Abstract
The principle role of the vascular endothelium is to present a semi-impermeable barrier to soluble factors and circulating cells, while still permitting the passage of leukocytes from the bloodstream into the tissue. The process of diapedesis involves the selective disruption of endothelial cell junctions, an event that could in theory compromise vascular integrity. It is therefore somewhat surprising that neutrophil transmigration does not significantly impair endothelial barrier function. We examined whether neutrophils might secrete factors that promote vascular integrity during the latter stages of neutrophil transmigration, and found that neutrophil proteinase 3 (PR3) – a serine protease harbored in azurophilic granules – markedly enhanced barrier function in endothelial cells. PR3 functioned in this capacity both in its soluble form and in a complex with cell-surface NB1. PR3-mediated enhancement of endothelial cell junctional integrity required its proteolytic activity, as well as endothelial cell expression of the protease-activated receptor, PAR-2. Importantly, PR3 suppressed the vascular permeability changes and disruption of junctional proteins induced by the action of PAR-1 agonists. These findings establish the potential for neutrophil-derived PR3 to play a role in reestablishing vascular integrity following leukocyte transmigration, and in protecting endothelial cells from PAR-1-induced permeability changes that occur during thrombotic and inflammatory events.
Keywords: NB1, PECAM-1, calcium signaling, serine protease
Introduction
The vascular endothelium plays a critical role in maintaining hemostasis, preserving the integrity of the circulatory system and regulating the inflammatory response.1, 2 The latter role produces particular challenges for endothelial cells, which must permit the emigration of leukocytes from the bloodstream into the sub-endothelium, while at the same time preserving vascular integrity.
One of the classical hallmarks of inflammation is edema and localized swelling. The contribution of emigrating leukocytes to vascular leakage has long been debated. Previous evidence has indicated that leukocytes can promote an increase in vascular permeability3–7. However, despite the fact that leukocytes are capable of releasing cytokines, proteolytic enzymes, and reactive oxygen intermediates that promote local tissue damage, induce endothelial cell contraction and recruit additional leukocytes,8, 9 a number of studies have found that leukocyte transmigration results in only a transient, negligible opening of adherens junctions10–12, with little induction of macromolecule leakage.10, 12–15 Further evidence that neutrophil transmigration does not cause increased endothelial cell permeability during the inflammatory response is suggested by both in vivo and in vitro studies16 showing a general lack of correlation between neutrophil transmigration and increased vascular permeability. For example, in an aseptic model of wound healing, Kim et al. reported that the maximal increase in vascular permeability occurred a full six hours prior to peak neutrophil influx17, with the magnitude of vascular leakage unaffected by neutrophil depletion17. Burns et al.18 and Inglis et al.,19 found that neutrophil transmigration across IL-1β- and fMLP-stimulated endothelial cell monolayers actually improved vascular cell barrier function. Despite considerable progress in this area, however, it is still not clear how leukocytes, particularly neutrophils, might be able to preserve vascular integrity during the process of transmigration.
Neutrophils contain in their cytoplasmic granules a number of serine proteases, including cathepsin G, neutrophil elastase and proteinase 3 (PR3). Once released, these proteolytic enzymes can be concentrated in neutrophil extracellular traps (NETs)20 or rebound to the cell surface, where they can exert widespread effects, including induction of bactericidal activity,21–24 degradation of extracellular matrix proteins,25–27 promotion of neutrophil transmigration,28–33 and regulation of vascular integrity. 34 In vivo, the activity of cathepsin G and neutrophil elastase is inhibited by secretory leukocyte protease inhibitor (SLP). Cathepsin G is also inhibited by anti-chymotrypsin.35 Neutrophil elastase and PR3 are inhibited by α1-antitrypsin and elafin.36 The rapid inhibition of these proteases in vivo has the effect of restricting their activity to areas of local neutrophil accumulation.
Of particular interest is PR3, also known as elastin degrading protease, the most abundant serine protease in neutrophils.37 Following neutrophil activation, PR3 is secreted from azurophil granules and rebinds to the neutrophil surface through an association with NB1 (CD177, HNA- 2a) – an 60 kDa glycosyl-phosphatidylinositol (GPI)-linked, cell surface glycoprotein that is expressed on a subpopulation of neutrophils in 97% of healthy individuals.33, 38 This interaction is unique to PR3, and does not occur for other neutrophil serine proteases. PR3, in association with NB1, is partially protected from proteolytic inactivation,32,37 – a property that may significantly increase its efficacy. In addition, NB1 has been reported to be a heterophilic binding partner for endothelial cell PECAM-1, and disrupting NB1-PECAM-1 interactions has been shown to significantly inhibit neutrophil transmigration.33, 38 As PECAM-1 is expressed at endothelial cell junctions where transmigration occurs,39 it is possible that NB1 directs at least a subpopulation of PR3 molecules to these areas to aid in neutrophil diapedesis, perhaps through degrading junctional proteins or the extracellular matrix. Another possibility is that PR3 acts, with or without NB1, at the endothelial cell apical surface, where it can interact with endothelial cell receptors proximal to PECAM-1.
Similar to other serine proteases, PR3 has been reported to interact with protease activated receptors (PARs). PR3 has been shown to activate platelets,40 dendritic cells41 and endothelial cells42 through PAR-1 and PAR-2. Because members of the PAR family are associated with regulating vascular permeability, the potential for PR3 to act on these receptors suggests a possible mechanism for neutrophil regulation of barrier function. In the present study, we demonstrate that the serine protease PR3 is able to significantly enhance endothelial cell barrier function through a PAR-2-dependent pathway. In addition, we show that PR3 induces sustained endothelial cell calcium signaling, while at the same time inhibiting the permeability changes and disruption of endothelial cell junctional proteins induced by PAR-1 agonists.
Materials and Methods
Cell lines
Primary isolated human umbilical vein endothelial cells (HUVEC) were maintained in RPMI (Invitrogen) with 10% FBS, 2 mM L-glutamine and 500 µg/ml gentamycin. Cells were used between passages 3–4.
Antibodies
Antibodies against NB1 (MEM166), VE-cadherin (H-72), PR3 (PR3G-2), β-actin, PAR-1, and PAR-2 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). All secondary antibodies were obtained from Jackson Immuno Research (West Grove, PA).
Reagents
IL-1β was purchased from Peprotech (Rocky Hill, NJ). The protease inhibitors elafin and AEBSF were purchased from AnaSpec (San Jose, CA) and Roche (Mannheim, Germany), respectively. Peptide agonists for PAR-1 (SFLLRN) and PAR-2 (SLIGKV) were purchased from Peptides International (Louisville, KY). PR3 was purchased from Enzo LifeScience (Farmingdale, NY) and was isolated from human sputum with a reported purity of >95%. HNE and CG were purchased from EMD Millipore (Darmstadt, Germany). Soluble recombinant NB1 and NB1-IgG were kindly provided by Dr. Sentot Santoso (Justus Liebig University, Giessen, Germany). Fluo4 and BAPTA-AM were purchased from Invitrogen. Thapsigargin and ionomycin were purchased from Sigma-Aldrich.
Neutrophil isolation
Neutrophils were isolated as previously described 43. Briefly, blood from healthy, consenting adult donors was collected in vacutainer tubes (BD Bioscience, Franklin Lakes, NJ) using 2 mM EDTA as an anti-coagulant. Donors were later characterized as either NB1 positive or negative by flow cytometry. Whole blood was layered over a ficoll-histopaque gradient (Sigma) and centrifuged for 30 minutes at 1000×g. Neutrophils were isolated from the buffy coat layer and the volume brought to 10 ml with PBSA (Dulbecco’s PBS without calcium or magnesium with 0.1% BSA). Neutrophils were then washed twice with PBSA at 200 xg for 10 minutes before being quantified.
Electric Cell-Substrate Impedance Sensing (ECIS) measurements
ECIS measurements were performed in 8W10E+ electrode arrays on an ECIS Zè instrument (Applied Biophysics, Troy, NY). Cells were plated on arrays coated with 0.2% collagen (Sigma-Aldrich) and allowed to grow to confluence for 1–2 days. On the day of the experiment, the culture media was replaced with media containing 1% FBS. Resistance (in units of Ω) at 4000 Hz was recorded and baseline barrier function was normalized within each experiment to combine results from multiple experiments for analysis. Within each experiment all treatments were done in duplicate and the mean value was collected. Cells were stimulated with agonists for PAR-1 (SFLLRN, 1–10 µM), PAR-2 (SLIGKV, 1–10 µM) or serine proteases including PR3 (1-0.1 U/ml), cathepsin G (CG, 1-0.1 U/ml), human neutrophil elastase (HNE, 1-0.1 U/ml) or thrombin (1 U/ml).
ECIS with neutrophils
In some ECIS experiments endothelial cells were stimulated four hours with IL-1β (1 ng/ml) before the addition of neutrophils. This stimulation alone did not result in a significant change in vascular permeability (data not shown). Stimulated endothelial cells were then incubated with neutrophils from either NB1-positive or NB1-null individuals (1x106 cells). In some experiments neutrophils were incubated five minutes with the serine protease inhibitors AEBSF (10 µM) or elafin (2 µM) before being added to the endothelial cells. Incubation of these inhibitors alone did not significantly affect endothelial cell electrical resistance (data not shown).
Transfection of siRNA
HUVEC were transfected with siRNA for PAR-1 (100 nM, sc-36663), PAR-2 (100 nM, sc-36188) or control siRNA (Santa Cruz Biotechnologies, Santa Cruz, CA) using Lipofectamine (Invitrogen, Grand Island, NY) and following the manufacture’s protocol. After 48 hours siRNA inhibition of protein expression was determined by western blot analysis. Transfections were done in both 12 well plates as well as in 8W10E+ arrays used for ECIS.
Calcium measurements
Calcium signaling in endothelial cells was detected using the calcium sensitive dye Fluo4 (Invitrogen). Endothelial cells cultured in 2 well chamber slides were incubated 15 minutes with Fluo4 (10 µM). After Fluo4 loading endothelial cells were washed once, fresh media was added and the cells were transferred to a heated incubation chamber and observed using an Olympus FV1000 MPE confocal microscope (Center Valley, PA). After a baseline was established cells were treated with ionomycin, PAR-1 or PAR-2 peptide agonists or PR3. In some experiments, endothelial cells were pre-loaded with BAPTA-AM (10 µM) for 30 minutes. In other experiments cells were pre-treated with thapsigargin (1–10 µM) immediately prior to agonist stimulation. Calcium fluxes were recorded for ten minutes and later analyzed off- line using Olympus Fluoview software. For every experiment ten cells were randomly selected from each field of view for line scan analysis.
Confocal Microscopy
Confocal microscopy was used to identify morphological changes in endothelial cell VE-cadherin localization and disruption of cell-cell junctions. Endothelial cells were cultured on 8 chamber slides (1x105 cell/ml) until confluent. Monolayers were first pre- treated with PR3 (0.1 U/ml) or a PAR-2 peptide agonist (SLIGKV, 1 µM) for 15 minutes, then treated with a PAR-1 peptide agonist (SFLLRN, 1 µM). After 30 minutes cells were fixed with 2% paraformaldehyde for 2 hours at 4°C. The cells were then washed in PBS and permeabilized with ice-cold 0.5% Triton X-100 in PBS for 3 minutes and then washed with PBS. The cells were next blocked one hour at 37°C with 5% FBS in PBS. Cells were then stained with antibodies against VE-cadherin overnight at 4°C in PBS containing 0.3% bovine albumin (PBSA). After being washed with PBS the cells were stained one hour with Texas Red conjugated anti-rabbit antibodies at room temperature. The slides were then stained with DAPI (10 nM) for 10 minutes at room temperature. Slides were coverslipped using Prolong Gold antifade (Invitrogen; Eugene, OR) and analyzed for VE-cadherin localization using an Olympus FV1000 MPE confocal microscope.
Statistical analysis
Results, where applicable, are expressed as mean ± SEM. Statistical analysis was performed on GraphPad Prism 5 software (GraphPad Software, Inc., La Jolla, CA) and significance was determined using ANOVA and the Bonferroni post hoc test.
Results
Neutrophils and PR3 enhance endothelial cell barrier function
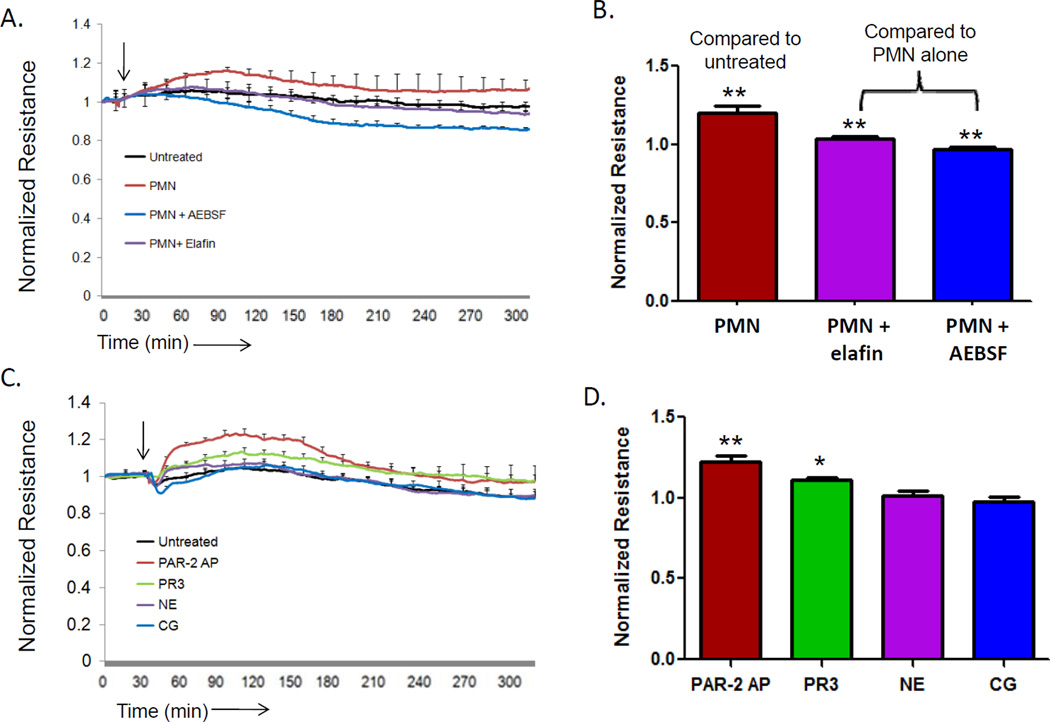
Neutrophils in contact with endothelial cells have been reported to enhance endothelial cell barrier function.18, 44 To confirm these findings, we cultured endothelial cells on gold-coated chamber slides, added neutrophils, and measured neutrophil-induced changes in barrier function using Electric-Cell substrate Impedance Sensing (ECIS). As shown in Figures 1A and B, addition of neutrophils induced an immediate enhancement in endothelial cell barrier function, as expected. In contrast, neutrophils added to endothelial cells in the presence of serine protease inhibitors (AEBSF, elafin) failed to augment junctional integrity. To explore directly the ability of neutrophil-derived serine proteases to mediate these effects, we incubated endothelial cells with cathepsin G (CG), neutrophil elastase (NE), proteinase 3 (PR3), or a positive control PAR-2 peptide agonist (SLIGKV) previously shown to enhance barrier function. 45 As shown in Figures 1C and D, both PR3 and the PAR-2 peptide agonist significantly increased endothelial cell monolayer integrity, while CG and NE had little effect. To determine if PR3 activity was required for the change in endothelial cell barrier function, PR3 was either heat-inactivated by boiling for 30 minutes or pre-treated with elafin (2 µM) for 15 minutes. In these conditions PR3 was unable to produce a change in barrier function compared to untreated cells (data not shown). These data demonstrate that both neutrophils and neutrophil-derived PR3 are able to enhance endothelial cell barrier function.
Figure 1. Neutrophil PR3 induces an increase in endothelial cell barrier function.
HUVEC (1x105) cultured on 8W10E+ arrays and barrier function was measured by ECIS. The arrow indicates the time of endothelial cell treatment. (A) HUVEC were stimulated four hours with IL-1β (1 ng/ml) before the addition of neutrophils (1x106) in the presence or absence of serine protease inhibitors AEBSF (10 µM) or elafin 2 µM). Results represent the mean ± SEM of two wells from one of four representative experiments. (B) Neutrophils caused a significant increase in endothelial cell barrier function compared to untreated endothelial cells. Inhibition of neutrophil serine proteases with elafin or AEBSF significantly reduced the increase in endothelial cell barrier function induced by neutrophils (mean ± SEM from four separate experiments, ** p <0.01). (C) Endothelial cells treated with a positive control PAR-2 activating peptide (SLIGKV, 10 µM), proteinase 3 (PR3, 0.1 U/ml), neutrophil elastase (NE, 0.1 U/ml) or cathepsin G (CG, 0.1 U/ml). Results represent the mean ± SEM of two wells from one of four representative experiments. (D) PAR-2 and PR3 induced a significant increase in endothelial cell barrier function 60 minutes after treatment (mean ± SEM from four separate experiments, ** p <0.01).
Role of neutrophil NB1 (CD177) in endothelial cell barrier function
Only 40–60% of the neutrophils in healthy individuals express NB1 on their surface, while 3–5% of the population (henceforth termed NB1-null individuals) do not express NB1 at all due to a frame-shift mutation leading to early termination of the NB1 transcript.46 Activated neutrophils secrete PR3, some of which rebinds to the cell surface in an NB1-dependent manner.
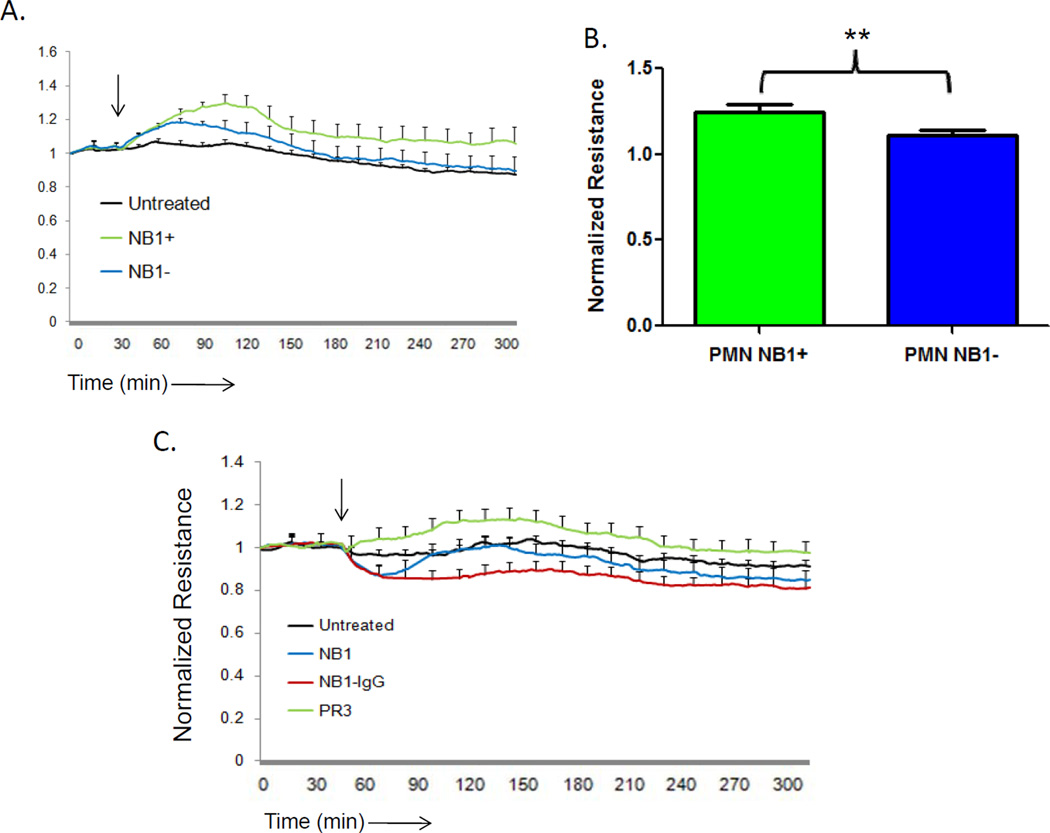
PR3/NB1 complex formation has also been shown to significantly increase on the neutrophil surface during the process of transendothelial migration. Although NB1-null individuals are unable to present PR3 on the neutrophil surface, they still express normal levels of PR3 in their intracellular granules (43). To determine whether NB1 plays a role in the ability of PR3 to promote an increase in endothelial cell barrier function, we incubated neutrophils derived from NB1-positive and NB1-null individuals with IL-1β -stimulated endothelial cells, and measured their impact on barrier function using ECIS. As shown in Figure 2A, although addition of neutrophils from either NB1-positive and NB1-null individuals increased endothelial cell barrier function, the increase was significantly greater with NB1-positive neutrophils (Figure 2B). To determine whether NB1 itself might be responsible for this enhancement of monolayer integrity, we incubated endothelial cells with soluble monovalent NB1 or bivalent NB1-IgG. As shown in Figure 2C, although PR3 was able to increase barrier function, NB1 alone, in either form was without an effect. These data demonstrate that NB1-bound PR3 can function as an effective positive modulator of vascular integrity.
Figure 2. Neutrophil expressed NB1 alone does not increase endothelial cell barrier function.
HUVEC (1x105) cultured on 8W10E+ arrays and barrier function was measured by ECIS. The arrow indicates the time of endothelial cell treatment. HUVEC were stimulated four hours with IL-1β (1 ng/ml) before the addition of neutrophils (1x106) from either NB1-positive or NB1-null individuals. Results represent the mean ± SEM of two wells from one of four representative experiments. (B) NB1-positive neutrophils promoted a significant increase in endothelial cell barrier function compared to NB1-negative neutrophils after 60 minutes of incubation (mean ± SEM from four separate experiments, ** p <0.01). (C) Monomeric NB1 (20 µg/ml) or bivalent NB1-IgG (20 µg/ml) did not promote an increase in endothelial cell barrier function. Results represent the mean ± SEM of two wells from one of three representative experiments.
PR3 promotes barrier function via its action on endothelial cell PAR-2
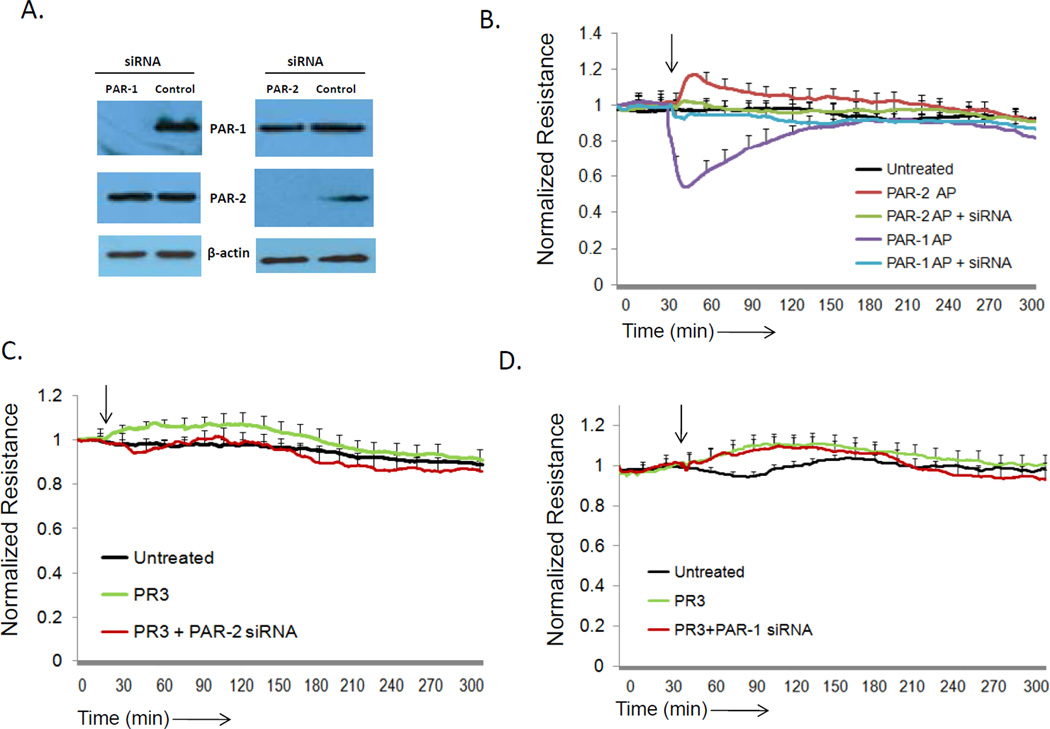
Endothelial cells express four protease activated receptors, but of these, only PAR-1 and PAR-2 have been shown to play a role in regulating vascular barrier function in response to serine proteases. Activation of PAR-1 by thrombin or thrombin agonist peptides induces rapid endothelial cell contraction and vascular permeability, however thrombin does not activate PAR-247. In contrast, PAR-2 can become activated by coagulation Factor Xa, and promotes endothelial cell barrier protection.48, 49 Factor VIIa has also been reported to enhance endothelial cell barrier function, but acts on PAR-1, rather than PAR-2, to exert its effects.50 To examine whether PR3 augments endothelial cell barrier function by acting on either PAR-1 or PAR-2, we examined its effects on endothelial cells in which protein expression of PAR-1 and PAR-2 had been knocked down using PAR-specific siRNAs (Figure 3A). As shown in Figure 3B, a PAR-1 agonist peptide was effective in disrupting endothelial cell monolayer integrity in wild-type endothelial cells and in endothelial cells in which PAR-2 had been knocked down, but had no effect in PAR-1-deficient endothelial cells, as expected. Siilarly, PAR-2-induced barrier protection was abrogated only in endothelial cells lacking PAR-2 expression, demonstrating the specificity of the assay system. Importantly, while PR3 was effective in augmenting barrier function in wild-type and PAR-1-deficient endothelial cell monolayers, it failed to have an effect on endothelial cells in which PAR-2 had been knocked down (Figures 3C and 3D). Although these experiments demonstrate the importance of endothelial PAR-2 expression for PR3- mediated barrier function, it was not clear if PR3 could cleave PAR-2 on the cell surface, a process required for physiological PAR-2 activation. To investigate this, human embryonic kidney (HEK) 293 cells were transfected with a PAR-2 alkaline phosphatase reporter construct (supplemental Figure 1). In cells incubated with PR3 we found significant PAR-2 cleavage which was completed abrogated in the presence of the inhibitor elafin. Therefore cell surface expressed PAR-2 is cleaved by PR3. These data demonstrate that the ability of PR3 to exert barrier protective effects is dependent upon endothelial cell expression of PAR-2, and not PAR-1.
Figure 3. PR3 promotes barrier function through PAR-2 activation on endothelial cells.
(A) HUVEC cultured on 12 well plates were treated with siRNA for PAR-1, PAR-2 or control siRNA. After 48 hours expression of PAR-1, PAR-2 and β-actin was determined by western blot. The siRNA for PAR-1 and PAR-2 specifically inhibited expression of their targets. (B-D) HUVEC (1x105) cultured on 8W10E+ arrays were treated with siRNA for PAR-1 or PAR- 2 and barrier function was measured by ECIS. The arrow indicates the time of endothelial cell treatment. (B) After 48 hours of siRNA knockdown endothelial cells were treated with PAR-1 agonist peptides (AP) (SFLLRN, 1 µM) or PAR-2 (SLIGKV, 1 µM) peptide agonists to determine the functional efficiency of PAR expression knockdown. (C) Endothelial cells treated with PAR-2 siRNA were stimulated with PR3. (D) Endothelial cells treated with PAR-1 siRNA were stimulated with PR3. Results represent the mean ± SEM of two wells from one of four representative experiments.
PR3 induces calcium signaling in endothelial cells
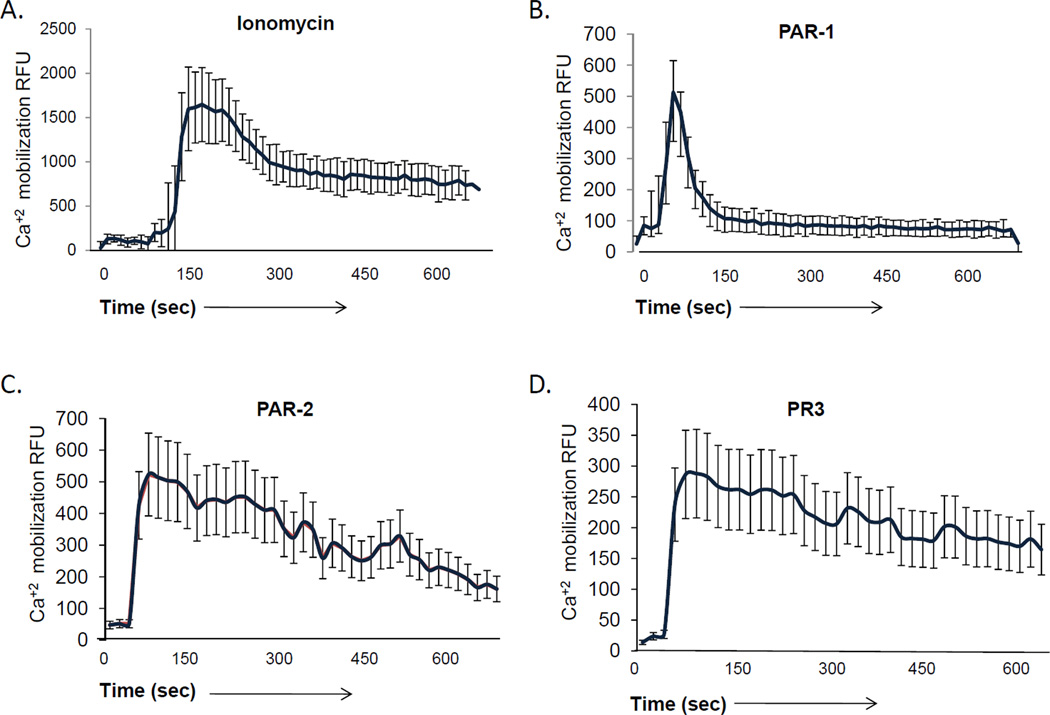
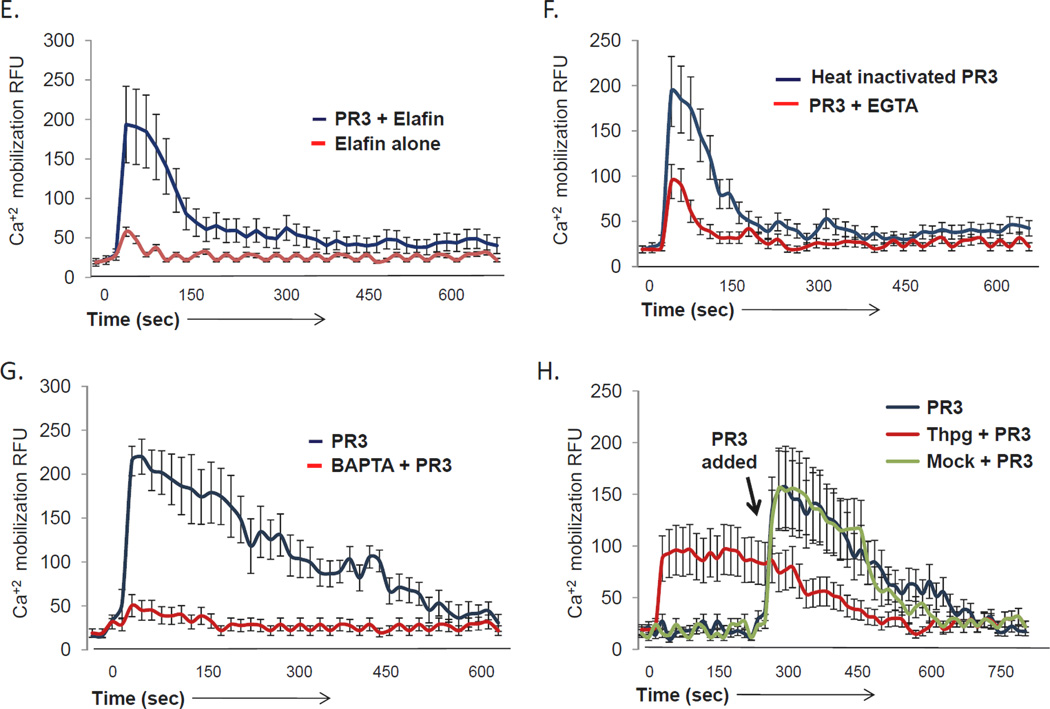
Many signaling events, including those downstream of PAR-1 and PAR-2 activation, require intracellular calcium signaling. To determine whether PR3 stimulation of endothelial cell PAR-2 results in release of calcium from intracellular stores, we loaded endothelial cell monolayers with Fluo4 and examined their responses to a series of agonists using confocal microscopy. As shown in Figure 4A, addition of the calcium ionophore ionomycin produced an immediate, robust, calcium flux, while stimulation with a PAR-1 agonist produced a characteristic, short-lived response (Figure 4B). In contrast, stimulation with a PAR-2 agonist (Figure 4C) promoted a sustained calcium flux, consistent with previous reports.51, 52 As shown in Figure 4D, PR3 produced both a rapid primary calcium signal as well as a sustained calcium response, consistent with its action on PAR-2 and not PAR-1. Chelating intracellular calcium with BAPTA-AM blocked both the primary and sustained responses (Figure 4G), while depletion of extracellular calcium with extracellular EGTA blocked only the sustained calcium signal induced by PR3 (Figure 4F), suggesting that the sustained signal required influx of extracellular stores. To determine if the proteolytic activity of PR3 was required for its ability to induce sustained calcium signaling, we inactivated PR3 by heat denaturing, or by pre-incubating it with the serine protease inhibitor, elafin. As shown in Figures 4E and 4F, although PR3 inactivated by either method was able to induce a primary calcium flux, both the amplitude and duration of the response were markedly blunted. However, inhibiting intracellular calcium reuptake with thapsigargin prior to addition of PR3 allowed cells to retain a sustained, albeit blunted, calcium flux (Figure 4H). Therefore PR3 signaling requires both intracellular and extracellular calcium stores to induce a sustained calcium response.
Figure 4. PR3 induces calcium signaling in endothelial cells.
HUVEC cultured on 2 chamber slides until confluent were loaded with the calcium sensitive dye Fluo4 (5 µM) for 30 minutes. The HUVEC were then examined for calcium signaling using confocal microscopy. Endothelial cells were treated with: (A) the calcium ionophore ionomycin (5 µM), agonist peptides (AP) for (B) PAR-1 (SFLLRN, 1 µM) or (C) PAR-2 (SLIGKV, 1 µM) or (D) PR3 (0.1 U/ml). In some experiments PR3 was inactivated by a 15 minute pre-incubation with elafin (E) or 30 minutes of boiling (F) before being added to the endothelial cells. In other experiments endothelial cells were treated with PR3 in presence of the calcium chelator EGTA (F) or with the intracellular calcium chelator BAPTA (G). Alternatively intracellular calcium was depleted using thapsigargin (Thpg) (H). Results represent the mean ± SEM of ten cells from one of six representative experiments.
PR3 signaling partially abrogates vascular permeability changes induced by PAR-1
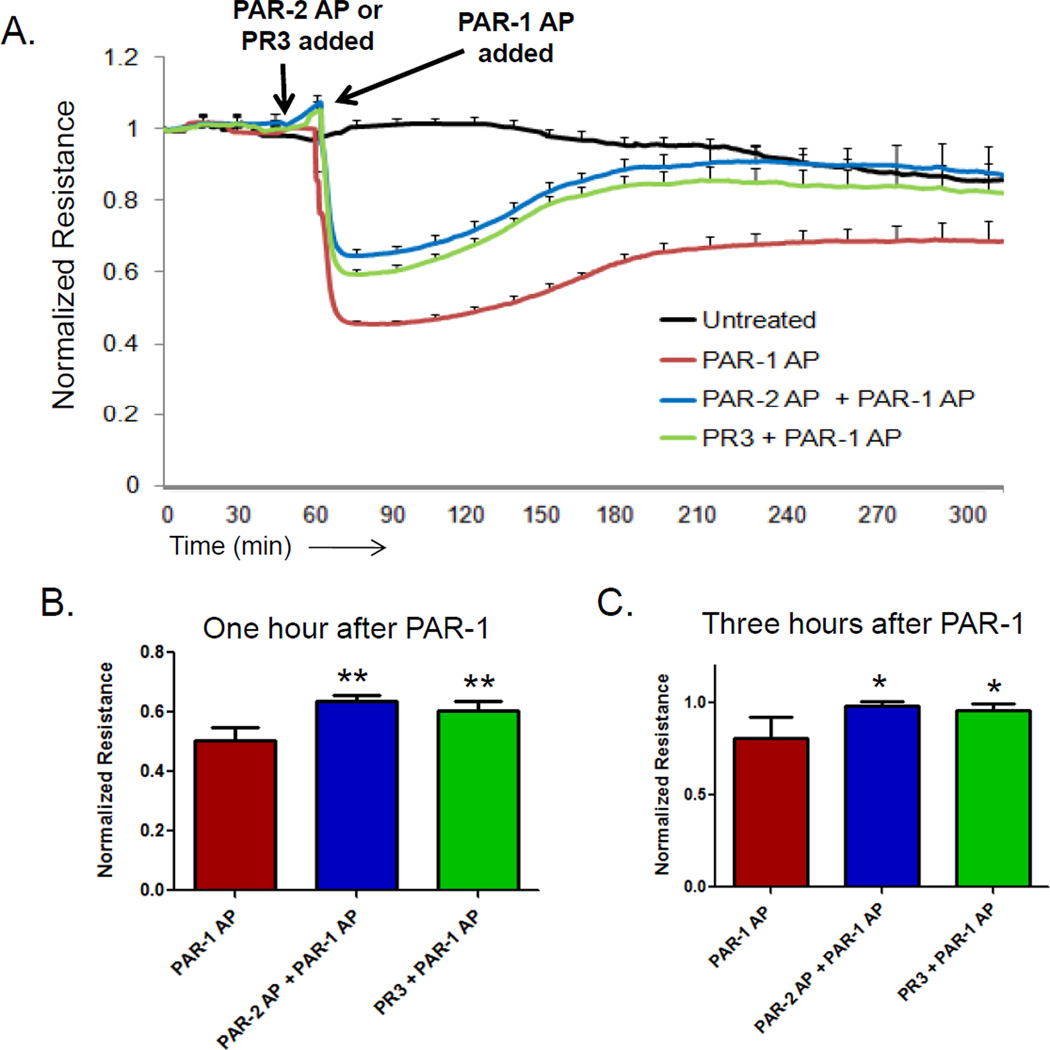
To determine whether PR3-induced activation of PAR-2 might be able to reverse or moderate the junctional disruption effects induced by PAR-1 agonists, we pretreated endothelial cell monolayers with PR3 or a PAR-2 peptide agonist 15 minutes before challenge with a PAR-1 agonist. As shown in Figure 5, PR3 or a PAR-2 agonist significantly inhibited PAR-1-induced permeability, blunting both the immediate increase in (Figures 5A and 5B), as well as markedly improving the rate at which vascular integrity was restored (Figure 5C). Therefore, neutrophil- derived PR3 not only enhances baseline endothelial cell barrier function (Figures 1–3), but can also ameliorate the loss of junctional integrity induced by agonists that act on PAR-1. One mechanism by which PAR-2 activation could inhibit PAR-1 induced permeability is by regulating the small GTPases Rac1 and RhoA. Rac1 activation downstream of PAR-2 is linked to increased barrier function, while RhoA activity following PAR-1 stimulation promotes vascular permeability. Using an assay specific for the active, GTP-bound forms of Rac1 and RhoA (Cytoskeleton Inc, Denver, CO) we observed active Rac1-GTP in endothelial cells treated with a PAR-2 agonist peptide or PR3, but no increase in RhoA-GTP (supplemental Figure 2) In contrast, a PAR-1 agonist peptide was able to significantly increase RhoA-GTP, but it did not increase Rac1-GTP binding. Therefore, the activation of Rac1 and inhibition of RhoA may be one pathway that PR3 incorporates to inhibit PAR-1 mediated vascular permeability.
Figure 5. PR3 inhibits PAR1 mediated vascular permeability.
HUVEC (1x105) were cultured on 8W10E+ arrays and barrier function was measured by ECIS. The arrows indicate the time of endothelial cell treatment. (A) Endothelial cells pre-treated with PAR-2 agonist peptide (AP) (SLIGKV, 1 µM) or PR3 (0.1 U/ml) for 15 minutes before being treated with PAR-1 agonist peptide (AP) (SFLLRN, 1 µM). Endothelial cell pre-treatment with PAR-2 AP or PR3 significantly inhibited PAR-1 AP induced barrier function after 1 hour (B) and promoted barrier function as long as 3 hours (C) after treatment. Results represent the mean ± SEM of two wells from one of four representative experiments. (mean ± SEM from four separate experiments, ** p <0.01).
PR3 inhibits PAR-1 cytoskeletal changes in endothelial cells
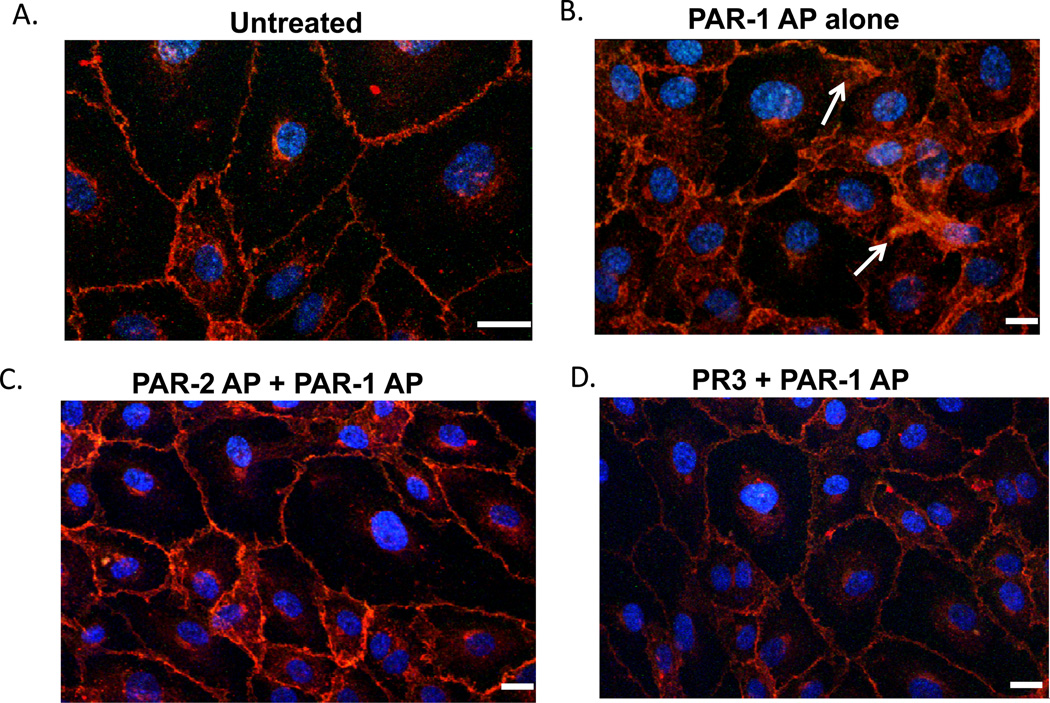
Vascular permeability involves both cytoskeletal changes and reorganization of intracellular junctions, both of which contribute to morphological changes, including formation of gaps and interdigitating membrane protrusions at cell-cell borders that can be easily visualized using fluorescent microscopy. To further explore the ability of PR3 to inhibit PAR-1 signaling, we examined the localization of VE-cadherin in resting and agonist-treated endothelial cell monolayers. As shown in Figure 6A, VE-cadherin is present at cell junctions as a tight, uninterrupted band in untreated endothelial cell monolayers. Following stimulation with a PAR-1 agonist, VE-cadherin localizes to membrane ruffles and cellular protrusions, indicative of morphological changes that result in increased vascular permeability (Figure 6B). When endothelial cells were pre-treated with PR3 or a PAR-2 agonist before PAR-1 stimulation, however, we observed a dramatic inhibition of gap formation and membrane ruffling (Figures 6C, D). This data suggests that the improvement in barrier function conferred by PR3 or PAR-2 agonist peptide treatment is due to stabilization of VE-cadherin-containing endothelial cell-cell junctions.
Figure 6. PR3 inhibits PAR1 changes in VE-cadherin localization.
Confluent monolayers of HUVEC cultured on 8 chamber glass slides were stimulated with a PAR-1 peptide agonist (SFLLR, 1 µM) for 30 minutes. In some experiments, HUVEC were pre-treated 15 minutes with PR3 (0.1 U/ml) before PAR-1 stimulation. After 30 minutes cells were fixed and stained with antibodies against the junction protein VE-cadherin (red) and nuclei were stained with DAPI (Blue). PAR-1-stimulated HUVEC demonstrated intercellular gap formation and cellular interdigitations characteristic of decreased vascular barrier function (indicated by arrows). However, endothelial cells pre-treated with PR3 or PAR-2 peptide agonist before PAR-1 stimulation showed an absence of VE-cadherin disruption. Results are representative of five separate experiments. Scale 10 microns.
Discussion
Though transmigrating leukocytes disrupt endothelial cell junctions and degrade extracellular matrix proteins during the process of diapedesis,53 endothelial cell barrier function has been reported to actually increase during the process.16, 18, 44 The mechanisms by which neutrophils maintain, and later augment, vascular integrity during transmigration are not well understood. In the present study, we demonstrate that the neutrophil serine protease PR3 acts on PAR-2 to enhance endothelial cell barrier function, revealing a novel pathway for the maintenance of vascular integrity. Furthermore, PR3 stimulation of endothelial cells can counter vascular permeability induced by stimulating the thrombin receptor, PAR-1. These data suggest a heretofore unappreciated mechanism that neutrophils employ to maintain the integrity of the vascular endothelium during diapedesis during the inflammatory response.
Activation of endothelial cells by PR3 was found to require endothelial cell expression of PAR-2, a protease-activated receptor capable of enhancing barrier function. Endothelial cells have been reported to express all four protease activated receptors54, with PAR-2 functioning to promote endothelial cell barrier function55, while PAR-1 either increasing or decreasing endothelial cell barrier function, depending on the stimulus49. The role of endothelial cell PAR-3 and PAR-4 in regulating endothelial cell barrier integrity are less clear. PAR-2 can be activated by Factor Xa, Tissue Factor/VIIa, or tertiary complexes of these molecules56, 57 as well as the serine proteases trypsin58 and tryptase59. Neutrophil serine proteases have been reported to interact with PAR-1 receptors; NE and CG have been shown to cleave and inactivate PAR-1.60, 61 NE and CG have been shown previously to promote vascular permeability,62–65 but this may be through PAR-1 independent pathways. For example, CG has been reported to activate PAR-466, but whether this contributes to vascular permeability is unknown. Similar to CG and NE, PR3 has been reported to inactivate PAR-1, in addition to activating PAR-2. PR3 cleavage of PAR-1 between Val72-Ser73 inhibits activation of the receptor by thrombin, although PAR-1 remains responsive to peptide agonists.61 In the present study, we found that PR3 was an efficient promoter of barrier function, and its effects required PAR-2, but not PAR-1, expression by endothelial cells. We also confirmed that PR3 could cleave PAR-2 when it is expressed on the cell surface (supplemental Figure 1). However, we do not know at this time if PR3 cleaves at the canonical Arg36-Ser37 PAR-2 activation site. It may be that PR3 activation of PAR-2 occurs outside of this region. Neutrophil elastase has been reported to activate PAR-2 by cleaving outside the Arg36-Ser37 cleavage site, but this does not promote endothelial cell barrier function67. Therefore it is possible that PR3 may also activate PAR-2 by cleaving at alternative cleavage sites and this deserves future study. In addition to its action on PAR-2, PR3 has also been reported to inactivate the Endothelial Cell Protein C Receptor (EPCR).68 This is interesting since activated Protein C (aPC) has been shown to promote endothelial cell barrier function through the EPCR/PAR-1 axis.69 It is unlikely that PR3 modulates EPCR function, since loss of PAR-1 had little effect on the ability of PR3 to modulate endothelial cell barrier integrity (Figure 3). Should PR3 cleave EPCR in vivo, however, it would produce the interesting scenario in which it both increases endothelial barrier function by activating PAR-2 while simultaneously inhibiting the ability of APC to activate PAR-1 activity. Whether these events occur in vivo may depend on temporal and spatially-dependant conditions.
We also observed that PR3 promotes sustained calcium signaling in endothelial cells (Figure 4). The observation that protease inhibitors or heat inactivation blocks the ability of PR3 to induce sustained cytosolic calcium flux, with only a moderate effect on immediate PR3-induced calcium transients (Figures 4E, 4F), suggests that PR3 might also be interacting with endothelial cells in a manner independent of its proteolytic. PR3 has been reported to bind to integrins70, 71 and the endothelial cell protein C receptor.71 Precisely how PR3 interacts with endothelial cells to exert enzyme activity-independent responses may be an interesting topic of future investigation.
Thrombin generation occurs in many inflammatory conditions such as sepsis where increased vascular permeability is associated with disease severity. In some of these inflammatory conditions there is a large influx of neutrophils, therefore, neutrophils recruited to these areas may play a role in helping block or mitigate endothelial cell permeability induced by thrombin signaling through PAR-1. There is also a significant increase in endothelial cell expression of PAR-2 during sepsis, while PAR-1 levels are not affected.72 Likewise, NB1 expression on circulating neutrophils has been reported to significantly increased during sepsis.73 Since NB1 is required for expression of PR3 on the neutrophil cell surface, this suggests that during sepsis there may be increased PR3 surface expression on neutrophils. Coupled with increased endothelial cell PAR-2 expression, this could play a role in helping neutrophils promote vascular integrity during inflammatory responses or sepsis. Whether PR3 and NB1 play a role in this regard is an area for future investigation.
Both soluble and NB1-bound PR3 can enhance barrier function (Figure 1). The significance of PR3 interacting with NB1 is two-fold. First, PR3 interacting with NB1 is protected from proteolytic inactivation by inhibitors such as α1-antitrypsin37. PR3 is therefore able to have a sustained affect, under conditions where other serine proteases (e.g. NE and CG) become inactivated. Secondly, NB1 is a receptor for endothelial cell PECAM-1, suggesting that PR3 may be presented to the endothelium at cell-cell junctions where PECAM-1 is localized. In this regard, PR3 might play a role in neutrophil transmigration by degrading proteins at cell borders.(28) Alternatively NB1, through its interaction with PECAM-1, may present PR3 to endothelial receptors in the vicinity of PECAM-1. For example, PECAM-1 and PAR-1 have been shown to localize to lipid rafts 74 where PR3 may be able to interact with and inactivate PAR-1. Likewise, if PAR-2 is also found in lipid rafts it may be in close proximity to PECAM-1 and be activated by the NB1-PR3 complex. In future studies we hope to explore the role of NB1- PECAM-1 interactions in promoting PR3 activation of PAR-2.
Approximately 3–5% of the population do not express NB1 on their neutrophils.75 We found that although NB1-null neutrophils were able to enhance barrier function (Figure 1), the degree to which it had an effect was significantly diminished compared with the effects of neutrophils from NB1+ individuals. The observation that NB1 is required for PR3 surface expression suggests either that 1) PR3 secreted from NB1-negative neutrophils becomes sufficiently concentrated at the endothelial cell surface to retain some of its barrier-promoting activities even in the absence of NB1, or 2) NB1- neutrophils have the capacity to employ an alternative, PR3- independent, mechanism to re-seal endothelial cell junctions during diapedesis. Because no other serine proteases have been shown to promote barrier function, PR3 is likely to be the major contributor to vascular integrity during neutrophil transmigration. However, we cannot discount the possibility that a compensatory mechanism exists in NB1-null individuals.
One of the most intriguing results from the current study is the observation that PR3 reduced the extent to which PAR-1 agonists disrupt the vascular permeability barrier. Thus, pre-treatment of endothelial cells with PR3 or a PAR-2 agonist not only suppressed the initial permeability change following PAR-1 stimulation, they also restored barrier function significantly faster that cells stimulated with PAR-1 agonists (Figure 5). The mechanism by which PR3 exerted these effects is suggested by the observation that PR3 inhibited PAR-1-mediated cytoskeletal reorganization and endothelial cell gap formation (Figure 6). The signaling pathway from PR3 to the cytoskeleton remains to be investigated.
In summary, we have found that PR3 plays a significant role in promoting vascular integrity by signaling through endothelial cell PAR-2. Our study describes a unique mechanism in which neutrophils promote endothelial cell barrier function during transmigration as well as inhibiting permeability induced by thrombin receptor signaling. These findings suggest that neutrophils may play a much more significant role in regulating hemostasis and vascular function than is currently believed.
Supplementary Material
Acknowledgments
We want to thank Dr. Sentot Santoso at Justus Liebig University, Giessen, Germany for providing our lab with soluble NB1. We thank Dr. Brian Curtis, Director of the Platelet and Neutrophil Immunology Lab, for providing us with neutrophils from NB1-null patients. We also wish to thank Dr. Ali Rezaie at the Saint Louis University School of Medicine for providing our lab with the PAR-2-ALP constructs.
Sources of Funding
This work was funded by the NIH Training Grant HL-07209 and grant HL40926 from the National Heart, Lung and Blood Institute of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors have nothing to disclose.
Reference List
- 1.Pober JS, Sessa WC. Evolving functions of endothelial cells in inflammation. Nat Rev Immunol. 2007 Oct;7(10):803–815. doi: 10.1038/nri2171. [DOI] [PubMed] [Google Scholar]
- 2.Cines DB, Pollak ES, Buck CA, Loscalzo J, Zimmerman GA, McEver RP, Pober JS, Wick TM, Konkle BA, Schwartz BS, Barnathan ES, McCrae KR, Hug BA, Schmidt AM, Stern DM. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood. 1998 May 15;91(10):3527–3561. [PubMed] [Google Scholar]
- 3.Zhu L, Castranova V, He P. fMLP-stimulated neutrophils increase endothelial (Ca2+)i and microvessel permeability in the absence of adhesion: role of reactive oxygen species. Am J Physiol Heart Circ Physiol. 2005 Mar;288(3):H1331–H1338. doi: 10.1152/ajpheart.00802.2004. [DOI] [PubMed] [Google Scholar]
- 4.DiStasi MR, Ley K. Opening the flood-gates: how neutrophil-endothelial interactions regulate permeability. Trends Immunol. 2009 Nov;30(11):547–556. doi: 10.1016/j.it.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Breslin JW, Sun H, Xu W, Rodarte C, Moy AB, Wu MH, Yuan SY. Involvement of ROCK-mediated endothelial tension development in neutrophil-stimulated microvascular leakage. Am J Physiol Heart Circ Physiol. 2006 Feb;290(2):H741–H750. doi: 10.1152/ajpheart.00238.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Breslin JW, Yuan SY. Involvement of RhoA and Rho kinase in neutrophil-stimulated endothelial hyperpermeability. Am J Physiol Heart Circ Physiol. 2004 Mar;286(3):H1057–H1062. doi: 10.1152/ajpheart.00841.2003. [DOI] [PubMed] [Google Scholar]
- 7.Gautam N, Herwald H, Hedqvist P, Lindbom L. Signaling via beta(2) integrins triggers neutrophil-dependent alteration in endothelial barrier function. J Exp Med. 2000 Jun 5;191(11):1829–1839. doi: 10.1084/jem.191.11.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hernandez LA, Grisham MB, Twohig B, Arfors KE, Harlan JM, Granger DN. Role of neutrophils in ischemia-reperfusion-induced microvascular injury. Am J Physiol. 1987 Sep;253(3 Pt 2):H699–H703. doi: 10.1152/ajpheart.1987.253.3.H699. [DOI] [PubMed] [Google Scholar]
- 9.Del MA, Zanetti A, Corada M, Rival Y, Ruco L, Lampugnani MG, Dejana E. Polymorphonuclear leukocyte adhesion triggers the disorganization of endothelial cell-tocell adherens junctions. J Cell Biol. 1996 Oct;135(2):497–510. doi: 10.1083/jcb.135.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lewis RE, Granger HJ. Diapedesis and the permeability of venous microvessels to protein macromolecules: the impact of leukotriene B4 (LTB4) Microvasc Res. 1988 Jan;35(1):27–47. doi: 10.1016/0026-2862(88)90048-9. [DOI] [PubMed] [Google Scholar]
- 11.Shaw SK, Bamba PS, Perkins BN, Luscinskas FW. Real-time imaging of vascular endothelial-cadherin during leukocyte transmigration across endothelium. J Immunol. 2001 Aug 15;167(4):2323–2330. doi: 10.4049/jimmunol.167.4.2323. [DOI] [PubMed] [Google Scholar]
- 12.Phillipson M, Kaur J, Colarusso P, Ballantyne CM, Kubes P. Endothelial domes encapsulate adherent neutrophils and minimize increases in vascular permeability in paracellular and transcellular emigration. PLoS One. 2008;3(2):e1649. doi: 10.1371/journal.pone.0001649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hurley JV. Acute Inflammation: The effect of concurrent leucocytic emigration and increased permeability on particle retention by the vascular wall. Br J Exp Pathol. 1964 Dec;45:627–633. [PMC free article] [PubMed] [Google Scholar]
- 14.Thureson-Klein A, Hedqvist P, Lindbom L. Leukocyte diapedesis and plasma extravasation after leukotriene B4: lack of structural injury to the endothelium. Tissue Cell. 1986;18(1):1–12. doi: 10.1016/0040-8166(86)90002-9. [DOI] [PubMed] [Google Scholar]
- 15.Zeng M, Zhang H, Lowell C, He P. Tumor necrosis factor-alpha-induced leukocyte adhesion and microvessel permeability. Am J Physiol Heart Circ Physiol. 2002 Dec;283(6):H2420–H2430. doi: 10.1152/ajpheart.00787.2001. [DOI] [PubMed] [Google Scholar]
- 16.He P. Leucocyte/endothelium interactions and microvessel permeability: coupled or uncoupled? Cardiovasc Res. 2010 Jul 15;87(2):281–290. doi: 10.1093/cvr/cvq140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim MH, Liu W, Borjesson DL, Curry FR, Miller LS, Cheung AL, Liu FT, Isseroff RR, Simon SI. Dynamics of neutrophil infiltration during cutaneous wound healing and infection using fluorescence imaging. J Invest Dermatol. 2008 Jul;128(7):1812–1820. doi: 10.1038/sj.jid.5701223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burns AR, Bowden RA, MacDonell SD, Walker DC, Odebunmi TO, Donnachie EM, Simon SI, Entman ML, Smith CW. Analysis of tight junctions during neutrophil transendothelial migration. J Cell Sci. 2000 Jan;113(Pt 1):45–57. doi: 10.1242/jcs.113.1.45. [DOI] [PubMed] [Google Scholar]
- 19.Inglis VI, Jones MP, Tse AD, Easton AS. Neutrophils both reduce and increase permeability in a cell culture model of the blood-brain barrier. Brain Res. 2004 Feb 20;998(2):218–229. doi: 10.1016/j.brainres.2003.11.031. [DOI] [PubMed] [Google Scholar]
- 20.Massberg S, Grahl L, von Bruehl ML, Manukyan D, Pfeiler S, Goosmann C, Brinkmann V, Lorenz M, Bidzhekov K, Khandagale AB, Konrad I, Kennerknecht E, Reges K, Holdenrieder S, Braun S, Reinhardt C, Spannagl M, Preissner KT, Engelmann B. Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases. Nat Med. 2010 Aug;16(8):887–896. doi: 10.1038/nm.2184. [DOI] [PubMed] [Google Scholar]
- 21.Hirche TO, Benabid R, Deslee G, Gangloff S, Achilefu S, Guenounou M, Lebargy F, Hancock RE, Belaaouaj A. Neutrophil elastase mediates innate host protection against Pseudomonas aeruginosa. J Immunol. 2008 Oct 1;181(7):4945–4954. doi: 10.4049/jimmunol.181.7.4945. [DOI] [PubMed] [Google Scholar]
- 22.Standish AJ, Weiser JN. Human neutrophils kill Streptococcus pneumoniae via serine proteases. J Immunol. 2009 Aug 15;183(4):2602–2609. doi: 10.4049/jimmunol.0900688. [DOI] [PubMed] [Google Scholar]
- 23.Belaaouaj A, McCarthy R, Baumann M, Gao Z, Ley TJ, Abraham SN, Shapiro SD. Mice lacking neutrophil elastase reveal impaired host defense against gram negative bacterial sepsis. Nat Med. 1998 May;4(5):615–618. doi: 10.1038/nm0598-615. [DOI] [PubMed] [Google Scholar]
- 24.Weinrauch Y, Drujan D, Shapiro SD, Weiss J, Zychlinsky A. Neutrophil elastase targets virulence factors of enterobacteria. Nature. 2002 May 2;417(6884):91–94. doi: 10.1038/417091a. [DOI] [PubMed] [Google Scholar]
- 25.Rao NV, Wehner NG, Marshall BC, Gray WR, Gray BH, Hoidal JR. Characterization of proteinase-3 (PR-3), a neutrophil serine proteinase. Structural and functional properties. J Biol Chem. 1991 May 25;266(15):9540–9548. [PubMed] [Google Scholar]
- 26.Steadman R, Irwin MH, St John PL, Blackburn WD, Heck LW, Abrahamson DR. Laminin cleavage by activated human neutrophils yields proteolytic fragments with selective migratory properties. J Leukoc Biol. 1993 Apr;53(4):354–365. doi: 10.1002/jlb.53.4.354. [DOI] [PubMed] [Google Scholar]
- 27.Capodici C, Berg RA. Cathepsin G degrades denatured collagen. Inflammation. 1989 Apr;13(2):137–145. doi: 10.1007/BF00924785. [DOI] [PubMed] [Google Scholar]
- 28.Young RE, Voisin MB, Wang S, Dangerfield J, Nourshargh S. Role of neutrophil elastase in LTB4-induced neutrophil transmigration in vivo assessed with a specific inhibitor and neutrophil elastase deficient mice. Br J Pharmacol. 2007 Jul;151(5):628–637. doi: 10.1038/sj.bjp.0707267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Voisin MB, Woodfin A, Nourshargh S. Monocytes and neutrophils exhibit both distinct and common mechanisms in penetrating the vascular basement membrane in vivo. Arterioscler Thromb Vasc Biol. 2009 Aug;29(8):1193–1199. doi: 10.1161/ATVBAHA.109.187450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Woodman RC, Johnston B, Hickey MJ, Teoh D, Reinhardt P, Poon BY, Kubes P. The functional paradox of CD43 in leukocyte recruitment: a study using CD43-deficient mice. J Exp Med. 1998 Dec 7;188(11):2181–2186. doi: 10.1084/jem.188.11.2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cepinskas G, Sandig M, Kvietys PR. PAF-induced elastase-dependent neutrophil transendothelial migration is associated with the mobilization of elastase to the neutrophil surface and localization to the migrating front. J Cell Sci. 1999 Jun;112(Pt 12):1937–1945. doi: 10.1242/jcs.112.12.1937. [DOI] [PubMed] [Google Scholar]
- 32.Young RE, Thompson RD, Larbi KY, La M, Roberts CE, Shapiro SD, Perretti M, Nourshargh S. Neutrophil elastase (NE)-deficient mice demonstrate a nonredundant role for NE in neutrophil migration, generation of proinflammatory mediators, and phagocytosis in response to zymosan particles in vivo. J Immunol. 2004 Apr 1;172(7):4493–4502. doi: 10.4049/jimmunol.172.7.4493. [DOI] [PubMed] [Google Scholar]
- 33.Kuckleburg CJ, Tilkens SB, Santoso S, Newman PJ. Proteinase 3 Contributes to Transendothelial Migration of NB1-Positive Neutrophils. J Immunol. 2012 Mar 1;188(5):2419–2426. doi: 10.4049/jimmunol.1102540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hermant B, Bibert S, Concord E, Dublet B, Weidenhaupt M, Vernet T, Gulino-Debrac D. Identification of proteases involved in the proteolysis of vascular endothelium cadherin during neutrophil transmigration. J Biol Chem. 2003 Apr 18;278(16):14002–14012. doi: 10.1074/jbc.M300351200. [DOI] [PubMed] [Google Scholar]
- 35.Rest RF. Human neutrophil and mast cell proteases implicated in inflammation. Methods Enzymol. 1988;163:309–327. doi: 10.1016/0076-6879(88)63030-8. [DOI] [PubMed] [Google Scholar]
- 36.Williams SE, Brown TI, Roghanian A, Sallenave JM. SLPI and elafin: one glove, many fingers. Clin Sci (Lond) 2006 Jan;110(1):21–35. doi: 10.1042/CS20050115. [DOI] [PubMed] [Google Scholar]
- 37.Campbell EJ, Campbell MA, Owen CA. Bioactive proteinase 3 on the cell surface of human neutrophils: quantification, catalytic activity, and susceptibility to inhibition. J Immunol. 2000 Sep 15;165(6):3366–3374. doi: 10.4049/jimmunol.165.6.3366. [DOI] [PubMed] [Google Scholar]
- 38.Sachs UJ, Andrei-Selmer CL, Maniar A, Weiss T, Paddock C, Orlova VV, Choi EY, Newman PJ, Preissner KT, Chavakis T, Santoso S. The neutrophil-specific antigen CD177 is a counter-receptor for platelet endothelial cell adhesion molecule-1 (CD31) J Biol Chem. 2007 Aug 10;282(32):23603–23612. doi: 10.1074/jbc.M701120200. [DOI] [PubMed] [Google Scholar]
- 39.Sun J, Paddock C, Shubert J, Zhang HB, Amin K, Newman PJ, Albelda SM. Contributions of the extracellular and cytoplasmic domains of platelet-endothelial cell adhesion molecule-1 (PECAM-1/CD31) in regulating cell-cell localization. J Cell Sci. 2000 Apr;113(Pt 8):1459–1469. doi: 10.1242/jcs.113.8.1459. [DOI] [PubMed] [Google Scholar]
- 40.Renesto P, Halbwachs-Mecarelli L, Nusbaum P, Lesavre P, Chignard M. Proteinase 3. A neutrophil proteinase with activity on platelets. J Immunol. 1994 May 1;152(9):4612–4617. [PubMed] [Google Scholar]
- 41.Jiang B, Grage-Griebenow E, Csernok E, Butherus K, Ehlers S, Gross WL, Holle JU. The role of proteinase 3 (PR3) and the protease-activated receptor-2 (PAR-2) pathway in dendritic cell (DC) maturation of human-DC-like monocytes and murine DC. Clin Exp Rheumatol. 2010 Jan;28(1 Suppl 57):56–61. [PubMed] [Google Scholar]
- 42.Steppich BA, Seitz I, Busch G, Stein A, Ott I. Modulation of tissue factor and tissue factor pathway inhibitor-1 by neutrophil proteases. Thromb Haemost. 2008 Dec;100(6):1068–1075. [PubMed] [Google Scholar]
- 43.Buttrum SM, Hatton R, Nash GB. Selectin-mediated rolling of neutrophils on immobilized platelets. Blood. 1993 Aug 15;82(4):1165–1174. [PubMed] [Google Scholar]
- 44.Inglis VI, Jones MP, Tse AD, Easton AS. Neutrophils both reduce and increase permeability in a cell culture model of the blood-brain barrier. Brain Res. 2004 Feb 20;998(2):218–229. doi: 10.1016/j.brainres.2003.11.031. [DOI] [PubMed] [Google Scholar]
- 45.McLaughlin JN, Shen L, Holinstat M, Brooks JD, Dibenedetto E, Hamm HE. Functional selectivity of G protein signaling by agonist peptides and thrombin for the protease-activated receptor-1. J Biol Chem. 2005 Jul 1;280(26):25048–25059. doi: 10.1074/jbc.M414090200. [DOI] [PubMed] [Google Scholar]
- 46.Wolff J, Brendel C, Fink L, Bohle RM, Kissel K, Bux J. Lack of NB1 GP (CD177/HNA-2a) gene transcription in NB1 GP-neutrophils from NB1 GP-expressing individuals and association of low expression with NB1 gene polymorphisms. Blood. 2003 Jul 15;102(2):731–733. doi: 10.1182/blood-2002-09-2831. [DOI] [PubMed] [Google Scholar]
- 47.Fox MT, Harriott P, Walker B, Stone SR. Identification of potential activators of proteinase-activated receptor-2. FEBS Lett. 1997 Nov 17;417(3):267–269. doi: 10.1016/s0014-5793(97)01298-2. [DOI] [PubMed] [Google Scholar]
- 48.Bae JS, Yang L, Rezaie AR. Lipid raft localization regulates the cleavage specificity of protease activated receptor 1 in endothelial cells. J Thromb Haemost. 2008 Jun;6(6):954–961. doi: 10.1111/j.1538-7836.2008.02924.x. [DOI] [PubMed] [Google Scholar]
- 49.Feistritzer C, Lenta R, Riewald M. Protease-activated receptors-1 and -2 can mediate endothelial barrier protection: role in factor Xa signaling. J Thromb Haemost. 2005 Dec;3(12):2798–2805. doi: 10.1111/j.1538-7836.2005.01610.x. [DOI] [PubMed] [Google Scholar]
- 50.Sen P, Gopalakrishnan R, Kothari H, Keshava S, Clark CA, Esmon CT, Pendurthi UR, Rao LV. Factor VIIa bound to endothelial cell protein C receptor activates protease activated receptor-1 and mediates cell signaling and barrier protection. Blood. 2011 Mar 17;117(11):3199–3208. doi: 10.1182/blood-2010-09-310706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Domotor E, Bartha K, Machovich R, Adam-Vizi V. Protease-activated receptor-2 (PAR-2) in brain microvascular endothelium and its regulation by plasmin and elastase. J Neurochem. 2002 Mar;80(5):746–754. doi: 10.1046/j.0022-3042.2002.00759.x. [DOI] [PubMed] [Google Scholar]
- 52.Molino M, Woolkalis MJ, Reavey-Cantwell J, Pratico D, Andrade-Gordon P, Barnathan ES, Brass LF. Endothelial cell thrombin receptors and PAR-2. Two protease-activated receptors located in a single cellular environment. J Biol Chem. 1997 Apr 25;272(17):11133–11141. doi: 10.1074/jbc.272.17.11133. [DOI] [PubMed] [Google Scholar]
- 53.Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol Rev. 2006 Jan;86(1):279–367. doi: 10.1152/physrev.00012.2005. [DOI] [PubMed] [Google Scholar]
- 54.Jesmin S, Gando S, Zaedi S, Sakuraya F. Differential expression, time course and distribution of four PARs in rats with endotoxin-induced acute lung injury. Inflammation. 2007 Apr;30(1–2):14–27. doi: 10.1007/s10753-006-9017-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Klarenbach SW, Chipiuk A, Nelson RC, Hollenberg MD, Murray AG. Differential actions of PAR2 and PAR1 in stimulating human endothelial cell exocytosis and permeability: the role of Rho-GTPases. Circ Res. 2003 Feb 21;92(3):272–278. doi: 10.1161/01.res.0000057386.15390.a3. [DOI] [PubMed] [Google Scholar]
- 56.Riewald M, Ruf W. Mechanistic coupling of protease signaling and initiation of coagulation by tissue factor. Proc Natl Acad Sci U S A. 2001 Jul 3;98(14):7742–7747. doi: 10.1073/pnas.141126698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Camerer E, Kataoka H, Kahn M, Lease K, Coughlin SR. Genetic evidence that protease-activated receptors mediate factor Xa signaling in endothelial cells. J Biol Chem. 2002 May 3;277(18):16081–16087. doi: 10.1074/jbc.M108555200. [DOI] [PubMed] [Google Scholar]
- 58.Kong W, McConalogue K, Khitin LM, Hollenberg MD, Payan DG, Bohm SK, Bunnett NW. Luminal trypsin may regulate enterocytes through proteinase-activated receptor 2. Proc Natl Acad Sci U S A. 1997 Aug 5;94(16):8884–8889. doi: 10.1073/pnas.94.16.8884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Molino M, Barnathan ES, Numerof R, Clark J, Dreyer M, Cumashi A, Hoxie JA, Schechter N, Woolkalis M, Brass LF. Interactions of mast cell tryptase with thrombin receptors and PAR-2. J Biol Chem. 1997 Feb 14;272(7):4043–4049. doi: 10.1074/jbc.272.7.4043. [DOI] [PubMed] [Google Scholar]
- 60.Molino M, Blanchard N, Belmonte E, Tarver AP, Abrams C, Hoxie JA, Cerletti C, Brass LF. Proteolysis of the human platelet and endothelial cell thrombin receptor by neutrophil-derived cathepsin G. J Biol Chem. 1995 May 12;270(19):11168–11175. doi: 10.1074/jbc.270.19.11168. [DOI] [PubMed] [Google Scholar]
- 61.Renesto P, Si-Tahar M, Moniatte M, Balloy V, Van DA, Pidard D, Chignard M. Specific inhibition of thrombin-induced cell activation by the neutrophil proteinases elastase, cathepsin G, and proteinase 3: evidence for distinct cleavage sites within the aminoterminal domain of the thrombin receptor. Blood. 1997 Mar 15;89(6):1944–1953. [PubMed] [Google Scholar]
- 62.Suttorp N, Nolte A, Wilke A, Drenckhahn D. Human neutrophil elastase increases permeability of cultured pulmonary endothelial cell monolayers. Int J Microcirc Clin Exp. 1993 Dec;13(3):187–203. [PubMed] [Google Scholar]
- 63.Killackey JJ, Killackey BA. Neutrophil-mediated increased permeability of microcarrier-cultured endothelial monolayers: a model for the in vitro study of neutrophil-dependent mediators of vasopermeability. Can J Physiol Pharmacol. 1990 Jul;68(7):836–844. doi: 10.1139/y90-127. [DOI] [PubMed] [Google Scholar]
- 64.Carl VS, Moore EE, Moore FA, Whalley ET. Involvement of bradykinin B1 and B2 receptors in human PMN elastase release and increase in endothelial cell monolayer permeability. Immunopharmacology. 1996 Jun;33(1–3):325–329. doi: 10.1016/0162-3109(96)00055-0. [DOI] [PubMed] [Google Scholar]
- 65.Suttorp N, Nolte A, Wilke A, Drenckhahn D. Human neutrophil elastase increases permeability of cultured pulmonary endothelial cell monolayers. Int J Microcirc Clin Exp. 1993 Dec;13(3):187–203. [PubMed] [Google Scholar]
- 66.Sambrano GR, Huang W, Faruqi T, Mahrus S, Craik C, Coughlin SR. Cathepsin G activates protease-activated receptor-4 in human platelets. J Biol Chem. 2000 Mar 10;275(10):6819–6823. doi: 10.1074/jbc.275.10.6819. [DOI] [PubMed] [Google Scholar]
- 67.Ramachandran R, Mihara K, Chung H, Renaux B, Lau CS, Muruve DA, DeFea KA, Bouvier M, Hollenberg MD. Neutrophil elastase acts as a biased agonist for proteinase-activated receptor-2 (PAR2) J Biol Chem. 2011 Jul 15;286(28):24638–24648. doi: 10.1074/jbc.M110.201988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Villegas-Mendez A, Montes R, Ambrose LR, Warrens AN, Laffan M, Lane DA. Proteolysis of the endothelial cell protein C receptor by neutrophil proteinase 3. J Thromb Haemost. 2007 May;5(5):980–988. doi: 10.1111/j.1538-7836.2007.02480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rezaie AR. The occupancy of endothelial protein C receptor by its ligand modulates the par-1 dependent signaling specificity of coagulation proteases. IUBMB Life. 2011 Jun;63(6):390–396. doi: 10.1002/iub.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.David A, Kacher Y, Specks U, Aviram I. Interaction of proteinase 3 with CD11b/CD18 (beta2 integrin) on the cell membrane of human neutrophils. J Leukoc Biol. 2003 Oct;74(4):551–557. doi: 10.1189/jlb.1202624. [DOI] [PubMed] [Google Scholar]
- 71.Kurosawa S, Esmon CT, Stearns-Kurosawa DJ. The soluble endothelial protein C receptor binds to activated neutrophils: involvement of proteinase-3 and CD11b/CD18. J Immunol. 2000 Oct 15;165(8):4697–4703. doi: 10.4049/jimmunol.165.8.4697. [DOI] [PubMed] [Google Scholar]
- 72.Kaneider NC, Leger AJ, Agarwal A, Nguyen N, Perides G, Derian C, Covic L, Kuliopulos A. 'Role reversal' for the receptor PAR1 in sepsis-induced vascular damage. Nat Immunol. 2007 Dec;8(12):1303–1312. doi: 10.1038/ni1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gohring K, Wolff J, Doppl W, Schmidt KL, Fenchel K, Pralle H, Sibelius U, Bux J. Neutrophil CD177 (NB1 gp, HNA-2a) expression is increased in severe bacterial infections and polycythaemia vera. Br J Haematol. 2004 Jul;126(2):252–254. doi: 10.1111/j.1365-2141.2004.05027.x. [DOI] [PubMed] [Google Scholar]
- 74.Gratzinger D, Canosa S, Engelhardt B, Madri JA. Platelet endothelial cell adhesion molecule-1 modulates endothelial cell motility through the small G-protein Rho. FASEB J. 2003 Aug;17(11):1458–1469. doi: 10.1096/fj.02-1040com. [DOI] [PubMed] [Google Scholar]
- 75.Kissel K, Santoso S, Hofmann C, Stroncek D, Bux J. Molecular basis of the neutrophil glycoprotein NB1 (CD177) involved in the pathogenesis of immune neutropenias and transfusion reactions. Eur J Immunol. 2001 May;31(5):1301–1309. doi: 10.1002/1521-4141(200105)31:5<1301::AID-IMMU1301>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.