPFC from a meta-analysis of 192 neuroimaging studies. Top panel: locations of individual activated foci associated with delivery of physically painful stimuli, such as heat, cold or electric shock. Bottom panel: thresholded activation likelihood estimate (Shackman et al., 2011). (B) Changes in blood flow in the rat brain during exposure to an environment previously associated with painful colorectal distention. Cerebral blood flow was imaged via radioactively labeled [14C]-iodoantipyrine. Colors indicate statistically significant differences between conditioned and control rats in positive (red) and negative (blue) directions (Wang et al., 2011). Abbreviations: fmi – forceps minor of the corpus callosum, S1, S2 – primary and secondary somatosensory cortex. (C) Development of shock-anticipatory activity in mPFC during trace eye-blink conditioning. In Box A, rats were exposed to a tone as a conditioned stimulus (CS) and, after a 500 ms delay, mild eye-shock as an unconditioned stimulus (US). In Box B, CS and US were presented randomly so that the tone was not predictive of shock (pseudo-conditioning). Within each of the two sub-plot on the left, each row shows a z-score value for averaged population activity from all neurons showing an excitatory response during CS and trace interval. The horizontal axis indicates time within a trial and spans 1800 milliseconds. Early in training (rows below “Early”), mPFC cells respond primarily during the tone. After successful acquisition (rows above “Late”), cells maintain responses throughout the delay until delivery of shock. No such shock-anticipatory activity is evident in pseudo-conditioned Box B. The two panels on the right show similar results for all neurons showing an inhibitory response during the CS and trace interval (Takehara-Nishiuchi and McNaughton, 2008).
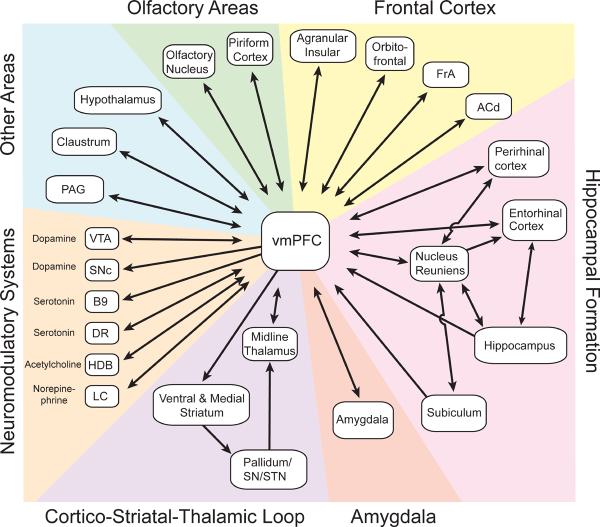
The involvement of mPFC, especially its ventral division, in motivationally salient events is also supported by anatomy. There appears to be a dorsal-ventral gradient in rodent mPFC, where ventral regions, including ventral prelimbic and infralimbic cortex, are specialized for autonomic/emotional control and dorsal regions, including anterior cingulate and dorsal prelimbic cortex, are specialized for the control of actions (Gabbott et al., 2005; Heidbreder and Groenewegen, 2003). In fact, based on its connectivity, the ventral mPFC has been characterized as “visceral motor cortex.” (Figure 3) (Neafsey et al., 1993). Prominent among its connections are reciprocal projections to and from the amygdala and a unilateral projection to dorso- and ventromedial striatum. The ventral mPFC is strongly interconnected with anterior insular areas, known to be involved in both interoception (Allen et al., 1991) and pain perception (Jasmin et al., 2004). The ventral mPFC communicates with the hypothalamus, which mediates intrinsic homeostatic drives, such as hunger and thirst, and coordinates the autonomic and endocrine systems (Saper, 2003). Another prominent connection is with the periaqueductal gray, a region involved in aggression, defensive behavior, and modulation of pain (Nelson and Trainor, 2007; Sewards and Sewards, 2002). The ventral mPFC also provides the primary cortical input to the lateral habenula, an area involved in learned responses to pain, stress, anxiety, and reward (Hikosaka, 2010; Li et al., 2011). Finally, the ventral mPFC has bidirectional connections with a wide range of neuromodulatory systems, including the dorsal raphe, ventral tegmental area, and locus coeruleus which, among other things, play an important role in adaptive responses to rewarding and stressful events (Itoi and Sugimoto, 2010; Kranz et al., 2010; Maier and Watkins, 2005; Schultz, 2001). The connections of the dorsal mPFC are similar to those of the ventral mPFC except that the dorsal mPFC has weaker connectivity with emotional and autonomic centers and stronger connectivity with motor and pre-motor areas (Gabbott et al., 2005; Heidbreder and Groenewegen, 2003; Hoover and Vertes, 2007). The dorsal mPFC in rats also projects directly to the spinal cord (Gabbott et al., 2005). In sum, the mPFC has access to information about motivational stimuli, including both pain and reward, as well as control over autonomic and skeletal-muscle activity.
Figure 3.
Major Anatomical Connections of Ventral mPFC. Arrows indicate directionality. Connections are derived from recent surveys of efferents and afferents of the mPFC and only the anatomically most dense projections are represented (Gabbott et al., 2005; Heidbreder and Groenewegen, 2003; Hoover and Vertes, 2007; Vertes et al., 2007). Hence, some potentially important connections, such as those to the lateral habenula, are not shown. Abbreviations: ACd – dorsal anterior cingulate cortex, B9 – B9 serotonin cells, DR – dorsal raphe, FrA - frontal association cortex, HDB - horizontal limb of the diagonal band of Broca, LC – locus coeruleus, PAG – periaqueductal gray, SN – substatia nigra, STN – sub-thalamic nucleus, VTA – ventral tegmental area.
Based on this evidence, we suggest that the inputs to mPFC are context and events and its output is the response which past experience predicts will lead to the most favorable outcome in a given situation (Figure 4). The term “context” often refers to any set of cues which situate the animal in place and time, a type of information thought to be encoded by the hippocampus (Nadel, 2008). Here, we broaden the definition to additionally encompass the animal's emotional state (e.g., anger, fear). “Events” constitute both sensory cues and actions. In situations associated with aversive experiences, the most adaptive response may be a release of stress hormones and freezing. Conversely, appetitive situations might require approach towards a reward location. These outputs are trained by evaluative feedback signals which serve as inputs to mPFC. Just as visual cortex might map a pattern of visual inputs onto a particular object percept, the mPFC maps events onto the emotional or motoric response that will be most adaptive within a given context. Hence, what differentiates mPFC from other cortical areas is not its underlying functional architecture, but rather, its unique inputs and outputs. As with other cortical areas, memories in mPFC are probably schematic (i.e., they represent the gist or central tendency over a collection of experiences) rather than representing a single episodic event (McClelland et al., 1995; Winocur et al., 2010).
Figure 4.
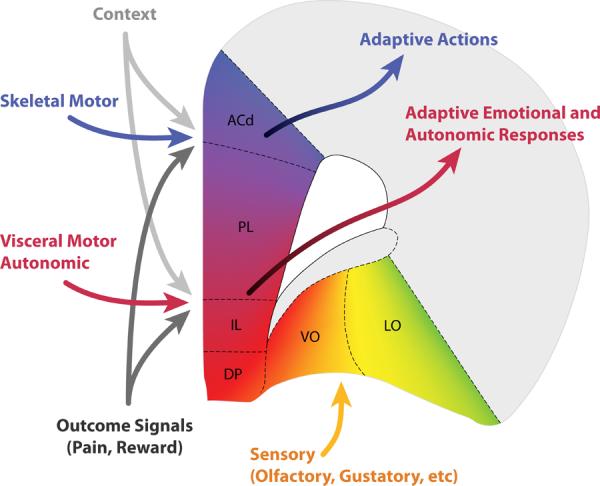
Schematic view of hypothesized inputs to and outputs from different regions of prefrontal cortex. The mPFC is conceived as a network mapping events within a given spatial and emotional context with the most adaptive response, which can be either action or an emotional response, depending on the area. A separate set of inputs carries information about outcomes of actions, which modulate plasticity. All frontal areas are strongly inter-connected, meaning that information about actions, emotions, and stimuli is available to all prefrontal areas. (Paxinos and Watson, 2007). Abbreviations: ACd – dorsal anterior cingulate, PL – prelimbic cortex, IL – infralimbic cortex, DP –dorsal peduncular cortex, VO – ventral orbital cortex, LO – lateral orbital cortex.
The inclusion of context and events as mPFC inputs is supported by electrophysiological evidence. Cells in mPFC are strongly modulated by which room an animal is in (Hyman et al., 2012). Further, location can modulate the responses to other events such as receipt of reward or lever pressing (Hyman et al., 2012; Hyman et al., 2005; Miyazaki et al., 2004). Even subtle differences in position, as little as 1 cm, can change the firing of mPFC cells (Cowen and McNaughton, 2007; Euston and McNaughton, 2006). The temporal context of a task can also modulate mPFC firing; some cells respond selectively to specific task phases, such as the inter-trial interval (Jung et al., 1998; Lapish et al., 2008). Another aspect of context is task rules. Two studies have imposed a situation in which a rat is doing the same behavior (i.e., pressing the right lever) for different reasons (i.e., because it is visually cued or because all right presses are rewarded) and shown that the firing of some mPFC cells changes depending on why the animal is performing the action (Durstewitz et al., 2010; Rich and Shapiro, 2009).
Cells in mPFC also respond robustly to events, both motoric and sensory. The activity of single mPFC cells is often related to specific behaviors such as turning, running one direction on a path, and lever pressing (Cowen and McNaughton, 2007; Hyman et al., 2012; Jung et al., 1998; Narayanan and Laubach, 2006). When learning is involved, cells in mPFC can develop responses to cues or actions which predict reward (Mulder et al., 2000; Peters et al., 2005) or punishment (Gilmartin and McEchron, 2005; Laviolette et al., 2005; Takehara-Nishiuchi and McNaughton, 2008). The mPFC can also respond to salient cues, such as a tone, that are not tied to reward or punishment (e.g., Takehara-Nishiuchi and McNaughton, 2008).
In many cases, the response of mPFC to motivationally salient events may reflect the adaptive anticipatory response, such as autonomic arousal in expectation of reward. However, the mPFC also exhibits robust responses to outcomes, both positive and negative. In fact, in both monkeys and rats, anticipated reward value and actual reward value have been shown to be encoded by separate groups of neurons (Amiez et al., 2006; Cowen et al., 2012; Pratt and Mizumori, 2001; Shidara and Richmond, 2002; Sul et al., 2010). A similar picture exists for negative outcomes, though it is not clear that anticipated and actual outcomes are encoded by separate pools of neurons (Baeg et al., 2001; Gilmartin and McEchron, 2005; Takehara-Nishiuchi and McNaughton, 2008). In the framework presented here, the outcome-anticipatory signals are part of the mPFC output whereas outcome evaluative signals serve to drive learning and as such are part of the mPFC input. Outcome feedback signals, from areas such as ventral tegmental area, insular cortex, and hypothalamus, may drive synaptic changes via some form of reinforcement learning (Holroyd et al., 2002). Alternatively, it has been suggested that the mPFC compares actual and expected outcomes and computes the degree of expectancy violation (i.e., “surprise”) (Alexander and Brown, 2011). These surprise signals then drive learning within mPFC and elsewhere.
As previously mentioned, anatomical evidence suggests a dorsal-ventral gradient in which dorsal mPFC is action-related whereas ventral mPFC is more emotion-related. Consistent with this anatomical gradient, a recent rodent electrophysiology study showed that responses in dorsal mPFC were strongly driven by what the animal was doing (i.e., traveling down left or right arm of a maze) while responses in ventral mPFC showed greater sensitivity to reward outcomes (Sul et al., 2010). The dorsal mPFC also supports sustained responses in motor cortex during a delay, demonstrating a direct functional link to motor systems (Narayanan and Laubach, 2006). The medial OFC has connectivity similar to the adjacent ventral mPFC while more lateral OFC receives sensory inputs, including visual, somatosensory, and olfactory (Hoover and Vertes, 2011; Reep et al., 1996). Because all frontal regions are interconnected, OFC may provide sensory inputs to mPFC (Hoover and Vertes, 2011; Jones et al., 2005). The evidence thus suggests the functional organization depicted in Figure 4. Dorsal mPFC receives information from motor regions and outputs adaptive actions. Ventral mPFC receives information from emotion-related structures and outputs adaptive emotional responses. Finer distinctions also exist. For example, the prelimbic and infralimbic cortex project to distinct regions of amygdala, with potentially important consequences for their role in fear learning and extinction (Peters et al., 2008).
The mPFC of primates follows a similar organizational scheme. Comparisons between rodents and primates are complicated by the debate over the homology of prefrontal regions (Brown and Bowman, 2002; Chudasama, 2011; Preuss, 1995; Seamans et al., 2008; Uylings et al., 2003). Based on functional evidence, some have claimed that mPFC in rodents represents an undifferentiated proto-PFC with functional aspects of both medial and lateral PFC in primates (Brown and Bowman, 2002; Uylings et al., 2003). The anatomical evidence, however, strongly suggests that mPFC in rodents is more similar to primate mPFC than lateral PFC (Preuss, 1995; Wise, 2008). Supporting this mapping, lesion studies in rats, monkeys and humans suggest that dorsal anterior cingulate supports action-value associations while OFC supports stimulus-value associations (Camille et al., 2011; Ostlund and Balleine, 2007; Rushworth et al., 2011).
ROLE OF MPFC IN LONG-TERM MEMORY
Without other stipulations, the framework presented above predicts that the mPFC will be needed whenever context and events guide behavior (i.e., during memory acquisition, as well as recent and remote recall). However, several studies suggest that mPFC plays a selective role in remote but not recent memory. We consider this evidence and discuss extant theories, but conclude that mPFC is likely needed for both recent and remote memory.
Imaging studies were among the first to indicate a specific role for mPFC in long-term memory. An early study examined metabolic activity in 74 mouse brain regions during memory-guided retrieval of rewards on an eight-arm maze either 5 or 25 days after learning (Bontempi et al., 1999). The mPFC, along with parts of frontal motor cortex and temporal cortex, showed significantly more activity during remote retrieval compared with recent. The selective activation of mPFC in remote memory has now been replicated using tests of both spatial and fear memory (Frankland et al., 2004; Maviel et al., 2004; Teixeira et al., 2006). Further, the density of dendritic spine growth in mPFC promoted by contextual fear conditioning is greater when measured at a remote time point as compared to a recent time point (Restivo et al., 2009). Imaging studies of socially transmitted food preference have also shown greater frontal cortex activity for remote memory, but in this task the effect is strongest in the OFC (Lesburgueres et al., 2011; Ross and Eichenbaum, 2006).
The aforementioned findings in rodents have parallels in the human imaging literature (see also Nieuwenhuis and Takashima, 2011). One study compared brain activation during recall of a recently learned stimuli (i.e., visual scenes) versus recall of a stimuli learned several weeks earlier. A small area in the subgenual anterior cingulate was the only brain region to show increasing activation with increasing memory retention intervals up to 90 days (Takashima et al., 2006a). Human imaging studies also suggest that mPFC plays a special role in memory consolidation during sleep. In one representative study, subjects studied word pairs and then were either deprived of the subsequent night of sleep or allowed to sleep normally. When tested for the words 6 month later, activity in the ventromedial PFC and occipital cortex was specifically elevated in subjects allowed to sleep when compared to subjects who were sleep deprived (Gais et al., 2007).
Consistent with these imaging results, inactivating mPFC leads to deficits in retrieval of remote memories while apparently leaving recent memory intact. This effect has been demonstrated across a range of tasks including the radial arm maze (Maviel et al., 2004), the Morris water maze (Teixeira et al., 2006), contextual fear conditioning (Frankland et al., 2004; Holahan and Routtenberg, 2007) and conditioned taste aversion (Ding et al., 2008). Corroborating evidence comes from drug addiction studies, which have shown that the mPFC is necessary for reinstatement of cocaine seeking at remote but not recent time points (Koya et al., 2009). While remote memory is usually examined roughly 30 days after learning, the selective involvement of mPFC in retrieval of remote trace fear memories has been shown at 200 days (Quinn et al., 2008). A final task showing a specific role for mPFC in remote memory is trace eye blink conditioning, in which an animal is conditioned to blink to a tone by pairing the tone, after a brief delay, with a mild eye shock. Lesions or inactivation of ventral mPFC in both rats and rabbits selectively impair remote but not recent memories (Oswald et al., 2010; Takehara-Nishiuchi et al., 2006; Takehara et al., 2003).
Two theories have been forwarded to account for the specific involvement of mPFC in remote, but not recent, memory. It has been suggested that remote memories, being more difficult to recall, require stronger top-down cognitive control which is provided by the mPFC (Rudy et al., 2005). One issue with this approach is that top-down control over memory processes typically involves lateral prefrontal cortex rather than mPFC (e.g., Anderson et al., 2004). The other theory suggests that the mPFC takes over the role of the hippocampus in orchestrating the recall of remote memory (Frankland and Bontempi, 2005; Takashima et al., 2006b; Takehara-Nishiuchi and McNaughton, 2008). In other words, for recent memories, coherent patterns in hippocampus are sufficient to reinstate memories distributed across the neocortex. For remote memories, however, the mPFC supplies the necessary signals driving reinstatement. The necessary retrieval codes would presumably be transferred from hippocampus to mPFC during consolidation. In support of this model, it has been demonstrated that the hippocampus plays a role complimentary to the mPFC, in that it is strongly activated during retrieval of recent memories but not remote memories (Frankland et al., 2004; Takashima et al., 2006b). Similarly, several studies have shown that the hippocampus is necessary for recent but not remote memory retrieval (Maviel et al., 2004; Takehara et al., 2003), although not all studies are consistent (Quinn et al., 2008; Teixeira et al., 2006). The primary weakness of this view, in our opinion, is that it does not naturally extend to other domains of mPFC function (e.g., decision making).
We propose that memories in mPFC consolidate like other cortical memories. During the initial encoding, mPFC starts to map between contexts, events, and adaptive responses, relying on hippocampus to support rapid learning. During consolidation, repeated replay of the memory results in a strengthening of synapses supporting the memory within mPFC. As mentioned previously, the mPFC (and the cortex in general) is likely extracting the regularities over a range of experiences rather than the details of a specific episode (McClelland et al., 1995; Winocur et al., 2010). The hippocampus has been hypothesized to encode memories via an arbitrarily assigned pattern of activity which does not itself contain the memory contents, but rather, is capable of reactivating the neocortical activity patterns that constitute the content of the memory (McClelland et al., 1995). Thus, during recent retrieval, mPFC represents the context, events and adaptive responses but not the mapping between them. After consolidation, mPFC stores both the inputs and outputs as well as the means to generate the former from the later. It follows that if the mPFC is needed for the retrieval of remote memory on a particular task, it should also be needed for the retrieval of recent memory.
Several lines of evidence support the involvement of mPFC in recent memory. First, at least two studies found that mPFC lesion or inactivation affected both recent and remote memory for fear conditioning (Blum et al., 2006; Quinn et al., 2008). Second, as discussed below, a large body of studies demonstrated that disruption of mPFC activity immediately after a task can impair performance on that task the following day. In some cases, these later studies focus on the same task and mPFC subregion as those used in remote memory studies suggesting no mPFC involvement in recent memory (e.g., compare Frankland et al., 2004; Zhao et al., 2005). Third, other studies have directly demonstrated the necessity of mPFC for the retrieval of recent navigational (Churchwell et al., 2010), object-place (Lee and Solivan, 2008) and fear memories (Corcoran and Quirk, 2007), learned one or two days before testing.
Despite strong evidence that mPFC is needed for both recent and remote memory, the many studies showing greater involvement of mPFC in remote memory cannot be ignored (see supplemental Table 1). The most straightforward explanation is that mPFC participates in recent memory but plays an even greater role in retrieval of remote memory. Indeed, one study of contextual fear memory after mPFC lesion found a weak but significant impairment in recent memory and a stronger impairment in remote memory (Quinn et al., 2008). While our framework doesn't predict this phenomenon, it can be extended to accommodate the data. During the recall of recent memory, the role of mPFC is to represent context, events and responses while the mapping between them is stored within the hippocampus. During remote recall, on the other hand, the mPFC both represents and stores context-event-response mappings while the hippocampus becomes disengaged. Because mPFC serves for both storage and representation, the brain may be less able to compensate for its loss during remote retrieval than during recent.
ROLE OF MPFC IN MEMORY CONSOLIDATION
While the preceding section emphasized the role of mPFC in the retrieval of long-term memories, there is now considerable evidence that mPFC plays an important role in the consolidation of a wide range of memories. These studies demonstrate that activity in mPFC immediately after a task is needed for retrieval on subsequent days.
Evidence that mPFC is needed for stabilization of recently acquired memories spans a wide range of appetitive tasks. One study used an odor-reward association, acquired in just a few trials. When disruptive agents were injected into mPFC immediately after learning, subsequent testing 48 hours later revealed a severe memory impairment (Carballo-Marquez et al., 2007; Tronel et al., 2004; Tronel and Sara, 2003). Similar effects have been observed in lever-press for reward (Izaki et al., 2000), socially transmitted food preference (Carballo-Marquez et al., 2009), object recognition (Akirav and Maroun, 2006), and the Morris water maze (Leon et al., 2010).
Activity in mPFC immediately after learning is also important for the consolidation of fear memory. For example, interfering with mPFC plasticity immediately after trace fear conditioning (i.e., with a delay between tone and shock) has been shown to cause deficits in memory retrieval both 24 and 72 hours later (Runyan et al., 2004); however, the results for simple tone-shock fear conditioning are equivocal (Morrow et al., 1999; Zhao et al., 2005). Like trace fear conditioning, the consolidation of contextual fear conditioning is also dependent upon mPFC (Zhao et al., 2005). Contextual fear has also been examined using inhibitory avoidance. Interference with mPFC plasticity immediately after inhibitory avoidance training leads to deficits at 24 and 48 hours (Holloway and McIntyre, 2011; Zhang et al., 2011). Interestingly, application of glucocorticoid receptor agonists to mPFC immediately after training actually enhances inhibitory avoidance (Roozendaal et al., 2009).
The ventral region of mPFC also plays a critical role in the consolidation of extinction of both fear and drug-related memories (Peters et al., 2009). Extinction is now known to be an active learning process involving the association between a conditioned stimulus and the absence of the unconditioned stimulus that was formerly associated with it. As with many other types of learning, disruption of synaptic plasticity in ventral mPFC after extinction training impairs memory for extinction of fear when tested 1–2 days later (Mamiya et al., 2009; Sotres-Bayon et al., 2009). Likewise, inhibiting mPFC after each daily extinction session leads to impaired extinction of drug craving (LaLumiere et al., 2010). Intriguingly, a recent study demonstrated enhanced fear extinction when the ventral mPFC was treated with a plasticity enhancing agent after extinction training (Marek et al., 2011).
There appears to be a critical window for consolidation in that chemical disruption of mPFC one to two hours after learning causes memory impairment whereas disruption outside this window does not (Carballo-Marquez et al., 2007; Izaki et al., 2000; LaLumiere et al., 2010; Takehara-Nishiuchi et al., 2005; Tronel and Sara, 2003) (see supplementary Table 1). What is the nature of mPFC activity during this critical post-task period? Consolidation theory suggests that during off-line periods, most notably sleep, the hippocampus reactivates recently learned experiences which, in turn, causes replay of these events in the neocortex. Replay allows new memories to become integrated with previous cortical memories and hence, more robust to interference (i.e., “consolidated”) (McClelland et al., 1995). In support of this theory, spike patterns corresponding to task activity have been shown to replay in hippocampus and several cortical areas during the rest period immediately following a task (Hoffman and McNaughton, 2002; Ji and Wilson, 2007; Wilson and McNaughton, 1994). Recently, robust replay has been observed in mPFC and an associated structure, the nucleus accumbens (Euston et al., 2007; Lansink et al., 2009). In both structures, replay occurs at an accelerated rate relative to that seen during behavior. Further, this replay is selective for recently learned events, suggesting a causal link in memory formation (Peyrache et al., 2009).
A critical issue is whether replay in mPFC is orchestrated by the hippocampus. Considerable evidence suggests that it is. Reactivation in hippocampus is tied to local field potential features called “sharp waves” (Kudrimoti et al., 1999). Likewise, reactivation in mPFC is strongest during periods with a high density of field potential oscillations known as “low-voltage spindles” (Johnson et al., 2010). These two hallmarks of memory reactivation, hippocampal sharp waves and cortical low-voltage spindles, tend to occur within a few hundred milliseconds of one another (Battaglia et al., 2004; Molle et al., 2006; Siapas and Wilson, 1998; Sirota et al., 2003). Further strengthening the link, sharp waves in hippocampus have recently been shown to be correlated with memory replay in mPFC (Peyrache et al., 2009). The directionality of the mPFC-hippocampal interaction during sleep has been difficult to discern, with some results suggesting events in hippocampus precede those in cortex (Battaglia et al., 2004; Wierzynski et al., 2009) while others suggest the opposite (Isomura et al., 2006; Molle et al., 2006). Perhaps both sides are correct. It has been suggested that cortical events initiate hippocampal replay, which in turn reinforces the on-going replay of patterns in the neocortex (Sirota et al., 2003). Alternatively, the directionality of information flow from hippocampus to mPFC may depend on whether the information being processed is newly learned.
One puzzling aspect of these consolidation findings is that tasks affected by post-task mPFC disruption are not necessarily mPFC-dependent during initial learning. For example, odor-reward associations tested 48 hours after learning are impaired by consolidation block, yet rats with prelimbic lesions can easily acquire and retrieve odor-reward associations within a single session (Birrell and Brown, 2000; Tronel and Sara, 2003). Likewise, a NMDA antagonist injected into infralimbic cortex after extinction training interferes with the consolidation of fear extinction but NMDA receptor block during extinction training has no effect on within-session acquisition of extinction or subsequent recall (Mamiya et al., 2009). Instrumental conditioning of lever pressing for food reward shows a similar pattern (Izaki et al., 2000; Ostlund and Balleine, 2005) as does object recognition (Akirav and Maroun, 2006; Ennaceur et al., 1997). The framework presented here predicts that mPFC will be involved in initial acquisition, consolidation and retrieval of context-event-response associations. Hence, it cannot fit these data without additional stipulations. One possible explanation is that other prefrontal cortical areas can compensate for mPFC loss during learning but not during consolidation or recall. For example, aspects of odor-reward association may be mediated by both the OFC as well as ventral mPFC. If ventral mPFC is off-line during learning, OFC may become more heavily involved to the point that it can support learning independently. If mPFC is on-line during learning, however, the mPFC remains essential for consolidation and recall.
ROLE OF MPFC IN SHORT-TERM MEMORY
Numerous research paradigms implicate mPFC in short-term memory, operationally defined here as memory spanning seconds to minutes. Historically, considerable emphasis has been placed on the role of mPFC in memory spanning intervals less than a minute, a capacity referred to as “working” memory (Uylings et al., 2003). Similar to primates with damage to dorsolateral prefrontal cortex, rats with mPFC damage often show deficits in tasks requiring a delayed response (e.g., Horst and Laubach, 2009). The functional similarity between rodent mPFC and primate dorsolateral prefrontal cortex is further bolstered by demonstrations that both exhibit persistent cellular activity during delay periods that is selective for a prior or upcoming target location (Baeg et al., 2003; Batuev et al., 1990; Funahashi, 2006). The idea that mPFC is specialized for working memory, however, has been undermined by recent findings. First, some of the most compelling evidence that mPFC plays a role in working memory are studies demonstrating that performance of rats with mPFC lesions gets worse with longer retention delays. However, in some of these studies, delay length is confounded with task novelty (Gisquet-Verrier and Delatour, 2006). In one example, mPFC-lesioned rats trained using a 5 second delay show impairment when switched to a 20 second delay (Delatour and Gisquet-Verrier, 1999); however, rats trained from the beginning on a randomly shuffled range of delays fail to show deficits (Gisquet-Verrier et al., 2000). Second, neurons in mPFC are highly selective to slight changes in position or trajectory (Cowen and McNaughton, 2007; Euston and McNaughton, 2006; Fujisawa et al., 2008). It is difficult to rule out the possibility that some, if not all, delay-related neural activity is entirely reflective of an “embodied memory” strategy involving differential behavior during the delay, rather than working memory per se. Indeed, it has been suggested that the primary deficit in rats with mPFC lesions is not information storage but rather the implementation of mediating strategies (Chudasama and Muir, 1997). Finally, working memory in some studies is confounded with memory for the rules of the task (i.e., reference memory). As an illustration, Touzani et al. (2007) trained mice on a spatial win-shift task in which the correct choice depended on which maze arm was rewarded two trials back. Consistent with the hypothesis that mPFC supports working memory, mice with mPFC lesions were incapable of acquiring this task. However, mice given mPFC injections of a protein synthesis blocker after each daily training session were also impaired. The lack of treatment during the task reduces the likelihood of interference with working memory. Instead, the impairment is likely due to disruption of consolidation which precluded acquisition of the task rules. In summary, many studies of working memory implicate the mPFC. Unfortunately, it is often difficult to determine whether the observed deficits are due to a breakdown in trial-specific working memory, mediating strategies, or a deficit in reference memory.
Despite these concerns, a few well-controlled studies do support a role for rodent dorsal mPFC in working memory for actions. One study showed impairments in delayed spatial alternation despite the researchers' careful efforts to rule out mediating strategies (Horst and Laubach, 2009). Another showed that when rats were required to hold down a lever until cued, one-third of dorsal mPFC cells were significantly modulated during the delay (Narayanan and Laubach, 2006). Further, half of these were predictive of errors (i.e., premature release). A follow-up study showed that one fifth of dorsal mPFC neurons respond differently after error trials and maintain this activity into the next trial (Narayanan and Laubach, 2008). Hence, mPFC cells exhibit properties consistent with short-term maintenance of memory for action and errors.
There is also evidence that mPFC plays a role in memory spanning minutes to hours, but only in certain circumstances. In general, forming a short-term memory for locations, odors, or objects does not require the mPFC (Birrell and Brown, 2000; Ennaceur et al., 1997; Seamans et al., 1995). For example, rodents with mPFC inactivation show normal performance in free foraging in an 8-arm maze (Seamans et al., 1995). However, the task does become mPFC dependent if run as a spatial “win-shift” task (Seamans et al., 1995). In this variant, rats are initially rewarded on four arms and, after a delay of 30 minutes, are tested for their ability to locate the previously non-rewarded arms. Surprisingly, the role of mPFC is limited to the retrieval phase; inactivation of the mPFC before training or the delay has no effect on test performance (Floresco et al., 1997; Seamans et al., 1995). Short-term memory for rewarded odors depends on mPFC when either a large number of odors must be remembered or odor associations must be acquired via social interaction (Boix-Trelis et al., 2007). In one example, rats with mPFC lesions were impaired when required to remember 10 sample odors over a 10 minute delay (Farovik et al., 2008). In comparison, short-term memory for objects, tested via novel object preference, does not require the mPFC (Ennaceur et al., 1997). To our knowledge, no within-session object-recognition task has shown mPFC dependence.
INTERACTIONS WITH HIPPOCAMPUS
Given the prominent role of hippocampus in memory, it is no surprise that the hippocampus and mPFC are anatomically related. Compared to other cortical areas, projections from the ventral half of the hippocampus and subiculum to mPFC are particularly strong (Cenquizca and Swanson, 2007; Jay and Witter, 1991). The pathway is unidirectional but may be reciprocated via a bi-synaptic route through the nucleus reunions or lateral entorhinal cortex (see Figure 3) (Burwell and Amaral, 1998; Vertes et al., 2007).
The evidence supports two possible roles for the hippocampal input to mPFC: to provide context or to enable rapid associative learning. The ability of the hippocampus to encode spatial location via “place fields” is well known (Wilson and McNaughton, 1993). However, as one moves along the septal (dorsal) – temporal (ventral) axis, place fields become progressively larger (Jung et al., 1994). Ventral hippocampal fields are so large that they probably encode global context (i.e., which room the animal is in) (Kjelstrup et al., 2008). Accordingly, the mPFC, whose inputs arise mostly from ventral and intermediate hippocampus, exhibits no evidence of place cell-like responses but does discriminate between rooms (Hyman et al., 2012; Jung et al., 1998; Poucet, 1997). Recent evidence has established that the firing of hippocampal place cells is modulated by environmental stimuli (Leutgeb et al., 2005). Given its strong connectivity with limbic structures, ventral hippocampus may encode non-spatial contextual signals for such things as odors, bodily states, and emotions (Pennartz et al., 2011). Hence, as has been previously suggested, the hippocampal input is a plausible source of spatial and emotional context (Jung et al., 1998; Pennartz et al., 2011). The other possible role for hippocampal input to mPFC is to support rapid learning. Wise and Murray (Wise and Murray, 2000) have provided evidence that arbitrary visual-motor mappings formed within premotor cortex initially depend on rapid associative mechanisms within the hippocampus but, through consolidation, become hippocampally-independent. A similar principle may apply to the mPFC. To wit, the rapid formation and consolidation of associations between contexts, events, and responses within mPFC may depend on hippocampus whereas long-term storage may be mediated mostly by mPFC. The aforementioned evidence for coordinated memory replay in mPFC and hippocampus during consolidation supports this claim.
The role of communication between hippocampus and mPFC has been studied via functional disconnection, in which the mPFC is inactivated in one hemisphere and the hippocampus is inactivated in the other. Because the connections between hippocampus and mPFC are unilateral, the animal is left with one intact hippocampus and one intact mPFC but no pathway between them (Floresco et al., 1997). This technique has been used to demonstrate that mPFC-hippocampal communication is necessary for short-term memory in paradigms including the water maze (Wang and Cai, 2008), the T-maze (Wang and Cai, 2006), spatial win-shift on the radial arm maze (Floresco et al., 1997; Goto and Grace, 2008), and the Hebb-Williams maze, a spatial maze requiring a specific set of turns to reach reward (Churchwell et al., 2010). In fact, the effects of mPFC-hippocampal disconnection are nearly the same as those seen after bilateral mPFC inactivation, supporting the claim that mPFC is dependent upon the hippocampal-mPFC pathway either for context or for rapid learning.
As further evidence for a functional interaction between mPFC and hippocampus, electrophysiological rhythms in these two structures are coupled, particularly in the theta range. Roughly half of mPFC cells exhibit phase locking to hippocampal theta while rats engage in spatial tasks (Hyman et al., 2005; Siapas et al., 2005). More importantly, this synchronous activity is associated with memory acquisition and retrieval. Theta coherence between mPFC and dorsal hippocampus, measured by both spike-theta phase locking and local field potential coherence, increases as the animal approaches a memory-guided choice point (Benchenane et al., 2010; Fujisawa and Buzsaki, 2011; Jones and Wilson, 2005). Further, this choice-related activity increases after acquisition of a new rule (Benchenane et al., 2010). Reductions in phase locking between mPFC spikes and hippocampal theta are also predictive of errors, suggesting that mPFC-hippocampal synchrony is either necessary for correct retrieval or, alternatively, reflects decision confidence (Hyman et al., 2010).
DISCUSSION AND CONCLUSION
As has been previously suggested, the mPFC likely forms and stores schema which map context and events onto appropriate actions (Alexander and Brown, 2011; Miller and Cohen, 2001). The purpose of these schema is to direct the correct emotional or motoric response to a given set of events in light of past experience (Bechara and Damasio, 2005; Fellows, 2007). Here, we have explored how these schema are stored and retrieved on time scales ranging from seconds to weeks. Compared to primary motor cortex, the mPFC may have more capacity to maintain responses over brief periods of time (i.e., seconds). As such, it may provide a source of top-down control over motor cortex when action sets must be maintained (Narayanan and Laubach, 2006). For memories spanning more than a few seconds, the mPFC probably requires support from the hippocampus. With regard to consolidation of memory, the framework presented here suggests that the mPFC functions no different than any other area of the cortex. The hypothesized role of mPFC at different times after learning is depicted in Figure 5. During a phase of rapid consolidation occurring during the first few hours after learning, the hippocampus and mPFC replay the memories and, in so doing, synapses supporting that memory are strengthened in mPFC while they degrade in hippocampus. There is also likely a transformation from a memory for specific episodes to a more schematic representation (McClelland et al., 1995; Winocur et al., 2010), though in most rodent studies, these two forms of memory are difficult to separate. In rats, this process of consolidation of the memory within mPFC continues for about two weeks (Takehara-Nishiuchi et al., 2006). A concomitant weakening of episodic traces in the hippocampus during this period might progressively shift the burden of remote recall to mPFC, hence explaining the enhanced dependence of remote memory on mPFC.
Figure 5.
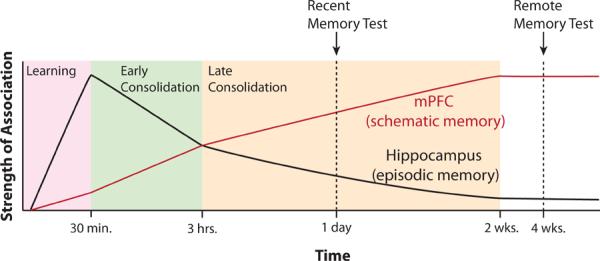
Proposed Timeline of mPFC's Role in Memory. Vertical axis is the strength of the memory stored in either mPFC or hippocampus while horizontal axis indicates time on a log scale. During 30 minutes of task learning, the hippocampus learns more quickly than mPFC. During early consolidation, the hippocampus supports memory replay and the development of schematic representations within mPFC. Concurrently, episodic memory traces within hippocampus decay. This process continues during late consolidation, albeit at a slower rate.
Why aren't all tasks involving motivated behavior impaired by mPFC lesions? Many simple tasks, such as instrumental conditioning or place-reward association are not dependent upon mPFC (Coutureau et al., 2012; Ragozzino et al., 1999). We suppose that the mPFC is one of many learning systems which operate in parallel. These other learning systems likely include the amygdala as well as other mPFC sub-regions and their associated striatal projection zones. In some cases, one system may be able to compensate for the loss of another. In other cases, learning in one system may forestall learning in another (Gruber and McDonald, 2012). Further complicating the search for mPFC function, loss of one area may shift learning to another area that would not otherwise be engaged. Hence, whether a task will depend on mPFC hinges on whether mPFC makes a unique contribution to that particular type of learning which cannot be handled by other areas. Exactly what that unique contribution is remains unclear; as suggested by Miller and Cohen (2001), contextual control of action is no doubt part of mPFC's role. However, the necessity of mPFC for contingency detection suggests that mPFC's role may be more specific, perhaps involving the extraction of temporal relationships between antecedents and outcomes (Coutureau et al., 2012). We contend that the functional lesion data are insufficient to fully constrain a theory of mPFC function at this time.
In our view, the strength of the framework presented here is that it provides a unified explanation for a broad range of memory studies. It also makes the specific prediction that in tasks involving contextual control of affect or action, the mPFC should be necessary for initial encoding, recent recall, and remote recall. The one caveat is that, during learning, other brain areas may be able to compensate for the lack of one or more mPFC sub-regions. This supposition is itself testable. Imaging or inactivation studies should show that other areas, such as OFC, can compensate for the loss of mPFC during on-line learning. Once a task has been learned with mPFC intact, however, the mPFC will be needed for early consolidation as well as recent and remote retrieval. Our claim that mPFC is needed for recent memory is at odds with several studies showing a selective involvement of mPFC in remote but not recent memory (e.g., Frankland et al., 2004). However, as previously noted, our claim is supported by a few studies showing the necessity of mPFC for recent memory. We predict that closer examination of experiments demonstrating a selective mPFC role in remote memory will also show a weak involvement of mPFC in recent memory.
The framework presented here also makes specific predictions about the interactions between mPFC and the hippocampus. While the role of mPFC in working memory over a period of seconds remains a possibility, we suggest that any trial specific information maintained for minutes or hours is supported by the hippocampus. This is consistent with the finding that mPFC-hippocampal theta phase locking increases during the retrieval of short-term memory (e.g., Jones and Wilson, 2005). Further, both mPFC and hippocampus should be necessary for rapid consolidation after learning. Hence, we predict that every task which shows sensitivity to mPFC disruption during consolidation should similarly be sensitive to mPFC-hippocampal disconnection during the same interval.
Supplementary Material
ACKNOWLEDGMENTS
Supported by grants from Alberta Innovates Health Solutions (Polaris Award to B.L.M.), the Canadian Natural Sciences and Engineering Research Council to D.R.E and A.J.G., and the United States National Institute of Neurological Disorders and Stroke to B.L.M. and D.R.E. (NS020331-26). Thanks to Drs. Cyriel Pennartz, Jeremy Seamans, Rob McDonald, Hendrik Steenland, and Rob Sutherland for helpful comments on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Akirav I, Maroun M. Ventromedial prefrontal cortex is obligatory for consolidation and reconsolidation of object recognition memory. Cereb Cortex. 2006;16:1759–1765. doi: 10.1093/cercor/bhj114. [DOI] [PubMed] [Google Scholar]
- Alexander WH, Brown JW. Medial prefrontal cortex as an action-outcome predictor. Nat Neurosci. 2011;14:1338–1344. doi: 10.1038/nn.2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen GV, Saper CB, Hurley KM, Cechetto DF. Organization of visceral and limbic connections in the insular cortex of the rat. J Comp Neurol. 1991;311:1–16. doi: 10.1002/cne.903110102. [DOI] [PubMed] [Google Scholar]
- Amiez C, Joseph JP, Procyk E. Reward encoding in the monkey anterior cingulate cortex. Cereb Cortex. 2006;16:1040–1055. doi: 10.1093/cercor/bhj046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson MC, Ochsner KN, Kuhl B, Cooper J, Robertson E, Gabrieli SW, Glover GH, Gabrieli JD. Neural systems underlying the suppression of unwanted memories. Science. 2004;303:232–235. doi: 10.1126/science.1089504. [DOI] [PubMed] [Google Scholar]
- Baeg EH, Kim YB, Huh K, Mook-Jung I, Kim HT, Jung MW. Dynamics of population code for working memory in the prefrontal cortex. Neuron. 2003;40:177–188. doi: 10.1016/s0896-6273(03)00597-x. [DOI] [PubMed] [Google Scholar]
- Baeg EH, Kim YB, Jang J, Kim HT, Mook-Jung I, Jung MW. Fast spiking and regular spiking neural correlates of fear conditioning in the medial prefrontal cortex of the rat. Cereb Cortex. 2001;11:441–451. doi: 10.1093/cercor/11.5.441. [DOI] [PubMed] [Google Scholar]
- Battaglia FP, Sutherland GR, McNaughton BL. Hippocampal sharp wave bursts coincide with neocortical “up-state” transitions. Learn. Mem. 2004;11:697–704. doi: 10.1101/lm.73504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batuev AS, Kursina NP, Shutov AP. Unit activity of the medial wall of the frontal cortex during delayed performance in rats. Behav Brain Res. 1990;41:95–102. doi: 10.1016/0166-4328(90)90145-5. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio AR. The somatic marker hypothesis: A neural theory of economic decision. Games and Economic Behavior. 2005;52:336–372. [Google Scholar]
- Benchenane K, Peyrache A, Khamassi M, Tierney PL, Gioanni Y, Battaglia FP, Wiener SI. Coherent theta oscillations and reorganization of spike timing in the hippocampal- prefrontal network upon learning. Neuron. 2010;66:921–936. doi: 10.1016/j.neuron.2010.05.013. [DOI] [PubMed] [Google Scholar]
- Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci. 2000;20:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum S, Hebert AE, Dash PK. A role for the prefrontal cortex in recall of recent and remote memories. Neuroreport. 2006;17:341–344. doi: 10.1097/01.wnr.0000201509.53750.bc. [DOI] [PubMed] [Google Scholar]
- Boix-Trelis N, Vale-Martinez A, Guillazo-Blanch G, Marti-Nicolovius M. Muscarinic cholinergic receptor blockade in the rat prelimbic cortex impairs the social transmission of food preference. Neurobiol Learn Mem. 2007;87:659–668. doi: 10.1016/j.nlm.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Bontempi B, Laurent-Demir C, Destrade C, Jaffard R. Time-dependent reorganization of brain circuitry underlying long-term memory storage. Nature. 1999;400:671–675. doi: 10.1038/23270. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn Sci. 2004;8:539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Brown VJ, Bowman EM. Rodent models of prefrontal cortical function. Trends Neurosci. 2002;25:340–343. doi: 10.1016/s0166-2236(02)02164-1. [DOI] [PubMed] [Google Scholar]
- Burton BG, Hok V, Save E, Poucet B. Lesion of the ventral and intermediate hippocampus abolishes anticipatory activity in the medial prefrontal cortex of the rat. Behav Brain Res. 2009;199:222–234. doi: 10.1016/j.bbr.2008.11.045. [DOI] [PubMed] [Google Scholar]
- Burwell RD, Amaral DG. Cortical afferents of the perirhinal, postrhinal, and entorhinal cortices of the rat. J Comp Neurol. 1998;398:179–205. doi: 10.1002/(sici)1096-9861(19980824)398:2<179::aid-cne3>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Camille N, Tsuchida A, Fellows LK. Double dissociation of stimulus-value and action-value learning in humans with orbitofrontal or anterior cingulate cortex damage. J Neurosci. 2011;31:15048–15052. doi: 10.1523/JNEUROSCI.3164-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carballo-Marquez A, Vale-Martinez A, Guillazo-Blanch G, Marti-Nicolovius M. Muscarinic receptor blockade in ventral hippocampus and prelimbic cortex impairs memory for socially transmitted food preference. Hippocampus. 2009;19:446–455. doi: 10.1002/hipo.20530. [DOI] [PubMed] [Google Scholar]
- Carballo-Marquez A, Vale-Martinez A, Guillazo-Blanch G, Torras-Garcia M, Boix-Trelis N, Marti-Nicolovius M. Differential effects of muscarinic receptor blockade in prelimbic cortex on acquisition and memory formation of an odor-reward task. Learn Mem. 2007;14:616–624. doi: 10.1101/lm.597507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenquizca LA, Swanson LW. Spatial organization of direct hippocampal field CA1 axonal projections to the rest of the cerebral cortex. Brain Res Rev. 2007;56:1–26. doi: 10.1016/j.brainresrev.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudasama Y. Animal Models of Prefrontal-Executive Function. Behav. Neurosci. 2011;125:327–343. doi: 10.1037/a0023766. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Muir JL. A behavioural analysis of the delayed non-matching to position task: the effects of scopolamine, lesions of the fornix and of the prelimbic region on mediating behaviours by rats. Psychopharmacology (Berl) 1997;134:73–82. doi: 10.1007/s002130050427. [DOI] [PubMed] [Google Scholar]
- Churchwell JC, Morris AM, Musso ND, Kesner RP. Prefrontal and hippocampal contributions to encoding and retrieval of spatial memory. Neurobiol Learn Mem. 2010;93:415–421. doi: 10.1016/j.nlm.2009.12.008. [DOI] [PubMed] [Google Scholar]
- Corcoran KA, Quirk GJ. Activity in Prelimbic Cortex Is Necessary for the Expression of Learned, But Not Innate, Fears. J. Neurosci. 2007;27:840–844. doi: 10.1523/JNEUROSCI.5327-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutureau E, Esclassan F, Di Scala G, Marchand AR. The role of the rat medial prefrontal cortex in adapting to changes in instrumental contingency. PLoS One. 2012;7:e33302. doi: 10.1371/journal.pone.0033302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowen SL, Davis GA, Nitz DA. Anterior cingulate neurons in the rat map anticipated effort and reward to their associated action sequences. J Neurophysiol. 2012;107:2393–2407. doi: 10.1152/jn.01012.2011. [DOI] [PubMed] [Google Scholar]
- Cowen SL, McNaughton BL. Selective delay activity in the medial prefrontal cortex of the rat: contribution of sensorimotor information and contingency. J Neurophysiol. 2007;98:303–316. doi: 10.1152/jn.00150.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delatour B, Gisquet-Verrier P. Lesions of the prelimbic-infralimbic cortices in rats do not disrupt response selection processes but induce delay-dependent deficits: evidence for a role in working memory? Behav Neurosci. 1999;113:941–955. doi: 10.1037//0735-7044.113.5.941. [DOI] [PubMed] [Google Scholar]
- Ding HK, Teixeira CM, Frankland PW. Inactivation of the anterior cingulate cortex blocks expression of remote, but not recent, conditioned taste aversion memory. Learn Mem. 2008;15:290–293. doi: 10.1101/lm.905008. [DOI] [PubMed] [Google Scholar]
- Durstewitz D, Vittoz NM, Floresco SB, Seamans JK. Abrupt transitions between prefrontal neural ensemble states accompany behavioral transitions during rule learning. Neuron. 2010;66:438–448. doi: 10.1016/j.neuron.2010.03.029. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Neave N, Aggleton JP. Spontaneous object recognition and object location memory in rats: the effects of lesions in the cingulate cortices, the medial prefrontal cortex, the cingulum bundle and the fornix. Exp Brain Res. 1997;113:509–519. doi: 10.1007/pl00005603. [DOI] [PubMed] [Google Scholar]
- Euston DR, McNaughton BL. Apparent encoding of sequential context in rat medial prefrontal cortex is accounted for by behavioral variability. J Neurosci. 2006;26:13143–13155. doi: 10.1523/JNEUROSCI.3803-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euston DR, Tatsuno M, McNaughton BL. Fast-forward playback of recent memory sequences in prefrontal cortex during sleep. Science. 2007;318:1147–1150. doi: 10.1126/science.1148979. [DOI] [PubMed] [Google Scholar]
- Farovik A, Dupont LM, Arce M, Eichenbaum H. Medial prefrontal cortex supports recollection, but not familiarity, in the rat. J Neurosci. 2008;28:13428–13434. doi: 10.1523/JNEUROSCI.3662-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellows LK. Advances in understanding ventromedial prefrontal function: the accountant joins the executive. Neurology. 2007;68:991–995. doi: 10.1212/01.wnl.0000257835.46290.57. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Seamans JK, Phillips AG. Selective roles for hippocampal, prefrontal cortical, and ventral striatal circuits in radial-arm maze tasks with or without a delay. J Neurosci. 1997;17:1880–1890. doi: 10.1523/JNEUROSCI.17-05-01880.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankland PW, Bontempi B. The organization of recent and remote memories. Nat Rev Neurosci. 2005;6:119–130. doi: 10.1038/nrn1607. [DOI] [PubMed] [Google Scholar]
- Frankland PW, Bontempi B, Talton LE, Kaczmarek L, Silva AJ. The involvement of the anterior cingulate cortex in remote contextual fear memory. Science. 2004;304:881–883. doi: 10.1126/science.1094804. [DOI] [PubMed] [Google Scholar]
- Fujisawa S, Amarasingham A, Harrison MT, Buzsaki G. Behavior-dependent short-term assembly dynamics in the medial prefrontal cortex. Nat Neurosci. 2008;11:823–833. doi: 10.1038/nn.2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa S, Buzsaki G. A 4 Hz oscillation adaptively synchronizes prefrontal, VTA, and hippocampal activities. Neuron. 2011;72:153–165. doi: 10.1016/j.neuron.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funahashi S. Prefrontal cortex and working memory processes. Neuroscience. 2006;139:251–261. doi: 10.1016/j.neuroscience.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Gabbott PL, Warner TA, Jays PR, Salway P, Busby SJ. Prefrontal cortex in the rat: projections to subcortical autonomic, motor, and limbic centers. J Comp Neurol. 2005;492:145–177. doi: 10.1002/cne.20738. [DOI] [PubMed] [Google Scholar]
- Gais S, Albouy G, Boly M, Dang-Vu TT, Darsaud A, Desseilles M, Rauchs G, Schabus M, Sterpenich V, Vandewalle G, et al. Sleep transforms the cerebral trace of declarative memories. Proc Natl Acad Sci U S A. 2007;104:18778–18783. doi: 10.1073/pnas.0705454104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmartin MR, McEchron MD. Single neurons in the medial prefrontal cortex of the rat exhibit tonic and phasic coding during trace fear conditioning. Behav Neurosci. 2005;119:1496–1510. doi: 10.1037/0735-7044.119.6.1496. [DOI] [PubMed] [Google Scholar]
- Gisquet-Verrier P, Delatour B. The role of the rat prelimbic/infralimbic cortex in working memory: Not involved in the short-term maintenance but in monitoring and processing functions. Neuroscience. 2006;141:585–596. doi: 10.1016/j.neuroscience.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Gisquet-Verrier P, Winocur G, Delatour B. Functional dissociation between dorsal and ventral regions of the medial prefrontal cortex in rats. Psychobiology. 2000;28:248–260. [Google Scholar]
- Goto Y, Grace AA. Dopamine modulation of hippocampal-prefrontal cortical interaction drives memory-guided behavior. Cereb Cortex. 2008;18:1407–1414. doi: 10.1093/cercor/bhm172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber AJ, Calhoon GG, Shusterman I, Schoenbaum G, Roesch MR, O'Donnell P. More is less: a disinhibited prefrontal cortex impairs cognitive flexibility. J Neurosci. 2010;30:17102–17110. doi: 10.1523/JNEUROSCI.4623-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber AJ, McDonald RJ. Context, emotion, and the strategic pursuit of goals: interactions among multiple brain systems controlling motivated behavior. Front Behav Neurosci. 2012;6 doi: 10.3389/fnbeh.2012.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidbreder CA, Groenewegen HJ. The medial prefrontal cortex in the rat: evidence for a dorso-ventral distinction based upon functional and anatomical characteristics. Neurosci Biobehav Rev. 2003;27:555–579. doi: 10.1016/j.neubiorev.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Hikosaka O. The habenula: from stress evasion to value-based decision-making. Nat Rev Neurosci. 2010;11:503–513. doi: 10.1038/nrn2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman KL, McNaughton BL. Coordinated reactivation of distributed memory traces in primate neocortex. Science. 2002;297:2070–2073. doi: 10.1126/science.1073538. [DOI] [PubMed] [Google Scholar]
- Holahan MR, Routtenberg A. Post-translational synaptic protein modification as substrate for long-lasting, remote memory: an initial test. Hippocampus. 2007;17:93–97. doi: 10.1002/hipo.20245. [DOI] [PubMed] [Google Scholar]
- Holloway CM, McIntyre CK. Post-training disruption of Arc protein expression in the anterior cingulate cortex impairs long-term memory for inhibitory avoidance training. Neurobiol Learn Mem. 2011;95:425–432. doi: 10.1016/j.nlm.2011.02.002. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Coles MG, Nieuwenhuis S. Medial prefrontal cortex and error potentials. Science. 2002;296:1610–1611. doi: 10.1126/science.296.5573.1610. author reply 1610-1611. [DOI] [PubMed] [Google Scholar]
- Hoover WB, Vertes RP. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct Funct. 2007;212:149–179. doi: 10.1007/s00429-007-0150-4. [DOI] [PubMed] [Google Scholar]
- Hoover WB, Vertes RP. Projections of the medial orbital and ventral orbital cortex in the rat. J Comp Neurol. 2011;519:3766–3801. doi: 10.1002/cne.22733. [DOI] [PubMed] [Google Scholar]
- Horst NK, Laubach M. The role of rat dorsomedial prefrontal cortex in spatial working memory. Neuroscience. 2009;164:444–456. doi: 10.1016/j.neuroscience.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman JM, Ma L, Balaguer-Ballester E, Durstewitz D, Seamans JK. Contextual encoding by ensembles of medial prefrontal cortex neurons. Proc Natl Acad Sci U S A. 2012;109:5086–5091. doi: 10.1073/pnas.1114415109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman JM, Zilli EA, Paley AM, Hasselmo ME. Medial prefrontal cortex cells show dynamic modulation with the hippocampal theta rhythm dependent on behavior. Hippocampus. 2005;15:739–749. doi: 10.1002/hipo.20106. [DOI] [PubMed] [Google Scholar]
- Hyman JM, Zilli EA, Paley AM, Hasselmo ME. Working Memory Performance Correlates with Prefrontal-Hippocampal Theta Interactions but not with Prefrontal Neuron Firing Rates. Front Integr Neurosci. 2010;4:2. doi: 10.3389/neuro.07.002.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isomura Y, Sirota A, Ozen S, Montgomery S, Mizuseki K, Henze DA, Buzsaki G. Integration and segregation of activity in entorhinal-hippocampal subregions by neocortical slow oscillations. Neuron. 2006;52:871–882. doi: 10.1016/j.neuron.2006.10.023. [DOI] [PubMed] [Google Scholar]
- Itoi K, Sugimoto N. The brainstem noradrenergic systems in stress, anxiety and depression. J Neuroendocrinol. 2010;22:355–361. doi: 10.1111/j.1365-2826.2010.01988.x. [DOI] [PubMed] [Google Scholar]
- Izaki Y, Hori K, Nomura M. Disturbance of rat lever-press learning by hippocampoprefrontal disconnection. Brain Res. 2000;860:199–202. doi: 10.1016/s0006-8993(00)02039-4. [DOI] [PubMed] [Google Scholar]
- Jasmin L, Burkey AR, Granato A, Ohara PT. Rostral agranular insular cortex and pain areas of the central nervous system: a tract-tracing study in the rat. J Comp Neurol. 2004;468:425–440. doi: 10.1002/cne.10978. [DOI] [PubMed] [Google Scholar]
- Jay TM, Witter MP. Distribution of hippocampal CA1 and subicular efferents in the prefrontal cortex of the rat studied by means of anterograde transport of Phaseolus vulgaris-leucoagglutinin. J Comp Neurol. 1991;313:574–586. doi: 10.1002/cne.903130404. [DOI] [PubMed] [Google Scholar]
- Ji D, Wilson MA. Coordinated memory replay in the visual cortex and hippocampus during sleep. Nat Neurosci. 2007;10:100–107. doi: 10.1038/nn1825. [DOI] [PubMed] [Google Scholar]
- Johansen JP, Fields HL, Manning BH. The affective component of pain in rodents: direct evidence for a contribution of the anterior cingulate cortex. Proc Natl Acad Sci U S A. 2001;98:8077–8082. doi: 10.1073/pnas.141218998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LA, Euston DR, Tatsuno M, McNaughton BL. Stored-trace reactivation in rat prefrontal cortex is correlated with down-to-up state fluctuation density. J Neurosci. 2010;30:2650–2661. doi: 10.1523/JNEUROSCI.1617-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BF, Groenewegen HJ, Witter MP. Intrinsic connections of the cingulate cortex in the rat suggest the existence of multiple functionally segregated networks. Neuroscience. 2005;133:193–207. doi: 10.1016/j.neuroscience.2005.01.063. [DOI] [PubMed] [Google Scholar]
- Jones MW, Wilson MA. Theta rhythms coordinate hippocampal-prefrontal interactions in a spatial memory task. PLoS Biol. 2005;3:e402. doi: 10.1371/journal.pbio.0030402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung MW, Qin Y, McNaughton BL, Barnes CA. Firing characteristics of deep layer neurons in prefrontal cortex in rats performing spatial working memory tasks. Cereb Cortex. 1998;8:437–450. doi: 10.1093/cercor/8.5.437. [DOI] [PubMed] [Google Scholar]
- Jung MW, Wiener SI, McNaughton BL. Comparison of spatial firing characteristics of units in dorsal and ventral hippocampus of the rat. J Neurosci. 1994;14:7347–7356. doi: 10.1523/JNEUROSCI.14-12-07347.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kable JW, Glimcher PW. The neural correlates of subjective value during intertemporal choice. Nat Neurosci. 2007;10:1625–1633. doi: 10.1038/nn2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjelstrup KB, Solstad T, Brun VH, Hafting T, Leutgeb S, Witter MP, Moser EI, Moser MB. Finite scale of spatial representation in the hippocampus. Science. 2008;321:140–143. doi: 10.1126/science.1157086. [DOI] [PubMed] [Google Scholar]
- Koya E, Uejima JL, Wihbey KA, Bossert JM, Hope BT, Shaham Y. Role of ventral medial prefrontal cortex in incubation of cocaine craving. Neuropharmacology. 2009;56(Suppl 1):177–185. doi: 10.1016/j.neuropharm.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranz GS, Kasper S, Lanzenberger R. Reward and the serotonergic system. Neuroscience. 2010;166:1023–1035. doi: 10.1016/j.neuroscience.2010.01.036. [DOI] [PubMed] [Google Scholar]
- Kudrimoti HS, Barnes CA, McNaughton BL. Reactivation of hippocampal cell assemblies: effects of behavioral state, experience, and EEG dynamics. J Neurosci. 1999;19:4090–4101. doi: 10.1523/JNEUROSCI.19-10-04090.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaLumiere RT, Niehoff KE, Kalivas PW. The infralimbic cortex regulates the consolidation of extinction after cocaine self-administration. Learn Mem. 2010;17:168–175. doi: 10.1101/lm.1576810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansink CS, Goltstein PM, Lankelma JV, McNaughton BL, Pennartz CM. Hippocampus leads ventral striatum in replay of place-reward information. PLoS Biol. 2009;7:e1000173. doi: 10.1371/journal.pbio.1000173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapish CC, Durstewitz D, Chandler LJ, Seamans JK. Successful choice behavior is associated with distinct and coherent network states in anterior cingulate cortex. Proc Natl Acad Sci U S A. 2008;105:11963–11968. doi: 10.1073/pnas.0804045105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laviolette SR, Lipski WJ, Grace AA. A subpopulation of neurons in the medial prefrontal cortex encodes emotional learning with burst and frequency codes through a dopamine D4 receptor-dependent basolateral amygdala input. J Neurosci. 2005;25:6066–6075. doi: 10.1523/JNEUROSCI.1168-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I, Solivan F. The roles of the medial prefrontal cortex and hippocampus in a spatial paired-association task. Learn Mem. 2008;15:357–367. doi: 10.1101/lm.902708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon WC, Bruno MA, Allard S, Nader K, Cuello AC. Engagement of the PFC in consolidation and recall of recent spatial memory. Learn Mem. 2010;17:297–305. doi: 10.1101/lm.1804410. [DOI] [PubMed] [Google Scholar]
- Lesburgueres E, Gobbo OL, Alaux-Cantin S, Hambucken A, Trifilieff P, Bontempi B. Early tagging of cortical networks is required for the formation of enduring associative memory. Science. 2011;331:924–928. doi: 10.1126/science.1196164. [DOI] [PubMed] [Google Scholar]
- Leutgeb S, Leutgeb JK, Barnes CA, Moser EI, McNaughton BL, Moser MB. Independent codes for spatial and episodic memory in hippocampal neuronal ensembles. Science. 2005;309:619–623. doi: 10.1126/science.1114037. [DOI] [PubMed] [Google Scholar]
- Li B, Piriz J, Mirrione M, Chung C, Proulx CD, Schulz D, Henn F, Malinow R. Synaptic potentiation onto habenula neurons in the learned helplessness model of depression. Nature. 2011;470:535–539. doi: 10.1038/nature09742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier SF, Watkins LR. Stressor controllability and learned helplessness: the roles of the dorsal raphe nucleus, serotonin, and corticotropin-releasing factor. Neurosci Biobehav Rev. 2005;29:829–841. doi: 10.1016/j.neubiorev.2005.03.021. [DOI] [PubMed] [Google Scholar]
- Mamiya N, Fukushima H, Suzuki A, Matsuyama Z, Homma S, Frankland PW, Kida S. Brain region-specific gene expression activation required for reconsolidation and extinction of contextual fear memory. J Neurosci. 2009;29:402–413. doi: 10.1523/JNEUROSCI.4639-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek R, Coelho CM, Sullivan RK, Baker-Andresen D, Li X, Ratnu V, Dudley KJ, Meyers D, Mukherjee C, Cole PA, et al. Paradoxical Enhancement of Fear Extinction Memory and Synaptic Plasticity by Inhibition of the Histone Acetyltransferase p300. J Neurosci. 2011;31:7486–7491. doi: 10.1523/JNEUROSCI.0133-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maviel T, Durkin TP, Menzaghi F, Bontempi B. Sites of neocortical reorganization critical for remote spatial memory. Science. 2004;305:96–99. doi: 10.1126/science.1098180. [DOI] [PubMed] [Google Scholar]
- McClelland JL, McNaughton BL, O'Reilly RC. Why there are complementary learning systems in the hippocampus and neocortex: insights from the successes and failures of connectionist models of learning and memory. Psychol Rev. 1995;102:419–457. doi: 10.1037/0033-295X.102.3.419. [DOI] [PubMed] [Google Scholar]
- Miller EK. The prefrontal cortex and cognitive control. Nat Rev Neurosci. 2000;1:59–65. doi: 10.1038/35036228. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Miyazaki K, Miyazaki KW, Matsumoto G. Different representation of forthcoming reward in nucleus accumbens and medial prefrontal cortex. Neuroreport. 2004;15:721–726. doi: 10.1097/00001756-200403220-00030. [DOI] [PubMed] [Google Scholar]
- Molle M, Yeshenko O, Marshall L, Sara SJ, Born J. Hippocampal sharp wave-ripples linked to slow oscillations in rat slow-wave sleep. J Neurophysiol. 2006;96:62–70. doi: 10.1152/jn.00014.2006. [DOI] [PubMed] [Google Scholar]
- Morrow BA, Elsworth JD, Inglis FM, Roth RH. An antisense oligonucleotide reverses the footshock-induced expression of fos in the rat medial prefrontal cortex and the subsequent expression of conditioned fear-induced immobility. J Neurosci. 1999;19:5666–5673. doi: 10.1523/JNEUROSCI.19-13-05666.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder AB, Nordquist R, Orgut O, Pennartz CM. Plasticity of neuronal firing in deep layers of the medial prefrontal cortex in rats engaged in operant conditioning. Prog Brain Res. 2000;126:287–301. doi: 10.1016/S0079-6123(00)26020-2. [DOI] [PubMed] [Google Scholar]
- Nadel L. The Hippocampus and Context Revisited. In: Mizumori SJY, editor. Hippocampal Place Fields: Relevance to Learning and Memory. Oxford University Press; New York, NY: 2008. pp. 3–15. [Google Scholar]
- Narayanan NS, Horst NK, Laubach M. Reversible inactivations of rat medial prefrontal cortex impair the ability to wait for a stimulus. Neuroscience. 2006;139:865–876. doi: 10.1016/j.neuroscience.2005.11.072. [DOI] [PubMed] [Google Scholar]
- Narayanan NS, Laubach M. Top-down control of motor cortex ensembles by dorsomedial prefrontal cortex. Neuron. 2006;52:921–931. doi: 10.1016/j.neuron.2006.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan NS, Laubach M. Neuronal Correlates of Post-Error Slowing in the Rat Dorsomedial Prefrontal Cortex. J Neurophysiol. 2008;100:520–525. doi: 10.1152/jn.00035.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neafsey EJ, Terreberry RR, Hurley KM, Ruit KG, Frysztak RJ. Anterior cingulate cortex in rodents: Connections, visceral control functions, and implications for emotion. In: Vogt BA, Gabriel M, editors. Neurobiology of cingulate cortex and limbic thalamus: A comprehensive handbook. Birkhaeuser; Cambridge, MA: 1993. pp. 206–223. [Google Scholar]
- Nelson RJ, Trainor BC. Neural mechanisms of aggression. Nat Rev Neurosci. 2007;8:536–546. doi: 10.1038/nrn2174. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis IL, Takashima A. The role of the ventromedial prefrontal cortex in memory consolidation. Behav Brain Res. 2011;218:325–334. doi: 10.1016/j.bbr.2010.12.009. [DOI] [PubMed] [Google Scholar]
- Ostlund SB, Balleine BW. Lesions of medial prefrontal cortex disrupt the acquisition but not the expression of goal-directed learning. J Neurosci. 2005;25:7763–7770. doi: 10.1523/JNEUROSCI.1921-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostlund SB, Balleine BW. Orbitofrontal cortex mediates outcome encoding in Pavlovian but not instrumental conditioning. J Neurosci. 2007;27:4819–4825. doi: 10.1523/JNEUROSCI.5443-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswald BB, Maddox SA, Tisdale N, Powell DA. Encoding and retrieval are differentially processed by the anterior cingulate and prelimbic cortices: a study based on trace eyeblink conditioning in the rabbit. Neurobiol Learn Mem. 2010;93:37–45. doi: 10.1016/j.nlm.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain. 6th edn Academic Press; San Diego, CA: 2007. [Google Scholar]
- Pennartz CM, Ito R, Verschure PF, Battaglia FP, Robbins TW. The hippocampal-striatal axis in learning, prediction and goal-directed behavior. Trends Neurosci. 2011;34:548–559. doi: 10.1016/j.tins.2011.08.001. [DOI] [PubMed] [Google Scholar]
- Peters J, Kalivas PW, Quirk GJ. Extinction circuits for fear and addiction overlap in prefrontal cortex. Learn Mem. 2009;16:279–288. doi: 10.1101/lm.1041309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Vallone J, Laurendi K, Kalivas PW. Opposing roles for the ventral prefrontal cortex and the basolateral amygdala on the spontaneous recovery of cocaine-seeking in rats. Psychopharmacology (Berl) 2008;197:319–326. doi: 10.1007/s00213-007-1034-2. [DOI] [PubMed] [Google Scholar]
- Peters YM, O'Donnell P, Carelli RM. Prefrontal cortical cell firing during maintenance, extinction, and reinstatement of goal-directed behavior for natural reward. Synapse. 2005;56:74–83. doi: 10.1002/syn.20129. [DOI] [PubMed] [Google Scholar]
- Peyrache A, Khamassi M, Benchenane K, Wiener SI, Battaglia FP. Replay of rule-learning related neural patterns in the prefrontal cortex during sleep. Nat Neurosci. 2009;12:919–926. doi: 10.1038/nn.2337. [DOI] [PubMed] [Google Scholar]
- Posner MI, Rothbart MK, Sheese BE, Tang Y. The anterior cingulate gyrus and the mechanism of self-regulation. Cogn Affect Behav Neurosci. 2007;7:391–395. doi: 10.3758/cabn.7.4.391. [DOI] [PubMed] [Google Scholar]
- Poucet B. Searching for spatial unit firing in the prelimbic area of the rat medial prefrontal cortex. Behav Brain Res. 1997;84:151–159. doi: 10.1016/s0166-4328(96)00144-1. [DOI] [PubMed] [Google Scholar]
- Pratt WE, Mizumori SJ. Neurons in rat medial prefrontal cortex show anticipatory rate changes to predictable differential rewards in a spatial memory task. Behav Brain Res. 2001;123:165–183. doi: 10.1016/s0166-4328(01)00204-2. [DOI] [PubMed] [Google Scholar]
- Preuss TM. Do rats have prefrontal cortex? The Rose-Woolsey-Akert program reconsidered. Journal of Cognitive Neuroscience. 1995;7(1):1–24. doi: 10.1162/jocn.1995.7.1.1. [DOI] [PubMed] [Google Scholar]
- Quilodran R, Rothe M, Procyk E. Behavioral shifts and action valuation in the anterior cingulate cortex. Neuron. 2008;57:314–325. doi: 10.1016/j.neuron.2007.11.031. [DOI] [PubMed] [Google Scholar]
- Quinn JJ, Ma QD, Tinsley MR, Koch C, Fanselow MS. Inverse temporal contributions of the dorsal hippocampus and medial prefrontal cortex to the expression of long-term fear memories. Learn Mem. 2008;15:368–372. doi: 10.1101/lm.813608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino ME, Detrick S, Kesner RP. Involvement of the prelimbic-infralimbic areas of the rodent prefrontal cortex in behavioral flexibility for place and response learning. J Neurosci. 1999;19:4585–4594. doi: 10.1523/JNEUROSCI.19-11-04585.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reep RL, Corwin JV, King V. Neuronal connections of orbital cortex in rats: topography of cortical and thalamic afferents. Exp Brain Res. 1996;111:215–232. doi: 10.1007/BF00227299. [DOI] [PubMed] [Google Scholar]
- Restivo L, Vetere G, Bontempi B, Ammassari-Teule M. The formation of recent and remote memory is associated with time-dependent formation of dendritic spines in the hippocampus and anterior cingulate cortex. J Neurosci. 2009;29:8206–8214. doi: 10.1523/JNEUROSCI.0966-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich EL, Shapiro M. Rat prefrontal cortical neurons selectively code strategy switches. J Neurosci. 2009;29:7208–7219. doi: 10.1523/JNEUROSCI.6068-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306:443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, McReynolds JR, Van der Zee EA, Lee S, McGaugh JL, McIntyre CK. Glucocorticoid Effects on Memory Consolidation Depend on Functional Interactions between the Medial Prefrontal Cortex and Basolateral Amygdala. J. Neurosci. 2009;29:14299–14308. doi: 10.1523/JNEUROSCI.3626-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross RS, Eichenbaum H. Dynamics of hippocampal and cortical activation during consolidation of a nonspatial memory. J Neurosci. 2006;26:4852–4859. doi: 10.1523/JNEUROSCI.0659-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy JW, Biedenkapp JC, O'Reilly RC. Prefrontal cortex and the organization of recent and remote memories: an alternative view. Learn Mem. 2005;12:445–446. doi: 10.1101/lm.97905. [DOI] [PubMed] [Google Scholar]
- Runyan JD, Moore, Dash PK. A role for prefrontal cortex in memory storage for trace fear conditioning. J Neurosci. 2004;24:1288–1295. doi: 10.1523/JNEUROSCI.4880-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushworth MF, Noonan MP, Boorman ED, Walton ME, Behrens TE. Frontal cortex and reward-guided learning and decision-making. Neuron. 2011;70:1054–1069. doi: 10.1016/j.neuron.2011.05.014. [DOI] [PubMed] [Google Scholar]
- Saper CB. The Hypothalamus. In: Paxinos G, editor. The Human Nervous System. Academic Press; San Diego, CA: 2003. pp. 513–550. [Google Scholar]
- Schroeder BE, Binzak JM, Kelley AE. A common profile of prefrontal cortical activation following exposure to nicotine- or chocolate-associated contextual cues. Neuroscience. 2001;105:535–545. doi: 10.1016/s0306-4522(01)00221-4. [DOI] [PubMed] [Google Scholar]
- Schultz W. Reward signaling by dopamine neurons. Neuroscientist. 2001;7:293–302. doi: 10.1177/107385840100700406. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Floresco SB, Phillips AG. Functional differences between the prelimbic and anterior cingulate regions of the rat prefrontal cortex. Behav Neurosci. 1995;109:1063–1073. doi: 10.1037//0735-7044.109.6.1063. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Lapish CC, Durstewitz D. Comparing the prefrontal cortex of rats and primates: insights from electrophysiology. Neurotox Res. 2008;14:249–262. doi: 10.1007/BF03033814. [DOI] [PubMed] [Google Scholar]
- Sewards TV, Sewards MA. The medial pain system: Neural representations of the motivational aspect of pain. Brain Research Bulletin. 2002;59:163–180. doi: 10.1016/s0361-9230(02)00864-x. [DOI] [PubMed] [Google Scholar]
- Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat Rev Neurosci. 2011;12:154–167. doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shidara M, Richmond BJ. Anterior cingulate: single neuronal signals related to degree of reward expectancy. Science. 2002;296:1709–1711. doi: 10.1126/science.1069504. [DOI] [PubMed] [Google Scholar]
- Siapas AG, Lubenov EV, Wilson MA. Prefrontal phase locking to hippocampal theta oscillations. Neuron. 2005;46:141–151. doi: 10.1016/j.neuron.2005.02.028. [DOI] [PubMed] [Google Scholar]
- Siapas AG, Wilson MA. Coordinated interactions between hippocampal ripples and cortical spindles during slow-wave sleep. Neuron. 1998;21:1123–1128. doi: 10.1016/s0896-6273(00)80629-7. [DOI] [PubMed] [Google Scholar]
- Sirota A, Csicsvari J, Buhl D, Buzsaki G. Communication between neocortex and hippocampus during sleep in rodents. Proc Natl Acad Sci U S A. 2003;100:2065–2069. doi: 10.1073/pnas.0437938100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotres-Bayon F, Diaz-Mataix L, Bush DE, LeDoux JE. Dissociable roles for the ventromedial prefrontal cortex and amygdala in fear extinction: NR2B contribution. Cereb Cortex. 2009;19:474–482. doi: 10.1093/cercor/bhn099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sul JH, Kim H, Huh N, Lee D, Jung MW. Distinct roles of rodent orbitofrontal and medial prefrontal cortex in decision making. Neuron. 2010;66:449–460. doi: 10.1016/j.neuron.2010.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashima A, Jensen O, Oostenveld R, Maris E, van de Coevering M, Fernandez G. Successful declarative memory formation is associated with ongoing activity during encoding in a distributed neocortical network related to working memory: a magnetoencephalography study. Neuroscience. 2006a;139:291–297. doi: 10.1016/j.neuroscience.2005.05.067. [DOI] [PubMed] [Google Scholar]
- Takashima A, Petersson KM, Rutters F, Tendolkar I, Jensen O, Zwarts MJ, McNaughton BL, Fernandez G. Declarative memory consolidation in humans: a prospective functional magnetic resonance imaging study. Proc Natl Acad Sci U S A. 2006b;103:756–761. doi: 10.1073/pnas.0507774103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takehara-Nishiuchi K, Kawahara S, Kirino Y. NMDA receptor-dependent processes in the medial prefrontal cortex are important for acquisition and the early stage of consolidation during trace, but not delay eyeblink conditioning. Learn Mem. 2005;12:606–614. doi: 10.1101/lm.5905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takehara-Nishiuchi K, McNaughton BL. Spontaneous changes of neocortical code for associative memory during consolidation. Science. 2008;322:960–963. doi: 10.1126/science.1161299. [DOI] [PubMed] [Google Scholar]
- Takehara-Nishiuchi K, Nakao K, Kawahara S, Matsuki N, Kirino Y. Systems consolidation requires postlearning activation of NMDA receptors in the medial prefrontal cortex in trace eyeblink conditioning. J Neurosci. 2006;26:5049–5058. doi: 10.1523/JNEUROSCI.4381-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takehara K, Kawahara S, Kirino Y. Time-dependent reorganization of the brain components underlying memory retention in trace eyeblink conditioning. J Neurosci. 2003;23:9897–9905. doi: 10.1523/JNEUROSCI.23-30-09897.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira CM, Pomedli SR, Maei HR, Kee N, Frankland PW. Involvement of the anterior cingulate cortex in the expression of remote spatial memory. J Neurosci. 2006;26:7555–7564. doi: 10.1523/JNEUROSCI.1068-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touzani K, Puthanveettil SV, Kandel ER. Consolidation of learning strategies during spatial working memory task requires protein synthesis in the prefrontal cortex. Proc Natl Acad Sci U S A. 2007;104:5632–5637. doi: 10.1073/pnas.0611554104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronel S, Feenstra MG, Sara SJ. Noradrenergic action in prefrontal cortex in the late stage of memory consolidation. Learn Mem. 2004;11:453–458. doi: 10.1101/lm.74504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronel S, Sara SJ. Blockade of NMDA receptors in prelimbic cortex induces an enduring amnesia for odor-reward associative learning. J Neurosci. 2003;23:5472–5476. doi: 10.1523/JNEUROSCI.23-13-05472.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uylings HBM, Groenewegen HJ, Kolb B. Do rats have a prefrontal cortex? Behavioural Brain Research. 2003;146:3–17. doi: 10.1016/j.bbr.2003.09.028. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Hoover WB, Szigeti-Buck K, Leranth C. Nucleus reuniens of the midline thalamus: link between the medial prefrontal cortex and the hippocampus. Brain Res Bull. 2007;71:601–609. doi: 10.1016/j.brainresbull.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GW, Cai JX. Disconnection of the hippocampal-prefrontal cortical circuits impairs spatial working memory performance in rats. Behav Brain Res. 2006;175:329–336. doi: 10.1016/j.bbr.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Wang GW, Cai JX. Reversible disconnection of the hippocampal-prelimbic cortical circuit impairs spatial learning but not passive avoidance learning in rats. Neurobiol Learn Mem. 2008;90:365–373. doi: 10.1016/j.nlm.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Wang Z, Bradesi S, Charles JR, Pang RD, Maarek JM, Mayer EA, Holschneider DP. Functional brain activation during retrieval of visceral pain-conditioned passive avoidance in the rat. Pain. 2011;152:2746–2756. doi: 10.1016/j.pain.2011.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierzynski CM, Lubenov EV, Gu M, Siapas AG. State-dependent spike-timing relationships between hippocampal and prefrontal circuits during sleep. Neuron. 2009;61:587–596. doi: 10.1016/j.neuron.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MA, McNaughton BL. Dynamics of the hippocampal ensemble code for space. Science. 1993;261:1055–1058. doi: 10.1126/science.8351520. [DOI] [PubMed] [Google Scholar]
- Wilson MA, McNaughton BL. Reactivation of hippocampal ensemble memories during sleep. Science. 1994;265:676–679. doi: 10.1126/science.8036517. [DOI] [PubMed] [Google Scholar]
- Winocur G, Moscovitch M, Bontempi B. Memory formation and long-term retention in humans and animals: Convergence towards a transformation account of hippocampal-neocortical interactions. Neuropsychologia. 2010 doi: 10.1016/j.neuropsychologia.2010.04.016. [DOI] [PubMed] [Google Scholar]
- Wise SP. Forward frontal fields: phylogeny and fundamental function. Trends Neurosci. 2008;31:599–608. doi: 10.1016/j.tins.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise SP, Murray EA. Arbitrary associations between antecedents and actions. Trends Neurosci. 2000;23:271–276. doi: 10.1016/s0166-2236(00)01570-8. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Fukushima H, Kida S. Induction and requirement of gene expression in the anterior cingulate cortex and medial prefrontal cortex for the consolidation of inhibitory avoidance memory. Mol Brain. 2011;4:4. doi: 10.1186/1756-6606-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao MG, Toyoda H, Lee YS, Wu LJ, Ko SW, Zhang XH, Jia Y, Shum F, Xu H, Li BM, et al. Roles of NMDA NR2B subtype receptor in prefrontal long-term potentiation and contextual fear memory. Neuron. 2005;47:859–872. doi: 10.1016/j.neuron.2005.08.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.