Abstract
Multidrug chemotherapy for 6–9-months is one of the primary treatments in effective control of tuberculosis, although the mechanisms underlying the persistence of its etiological agent, Mycobacterium tuberculosis, against antibiotics remain unclear. Ever-mounting evidence indicates that the survival of many environmental and pathogenic microbial species against antibiotics is influenced by their ability to grow as surface-associated multicellular communities called biofilms. In recent years, several mycobacterial species, including M. tuberculosis, have been found to form drug-tolerant biofilms in vitro through genetically controlled mechanisms. In this review, the authors discuss the relevance of the in vitro mycobacterial biofilms in understanding the antibiotic recalcitrance of tuberculosis infections.
Keywords: biofilms, drug tolerance, Mycobacterium tuberculosis, tuberculosis
Mycobacterial infections are uniquely recalcitrant to antibiotics
Treatment of a typical infection of Mycobacterium tuberculosis – the causative pathogen of tuberculosis (TB) – requires 6–9 months of chemotherapy with isoniazid, pyrazinamide, rifampicin and often ethambutol – a multidrug regimen developed nearly 40 years ago [1,2]. This extended treatment has had a devastating impact on the global burden of TB, which is estimated to be prevalent in either clinical or subclinical states in about a third of the global human population, and kills approximately 1.7 million people every year [3]. In particular, the long chemotherapy regimen can be attributed to patients’ noncompliance and poor case management in resource-limited countries with a high burden of the disease. An incomplete course of treatment is considered to be one of the primary contributors to the emergence of multidrug-resistant TB and extremely drug-resistant TB [4,5]. A long and complex chemotherapy of TB poses further challenges to the treatment of HIV–TB coinfections – a leading cause of death in AIDS patients [6,7]. The extended chemotherapy is necessary to sterilize a small subpopulation of drug-tolerant M. tuberculosis bacilli, although it remains unclear where and how these persisters survive in the tissues [8]. Overall, an improvement in case management and subsequent control of active TB require a new generation of drugs that can clear the infection efficiently in a shorter period of time. Thus, it is time to evaluate the perspectives of mycobacterial persistence against antibiotics in the context of existing broader concepts emerging from studies on other bacterial pathogens. In this review, the authors discuss how the established paradigm of microbial growth and persistence in organized multicellular communities, called biofilms, could be relevant to the understanding of drug recalcitrance in TB infections.
Mycobacteria have a strong propensity to grow in multicellular structures
Cultures of mycobacteria in vitro spontaneously produce macroscopic structures, leading to the development of pellicles on the air–media interface. To circumvent the challenges of these growth characteristics in clonal purification of the strains, Dubos and Middlebrook described a method of growing M. tuberculosis in the presence of polysorbate-80, which produced a homogeneously dispersed suspension without any signif icant impact on the virulence properties of the pathogen [9]. The detergent is now widely used in the mycobacterial growth medium, although there are potentially significant pitfalls in the interpretations of biological characteristics of the bacilli grown in such a condition. First, the interaction of a detergent with surface-exposed, noncovalently-associated lipids of the bacteria, and subsequent alteration in the structure of the envelope, can potentially have a profound impact on the functional properties of the envelope, including altered permeability to small molecules. For instance, mycobacteria grown in media with polysorbate-80 are significantly more sensitive to antibiotics than those grown without the detergent [10]. Second, bacteria in a planktonic culture are homogeneously exposed to nutrients and oxygen, unlike those in the clusters exposed to heterogeneous microenvironments, with a self-generated gradient of nutrients and oxygen from the surface to the interior core. While cells on the surface of the clusters would presumably be exposed to rich growth conditions, those at the core could possibly encounter bacteriostatic conditions. The resulting nonuniform adaptive responses in the population can foster unique phenotypic diversity, most likely leading to the emergence of a stress tolerant subpopulation.
Thus, structured growth of mycobacteria in clusters can promote phenotypic persistence of constituent bacilli through physical protection from the environmental threats and self-formed bacteriostatic microenvironments, adaptation to which could facilitate the development of stress-tolerant physiology. However, complexities of mycobacterial growth in structured aggregates and involvement of biological mechanisms in the formation of the structures is under-appreciated by the long-held simplistic perspective in which cellular aggregation is believed to be a likely consequence of hydrophobic interactions between the waxy surfaces of the bacilli.
Biofilms: a genetically programmed lifestyle of microbes
In a landmark microscopic study of cystic fibrosis lung tissues, Costerton and colleagues observed that the growth of Pseudomonas aeruginosa, the causative bacterial pathogen, occurred in microcolonies encapsulated by extracellular polysaccharides (EPS) [11]. Subsequently, a similar pattern of clustered growth of Staphylococcus aureus was observed upon microscopic examination of infected implantation devices [12]. These studies initiated a shift in the view of microbes, from unicellular, motile and planktonic organisms to surface-attached sessile biofilm communities, coexisting along with many other species [13–24]. In biofilms, the resident microbes are self-organized into 3D, matrix-encapsulated structures, with a differentiated interior consisting of water channels and cavities [14,15,18,20,25,26]. Development of microbial biofilms proceeds through genetically controlled specific stages that typically start with surface attachment and colonization of planktonic cells, followed by sessile growth, differentiation and encapsulation of structures [13–15,17,18,21,27–30] (Figure 1). Attachment of planktonic cells is the most critical step in biofilm formation and is dependent on surface properties of both the substratum and the cells. While most biotic and abiotic surfaces are suitable for microbial colonization, certain patterns such as sharklet, or diamond-shaped nanodenticles of shark, are inhibitory [31]. From the microbial perspective, surface attachment is facilitated by both the environmental cues like nutrient limitation, as well as genetic factors. Species of proteobacteria and cyanobacteria utilize a noncovalent assembly of proteinaceous structure, called pili, for surface attachment [32,33].
Figure 1. Schematic model representing distinct developmental stages of microbial biofilms.
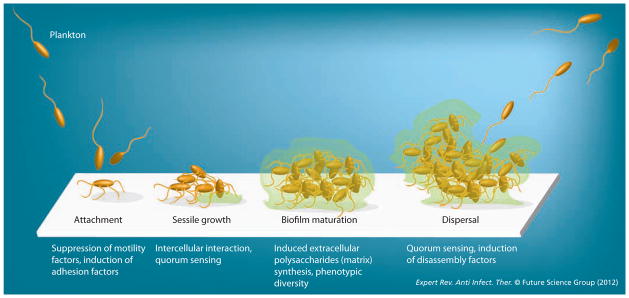
Each stage is associated with specific sets of phenotypic switches, facilitated by tightly regulated changes in gene expression patterns.
Surface attachment triggers changes in gene expression that facilitate the growth of biofilms. For example, a master regulator (CsgD) in Escherichia coli promotes biofilm formation by repressing flagellar synthesis and motility, and inducing cyclic diGMP – a secondary messenger for EPS synthesis – through activation of adrA [34]. While characterization of CsgD is limited to E. coli and Salmonella spp., cyclic diGMP-dependent induction of EPS synthesis and biofilm formation has been found in several Gram-negative species [35]. In Vibrio cholerae, cyclic diGMP, and therefore biofilms and virulence genes, are negatively regulated, but quorum sensing – a phenomenon of high-density, specific intercellular communication in bacteria – is positively regulated by hapR [36]. The inverse correlation between quorum sensing and biofilms in V. cholerae suggests biofilm formation as a colonization strategy, while quorum sensing and subsequent suppression of virulence indicate dispersal activity [36,37]. The quorum sensing in P. aeruginosa, however, appears to positively induce maturation of biofilms [38].
Biofilm development in Gram-positive species is modeled around the extensively studied mechanisms in Bacillus subtilis. The EPS synthesis and matrix-formation genes are under negative and positive regulation of SinR and SinI regulators, respectively [39,40]. Furthermore, biofilm formation is negatively controlled by the regulators that trigger sporulation, spo0A-P, indicating that sporulation and biofilm formation are the mutually exclusive choices made by B. subtilis in response to environmental cues [41].
Reconstructing these studies reveals a fascinating overall picture of the microbial world, in which bacteria switch from a motile planktonic form to sessile, surface-attached biofilms by reprogramming the patterns of gene expression (Figure 1). While motility in the planktonic state could be imagined to facilitate the search of individual cells for new niches, development of robust architecture in biofilms and matrix-encapsulation of cells presumably contribute to the phenotypic tolerance against environmental stress, including antibiotics [17,18,20,24,42–47]. The tolerance is believed to be a cumulative effect of the physical protection from the matrix and the physiological adaptation in the resident population in response to the nutrients and oxygen gradients that generate heterogeneity in the microenvironments [13,42–44,48]. The latter process can lead to extensive phenotypic diversity in the population, as elegantly demonstrated by several transcriptomics and genetic studies of B. subtilis biofilms [23,49]. The expression patterns of genes involved in motility, matrix production and sporulation in B. subtilis biofilms are associated with nonoverlapping subpopulations, which are spatially and temporally separated from each other in the multicellular structure [23].
Biofilms: the predominant manifestation of chronic microbial infection
Biofilms are the common clinical manifestation of pathogenic colonization in host tissues and implant devices [16,20,24,25,42,50–53]. Besides the biofilms of P. aeruginosa in cystic fibrosis, other well-documented examples of pathogenic biofilms include: polymicrobial growth in dental plaques and otitis media, mono-infections of Staphylococcus and Streptococcus spp. in implants, pneumonia, osteomyelitis, infective endocarditis, meningitis, dermatitis and in nasal passages, as well as uropathogenic E. coli infection in the urinary tract [12,51,52,54,55].
Regardless of the causative pathogen, infectious biofilms display extraordinary tolerance to antibiotics and subversion to the host immune system [20,24,56,57]. For example, a matrix component of P. aeruginosa biofilms, rhamnolipid, triggers efficient lysis of neutrophils [58]. While the process directly protects the population from the antimicrobial activities of the neutrophils, it also indirectly creates a protected niche by causing necrosis of the tissues through the release of peroxides and proteases from the lysed cells. Modulation of the immune response by biofilms is further evident in the skewed Th1/Th2 response during infections of P. aeruginosa and S. aureus. The early infections of these extracellular pathogens produce a strong Th1-dependent cell-mediated adaptive immune response that is effective against the intracellular pathogen, whereas the later stages of infection produce Th2-mediated humoral immunity [59–62]. The antibodies produced at high bacterial burden are perhaps inadequate at clearing the infection. Although the precise molecular components responsible for the skewed Th1/Th2 response remain unidentified, factors influencing the development of biofilms, such as quorum sensing, have been implicated in the host response [57]. From a broader perspective, the evidence of misdirection and evasion of immune response by biofilms are generally consistent with their subclinical pathology.
Treatment of biofilm infections is extremely difficult. The planktonic cultures of clinical isolates of Staphylococci have been found to be approximately 20–50 times more sensitive to antibiotics than their biofilms [63]. Similarly, biofilms of pathogenic E. coli and P. aeruginosa are 100–1000 times more tolerant to all tested antibiotics than their planktonic counterpart [64]. The drug tolerance in biofilms, acquired through the mechanisms described in the previous section, necessitates a very aggressive prophylactic and therapeutic regimen [56]. Although several preventive measures, such as the coating of implant devices with antimicrobial agents such as metal chelators, are proposed as effective control measures against biofilms, there is no clear therapeutic strategy as yet that involves dispersal of biofilms prior to treatment [65]. However, in one of the most promising developments in dispersal strategies of biofilms, Losick and colleagues recently discovered that biofilms of Gram-positive and Gram-negative species could be efficiently dispersed by nanomolar concentrations of D-amino-acids, although their clinical efficacies in conjunction with other antibiotics remain untested [66].
In summary, biofilm formation is a highly effective and ubiquitous strategy for the pathogen to proliferate as a stress-tolerant community in protected host niches, with limited invasion from the immune system. Thus, biofilm infections can potentially pose significant diagnostic and therapeutic challenges in clinical settings.
Do infections of M. tuberculosis display characteristics of biofilms?
Antibiotic recalcitrance & chronicity of M. tuberculosis infection: the common features associated with pathogenic biofilm infections
Infections of M. tuberculosis are often asymptomatic, chronic in a clinically symptomatic state and highly recalcitrant to antibiotics. Although molecular mechanisms underlying persistence of the pathogen against the host immune system and chemotherapy remain unclear, these clinical features bear similarities with the characteristics of infections associated with microbial biofilms; thereby raising the question as to whether or not M. tuberculosis forms biofilms in vivo, and whether biofilm formation contributes to their persistence. While reminiscence of M. tuberculosis growth in large multicellular clusters has been previously described in histopathological studies of infected lungs, it is unclear if these structures represent a genetically programmed growth pattern of persistent biofilms [67]. However, preliminary evidence suggests that biofilms could perhaps be an in vivo lifestyle of M. tuberculosis, contributing to their persistence against antibiotics. In a pathological study by Lenaerts et al., the surviving population of the pathogen after drug treatment of infected guinea pigs was found to be in microcolonies of bacteria located around the acellular rim in the granulomas [68]. Furthermore, the discovery of a M. tuberculosis-encoded pilin-like protein, which is not only expressed in vivo but also binds strongly to the eukaryotic extracellular matrix, supports the notion that the pathogen’s surface could be actively engaged in surface attachment [69]. The idea of M. tuberculosis persistence in biofilms is supported by the recent findings that portray a highly complex and dynamic state of host–pathogen interface in both acute and latent TB [70–77]. Regardless of the phase of TB infection in nonhuman primates, or murine models, replication of bacilli and engagement of immune cells most likely remain active within a localized host–pathogen interface [70,74,76,77]. Furthermore, the TB lesions representing the host–pathogen interfaces are pathologically heterogeneous, distributed over a wide range of sizes and morphologies in a single individual, irrespective of the clinical symptoms of infection [71,78].
The implications of these findings are that in any given infection of M. tuberculosis, the pathogen thrives in diverse micro-environments, possibly in a wide range of morphological and physiological states that could include biofilms. The question then arises as to which of these states can give rise to drug-tolerant persisters. The drug-tolerant persisters of M. tuberculosis are widely understood to be a small subpopulation of either nonreplicating or slow-replicating variants, presumably emerging through the adaptive processes in a bacteriostatic environment of calcified or closed lesions [79,80]. Persistence of M. tuberculosis against antibiotics in a nonreplicative state has been inferred from the microscopic detection of acid-fast bacilli in culture-negative smears of drug-treated patients [81–83]. This was later strengthened by the Cornell model of TB reactivation, in which the disease relapsed at higher frequencies when drug treatments in infected mice were terminated soon after the sterilization of culturable bacilli [84,85]. Furthermore, low concentrations of oxygen were found in lesions associated with acid-fast bacilli in drug-treated infections, leading to a notion that hypoxia could likely be the primary inducer of the nonreplicating physiology in M. tuberculosis [86–88]. Wayne and colleagues demonstrated the emergence of nonreplicating persisters during depletion of oxygen from in vitro M. tuberculosis cultures, which has been extensively investigated for molecular insight into mycobacterial adaption to limiting oxygen and its influence on development of drug-tolerance physiology [89–91]. Besides physiological adaptation to hypoxia, several additional cellular and physiological factors have also been attributed to the intrinsic antibiotic tolerance in mycobacteria. These include: restricted permeability of a thick mycobacterial envelope, metabolic plasticity under nonhypoxic stress and asymmetric growth pattern [78,92–95]. Thus it is plausible that the drug-tolerant sub-population in any single TB patient could emerge through multiple mechanisms based on the growth and adaptation of the pathogen to its immediate microenvironment, including those within the core of its large multicellular aggregates.
Mycobacterial species form biofilms in vitro
Multiple mycobacterial species, most notably Mycobacterium avium, have been found to exist as multicellular communities in the environment, as well as in clinical settings [96–99]. The prevalence of these mycobacterial communities in their natural habitat can be further appreciated by evidence that the aggregates and pellicles of mycobacteria, routinely observed in detergent-free in vitro cultures, represent a genetically programmed development of organized, drug-tolerant communities – the key features of biofilms. Kolter and colleagues first reported that surface attachment, sliding motility and biofilm formation in a nonpathogenic mycobacteria, Mycobacterium smegmatis, require an acetylated derivative of glycopeptidolipids (GPL) [100]. While the mutant of GPL acetyl-transferase produced defective biofilms, it had no growth defect in planktonic form [100]. GPL biosynthesis was also induced during multicellular growth of M. avium, suggesting that the two species share the mechanisms for biofilm development [101]. Besides GPL, mycolyl-diacylglycerol (MDAG) and free mycolic acids (FM) are the other two surface molecules known so far that have an important role in biofilm development of M. smegmatis [27,102,103]. Thus, lipids could likely have critical roles in intercellular and cell-to-substratum interactions in mycobacterial biofilms. While MDAG synthesis is regulated by a nucleoid-associated protein, Lsr2, FM synthesis is induced during the maturation of biofilms through a GroEL1-dependent modulation of type II fatty acid synthases [27,102–104]. The interaction between GroEL1 and β-keto-acyl ACP synthases (KasA and KasB), the enzymes of FASII, is specifically induced during the later stages of biofilm formation, indicating that biofilm development in M. smegmatis proceeds through distinct stages not associated with planktonic growth [104]. The development of M. smegmatis biofilms also requires a greater abundance of intracellular iron, which is facilitated by induced activity of siderophore synthesis [105]. Interestingly, a strict dependence on iron availability for biofilm formation is also found in P. aeruginosa [106].
In vitro growth of M. tuberculosis in detergent-free media also proceeds through genetically controlled developmental stages, ultimately maturing into FM-rich biofilms on the air–media interface (Figure 2A & 2B) [102]. There are at least three genetic loci, pks16, helY and pks1, implicated in the process of M. tuberculosis biofilm formation [102,107]. Mutants of all three genes fail to produce matured biofilms while growing indistinguishably from the wild-type in planktonic form. Intriguingly, biofilm formation in M. tuberculosis is also sensitive to the gaseous environment on the air–media interface, consistent with the idea that a distinct gaseous composition could induce intercellular or cell-surface interactions in slow growing mycobacteria [102].
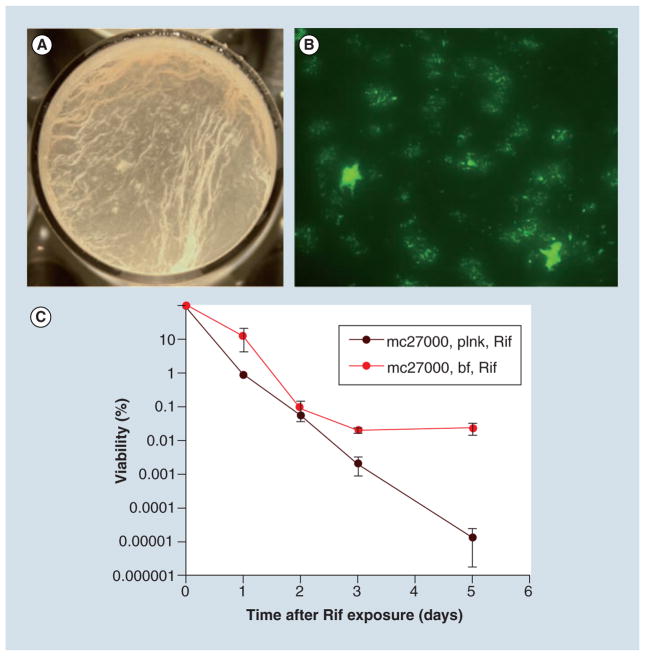
Figure 2. In vitro biofilms of Mycobacterium tuberculosis are drug tolerant.
(A) Biofilms of Mycobacterium tuberculosis on the air–media interface grown in a 12-well plate. (B) Biofilms of green fluorescent protein expressing M. tuberculosis on a polycarbonate surface, showing distinct microcolonies. (C) Presence of rifampicin-tolerant persisters in M. tuberculosis biofilms formed on air–media interface. The data from the original article are reproduced in accordance with the policy of the journal publishing the original article.
bf: Biofilm; plnk: Planktonic; Rif: Rifampicin.
Adapted from [102].
Nontuberculous mycobacteria colonize as biofilms
At least two nontuberculous mycobacterial species, Mycobacterium ulcerans and M. avium, have been reported to colonize in the host as multicellular communities [108,109]. Infection of an aquatic insect, Naucoris cimicoides, by M. ulcerans produces a multicellular structure of the pathogen encapsulated by an extracellular matrix [108]. Interestingly, the matrix of M. ulcerans multicellular structures are laden with the toxin mycolactone, which is required for the colonization and virulence of the pathogen [108,110]. Thus, formation of the extracellular matrix, the hallmark of biofilms, by M. ulcerans has a direct influence on its virulence properties. Implication of biofilms in M. avium infection is demonstrated by the inability of the biofilm-defective mutant strain to invade and translocate the bronchial epithelial cells [109].
Mycobacterial growth in biofilms: a relevant in vitro growth model
While the questions as to how, when and where M. tuberculosis forms biofilms in vivo remain open to investigation, it is clear that the pathogen, along with other mycobacteria form these structures in vitro through dedicated genetic pathways. This provides a compelling argument that the in vitro biofilms represent a spontaneous growth characteristic and therefore should be adopted as a growth model for basic mycobacteriology. Moreover, growth of mycobacteria in planktonic form, usually in the presence of detergent, grossly misrepresents their natural behavior and metabolic activities, which can only be revealed by biofilms. Besides the dedicated genetic pathways for structural development, mycobacterial biofilms also acquire unique phenotypes not associated with planktonic forms. The biofilms of M. tuberculosis and M. smegmatis harbor a subpopulation of drug tolerant persisters at several orders of magnitude higher than both defective biofilms of the mutants, as well as the planktonic cultures of the wild-type (Figure 2C) [102,103,111]. Interestingly, presence of antibiotics has been found to influence phenotypic heterogeneity in the biofilms of M. avium [112]. Growth of M. smegmatis in biofilms has been found to be critical for the conjugal transfer of DNA, suggesting that cell-to-cell contact in biofilms could provide a unique opportunity for intercellular transfer of genetic material [113].
The distinction between phenotypes of planktonic and biofilm mycobacteria is further represented by the fact that 82 genes are exclusively induced during maturation of M. smegmatis biofilms (Table 1) [105]. Based on sequence homology, these genes belong to diverse functional categories, including transport of small molecules across the envelope, DNA replication and repair, adaptation to carbon and oxidative stresses, as well as lipid biosynthesis and envelope remodeling (Table 1). The overall pattern of induction is consistent with the idea that the microenvironments of mycobacterial biofilms pose unique challenges to which the constituent cells must adapt. These adaptive processes could possibly be the origin of their drug tolerance behavior. For example, bacterial multidrug efflux pumps have been implicated in physiological export of lipids, raising a possibility that the biofilm-induced transporters could also confer drug tolerance behavior to the bacilli [114]. Induction in fatty acid biosynthesis pathways, perhaps to facilitate the synthesis of biofilm-associated waxy extracellular components such as MDAG and FM, could reduce the permeability of the envelope and induce the drug tolerance properties. Interestingly, Lsr-2, the regulator of MDAG synthesis in M. smegmatis, has also been implicated in regulating the expression of multidrug-tolerance operon, iniABC, in M. tuberculosis [115].
Table 1.
Genes exclusively induced during later developmental stages (4-day) of Mycobacterium smegmatis biofilms.
| Locus tag | Annotation | Mycobacterium tuberculosis homologs |
|---|---|---|
| MSMEG_0011 | Iron utilization protein, putative | Rv2895c |
| MSMEG_0013 | Ferricexochelin uptake | |
| MSMEG_0015 | Ferricexochelin uptake | |
| MSMEG_0020 | FxuD protein | Rv0265c |
| MSMEG_0225 | Membrane protein, MmpL family | Rv2339 |
| MSMEG_0313 | 6-phosphogluconate dehydratase | Rv0189c |
| MSMEG_0316 | Transcription regulator ROK family VC2007 (imported), putative | |
| MSMEG_0422 | Carboxyphosphonoenolpyruvatephosphonomutase-like protein | Rv1998c |
| MSMEG_0457 | DNA gyrase subunit B, putative | Rv0005 |
| MSMEG_0504 | Ribokinase | Rv2436 |
| MSMEG_0534 | Multidrug-resistance protein, putative | |
| MSMEG_0550 | ABC transporter, periplasmic substrate-binding protein | |
| MSMEG_0782 | Aminotransferase, class III | Rv2598 |
| MSMEG_0790 | MUTT3 | Rv0413 |
| MSMEG_0911 | Isocitratelyase | Rv0467 |
| MSMEG_1123 | Putativecobalamin synthesis protein | |
| MSMEG_1129 | D-amino acid dehydrogenase, small subunit, putative | |
| MSMEG_1268 | MutT-nudix family protein | Rv1593c |
| MSMEG_1269 | Serine-threonine protein phosphatase | |
| MSMEG_1332 | Conserved hypothetical protein | Rv0633c |
| MSMEG_1336 | Flavohemoprotein, putative | Rv3571 |
| MSMEG_1419 | Conserved hypothetical protein | |
| MSMEG_1515 | Sensor histidine kinase | |
| MSMEG_1742 | Oxidoreductase | Rv3554 |
| MSMEG_1743 | DESA3 | Rv3229c |
| MSMEG_1757 | Lhr | Rv3296 |
| MSMEG_1953 | Unnamed protein product | Rv3260c |
| MSMEG_2188 | Putative integral membrane protein | |
| MSMEG_2238 | AldA | Rv0768 |
| MSMEG_2268 | Thioredoxin-like protein | |
| MSMEG_2269 | Conserved hypothetical protein | |
| MSMEG_2293 | Conserved hypothetical protein | |
| MSMEG_2311 | Predicted protein, putative | |
| MSMEG_2350 | Conserved hypothetical protein | Rv3030 |
| MSMEG_2380 | Sugar transporter family protein | Rv2994 |
| MSMEG_2601 | Protocatechuate 3,4-dioxygenase β subunit | |
| MSMEG_2735 | Diaminopimelateepimerase | Rv2726c |
| MSMEG_2740 | LexA repressor | Rv2720 |
| MSMEG_2743 | Conserved hypothetical protein TIGR00244 | Rv2718c |
| MSMEG_2774 | GGDEF domain protein | |
| MSMEG_2945 | Holliday junction DNA helicase RuvB | Rv2592c |
| MSMEG_3183 | Threoninedehydratase, biosynthetic | |
| MSMEG_3194 | Biotin synthase | Rv1589 |
| MSMEG_3297 | Adenylatecyclase | Rv1900c |
| MSMEG_3300 | Oxidoreductase, Gfo-Idh-MocA family | |
| MSMEG_3301 | Oxidoreductase, putative | Rv0791c |
| MSMEG_3305 | Integral membrane protein domain protein | |
| MSMEG_3568 | BFD-like (2Fe–2S) binding domain family | |
| MSMEG_4038 | Vanillin dehydrogenase | |
| MSMEG_4057 | Transcriptional regulator, GntR family | Rv0165 |
| MSMEG_4383 | Membrane protein, MmpL family | Rv0676c |
| MSMEG_4477 | Hydrolase, α-β hydrolase fold family | Rv1900c |
| MSMEG_4512 | Polyketide synthase, putative | Rv2381c |
| MSMEG_4532 | Sulfate ABC transporter, permease protein CysT | Rv2399c |
| MSMEG_5004 | Ser-Thr protein phosphatase family | Rv1277 |
| MSMEG_5048 | Conserved hypothetical protein | Rv1249c |
| MSMEG_5102 | ABC transporter domain protein | |
| MSMEG_5130 | LpqW | Rv1166 |
| MSMEG_5216 | Glyoxalase family protein superfamily | |
| MSMEG_5308 | Surface antigen, putative | RV1057 |
| MSMEG_5310 | SAM-dependent methyltransferases | Rv2622 |
| MSMEG_5312 | ABC transport protein | |
| MSMEG_5556 | Major facilitator family transporter | |
| MSMEG_5659 | ABC transporter, ATP-binding-permease protein | Rv0194 |
| MSMEG_5680 | Glyoxalase family protein | Rv0887c |
| MSMEG_5716 | Conserved hypothetical protein | |
| MSMEG_5732 | N5,N10-methylene-tetrahydromethanopterin reductase (mer), putative | Rv3093c |
| MSMEG_6030 | P450 heme-thiolate protein, putative | Rv1777 |
| MSMEG_6060 | Predicted permease superfamily | |
| MSMEG_6255 | Conserved hypothetical protein | Rv3707c |
| MSMEG_6476 | Putative large secreted protein | |
| MSMEG_6487 | Amidohydrolase family superfamily | |
| MSMEG_6540 | Virulence factor | Rv0589 |
| MSMEG_6555 | Transcriptional regulator, TetR family, putative | |
| MSMEG_6567 | COG2837: predicted iron-dependent peroxidase | |
| MSMEG_6569 | Uncharacterized BCR | |
| MSMEG_6570 | Membrane protein, putative | |
| MSMEG_6618 | Superfamily II DNA and RNA helicases | Rv2092c |
| MSMEG_6619 | Conserved hypothetical protein | Rv3096 |
| MSMEG_6742 | Conserved hypothetical protein | |
| MSMEG_6783 | Putative integral membrane protein | |
| MSMEG_6923 | Conserved hypothetical protein | Rv0036c |
The list was originally published by Ojha et al. in a transcriptomic analysis of planktonic and biofilm cultures of the nonpathogenic mycobacterial species using microarrays [105]. The Mycobacterium tuberculosis homologs denote the open reading frames with at least 25% identity. The data from the original article are reproduced in accordance with the policy of the journal publishing the original article.
Data taken from [105].
Adaptation to oxidative stress in biofilms, as indicated by the induction of multiple oxidoreductases, LexA and thioredoxin-like proteins, could also influence the tolerance to antibiotics that generate high concentrations of intracellular free radicals (Table 1). This is supported by a correlation between isoniazid tolerance and induced biosynthesis of mycothiol – a predominant reducing agent that maintains redox homeostasis [116].
In conclusion, in vitro biofilms represent a complex but spontaneously self-assembled multicellular architecture of mycobacteria, with a treasury of unexplored knowledge about the mechanisms that shape their stress tolerance behavior.
Expert commentary & five-year view
The in vitro growth of M. tuberculosis in biofilms, together with the evidence of in vivo biofilms of nontuberculous mycobacteria, opens up the question as to whether biofilm formation could be a survival strategy of M. tuberculosis in chronic infection. Moreover, this question is timely with the recent emerging shift in pathological and bacteriological understanding of chronic TB. In the coming years, it will most likely become clear if the multicellular clusters of M. tuberculosis in infected lungs indeed represent the drug tolerant biofilms, held together by an extracellular matrix. It is noteworthy that such in vivo studies would heavily rely upon the basic understanding of intercellular interactions and matrix synthesis of the pathogen in cultures in vitro. The fundamental mechanisms of surface attachment, intercellular signaling and matrix production, identif ied in vitro, can be argued to have reasonable predictability under in vivo conditions, regardless of the differences between the two growth environments. Because the development of drug-tolerant persisters is intricately linked to the physical integrity of the matured biofilms, factors influencing the formation or dissociation of biofilms can serve as potential targets for faster clearance of mycobacterial infections [102].
Key issues.
Mycobacterium tuberculosis infections are uniquely recalcitrant to antibiotics and are sterilized with 6–9 months of multidrug chemotherapy.
Although extended therapy is necessary to eliminate a minor subpopulation of drug-tolerant persisters, it is not clear how and where these persisters develop in the host.
Most microbial species naturally persist through a sessile growth mechanism that leads to the formation of robust, multicellular communities called biofilms.
Mycobacterial species grown in vitro produce biofilms developed through dedicated genetic pathways, held together by waxy extracellular materials, and harbor drug-tolerant persisters.
Histopathological studies from the autopsies of tuberculosis lesions have revealed multicellular clusters of M. tuberculosis.
This raises the possibility that the clustered growth of mycobacteria could represent biofilms harboring drug-tolerant persisters.
Acknowledgments
The excellent technical support of Kathleen Kulkain proofreading this review is acknowledged. The authors also thank John Wiley & Sons for their policy to reproduce some of the figures originally published by AK Ojha in one of their journals.
Footnotes
For reprint orders, please contact reprints@expert-reviews.com
Financial & competing interests disclosure
The work in the Ojha laboratory is funded by the NIH (AI079288). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Mitchison DA. The diagnosis and therapy of tuberculosis during the past 100 years. Am J Respir Crit Care Med. 2005;171(7):699–706. doi: 10.1164/rccm.200411-1603OE. [DOI] [PubMed] [Google Scholar]
- 2.BMC. Controlled clinical trial of short-course (6-month) regimens of chemotherapy for treatment of pulmonary tuberculosis. Lancet. 1972;1(7760):1079–1085. [PubMed] [Google Scholar]
- 3.Dye C, Lönnroth K, Jaramillo E, Williams BG, Raviglione M. Trends in tuberculosis incidence and their determinants in 134 countries. Bull World Health Organ. 2009;87(9):683–691. doi: 10.2471/BLT.08.058453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mitchison DA. How drug resistance emerges as a result of poor compliance during short course chemotherapy for tuberculosis. Int J Tuberc Lung Dis. 1998;2(1):10–15. [PubMed] [Google Scholar]
- 5.Ormerod LP. Multidrug-resistant tuberculosis (MDR-TB): epidemiology, prevention and treatment. Br Med Bull. 2005;73:17–24. doi: 10.1093/bmb/ldh047. [DOI] [PubMed] [Google Scholar]
- 6.Harrington M. From HIV to tuberculosis and back again: a tale of activism in 2 pandemics. Clin Infect Dis. 2010;50(Suppl 3):S260–S266. doi: 10.1086/651500. [DOI] [PubMed] [Google Scholar]
- 7.Aaron L, Saadoun D, Calatroni I, et al. Tuberculosis in HIV-infected patients: a comprehensive review. Clin Microbiol Infect. 2004;10(5):388–398. doi: 10.1111/j.1469-0691.2004.00758.x. [DOI] [PubMed] [Google Scholar]
- 8.Jindani A, Doré CJ, Mitchison DA. Bactericidal and sterilizing activities of antituberculosis drugs during the first 14 days. Am J Respir Crit Care Med. 2003;167(10):1348–1354. doi: 10.1164/rccm.200210-1125OC. [DOI] [PubMed] [Google Scholar]
- 9.Dubos RJ, Middlebrook G. The effect of wetting agents on the growth of tubercle bacilli. J Exp Med. 1948;88(1):81–88. doi: 10.1084/jem.88.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Boxtel RM, Lambrecht RS, Collins MT. Effects of colonial morphology and Tween 80 on antimicrobial susceptibility of Mycobacterium paratuberculosis. Antimicrob Agents Chemother. 1990;34(12):2300–2303. doi: 10.1128/aac.34.12.2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11••.Lam J, Chan R, Lam K, Costerton JW. Production of mucoid microcolonies by Pseudomonas aeruginosa within infected lungs in cystic fibrosis. Infect Immun. 1980;28(2):546–556. doi: 10.1128/iai.28.2.546-556.1980. Discussion on microbial existence in multicellular communities. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Costerton JW, Irvin RT, Cheng KJ. The role of bacterial surface structures in pathogenesis. Crit Rev Microbiol. 1981;8(4):303–338. doi: 10.3109/10408418109085082. [DOI] [PubMed] [Google Scholar]
- 13.Kolter R, Losick R. One for all and all for one. Science. 1998;280(5361):226–227. doi: 10.1126/science.280.5361.226. [DOI] [PubMed] [Google Scholar]
- 14.Blankenship JR, Mitchell AP. How to build a biofilm: a fungal perspective. Curr Opin Microbiol. 2006;9(6):588–594. doi: 10.1016/j.mib.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 15.Stoodley P, Sauer K, Davies DG, Costerton JW. Biofilms as complex differentiated communities. Annu Rev Microbiol. 2002;56:187–209. doi: 10.1146/annurev.micro.56.012302.160705. [DOI] [PubMed] [Google Scholar]
- 16.Hogan DA, Kolter R. Pseudomonas candida interactions: an ecological role for virulence factors. Science. 2002;296(5576):2229–2232. doi: 10.1126/science.1070784. [DOI] [PubMed] [Google Scholar]
- 17.Allison DG. The biofilm matrix. Biofouling. 2003;19(2):139–150. doi: 10.1080/0892701031000072190. [DOI] [PubMed] [Google Scholar]
- 18.Branda SS, Vik S, Friedman L, Kolter R. Biofilms: the matrix revisited. Trends Microbiol. 2005;13(1):20–26. doi: 10.1016/j.tim.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 19.Feazel LM, Baumgartner LK, Peterson KL, Frank DN, Harris JK, Pace NR. Opportunistic pathogens enriched in showerhead biofilms. Proc Natl Acad Sci USA. 2009;106(38):16393–16399. doi: 10.1073/pnas.0908446106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fux CA, Costerton JW, Stewart PS, Stoodley P. Survival strategies of infectious biofilms. Trends Microbiol. 2005;13(1):34–40. doi: 10.1016/j.tim.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 21.Hall-Stoodley L, Stoodley P. Biofilm formation and dispersal and the transmission of human pathogens. Trends Microbiol. 2005;13(1):7–10. doi: 10.1016/j.tim.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 22.Stewart PS, Franklin MJ. Physiological heterogeneity in biofilms. Nat Rev Microbiol. 2008;6(3):199–210. doi: 10.1038/nrmicro1838. [DOI] [PubMed] [Google Scholar]
- 23.Vlamakis H, Aguilar C, Losick R, Kolter R. Control of cell fate by the formation of an architecturally complex bacterial community. Genes Dev. 2008;22(7):945–953. doi: 10.1101/gad.1645008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284(5418):1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 25.Donlan RM, Costerton JW. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev. 2002;15(2):167–193. doi: 10.1128/CMR.15.2.167-193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Danese PN, Pratt LA, Dove SL, Kolter R. The outer membrane protein, antigen 43, mediates cell-to-cell interactions within Escherichia coli biofilms. Mol Microbiol. 2000;37(2):424–432. doi: 10.1046/j.1365-2958.2000.02008.x. [DOI] [PubMed] [Google Scholar]
- 27.Chen JM, German GJ, Alexander DC, Ren H, Tan T, Liu J. Roles of Lsr2 in colony morphology and biofilm formation of Mycobacterium smegmatis. J Bacteriol. 2006;188(2):633–641. doi: 10.1128/JB.188.2.633-641.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hinsa SM, Espinosa-Urgel M, Ramos JL, O’Toole GA. Transition from reversible to irreversible attachment during biofilm formation by Pseudomonas fluorescens WCS365 requires an ABC transporter and a large secreted protein. Mol Microbiol. 2003;49(4):905–918. doi: 10.1046/j.1365-2958.2003.03615.x. [DOI] [PubMed] [Google Scholar]
- 29.Kolter R, Greenberg EP. Microbial sciences: the superficial life of microbes. Nature. 2006;441(7091):300–302. doi: 10.1038/441300a. [DOI] [PubMed] [Google Scholar]
- 30.O’Toole GA, Pratt LA, Watnick PI, Newman DK, Weaver VB, Kolter R. Genetic approaches to study of biofilms. Meth Enzymol. 1999;310:91–109. doi: 10.1016/s0076-6879(99)10008-9. [DOI] [PubMed] [Google Scholar]
- 31.Carman ML, Estes TG, Feinberg AW, et al. Engineered antifouling microtopographies – correlating wettability with cell attachment. Biofouling. 2006;22(1–2):11–21. doi: 10.1080/08927010500484854. [DOI] [PubMed] [Google Scholar]
- 32.Nudleman E, Kaiser D. Pulling together with type IV pili. J Mol Microbiol Biotechnol. 2004;7(1–2):52–62. doi: 10.1159/000077869. [DOI] [PubMed] [Google Scholar]
- 33.Proft T, Baker EN. Pili in Gram-negative and Gram-positive bacteria – structure, assembly and their role in disease. Cell Mol Life Sci. 2009;66(4):613–635. doi: 10.1007/s00018-008-8477-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ogasawara H, Yamamoto K, Ishihama A. Role of the biofilm master regulator CsgD in cross-regulation between biofilm formation and flagellar synthesis. J Bacteriol. 2011;193(10):2587–2597. doi: 10.1128/JB.01468-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cotter PA, Stibitz S. c-di-GMP-mediated regulation of virulence and biofilm formation. Curr Opin Microbiol. 2007;10(1):17–23. doi: 10.1016/j.mib.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 36.Hammer BK, Bassler BL. Quorum sensing controls biofilm formation in Vibrio cholerae. Mol Microbiol. 2003;50(1):101–104. doi: 10.1046/j.1365-2958.2003.03688.x. [DOI] [PubMed] [Google Scholar]
- 37.Higgins DA, Pomianek ME, Kraml CM, Taylor RK, Semmelhack MF, Bassler BL. The major Vibrio cholerae autoinducer and its role in virulence factor production. Nature. 2007;450(7171):883–886. doi: 10.1038/nature06284. [DOI] [PubMed] [Google Scholar]
- 38.Davies DG, Parsek MR, Pearson JP, Iglewski BH, Costerton JW, Greenberg EP. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science. 1998;280(5361):295–298. doi: 10.1126/science.280.5361.295. [DOI] [PubMed] [Google Scholar]
- 39.Kearns DB, Chu F, Branda SS, Kolter R, Losick R. A master regulator for biofilm formation by Bacillus subtilis. Mol Microbiol. 2005;55(3):739–749. doi: 10.1111/j.1365-2958.2004.04440.x. [DOI] [PubMed] [Google Scholar]
- 40.Chu F, Kearns DB, Branda SS, Kolter R, Losick R. Targets of the master regulator of biofilm formation in Bacillus subtilis. Mol Microbiol. 2006;59(4):1216–1228. doi: 10.1111/j.1365-2958.2005.05019.x. [DOI] [PubMed] [Google Scholar]
- 41.Chai Y, Chu F, Kolter R, Losick R. Bistability and biofilm formation in Bacillus subtilis. Mol Microbiol. 2008;67(2):254–263. doi: 10.1111/j.1365-2958.2007.06040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hall-Stoodley L, Costerton JW, Stoodley P. Bacterial biofilms: from the natural environment to infectious diseases. Nat Rev Microbiol. 2004;2(2):95–108. doi: 10.1038/nrmicro821. [DOI] [PubMed] [Google Scholar]
- 43.Keren I, Shah D, Spoering A, Kaldalu N, Lewis K. Specialized persister cells and the mechanism of multidrug tolerance in Escherichia coli. J Bacteriol. 2004;186(24):8172–8180. doi: 10.1128/JB.186.24.8172-8180.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mah TF, O’Toole GA. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001;9(1):34–39. doi: 10.1016/s0966-842x(00)01913-2. [DOI] [PubMed] [Google Scholar]
- 45.McNeill K, Hamilton IR. Acid tolerance response of biofilm cells of Streptococcus mutans. FEMS Microbiol Lett. 2003;221(1):25–30. doi: 10.1016/S0378-1097(03)00164-2. [DOI] [PubMed] [Google Scholar]
- 46.Teitzel GM, Parsek MR. Heavy metal resistance of biofilm and planktonic Pseudomonas aeruginosa. Appl Environ Microbiol. 2003;69(4):2313–2320. doi: 10.1128/AEM.69.4.2313-2320.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walters MC, 3rd, Roe F, Bugnicourt A, Franklin MJ, Stewart PS. Contributions of antibiotic penetration, oxygen limitation, and low metabolic activity to tolerance of Pseudomonas aeruginosa biofilms to ciprofloxacin and tobramycin. Antimicrob Agents Chemother. 2003;47(1):317–323. doi: 10.1128/AAC.47.1.317-323.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hall-Stoodley L, Stoodley P. Developmental regulation of microbial biofilms. Curr Opin Biotechnol. 2002;13(3):228–233. doi: 10.1016/s0958-1669(02)00318-x. [DOI] [PubMed] [Google Scholar]
- 49.Lazazzera BA. Lessons from DNA microarray analysis: the gene expression profile of biofilms. Curr Opin Microbiol. 2005;8(2):222–227. doi: 10.1016/j.mib.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 50.Gilbert P, Allison DG, McBain AJ. Biofilms in vitro and in vivo: do singular mechanisms imply cross-resistance? Symp Ser Soc Appl Microbiol. 2002;31:98S–110S. [PubMed] [Google Scholar]
- 51.Post JC, Stoodley P, Hall-Stoodley L, Ehrlich GD. The role of biofilms in otolaryngologic infections. Curr Opin Otolaryngol Head Neck Surg. 2004;12(3):185–190. doi: 10.1097/01.moo.0000124936.46948.6a. [DOI] [PubMed] [Google Scholar]
- 52.Sbordone L, Bortolaia C. Oral microbial biofilms and plaque-related diseases: microbial communities and their role in the shift from oral health to disease. Clin Oral Investig. 2003;7(4):181–188. doi: 10.1007/s00784-003-0236-1. [DOI] [PubMed] [Google Scholar]
- 53.Yoon SS, Hennigan RF, Hilliard GM, et al. Pseudomonas aeruginosa anaerobic respiration in biofilms: relationships to cystic fibrosis pathogenesis. Dev Cell. 2002;3(4):593–603. doi: 10.1016/s1534-5807(02)00295-2. [DOI] [PubMed] [Google Scholar]
- 54.Marrie TJ, Nelligan J, Costerton JW. A scanning and transmission electron microscopic study of an infected endocardial pacemaker lead. Circulation. 1982;66(6):1339–1341. doi: 10.1161/01.cir.66.6.1339. [DOI] [PubMed] [Google Scholar]
- 55.Anderson GG, Dodson KW, Hooton TM, Hultgren SJ. Intracellular bacterial communities of uropathogenic Escherichia coli in urinary tract pathogenesis. Trends Microbiol. 2004;12(9):424–430. doi: 10.1016/j.tim.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 56.Høiby N, Bjarnsholt T, Givskov M, Molin S, Ciofu O. Antibiotic resistance of bacterial biofilms. Int J Antimicrob Agents. 2010;35(4):322–332. doi: 10.1016/j.ijantimicag.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 57.Jensen PØ, Givskov M, Bjarnsholt T, Moser C. The immune system vs. Pseudomonas aeruginosa biofilms. FEMS Immunol Med Microbiol. 2010;59(3):292–305. doi: 10.1111/j.1574-695X.2010.00706.x. [DOI] [PubMed] [Google Scholar]
- 58.Van Gennip M, Christensen LD, Alhede M, et al. Inactivation of the rhlA gene in Pseudomonas aeruginosa prevents rhamnolipid production, disabling the protection against polymorphonuclear leukocytes. APMIS. 2009;117(7):537–546. doi: 10.1111/j.1600-0463.2009.02466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moss RB, Hsu YP, Olds L. Cytokine dysregulation in activated cystic fibrosis (CF) peripheral lymphocytes. Clin Exp Immunol. 2000;120(3):518–525. doi: 10.1046/j.1365-2249.2000.01232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Leid JG, Shirtliff ME, Costerton JW, Stoodley P. Human leukocytes adhere to, penetrate, and respond to Staphylococcus aureus biofilms. Infect Immun. 2002;70(11):6339–6345. doi: 10.1128/IAI.70.11.6339-6345.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Prabhakara R, Harro JM, Leid JG, Harris M, Shirtliff ME. Murine immune response to a chronic Staphylococcus aureus biofilm infection. Infect Immun. 2011;79(4):1789–1796. doi: 10.1128/IAI.01386-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moser C, Kjaergaard S, Pressler T, Kharazmi A, Koch C, Høiby N. The immune response to chronic Pseudomonas aeruginosa lung infection in cystic fibrosis patients is predominantly of the Th2 type. APMIS. 2000;108(5):329–335. doi: 10.1034/j.1600-0463.2000.d01-64.x. [DOI] [PubMed] [Google Scholar]
- 63.Saginur R, Stdenis M, Ferris W, et al. Multiple combination bactericidal testing of staphylococcal biofilms from implant-associated infections. Antimicrob Agents Chemother. 2006;50(1):55–61. doi: 10.1128/AAC.50.1.55-61.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ceri H, Olson ME, Stremick C, Read RR, Morck D, Buret A. The Calgary Biofilm Device: new technology for rapid determination of antibiotic susceptibilities of bacterial biofilms. J Clin Microbiol. 1999;37(6):1771–1776. doi: 10.1128/jcm.37.6.1771-1776.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Banin E, Brady KM, Greenberg EP. Chelator-induced dispersal and killing of Pseudomonas aeruginosa cells in a biofilm. Appl Environ Microbiol. 2006;72(3):2064–2069. doi: 10.1128/AEM.72.3.2064-2069.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66••.Kolodkin-Gal I, Romero D, Cao S, Clardy J, Kolter R, Losick R. D-amino acids trigger biofilm disassembly. Science. 2010;328(5978):627–629. doi: 10.1126/science.1188628. It describes a very effective strategy for dispersing microbial biofilms. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Canetti G, Gay P, Le Lirzin M. Trends in the prevalence of primary drug resistance in pulmonary tuberculosis in France from 1962 to 1970: a national survey. Tubercle. 1972;53(2):57–83. doi: 10.1016/0041-3879(72)90022-0. [DOI] [PubMed] [Google Scholar]
- 68•.Lenaerts AJ, Hoff D, Aly S, et al. Location of persisting mycobacteria in a Guinea pig model of tuberculosis revealed by r207910. Antimicrob Agents Chemother. 2007;51(9):3338–3345. doi: 10.1128/AAC.00276-07. Pathological evidence for the presence of drug-tolerant persisters of Mycobacterium tuberculosis in multicellular microcolonies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Alteri CJ, Xicohténcatl-Cortes J, Hess S, Caballero-Olín G, Girón JA, Friedman RL. Mycobacterium tuberculosis produces pili during human infection. Proc Natl Acad Sci USA. 2007;104(12):5145–5150. doi: 10.1073/pnas.0602304104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70•.Gill WP, Harik NS, Whiddon MR, Liao RP, Mittler JE, Sherman DR. A replication clock for Mycobacterium tuberculosis. Nat Med. 2009;15(2):211–214. doi: 10.1038/nm.1915. Evidence of a dynamic state of M. tuberculosis population in chronic phase of infection in murine model; challenging a long-standing dogma of nonreplicating bacilli in subclinical infections. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Barry CE, 3rd, Boshoff HI, Dartois V, et al. The spectrum of latent tuberculosis: rethinking the biology and intervention strategies. Nat Rev Microbiol. 2009;7(12):845–855. doi: 10.1038/nrmicro2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rhoades ER, Geisel RE, Butcher BA, McDonough S, Russell DG. Cell wall lipids from Mycobacterium bovis BCG are inflammatory when inoculated within a gel matrix: characterization of a new model of the granulomatous response to mycobacterial components. Tuberculosis (Edinb) 2005;85(3):159–176. doi: 10.1016/j.tube.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 73.Lin PL, Flynn JL. Understanding latent tuberculosis: a moving target. J Immunol. 2010;185(1):15–22. doi: 10.4049/jimmunol.0903856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74•.Ford CB, Lin PL, Chase MR, et al. Use of whole genome sequencing to estimate the mutation rate of Mycobacterium tuberculosis during latent infection. Nat Genet. 2011;43(5):482–486. doi: 10.1038/ng.811. Findings of murine model in nonhuman primates, providing a compelling argument for a complex host–pathogen interaction in latent tuberculosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yong DB, Gideon HP, Wilkinson RJ. Eliminating latent tuberculosis. Trends Microbiol. 2009;17(15):183–188. doi: 10.1016/j.tim.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 76.Ulrichs T, Kosmiadi GA, Jörg S, et al. Differential organization of the local immune response in patients with active cavitary tuberculosis or with nonprogressive tuberculoma. J Infect Dis. 2005;192(1):89–97. doi: 10.1086/430621. [DOI] [PubMed] [Google Scholar]
- 77.Lazarevic V, Nolt D, Flynn JL. Long-term control of Mycobacterium tuberculosis infection is mediated by dynamic immune responses. J Immunol. 2005;175(2):1107–1117. doi: 10.4049/jimmunol.175.2.1107. [DOI] [PubMed] [Google Scholar]
- 78.Young DB, Gideon HP, Wilkinson RJ. Eliminating latent tuberculosis. Trends Microbiol. 2009;17(5):183–188. doi: 10.1016/j.tim.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 79.Parrish NM, Dick JD, Bishai WR. Mechanisms of latency in Mycobacterium tuberculosis. Trends Microbiol. 1998;6(3):107–112. doi: 10.1016/s0966-842x(98)01216-5. [DOI] [PubMed] [Google Scholar]
- 80.Gomez JE, McKinney JD. M. tuberculosis persistence, latency, and drug tolerance. Tuberculosis (Edinb) 2004;84(1–2):29–44. doi: 10.1016/j.tube.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 81.Medlar EM, Bernstein S, Steward DM. A bacteriologic study of resected tuberculous lesions. Am Rev Tuberc. 1952;66(1):36–43. doi: 10.1164/art.1952.66.1.36. [DOI] [PubMed] [Google Scholar]
- 82.Loring WE, Vandiviere HM. The treated pulmonary lesion and its tubercle bacillus. I Pathology and pathogenesis. Am J Med Sci. 1956;232(1):20–29. doi: 10.1097/00000441-195607000-00005. [DOI] [PubMed] [Google Scholar]
- 83.Loring WW, Melvin I, Vandiviere HM, Willis HS. The death and resurrection of the tubercle bacillus. Trans Am Clin Climatol Assoc. 1955;67:132–138. [PMC free article] [PubMed] [Google Scholar]
- 84.McCune RM, Feldmann FM, Lambert HP, McDermott W. Microbial persistence. I The capacity of tubercle bacilli to survive sterilization in mouse tissues. J Exp Med. 1966;123(3):445–468. doi: 10.1084/jem.123.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mccune RM, Jr, Mcdermott W, Tompsett R. The fate of Mycobacterium tuberculosis in mouse tissues as determined by the microbial enumeration technique. II The conversion of tuberculous infection to the latent state by the administration of pyrazinamide and a companion drug. J Exp Med. 1956;104(5):763–802. doi: 10.1084/jem.104.5.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Haapanen JH, Kass I, Gensini G, Middlebrook G. Studies on the gaseous content of tuberculous cavities. Am Rev Respir Dis. 1959;80(Part 1):1–5. doi: 10.1164/arrd.1959.80.1P1.1. [DOI] [PubMed] [Google Scholar]
- 87.Vandiviere HM, Loring WE, Melvin I, Willis S. The treated pulmonary lesion and its tubercle bacillus. II. The death and resurrection. Am J Med Sci. 1956;232(1):30–37. doi: 10.1097/00000441-195607000-00006. passim. [DOI] [PubMed] [Google Scholar]
- 88.Via LE, Lin PL, Ray SM, et al. Tuberculous granulomas are hypoxic in guinea pigs, rabbits, and nonhuman primates. Infect Immun. 2008;76(6):2333–2340. doi: 10.1128/IAI.01515-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wayne LG, Hayes LG. An in vitro model for sequential study of shiftdown of Mycobacterium tuberculosis through two stages of nonreplicating persistence. Infect Immun. 1996;64(6):2062–2069. doi: 10.1128/iai.64.6.2062-2069.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rustad TR, Harrell MI, Liao R, Sherman DR. The enduring hypoxic response of Mycobacterium tuberculosis. PLoS ONE. 2008;3(1):e1502. doi: 10.1371/journal.pone.0001502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Park HD, Guinn KM, Harrell MI, et al. Rv3133c/dosR is a transcription factor that mediates the hypoxic response of Mycobacterium tuberculosis. Mol Microbiol. 2003;48(3):833–843. doi: 10.1046/j.1365-2958.2003.03474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Morris RP, Nguyen L, Gatfield J, et al. Ancestral antibiotic resistance in Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 2005;102(34):12200–12205. doi: 10.1073/pnas.0505446102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Baek SH, Li AH, Sassetti CM. Metabolic regulation of mycobacterial growth and antibiotic sensitivity. PLoS Biol. 2011;9(5):e1001065. doi: 10.1371/journal.pbio.1001065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Aldridge BB, Fernandez-Suarez M, Heller D, et al. Asymmetry and aging of mycobacterial cells lead to variable growth and antibiotic susceptibility. Science. 2012;335(6064):100–104. doi: 10.1126/science.1216166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nikaido H, Jarlier V. Permeability of the mycobacterial cell wall. Res Microbiol. 1991;142(4):437–443. doi: 10.1016/0923-2508(91)90117-s. [DOI] [PubMed] [Google Scholar]
- 96.Hall-Stoodley L, Brun OS, Polshyna G, Barker LP. Mycobacterium marinum biofilm formation reveals cording morphology. FEMS Microbiol Lett. 2006;257(1):43–49. doi: 10.1111/j.1574-6968.2006.00143.x. [DOI] [PubMed] [Google Scholar]
- 97.Bosio S, Leekha S, Gamb SI, Wright AJ, Terrell CL, Miller DV. Mycobacterium fortuitum prosthetic valve endocarditis: a case for the pathogenetic role of biofilms. Cardiovasc Pathol. 2012;21(4):361–364. doi: 10.1016/j.carpath.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 98.Feazel LM, Baumgartner LK, Peterson KL, et al. Opportunistic pathogens enriched in showerhead biofilms. Proc Natl Acad Sci USA. 2009;106(38):16393–16399. doi: 10.1073/pnas.0908446106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cook KL, Britt JS, Bolster CH. Survival of Mycobacterium avium subsp paratuberculosis in biofilms on livestock watering trough materials. Vet Microbiol. 2010;141(1–2):103–109. doi: 10.1016/j.vetmic.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 100.Recht J, Kolter R. Glycopeptidolipid acetylation affects sliding motility and biofilm formation in Mycobacterium smegmatis. J Bacteriol. 2001;183(19):5718–5724. doi: 10.1128/JB.183.19.5718-5724.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yamazaki Y, Danelishvili L, Wu M, Macnab M, Bermudez LE. Mycobacterium avium genes associated with the ability to form a biofilm. Appl Environ Microbiol. 2006;72(1):819–825. doi: 10.1128/AEM.72.1.819-825.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ojha AK, Baughn AD, Sambandan D, et al. Growth of Mycobacterium tuberculosis biofilms containing free mycolic acids and harbouring drug-tolerant bacteria. Mol Microbiol. 2008;69(1):164–174. doi: 10.1111/j.1365-2958.2008.06274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ojha AK, Trivelli X, Guerardel Y, Kremer L, Hatfull GF. Enzymatic hydrolysis of trehalose dimycolate releases free mycolic acids during mycobacterial growth in biofilms. J Biol Chem. 2010;285(23):17380–17389. doi: 10.1074/jbc.M110.112813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ojha A, Anand M, Bhatt A, Kremer L, Jacobs WR, Jr, Hatfull GF. GroEL1: a dedicated chaperone involved in mycolic acid biosynthesis during biofilm formation in mycobacteria. Cell. 2005;123(5):861–873. doi: 10.1016/j.cell.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 105.Ojha A, Hatfull GF. The role of iron in Mycobacterium smegmatis biofilm formation: the exochelin siderophore is essential in limiting iron conditions for biofilm formation but not for planktonic growth. Mol Microbiol. 2007;66(2):468–483. doi: 10.1111/j.1365-2958.2007.05935.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Banin E, Vasil ML, Greenberg EP. Iron and Pseudomonas aeruginosa biofilm formation. Proc Natl Acad Sci USA. 2005;102(31):11076–11081. doi: 10.1073/pnas.0504266102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pang JM, Layre E, Sweet L, et al. The polyketide Pks1 contributes to biofilm formation in Mycobacterium tuberculosis. J Bacteriol. 2012;194(3):715–721. doi: 10.1128/JB.06304-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Marsollier L, Aubry J, Coutanceau E, et al. Colonization of the salivary glands of Naucoris cimicoides by Mycobacterium ulcerans requires host plasmatocytes and a macrolide toxin, mycolactone. Cell Microbiol. 2005;7(7):935–943. doi: 10.1111/j.1462-5822.2005.00521.x. [DOI] [PubMed] [Google Scholar]
- 109.Carter G, Wu M, Drummond DC, Bermudez LE. Characterization of biofilm formation by clinical isolates of Mycobacterium avium. J Med Microbiol. 2003;52(Pt 9):747–752. doi: 10.1099/jmm.0.05224-0. [DOI] [PubMed] [Google Scholar]
- 110.George KM, Chatterjee D, Gunawardana G, et al. Mycolactone: a polyketide toxin from Mycobacterium ulcerans required for virulence. Science. 1999;283(5403):854–857. doi: 10.1126/science.283.5403.854. [DOI] [PubMed] [Google Scholar]
- 111.Teng R, Dick T. Isoniazid resistance of exponentially growing Mycobacterium smegmatis biofilm culture. FEMS Microbiol Lett. 2003;227(2):171–174. doi: 10.1016/S0378-1097(03)00584-6. [DOI] [PubMed] [Google Scholar]
- 112.McNabe M, Tennant R, Danelishvili L, Young L, Bermudez LE. Mycobacterium avium ssp hominissuis biofilm is composed of distinct phenotypes and influenced by the presence of antimicrobials. Clin Microbiol Infect. 2011;17(5):697–703. doi: 10.1111/j.1469-0691.2010.03307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nguyen KT, Piastro K, Gray TA, Derbyshire KM. Mycobacterial biofilms facilitate horizontal DNA transfer between strains of Mycobacterium smegmatis. J Bacteriol. 2010;192(19):5134–5142. doi: 10.1128/JB.00650-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.da Silva PE, Von Groll A, Martin A, Palomino JC. Efflux as a mechanism for drug resistance in Mycobacterium tuberculosis. FEMS Immunol Med Microbiol. 2011;63(1):1–9. doi: 10.1111/j.1574-695X.2011.00831.x. [DOI] [PubMed] [Google Scholar]
- 115.Colangeli R, Helb D, Vilchèze C, et al. Transcriptional regulation of multi-drug tolerance and antibiotic-induced responses by the histone-like protein Lsr2 in M. tuberculosis. PLoS Pathog. 2007;3(6):e87. doi: 10.1371/journal.ppat.0030087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Vilchèze C, Av-Gay Y, Barnes SW, et al. Coresistance to isoniazid and ethionamide maps to mycothiol biosynthetic genes in Mycobacterium bovis. Antimicrob Agents Chemother. 2011;55(9):4422–4423. doi: 10.1128/AAC.00564-11. [DOI] [PMC free article] [PubMed] [Google Scholar]