Abstract
In 2004, chikungunya virus (CHIKV) re-emerged from East Africa to cause devastating epidemics of debilitating and often chronic arthralgia that have affected millions of people in the Indian Ocean Basin and Asia. More limited epidemics initiated by travelers subsequently occurred in Italy and France, as well as human cases exported to most regions of the world, including the Americas where CHIKV could become endemic. Because CHIKV circulates during epidemics in an urban mosquito–human cycle, control of transmission relies on mosquito abatement, which is rarely effective. Furthermore, there is no antiviral treatment for CHIKV infection and no licensed vaccine to prevent disease. Here, we discuss the challenges to the development of a safe, effective and affordable chikungunya vaccine and recent progress toward this goal.
Keywords: alphavirus, antibody, chikungunya, emergence, evolution, mosquito, vaccine
Chikungunya virus (CHIKV), first isolated in 1953 during an epidemic in Tanzania among the Makonde tribe [1], is a mosquito-borne alphavirus in the family Togaviridae [2]. Its name derives from a Makonde word that translates to ‘disease that bends up the joints’, describing the posture of infected persons experiencing severe joint pain. As described initially by Ross, CHIKV infection typically causes a “very sharp onset of crippling joint pains, severe fever and eventually the conspicuous rash” [1]. It is easily confused with dengue, and Ross described chikungunya fever (CHIK) as “a clinical variant of classical dengue differing in the absence of headache, of tenderness on pressure to the eyeballs, and of pain on eye movements” [1]. Synovitis can be severe and highly destructive, as demonstrated in a case report documenting chronic arthritis leading to destroyed metatarsal heads and osteoarthritic lesions in the ankles [3]. However, unlike dengue, attack rates during epidemics often exceed 50% with few inapparent infections.
Although CHIK is usually self-limiting and is rarely fatal, the arthralgia is extremely painful and debilitating, typically lasting for 1 week but often much longer. For example, on La Réunion Island, 33% of joint pains, 10% of cerebral disorders and 7.5% of sensory and neural impairments among all residents were attributed to CHIKV infection during an epidemic that occurred 18 months earlier [4]. One study in India indicated that over 72% of patients suffered from arthralgia 1 month after onset [5], and another estimated the mean duration of arthritic pain at 89 days [6]. Chronic arthralgia has been associated with high levels of IL-6 and granulocyte macrophage colony-stimulating factor [7], and older patients and persons with high viremia during the acute phase are more likely to develop chronic symptoms. The latter study detected CHIKV in perivascular synovial macrophages in one chronic patient 18 months after onset [8]. Both the acute arthralgia as well as the chronic disease result in major losses in productivity; in one affected region of India, approximately two-thirds of the disability measured in the population was attributed to CHIKV infections [9].
CHIKV genome, structure & replication
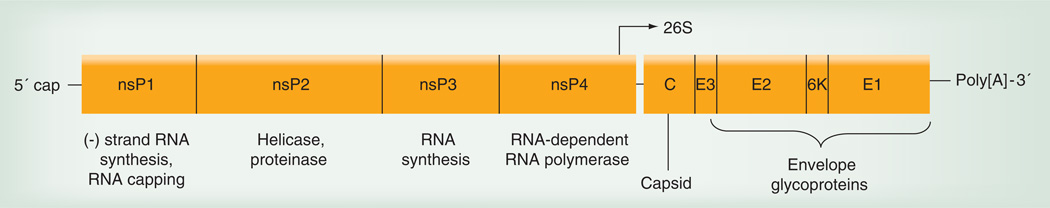
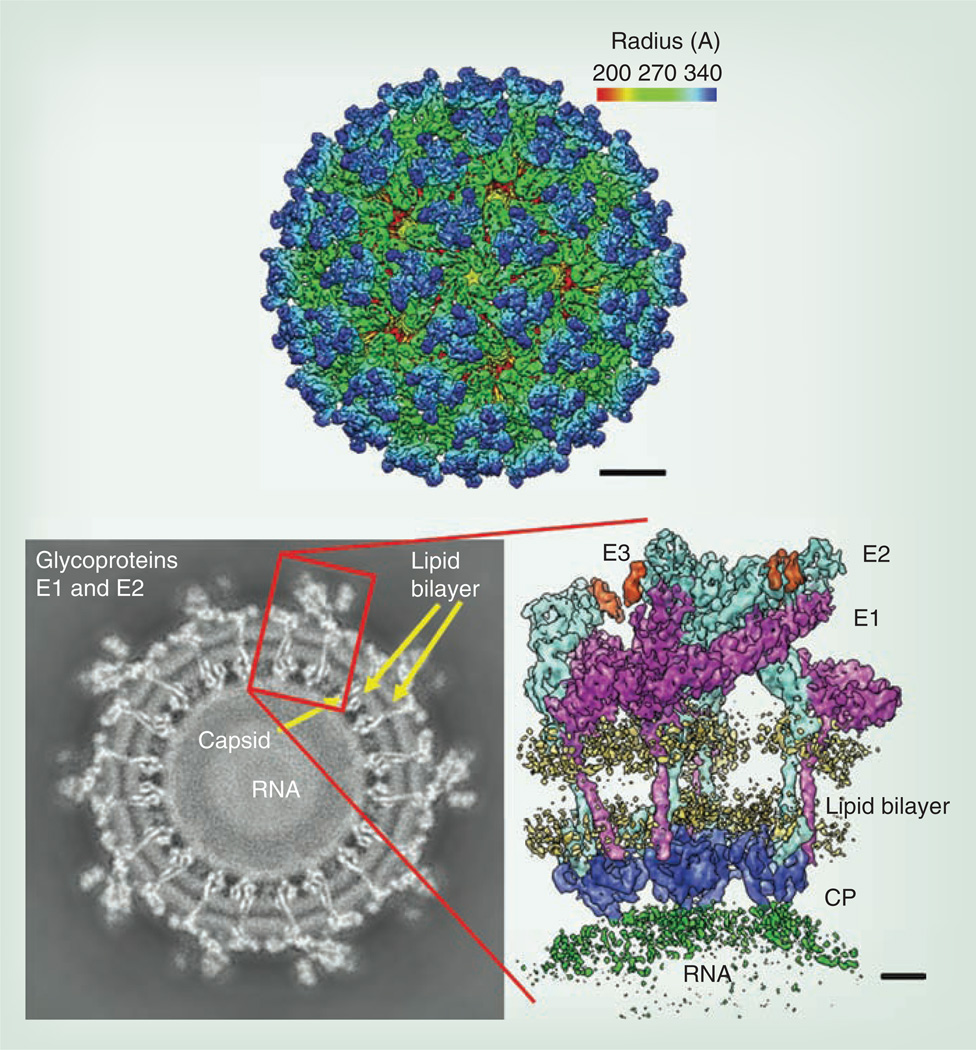
Like other alphaviruses, CHIKV contains an RNA genome of approximately 11.8 kB that is single stranded and messenger (‘positive strand’) sense (Figure 1). The genomic RNA is capped and polyadenylated, and encodes two open reading frames (ORFs). The 5´ ORF comprises nearly two-thirds of the genome and encodes four nonstructural proteins that participate in genome replication, RNA capping, polyprotein cleavage and other functions required for viral replication. It is expressed via cap-dependent translation as an nsP1–3 or nsP1–4 polyprotein that is cleaved by an nsP2-encoded protease. By contrast, the structural protein ORF is included in a subgenomic message, which is then translated via a cap-dependent mechanism. The structural ORF polyprotein is eventually cleaved into the three main structural proteins: the capsid and the envelope glycoproteins E2 and E1 [10]. The mature virion is 70 nm in diameter and contains 240 heterodimers of E2/E1 arranged as trimeric spikes on its surface (Figure 2). These heterodimer spikes are inserted into the plasma membrane of infected cells after transport through the secretory pathway. Cytoplasmic nucleocapsids containing the genomic RNA and 240 copies of the capsid protein bud from the cell surface to acquire the virion envelope and envelope protein spikes.
Figure 1. Organization of the chikungunya virus genome, including the nonstructural and structural polyprotein open reading frames, and the 26S or subgenomic promoter.
Figure 2. Cryoelectron microscopic reconstruction of the alphavirus virion.
Enlarged image on the right shows the orientation of the E2 and E1 envelope glycoproteins in the virion spikes.
CP: Capsid protein.
Reproduced with permission from [116].
Transmission cycles of CHIKV
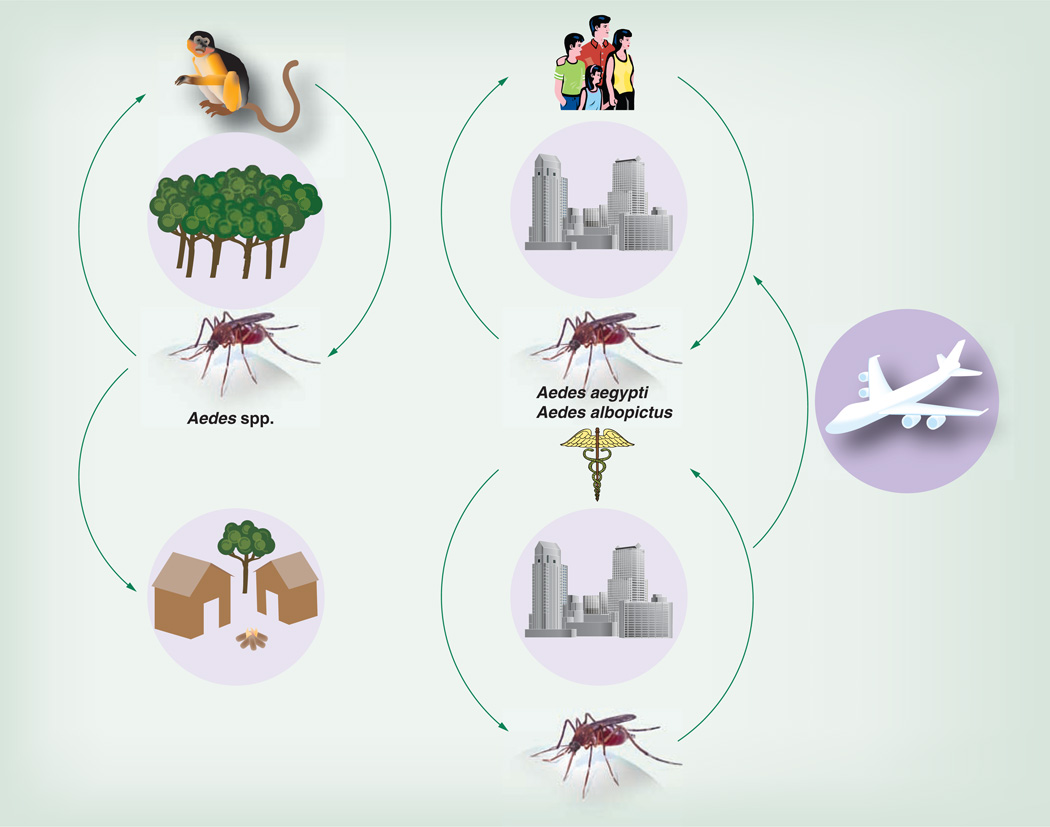
Like most alphaviruses, CHIKV is a mosquito-borne arbovirus. Two distinct transmission cycles have been well documented. In Africa, an enzootic cycle occurs in forested habitats where arboreal mosquitoes, principally Aedes furcifer, Aedes taylori, Aedes africanus and/or Aedes luteocephalus, serve as vectors (Figure 3) [11–14]. Most evidence points to nonhuman primates (NHPs) as the principal reservoir and amplification hosts in the enzootic cycle based on their high rates of seroprevalence [15], documented infection and viremia in nature, and viremia levels in response to experimental infection [16,17]. Enzootic transmission has not been described on other continents despite the presence of endemic CHIK in Southeast Asia at least since the 1950s [18]. However, seroprevalence in some Asian NHPs [19] and CHIKV isolation from a monkey in Malaysia [20] suggest the possibility of such an Asian enzootic cycle.
Figure 3. Chikungunya virus transmission cycles, including the progenitor sylvatic, enzootic cycle on the left and the emergent epidemic cycle on the right.
Reproduced with permission from [32].
In Africa, the enzootic transmission cycle can spill over to infect people who live nearby, and enzootic mosquito vectors may be involved in interhuman transmission during small outbreaks. Aedes furcifer, probably a principal enzootic vector, is known to enter human villages [11], where it presumably transmits from monkeys to humans. Epidemics also occur in Africa when CHIKV is introduced into urban areas where the more anthropophilic vectors, Aedes aegypti and Aedes albopictus, can initiate human–mosquito–human transmission [21,22].
Like only two other mosquito-borne viruses, dengue and yellow fever, CHIKV is capable of initiating a sustained, urban transmission cycle that relies only on A. aegypti and/or A. albopictus and human amplification hosts [23,24]. This endemic/epidemic cycle results in high levels of human exposure to mosquito transmission, particularly because these vectors live in close proximity to people. The behavior and ecology of A. aegypti, in particular, are ideal for epidemic transmission because adult females prefer to feed on humans, often take several partial blood meals during a single gonotrophic cycle, oviposit in artificial containers as their preferred larval sites, and rest inside houses with ready access to human hosts [25]. Although the infectivity of various CHIKV strains varies widely for both A. aegypti and A. albopictus, humans develop high-titer viremias that generally persist during the first 4 days after the onset of symptoms, with the peak estimated on the day of onset at approximately 109 viral RNA copies/ml [26] and infectious titers sometimes exceeding 107 PFU/ml [27]. These titers generally exceed the oral infectious dose 50% levels for both epidemic vector species [28–31], permitting efficient transmission among humans by mosquitoes. However, as discussed below, adaptive mutations for A. albopictus infection and transmission have dramatically affected the intensity of epidemic transmission during outbreaks that began in 2005, as well as the spread of the disease to more temperate climates [24,32].
History of chikungunya outbreaks
Phylogenetic studies of CHIKV suggest that the virus has been circulating enzootically in Africa for centuries or longer [33,34]. Although CHIKV infection is difficult to distinguish from dengue and other tropical diseases, Carey suggested that CHIK outbreaks occurred in Indonesia during the 18th century, presumably transported from Africa on sailing ships that harbored both the vector, A. aegypti, and susceptible humans to allow onboard transmission [18]. This mechanism is also suspected for the introduction of yellow fever virus from Africa into the New World during the 17th or 18th century [35]. The possibility of transient, imported CHIK epidemics in the Americas has also been proposed [18]. In addition to evidence of CHIK outbreaks in the 18th century, there are descriptions of serial epidemics consistent with CHIK in India, dating from the 1820s, 1853, 1871 and 1923, especially in Calcutta but also extending to Burma [18,36]. Carey suspected that many of these outbreaks were transported aboard sailing ships embarking from Zanzibar, off the coast of East Africa [18].
Evolution & emergence mechanisms
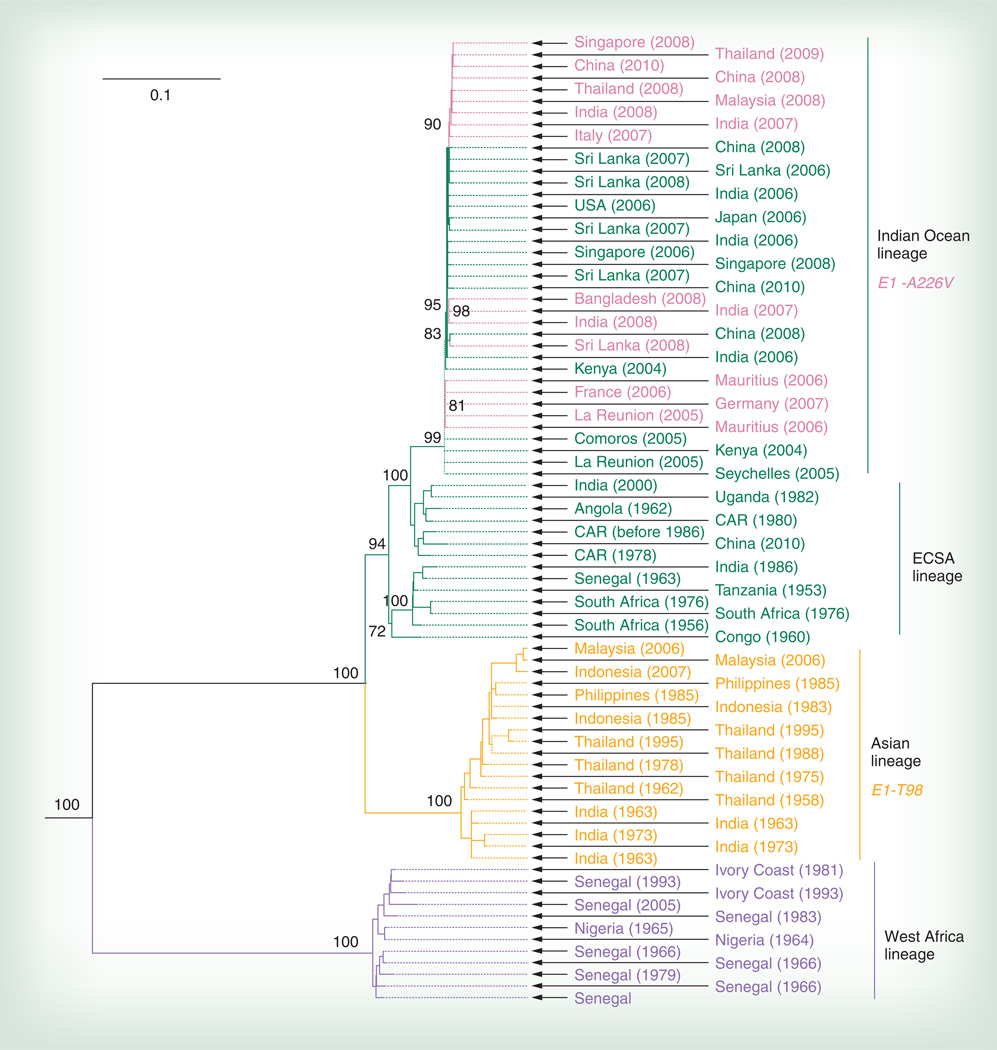
The historical accounts of CHIKV spread from Africa to Asia and perhaps the Americas were supported by the first phylogenetic studies of the virus [33]. These studies concluded that CHIKV originated in Africa, where two major enzootic lineages circulate principally in West and East/Central/South Africa (ECSA), respectively. The oldest Asian CHIKV isolates, dating from the late 1950s or earlier, fell within the ECSA lineage, indicating emergence and transport from this region of Africa. By contrast, the West African lineage was not associated with any CHIKV strains isolated during major epidemics outside of Africa.
More recent phylogenetic analyses using complete genomic sequences (Figure 4) largely confirm these earlier results and place more precise dates on CHIKV divergence events. Although the accuracy of ancient divergence estimates using coalescent methods is difficult to verify, these studies conclude that all CHIKV strains sampled to date evolved from a common ancestor during the past 500 years, and that the endemic/epidemic lineage first sampled in Asia during the late 1950s was introduced from East Africa at least 70 years ago [34]. The ECSA CHIKV lineage was also identified in West Africa (a bat isolate from Senegal), indicating that the two main enzootic clades overlap spatially, at least on occasion.
Figure 4. Phylogenetic tree of chikungunya virus strains derived from complete concatenated open reading frames for the nonstructural and structural polyproteins.
Key E1 amino acid substitutions that facilitated (Indian Ocean lineage) or prevented (Asian lineage) adaptation to Aedes albopictus are shown on the right.
CAR: Central African republic; ECSA: East/Central/South Africa.
In 2004, a CHIK epidemic began in East Africa that resulted in its emergence both in the literal sense and in the figurative sense from relative obscurity. Initially, epidemics were observed in coastal Kenya, first in Lamu and then in Mombasa [37,38], followed by spread to Comoros, La Réunion and other islands in the Indian Ocean during 2005 (Figure 5) [39]. Many tourists returning to Europe from vacations in this region were afflicted, raising awareness of the epidemic [26]. Then, in late 2005, epidemics ensued in India [40], followed by exportation via infected travelers to nearly all regions of the world including the USA [41]. Autochthonous transmission of these new epidemic strains subsequently occurred in Southeast Asia [42] and Europe [43,44], but fortunately not in the Americas. Many of these epidemics in the Indian Ocean Basin, India and Southeast Asia continued into 2012.
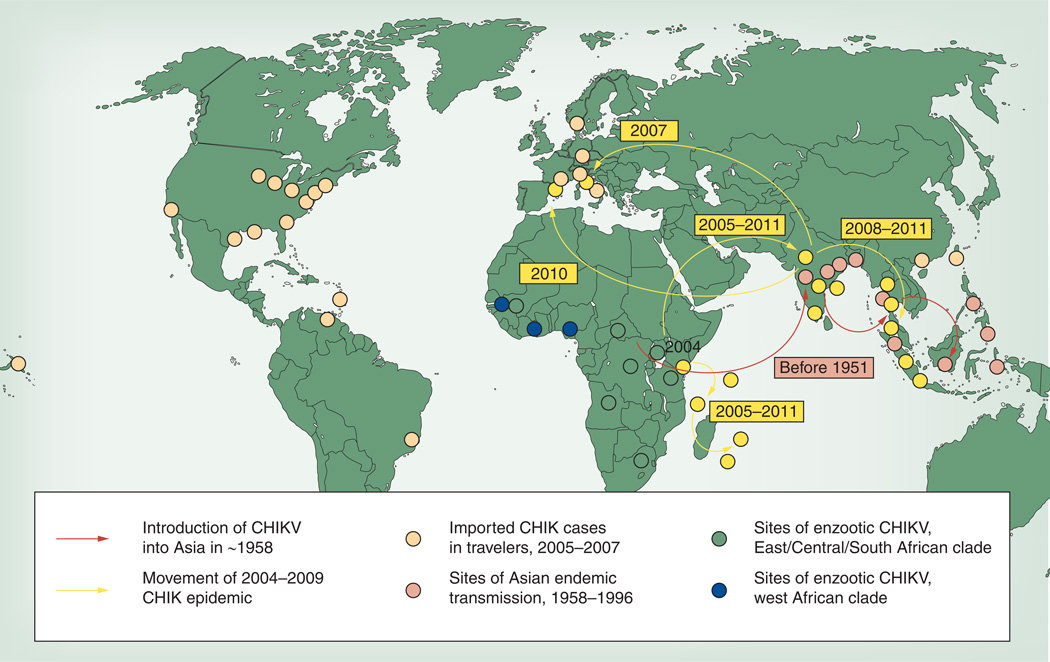
Figure 5. Distribution of chikungunya virus strains and movement of outbreaks inferred from the phylogenetic analysis depicted in Figure 4.
CHIK: Chikungunya fever; CHIKV: Chikungunya virus.
Reproduced with permission from [24].
The source of the 2005 Indian Ocean Basin epidemic was first traced by Schuffenecker et al. to the ECSA enzootic lineage, like the earlier Asian emergence before 1958 [45]. The Indian epidemic was later shown to have resulted from an independent emergence from the mainland of East Africa [34]. The first CHIKV isolates from the La Réunion epidemic exhibited an alanine at E1 envelope glycoprotein residue 226, but later isolates showed an A226V substitution near the fusion peptide that mediates viral entry via endosomes. On the basis of earlier studies implicating this residue in cholesterol dependence for replication of a closely related alphavirus, Semliki Forest virus [46], Schuffenecker et al. hypothesized that this CHIKV E1 substitution affected infection of mosquitoes, which are cholesterol auxotrophs [45]. This hypothesis was later supported by experimental infections of A. albopictus, which demonstrated that this mutation caused a dramatic increase in CHIKV infectivity [28,31]. The A226V substitution, which confers an approximate 100-fold reduction in the infectious dose 50% for A. albopictus but has little or no effect on infection of A. aegypti [28,30], appeared to result in highly efficient transmission in regions where the latter mosquito, previously considered the only principal vector, was not abundant. This substitution was shown to have occurred independently or convergently in several locations of India and in La Réunion and Gabon [47], and was consistently found in locations where A. albopictus was the predominant vector, consistent with convergent selection by vector susceptibility [34,48].
Finally, beginning in 2009, an additional envelope glycoprotein substitution, L210Q in the E2 protein, was detected in regions of India where A. albopictus was the main vector [49]. This mutation has been shown to further increase infectivity for A. albopictus, albeit to a lesser extent that E1-A226V, but again has no effect on infectivity for A. aegypti [30]. This additional adaptive mutation suggests even more efficient transmission of current Indian strains, with major public health implications not only for that country but also due to the threat of CHIKV exportation to other regions. Since 1985, A. albopictus has colonized many parts of the world from its native Asia and, unlike A. aegypti, it can survive winters in temperate climates [50]. Thus, the adaptation of CHIKV to this vector puts many new temperate regions of the world, including the Americas, at risk for epidemics and endemicity.
A surprising finding of recent CHIKV sequencing efforts was the lack of either of the A. albopictus-adaptive envelope glycoprotein mutations in strains of the old endemic/epidemic Asian lineage. This CHIKV genotype has been circulating in regions native to A. albopictus for at least 64 years yet did not undergo comparable adaptive selection for more efficient infection of this vector. This was particularly surprising because these mutations, while conferring a strong fitness advantage in A. albopictus, have little or no effect on infection of A. aegypti, assumed to be the principal vector in Southeast Asia. Aedes albopictus tends to be more common in rural and forested areas while A. aegypti predominates in urban settings of many tropical regions [51–53]. In India, A. aegypti predominates in many regions during the dry season, but A. albopictus populations in the South are highest during the rainy season [54,55]. Tsetsarkin et al. hypothesized that the E1-A226V substitutions had CHIKV lineage-specific penetrance, and showed that, in the genetic background of the old Asian strains, they had no effect on infectivity for either urban vector [56]. An additional E1 substitution, already present in ECSA strains but not in the old Asian lineage, was required for E1–226V to exert its effect on A. albopictus infection. Thus, a single mutation with an apparently neutral phenotype prevented the Asian CHIKV strain from adapting to A. albopictus for decades, underscoring the dramatic impact of epistatic effects on CHIKV phenotypes [32,56].
Human chikungunya disease
In humans, CHIK is characterized by an abrupt febrile illness (>38.9°C), polyarthralgia and maculopapular rash. In some cases, the clinical symptoms are indistinguishable from dengue fever. The incubation period for CHIKV ranges from 3 to 7 days and asymptomatic infections occur in 5–15% of the cases. The case–fatality ratio has been estimated to be 1:1000, with most deaths occurring in neonates, adults with underlying conditions and older people [57]. Following transmission, CHIKV replicates in the skin and then disseminates to the liver and joints, presumably through the blood. The acute infection can last 1–10 days and is characterized by high levels of viremia (105–1012 RNA copies per milliliter of blood as established by real-time PCR) that can last up to 12 days [58]. Interestingly, higher viremias are detected in newborns and older patients with CHIKV disease, but their effect on increased disease severity is unclear [59].
Recent studies during the La Reunion outbreak revealed that the most common clinical signs and symptoms observed in patients with CHIK included asymmetrical bilateral polyarthralgia (96%) affecting the lower (98%) and small joints (75%), asthenia (89%), headache (70%), digestive trouble (63%), myalgia (59%), exanthems (48%), conjunctival hyperhemia (23%) and adenopathy (9%) [57,59,60]. Vertigo, cutaneous dysesthesia, pharyngitis and hemorrhages were seldom observed. The pain can be excruciating and can involve more than one joint; fingers, wrists, elbows, toes, ankles and knees are most commonly affected. Previously injured joints are especially susceptible. Paresthesia (numbness and tingling) in the skin covering affected joints has also been described, although the joints are often swollen there and usually have no other signs of inflammation.
Usually, CHIK is a self-limiting disease, but unusual clinical presentations were observed during the recent La Reunion outbreak, including hepatitis, autoimmune neurologic pathologies (Guillain–Barré), cardiologic manifestations and deaths [60]. Neurological manifestations can include encephalitis, myelopathy, peripheral neuropathy and myopathy. Mother-to-child transmission is also being reported with high rates of morbidity. Vertical transmission from CHIKV-infected mother to fetus can also occur and can lead to congenital illness and fetal death. Encephalopathy appears to represent the most common neurologic manifestation among CHIKV-infected neonates through mother-to-child transmission. The neurological manifestations described in adults involve cases of encephalopathy frequently associated with the presence of IgM anti-CHIKV antibodies in cerebrospinal fluid, encephalitis and encephalomyeloradiculitis. Frequent clinical laboratory abnormalities observed during CHIK infection include leukopenia (38%), thrombocytopenia (37%), increased aspartate aminotransferase and alanine aminotransferase blood levels (32 and 7%, respectively) and hypocalcemia [60] (39%). Lymphopenia (<1000/mm3) was very closely associated with viremic patients.
The acute signs and symptoms usually resolve in less than 2 weeks, but arthralgia may linger for weeks, months or even years, and this is a clinical symptom that may distinguish CHIKV from dengue virus infection. Reports suggest that the nature of the joint pain during and after CHIKV infection might be inflammatory [61,62]. Increased levels of urinary proline, hydroxyproline and mucopolysaccharides have been reported among CHIKV-infected patients [62], which indicates an inflammatory response in the connective tissues. Although patients are positive for rheumatoid factor and anticyclic citrullinated peptide, there are generally no rheumatoid arthritis (RA)-related classic symptoms such as erosion of cartilages and bones. Manimunda et al. studied CHIK-infected patients with arthritic disease, and they tested negative for rheumatoid factor and positive for anticyclic citrullinated peptide [62]. Their MRI findings showed joint effusion, erosion marrow edema, synovial thickening, tendinitis and tenosynovitis, suggesting that CHIK arthritis is chronic inflammatory erosive arthritis. The molecular mechanism for this chronic arthralgia is still not well understood. Involvement of many factors have been reported in long-term persistence of arthralgia. These factors include viral persistence in tissue sanctuaries, evasion of immune responses, re-activation of virus, uncontrolled proinflammatory cytokine response and/or crossreactivity with self-antigens [58]. Rheumatic manifestations in 10–20% of the patients with CHIKV typically consist of a febrile arthritis mainly affecting the extremities (ankles, wrists or phalanges) [60]. A recent study has reported high levels of CHIKV IgM in a cohort of Indian patients with post-CHIKV RA-like illnesses [63]. These patients were clearly naive for musculoskeletal disorders before CHIKV infection. Interestingly, 5–10% of patients with CHIKV arthritis were also positive for rheumatoid factor and anticyclic citrullinated peptide. This study did not reveal any major RA classic erosions of the cartilage and bones, and hence the post-CHIKV RA is reminiscent of, but distinguishable from, autoimmune RA. Another study suggested that rapid neutralization and resolution of acute viremia with higher levels of neutralizing IgG3 antibodies were associated with lack of long-term arthralgia, whereas later development of an IgG3-neutralizing antibody response was associated with persistent arthralgia [64,65].
The pathogenesis of CHIKV infection in humans is still poorly understood, but recent outbreaks have provided insights into the cells and organs involved in viral replication. CHIKV can replicate in human epithelial and endothelial cells, primary fibroblasts and to a lesser extent, monocyte-derived macrophages [66]. By contrast, CHIKV does not replicate in lymphoid and monocytoid cell lines, primary lymphocytes and monocytes, or monocyte-derived dendritic cells. Immunohistology studies on muscle biopsies from CHIKV-infected patients with myositic syndrome show viral antigens exclusively inside skeletal muscle progenitor cells (designed as satellite cells), and not in muscle fibers. CHIKV can replicate and induce cytopathic effect in human satellite cells, whereas myotubes are essentially refractory to infection. Interestingly, CHIKV was detected in these cells during the acute and recurrent phase (3 months post-initial infection). Although CHIKV is not a neurotropic virus, there is evidence supporting neurological involvement, although the molecular mechanisms have not been investigated [67].
CHIKV infection elicits strong innate responses, principally involving the production of antiviral IFN-α as well as many proinflammatory cytokines, chemokines and growth factors [68]. This innate response is followed by activation of adaptive immunity through activation and proliferation of CD8+ T cells in the early stages of the disease. Later stages of the acute phase are characterized by a classical switch to CD4+ T-cell response and the production of anti-inflammatory proteins IL-1RA and IL-2RA. Contrary results have been found between the correlation of viremia and levels of IFN-α. During typical CHIKV infection, strong IFN-α production could be crucial in the rapid control of the viremia. CHIKV infection induces a strong inflammatory response that may be orchestrated by the production of IL-16, IL-17, monocyte chemoattractant protein 1 (MCP-1), IP-10 and MIP-1α. The end of the acute phase is characterized by the production of proinflammatory MIF, MIP-1b, SDF-1a, and IL-6 and IL-8. CCL5 levels also were high in all patients during the first week after symptom onset. CCL5, MCP-1, IP-10, MIP-1b and IL-8 are produced by activated macrophages that are susceptible to CHIKV infection [66]. These chemokines play a major role in recruiting leucocytes to sites of infection, as well as orchestrating the deployment of efficient antiviral defenses.
CHIKV infection also induces strong cellular immune responses. High plasma levels of IFN-γ, IL-4, IL-7 and IL-12p40, cytokines that promote adaptive immunity, suggested the involvement of cellular responses [68]. A key role for natural killer cells in the clearance of infected cells and in the development of CHIKV arthralgia has also been suggested. The B cell-promoting cytokines IL-4 and in some cases IL-10, are upregulated in the first few days after symptom onset and may enhance the production of CHIKV-specific IgG. CD4+ T lymphocytes, which are also involved in the promotion of humoral responses, are strongly activated toward the end of the acute phase. IgG antibodies are detected in the first week after infection, indicating rapid seroconversion and high levels of antibody responses among CHIKV-infected individuals [64]. CHIKV-specific IgM and IgGs last for 4–6 months in CHIKV-infected patients [69]. However, their role in chronic arthralgia is not very well understood. Neutralizing antibodies seem to be predominantly IgG3 subtype [64,65]. The persistence of the specific IgM response months after the initial infection has already been observed for several alphaviruses and may be related to viral persistence but through poorly understood mechanisms.
Animal models & pathogenesis
For many years the study of the pathogenesis of CHIKV has been hampered by the lack of a small animal model of disease. Early studies of CHIKV isolation used young albino Swiss mice [1]. More recently, several mouse models have been investigated that mimic varying aspects of human clinical disease. In models including C57BL/6, ICR and CD-1, mice display age-dependent susceptibility to CHIKV infection and disease [70,71]. After CHIKV infection, newborn and 14-day-old outbred ICR and CD-1 mice develop lethargy, difficulty in walking, dragging of hind limbs and reduced weight gain. Infected animals develop high viremia levels (106–108 PFU) and the virus can be detected in the leg muscle even for several weeks postinfection. The major histopathologic changes were in skeletal muscle (focal necrosis and inflammation), followed by fibrosis and dystrophic calcification. Most of the animals eventually recovered. In more recent studies, biophotonic imaging of 3–4-week-old CD-1 mice infected with a recombinant CHIKV expressing the luciferase gene show viral detection for up to 5 days postinfection at the site of inoculation (footpad) with limited dissemination to the skeletal muscle [72]. 6-day-old C57/BL/6 mice can develop flaccid paralysis and die whereas 9-day-old mice recover from CHIKV infection [70]. In susceptible 9-day-old mice, CHIKV can be detected in serum, liver, muscle, joint, skin and to a lesser extent in the brain. Strikingly, by 12 days of age, C57BL/6 mice are no longer susceptible to CHIK disease, and show neither morbidity nor mortality. The requirement of young mice makes the testing of prophylactic vaccines difficult, as there is insufficient time for repeat dose vaccination or challenge. Recent work has resulted in the development of an arthritic model in adult wild-type (wt; C57BL/6) mice [73,74]. This model recapitulates the self-limiting arthritis, tenosynovitis and myositis seen in humans. Rheumatic disease was associated with a prolific infiltrate of monocytes, macrophages and natural killer cells, and the production of MCP-1, TNF-α and IFN-γ) [70]. Infection with CHIKV induced significant mononuclear infiltrates, proinflammatory mediators and foot swelling. The studies showed primary mouse macrophages to be productively infected with CHIKV and their depletion ameliorated rheumatic disease and prolonged the viremia. Although the C57BL/6 model has led to important insights into arthralgic manifestations of CHIKV, efficacy and safety testing need to be performed in a mouse model that mimics signs of acute CHIKV infection, including viral replication.
Several interferon-deficient mouse models have been developed to study CHIKV infection. Adult mice with a partially (IFN-α/βR+/−) or totally (IFN-α/βR−/−) abrogated type-I IFN pathway develop a mild or severe infection, respectively [70,75]. The A129 mouse model reproduces the clinical and pathologic findings in humans. The mice display clinical symptoms by 2 days postinfection, which include lethargy, rapid weight loss, hunched posture, ruffling of the fur and polyarthralgia [75,76]. In mice with a mild infection, after a burst of viral replication in the liver, CHIKV primarily targets muscle, joint and skin fibroblasts, a cell and tissue tropism similar to that observed in biopsy samples of CHIKV-infected humans. In case of severe infections, CHIKV also disseminates to other tissues including the CNS, where it specifically targets the choroid plexuses and the leptomeninges [76]. Infection of A129 mice with 103 PFU of wt CHIKV-LR strain resulted in 100% mortality by day 5 [76].
Despite the absence of adequate interferon signaling, this adult mouse model has been useful in the evaluation of vaccines against CHIKV as they can mount appropriate B- and T-cell responses. The A129 mouse model has been used to demonstrate the role of antibody responses in protecting the mice against experimentally induced CHIKV infection. Neutralizing antibodies have been detected following infection, and passive immunization with antiserum raised against CHIKV in vaccinated mice can protect naive mice from subsequent challenge [75]. This is in agreement with an earlier publication describing a related vaccine (protecting against Ross River virus), which also demonstrated the production of neutralizing antibodies, and their ability to protect after passive transfer to a naive host [77]. A neutralizing titer of 1:50 was shown to exert 100% protection in this model, while naive serum provided no protection [75]. The different strains of CHIKV are antigenically closely related such that infection with one strain leads to protection from all CHIKV strains [78].
In addition to studies in mice, CHIKV pathogenesis has been investigated in cynomolgus macaques. NHPs are particularly relevant for studies of pathogenesis and assessment of therapies because their physiology and immune system are similar to those in humans [79–82]. NHPs are susceptible to CHIKV infection and they are believed to act as natural reservoirs in Africa and Asia [83–85]. In macaques, the viral dose used for experimental infection can affect the clinical signs. A low viral dose (101 PFU), can infect animals without clinical signs being observed. An intermediate dose (102–106 PFU) results in viremia with fever and rash. A high dose (≥107 PFU) results in swelling in wrist and ankle joints as well as clinical signs of meningoencephalitis (hunching and wobbling, asthenia and ataxia) and/or mortality. The neurological complications and fatalities were similar to the severe forms of disease described in humans during the La Réunion Island outbreak. In the first week after inoculation, all animals developed skin rashes of varying intensities. Gingival bleeding was also observed in half of the infected animals. It is unclear whether other substantial manifestations observed during the CHIKV clinical disease in humans such as arthralgia, headache or myalgia can be detected in the monkey animal model.
After infection in NHPs, major histological abnormalities can be detected in the spleen including a severe and persistent histiocytosis that appears as an infiltrate in the red pulp consisting of mononuclear cells, with abundant eosinophilic cytoplasm, euchromatic nucleus and prominent nucleolus, characteristic of macrophages [83]. In lymph nodes, an extensive infiltration of mononuclear cells, mainly in the cortex and, to a lesser extent, in the medulla can be observed. In the liver, significant changes include a mild to marked centrolobular hepatocytic hydropic degeneration and hepatocyte death, and large amounts of intracytoplasmic granular pigment in the Kupffer cells. In addition, increased serum levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were detected, indicating further hepatic pathogenesis. In the muscle, moderate focal mononuclear cell infiltration associated with isolated muscle fiber necrosis was detected. Mononuclear cell infiltration and moderate amounts of fibrinous exudates, indicative of joint inflammation, were observed in synovial tissues from animals showing swelling of joints [83].
The cytokine and chemokine profiles of CHIKV macaques inoculated with 103 PFU CHIKV shows that IFN-α/β concentrations increased substantially as early as 2 days post infection [83]. Other changes include rapid and large increases in CCL2 (MCP-1), IL-6 and IFN-γ levels.
History & current status of chikungunya vaccine development
Spread of arboviruses could be prevented with effective mosquito control, but this has proven to be difficult even in well-developed areas of the world. Because humans appear to be the only amplification hosts during urban transmission, the most effective means of controlling the spread of the infection is by vaccination. Currently, there is no licensed vaccine available. Several technologies have been used to develop CHIK vaccines, including inactivated viral vaccines, live-attenuated viruses, alphavirus chimeras, recombinant viral vaccines, consensus-based DNA vaccines, recombinant subunit vaccines and more recently, a virus-like particle (VLP) vaccine (Table 1) [86–95].
Table 1.
Representative vaccine candidates for chikungunya.
| Vaccine candidate | Technology | Stage of development | Ref. |
|---|---|---|---|
| 181/clone25 | Live-attenuated | Halted after clinical Phase II | [87] |
| ESCA | Formalin inactivated | Preclinical | [88] |
| DNA | DNA: E1, E2, E3 | Preclinical | [93,100] |
| VLP | Transfection of HEK293 | Clinical Phase I | [92] |
| CHIK-IRES | Live-attenuated | Preclinical | [101] |
| VEE/EEE/CHIK | Live-attenuated chimeric | Preclinical | [90,95] |
| E2 | Subunit | Preclinical | [94] |
CHIK-IRES: Chikungunya fever internal ribosome entry sequence; ECSA: East/Central/South Africa; VEE/EEE/CHIK: Venezuelan equine encephalitis, eastern equine encephalitis and chikungunya; VLP: Virus-like particle.
The first CHIKV vaccines described were formalin-inactivated vaccines prepared from a variety of cell substrates including African green monkey kidney and chick embryo cells [96]. Interestingly, formalin-inactivated CHIKV prepared from chicken embryos did not induce potent, protective immune responses. However, formalin-inactivated vaccines prepared from infected mouse brains or African green monkey kidney cells induced neutralizing antibody responses and protection in a mouse cerebral challenge model and induced neutralizing antibodies against CHIKV with no observed adverse events in human volunteers [88].
A live-attenuated CHIKV vaccine candidate (termed strain 181/clone25) was developed at the US Army Medical Research Institute of Infectious Diseases (USAMRIID) [97]. The parent virus, strain 15561, was isolated from a viremic patient during a CHIKV outbreak in Thailand in 1962. The 181/25 strain was derived from 15561 by 18 serial plaque-to-plaque passages in MRC-5 cells. This strain exhibited several attenuated characteristics including: small plaque phenotype, temperature sensitivity, decreased neurovirulence in newborn mice, and reduced levels of viremia in monkeys. CHIKV 181/25 also demonstrated protection of weanling mice from lethality after wt CHIKV challenge and protection of rhesus macaques from viremia after CHIKV infection [97]. After GMP production in MRC-5 cells, the vaccine was tested in Phase I studies [98] and a randomized, placebo-controlled Phase II study [87]. In all studies, the vaccine was demonstrated to be safe and well tolerated. In the Phase II study, 59 individuals were vaccinated with a single subcutaneous administration of approximately 105 PFU of the 181/25 strain; 14 individuals received placebo. Only one vaccinee developed a local rash at the injection site and no volunteer experienced fever. Most adverse events were observed in both vaccinated and placebo groups. However, five vaccinees developed transient (from 10 min to 24 h), mild arthralgias within the first 14 days after vaccination while no placebo recipients experienced such symptoms. Although it is a promising vaccine candidate, the US Army has not pursued further development of the strain. One commercial organization, Indian Immunologicals, has received vaccine materials from the Walter Reed Army Institute of Research and is reportedly reinitiating vaccine development [201]. However, recent studies indicate that the 181/25 strain is attenuated by only two point mutations, and reversions in vaccinated mice suggest that genetic instability explains its underlying reactogenicity [99].
In addition to the USAMRIID vaccine, there are a number of other CHIKV vaccine candidates that have been developed and tested. A DNA vaccine was designed based on consensus envelope protein (E1, E2 and E3) sequences, adapted for mammalian cell expression by codon and RNA optimization [93]. The protein coding regions were linked, separated by furin protease sites to permit cleavage of the polyprotein into its constituent antigens in the absence of the viral protease. Expression of the ORF was directed by a CMV promoter, translation initiation was facilitated with a Kozak consensus sequence and secretion of the proteins promoted by IgE secretory leader sequences. The expression cassette was inserted into a DNA vaccine plasmid vector, and DNA manufactured for preclinical studies. The DNA vaccine was injected intramuscularly into mice followed by intramuscular electroporation three-times at biweekly intervals. The DNA-electroporated mice were protected from subsequent lethal intranasal challenge with a CHIKV wt clinical isolate. A capsid-expressing DNA construct failed to protect the mice, but the DNA vaccine expressing the envelope protein induced neutralizing antibody titers after intramuscular electroporation (three administrations separated by 4 weeks) in NHPs [100].
A VLP-based vaccine expressing the CHIKV envelope proteins produced high-titered neutralizing antibodies in monkeys after three doses, and protected them against viremia after challenge [92]. Viral structural proteins from CHIKV strain 37997 were expressed after transfection of DNA into HEK293 cells, forming VLPs. These VLPs were purified by buoyant density gradient sedimentation of clarified supernatants. To facilitate vaccine development, the investigators studied entry mechanisms, characterization of in vitro infection, neutralization capabilities of serum from CHIKV-infected NHPs, and cryo-electron microscopy. This vaccine has recently entered a Phase I clinical trial in healthy adults (ClinicalTrials.gov identifier: NCT01489358).
Because all of the vaccine candidates described above require multiple immunizations and some will be expensive to manufacture, we sought to develop a live-attenuated vaccine with greater safety than the 181/25 strain [101]. Initially, in this study chimeric alphavirus strains were engineered using cDNA clones, which include the genetic backbones from Sindbis virus, a naturally attenuated strain of eastern equine encephalitis virus, or the TC-83 vaccine strain of Venezuelan equine encephalitis virus [90,91]. These chimeric vaccine strains were highly attenuated and immunogenic in mice, and protected against lethal intranasal challenge with a neuroadapted CHIKV strain. Furthermore, these chimeric virus strains exhibited reduced infectivity for potential mosquito vectors than the parental viruses, a useful safety feature [102].
More recently, together with this study collaborators, a vaccine strain based on the La Reunion isolate of CHIKV collected in 2006 was developed, which is in a genotype group that is responsible for recent CHIKV outbreaks and contains the envelope gene mutation that has been found in most of these outbreaks [32,45,103]. The construct contains an encephalomyocarditis virus internal ribosome entry sequence (IRES) between the CHIKV nonstructural and structural protein genes to attenuate the virus and prevent the virus from infecting mosquitoes. As insects lack the ability to efficiently translate proteins from this IRES element, the CHIK vaccine does not infect the relevant A. albopictus mosquito, ensuring that it cannot be transmitted between people by mosquitoes. Attenuation is also achieved by mutations that inactivate the ability of the viruses to produce subgenomic RNA for its structural genes, and replaces the nonfunctional subgenomic promoter with an IRES element to drive the production of structural proteins. In addition, this CHIK vaccine virus showed genetic stability after ten passages in Vero cell culture. This attenuated virus replicates more slowly in cell culture and produces smaller plaque phenotypes, but can be grown to high titer on Vero cells and requires minimal downstream processing [101].
The CHIK-IRES vaccine has been tested for safety and efficacy in multiple mouse models [101]. Studies in CD-1 mice demonstrate that infection of 6-day-old animals vaccinated with CHIK-IRES produce no detectable virus in the serum, brain or leg tissue as compared with the 181/25 and wt CHIK-LR strains. Furthermore, infection of 3-week-old A129 mice with the 181/25 strain result in rapid morbidity and by day 8 all succumbed to infection. By contrast, all adult A129 mice infected with 181/25 survived. By contrast, both young and adult A129 mice receiving the CHIK-IRES vaccine exhibited no signs of morbidity or mortality. In addition, this CHIK vaccine induces high levels of neutralizing antibodies against CHIKV in mice and protects them from CHIKV challenge [101]. The role of neutralizing antibodies in protection was also demonstrated by performing a series of passive transfer studies of vaccine immune serum to naive A129 mice followed by challenge with wt CHIKV-LR virus [9,26]. All mice that received immune serum exhibited increased survival compared with those that received normal mouse serum [26]. Subcutaneous vaccination of NHPs with the CHIK-IRES produced high levels of neutralizing antibodies after one injection, and immunized animals were protected from wt CHIKV challenge [Roy C, Weaver S, Unpublished Data].
Potential markets for a chikungunya vaccine
The epidemiology of CHIKV makes it difficult to identify large, long-lasting markets for a safe and effective vaccine. An estimated 1 million cases per year globally (0.5–6 million cases were estimated from 2005 to 2007) and a 0.1% case–fatality rate would lead to 1000 deaths worldwide. This low mortality suggests the CHIK will be of lower priority to public health professionals than other arboviral diseases, such as dengue (>20,000 deaths per year), Japanese encephalitis (16,000 deaths per year) and even yellow fever (10,000 deaths per year). The military markets are significant, as many military bases and personnel are stationed in CHIKV-endemic areas. Similarly, travelers and tourists would constitute a market for a CHIKV vaccine. The economies of many of these tropical endemic areas, like La Réunion, depend heavily on tourism. The economic impact of a CHIK outbreak is wide ranging, as the threat of infection could deter tourists from visiting these endemic destinations. Chikungunya tends to be episodic and epidemic by nature, and the lack of multiple antigenic CHIKV subtypes suggests that immunity is life long. As the virus enters a naive population, human-to-mosquito-to-human transmission can cause epidemic numbers of cases. However, once the population has been exposed and more individuals are protected from developing viremia due to their adaptive immune responses, herd immunity naturally limits the case load. The history of the La Réunion Island epidemic provides an illustrative case study. When CHIKV re-emerged there in 2009–2010, the virus was much more limited in its spread than 4 years earlier due to many factors including greater human herd immunity, improved surveillance and more effective mosquito control measures.
However, given the growing populations and growing economies in these countries, the demand for a CHIK vaccine could explode after another outbreak similar to La Reunion in 2006. In addition, the potential for introduction and dissemination of CHIKV to more temperate climates including Europe and North America highlight the importance and market potential for a safe and effective CHIK vaccine. Currently, the highest priority markets are the private sectors in CHIKV-endemic regions, and the travelers market to CHIK-endemic countries. Military use and public markets are smaller, but still contribute substantially to a projected market for a CHIK vaccine. Most of the countries listed as endemic for CHIKV are Global Alliance for Vaccines and Immunisation (GAVI)-eligible countries. Development of a CHIKV vaccine estimates a potential of 6 million doses per year. Since CHIK is often misdiagnosed as dengue fever, increased surveillance and reporting may improve the understanding of disease burden, thus increasing the market potential.
Challenges to clinical trials & deployment of a chikungunya vaccine
Vaccination is the most cost-effective means of protecting the at-risk populations in CHIK-endemic developing countries. CHIK epidemics are explosive and rapidly moving, but not predictable. As CHIKV outbreaks occur only sporadically and unpredictably in affected countries, CHIK vaccine efficacy cannot be proven in Phase III studies, a requirement for traditional regulatory approval by the US FDA. Design of clinical trials, especially Phase II and III human trials, is difficult due to the epidemiology of CHIKV infection and disease. An option to seek licensure based on neutralizing antibody levels as a correlate of immune protection would be valuable. Data obtained so far have clearly shown that protective antibodies play a role in control of CHIKV infection. Adoptive transfer studies indicate that neutralization of viruses by anti-CHIKV antibodies protects against CHIK infection. Antibody titers are acceptable correlates of human protection for several licensed vaccines, such as the hepatitis A vaccine [104], pneumococcal vaccine [105,106], measles [107], influenza [108] and others. For protecting against viruses transmitted by arthropods, such as the yellow fever vaccine, neutralizing antibodies induced by immunization correlate well with protection; a level of 0.7 neutralization units equivalent to a 1:5 titer is considered protective [109]. For Japanese encephalitis vaccines a neutralizing titer of 1:10 is thought to be protective [110]. For tick-borne encephalitis an antibody level of 125 ELISA units is protective [111,112]. Interestingly, natural CHIKV infections induce high neutralizing antibody levels varying in titer from 40 to 20,000 [41]. These neutralizing antibodies have been detected in patients with CHIK early during the course of infection, and the presence of IgG antibodies correlates with viral clearance and long-term protection [64,65]. The E2 domain of the CHIKV envelope glycoprotein has been shown to bind with high affinity to CHIKV, suggesting that this epitope is responsible for virus neutralization. These data provide convincing evidence that levels of neutralizing antibodies are strongly correlated with a protective immune response. Establishing a correlate of protection would ease the regulatory path to CHIKV licensure, and accelerate the development accessibility of this important vaccine.
Expert commentary & five-year view
Although past experience suggests that the recent CHIK epidemic could subside to endemicity in Southeast Asia and perhaps to extinction in India, the highly efficient transmission of currently circulating strains by two different mosquito vectors, A. aegypti and A. albopictus, combined with further urbanization indicates that CHIKV transmission may continue at high levels for the forseeable future. Regardless, CHIKV will probably continue to emerge from enzootic African cycles into Asia and probably will eventually reach naive populations in the Americas to dramatically extend its geographic range and public health importance. Thus, the need for a CHIK vaccine will continue indefinitely. Most major vaccine approaches have been applied recently to CHIK, and the selection of an optimal vaccine may differ depending on the goals and markets targeted. While inactivated, VLP or DNA vaccines with the highest safety profiles may be ideal for traveler markets where cost and rapid protection are not critical, the advantages of rapid immunity via a single dose and the low cost of live-attenuated vaccines may outweigh these factors in resource-poor endemic locations or to control explosive epidemics. Vaccines are cost-effective methods to prevent infectious diseases [113]. The economic impact of vaccination is very minimal compared with postexposure treatment and disease management [114,115]. Live-attenuated vaccines are cost-effective to produce, as they can be easily grown and scaled-up on certified cell substrates, and require minimal downstream processing as compared with subunit or VLP vaccines. Regardless of which type(s) of vaccines ultimately progress forward towards licensure, clinical efficacy trials will represent a challenge because the burden of endemic disease remains poorly characterized in most locations of Asia and Africa due to a lack of widespread laboratory diagnostics and the difficulty in distinguishing CHIK from dengue and other tropical diseases. Thus, more systematic, laboratory-assisted surveillance is needed to detect and control CHIK, as well as to design the ultimate testing of vaccines, or animal testing will need to be substituted for human efficacy.
Key issues.
Chikungunya virus (CHIKV) causes an acute febrile disease characterized by severe arthralgia in the small joints. This arthralgia is highly debilitating and can be chronic.
CHIKV circulates continuously throughout sub-Saharan Africa in enzootic cycles involving arboreal mosquito vectors and nonhuman primates as reservoir hosts.
Humans become infected in Africa via direct contact with the sylvatic, enzootic cycles or when CHIKV enters into a human–mosquito–human urban cycle.
CHIKV periodically emerges from eastern African enzootic cycles and is transported via human travelers to Asia, where major epidemics ensue.
An epidemic that began in East Africa in 2004 has spread into the Indian Ocean Basin, India and Southeast Asia, where millions of cases have occurred.
Adaptation of CHIKV to the mosquito vector, Aedes albopictus, via two envelope glycoprotein substitutions, resulted in more efficient urban transmission and spread into temperate climates.
The frequent transport of CHIKV via infected travelers has demonstrated the risk of introduction and subsequent local transmission in Europe, and also puts the Western Hemisphere at risk for endemicity because the urban vectors are widespread in the Americas and the human population there is naive.
Currently, there is no effective antiviral treatment for CHIKV infection or disease. However, there are several vaccine candidates in preclinical and clinical development.
-
Past vaccine development included the 181/clone25 strain, which relies on only two point mutations for its attenuation and which proved mildly reactogenic in clinical trials.
Recent vaccine development efforts have included inactivated virus, recombinant subunit, DNA, adenoviral vectored and virus-like particle formulations. These vaccine candidates emphasize safety but may not be ideal for markets in developing countries due to their high cost and/or the requirement for multiple doses.
A live, rationally attenuated CHIK vaccine candidate based on attenuation using a picornavirus internal ribosome entry site to replace the subgenomic promoter appears to be safe and efficacious after a single dose. If this product proves safe in clinical trials it may offer the advantages of a live vaccine (rapid and long-lasting immunity) with a more stable attenuation phenotype than traditional, empirically derived attenuation methods.
Acknowledgments
Research by the authors on CHIKV is supported by NIH grants AI069145, AI082202, AI093491 and by a grant from the National Institute of Allergy and Infectious Disease (NIAID) through the Western Regional Center of Excellence for Biodefense and Emerging Infectious Disease Research, NIH grant U54 AIO57156. SC Weaver is an inventor on a UTMB patent application describing IRES-attenuated alphavirus vaccines. Inviragen has obtained license to the patent for commercial development of a CHIK vaccine.
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Ross RW. The Newala epidemic. III. The virus: isolation, pathogenic properties and relationship to the epidemic. J. Hyg. (Lond.) 1956;54(2):177–191. doi: 10.1017/s0022172400044442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weaver SC, Frey TK, Huang HV, et al. Togaviridae. In: Fauquet CM, Mayo MA, Maniloff J, Desselberger U, Ball LA, editors. Virus Taxonomy, VIIIth Report of the ICTV. London, UK: Elsevier/Academic Press; 2005. pp. 999–1008. [Google Scholar]
- 3.Brighton SW, Simson IW. A destructive arthropathy following chikungunya virus arthritis – a possible association. Clin. Rheumatol. 1984;3(2):253–258. doi: 10.1007/BF02030766. [DOI] [PubMed] [Google Scholar]
- 4.Gérardin P, Fianu A, Malvy D, et al. Perceived morbidity and community burden after a chikungunya outbreak: the TELECHIK survey, a population-based cohort study. BMC Med. 2011;9:5. doi: 10.1186/1741-7015-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vijayakumar KP, Nair Anish TS, George B, Lawrence T, Muthukkutty SC, Ramachandran R. Clinical profile of chikungunya patients during the epidemic of 2007 in Kerala, India. J. Glob. Infect. Dis. 2011;3(3):221–226. doi: 10.4103/0974-777X.83526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Andrade DC, Jean S, Clavelou P, Dallel R, Bouhassira D. Chronic pain associated with the chikungunya fever: long-lasting burden of an acute illness. BMC Infect. Dis. 2010;10:31. doi: 10.1186/1471-2334-10-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chow A, Her Z, Ong EK, et al. Persistent arthralgia induced by chikungunya virus infection is associated with interleukin-6 and granulocyte macrophage colony-stimulating factor. J. Infect. Dis. 2011;203(2):149–157. doi: 10.1093/infdis/jiq042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoarau JJ, Jaffar Bandjee MC, Krejbich Trotot P, et al. Persistent chronic inflammation and infection by chikungunya arthritogenic alphavirus in spite of a robust host immune response. J. Immunol. 2010;184(10):5914–5927. doi: 10.4049/jimmunol.0900255. [DOI] [PubMed] [Google Scholar]
- 9.Krishnamoorthy K, Harichandrakumar KT, Krishna Kumari A, Das LK. Burden of chikungunya in India: estimates of disability adjusted life years (DALY) lost in 2006 epidemic. J. Vector Borne Dis. 2009;46(1):26–35. [PubMed] [Google Scholar]
- 10.Kuhn RJ. Togaviridae: The viruses and their replication. In: Knipe DM, Howley PM, editors. Fields’ Virology. 5th Edition. NY, USA: Lippincott, Williams and Wilkins; 2007. pp. 1001–1022. [Google Scholar]
- 11.Diallo M, Thonnon J, Traore-Lamizana M, Fontenille D. Vectors of chikungunya virus in Senegal: current data and transmission cycles. Am. J. Trop. Med. Hyg. 1999;60(2):281–286. doi: 10.4269/ajtmh.1999.60.281. [DOI] [PubMed] [Google Scholar]
- 12.Jupp PG, McIntosh BM, Dos Santos I, DeMoor P. Laboratory vector studies on six mosquito and one tick species with chikungunya virus. Trans. R. Soc. Trop. Med. Hyg. 1981;75(1):15–19. doi: 10.1016/0035-9203(81)90005-5. [DOI] [PubMed] [Google Scholar]
- 13.Jupp PG, McIntosh BM. Aedes furcifer and other mosquitoes as vectors of chikungunya virus at Mica, northeastern Transvaal, South Africa. J. Am. Mosq. Control Assoc. 1990;6(3):415–420. [PubMed] [Google Scholar]
- 14.Paterson HE, Mcintosh BM. Further studies on the chikungunya outbreak in southern rhodesia in 1962. ii. transmission experiments with the Aedes furcifer-taylori group of mosquitoes and with a member of the anopheles gambiae complex. Ann. Trop. Med. Parasitol. 1964;58:52–55. doi: 10.1080/00034983.1964.11686214. [DOI] [PubMed] [Google Scholar]
- 15.Mcintosh BM, Paterson HE, Mcgillivray G, Desousa J. Further studies on the chikungunya outbreak in southern rhodesia in 1962. I mosquitoes, wild primates and birds in relation to the epidemic. Ann. Trop. Med. Parasitol. 1964;58:45–51. doi: 10.1080/00034983.1964.11686213. [DOI] [PubMed] [Google Scholar]
- 16.McIntosh BM, Paterson HE, Donaldson JM, De Sousa J. Chikungunya virus: viral susceptibility and transmission studies with some vertebrates and mosquitoes. S. African. J. Med. Sci. 1963;28:45–50. [Google Scholar]
- 17.Paul SD, Singh KR. Experimental infection of Macaca radiata with chikungunya virus and transmission of virus by mosquitoes. Indian J. Med. Res. 1968;56(6):802–811. [PubMed] [Google Scholar]
- 18.Carey DE. Chikungunya and dengue: a case of mistaken identity? J. Hist. Med. Allied Sci. 1971;26(3):243–262. doi: 10.1093/jhmas/xxvi.3.243. [DOI] [PubMed] [Google Scholar]
- 19.Wolfe ND, Kilbourn AM, Karesh WB, et al. Sylvatic transmission of arboviruses among Bornean orangutans. Am. J. Trop. Med. Hyg. 2001;64(5–6):310–316. doi: 10.4269/ajtmh.2001.64.310. [DOI] [PubMed] [Google Scholar]
- 20.Apandi Y, Nazni WA, Noor Azleen ZA, et al. The first isolation of chikungunya virus from nonhuman primates in Malaysia. J. Gen. Mol. Virol. 2009;1:35–39. [Google Scholar]
- 21.Peyrefitte CN, Bessaud M, Pastorino BA, et al. Circulation of chikungunya virus in Gabon, 2006–2007. J. Med. Virol. 2008;80(3):430–433. doi: 10.1002/jmv.21090. [DOI] [PubMed] [Google Scholar]
- 22.Peyrefitte CN, Rousset D, Pastorino BA, et al. Chikungunya virus, Cameroon, 2006. Emerging Infect. Dis. 2007;13(5):768–771. doi: 10.3201/eid1305.061500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weaver SC. Evolutionary influences in arboviral disease. Curr. Top. Microbiol. Immunol. 2006;299:285–314. doi: 10.1007/3-540-26397-7_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weaver SC, Reisen WK. Present and future arboviral threats. Antiviral Res. 2010;85(2):328–345. doi: 10.1016/j.antiviral.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ooi EE, Gubler D. Dengue virus – mosquito interactions. In: Hanley KA, Weaver SC, editors. Frontiers in Dengue Virus Research. Norwich, UK: Horizon Press; 2010. [Google Scholar]
- 26.Panning M, Grywna K, van Esbroeck M, Emmerich P, Drosten C. Chikungunya fever in travelers returning to Europe from the Indian Ocean region-2006. Emerging Infect. Dis. 2008;14(3):416–422. doi: 10.3201/eid1403.070906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leo YS, Chow AL, Tan LK, Lye DC, Lin L, Ng LC. Chikungunya outbreak, Singapore, 2008. Emerging Infect. Dis. 2009;15(5):836–837. doi: 10.3201/eid1505.081390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tsetsarkin KA, Vanlandingham DL, McGee CE, Higgs S. A single mutation in chikungunya virus affects vector specificity and epidemic potential. PLoS Pathog. 2007;3(12):e201. doi: 10.1371/journal.ppat.0030201. • Using reverse genetic approaches, this paper demonstrated the dramatic effect of the A226V amino acid substitution in the E1 protein on increasing chikungunya virus infectivity for the vector mosquito Aedes albopictus.
- 29.Tsetsarkin KA, McGee CE, Volk SM, Vanlandingham DL, Weaver SC, Higgs S. Epistatic roles of E2 glycoprotein mutations in adaption of chikungunya virus to Aedes albopictus and Ae. aegypti mosquitoes. PLoS ONE. 2009;4(8):e6835. doi: 10.1371/journal.pone.0006835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsetsarkin KA, Weaver SC. Sequential adaptive mutations enhance efficient vector switching by Chikungunya virus and its epidemic emergence. PLoS Pathog. 2011;7(12):e1002412. doi: 10.1371/journal.ppat.1002412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vazeille M, Moutailler S, Coudrier D, et al. Two Chikungunya isolates from the outbreak of La Reunion (Indian Ocean) exhibit different patterns of infection in the mosquito Aedes albopictus. PLoS ONE. 2007;2(11):e1168. doi: 10.1371/journal.pone.0001168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsetsarkin KA, Chen R, Sherman MB, Weaver SC. Chikungunya virus: evolution and genetic determinants of emergence. Curr. Opin. Virol. 2011;1(4):310–317. doi: 10.1016/j.coviro.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Powers AM, Brault AC, Tesh RB, Weaver SC. Re-emergence of Chikungunya and O’nyong-nyong viruses: evidence for distinct geographical lineages and distant evolutionary relationships. J. Gen. Virol. 2000;81(Pt 2):471–479. doi: 10.1099/0022-1317-81-2-471. [DOI] [PubMed] [Google Scholar]
- 34.Volk SM, Chen R, Tsetsarkin KA, et al. Genome-scale phylogenetic analyses of chikungunya virus reveal independent emergences of recent epidemics and various evolutionary rates. J. Virol. 2010;84(13):6497–6504. doi: 10.1128/JVI.01603-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bryant JE, Holmes EC, Barrett AD. Out of Africa: a molecular perspective on the introduction of yellow fever virus into the Americas. PLoS Pathog. 2007;3(5):e75. doi: 10.1371/journal.ppat.0030075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ng LC, Hapuarachchi HC. Tracing the path of chikungunya virus–evolution and adaptation. Infect. Genet. Evol. 2010;10(7):876–885. doi: 10.1016/j.meegid.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 37.Chretien JP, Anyamba A, Bedno SA, et al. Drought-associated chikungunya emergence along coastal East Africa. Am. J. Trop. Med. Hyg. 2007;76(3):405–407. [PubMed] [Google Scholar]
- 38.Powers AM, Logue CH. Changing patterns of chikungunya virus: re-emergence of a zoonotic arbovirus. J. Gen. Virol. 2007;88(Pt 9):2363–2377. doi: 10.1099/vir.0.82858-0. [DOI] [PubMed] [Google Scholar]
- 39.Sang RC, Ahmed O, Faye O, et al. Entomologic investigations of a chikungunya virus epidemic in the Union of the Comoros, 2005. Am. J. Trop. Med. Hyg. 2008;78(1):77–82. [PubMed] [Google Scholar]
- 40.Arankalle VA, Shrivastava S, Cherian S, et al. Genetic divergence of chikungunya viruses in India (1963–2006) with special reference to the 2005–2006 explosive epidemic. J. Gen. Virol. 2007;88(Pt 7):1967–1976. doi: 10.1099/vir.0.82714-0. [DOI] [PubMed] [Google Scholar]
- 41.Lanciotti RS, Kosoy OL, Laven JJ, et al. Chikungunya virus in US travelers returning from India, 2006. Emerg. Infect. Dis. 2007;13(5):764–767. doi: 10.3201/eid1305.070015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ng LC, Tan LK, Tan CH, et al. Entomologic and virologic investigation of chikungunya, Singapore. Emerg. Infect. Dis. 2009;15(8):1243–1249. doi: 10.3201/eid1508.081486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rezza G, Nicoletti L, Angelini R, et al. CHIKV study group. Infection with chikungunya virus in Italy: an outbreak in a temperate region. Lancet. 2007;370(9602):1840–1846. doi: 10.1016/S0140-6736(07)61779-6. • Documents an outbreak of chikungunya fever in Italy, the first such outbreak in a temperate region, which underscores the risk of A. albopictus-adapted virus strains to geographic expansion.
- 44.Grandadam M, Caro V, Plumet S, et al. Chikungunya virus, southeastern France. Emerging Infect. Dis. 2011;17(5):910–913. doi: 10.3201/eid1705.101873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schuffenecker I, Iteman I, Michault A, et al. Genome microevolution of chikungunya viruses causing the Indian Ocean outbreak. PLoS Med. 2006;3(7):e263. doi: 10.1371/journal.pmed.0030263. • Determined the origin of the chikungunya virus strains that caused epidemics in the Indian Ocean Basin from 2005 to 2011 and first hypothesized the role of the A226V amino acid substitution in the E1 protein on A. albopictus infectivity.
- 46.Vashishtha M, Phalen T, Marquardt MT, Ryu JS, Ng AC, Kielian M. A single point mutation controls the cholesterol dependence of Semliki Forest virus entry and exit. J. Cell Biol. 1998;140(1):91–99. doi: 10.1083/jcb.140.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pagès F, Peyrefitte CN, Mve MT, et al. Aedes albopictus mosquito: the main vector of the 2007 chikungunya outbreak in Gabon. PLoS ONE. 2009;4(3):e4691. doi: 10.1371/journal.pone.0004691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de Lamballerie X, Leroy E, Charrel RN, Ttsetsarkin K, Higgs S, Gould EA. Chikungunya virus adapts to tiger mosquito via evolutionary convergence: a sign of things to come? Virol. J. 2008;5:33. doi: 10.1186/1743-422X-5-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Niyas KP, Abraham R, Unnikrishnan RN, et al. Molecular characterization of chikungunya virus isolates from clinical samples and adult Aedes albopictus mosquitoes emerged from larvae from Kerala, South India. Virol. J. 2010;7:189. doi: 10.1186/1743-422X-7-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lambrechts L, Scott TW, Gubler DJ. Consequences of the expanding global distribution of Aedes albopictus for dengue virus transmission. PLoS Negl. Trop. Dis. 2010;4(5):e646. doi: 10.1371/journal.pntd.0000646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Honório NA, Castro MG, Barros FS, Magalhães Mde A, Sabroza PC. The spatial distribution of Aedes aegypti and Aedes albopictus in a transition zone, Rio de Janeiro, Brazil. Cad. Saude. Publica. 2009;25(6):1203–1214. doi: 10.1590/s0102-311x2009000600003. [DOI] [PubMed] [Google Scholar]
- 52.Rey JR, Nishimura N, Wagner B, Braks MA, O’Connell SM, Lounibos LP. Habitat segregation of mosquito arbovirus vectors in South Florida. J. Med. Entomol. 2006;43(6):1134–1141. doi: 10.1603/0022-2585(2006)43[1134:hsomav]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Braks MA, Honório NA, Lourençqo-De-Oliveira R, Juliano SA, Lounibos LP. Convergent habitat segregation of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) in southeastern Brazil and Florida. J. Med. Entomol. 2003;40(6):785–794. doi: 10.1603/0022-2585-40.6.785. [DOI] [PubMed] [Google Scholar]
- 54.Tewari SC, Thenmozhi V, Katholi CR, Manavalan R, Munirathinam A, Gajanana A. Dengue vector prevalence and virus infection in a rural area in South India. Trop. Med. Int. Health. 2004;9(4):499–507. doi: 10.1111/j.1365-3156.2004.01103.x. [DOI] [PubMed] [Google Scholar]
- 55.Tandon N, Ray S. Breeding habitats and larval indices of Aedes aegypti and Ae. albopictus in the residential areas of Calcutta City. J. Commun. Dis. 2000;32(3):180–184. [PubMed] [Google Scholar]
- 56.Tsetsarkin KA, Chen R, Leal G, et al. Chikungunya virus emergence is constrained in Asia by lineage-specific adaptive landscapes. Proc. Natl Acad. Sci. USA. 2011;108(19):7872–7877. doi: 10.1073/pnas.1018344108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lemant J, Boisson V, Winer A, et al. Serious acute chikungunya virus infection requiring intensive care during the Reunion Island outbreak in 2005–2006. Crit. Care Med. 2008;36(9):2536–2541. doi: 10.1097/CCM.0b013e318183f2d2. [DOI] [PubMed] [Google Scholar]
- 58.Das T, Jaffar-Bandjee MC, Hoarau JJ, et al. Chikungunya fever: CNS infection and pathologies of a re-emerging arbovirus. Prog. Neurobiol. 2010;91(2):121–129. doi: 10.1016/j.pneurobio.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 59.Economopoulou A, Dominguez M, Helynck B, et al. Atypical Chikungunya virus infections: clinical manifestations, mortality and risk factors for severe disease during the 2005–2006 outbreak on Réunion. Epidemiol. Infect. 2009;137(4):534–541. doi: 10.1017/S0950268808001167. [DOI] [PubMed] [Google Scholar]
- 60.Borgherini G, Poubeau P, Staikowsky F, et al. Outbreak of chikungunya on Reunion Island: early clinical and laboratory features in 157 adult patients. Clin. Infect. Dis. 2007;44(11):1401–1407. doi: 10.1086/517537. [DOI] [PubMed] [Google Scholar]
- 61.Brighton SW, Prozesky OW, de la Harpe AL. Chikungunya virus infection. A retrospective study of 107 cases. S. Afr. Med. J. 1983;63(9):313–315. [PubMed] [Google Scholar]
- 62.Manimunda SP, Vijayachari P, Uppoor R, et al. Clinical progression of chikungunya fever during acute and chronic arthritic stages and the changes in joint morphology as revealed by imaging. Trans. R. Soc. Trop. Med. Hyg. 2010;104(6):392–399. doi: 10.1016/j.trstmh.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 63.Ganu MA, Ganu AS. Post-chikungunya chronic arthritis–our experience with DMARDs over two year follow-up. J. Assoc. Physicians India. 2011;59:83–86. [PubMed] [Google Scholar]
- 64.Kam YW, Lum FM, Teo TH, et al. Early neutralizing IgG response to chikungunya virus in infected patients targets a dominant linear epitope on the E2 glycoprotein. EMBO Mol. Med. 2012;4(4):330–343. doi: 10.1002/emmm.201200213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kam YW, Simarmata D, Chow A, et al. Early appearance of neutralizing immunoglobulin G3 antibodies is associated with chikungunya virus clearance and long-term clinical protection. J. Infect. Dis. 2012;205(7):1147–1154. doi: 10.1093/infdis/jis033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sourisseau M, Schilte C, Casartelli N, et al. Characterization of re-emerging chikungunya virus. PLoS Pathog. 2007;3(6):e89. doi: 10.1371/journal.ppat.0030089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Arpino C, Curatolo P, Rezza G. Chikungunya and the nervous system: what we do and do not know. Rev. Med. Virol. 2009;19(3):121–129. doi: 10.1002/rmv.606. [DOI] [PubMed] [Google Scholar]
- 68.Wauquier N, Becquart P, Nkoghe D, Padilla C, Ndjoyi-Mbiguino A, Leroy EM. The acute phase of chikungunya virus infection in humans is associated with strong innate immunity and T CD8 cell activation. J. Infect. Dis. 2011;204(1):115–123. doi: 10.1093/infdis/jiq006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Aoyama I, Uno K, Yumisashi T, et al. A case of chikungunya fever imported from India to Japan, follow-up of specific IgM and IgG antibodies over a 6-month period. Jpn. J. Infect. Dis. 2010;63(1):65–66. [PubMed] [Google Scholar]
- 70. Couderc T, Chrétien F, Schilte C, et al. A mouse model for chikungunya: young age and inefficient type-I interferon signaling are risk factors for severe disease. PLoS Pathog. 2008;4(2):e29. doi: 10.1371/journal.ppat.0040029. • First demonstration of the utility of mice with defective type-I interferon receptors as models of human disease that are useful for vaccine testing.
- 71.Ziegler SA, Lu L, da Rosa AP, Xiao SY, Tesh RB. An animal model for studying the pathogenesis of chikungunya virus infection. Am. J. Trop. Med. Hyg. 2008;79(1):133–139. [PubMed] [Google Scholar]
- 72.Ziegler SA, Nuckols J, McGee CE, et al. In vivo imaging of chikungunya virus in mice and Aedes mosquitoes using a Renilla luciferase clone. Vector Borne Zoonotic Dis. 2011;11(11):1471–1477. doi: 10.1089/vbz.2011.0648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Morrison TE, Oko L, Montgomery SA, et al. A mouse model of chikungunya virus-induced musculoskeletal inflammatory disease: evidence of arthritis, tenosynovitis, myositis, and persistence. Am. J. Pathol. 2011;178(1):32–40. doi: 10.1016/j.ajpath.2010.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gardner J, Anraku I, Le TT, et al. Chikungunya virus arthritis in adult wild-type mice. J. Virol. 2010;84(16):8021–8032. doi: 10.1128/JVI.02603-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Partidos CD, Weger J, Brewoo J, et al. Probing the attenuation and protective efficacy of a candidate chikungunya virus vaccine in mice with compromised interferon (IFN) signaling. Vaccine. 2011;29(16):3067–3073. doi: 10.1016/j.vaccine.2011.01.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gardner CL, Burke CW, Higgs ST, Klimstra WB, Ryman KD. Interferon-α/β deficiency greatly exacerbates arthritogenic disease in mice infected with wild-type chikungunya virus but not with the cell culture-adapted live-attenuated 181/25 vaccine candidate. Virology. 2012;425(2):103–112. doi: 10.1016/j.virol.2011.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Holzer GW, Coulibaly S, Aichinger G, et al. Evaluation of an inactivated Ross River virus vaccine in active and passive mouse immunization models and establishment of a correlate of protection. Vaccine. 2011;29(24):4132–4141. doi: 10.1016/j.vaccine.2011.03.089. [DOI] [PubMed] [Google Scholar]
- 78.Shah KV, Gibbs CJ, Jr, Banerjee G. Virological investigation of the epidemic of haemorrhagic fever in Calcutta: isolation of three strains of chikungunya virus. Indian. J. Med. Res. 1964;52:676–683. [PubMed] [Google Scholar]
- 79.Reed DS, Lind CM, Lackemeyer MG, Sullivan LJ, Pratt WD, Parker MD. Genetically engineered, live, attenuated vaccines protect nonhuman primates against aerosol challenge with a virulent IE strain of Venezuelan equine encephalitis virus. Vaccine. 2005;23(24):3139–3147. doi: 10.1016/j.vaccine.2004.12.023. [DOI] [PubMed] [Google Scholar]
- 80.Verrier B, Le Grand R, Ataman-Onal Y, et al. Evaluation in rhesus macaques of Tat and rev-targeted immunization as a preventive vaccine against mucosal challenge with SHIV-BX08. DNA Cell Biol. 2002;21(9):653–658. doi: 10.1089/104454902760330183. [DOI] [PubMed] [Google Scholar]
- 81.Vierboom MP, Jonker M, Tak PP, ‘t Hart BA. Preclinical models of arthritic disease in nonhuman primates. Drug Discov. Today. 2007;12(7–8):327–335. doi: 10.1016/j.drudis.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 82.Walsh GP, Tan EV, dela Cruz EC, et al. The Philippine cynomolgus monkey (Macaca fasicularis) provides a new nonhuman primate model of tuberculosis that resembles human disease. Nat. Med. 1996;2(4):430–436. doi: 10.1038/nm0496-430. [DOI] [PubMed] [Google Scholar]
- 83. Labadie K, Larcher T, Joubert C, et al. Chikungunya disease in nonhuman primates involves long-term viral persistence in macrophages. J. Clin. Invest. 2010;120(3):894–906. doi: 10.1172/JCI40104. • Describes extensive experimental infections of cynomolgus macaques with chikungunya virus and demonstrates their usefulness as models for vaccine testing.
- 84.Inoue S, Morita K, Matias RR, et al. Distribution of three arbovirus antibodies among monkeys (Macaca fascicularis) in the Philippines. J. Med. Primatol. 2003;32(2):89–94. doi: 10.1034/j.1600-0684.2003.00015.x. [DOI] [PubMed] [Google Scholar]
- 85.Kaschula VR, Van Dellen AF, de Vos V. Some infectious diseases of wild vervet monkeys (Cercopithecus aethiops pygerythrus) in South Africa. J. S. Afr. Vet. Assoc. 1978;49(3):223–227. [PubMed] [Google Scholar]
- 86.Eckels KH, Harrison VR, Hetrick FM. Chikungunya virus vaccine prepared by Tween-ether extraction. Appl. Microbiol. 1970;19(2):321–325. doi: 10.1128/am.19.2.321-325.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Edelman R, Tacket CO, Wasserman SS, Bodison SA, Perry JG, Mangiafico JA. Phase 2 safety and immunogenicity study of live chikungunya virus vaccine TSI-GSD-218. Am. J. Trop. Med. Hyg. 2000;62(6):681–685. doi: 10.4269/ajtmh.2000.62.681. • Describes Phase II testing of the live-attenuated chikungunya vaccine developed by the US Army and administered under an investigational new drug permit to scientists at risk for laboratory infection.
- 88.Harrison VR, Eckels KH, Bartelloni PJ, Hampton C. Production and evaluation of a formalin-killed chikungunya vaccine. J. Immunol. 1971;107(3):643–647. [PubMed] [Google Scholar]
- 89.Tiwari M, Parida M, Santhosh SR, Khan M, Dash PK, Rao PV. Assessment of immunogenic potential of Vero adapted formalin-inactivated vaccine derived from novel ECSA genotype of chikungunya virus. Vaccine. 2009;27(18):2513–2522. doi: 10.1016/j.vaccine.2009.02.062. [DOI] [PubMed] [Google Scholar]
- 90.Wang E, Volkova E, Adams AP, et al. Chimeric alphavirus vaccine candidates for chikungunya. Vaccine. 2008;26(39):5030–5039. doi: 10.1016/j.vaccine.2008.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang E, Kim DY, Weaver SC, Frolov I. Chimeric Chikungunya viruses are nonpathogenic in highly sensitive mouse models but efficiently induce a protective immune response. J. Virol. 2011;85(17):9249–9252. doi: 10.1128/JVI.00844-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Akahata W, Yang ZY, Andersen H, et al. A virus-like particle vaccine for epidemic chikungunya virus protects nonhuman primates against infection. Nat. Med. 2010;16(3):334–338. doi: 10.1038/nm.2105. •• Describes a virus-like particle chikungunya vaccine that was shown to be safe and efficacious in nonhuman primates and is now in Phase I clinical trials.
- 93.Muthumani K, Lankaraman KM, Laddy DJ, et al. Immunogenicity of novel consensus-based DNA vaccines against chikungunya virus. Vaccine. 2008;26(40):5128–5134. doi: 10.1016/j.vaccine.2008.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Metz SW, Geertsema C, Martina BE, et al. Functional processing and secretion of chikungunya virus E1 and E2 glycoproteins in insect cells. Virol. J. 2011;8:353. doi: 10.1186/1743-422X-8-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang D, Suhrbier A, Penn-Nicholson A, et al. A complex adenovirus vaccine against chikungunya virus provides complete protection against viraemia and arthritis. Vaccine. 2011;29(15):2803–2809. doi: 10.1016/j.vaccine.2011.01.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.White A, Berman S, Lowenthal JP. Comparative immunogenicities of chikungunya vaccines propagated in monkey kidney monolayers and chick embryo suspension cultures. Appl. Microbiol. 1972;23(5):951–952. doi: 10.1128/am.23.5.951-952.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Levitt NH, Ramsburg HH, Hasty SE, Repik PM, Cole FE, Jr, Lupton HW. Development of an attenuated strain of chikungunya virus for use in vaccine production. Vaccine. 1986;4(3):157–162. doi: 10.1016/0264-410x(86)90003-4. [DOI] [PubMed] [Google Scholar]
- 98.McClain DJ, Pittman PR, Ramsburg HH, et al. Immunologic interference from sequential administration of live-attenuated alphavirus vaccines. J. Infect. Dis. 1998;177(3):634–641. doi: 10.1086/514240. [DOI] [PubMed] [Google Scholar]
- 99.Gorchakov R, Wang E, Leal G, et al. Attenuation of chikungunya virus vaccine strain 181/clone 25 is determined by two amino acid substitutions in the E2 envelope glycoprotein. J. Virol. 2012;86(11):6084–6096. doi: 10.1128/JVI.06449-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mallilankaraman K, Shedlock DJ, Bao H, et al. A DNA vaccine against chikungunya virus is protective in mice and induces neutralizing antibodies in mice and nonhuman primates. PLoS Negl. Trop. Dis. 2011;5(1):e928. doi: 10.1371/journal.pntd.0000928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Plante K, Wang E, Partidos CD, et al. Novel chikungunya vaccine candidate with an IRES-based attenuation and host range alteration mechanism. PLoS Pathog. 2011;7(7):e1002142. doi: 10.1371/journal.ppat.1002142. •• Describes the development of a live, rationally attenuated chikungunya vaccine that was demonstrated to be safe and efficacious in mouse models after a single administration.
- 102.Darwin JR, Kenney JL, Weaver SC. Transmission potential of two chimeric chikungunya vaccine candidates in the urban mosquito vectors, Aedes aegypti and Ae. albopictus. Am. J. Trop. Med. Hyg. 2011;84(6):1012–1015. doi: 10.4269/ajtmh.2011.11-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sumathy K, Ella KM. Genetic diversity of chikungunya virus, India 2006–2010: evolutionary dynamics and serotype analyses. J. Med. Virol. 2012;84(3):462–470. doi: 10.1002/jmv.23187. [DOI] [PubMed] [Google Scholar]
- 104.Fiore AE, Finestone SM, Bell BP. Hepatitis A vaccine. In: Plotkin SA, Orenstein WA, Offit PA, editors. Vaccines. 5th Edition. USA: Elsevier-Saunders; 2008. pp. 177–203. [Google Scholar]
- 105.Siber GR, Chang I, Baker S, et al. Estimating the protective concentration of antipneumococcal capsular polysaccharide antibodies. Vaccine. 2007;25(19):3816–3826. doi: 10.1016/j.vaccine.2007.01.119. [DOI] [PubMed] [Google Scholar]
- 106.Jódar L, Butler J, Carlone G, et al. Serological criteria for evaluation and licensure of new pneumococcal conjugate vaccine formulations for use in infants. Vaccine. 2003;21(23):3265–3272. doi: 10.1016/s0264-410x(03)00230-5. [DOI] [PubMed] [Google Scholar]
- 107.Chen RT, Markowitz LE, Albrecht P, et al. Measles antibody: reevaluation of protective titers. J. Infect. Dis. 1990;162(5):1036–1042. doi: 10.1093/infdis/162.5.1036. [DOI] [PubMed] [Google Scholar]
- 108.Dowdle WR, Coleman MT, Mostow SR, Kaye HS, Schoenbaum SC. Inactivated influenza vaccines. 2. Laboratory indices of protection. Postgrad. Med. J. 1973;49(569):159–163. doi: 10.1136/pgmj.49.569.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mason RA, Tauraso NM, Spertzel RO, Ginn RK. Yellow fever vaccine: direct challenge of monkeys given graded doses of 17D vaccine. Appl. Microbiol. 1973;25(4):539–544. doi: 10.1128/am.25.4.539-544.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hombach J, Solomon T, Kurane I, Jacobson J, Wood D. Report on a WHO consultation on immunological endpoints for evaluation of new Japanese encephalitis vaccines, WHO, Geneva, 2–3 September, 2004. Vaccine. 2005;23(45):5205–5211. doi: 10.1016/j.vaccine.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 111.Kreil TR, Burger I, Bachmann M, Fraiss S, Eibl MM. Antibodies protect mice against challenge with tick-borne encephalitis virus (TBEV)-infected macrophages. Clin. Exp. Immunol. 1997;110(3):358–361. doi: 10.1046/j.1365-2249.1997.4311446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kreil TR, Eibl MM. Pre- and postexposure protection by passive immunoglobulin but no enhancement of infection with a flavivirus in a mouse model. J. Virol. 1997;71(4):2921–2927. doi: 10.1128/jvi.71.4.2921-2927.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.WHO, UNICEF, World Bank. 3rd Edition. Switzerland: WHO Press, Geneva; 2009. State of the World’s Vaccines and Immunization. [Google Scholar]
- 114.Ehreth J. The value of vaccination: a global perspective. Vaccine. 2003;21(27–30):4105–4117. doi: 10.1016/s0264-410x(03)00377-3. [DOI] [PubMed] [Google Scholar]
- 115.Tu HA, Woerdenbag HJ, Kane S, Riewpaiboon A, van Hulst M, Postma MJ. Economic evaluations of hepatitis B vaccination for developing countries. Expert Rev. Vaccines. 2009;8(7):907–920. doi: 10.1586/erv.09.53. [DOI] [PubMed] [Google Scholar]
- 116.Zhang R, Hryc CF, Cong Y, et al. 4.4 Å cryo-EM structure of an enveloped alphavirus Venezuelan equine encephalitis virus. EMBO J. 2011;30(18):3854–3863. doi: 10.1038/emboj.2011.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
Website
- 201.Indian Immunologicals developing vaccine for chikungunya. [9 February 2012];The Hindu Business Line. www.thehindubusinessline.com/companies/article2875901.ece.