Abstract
Members of the Mix/Bix family of Paired-class homeobox genes play important roles in the development of vertebrate mesoderm and endoderm. The single Mix/Bix family member identified in the mouse, Mix-like 1 (Mixl1), is required for mesendoderm patterning during gastrulation and promotes mesoderm formation and hematopoiesis in embryonic stem (ES) cell-derived embryoid bodies. Despite its crucial functions the transcriptional activity and targets of Mixl1 have not been well described. To investigate the molecular mechanisms of Mixl1-mediated transcriptional regulation, we have characterized the DNA-binding specificity and transcriptional properties of this homeodomain protein in differentiating ES cells. Mixl1 binds preferentially as a dimer to an 11 bp Mixl1 Binding Sequence (MBS) that contains two inverted repeats separated by a 3 bp spacer. The MBS mediates transcriptional activation by Mixl1 in both NIH 3T3 cells and in a new application of an inducible ES cell differentiation system. Consistent with our previous observation that early induction of Mixl1 expression in ES cells results in premature activation of Goosecoid (Gsc), we have found that Mixl1 occupies two variant MBSs within and activates transcription from the Gsc promoter in vitro and in vivo. These results strongly suggest that Gsc is a direct target gene of Mixl1 during embryogenesis.
Keywords: homeodomain, transcription factor, mouse embryonic stem cells, mesoderm induction, gastrulation
Introduction
The formation of the primary germ layers, ectoderm, mesoderm, and definitive endoderm, during vertebrate gastrulation is controlled through the interplay of signaling molecules and downstream transcriptional regulators (reviewed in refs. 1–3). Among the transcription factors that regulate mesoderm and endoderm development are those encoded by the Mix/Bix Paired class homeobox genes 4–18, which are regulated by Transforming Growth Factor (TGF)-β superfamily members such as Nodal/activin and Bone Morphogenetic Protein 4 (BMP4) 4, 6, 8, 13, 16, 19–24. Multiple members of the Mix/Bix gene family have been identified in Xenopus and zebrafish (reviewed by 10), but only a single Mix-like gene has been found in chicken 9, 15, mouse 10, 12, 14, and human 5, 10, 12. The expression of mouse Mix-like 1 (Mixl1), (also known as mMix and Mml) begins as early as 5.5 days postcoitum (dpc) in the visceral endoderm, prior to the onset of gastrulation 12, 14. From 6.5–8.0 dpc, Mixl1 is expressed in the primitive streak and nascent mesoderm 12, 14, 25. Targeted disruption of Mixl1 results in numerous embryonic defects, including a foreshortened body axis, absence of the heart tube and gut, deficient paraxial mesoderm, and sometimes an enlarged allantois, and Mixl1 mutant embryos die before 10.5 dpc 26. In addition, differentiating Mixl1-null embryonic stem (ES) cells show defects in hematopoiesis 21 and RNA interference-mediated Mixl1 knockdown blocks formation of definitive endoderm 27. Early expression of Mixl1 in doxycycline-inducible (i-Mixl1) ES cells accelerates the mesoderm developmental program, with increased numbers of mesodermal, hemangioblastic and hematopoietic progenitors, suggesting that Mixl1 plays a role in the recruitment and/or expansion of mesodermal progenitors to hemangioblastic and hematopoietic lineages 28. Together, these studies indicate that Mixl1 plays a critical role in mesoderm and endoderm development. Despite the importance of the Mix/Bix homeobox genes, including mouse Mixl1, in early embryogenesis, very little is known about the molecular mechanisms underlying their biological functions. Like other Mix/Bix proteins, Mixl1 contains a homeodomain for DNA binding and a C-terminal acidic region with potential transcriptional activation activity 10. Although several Mix/Bix family members have been reported to activate transcription in the frog 6, 8, 16, 29, 30, the transcriptional properties of mouse Mixl1 have not been characterized. The expression pattern of Mixl1 12, 14, 25 overlaps partially with that of the Paired class homeobox gene Goosecoid (Gsc) 31–34 in the primitive streak and node of the gastruling mouse embryo, and early induction of Mixl1 expression results in premature activation of Gsc in differentiating embryoid bodies 28. These observations suggest that Gsc may be a transcriptional target of Mixl1.
In this study, we have identified an optimal Mixl1 binding sequence (MBS), TAATTARATTA, to which Mixl1 binds preferentially as a dimer in vitro. In both NIH 3T3 cells and in a novel application of the i-Mixl1 ES system 28, Mixl1 function as a sequence-specific transcriptional activator. Moreover, Mixl1 binds specifically to and activates transcription via two variant MBSs within the Gsc promoter in vitro and occupies the Gsc promoter in vivo. These findings provide strong evidence that Gsc is a transcriptional target of Mixl1 during early mouse embryogenesis.
Materials and Methods
Plasmids and recombinant proteins
pGL2-promoter MT (a gift from Dr. Cory Abate-Shen; referred to as pGL2pro in this study) was derived by mutation of a putative homeodomain binding site (ATTA) in the SV40 promoter of pGL2-promoter (Promega). Plasmids constructed for this study are described in Table S1. For construction of pGL3-GscPro (Table S1), the −831 to +123 region of the Gsc gene was generated using polymerase chain reaction (PCR) amplification of a pSP73-Gsc3.1 template (a gift from Dr. Shin-Ichi Nishikawa) with GscP-5K and GscP-3N primers (Table S2) and was inserted between the Kpn I and Nhe I sites of pGL3-basic (Promega).
For mutational analysis of the variant MBSs in the mouse Gsc promoter region, the Gene Tailor Site-Directed Mutagenesis System (Invitrogen) was used as per manufacturer’s instructions. pGL3-GscProM1 and pGL3-GscProM2 (Table S1) were generated using primer pairs gMBSM1-A/B and gMBSM2-A/B (Table S2), respectively, with methylated pGL3-GscPro as template; pGL3-GscProM3 (Table S1) was generated using primer pair gMBSM1-A/B, with methylated pGL3-GscProM2 as template. Construction of pMT23-FLAG-Mixl1, pMT23-FLAG-Mixl1 P126I, and pMT23-FLAG-Mixl1 V132A has been described 10.
Recombinant GST-Mixl1 NHD and GST-Mixl1 HD fusion proteins were produced by the E coli strain BL21 transformed with pGEX5X1-Mixl1 NHD and pGEX5X1-Mixl1 HD, respectively. The recombinant proteins were expressed following induction using isopropyl β-D-1-thiogalactopyranoside (IPTG) and were purified using glutathione agarose (Sigma) as described 35. GST-Mixl1 HD protein immobilized to glutathione agarose was used for PCR-assisted binding site selection. Untagged Mixl1 HD and Mixl1 NHD were recovered by elution of GST-Mixl1 NHD and GST-Mixl1 HD from glutathione agarose with 50 mM Tris-HCl, pH 8.0, and 10 mM reduced glutathione followed by treatment with Factor Xa (New England BioLabs) as per manufacturer’s instructions.
Fluorescence activated cell sorting (FACS) analysis
i-Mixl1 ES cells were differentiated as described 36 in the presence or absence of DOX (0.4 μg/ml) and single cell suspensions were stained with the following antibodies in phosphate buffered saline (PBS) containing 10% FCS: biotin conjugated anti-mouse Flk1 (clone Avas12a1; eBioscience), phycoerythrin (PE) conjugated anti-mouse CD140a (PDGFRα) (clone APA5; eBioscience), PE labeled anti-mouse CD34 (RAM34) (no. 1387; BD Pharmingen), and PE-Cy7 conjugated anti-mouse CD41 (clone eBioMWReg30; eBioscience). Flk1 expression was detected using PE-Cy7 conjugated streptavidin (no. 557598; BD Pharmingen). FACS was performed using a BD LSR II system (Becton Dickinson). Flow cytometric data was analyzed using Flowjo software (Treestar).
PCR-assisted binding site selection
A pool of random sequence oligonucleotides, BNR76 (Table S2), was used for PCR-assisted binding site selection experiments based on modifications of previously published protocols 37, 38. The oligonucleotide pool contained 26 randomized residues in the central portion, flanked by two 25-nucleotide sequences containing Bam H1 (5′) and Eco RI (3′) sites, respectively. Double-stranded BNR76 was generated by PCR as described 37 and binding site selection for GST-Mixl1 HD (outlined in Fig. 1A) was performed using the protocol developed by Wilson et al.38. Selected DNA was amplified by PCR using primers B25 and R25 (Table S2) for the next round of selection. After five rounds of selection, the DNA was amplified by PCR, digested by Bam HI and Eco RI, and cloned into pBluescript SK (+) (Stratagene) for sequencing analysis. Consensus sequence was compiled from 32 aligned sequences and converted to the sequence logo by the WebLogo online program39 (http://weblogo.berkeley.edu. Accessed July 12, 2009).
Figure 1. Determination of optimal Mixl1 binding sequence by PCR-assisted binding site selection.
(A) Schematic diagram of the experimental procedure. (B) 32 sequenced oligonucleotides were aligned to the 11 bp TAATT-containing motif (underlined). A consensus sequence was compiled and converted to a sequence logo in which the height of letters represents the relative frequency of each nucleotide at each of the 11 positions 39.
Electrophoretic mobility shift assay (EMSA)
Top and bottom strands of oligonucleotide probes for EMSA were 5′-labeled individually with [γ-32P]-ATP (6000 Ci/mmol; Perkin Elmer Life Sciences) using T4 polynucleotide kinase (New England BioLabs) as per manufacturer’s instructions. The binding reaction was performed as described 40 with 2–50 nM Mixl1 HD or Mixl1 NHD and 0.7 nM [32P]-labeled, double stranded probe. For competition assays, Mixl1 HD or Mixl1 NHD was incubated with unlabeled, double-stranded oligonucleotides (35 nM) at RT prior to the addition of [32P]-labeled probe. Protein-DNA complexes were resolved by 6.5% polyacrylamide gel electrophoresis (PAGE) and visualized by autoradiography.
Cell culture, transient transfection and luciferase assays
NIH 3T3 cells were cultured in DMEM (Invitrogen) containing 10% (v/v) fetal calf serum (Hyclone) at 37°C in the presence of 5% CO2. Doxycycline (DOX) inducible Mixl1 (i-Mixl1) ES cells, which express cDNA encoding FLAG-tagged Mixl1, were maintained as described previously 36. Cells were plated in 24-well plates (Nunc) 24 hr prior to transfection in DMEM-10% fetal calf serum (for NIH 3T3 cells) or in ES cell maintenance medium (for i-Mixl1 ES cells). Transient transfection assays were performed in triplicate using FuGene 6 transfection reagent (Roche) as per manufacturer’s instructions. The pRL-TK plasmid (Promega), which expresses Renilla luciferase, was co-transfected as an internal control (20 ng per well) with other plasmids. To transfect NIH 3T3 cells (4 × 104 cells), 0.2 μg of test plasmid was co-transfected with 0.2 μg of pGL2pro- or pGL3-based reporter plasmid (Table S1). i-Mixl1 ES cells (4 × 104 cells) were transfected with 0.2 μg pGL2pro- or pGL3-based reporter plasmid (Table S1). After 6 hr, the culture medium was replaced with the maintenance medium without leukemia inhibitory factor (LIF), and DOX (0.4 μg/ml) was added to induce Mixl1 expression. At 48 hr post transfection, cells were rinsed twice with PBS, and lysed in Passive Lysis Buffer (Promega) at RT for 15 min. Luciferase assays were performed using the dual-luciferase assay system (Promega). Activities of both firefly and Renilla luciferases were measured using a Veritas™ microplate luminometer (Turner BioSystems). Relative luciferase activity was calculated by normalizing firefly luciferase activity (reporter) to Renilla luciferase activity (internal control).
Antibodies and Western blotting
Anti-Mixl1 polyclonal antibody was generated in rabbits using GST-Mixl1 NHD (residues 1–145) as the antigen, and anti-Mixl1 NHD IgG was purified by affinity chromatography using a protein A agarose column as described (http://www.millipore.com/immunodetection/id3/igg. Accessed July 12, 2009). To detect Mixl1 polypeptides transiently expressed in NIH 3T3 cells, lysates (10–15 μg protein) were resolved by sodium dodecyl sulfate (SDS)-PAGE as described 41, followed by Western blotting using anti-Mixl1 antibody (0.35 μg/ml) and chemiluminescent detection (Amersham ECL Plus Western Blotting Detection System, GE healthcare). β-Actin was used as a loading control for PAGE and was detected by an anti-β-Actin antibody (Sigma).
Quantitative real-time reverse transcription (RT)-PCR analysis
Total cellular RNA was isolated using the RNAeasy mini kit (Qiagen) as per manufacturer’s instructions. cDNA was synthesized from 1–5 μg of total RNA using the Superscript III First-Strand Synthesis System for RT-PCR (Invitrogen) as per manufacturer’s instructions. 1 μl of cDNA was analyzed in triplicate by quantitative real-time RT-PCR (QRT-PCR) as described, with Gapdh, Gpi1 and Mt2 as internal controls 28. The relative expression level of each gene was calculated from threshold cycle (Ct) values for the gene normalized to those of the internal controls. Normalization to each or all three internal controls generated comparable results, and data normalized to Gapdh are shown. It was possible to distinguish between the endogenous Mixl1 gene and the Mixl1 transgene through the use of specific primers that do or do not detect the 3′ UTR, respectively 28.
Chromatin immunoprecipitation (ChIP)
i-Mixl1ES cells were cultured in the maintenance medium without LIF in the presence or absence of DOX (0.4 μg/ml) for 48 24, 48, and 72 hr, respectively, prior to ChIP assays. ChIP assays were performed using the EZ ChIP chromatin immunoprecipitation kit (Millipore) as per manufacturer’s instructions. Immunoprecipitated chromatin was subjected to reverse crosslink and the purified DNA was resuspended in 50 μl of 10 mM Tris-HCl, pH 7.5. 3 μl of input (1%) or immunoprecipitated DNA was included in a 20 μl reaction containing 10 μl of SYBR Green Reaction Mix (Applied Biosystems) and 400 nM promoter-specific primers (Table S2), and quantitative real-time PCR was performed by an ABI PRISM 7900HT Sequence Detection System (SDS) running the following cycling program: 95°C, 3 min; 95°C, 20 sec., 59°C, 30 sec., 72°C, 30 sec., 40 cycles; 72°C, 2 min. The relative amount of immunoprecipitated DNA was determined by normalizing the Ct value for immunoprecipitated DNA to that for the input using the 2−ΔΔCt method as described 42. For conventional PCR, 4 μl of input or immunoprecipited DNA were included in a 20 μl reaction containing 200 nM promoter-specific primers and 2 units of Platinum Taq DNA polymerase (Invitrogen), running the following cycling program: 94°C, 3 min; 94°C, 20 sec., 59°C, 30 sec., 72°C, 30 sec., 32 cycles; 72°C, 2 min. PCR products were resolved using agarose gel (2.5%) electrophoresis.
Results
Determination of optimal Mixl1 target sequence in vitro
We used a DNA binding site selection assay to define the preferred Mixl1 recognition sequence, as outlined in Fig. 1A. A bipartite consensus sequence, TAATTARATTA, was compiled from 32 selected sequences, which contained two TAAT/ATTA inverted repeats separated by a 3 bp spacer (see the sequence logo, Fig. 1B). This consensus sequence, termed the Mixl1 Binding Sequence (MBS), is distinct from the consensus target sequence (TAATYGATTA) of the Drosophila Paired (Prd) homeodomain 38. In addition, the 3 bp spacer separating the two TAAT/ATTA half sites within the MBS is distinct from that of the “P3” recognition sequence (TAR for the MBS versus YNR for the P3 sequence). The P3 sequence was originally identified for a mutant Prd homeodomain containing a glutamine at position 9 (Q9) of the recognition helix 38 and is bound by Mixl1, also a Q9 type homeodomain protein, in vitro 10. Thus, the in vitro consensus target sequence of the Mixl1 homeodomain is very similar but not identical to that of the mutant Prd homeodomain.
Mixl1 binds its target sequence preferentially as a dimer
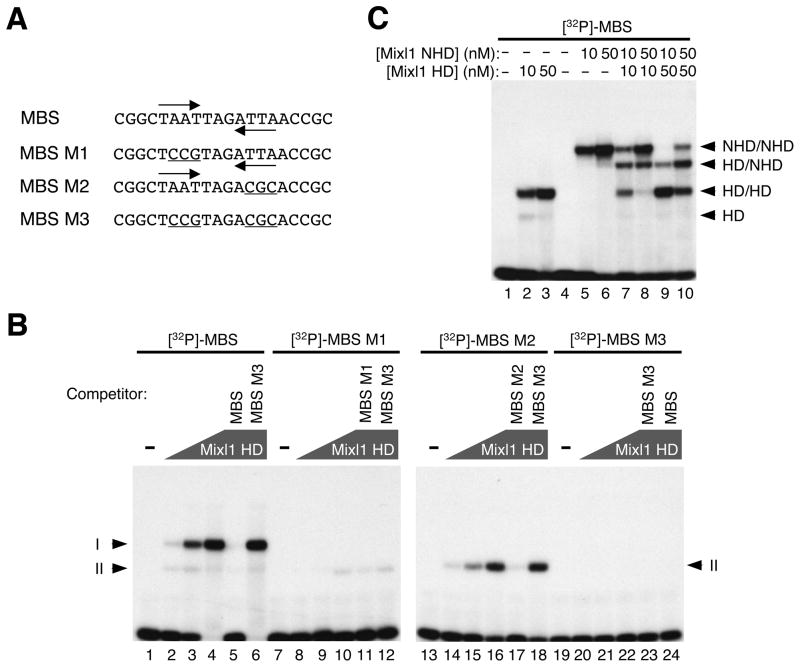
Paired class homeodomain proteins have been reported to bind DNA cooperatively as dimers 38. The dimerization status of Mixl1 was evaluated using EMSA, with increasing amounts of Mixl1 HD and [32P]-labeled, double-stranded 26 bp oligonucleotides containing the MBS (TAATTAGATTA) or one of its mutant forms as probes (Fig. 2A). Two complexes (here termed I and II) were detected at the lowest Mixl1 HD concentration tested (2 nM; Fig. 2B, lane 2). Complex I became dominant at the higher concentrations of Mixl1 HD (10 nM and 50 nM, respectively; Fig. 2B, lanes 3 and 4). The Mixl1 HD-MBS interaction was specific, as unlabeled MBS but not MBS M3 (both half sites mutated) competed efficiently with [32P]-labeled MBS for binding (Fig. 2B, compare lanes 5 and 6). Mixl1 HD formed only one complex (II), at lower efficiency, with the MBS M1 or MBS M2 probes, which contain a single half site (Fig. 2B, lanes 8–10 and 14–16). These findings suggest that Mixl HD can recognize each half site and binds to the palindromic MBS in a cooperative manner. Mixl1 HD bound more efficiently to the first (TAAT) half site than that to the second (ATTA), possibly due to differences in the residues flanking these sites (Fig. 2B, compare lanes 14–16 with 8–10). Binding of Mixl1 HD to the labeled MBS M1 and MBS M2 probes was competed by unlabeled MBS M1 (Fig. 2B, lane 11) and MBS M2 (Fig. 2B, lane 17). As expected, MBS M3 did not compete for binding of Mixl1 HD to labeled MBS M1 or M2 (Fig. 2B, lanes 12 and 18) and did not form complexes with Mixl1 HD (Fig. 2B, lanes 20–22).
Figure 2. Mixl1 binds the MBS preferentially as a dimer.
(A) Oligonucleotides containing the MBS or one of its mutant forms (MBS M1, MBS M2, and MBS M3) served as probes for EMSA (sense strands shown). The TAAT/ATTA half sites are marked by inverted arrows and mutations within the half sites are underlined. (B) EMSA of Mixl1 HD with [32P]-labeled, double-stranded probes containing the MBS, MBS M1, MBS M2, or MBS M3. Lanes 1, 7, 13 and 19 contained no protein. The concentration of Mixl1 HD was 2 nM (lanes 2, 8, 14, and 20), 10 nM (lanes 3, 9, 15, and 21), and 50 nM (lanes 4–6, 10–12, 16–18, and 22–24), respectively. Mixl1 HD and the MBS probe formed two complexes (Iand II). Mutations in either of the half sites (MBS M1 or MBS M2) abolished the formation of complex I. No complexes were formed when both half sites were mutated (MBS M3). Binding of Mixl1 HD to [32P]-labeled MBS, MBS M1 or MBS M2 was efficiently competed by each of the corresponding unlabeled, oligonucleotides (50X excess) (lanes 5, 11, and 17) but not by MBS M3 (lanes 6, 12 and 18). Polygons represent increasing concentrations of Mixl1 HD. The concentration for each of the unlabeled oligonucleotides (competitors; lanes 5, 6, 11, 12, 17, 18, 23 and 24) was 35 nM. (C) [32P]-labeled, double-stranded MBS was incubated with Mixl1 HD (10 and 50 nM, lanes 2 and 3), Mixl1 NHD (10 and 50 nM, lanes 5 and 6), or both (Mixl1 HD: 10 nM, lanes 7 and 8; 50 nM, lanes 9 and 10. Mixl1 NHD: 10 nM, lanes 7 and 9; 50 nM, lanes 8 and 10). Mixl1 HD formed two complexes with the MBS: HD/HD (complex I) and HD (complex II). Mixl1 NHD formed a dominant complex (NHD/NHD) with the MBS. A complex of intermediate mobility, HD/NHD, was formed by co-incubation of Mixl1 HD and Mixl1 NHD with the MBS probe.
To determine directly whether Mixl1 dimerizes on the MBS, EMSA was performed following co-incubation of MBS probe with Mixl1 HD and a larger polypeptide containing both the N-terminal domain and the homeodomain (residues 1–145, termed NHD; Fig. 2C). Again, Mixl1 HD alone formed two complexes with the MBS probe: HD/HD (complex I), and HD (complex II) (Fig. 2C, lanes 2 and 3). Mixl1 NHD alone formed a single, slowly migrating complex with the MBS probe (NHD/NHD, Fig. 2C, lanes 5 and 6). However, when Mixl1 HD and Mixl1 NHD were co-incubated with the MBS probe, a distinct complex of intermediate mobility was observed (HD/NHD, Fig. 2C, lanes 7–10), consistent with DNA binding by a heterodimer containing Mixl1 NHD and Mixl1 HD.
Mixl1 functions as a transcriptional activator in NIH 3T3 cells
To test whether Mixl1 can activate transcription through the MBS, we transfected NIH 3T3 cells with the Mixl1-expressing plasmid pMT23-FLAG-Mixl1, which encodes FLAG-tagged full-length Mixl1 protein 10, and pGL2pro (no MBS) or pGL2pro-6xMBS (6 tandem copies of the MBS upstream of the SV40 promoter and firefly luciferase reporter gene) (Fig. 3A). A modest (2-fold) activation of the SV40 promoter alone (pGL2pro) was detected (possibly mediated through the TAAT sequences within the promoter; Fig. 3B). In contrast, an ~18-fold activation of the promoter linked to the multimerized MBS was observed (Fig. 3B), indicating that Mixl1 can activate transcription through the MBS. To confirm that the DNA-binding activity of Mixl1 is required for its transcriptional activity, NIH 3T3 cells were co-transfected with pGL2pro-6xMBS and one of the two expression plasmids that encode FLAG-tagged Mixl1 polypeptides with point mutations in the homeodomain (Fig. 3C) that abolish its DNA binding activity 10. The expression of the wild-type and mutant FLAG-tagged Mixl1 polypepetides in NIH 3T3 cells was confirmed by Western blotting (Fig. 3D). As shown in Fig. 3E, the wild type Mixl1 activated the luciferase reporter by ~10-fold, whereas no activation was detected for either Mixl1 mutant. Therefore, the DNA-binding activity of the homeodomain is essential for transcriptional activation by Mixl1.
Figure 3. Mixl1 functions as a transcriptional activator in cultured NIH 3T3 cells.
(A) Diagram of the plasmids for transient transfection of NIH 3T3 cells. The promoterless pGL3-basic served as a negative control. pGL2pro contains a modified SV40 promoter (black box with arrow) driving the firefly luciferase gene as the reporter. pGL2pro-6xMBS contains six tandem copies of the MBS (6xMBS) upstream of the SV40 promoter and the reporter gene. (B) NIH 3T3 cells were co-transfected by pGL3-basic, pGL2pro or pGL2pro-6xMBS with either pMT23 or pMT23-FLAG-Mixl1, that encodes a FLAG-tagged Mixl1 protein. Mixl1 induced modest activation of the SV40 promoter alone (pGL2pro) but strong activation of the promoter-reporter gene linked to the multimerized MBS (pGL2pro-6xMBS). (C) Sequence of the Mixl1 homeodomain (residues 86–145) is shown. Either the P126I or V132A mutation abolished the DNA binding activity of Mixl1. (D) NIH 3T3 cells were transfected with pMT23 (vector), pMT23-FLAG-Mixl1 (Mixl1), pMT23-FLAG-Mixl1 P126I (P126I), or pMT23-FLAG-Mixl1 V132A (V132A), and the expression of FLAG-tagged Mixl1 and its mutant forms were detected by Western blotting using anti-Mixl1 antibody. The FLAG-Mixl1 (lane 2), FLAG-Mixl1 P126I (lane 3), and FLAG-Mixl1 V132A (lane 4) polypeptides are marked by an arrow. Endogenous β-Actin (loading control) was detected using an anti-β-Actin antibody. (E) NIH 3T3 cells were co-transfected with the reporter plasmid (pGL2pro or pGL2pro-6xMBS) and pMT23, pMT23-FLAG-Mixl1 (Mixl1), pMT23-FLAG-Mixl1 P126I (Mixl1 P126I), or pMT23-FLAG-Mixl1 V132A (Mixl1 V132A). Wild type Mixl1 but not either of the Mixl1 mutants (Mixl1 P126I and Mixl1 V132A) activated the reporter gene (pGL2pro-6xMBS).
Conditional activation of Mixl1 accelerates mesoderm formation in differentiating ES cells
Differentiating ES cells are an excellent model system for early mammalian development (reviewed in ref. 43). Under appropriate conditions, ES cells form structures known as embryoid bodies (EBs) that can differentiate along a number of distinct lineages representing each of the three embryonic germ layers. Mixl1 marks mesoderm in the mouse embryo and in ES cell-derived EBs 12, 14, 25. It is also expressed in a population of mesendoderm in differentiating ES cells 44. We chose to characterize the transcriptional properties of Mixl1 in differentiating i-Mixl1 ES cells, in which doxycycline (DOX) induction of a Mixl1 transgene accelerates mesodermal and hematopoietic development 28. DOX-induced Mixl1 expression results in premature activation of mesoderm (Twist, FoxA2, Nkx2 and Tbx6), hemangioblast (Runx1, Flk1, c-kit and Tal1), and hematopoietic (Cdx4, Hoxb4, Gata1 and eY-globin) marker genes 28. Moreover, induced Mixl1 expression expands Flk1+ and c-kit+ cell populations and hematopoietic progenitor numbers and accelerateds production of hemoglobin pigmentation in EBs, suggesting that Mixl1 may function in the recruitment and/or expansion of mesodermal progenitors to the hematopoietic lineage 28.
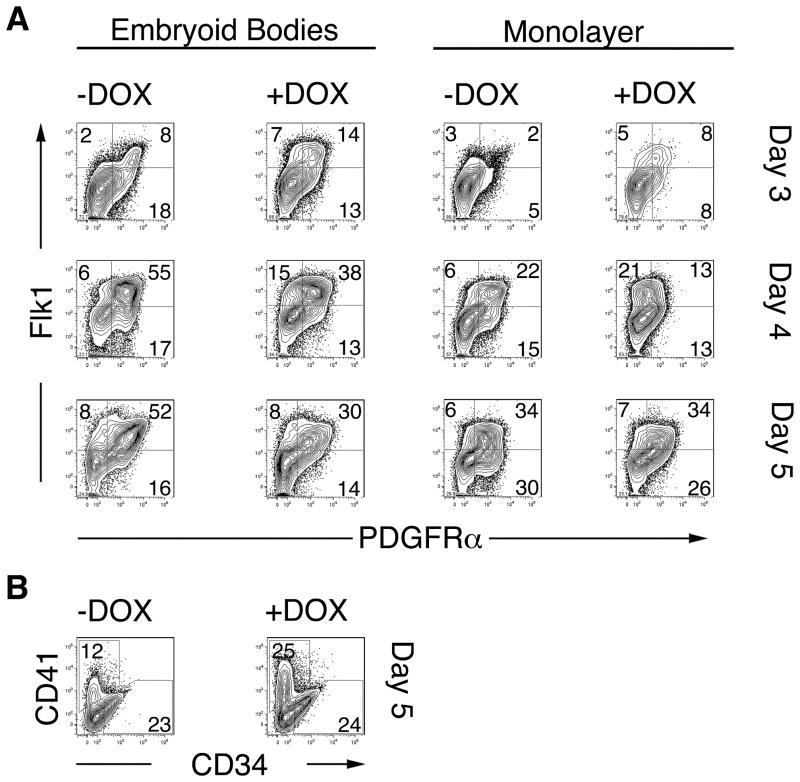
To characterize the activation of mesoderm in greater detail, i-Mixl1 ES cells were differentiated as EBs or in monolayer culture for 3 to 5 days. (Monolayer cultures were used to ensure uniform exposure of the cells to DOX and to transfection reagents). Expression of the mesoderm markers Flk1 and PDGFRα was examined. Induction of Mixl1 in EBs and monolayers consistently resulted in accelerated formation of Flk1+ cells at day 3 compared with uninduced cultures (1.5-to 2-fold for EBs and ~3.5 fold for monolayers; Fig. 4A), consistent with our earlier conclusion that activation of Mixl1 accelerates mesodermal development. Over the next two days, the numbers of Flk1+ cells continued to increase in both induced and uninduced cultures (Fig. 4A).
Figure 4. Enhanced mesoderm and hematopoietic development in response to conditional activation of Mixl1 during ES differentiation.
(A) i-Mixl1 ES cells were differentiated as EBs (left panels) or on gelatin-coated dishes as monolayers (right panels) for 3, 4 or 5 days in the presence or absence of DOX. The expression of the mesodermal markers Flk1 and PDGFRα was then examined by flow cytometry. (B) The frequency of CD41+ hematopoietic progenitors and CD34+ vascular endothelial progenitors in EBs cultured in the presence or absence of DOX for 5 days was assessed by FACS. Population frequencies from total, live, gated cells are shown. These data are representative of 3 independent experiments.
Flk1+ cells express PDGFRα early in their development but lose PDGFRα as they mature 45. A population of cells expressing PDGFRα but not Flk1 has been proposed to represent an ES-derived equivalent of paraxial mesoderm 45. A comparable PDGFRα+Flk1− population was present at lower frequency in DOX-induced than in uninduced cultures (Fig. 4A). DOX-induced Flk1+ cells expressed lower levels of PDGFRα on day 4 than did uninduced cells (Fig. 4A), again consistent with accelerated mesoderm development in response to Mixl1.
As i-Mixl1 EBs treated with DOX were previously found to produce larger numbers of hematopoietic progenitors (as measured by hematopoietic colony forming potential) 28, the expression of the earliest hematopoietic-specific surface marker, CD41 46, was assessed. We consistently observed a 1.5- to 2-fold increase in the frequency of CD41+ cells in DOX-treated EBs at day 5 of differentiation (Fig. 4B). These findings, together with the increase in generation of Flk1+ cells, suggest enhanced formation of hematopoietic mesoderm in response to over-expression of Mixl1 during ES cell differentiation. Generation of CD34+ vascular progenitors, however, was not altered by induction of Mixl1 (Fig. 4B).
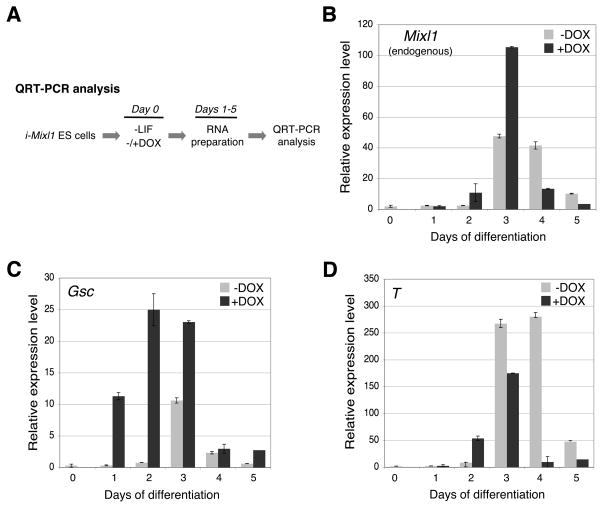
We next evaluated expression of mesodermal and mesendodermal genes in induced and uninduced cultures of i-Mixl1 ES cells (Fig. 5A). The expression of Mixl1 (mesoderm, mesendoderm), Goosecoid (Gsc, mesendoderm), and Brachyury (T, mesoderm) was activated early in response to induction of Mixl1, reaching a peak at day 3 of culture (shown for monolayer cultures, Fig. 5B–D). Comparable results were obtained for EBs, as previously reported 28 (data not shown). Activation of both T and endogenous Mixl1 was accelerated but the duration of their expression was not extended in response to induction of Mixl1. Robust activation of Gsc was detected as early as day 1 (Fig. 5C), suggesting that this gene might be a direct transcriptional target of Mixl1. Expression of T was also accelerated in response to induction of Mixl1 (Fig. 5D, days 2 and 3).
Figure 5. Mesendoderm and mesoderm marker genes are activated in differentiating i-Mixl1 ES cells.
(A) General experimental protocol for analysis of gene expression patterns of differentiating i-Mixl1 ES cell monolayer cultures using QRT-PCR. i-Mixl1 ES cells were allowed to differentiate in the LIF-free medium in the absence (−) or presence (+) of 0.4 μg/ml of DOX. Total cellular RNA was isolated at days 1 through 5 of culture and reverse transcribed to cDNA for QRT-PCR analysis. (B) The expression of endogenous Mixl1 reached a peak at day 3 and declined at day 5 of culture in uninduced cells (−DOX). Induction of Mixl1 (+DOX) accelerated endogenous Mixl1 expression. (C) The expression of Gsc was peaked at day 3 and declined at day 4 of culture in uninduced cells (−DOX). Induction of Mixl1 expression (+DOX) resulted in a premature activation of Gsc as early as day 1. (D) The expression of the Brachyury (T) gene was activated at days 3–4 and declined at day 5 of culture in uninduced cells (−DOX). Induction of Mixl1 (+DOX) accelerated T expression at day 2 of culture.
Mixl1 is a sequence-specific transcriptional activator in differentiating ES cells
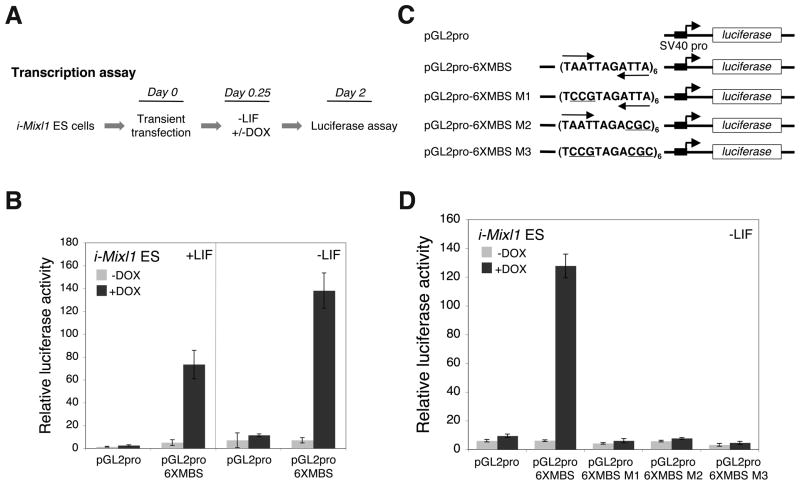
The transcriptional activation properties of Mixl1 were investigated in differentiating i-Mixl1 ES cell monolayer cultures using transient transfection assays (Fig. 6A). Activation of the 6xMBS reporter plasmid was detected in response to induction of Mixl1 in the presence or absence of LIF (Fig. 6B). Therefore, Mixl1 can activate transcription in undifferentiated as well as differentiating ES cells (Fig. 6B, compare the +LIF and −LIF panels).
Figure 6. Mixl1 functions as a sequence-specific transcriptional activator in differentiating i-Mix ES cells.
(A) General experimental protocol for transcription assays using i-Mixl1 ES cells. Undifferentiated i-Mixl1 ES cell monolayer cultures were transiently transfected with reporter plasmids in the LIF-containing medium at day 0. At 6 hr (day 0.25) post transfection, the LIF-containing medium in half of the cultures was replaced by LIF-free medium and the cells were cultured in the absence (−) or presence (+) of DOX (0.4 μg/ml). Cell lysates were prepared 48 hr post transfection for luciferase assays. (B) i-Mixl1 ES cells were transfected with the reporter plasmid (pGL2pro or pGL2pro-6xMBS) and then cultured in the LIF-containing or LIF-free medium in the presence or absence of DOX. Induction of Mixl1 (+DOX) resulted in the activation of the reporter luciferase gene linked to the multimerized MBS (pGLpro-6xMBS) in the presence or absence of LIF. (C) The reporter plasmids for analyzing the role of the TAAT/ATTA half sites in Mixl1-mediated transcriptional activation. Each plasmid except for pGL2pro contained six copies of the MBS or mutant MBS (MBS M1, MBS M2, or MBS M3). The TAAT/ATTA half sites are marked by inverted arrows and mutations are underlined. (D) i-Mixl1 ES cells were transfected with the individual reporter plasmids and then cultured in LIF-free medium in the presence or absence of DOX. Induction of Mixl1 (+DOX) resulted in the activation of the reporter luciferase gene linked to the multimerized MBS (pGL2pro-6xMBS) but not any of the mutant MBS (pGL2pro-6xMBS M1, -6xMBS M2, and -6xMBS M3).
A second series of experiments established that transcriptional activation by Mixl1 in i-Mixl1 ES cells was dependent on both half sites of the palindromic MBS recognition sequence (Fig. 6C). Significant luciferase reporter activity was detected in i-Mixl1 ES cells transfected with pGL2pro-6xMBS but not pGL2pro-6xMBS M1 (first half site mutated), M2 (second half site mutated), or M3 (both half sites mutated; Fig. 6D). Therefore, both TAAT/ATTA half sites within the palindromic MBS are required for transcriptional activation by Mixl1. The experiments shown in Figures 5 and 6 establish the i-Mixl1 ES cell system as an appropriate model for evaluating the transcriptional activity of Mixl1 in a biologically relevant context.
Gsc is a transcriptional target of Mixl1
The robust and rapid activation of Gsc in DOX-treated i-Mixl1 ES cells (Fig. 5C) 28 prompted us to investigate whether Gsc is a direct target gene of Mixl1. The Gsc promoter region contains two variant Mixl1 binding sequences (here termed gMBS1 and gMBS2, Fig. 7A). gMBS1 and gMBS2 are located within the distal element (DE) and proximal element (PE) conserved between the Xenopus and mouse Gsc genes 47, respectively. Mixl1 HD bound preferentially as a dimer to both gMBS1 and gMBS2 as shown by EMSA (Fig. 7B). Binding was specific, as unlabeled gMBS1 and gMBS2 but not the mutant gMBS (gMBS1M and gMBS2M; see Fig. 7A) sequences competed efficiently with [32P]-labeled gMBS1 and gMBS2 probes, respectively (Fig. 7B).
Figure 7. Gsc is a transcriptional target of Mixl1.
(A) Schematic diagram of the Gsc promoter region for transient transfection assays (−831 to +123). Boxes 1 and 2 represent the two pvariant MBSs, gMBS1 (−453 to −443) and gMBS2 (−370 to −360), respectively. gMBS1 is located within the conserved distal element (DE; from − 456 to −429), while gMBS2 is within the proximal element (PE; from −396 to −346). Below the diagram are the 26-bp EMSA probes (top strand sequences shown) that correspond to −461 to −436 (containing gMBS1 or its mutant, gMBS1M) and −378 to −353 (containing gMBS2 or its mutant, gMBS2M) regions of the Gsc gene, respectively. The TAAT/ATTA half sites are marked by inverted arrows and the mutations are underlined. (B) EMSA of [32P]-labeled, double-stranded MBS, gMBS1 and gMBS2 probes with increasing amounts of Mixl1 HD (10 nM for lanes 2, 7, and 12; 50 nM for lanes 3–5, 8–10, and 13–15). Lanes 1, 6, and 11 contained no protein. Mixl1 HD and each of the probes formed two complexes (I and II). Binding to [32P]-labeled MBS, gMBS1 and gMBS2 probes was efficiently competed by unlabeled MBS, gMBS1, and gMBS2 sequences, respectively (lanes 4, 9, and 14), but not by sequences containing MBS M3 (lane 5), gMBS1M (lane 10) or gMBS2M (lane 15). (C) pGL3-based luciferase reporter constructs for analyzing the promoter region (−831 to +123) of the Gsc gene (GscPro). Positions of gMBS1 and gMBS2 are marked by open boxes and mutant gMBS1 and gMBS2 marked by “X”. pGL3-GscProM1 and pGL3-GscProM2 contained mutations in gMBS1 and gMBS2, respectively, while pGL3-GscProM3 contained both mutations in both gMBS1 and gMBS2. (D) i-Mixl1 ES cells were transfected with pGL3-basic or the pGL3-GscPro based plasmid (GscPro, GscProM1, GscProM2, or GscProM3) and then cultured in LIF-free medium in the presence or absence of DOX (0.4 μg/ml). Induction of i-Mixl1 (+DOX) resulted in significant activation of the reporter luciferase gene in cells transfected with by pGL3-GscPro. Little or no activation of the reporter gene was detected in cells transfected by the other plasmids (pGL3-GscProM1, pGL3-GscProM2, and pGL3-GscProM3). (E) Specificity of the anti-Mixl1 antibody for the ChIP assay was validated by immunoprecipitation of FLAG-Mixl1 from COS cells transfected with an FLAG-Mixl1 expression plasmid (pMT23-FLAG-Mixl1). Cell lysates were incubated with the anti-Mixl1 antibody. Immunocomplexes were purified using protein A agarose and subjected to Western blotting with an anti-FLAG antibody. FLAG-Mixl1 was identified in cells transfected with pMT23-FLAG-Mixl1 (arrowhead) but not the empty pMT23 vector. IP, immunoprecipitation; Blot, Western blotting. (F and G) ChIP assays. i-Mixl1 ES cells were cultured in LIF-free medium in the presence or absence of DOX (0.4 μg/ml) for 1–3 days followed by in vivo crosslinking with formaldehyde. Cell lysates were sonicated and sheared chromatin was subjected to immunoprecipitation with a nonspecific rabbit IgG, anti-Mixl1 antibody (anti-Mixl1), or anti-acetyl-Histone H3 (anti-H3). (F) Quatitative real-time PCR analysis of immunoprecipitated DNA from DOX-induced (+DOX) and untreated (-DOX) differentiating (days 1–3) i-Mixl1 ES cells. The relative amount of immunoprecipitated DNA (relative IP) was determined using the 2−ΔΔ Ct method. (G) Conventional PCR analysis of immunoprecipitated DNA from DOX-induced (+DOX) and untreated (−DOX) differentiating (day 2) i-Mixl1 ES cells. PCR products were resolved by 2.5% agarose gel electrophoresis. In, input (1%); IP, immunoprecipitated DNA. PCR-amplified promoters are marked by arrows and the antibody or IgG for immunoprecipitation is listed by each panel.
We next investigated whether Mixl1 can activate transcription from gMBS1 and gMBS2 within the Gsc promoter, using transient transfection assays in differentiating i-Mixl1 ES cells. We analyzed the large Gsc promoter fragment (−831 to +123; Fig. 7A and C), which may contain other Gsc regulatory sequence motifs in addition to the PE and DE. Thus, regulation of the Gsc promoter could be evaluated in a cellular context that would include Mixl1 cofactors and other regulators of Gsc. Significant activation of the wild type but not the mutated Gsc promoter was detected in DOX-induced i-Mixl1 ES cells over that seen in the absence of induction by DOX (Fig. 7D), indicating that Mixl1 activates the Gsc promoter through gMBS1 and gMBS2 and that both sites are required for maximal activation.
To determine whether Mixl1 occupies the Gsc promoter in differentiating i-Mixl1 ES cells, we performed ChIP assays. The Gsc promoter fragments containing both gMBS1 and gMBS2 were precipitated from crosslinked and sonicated chromatin using a polyclonal anti-Mixl1 antibody whose specificity was validated by Western blotting (Fig. 3D), immunoprecipitation (Fig. 7E) and EMSA (Fig. S1). Precipitated fragments were detected using quantitative real-time PCR and Gsc promoter-specific primers (Table S2). As shown in Fig. 7F, the Gsc promoter fragments were immunoprecipitated at significant levels in DOX treated cells but not untreated cells (Fig. 7F, days 1–3), suggesting that the Gsc promoter was occupied by DOX-induced Mixl1 in vivo. The precipitated Gsc promoter fragments were also detectable by conventional PCR using the same primers followed by agarose gel electrophoresis (Fig. 7G). The acetyl-histone H3 bound Gapdh promoter (positive control) was detected by an anti-acetyl-histone H3 antibody (anti-H3) in differentiating i-Mixl1 ES cells in the presence or absence of DOX. The Gsc promoter was not detected by a nonspecific IgG (Fig. 7G). Thus, the ChIP assays revealed the occupancy of the Gsc promoter by Mixl1 in differentiating i-Mixl1 ES cells.
Discussion
Despite the crucial role of the Mixl1 transcription factor in mesoderm and endoderm development, little is known about its transcriptional activity and direct target genes. As an essential step toward elucidatings the molecular mechanisms underlying the biological functions of Mixl1 during development, we have taken a novel approach to characterize the transcriptional properties of Mixl1 in differentiating ES cells. We have shown that Mixl1 is a sequence-specific transcriptional activator and identified Gsc as a transcriptional target of Mixl1. These results represent the first detailed characterization of transcriptional properties for a Mix/Bix homeodomain protein.
DNA-binding specificity of Mixl1
In this study, we show that the optimal in vitro target sequence (MBS) of the Mixl1 homeodomain is very similar but not identical to the target sequence (P3) of the mutant (Q9) Prd homeodomain. Both of the 11 bp bipartite sequences contain the inverted TAAT/ATTA repeats but somewhat different 3 bp spacers (TAR for the MBS versus YNA for the P3 sequence). It has been proposed that the amino acid residue at position 9 of the recognition helix (i.e. position 50 of the homeodomain) influences binding specificity of the homeodomain 48. Since the Q9 type Mixl1 and mutant Prd homeodomains share only 60% identity, the differences in their in vitro binding specificity may result from residues other than Q9. It is noteworthy that Mixl1 is able to bind various TAATNNNATTA sequences in vitro and in vivo in addition to the consensus MBS determined in vitro. For example, Mixl1 binds the TAATAAGATTA (gMBS1) and TAATTTCATTA (gMBS2) sequences with relatively lower efficiency than for the MBS (see Fig. 7B) but the binding is specific and functional in mediating transcriptional activation by Mixl1. These findings suggest that binding efficiency may be determined by the 3 bp spacer. The variety of TAATNNNATTA sequences may serve as low- to high-affinity Mixl1 target sequences to mediate the transcriptional function of Mixl1. Finally, several Paired class homeodomain proteins have been reported to form heterodimers on TAATNNNATTA sequences 49–51. We show here that the Mixl1 homeodomain binds to the MBS preferentially as a dimer and in a cooperative manner, raising the possibility that heterodimerization of Mixl1 with other coexpressed Paired class homeodomain transcription factors may modulate its target specificity and/or transcriptional functions during embryogenesis.
Transcriptional properties of Mixl1 in differentiating ES cells
The in vitro differentiation of ES cells is a useful model for examining the role of transcriptional regulators in early developmental processes. We previously showed that conditional, premature induction of Mixl1 accelerates the mesodermal and hematopoietic developmental programs in differentiating ES cells 28. In this report, we have extended this analysis. In EBs treated with DOX to induce a Mixl1 transgene, accelerated development of lateral plate mesoderm-like (Flk1+ PDGFRα−) cells was observed. Interestingly, we consistently observed fewer paraxial mesoderm-like (Flk1−PDGFRα+) cells in DOX-treated than in untreated cultures. These results suggest that Mixl1 may play a role in the specification of distinct populations of mesoderm. Enhanced production of lateral plate-like (Flk1+PDGFRα−) mesoderm in response to Mixl1 is expected to result in an increase in commitment to the hematopoietic lineage. This prediction is supported by the increased frequency of CD41+ cells in DOX-treated cultures (this work) and by enhanced hematopoietic colony forming activity (as reported previously 28). Therefore, the differentiation of i-Mixl1 ES cells is a biologically relevant system for characterizing the transcriptional properties of Mixl1.
Mixl1 activates transcription through the multimerized MBS in both NIH 3T3 and differentiating i-Mixl1 ES cells in a manner that is dependent upon the DNA-binding homeodomain, suggesting that Mixl1 can function as a sequence-specific transcriptional activator. Although Mixl1 can bind to a mutant MBS containing a single half sites (albeit at a lower efficiency), the binding is not nfunctional, as mutations in either of the half sites abolish transcriptional activation. These findings suggest that dimeric binding of Mixl1 to its target sequences is a prerequisite for Mixl1 to activate transcription.
Gsc as a transcriptional target of Mixl1
In Xenopus, the organizer gene goosecoid is essential for patterning dorsal mesoderm and for the formation of the anteroposterior body axis 52, 53. Xenopus goosecoid expression is regulated by both TGF-β and Wnt signaling pathways through the DE and PE, respectively 47. In the presence of activin, Xenopus Mix.1 can activate transcription through a goosecoid promoter fragment containing both the DE and PE in animal caps, suggesting that goosecoid may be a target gene of Mix.1 29.
Mouse Gsc functions in craniofacial and skeletal development but its role in early stages of embryogenesis is not clear, in part because of the absence of a gastrulation phenotype in Gsc−/−embryos 54, 55. It has been proposed that early defects in Gsc mutant embryos might be compensated by the functions of other genes during mouse embryogenesis 56. Although a mouse Gsc promoter fragment containing both the DE and PE can mediate activin and XWnt-8 dependent transcriptional activation of a reporter gene in animal cap assays 47, the role of the DE and PE in the mouse embryo is not clear. Expression of Mixl1 and Gsc overlap spatially and temporally in embryos and in differentiating mouse ES cells 12, 14, 21, 25, 28, 31–34 and Gsc is activated in response to DOX-induced i-Mixl1 expression in differentiating ES cells, suggesting that Gsc is a direct transcriptional target of Mixl1. In this report, we show that Mixl1 binds to two variant MBSs in the Gsc promoter in vitro, occupies the native Gsc promoter in vivo, and activates transcription from the Gsc promoter through both gMBS1 and gMBS2 in differentiating i-Mixl1 ES cells. Taken together, these findings show that Gsc is a direct target gene of Mixl1. Potential MBSs are present in the promoters of several other genes whose expression overlaps temporally and spatially with that of Mixl1 (HZ and MHB, unpublished), but their physiological significance has not yet been evaluated.
Although both gMBS1 and gMBS2, located within the DE and PE, respectively, are required for maximal activation of the Gsc promoter by Mixl1, other sequences outside these regions may also be required for regulating Gsc expression. As a direct target gene of Mixl1, Gsc may interact with or function downstream of Mixl1. For example, it has been reported that FoxH1 represses Mixl1 through recruitment of Gsc during early embryogenesis 57. Thus, Mixl1 and Gsc might form a feedback loop that serves to control the relative expression levels of the two genes during normal development.
Supplementary Material
Acknowledgments
We thank Dr. Cory Abate-Shen for pGL2-promoter MT, Dr. Shin-Ichi Nishikawa for pSP73-Gsc3.1, and Dr. James Bieker for making his luminometer available for our use. We thank Dr. Zhiyong He for technical advice on real-time RT-PCR and Drs. Joan Isern, Rebecca Moore and Jumin Zhou for constructive comments on the manuscript. This work was supported by NIH grants KO1 DK070752 (to H. Z.) and RO1 HL62248 and EB02209 (to M. H. B.).
Footnotes
Author contributions: Conception and design, H.Z., S.T.F., M.E.F., M.H.B.; collection and assembly of data, H.Z., S.T.F., C.P.; data analysis and interpretation, H.Z., S.T.F., C.P., M.H.B.; provision of study material, M.E.H.; manuscript writing, H.Z., S.T.F., M.H.B.; financial support, H.Z. and M.H.B.; final approval of manuscript, M.H.B.
References
- 1.Rohde LA, Heisenberg CP. Zebrafish gastrulation: cell movements, signals, and mechanisms. Int Rev Cytol. 2007;261:159–192. doi: 10.1016/S0074-7696(07)61004-3. [DOI] [PubMed] [Google Scholar]
- 2.Tam PP, Loebel DA. Gene function in mouse embryogenesis: get set for gastrulation. Nat Rev Genet. 2007;8:368–381. doi: 10.1038/nrg2084. [DOI] [PubMed] [Google Scholar]
- 3.Tam PP, Loebel DA, Tanaka SS. Building the mouse gastrula: signals, asymmetry and lineages. Curr Opin Genet Dev. 2006;16:419–425. doi: 10.1016/j.gde.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 4.Ecochard V, Cayrol C, Rey S, et al. A novel Xenopus mix-like gene milk involved in the control of the endomesodermal fates. Development. 1998;125:2577–2585. doi: 10.1242/dev.125.14.2577. [DOI] [PubMed] [Google Scholar]
- 5.Guo W, Chan AP, Liang H, et al. A human Mix-like homeobox gene MIXL shows functional similarity to Xenopus Mix.1. Blood. 2002;100:89–95. doi: 10.1182/blood.v100.1.89. [DOI] [PubMed] [Google Scholar]
- 6.Henry GL, Melton DA. Mixer, a homeobox gene required for endoderm development. Science. 1998;281:91–96. doi: 10.1126/science.281.5373.91. [DOI] [PubMed] [Google Scholar]
- 7.Vize PD. DNA sequences mediating the transcriptional response of the Mix.2 homeobox gene to mesoderm induction. Dev Biol. 1996;177:226–231. doi: 10.1006/dbio.1996.0158. [DOI] [PubMed] [Google Scholar]
- 8.Tada M, Casey ES, Fairclough L, Smith JC. Bix1, a direct target of Xenopus T-box genes, causes formation of ventral mesoderm and endoderm. Development. 1998;125:3997–4006. doi: 10.1242/dev.125.20.3997. [DOI] [PubMed] [Google Scholar]
- 9.Stein S, Roeser T, Kessel M. CMIX, a paired-type homeobox gene expressed before and during formation of the avian primitive streak. Mech Dev. 1998;75:163–165. doi: 10.1016/s0925-4773(98)00088-4. [DOI] [PubMed] [Google Scholar]
- 10.Sahr K, Dias DC, Sanchez R, et al. Structure, upstream promoter region, and functional domains of a mouse and human Mix paired-like homeobox gene. Gene. 2002;291:135–147. doi: 10.1016/s0378-1119(02)00590-5. [DOI] [PubMed] [Google Scholar]
- 11.Rosa FM. Mix.1, a homeobox mRNA inducible by mesoderm inducers, is expressed mostly in the presumptive endodermal cells of Xenopus embryos. Cell. 1989;57:965–974. doi: 10.1016/0092-8674(89)90335-8. [DOI] [PubMed] [Google Scholar]
- 12.Robb L, Hartley L, Begley CG, et al. Cloning, expression analysis, and chromosomal localization of murine and human homologues of a Xenopus mix gene. Dev Dyn. 2000;219:497–504. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1070>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 13.Poulain M, Lepage T. Mezzo, a paired-like homeobox protein is an immediate target of Nodal signalling and regulates endoderm specification in zebrafish. Development. 2002;129:4901–4914. doi: 10.1242/dev.129.21.4901. [DOI] [PubMed] [Google Scholar]
- 14.Pearce JJ, Evans MJ. Mml, a mouse Mix-like gene expressed in the primitive streak. Mech Dev. 1999;87:189–192. doi: 10.1016/s0925-4773(99)00135-5. [DOI] [PubMed] [Google Scholar]
- 15.Peale FV, Jr, Sugden L, Bothwell M. Characterization of CMIX, a chicken homeobox gene related to the Xenopus gene mix.1. Mech Dev. 1998;75:167–170. doi: 10.1016/s0925-4773(98)00089-6. [DOI] [PubMed] [Google Scholar]
- 16.Mead PE, Brivanlou IH, Kelley CM, Zon LI. BMP-4-responsive regulation of dorsal-ventral patterning by the homeobox protein Mix.1. Nature. 1996;382:357–360. doi: 10.1038/382357a0. [DOI] [PubMed] [Google Scholar]
- 17.Mead PE, Zhou Y, Lustig KD, Huber TL, Kirschner MW, Zon LI. Cloning of Mix-related homeodomain proteins using fast retrieval of gel shift activities, (FROGS), a technique for the isolation of DNA-binding proteins. Proc Natl Acad Sci U S A. 1998;95:11251–11256. doi: 10.1073/pnas.95.19.11251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kikuchi Y, Trinh LA, Reiter JF, Alexander J, Yelon D, Stainier DY. The zebrafish bonnie and clyde gene encodes a Mix family homeodomain protein that regulates the generation of endodermal precursors. Genes Dev. 2000;14:1279–1289. [PMC free article] [PubMed] [Google Scholar]
- 19.Trinh LA, Meyer D, Stainier DY. The Mix family homeodomain gene bonnie and clyde functions with other components of the Nodal signaling pathway to regulate neural patterning in zebrafish. Development. 2003;130:4989–4998. doi: 10.1242/dev.00614. [DOI] [PubMed] [Google Scholar]
- 20.Pick M, Azzola L, Mossman A, Stanley EG, Elefanty AG. Differentiation of human embryonic stem cells in serum-free medium reveals distinct roles for bone morphogenetic protein 4, vascular endothelial growth factor, stem cell factor, and fibroblast growth factor 2 in hematopoiesis. Stem Cells. 2007;25:2206–2214. doi: 10.1634/stemcells.2006-0713. [DOI] [PubMed] [Google Scholar]
- 21.Ng ES, Azzola L, Sourris K, Robb L, Stanley EG, Elefanty AG. The primitive streak gene Mixl1 is required for efficient haematopoiesis and BMP4-induced ventral mesoderm patterning in differentiating ES cells. Development. 2005;132:873–884. doi: 10.1242/dev.01657. [DOI] [PubMed] [Google Scholar]
- 22.Lemaire P, Darras S, Caillol D, Kodjabachian L. A role for the vegetally expressed Xenopus gene Mix.1 in endoderm formation and in the restriction of mesoderm to the marginal zone. Development. 1998;125:2371–2380. doi: 10.1242/dev.125.13.2371. [DOI] [PubMed] [Google Scholar]
- 23.Kunwar PS, Zimmerman S, Bennett JT, Chen Y, Whitman M, Schier AF. Mixer/Bon and FoxH1/Sur have overlapping and divergent roles in Nodal signaling and mesendoderm induction. Development. 2003;130:5589–5599. doi: 10.1242/dev.00803. [DOI] [PubMed] [Google Scholar]
- 24.Hart AH, Willson TA, Wong M, Parker K, Robb L. Transcriptional regulation of the homeobox gene Mixl1 by TGF-beta and FoxH1. Biochem Biophys Res Commun. 2005;333:1361–1369. doi: 10.1016/j.bbrc.2005.06.044. [DOI] [PubMed] [Google Scholar]
- 25.Mohn D, Chen SW, Dias DC, et al. Mouse Mix gene is activated early during differentiation of ES and F9 stem cells and induces endoderm in frog embryos. Dev Dyn. 2003;226:446–459. doi: 10.1002/dvdy.10263. [DOI] [PubMed] [Google Scholar]
- 26.Hart AH, Hartley L, Sourris K, et al. Mixl1 is required for axial mesendoderm morphogenesis and patterning in the murine embryo. Development. 2002;129:3597–3608. doi: 10.1242/dev.129.15.3597. [DOI] [PubMed] [Google Scholar]
- 27.Izumi N, Era T, Akimaru H, Yasunaga M, Nishikawa S. Dissecting the molecular hierarchy for mesendoderm differentiation through a combination of embryonic stem cell culture and RNA interference. Stem Cells. 2007;25:1664–1674. doi: 10.1634/stemcells.2006-0681. [DOI] [PubMed] [Google Scholar]
- 28.Willey S, Ayuso-Sacido A, Zhang H, et al. Acceleration of mesoderm development and expansion of hematopoietic progenitors in differentiating ES cells by the mouse Mix-like homeodomain transcription factor. Blood. 2006;107:3122–3130. doi: 10.1182/blood-2005-10-4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Latinkic BV, Smith JC. Goosecoid and mix.1 repress Brachyury expression and are required for head formation in Xenopus. Development. 1999;126:1769–1779. doi: 10.1242/dev.126.8.1769. [DOI] [PubMed] [Google Scholar]
- 30.Germain S, Howell M, Esslemont GM, Hill CS. Homeodomain and winged-helix transcription factors recruit activated Smads to distinct promoter elements via a common Smad interaction motif. Genes Dev. 2000;14:435–451. [PMC free article] [PubMed] [Google Scholar]
- 31.Blum M, Gaunt SJ, Cho KW, et al. Gastrulation in the mouse: the role of the homeobox gene goosecoid. Cell. 1992;69:1097–1106. doi: 10.1016/0092-8674(92)90632-m. [DOI] [PubMed] [Google Scholar]
- 32.Faust C, Schumacher A, Holdener B, Magnuson T. The eed mutation disrupts anterior mesoderm production in mice. Development. 1995;121:273–285. doi: 10.1242/dev.121.2.273. [DOI] [PubMed] [Google Scholar]
- 33.Perea-Gomez A, Shawlot W, Sasaki H, Behringer RR, Ang S. HNF3beta and Lim1 interact in the visceral endoderm to regulate primitive streak formation and anterior-posterior polarity in the mouse embryo. Development. 1999;126:4499–4511. doi: 10.1242/dev.126.20.4499. [DOI] [PubMed] [Google Scholar]
- 34.Norris DP, Brennan J, Bikoff EK, Robertson EJ. The Foxh1-dependent autoregulatory enhancer controls the level of Nodal signals in the mouse embryo. Development. 2002;129:3455–3468. doi: 10.1242/dev.129.14.3455. [DOI] [PubMed] [Google Scholar]
- 35.Yeung KC, Inostroza JA, Mermelstein FH, Kannabiran C, Reinberg D. Structure-function analysis of the TBP-binding protein Dr1 reveals a mechanism for repression of class II gene transcription. Genes Dev. 1994;8:2097–2109. doi: 10.1101/gad.8.17.2097. [DOI] [PubMed] [Google Scholar]
- 36.Kennedy M, Keller GM. Hematopoietic commitment of ES cells in culture. Methods Enzymol. 2003;365:39–59. doi: 10.1016/s0076-6879(03)65003-2. [DOI] [PubMed] [Google Scholar]
- 37.Pollock RM. Determination of protein-DNA sequence specificity by PCR-assisted binding-site selection. Curr Protoc Mol Biol. 2001;Chapter 12(Unit 12):11. doi: 10.1002/0471142727.mb1211s33. [DOI] [PubMed] [Google Scholar]
- 38.Wilson D, Sheng G, Lecuit T, Dostatni N, Desplan C. Cooperative dimerization of paired class homeo domains on DNA. Genes Dev. 1993;7:2120–2134. doi: 10.1101/gad.7.11.2120. [DOI] [PubMed] [Google Scholar]
- 39.Crooks GE, Hon G, Chandonia JM, Brenner SE. WebLogo: a sequence logo generator. Genome Res. 2004;14:1188–1190. doi: 10.1101/gr.849004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Catron KM, Iler N, Abate C. Nucleotides flanking a conserved TAAT core dictate the DNA binding specificity of three murine homeodomain proteins. Mol Cell Biol. 1993;13:2354–2365. doi: 10.1128/mcb.13.4.2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. 2. Vol. 3. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 42.Aparicio O, Geisberg JV, Sekinger E, Yang A, Moqtaderi Z, Struhl K. Chromatin immunoprecipitation for determining the association of proteins with specific genomic sequences in vivo. Curr Protoc Mol Biol. 2005;Chapter 21(Unit 21):23. doi: 10.1002/0471142727.mb2103s69. [DOI] [PubMed] [Google Scholar]
- 43.Keller G. Embryonic stem cell differentiation: emergence of a new era in biology and medicine. Genes Dev. 2005;19:1129–1155. doi: 10.1101/gad.1303605. [DOI] [PubMed] [Google Scholar]
- 44.Tada S, Era T, Furusawa C, et al. Characterization of mesendoderm: a diverging point of the definitive endoderm and mesoderm in embryonic stem cell differentiation culture. Development. 2005;132:4363–4374. doi: 10.1242/dev.02005. [DOI] [PubMed] [Google Scholar]
- 45.Takebe A, Era T, Okada M, Martin Jakt L, Kuroda Y, Nishikawa S. Microarray analysis of PDGFR alpha+ populations in ES cell differentiation culture identifies genes involved in differentiation of mesoderm and mesenchyme including ARID3b that is essential for development of embryonic mesenchymal cells. Dev Biol. 2006;293:25–37. doi: 10.1016/j.ydbio.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 46.Mikkola HK, Fujiwara Y, Schlaeger TM, Traver D, Orkin SH. Expression of CD41 marks the initiation of definitive hematopoiesis in the mouse embryo. Blood. 2003;101:508–516. doi: 10.1182/blood-2002-06-1699. [DOI] [PubMed] [Google Scholar]
- 47.Watabe T, Kim S, Candia A, et al. Molecular mechanisms of Spemann’s organizer formation: conserved growth factor synergy between Xenopus and mouse. Genes Dev. 1995;9:3038–3050. doi: 10.1101/gad.9.24.3038. [DOI] [PubMed] [Google Scholar]
- 48.Wilson DS, Sheng G, Jun S, Desplan C. Conservation and diversification in homeodomain-DNA interactions: a comparative genetic analysis. Proc Natl Acad Sci U S A. 1996;93:6886–6891. doi: 10.1073/pnas.93.14.6886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mailhos C, Andre S, Mollereau B, Goriely A, Hemmati-Brivanlou A, Desplan C. Drosophila Goosecoid requires a conserved heptapeptide for repression of paired-class homeoprotein activators. Development. 1998;125:937–947. doi: 10.1242/dev.125.5.937. [DOI] [PubMed] [Google Scholar]
- 50.Qu S, Tucker SC, Zhao Q, deCrombrugghe B, Wisdom R. Physical and genetic interactions between Alx4 and Cart1. Development. 1999;126:359–369. doi: 10.1242/dev.126.2.359. [DOI] [PubMed] [Google Scholar]
- 51.Tucker SC, Wisdom R. Site-specific heterodimerization by paired class homeodomain proteins mediates selective transcriptional responses. J Biol Chem. 1999;274:32325–32332. doi: 10.1074/jbc.274.45.32325. [DOI] [PubMed] [Google Scholar]
- 52.Blumberg B, Wright CV, De Robertis EM, Cho KW. Organizer-specific homeobox genes in Xenopus laevis embryos. Science. 1991;253:194–196. doi: 10.1126/science.1677215. [DOI] [PubMed] [Google Scholar]
- 53.Cho KW, Blumberg B, Steinbeisser H, De Robertis EM. Molecular nature of Spemann’s organizer: the role of the Xenopus homeobox gene goosecoid. Cell. 1991;67:1111–1120. doi: 10.1016/0092-8674(91)90288-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yamada G, Mansouri A, Torres M, et al. Targeted mutation of the murine goosecoid gene results in craniofacial defects and neonatal death. Development. 1995;121:2917–2922. doi: 10.1242/dev.121.9.2917. [DOI] [PubMed] [Google Scholar]
- 55.Rivera-Perez JA, Mallo M, Gendron-Maguire M, Gridley T, Behringer RR. Goosecoid is not an essential component of the mouse gastrula organizer but is required for craniofacial and rib development. Development. 1995;121:3005–3012. doi: 10.1242/dev.121.9.3005. [DOI] [PubMed] [Google Scholar]
- 56.De Robertis EM. Goosecoid and gastrulation. In: Stern CD, editor. Gastrulation: From Cells to Embryos. 1. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 2004. pp. 581–589. [Google Scholar]
- 57.Izzi L, Silvestri C, von Both I, et al. Foxh1 recruits Gsc to negatively regulate Mixl1 expression during early mouse development. Embo J. 2007;26:3132–3143. doi: 10.1038/sj.emboj.7601753. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.