Abstract
Rationale
Early parental loss has been associated with neuroendocrine dysregulation in youth; however, the form of cortisol dysregulation varies widely. Identifying risk and protective factors that influence physiological regulation has important implications for understanding the development of mental health problems in parentally bereaved youth.
Objectives
The current study investigated the prospective effects of positive parenting on the relation between recent negative life events and cortisol activity in adolescents/young adults several years after bereavement.
Methods
Positive parenting was assessed an average of 18.5 months following parental death. Six years later, adolescents/young adults (N=55) reported on exposure to recent negative life events, and salivary cortisol was assessed before and after a conflict discussion task with their caregiver. The interaction between positive parenting and exposure to recent negative events was used to predict total cortisol output and response to the task.
Results
Multilevel modeling and the probing of the interaction effect demonstrated that total cortisol output increased with greater exposure to recent negative events among those with lower levels of past positive parenting. These relations were significant over and above current internalizing and externalizing symptoms.
Conclusions
The current results highlight the need to consider the interactive influence of proximal and distal factors on neuroendocrine functioning for youth exposed to early parental loss.
Keywords: Early parental death, Positive parenting, Cortisol, Negative life events
Introduction
Dysregulation of the hypothalamic–pituitary–adrenal (HPA) axis has been increasingly investigated to explain the negative psychological and physical health consequences of early life adversity. As one of the main effectors of the stress response system, the HPA axis prepares the body to respond adaptively to stress, in part through regulation of the release of cortisol. However, acute or chronic childhood stress can impair HPA axis functioning in the short- and long-term, resulting in relatively high or low basal cortisol, hypo- or hyper-reactivity to stress, or departures from the normative diurnal pattern of cortisol secretion (Gunnar 2007). Neuroendocrine dysregulation, in turn, has been implicated in the occurrence of a wide range of psychological disorders in children and adults (Davies et al. 2007; Heim et al. 2002; McEwen and Wingfield 2003; Raison and Miller 2003).
Youth who experience the death of a parent are at an increased risk of a range of mental health problems, including depression and other psychological and adjustment difficulties (Cerel et al. 2006; Dowdney 2000; Lutzke et al. 1997; Tremblay and Israel 1998). Research on children and adults who experienced the early death of a parent indicate that these psychological difficulties may be influenced by dysregulated neuroendocrine activity (Luecken and Lemery 2004; Pfeffer et al. 2007). Although the early loss of a parent has been found to disrupt children’s HPA axis functioning, the form of cortisol dysregulation varies widely. Higher basal cortisol has been observed among children and adults who experienced the loss of a parent during childhood (Nicolson 2004; Pfeffer et al. 2007). Conversely, other studies have documented lower basal cortisol in children (Flinn et al. 1996), as well as reduced cortisol awakening responses (Meinlschmidt and Heim 2005) and attenuated cortisol following corticotropin-releasing hormone stimulation (Bloch et al. 2007) in adults who experienced parental loss due to death, separation, or divorce.
Variability in the long-termimpact of early adversity on the HPA axis highlights the need to probe underlying processes. According to transitional events theory (Felner et al. 1988), the psychosocial adjustment of parentally bereaved youth is related to the cascade of stressful life events that follow the death and the resources youth possess to adapt to these experiences (Doyle et al. 2003). The quality of caregiving provided by the surviving parent is a critical resource that can promote positive adaptation following bereavement, as well as build the capacity to respond adaptively to future stressors (Haine et al. 2006; Kwok et al. 2005). Qualities of the family environment have been found to moderate the impact of childhood adversities on the physiological stress response system (Pendry and Adam 2007). For example, Luecken and colleagues found that childhood parental loss was associated with elevated cortisol only among young adults who also experienced low care, abuse, or high family conflict during childhood (Luecken 2000; Luecken and Appelhans 2006). A high-quality parent–child relationship characterized by warmth, acceptance, and effective discipline practices (i.e., positive parenting) is fundamental to the promotion of children’s psychosocial health following parental death; however, the prospective relation of positive parenting to cortisol activity in bereaved youth has not been examined.
One promising path to explore is the extent to which positive parenting following parental death influences the impact of future stressful life events on cortisol activity. It is theorized that positive parenting can help the child successfully navigate the emotional challenges associated with early life trauma in a manner that will prepare the child to manage later-life stress in a more emotionally and biologically adaptive manner. For example, one study demonstrated that higher warmth from the surviving parent during childhood predicted smaller increases in negative emotion during daily life stressors in young adulthood relative to participants reporting lower parental warmth (Luecken et al. 2009). Although recent exposure to negative life events has been associated with elevated basal cortisol in children (Bevans et al. 2008), parentally bereaved youth who garner emotional stress-buffering benefits from a warm and caring relationship with the surviving parent may be less physiologically affected by negative life events. Thus, the long-term impact of positive parenting, when considered in conjunction with recent exposure to negative life events, may help explain different patterns of cortisol activity observed among parentally bereaved youth.
The current study prospectively examines positive parenting during childhood/adolescence as a moderator of the effects of recent life stress on cortisol activity during late adolescence/young adulthood in a sample of parentally bereaved youth. As part of a longitudinal study of bereaved families, surviving caregivers’ use of positive parenting was assessed an average of 18.5 months after parental death. Six years later, cortisol was sampled from youth during a conflict discussion task with the surviving caregiver, and youth reported on the number of negative life events experienced over the previous year. It was expected that positive parenting would act as a stress-buffer such that higher exposure to recent negative life events would predict elevated cortisol during the task only among those who were vulnerable due to experiencing lower levels of positive parenting years earlier, shortly following the parental death. For those who received higher levels of positive parenting, no significant relation was expected between recent negative events and cortisol activity.
Material and methods
Participants
The sample consists of 55 adolescents and young adults (mean age=17.05, range 14–22; see Table 1 for sample characteristics) randomized to the control condition of an experimental trial of a prevention program for parentally bereaved families. Families were recruited through letters and presentations to community agencies in the Phoenix, Arizona metropolitan area that had contact with bereaved children. Eligibility criteria included (a) at least one child between 8 and16 years old, (b) willingness to be randomized to experimental or self-study control condition, (c) occurrence of parental death between 4 and 30 months prior to the program, (d) ability to complete the assessments in English, (e) neither caregiver nor youth were receiving mental health or other bereavement services, and (f) family had no plans to move out of the area in the next 6 months. For complete recruitment and eligibility details, see Sandler et al. (2003).
Table 1.
Sample characteristics and descriptive statistics for predictors and covariates
| Gender (N=55) | |
| Male | 35 |
| Female | 20 |
| Ethnicity (N=55) | |
| Hispanic | 7 |
| Caucasian | 34 |
| African–American | 6 |
| Native-American | 4 |
| Other | 4 |
| Participant age (M, SD) | 17.05 (2.17) |
| Recent negative life events (M, SD) | 12.85 (6.83) |
| Positive parenting (M, SD) | 0.02 (0.41) |
| Cortisol AUCG (M, SD) | 0.23 (0.15) |
| Current internalizing symptoms (M, SD) | 3.54 (1.32) |
| Current externalizing symptoms (M, SD) | 3.87 (0.84) |
Two hundred forty-four youth from 156 families completed a pre-assessment, following which families were randomly assigned to a dual-component preventive intervention for youth and their caregivers (N=135 children from 90 families) or a self-study control condition (N=109 children from 66 families). Of the 109 youth assigned to the control condition, 102 completed the 6-year follow-up; however, response burden was reduced by collecting cortisol samples from a maximum of two adolescents/ young adults within a family. Participants were only eligible for the discussion task in which cortisol was sampled if they reported at least three verbal contacts in the last month with the caregiver. Of the 88 adolescents/ young adults eligible for cortisol sampling, 12 refused and 15 could not be scheduled within the a priori time range (3–9 PM). Of the remaining 61 youth, complete data were available for 55, forming the final sample for analyses. Those who were eligible for cortisol sampling (n=88) did not differ from ineligible youth (n=14) with regard to ethnicity, gender, family income, cause of parental death, or time elapsed since parental death. Ineligible participants were 1 year older on average (p=0.05). There were no significant differences on these variables between eligible participants from whom cortisol was sampled and nonparticipating eligible youth (all p’s>0.10).
Measures and instruments
Positive parenting
Multiple methods were used to assess caregiver warmth and effective discipline at two time points, about 1 year after the death and 11 months later. To assess warmth, youth and caregivers completed the acceptance and rejection subscales from a revised version of the Child Report of Parenting Behavior Inventory, (CRPBI; 16 items in each subscale; Schaefer 1965; caregiver acceptance α= 0.911 and rejection α=87; youth acceptance α=0.92 and rejection α=0.87), a version of the Family Routines Inventory (seven items; Jensen et al. 1983; caregiver α=0.74; youth α=0.76), and a measure of stable positive family events (five items; Sandler et al. 1991; α was not computed because the events were not predicted to be correlated [Sandler and Guenther 1985]). Caregivers’ use of positive reinforcement was measured by caregiver and youth report on an adaptation of the Parent Perception Inventory (eight items; Hazzard et al. 1983; caregiver α=0.92; youth α= 0.91). Also, youth reported on sharing feelings with the caregiver (10 items; Ayers et al. 1998; α=0.85), and caregivers reported on how they communicated with their children around stressful family events (six items; Talk with Reassurance subscale of the Parent Expression of Emotion Questionnaire; Jones and Twohey 1998; α=0.74). Two behavioral observation measures of warmth during a dyadic interaction task were also used. Trained coders rated positive affective tone and paralinguistic (e.g., “uhhuhs”) and behavioral attending (e.g., head-nods); interrater reliability was assessed using Cohen’s (1960) kappa; k=0.77, 0.83 and 0.80 for affective tone, and paralinguistic and behavioral attending, respectively. Effective discipline was assessed by caregiver and youth report on the inconsistent discipline subscale of a revised version of the CRPBI (eight items; Schaefer 1965; youth α=0.80; caregiver α=0.86), and caregiver report on the Follow- Through subscale of the Oregon Discipline Scale (11 items; Oregon Social Learning Center, 1991; α=0.82). Ratings on all questionnaires were based on the past month; measures were coded such that high scores reflected high warmth and high effective discipline. Scores on these questionnaires have been shown to relate to youth mental health problems (e.g., Ayers et al. 1998; Kwok et al. 2005; Wolchik et al. 2005; Schaefer 1965; Wolchik et al. 2000).
The positive parenting composite used in the current study was based on a confirmatory factor analysis conducted by Kwok and colleagues (2005) in which the above measures were used to identify a second-order factor model of positive parenting (e.g., caregiver warmth and effective discipline). The standardized scores of the measures used as indicators by Kwok et al. (2005) were formed, and an average of the standardized scores at the two time points (a continuous variable) was used in the analyses.
Recent negative events
Experiences of negative events over the past year were assessed by self-report on an expanded version of the Negative Life Events Scale (NLES). The original NLES was derived from the General Life Events Schedule for Children (Sandler et al. 1986) and Parent Death Events List (Sandler et al. 1992) and included 51 items assessing different types of stressful events, such as exposure to interpersonal conflict, changes in the youth’s environment, caregiver distress, and loss events following parental death. Twenty items from the Life Experiences Survey (Sarason et al. 1978) were added to the NLES to capture life events common in young adulthood, such as trouble with one’s employer.
Current externalizing and internalizing symptoms
Current mental health problems were assessed by self-report on the externalizing and internalizing subscales of the Youth Self-Report form (if respondent was between 14–18 years of age) or the Young Adult Self-Report form (if respondent was over 18 years of age). To put scores from these two scales on a common metric, an equating transformation that was based on a large dataset obtained from Achenbach (n=800) was used. Higher scores correspond to a greater number of symptoms (Achenbach and Rescorla 2001).
Procedures
All procedures were approved by the University Institutional Review Board. Positive parenting was assessed during interviews conducted in the participants’ homes an average of 13 months following parental death and again 11 months later. Recent negative life events and current mental health symptoms were measured approximately 7 years following the death. Data were collected via interviews, with separate interviews conducted by trained interviewers with caregivers and youth. At the 7-year assessment, cortisol activity was assessed during a conflict discussion task between youth and their caregivers that was conducted between 3–9 PM on a weekday at the participants’ homes. The protocol was designed to avoid stressful interview questions or tasks prior to or in the 30 min following the discussion task. This videotaped task was a 12-min standardized behavioral observation. Prior to the interaction, caregivers and youth separately completed the Parent Issues Checklist (Prinz et al. 1979), which includes issues such as youth responsibilities and relationships, and rated these issues on the extent to which the issue made them angry. Interviewers selected the three issues that were endorsed by both caregiver and youth as engendering the highest level of anger. The dyad then discussed these issues. Adolescents/young adults provided a pre-task saliva sample, completed the task, and provided saliva samples immediately after the task (0-min post-task) and 15 and 30 min after the task. Samples were obtained with the Salivette device (Sarstedt, Rommelsdorf, Germany), and were stored frozen at 0°F for 1–3 months before being shipped on dry ice to Salimetrics (State College, PA, USA) for analysis of free cortisol using high-sensitive enzyme immunoassay. The test has a range of sensitivity from 0.007 to 1.8 μg/dl, and mean intra- and inter-assay coefficients of variation 4.13% and 8.89%.
Data analysis
Total cortisol output and cortisol reactivity have been shown to relate differentially to psychological variables (Pruessner et al. 2003); thus, both measures were of interest in the current study. For analyses of total cortisol, a trapezoidal formula was used for computing the overall intensity of the cortisol response (e.g., area under the curve with respect to ground; AUCG). AUCG, which has been described as reflecting “total hormonal output” (Pruessner et al. 2003, p. 928), encompasses the total area beneath the curve of all measurements, thus accounting for the difference between the single measurements and the distance of these measurements from the ground. Due to the nested nature of the data (i.e., multiple youth [max=2] within families), a two-level hierarchical linear model was applied (Raudenbush and Byrk 2002) using SAS PROC MIXED: level-one included the independent variables and covariates (“within-person variables”); level-two included family identification (the “between-person variable”). Cortisol AUCG was predicted from number of negative life events, positive parenting, and an interaction between negative life events and positive parenting, controlling for relevant covariates (described below).
Analyses of reactivity first examined a plot of each subject’s cortisol values at the four time points (i.e., pre-, immediately post-, 15-min post-, and 30-min postdiscussion). On average, cortisol declined across the task; only nine of the 55 subjects exhibited a rise in cortisol from pre-task assessment. The Wilcoxon rank-sum test (which is appropriate for unbalanced groups, count variables, and/or transformed variables) was used to examine differences between those who showed a cortisol increase from baseline (n=9) and those who did not (n=46). There were no significant differences between these two groups on the independent variables (i.e., negative life events, positive parenting), or total cortisol output; p’s ranged from 0.24–0.48. Thus, a three-level hierarchical model (i.e., cortisol values nested within individuals, individuals nested within families) was applied to the full sample to predict the slope of cortisol over time from the interaction between positive parenting and negative life events, controlling for relevant covariates (described below).
For all multilevel models, predictors were meancentered, and the interaction term was calculated as the product of mean-centered negative events and positive parenting to minimize non-essential multicollinearity that often occurs with higher order terms (Aiken and West 1991). The proportion of variance explained, pseudo-R2, was calculated as a measure of effect size for multilevel models (Raudenbush and Byrk 2002). Pseudo-R2 is analogous to R2, and it represents the amount of variance explained by the predictors in the model, ranging from 0 Psychopharmacology to 1. The reported pseudo-R2Δ represents the additional variance accounted for by positive parenting and negative life events.
Results
Preliminary analyses
Table 1 shows descriptive statistics for all study variables. Total cortisol (AUCG) did not correlate significantly with negative life events (r=−0.07, p=0.63) or positive parenting (r=0.01, p=0.93), and there were no main effects of negative events (B=0.002, p=0.39) or positive parenting (B=−0.03, p=0.54) on the slope of cortisol across the task. A number of factors can influence cortisol activity (see Nicolson 2008 for a brief review); therefore, the following variables were evaluated for inclusion as covariates: use of oral contraception, yearly income, participant age, participant gender, smoking status, caffeine intake, current externalizing symptoms, current internalizing symptoms, and time of day at which cortisol sampling began (between 3:45 PM and 8:15 PM; hereafter referred to as time of day).2 There was a marginally significant positive correlation between cortisol AUCG and participant age (r=0.25, p=0.06) and a significant negative correlation between cortisol AUCG and current externalizing symptoms (r= −0.29, p=0.03). There were also significant relations between cortisol slope and participant age (B=0.01, SE= 0.01, t=2.21, p=0.03), externalizing problems (B=0.04, SE=0.01, t=2.58, p=0.01), and internalizing problems (B= 0.02, SE=0.01, t=2.22, p=0.03). The correlation between cortisol and time of day was non-significant (r=−0.18, p=0.18); however, due to expected diurnal variability in cortisol secretion, time of day was included in the initial models. Thus, initial models included age, externalizing problems, internalizing problems, and time of day as covariates.
Primary analyses
The primary hypothesis that levels of post-bereavement positive parenting would moderate the relation between recent negative life events and total cortisol was tested within a multilevel framework, predicting cortisol AUCG from negative life events, positive parenting, and the interaction between negative life events and positive parenting, controlling for participant age, time of day, and internalizing and externalizing symptoms. The interaction term was significant (B=−0.095, SE=0.035, t=−2.72, p<0.01; see Table 2). Time of day (p=0.54) and internalizing symptoms (p=0.60) were not significant predictors and were dropped to simplify the model. The interaction term remained significant (B=−0.10, SE=0.03, t=−2.87, p<0.01). The predictors accounted for 12% of the variance above and beyond the covariates, indicating a medium to large effect size according to Cohen’s (1988) criteria for R2.
Table 2.
The regression of past positive parenting × recent negative life events on cortisol activity controlling for time of day of cortisol sampling, age, internalizing symptoms, and externalizing symptoms (full model) or age and externalizing symptoms (simplified model)
| Full model |
Simplified model |
|||||
|---|---|---|---|---|---|---|
| B | SE | t | B | SE | t | |
| Intercept | −1.18 | 1.28 | −0.93 | −1.67** | 0.078 | −21.52 |
| Time of day | −0.0007 | 0.001 | −0.69 | – | – | – |
| Participant age | 0.05 | 0.033 | 1.50 | 0.05 | 0.032 | 1.54 |
| Internalizing symptoms | 0.036 | 0.068 | 0.53 | – | – | – |
| Externalizing symptoms | −0.211* | 0.10 | −2.06 | −0.189* | 0.086 | −2.19 |
| Positive parenting | 1.18* | 0.45 | 2.63 | −0.066** | 0.194 | −0.34 |
| Negative life events | 0.01 | 0.011 | 0.95 | 0.011 | 0.01 | 1.03 |
| Negative life events × positive parenting | −0.095** | 0.035 | −2.72 | −0.098** | 0.034 | −2.87 |
p<=0.05,
p<0.01
Using procedures outlined in Aiken and West (1991), the significant interaction was probed by calculating simple slopes based on high and low levels of positive parenting, (i.e., one standard deviation above and below the mean, respectively). Tests of significance indicated that for those with low levels of positive parenting, cortisol AUCG increased as the number of recent negative events increased (B=0.05, SE=0.02, t=2.76, p<0.01). Conversely, there was a non-significant trend for those with higher levels of positive parenting; AUCG declined as the number of negative events increased (B=−0.03 SE=0.02, t=−1.79, p=0.08). See Fig. 1 for a graphical representation of these relations.
Fig. 1.
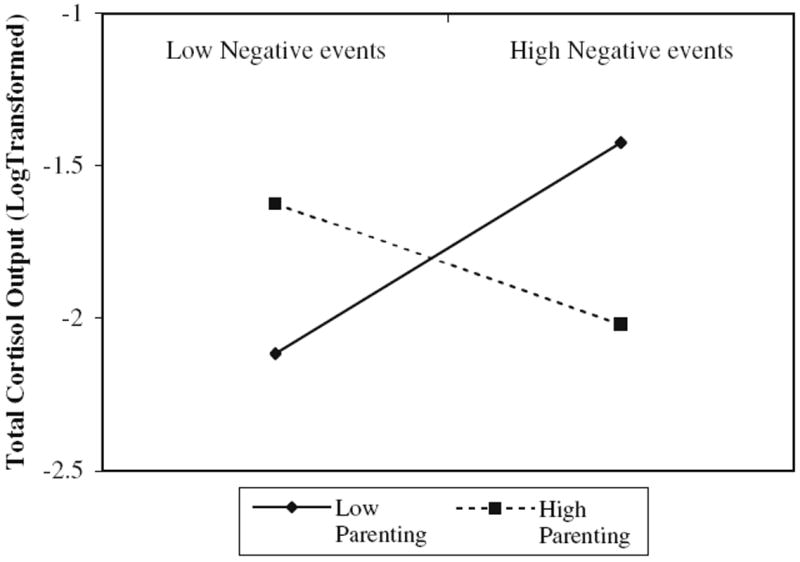
Plot of simple slopes depicting relation between negative life events and total cortisol output at 1 SD above (High) and below (Low) the mean of positive parenting
Finally, the slope of cortisol across the task was predicted from recent negative life events, positive parenting, and the interaction between the two, controlling for age and internalizing and externalizing problems. The interaction term was non-significant (p=0.64).
Discussion
Varied patterns of cortisol activity (e.g., higher and lower basal cortisol, attenuated or exaggerated cortisol reactivity) have been documented among individuals who experienced parental loss or chronic adversity in childhood (Bloch et al. 2007; Flinn et al. 1996; Hagan et al. 2010; Meinlschmidt and Heim 2005; Nicolson 2004; Pfeffer et al. 2007), with little understanding of the factors that explain these variable outcomes. The current study adds to the literature on the long-term consequences of childhood parental loss by highlighting the interaction of proximal (e.g., negative life events in the last year) and distal factors (e.g., quality of parenting several years prior) in predicting neuroendocrine functioning. As predicted, the relation between cortisol activity during a conflict discussion task and number of recent negative life events among parentally bereaved adolescents and young adults depended on the level of positive parenting 6 years earlier. For those who received lower levels of positive parenting, higher recent exposure to stressful events predicted elevated cortisol. These findings suggest that low positive parenting may represent a vulnerability factor for parentally bereaved children, increasing the effect of recent negative events on cortisol output during a conflict discussion. Importantly, although cortisol output was associated with current externalizing symptoms, the interaction between positive parenting and recent negative life events remained significant after controlling for current externalizing symptoms, suggesting that psychological symptoms do not explain the relation between positive parenting, recent stress, and cortisol activity.
It is interesting to speculate about the mechanisms that may account for the significant interaction. Warm and supportive interactions with the surviving caregiver may promote the acquisition of coping and mastery skills necessary to successfully meet the challenges of current and later stressors (Power 2004). Deficits in these skills may increase the emotional and physiological impact of stressors, resulting in higher cortisol responses. By creating a negative family environment that not only strains the stress response system but limits the youth’s capacity to cope effectively with stressors, parenting deficits may be especially problematic for recently bereaved youth who are exposed to a wide array of changes in their social and physical environments after the death. The allostatic load hypothesis (McEwen 1998) notes that consistent “wear and tear” on the body’s regulatory systems contributes to alteration of the HPA axis, which leaves the body illprepared to mount an adaptive response to future stressors. The current findings are supportive of prior evidence of elevated cortisol during lab-based stressors in bereaved youth who reported poor family relationships relative to those reporting higher quality relationships (Luecken 2000; Luecken and Appelhans 2006), but extend the findings to the relationship between naturalistic exposure to negative life events and elevated cortisol.
The relation between recent negative events and elevated cortisol activity among youth with a history of low levels of positive parenting may provide insight into treating psychopathology in youth exposed to early adversity. The physiological effects of early adversity may have implications for mental health long after adverse experiences have occurred. HPA dysregulation has been connected to the development and course of depression (Zobel et al. 2001), particularly in the context of current psychosocial stress (Burke et al. 2005). The current findings indicate that in a sample of youth who experienced the early trauma of parental death, cortisol activity during a conflict discussion task conducted during late adolescence/early adulthood depended on both recent negative events and prior levels of positive parenting. While pharmacologically lowering cortisol levels has shown some effectiveness in the treatment of depression (Murphy 1997), pharmacological treatment may be enhanced by consideration of both proximal and distal environmental/familial factors that may have an effect on cortisol levels. The current study highlights the need to evaluate psychosocial risk and protective factors as part of a more comprehensive biopsychosocial model that considers their relations to potential pharmacological approaches.
There are several limitations to the current study. First, cortisol and positive parenting were not measured prior to the death, precluding consideration of changes over time in the relations between parental loss, exposure to negative life events, positive parenting, and cortisol activity. Similarly, cause–effect relations cannot be drawn from this non-experimental study. Second, other aspects of the family environment (e.g., parental mental health problems) and characteristics of the youth (e.g., temperament) that may affect relations among negative life events, positive parenting, and cortisol activity were not included in the model. Third, on average, cortisol reactivity was not evident during the task. The observed decrease in cortisol may reflect anticipatory rise in cortisol across the sample prior to the discussion, or normal late afternoon/early evening decreases in cortisol. Alternatively, the task may have lacked the qualities necessary to arouse cortisol reactivity; recent research has suggested that such tasks lead to a pattern of declining cortisol (Adam et al. 2007). Fourth, because the discussion task only included youth who had regular contact with their caregiver, the findings cannot be generalized to youth with less contact. Lastly, due to the size of the sample, which is small for the detection of interaction effects, the findings should be viewed as suggestive and in need of replication.
Despite these limitations, the current study has important methodological strengths, and advances existing research on early parental loss and neuroendocrine functioning. As a prospective longitudinal study, the temporal precedence between the measurement of the proposed moderator (positive parenting) and measurement of the proposed moderated relation (recent negative life events to cortisol activity) provided a methodologically strong design (Kraemer et al. 2001). Also, the multi-method, multi-rater assessment of parenting circumvents the biases inherent in primary reliance on self-report or parent report of parenting experiences. The prospective design and multi-method evaluation of positive parenting is innovative and significantly advances a literature that has traditionally relied on retrospective, single-reporter accounts of early childhood environment.
Although environmental, familial, and neurobiological factors have been extensively researched separately as contributors to mental health problems in conjunction with early life stress, few studies have integrated them. This study integrated environmental, familial, and neurobiological factors by examining how parenting quality in earlier stages of development interacted with recent negative life events to impact current neuroendocrine functioning. Findings indicated that greater exposure to recent negative events predicted higher cortisol only among youth with lower levels of past positive parenting. Thus, high-quality caregiving following parental death may support positive adaptation in the face of subsequent negative life events.
Footnotes
Reliability coefficients for positive parenting scales were computed using the baseline sample (as reported in previous studies: Sandler et al. 2003; Kwok et al. 2005; Wolchik et al. 2006).
Use of psychotropic medication has also been shown to influence HPA axis functioning. Only two subjects reported current use of psychiatric medications. Analyses were run with and without these two subjects and results were the same.
Contributor Information
Melissa J. Hagan, Email: Melissa.Hagan@asu.edu, Department of Psychology, Arizona State University, Tempe, AZ 85287-1104, USA. Program for Prevention Research, Arizona State University, P.O. Box 876005, Tempe, AZ 85287-6005, USA.
Danielle S. Roubinov, Department of Psychology, Arizona State University, Tempe, AZ 85287-1104, USA
Jenna Gress-Smith, Department of Psychology, Arizona State University, Tempe, AZ 85287-1104, USA.
Linda J. Luecken, Department of Psychology, Arizona State University, Tempe, AZ 85287-1104, USA
Irwin N. Sandler, Department of Psychology, Arizona State University, Tempe, AZ 85287-1104, USA
Sharlene Wolchik, Department of Psychology, Arizona State University, Tempe, AZ 85287-1104, USA.
References
- Achenbach T, Rescorla L. Manual for the ASEBA school-age forms and profiles. University of Vermont, Research Center Children, Youth, and Families University of Vermont, Research Center Children, Youth, and Families; 2001. [Google Scholar]
- Adam EK, Klimes-Dougan B, Gunnar MR. Social regulation of the adrenocortical response to stress in infants, children, and adolescents: implications for psychopathology and education. In: Coch D, Dawson G, Fischer KW, editors. Human behavior, learning, and the developing brain: atypical development. Guilford; New York: 2007. pp. 264–304. [Google Scholar]
- Aiken LS, West SG. Multiple regression: testing and interpreting interactions. Sage; Thousand Oaks: 1991. [Google Scholar]
- Ayers TS, Sandler IN, Twohey JL. Conceptualization and measurement of coping in children and adolescents. Adv Clin Child Psych. 1998;20:243–301. [Google Scholar]
- Bevans K, Cerbone A, Overstreet S. Relations between recurrent trauma exposure and recent life stress and salivary cortisol among children. Dev Psychopathol. 2008;20:252–272. doi: 10.1017/S0954579408000126. [DOI] [PubMed] [Google Scholar]
- Bloch M, Peleg I, Koren D, Aner H, Klein E. Long-term effects of early parental loss due to divorce on the HPA axis. Horm Behav. 2007;51:516–523. doi: 10.1016/j.yhbeh.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Burke HM, Davis MC, Otte C, Mohr DC. Depression and cortisol responses to psychological stress: a meta-analysis. Psychoneuroendocrinology. 2005;30:846–856. doi: 10.1016/j.psyneuen.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Cerel J, Fristad MA, Verducci J, Weller EB, Weller RA. Childhood bereavement: psychopathology in the 2 years postparental death. J Am Acad Child Psy. 2006;45:681–690. doi: 10.1097/01.chi.0000215327.58799.05. [DOI] [PubMed] [Google Scholar]
- Cohen J. A coefficient of agreement for nominal scales. Educ Psychol Meas. 1960;20:37–46. [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2. Lawrence Earlbaum Associates; Hillsdale: 1988. [Google Scholar]
- Davies PT, Sturge-Apple ML, Cicchetti D, Cummings EM. The role of child adrenocortical functioning in pathways between interparental conflict and child maladjustment. Dev Psychol. 2007;43:918–930. doi: 10.1037/0012-1649.43.4.918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowdney L. Childhood bereavement following parental death. J Child Psychol Psyc. 2000;14:819–830. [PubMed] [Google Scholar]
- Doyle KW, Wolchik SA, Dawson-McClure SR, Sandler IN. Positive events as a stress buffer for children and adolescents in families in transition. J Clin Child Adolesc Psycho. 2003;32:536–545. doi: 10.1207/S15374424JCCP3204_6. [DOI] [PubMed] [Google Scholar]
- Felner RD, Terre L, Rowlison RT. A life transition framework for understanding marital dissolution and family reorganization. In: Wolchik SA, Karoly P, editors. Children of divorce: empirical perspectives on adjustment. Gardner; New York: 1988. pp. 35–65. [Google Scholar]
- Flinn MV, Quinlan RJ, Decker SA, Turner MT, England BG. Male–female differences in effects of parental absence on glucocorticoid stress response. Hum Nature-Int Bios. 1996;7:125–162. doi: 10.1007/BF02692108. [DOI] [PubMed] [Google Scholar]
- Gunnar MR. Stress effects on the developing brain. In: Romer D, Walker EF, editors. Adolescent psychopathology and the developing brain: integrating brain and prevention science. Oxford University Press; New York: 2007. pp. 127–147. [Google Scholar]
- Hagan MJ, Luecken LJ, Sandler IN, Tein J-Y. Prospective effects of post-bereavement negative events on cortisol activity in parentally bereaved youth. Dev Psychobiol. 2010 doi: 10.1002/dev.20433. [DOI] [PubMed] [Google Scholar]
- Haine RA, Wolchik SA, Sandler IN, Millsap RE, Ayers TS. Positive parenting as a protective resource for parentally bereaved children. Death Stud. 2006;30:1–28. doi: 10.1080/07481180500348639. [DOI] [PubMed] [Google Scholar]
- Hazzard A, Christensen A, Margolin G. Children’s perceptions of parental behaviors. J Abnorn Child Psych. 1983;11:49–59. doi: 10.1007/BF00912177. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Wagner D, Wilcox MM, Miller AH, Nemeroff CB. The role of early adverse experience and adulthood stress in the prediction of neuroendocrine stress reactivity in women: a multiple regression analysis. Depress Anxiety. 2002;15:117–125. doi: 10.1002/da.10015. [DOI] [PubMed] [Google Scholar]
- Jensen EW, James SA, Boyce WT, Hartnett SA. The family routines inventory: development and validation. Soc Sci Med. 1983;17:201–211. doi: 10.1016/0277-9536(83)90117-x. [DOI] [PubMed] [Google Scholar]
- Jones S, Twohey JL. Parents’ Expression of Emotions Questionnaire: Psychometric properties. San Francisco, CA. Poster presented at the Annual Convention of the American Psychological Association.1998. [Google Scholar]
- Kraemer HC, Stice E, Kazdin A, Offord D, Kupfer D. How do risk factors work together? Mediators, moderators, and independent, overlapping, and proxy risk factors. Am J Psychiatry. 2001;158:848–856. doi: 10.1176/appi.ajp.158.6.848. [DOI] [PubMed] [Google Scholar]
- Luecken LJ. Parental caring and loss during childhood and adult cortisol responses to stress. Psychol Health. 2000;15:841–851. [Google Scholar]
- Luecken LJ, Lemery KS. Early caregiving and physiological stress responses. Clin Psychol Rev. 2004;24:171–191. doi: 10.1016/j.cpr.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Luecken LJ, Appelhans BM. Early parental loss and salivary cortisol in young adulthood: the moderating role of family environment. Dev Psychopathol. 2006;18:295–308. doi: 10.1017/S0954579406060160. [DOI] [PubMed] [Google Scholar]
- Luecken LJ, Kraft A, Appelhans BM, Enders C. Emotional and cardiovascular sensitization to daily stress following childhood parental loss. Dev Psychol. 2009;45:296–302. doi: 10.1037/a0013888. [DOI] [PubMed] [Google Scholar]
- Lutzke JR, Ayers TS, Sandler IN, Barr A. Risks and interventions for the parentally bereaved child. In: Wolchik SA, Sandler IN, editors. Handbook of children’s coping: linking theory and intervention. Issues in clinical child psychology. Plenum; New York: 1997. pp. 215–243. [Google Scholar]
- McEwen BS. Stress, adaptation, and disease: allostasis and allostatic load. In: McCann SM, Lipton JM, Sternberg EM, Chrousos GP, Gold PW, Smith CC, editors. Neuroimmunomodulation: molecular aspects, integrative systems, and clinical advances (Annals of the New York Academy of Sciences) Academy of Sciences; New York: 1998. pp. 33–44. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Wingfield JC. The concept of allostasis in biology and biomedicine. Horm Behav. 2003;43:2–15. doi: 10.1016/s0018-506x(02)00024-7. [DOI] [PubMed] [Google Scholar]
- Meinlschmidt G, Heim C. Decreased cortisol awakening response after early loss experience. Psychoneuroendocrinology. 2005;30:568–576. doi: 10.1016/j.psyneuen.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Murphy BE. Antiglucocorticoid therapies in major depression: a review. Psychoneuroendocrinology. 1997;22(Suppl 1):S125–S132. [PubMed] [Google Scholar]
- Nicolson NA. Childhood parental loss and cortisol levels in adult men. Psychoneuroendocrinology. 2004;29:1012–1018. doi: 10.1016/j.psyneuen.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Nicolson NA. Measurement of cortisol. In: Luecken LJ, Gallo LC, editors. Handbook of physiological research methods in health psychology. Sage; Thousand Oaks: 2008. pp. 37–74. [Google Scholar]
- Kwok O-M, Haine RA, Sandler IN, Ayers TS, Wolchik SA, Tein J-Y. Positive parenting as a mediator of the relations between parental psychological distress and mental health problems of parentally bereaved children. J Clin Child Adolesc Psycho. 2005;34:260–271. doi: 10.1207/s15374424jccp3402_5. [DOI] [PubMed] [Google Scholar]
- Pendry P, Adam EK. Associations between parents’ marital functioning, maternal parenting quality, maternal emotion and child cortisol levels. Int J Behav Dev. 2007;31:218–231. [Google Scholar]
- Pfeffer CR, Altemus M, Heo M, Jiang H. Salivary cortisol and psychopathology in children bereaved by the september 11, 2001 terror attacks. Biol Psychiatry. 2007;61:957–965. doi: 10.1016/j.biopsych.2006.07.037. [DOI] [PubMed] [Google Scholar]
- Power TG. Stress and coping in childhood: the parents’ role. Parent Sci Pract. 2004;4:271–317. [Google Scholar]
- Prinz RJ, Foster SL, Kent RN, O’Leary KD. Multivariate assessment of conflict in distressed and nondistressed mother-adolescent dyads. J Appl Behav Anal. 1979;12:691–700. doi: 10.1901/jaba.1979.12-691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Raison CL, Miller AH. When not enough is too much: the role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. Am J Psychiat. 2003;160:1554–1565. doi: 10.1176/appi.ajp.160.9.1554. [DOI] [PubMed] [Google Scholar]
- Raudenbush SW, Byrk AS. Hierarchical linear models: applications and data analysis methods. Sage; Thousand Oaks: 2002. [Google Scholar]
- Sandler IN, Guenther R. Assessment of life stress. In: Karoly P, editor. Measurement strategies in health psychology. Wiley; New York: 1985. pp. 555–600. [Google Scholar]
- Sandler IN, Ramirez R, Reynolds K. Life stress for children of divorce, bereaved, and asthmatic children. Paper presented at the Annual Convention of the American Psychological Association.1986. [Google Scholar]
- Sandler IN, Wolchik SA, Braver S, Fogas B. Stability and quality of life events and psychological symptomatology in children of divorce. Am J Commun Psychol. 1991;19:501–520. doi: 10.1007/BF00937989. [DOI] [PubMed] [Google Scholar]
- Sandler IN, West SG, Baca L, Pillow DR, et al. Linking empirically based theory and evaluation: the Family Bereavement Program. Am J Commun Psychol. 1992;20:491–521. doi: 10.1007/BF00937756. [DOI] [PubMed] [Google Scholar]
- Sandler IN, Ayers TS, Wolchik SA, Tein J-Y, Kwok O-M, Haine RA, Twohey-Jacobs J, Suter J, Lin K, Padgett-Jones S, Weyer JL, Cole E, Kriege G, Griffin WA. The Family Bereavement Program: efficacy evaluation of a theory-based prevention program for parentally bereaved children and adolescents. J Consult Clin Psych. 2003;71:587–600. doi: 10.1037/0022-006x.71.3.587. [DOI] [PubMed] [Google Scholar]
- Sarason IG, Johnson JH, Siegel JM. Assessing the impact of life changes: development of the life experiences survey. J Consult Clin Psych. 1978;46:932–946. doi: 10.1037//0022-006x.46.5.932. [DOI] [PubMed] [Google Scholar]
- Schaefer ES. Children’s reports of parental behavior: an inventory. Child Dev. 1965;36:413–424. [PubMed] [Google Scholar]
- Tremblay GC, Israel AC. Children’s adjustment to parental death. Clin Psychol-Sci Pr. 1998;5:424–438. [Google Scholar]
- Wolchik SA, Wilcox KL, Tien J, Sandler IN. Maternal acceptance and consistency of discipline as buffers of divorce stressors on children’s psychological adjustment problems. J Abnorm Child Psych. 2000;28:87–102. doi: 10.1023/a:1005178203702. [DOI] [PubMed] [Google Scholar]
- Wolchik SA, Sandler IN, Winslow E, Smith-Daniels V. Programs for promoting parenting of residential parents: moving from efficacy to effectiveness. Fam Court Rev. 2005;43:65–80. [Google Scholar]
- Wolchik SA, Tien J, Sandler IN, Ayers TS. Stressors, quality of child-caregiver relationship, and children’s mental health problems after parental death: The mediating role of self-system beliefs. J Abnorm Child Psych. 2006;34:221–238. doi: 10.1007/s10802-005-9016-5. [DOI] [PubMed] [Google Scholar]
- Zobel AW, Nickel T, Sonntag A, Uhr M, Holsboer F, Ising M. Cortisol response in the combined dexamethasone/CRH test as predictor of relapse in patients with remitted depression: a prospective study. J Psychiat Res. 2001;35:83–94. doi: 10.1016/s0022-3956(01)00013-9. [DOI] [PubMed] [Google Scholar]


