Abstract
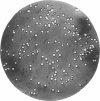
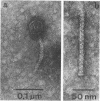
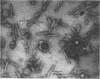
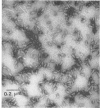
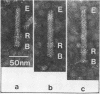
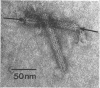
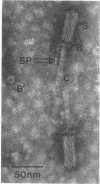
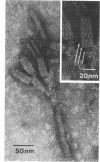
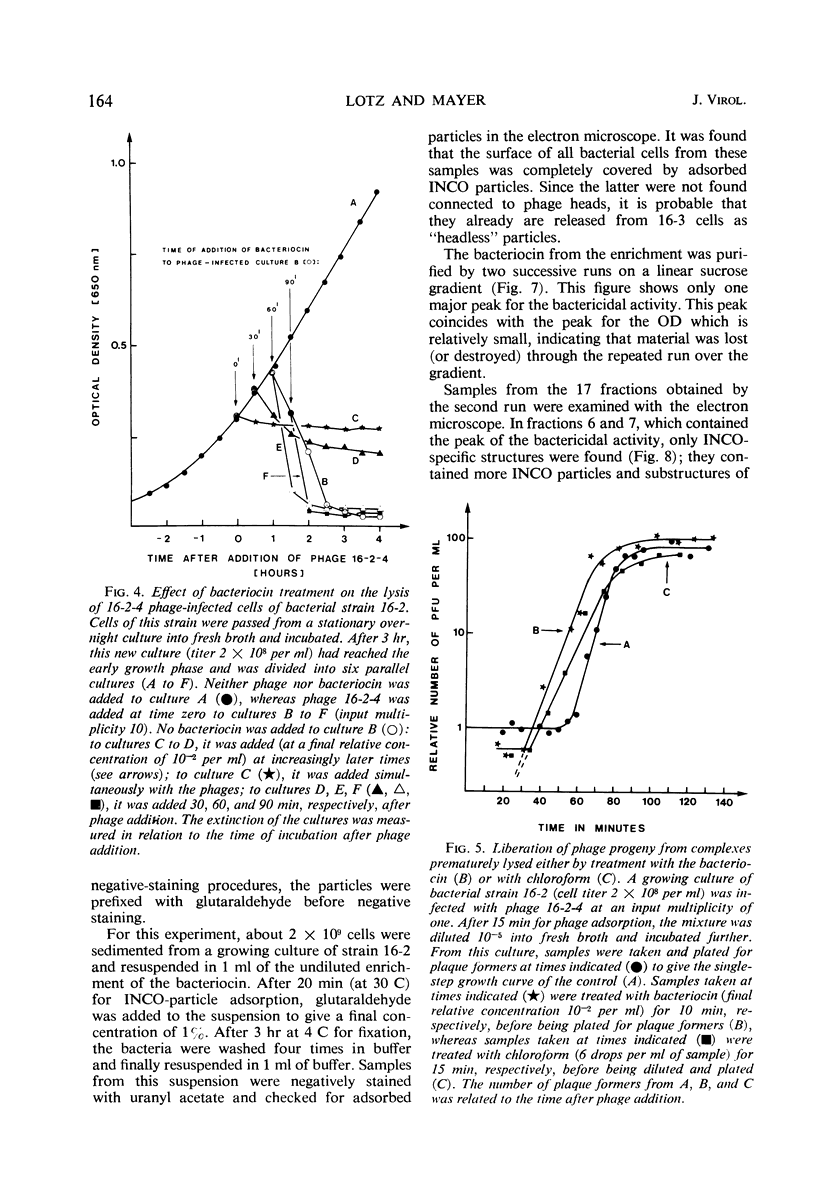
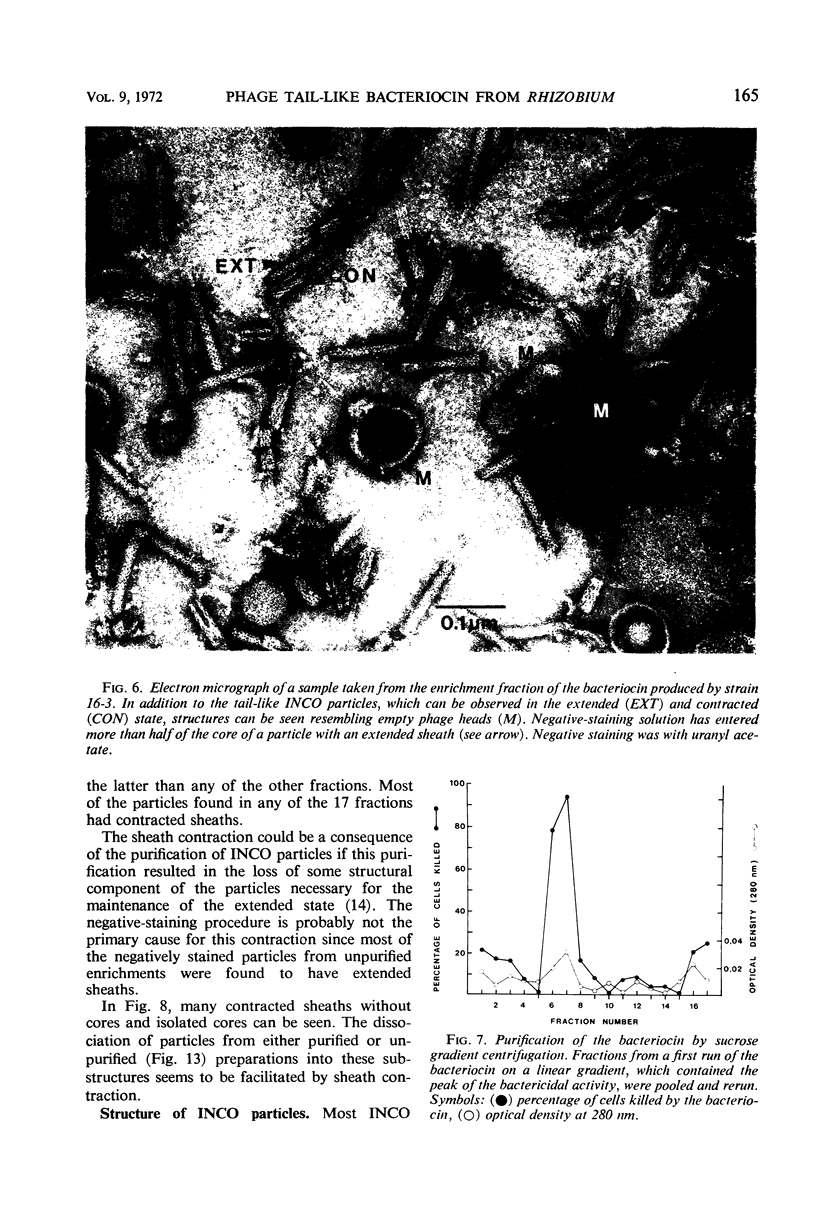
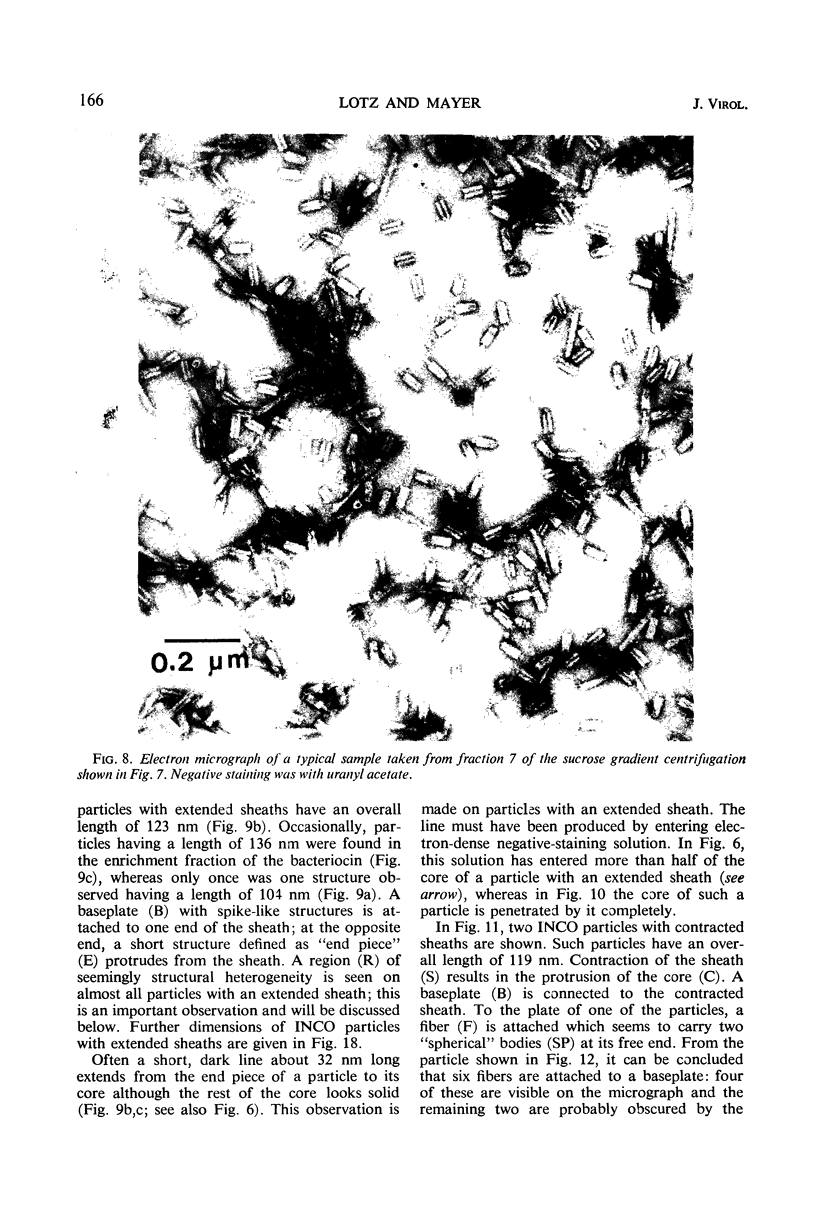
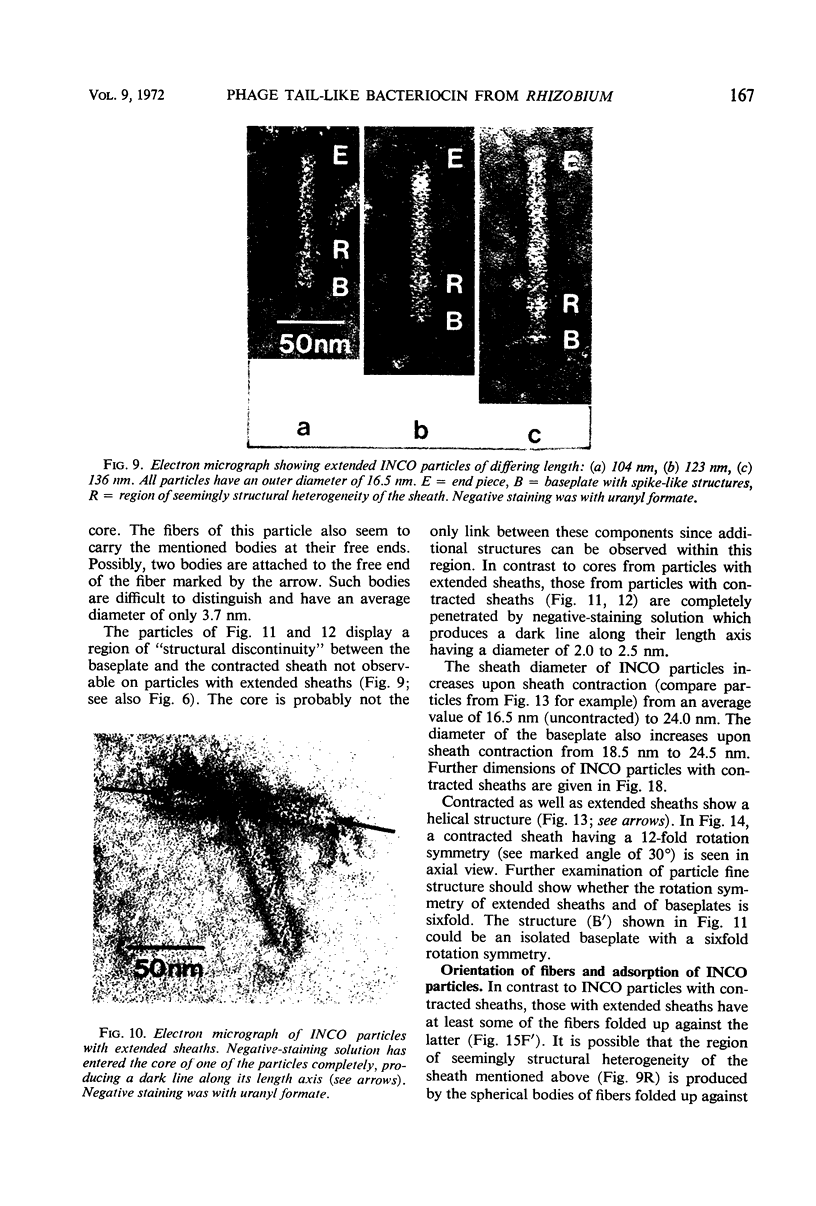
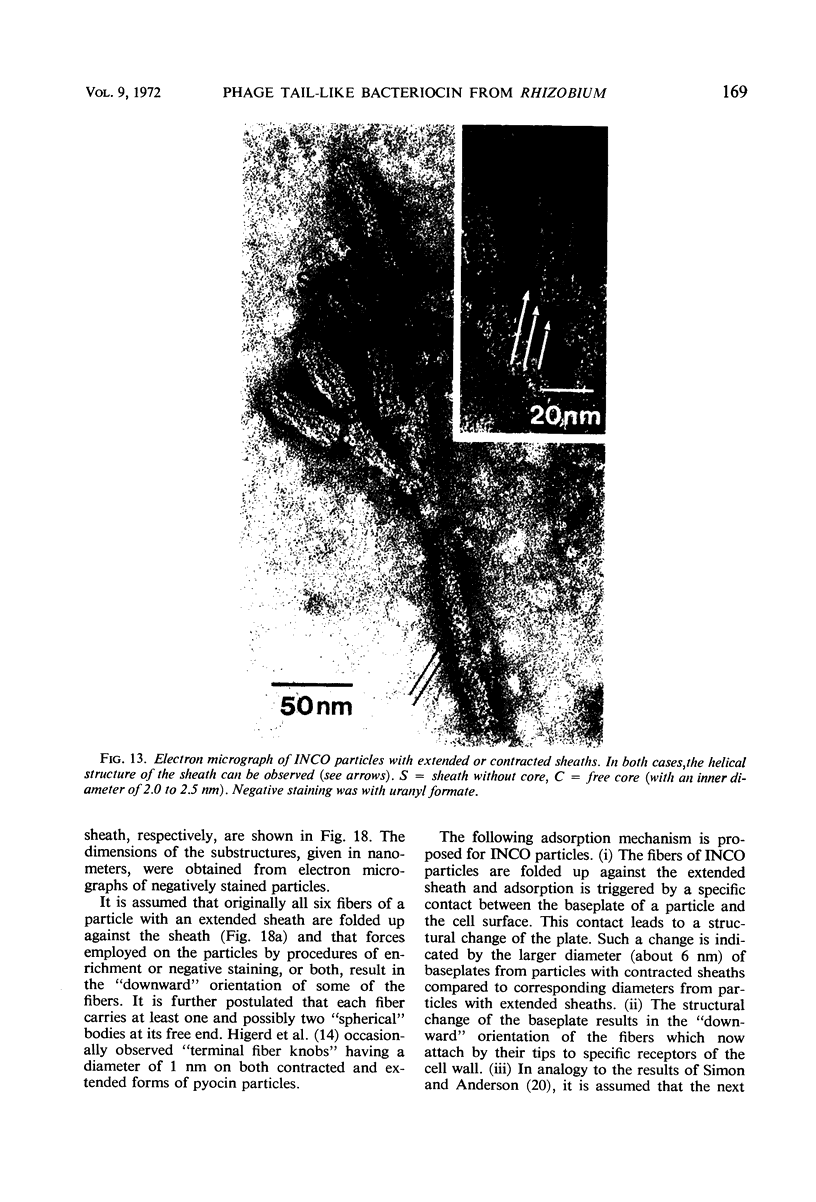
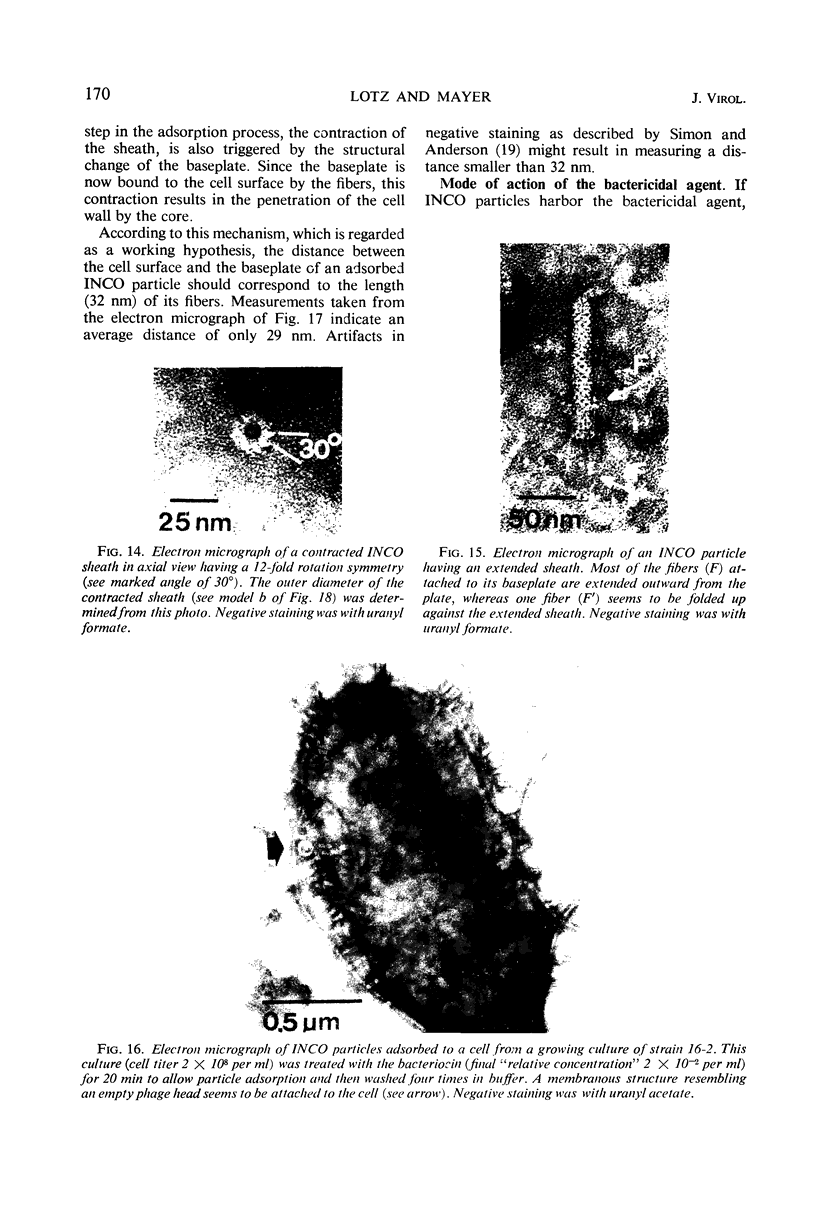
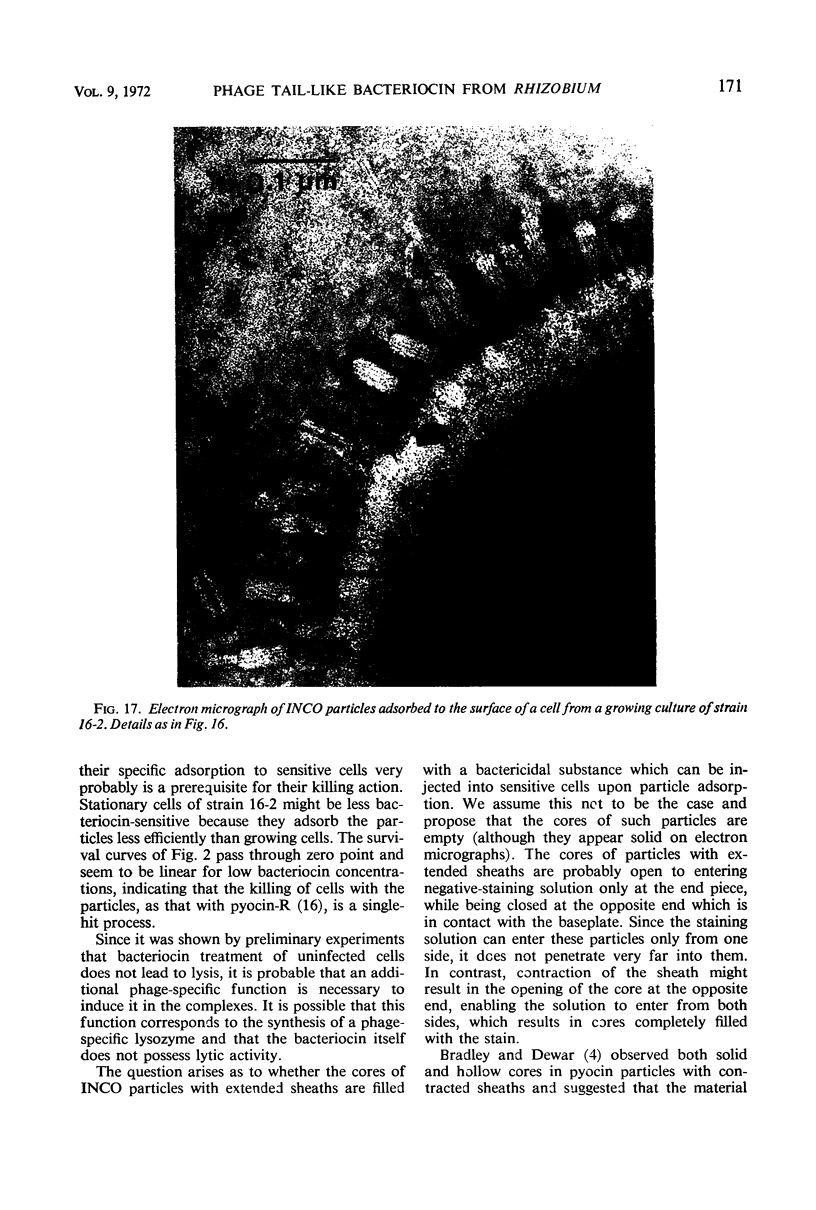
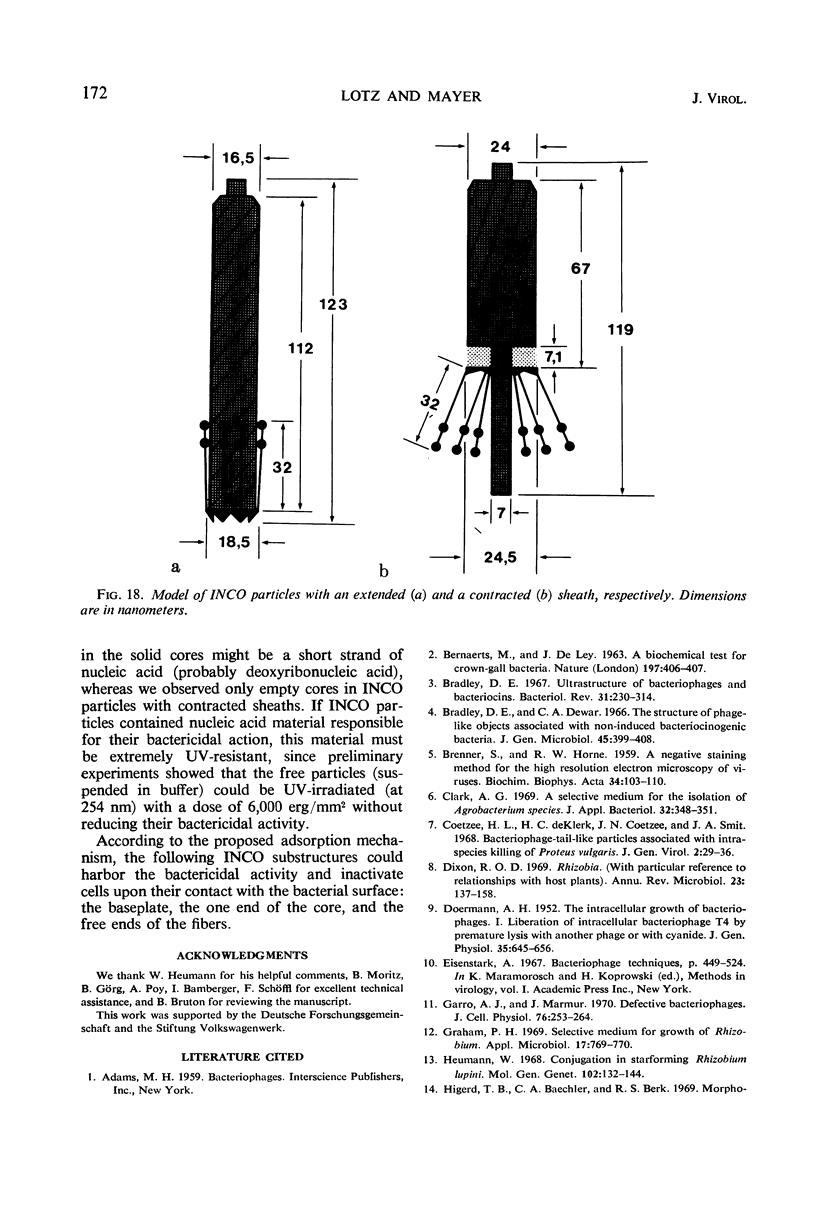
Bacterial strain 16-3 spontaneously produces a bacteriocin which inhibits the growth of closely related strain 16-2. Both strains were newly isolated from root nodules of lupines and probably belong to the species Rhizobium lupini. Production of infectious progeny of newly isolated virulent phage 16-2-4 in strain 16-2 is inhibited completely if complexes are bacteriocin-treated during the first half of the latent period. Treatment begun during the second half leads to premature lysis of complexes and inactivates only those progeny phages which were not yet fully matured at the moment of the particle-induced lysis. Examination by electron microscope of the bacteriocin enrichment revealed the presence of particles 123 nm in length which resemble the tails of T-even bacteriophages. Since the particles sediment together with the bactericidal activity in the sucrose gradient and adsorb specifically to bacteriocin-sensitive cells, it is concluded that they are identical with the bactericidal agent. The particles are not found attached to phage heads and cannot self-propagate; they are regarded as incomplete and are named INCO particles. INCO particles consist of a core enveloped by a contractile sheath. One end of the sheath is connected to a baseplate to which six fibers, each 32 nm in length, are attached. These connect the baseplate of an adsorbing particle to the cell surface. Since INCo cores are probably empty, it is concluded that specific adsorption of the particles to the bacterial surface is sufficient to inactive sensitive cells irreversibly.
Full text
PDF

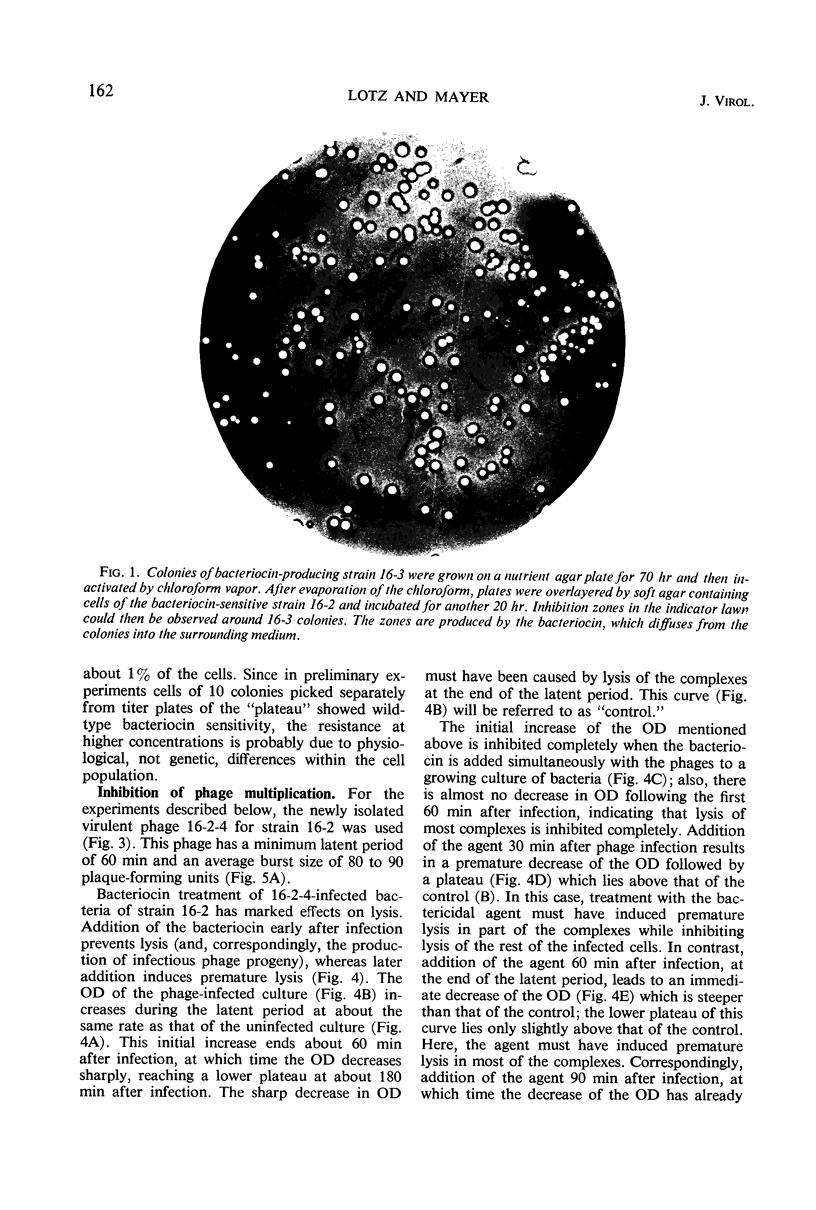
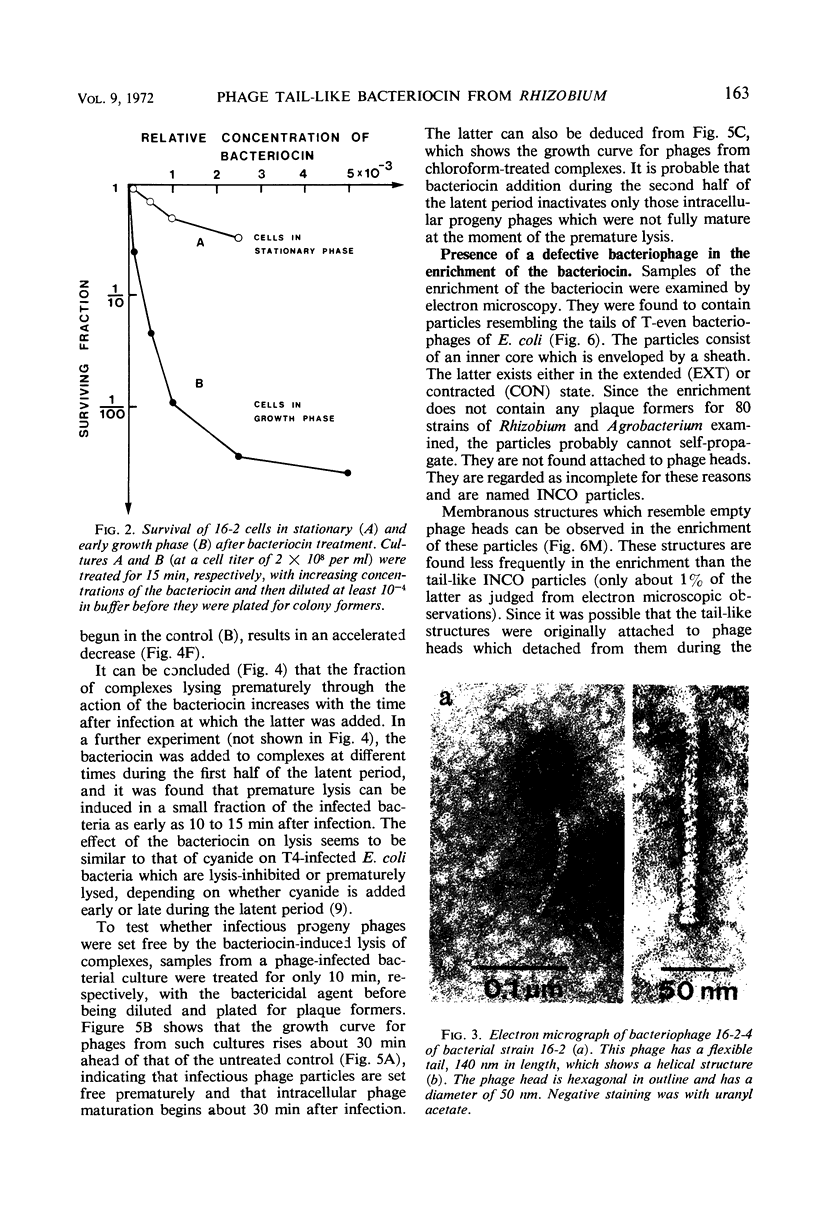


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRENNER S., HORNE R. W. A negative staining method for high resolution electron microscopy of viruses. Biochim Biophys Acta. 1959 Jul;34:103–110. doi: 10.1016/0006-3002(59)90237-9. [DOI] [PubMed] [Google Scholar]
- Bradley D. E. Ultrastructure of bacteriophage and bacteriocins. Bacteriol Rev. 1967 Dec;31(4):230–314. doi: 10.1128/br.31.4.230-314.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coetzee H. L., De Klerk H. C., Coetzee J. N., Smit J. A. Bacteriophage-tail-like particles associated with intra-species killing of Proteus vulgaris. J Gen Virol. 1968 Jan;2(1):29–36. doi: 10.1099/0022-1317-2-1-29. [DOI] [PubMed] [Google Scholar]
- DOERMANN A. H. The intracellular growth of bacteriophages. I. Liberation of intracellular bacteriophage T4 by premature lysis with another phage or with cyanide. J Gen Physiol. 1952 Mar;35(4):645–656. doi: 10.1085/jgp.35.4.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon R. O. Rhizobia (with particular reference to relationships with host plants). Annu Rev Microbiol. 1969;23:137–158. doi: 10.1146/annurev.mi.23.100169.001033. [DOI] [PubMed] [Google Scholar]
- Garro A. J., Marmur J. Defective bacteriophages. J Cell Physiol. 1970 Dec;76(3):253–263. doi: 10.1002/jcp.1040760305. [DOI] [PubMed] [Google Scholar]
- Graham P. H. Selective medium for growth of Rhizobium. Appl Microbiol. 1969 May;17(5):769–770. doi: 10.1128/am.17.5.769-770.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heumann W. Conjugation in starforming Rhizobium lupini. Mol Gen Genet. 1968;102(2):132–144. doi: 10.1007/BF01789140. [DOI] [PubMed] [Google Scholar]
- Higerd T. B., Baechler C. A., Berk R. S. Morphological studies on relaxed and contracted forms of purified pyocin particles. J Bacteriol. 1969 Jun;98(3):1378–1389. doi: 10.1128/jb.98.3.1378-1389.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii S. I., Nishi Y., Egami F. The fine structure of a pyocin. J Mol Biol. 1965 Sep;13(2):428–431. doi: 10.1016/s0022-2836(65)80107-3. [DOI] [PubMed] [Google Scholar]
- KAGEYAMA M., IKEDA K., EGAMI F. STUDIES OF A PYOCIN. III. BIOLOGICAL PROPERTIES OF THE PYOCIN. J Biochem. 1964 Jan;55:59–64. doi: 10.1093/oxfordjournals.jbchem.a127841. [DOI] [PubMed] [Google Scholar]
- Nomura M. Colicins and related bacteriocins. Annu Rev Microbiol. 1967;21:257–284. doi: 10.1146/annurev.mi.21.100167.001353. [DOI] [PubMed] [Google Scholar]
- Roslycky E. B. Bacteriocin production in the rhizobia bacteria. Can J Microbiol. 1967 Apr;13(4):431–432. doi: 10.1139/m67-057. [DOI] [PubMed] [Google Scholar]
- Simon L. D., Anderson T. F. The infection of Escherichia coli by T2 and T4 bacteriophages as seen in the electron microscope. I. Attachment and penetration. Virology. 1967 Jun;32(2):279–297. doi: 10.1016/0042-6822(67)90277-2. [DOI] [PubMed] [Google Scholar]
- Simon L. D., Anderson T. F. The infection of Escherichia coli by T2 and T4 bacteriophages as seen in the electron microscope. II. Structure and function of the baseplate. Virology. 1967 Jun;32(2):298–305. doi: 10.1016/0042-6822(67)90278-4. [DOI] [PubMed] [Google Scholar]