Abstract
It was previously demonstrated that transforming growth factor β (TGF-β) induces endothelial-to-mesenchymal transition (EndoMT) in murine lung endothelial cells (ECs) in vitro. Owing to the important role of caveolin-1 (CAV1) in TGF-β receptor internalization and TGF-β signaling, the participation of CAV1 in the induction of EndoMT in murine lung ECs was investigated. Pulmonary ECs were isolated from wild-type and Cav1 knockout mice using immunomagnetic methods with sequential anti-CD31 and anti-CD102 antibody selection followed by in vitro culture and treatment with TGF-β1. EndoMT was assessed by semiquantitative RT-PCR for Acta2, Col1a1, Snai1, and Snai2; by immunofluorescence for α-smooth muscle actin; and by Western blot analysis for α-smooth muscle actin, SNAIL1, SNAIL2, and the α2 chain of type I collagen. The same studies were performed in Cav1−/− pulmonary ECs after restoration of functional CAV1 domains using a cell-permeable CAV1 scaffolding domain peptide. Pulmonary ECs from Cav1 knockout mice displayed high levels of spontaneous Acta2, Col1A, Snai1, and Snai2 expression, which increased after TGF-β treatment. Spontaneous and TGF-β1–stimulated EndoMT were abrogated by the restoration of functional CAV1 domains using a cell-permeable peptide. The findings suggest that CAV1 regulation of EndoMT may play a role in the development of fibroproliferative vasculopathies.
Myofibroblasts are specialized mesenchymal cells that share features of fibroblasts and smooth muscle cells, display prominent cytoplasmic stress fibers, and express high levels of α-smooth muscle actin (α-SMA) RNA and protein. These cells also possess contractile properties and are capable of motion and tissue migration (reviewed by Hinz et al 1). Myofibroblasts are considered the main cellular elements responsible for normal and pathologic fibroproliferative responses owing to their ability to produce very high levels of interstitial collagens and other extracellular matrix components characteristic of fibrotic tissues (reviewed in several studies2–4). Myofibroblasts also represent the most abundant mesenchymal cells present in the stroma of malignant tumors, known as cancer-associated fibroblasts, which are essential for tumor growth and progression by providing a permissive and favorable microenvironment for tumorigenesis.5–7
Numerous studies have shown that myofibroblasts involved in tissue fibrosis can derive from epithelial cells in a process known as epithelial-to-mesenchymal transition (reviewed by Kalluri and Neilson8). Recently, a similar process occurring in endothelial cells (ECs), termed endothelial-to-mesenchymal transition (EndoMT), has been described. EndoMT is a process whereby ECs lose their endothelial-specific markers and acquire a mesenchymal cell myofibroblastic phenotype.9,10 Recent studies have demonstrated that this phenomenon may be an important source of myofibroblasts in cardiac, pulmonary, and kidney fibrosis (reviewed by Piera-Velazquez et al11,12).
Caveolin 1 (CAV1) is the most important member of a family of membrane proteins that are the major coating proteins of caveolae (reviewed in other articles13,14). CAV1 plays an important role in the endosomal degradation of transforming growth factor β (TGF-β) receptors, thus diminishing or abrogating TGF-β–mediated signaling.15,16 Owing to the crucial role of TGF-β and TGF-β pathways in the initiation, development, and persistence of fibrotic reactions, it has been suggested that CAV1 may participate in the pathogenesis of fibrotic disorders, such as systemic sclerosis (SSc) and idiopathic pulmonary fibrosis.17–19 Indeed, our previous studies have shown that mice lacking Cav1 expression [Cav1 knockout (Cav1 KO) mice] display a remarkable increase in the amount of interstitial collagen in the lung, as shown by histopathologic analysis of lung tissues, and a profound increase (>2.5-fold) in the amount of collagen per lung, as assessed by hydroxyproline assays.17 In accordance with these observations, we also found that Cav1 was down-regulated in affected SSc lungs and skin compared with normal Cav1 expression in histopathologically nonaffected areas.17 Moreover, selective restoration of functional CAV1 domains using a cell-permeable CAV1 scaffolding domain peptide20 reverted the profibrotic phenotype of dermal and lung fibroblasts cultured from patients with SSc.17,21
In a previous study, we demonstrated that TGF-β caused a potent induction of EndoMT in murine pulmonary ECs in vitro, a process that seemed to be mediated by a remarkable increase in the levels of the transcriptional repressor SNAIL1.22 Recently, it has been reported that TGF-β signaling through SMAD-dependent and SMAD-independent pathways in murine pulmonary ECs and in cultured human dermal microvascular cells22,23 initiate complex intracellular signaling cascades, which results in the phenotypic conversion of ECs into mesenchymal cells. A crucial molecule involved in this process is SNAIL1, a transcriptional repressor that was previously shown to be involved in the regulation of epithelial-to-mesenchymal transition by causing potent inhibition of E-cadherin expression.24,25 Owing to the previously demonstrated important regulatory role of CAV1 on TGF-β signaling, we investigated the participation of CAV1 in pulmonary EC transition into myofibroblasts using ECs isolated from Cav1 KO mice.26,27 The studies described herein demonstrate that pulmonary ECs isolated from Cav1-deficient mice display spontaneous EndoMT which is further increased by TGF-β stimulation. These observations suggest that the decreased levels of CAV1 demonstrated in certain human fibrotic disorders, including SSc and pulmonary fibrosis,17,21 may contribute to the pathogenesis of tissue fibrosis in these disorders through a marked increase in spontaneous and TGF-β–stimulated EndoMT.
Materials and Methods
Materials
Complete endothelial cell medium (ECM) consisting of basal EC medium supplemented with 5% fetal bovine serum, 10% EC growth supplement, 100 U/mL of penicillin, and 100 μg/mL of streptomycin was purchased from ScienCell Research Laboratories (Carlsbad, CA). Anti-CD31 and anti-CD102 were from BD Biosciences (Bedford, MA). The antibodies for Western blot analysis and indirect immunofluorescence were α-actin (α-SMA; clone SPM332; Santa Cruz Biotechnology, Santa Cruz, CA), anti–type I collagen (Rockland Immunochemicals, Gilbertsville, PA), SNAIL-1 (Santa Cruz Biotechnology), and Cy3-conjugated secondary antibodies (Sigma-Aldrich, St. Louis, MO). TGF-β was from R&D Systems (Minneapolis, MN), and EC growth supplement was from Sigma-Aldrich.
Isolation of Pulmonary ECs
Pulmonary ECs were isolated from wild-type (WT) and Cav1 KO mice by a modification of the Marelli-Berg procedure,28 as described previously.22 The generation and establishment of Cav1 KO mice were as described previously.26,27 Lungs from WT and Cav1 KO mice were minced and enzymatically digested with collagenase (30 mg/100 mL in 0.1% bovine serum albumin) in complete ED medium at 37°C for 1 hour with gentle mechanical agitation. The resulting cell mixture was filtered through a sterile 75-μm cell strainer. The single cell suspension obtained was then incubated with rat anti-mouse CD31 antibody (1:50) at 4°C for 45 minutes with gentle shaking, followed by magnetic bead separation using goat anti-rat IgG-conjugated microbeads (1:5; Miltenyi Biotec, Auburn, CA). The isolated ECs were cultured in complete EC medium in 2% gelatin–precoated 60-mm tissue culture dishes until 80% confluent. The cells were trypsinized and resuspended, and a second immunologic separation was performed using rat anti-mouse CD102 antibody. The resulting cells were plated, and the endothelial phenotype and purity of the preparation were confirmed by evaluating cellular uptake of DiI-labeled acetylated low-density lipoprotein (Biomedical Technologies Inc., Stoughton, MA) and assessing cell morphology as described previously.22 The purity of the EC preparation was quantitatively assessed by simultaneously staining with DiI-labeled acetylated low-density lipoprotein and the nuclear stain DAPI. Ninety-five percent to 98% of the cells (determined from DAPI nuclear staining) were ECs, as determined by their uptake of DiI-labeled acetylated low-density lipoprotein. All the animal studies were approved by the Institutional Animal Care and Use Committee of Thomas Jefferson University.
Culture and Treatment of Pulmonary ECs with TGF-β
Pulmonary ECs from WT and Cav1 KO mice were washed with serum-free medium and then incubated with 10 ng/mL of TGF-β1 in EC medium containing 0.5% fetal bovine serum. The medium was changed and TGF-β1 was replenished every 2 days. The cultured cells were visualized using an inverted microscope with a Nikon camera (Nikon Instruments, Melville, NY) and SPOT Advanced software version 4.0.9 (SPOT Imaging Solutions, a division of Diagnostic Instruments, Sterling Heights, MI). On day 3, the cells were either fixed for immunofluorescence staining or harvested for Western blot analysis or RT-PCR.
Selective Restoration of Functional CAV1 Domains in Vitro
Confluent cells between passages 4 and 9 were treated for 24 hours with either the penetratin peptide, a 16-amino acid cell-permeable peptide corresponding to the homeodomain of the Drosophila transcription factor Antennapedia (RQPKIWFPNRRKPWKK), or a peptide consisting of fusion of the Antennapedia peptide and the peptide corresponding to the scaffolding domain of CAV1 (DGIWKASFTTFTVTKYWFY), referred to as cav-1P.20,29 The peptides were custom synthesized and were used at 2.5- and 5-μM final concentrations. Pulmonary ECs were incubated with or without 10 ng/mL of TGF-β for 24 hours in triplicate for each experiment.
Immunofluorescence Staining
Pulmonary ECs were seeded onto glass culture slides and treated as described previously herein. After treatment, the cells were fixed with 3.7% formaldehyde and permeabilized with 0.1% Triton X-100 (Roche Diagnostics GmbH, Mannheim, Germany) in PBS for 3 minutes. Slides were washed with PBS and blocked with PBS containing 1% bovine serum albumin at room temperature for 1 hour, and then they were incubated with primary antibodies against α-SMA (1:200). Slides were then incubated with Cy3-conjugated secondary antibodies (1:500) followed by DAPI (Jackson ImmunoResearch Laboratories Inc., West Grove, PA) for nuclear staining.
Western Blot Analysis
Treated ECs from 6-well, gelatin-coated plastic tissue culture plates were washed with cold PBS, harvested using a cell lifter, centrifuged, and lysed with radioimmunoprecipitation assay lysis buffer (25 mmol/L Tris-HCl, pH 7.6, 150 mmol/L NaCl, 1% NP-40, 1% Na deoxycholate, and 0.1% SDS) supplemented with a complete protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN), homogenized using a pellet pestle motor (Kimble Chase, Vineland, NJ), and subsequently centrifuged at 13,000 × g for 15 minutes at 4°C. Cellular proteins were resolved by SDS-PAGE and were transferred to nitrocellulose membranes (Invitrogen, Carlsbad, CA). Blots were blocked for 1 hour in Tris-buffered saline–Tween (10 mmol/L Tris-HCl, pH 8.0, 150 mmol/L NaCl, 0.1% Tween 20) containing 5% nonfat dry milk (Bio-Rad Laboratories, Hercules, CA). For assessment of type I collagen, equal aliquots of media from each of the cultures were precipitated with ethanol; the pellets were resuspended in 0.4 mol/L NaCl, 0.5 mol/L Tris-HCl (pH 7.4) buffer and then were digested with a mixture of trypsin (1 μg/mL) and chymotrypsin (2.5 μg/mL) at room temperature for 5 minutes, followed by 5 minutes of treatment with a 1:10 volume of trypsin inhibitor (5 μg/mL in PBS), and then prepared for SDS-PAGE and transfer as described previously herein. The membranes were incubated overnight at 4°C with the primary antibodies in a 5% nonfat dry milk/Tris-buffered saline–Tween solution. Membranes were then washed with Tris-buffered saline–Tween and incubated for 1 hour with horseradish peroxidase–conjugated secondary antibodies diluted 3000-fold in 5% nonfat dry milk/Tris-buffered saline–Tween. Signal was detected using an enhanced chemiluminescence detection kit (Pierce, Rockford, IL) and was collected using autoradiography film (Denville Scientific, Metuchen, NJ). The signals were quantified using ImageJ software version 1.46 (NIH, Bethesda, MD).
Standard and Quantitative RT-PCR
WT and Cav1 KO mouse pulmonary ECs were treated in duplicate wells of 12-well, gelatin-treated plastic tissue culture dishes with control or cav-1p peptide for 24 hours, followed by 72 hours with or without TGF-β1 (10 ng/mL), and were harvested using a cell lifter, washed in cold PBS, and processed for RNA extraction (RNeasy kit; Qiagen Inc., Valencia, CA), including a genomic DNA digestion step. Total RNA (1 μg) was reverse transcribed using SuperScript II Reverse Transcriptase (Invitrogen) to generate first-strand cDNA. The primers were designed using Vector NTI software version 5 (Invitrogen) and were validated for specificity. The following primers were used for standard RT-PCR: Snai1: forward, 5′-ACCTTCCAGCAGCCCTACGA-3′; reverse, 5′-AGCGTGTGGCTTCGGATGTG-3′, and Gapdh: forward, 5′-TGTGGATGGCCCCTCTGGAA-3′; reverse, 5′-CGGCATCGAAGGTGGAAGAG-3′. Standard RT-PCR was performed following a standard amplification protocol. The reaction cycles were an initial denaturation at 95°C for 3 minutes and replicate for 35 cycles at 95°C for 30 seconds, 61°C (for Snai1) or 59°C (for Gapdh) for 45 seconds, and 72°C for 45 seconds, and followed by 72°C for 7 minutes. The products were electrophoresed on 1% agarose gels stained with ethidium bromide. The reaction products were visualized using a FluorImager system (Molecular Dynamics, Sunnyvale, CA). Images were quantitated by using ImageJ software, and the signals were normalized to those of Gapdh.
For quantitative RT-PCR, EC transcript levels were quantified using SYBR Green real-time PCR, as previously described.30 Primers were designed using Primer Express software version 3.0 (Applied Biosystems, Foster City, CA) and were validated for specificity. The following primers were used: Gapdh: forward, 5′-CATGGCCTTCCGTGTTCCTA-3′; reverse, 5′-CCTGCTTCACCACCTTCTTGA T-3′, Col1a1: forward, 5′-GCATGGCCAAGAAGACATCG-3′; reverse, 5′-TCCACGTCTCAGCATTGG G-3′, Snai1: forward, 5′-TTGTGTTCTGCACGACCTGTGGAAA-3′; reverse, 5′-TCTTCACATCCGAGTGGGTTTGGA-3′, Snai2: forward, 5′-ACTACAGCGAACTGGACACACACA-3′; reverse, 5′-AAAGGCCACTGGGTAAAGGAGAGT-3′, and Acta2: forward, 5′-ATTGTGCTGGACTCTGGAGATGGT-3′; reverse, 5′-TGATGTCACGGACAATCTCACGCT-3′. The differences in the number of mRNA copies in each PCR were corrected for Gapdh endogenous control transcript levels; levels in control experiments were set at 100%, and all other values are expressed as multiples of control values.
Statistical Analysis
Values reflect the mean ± SD of separate experiments, each performed in triplicate. The statistical significance of all the data was assessed by the two-tailed t-test. A P < 0.05 was considered statistically significant.
Results
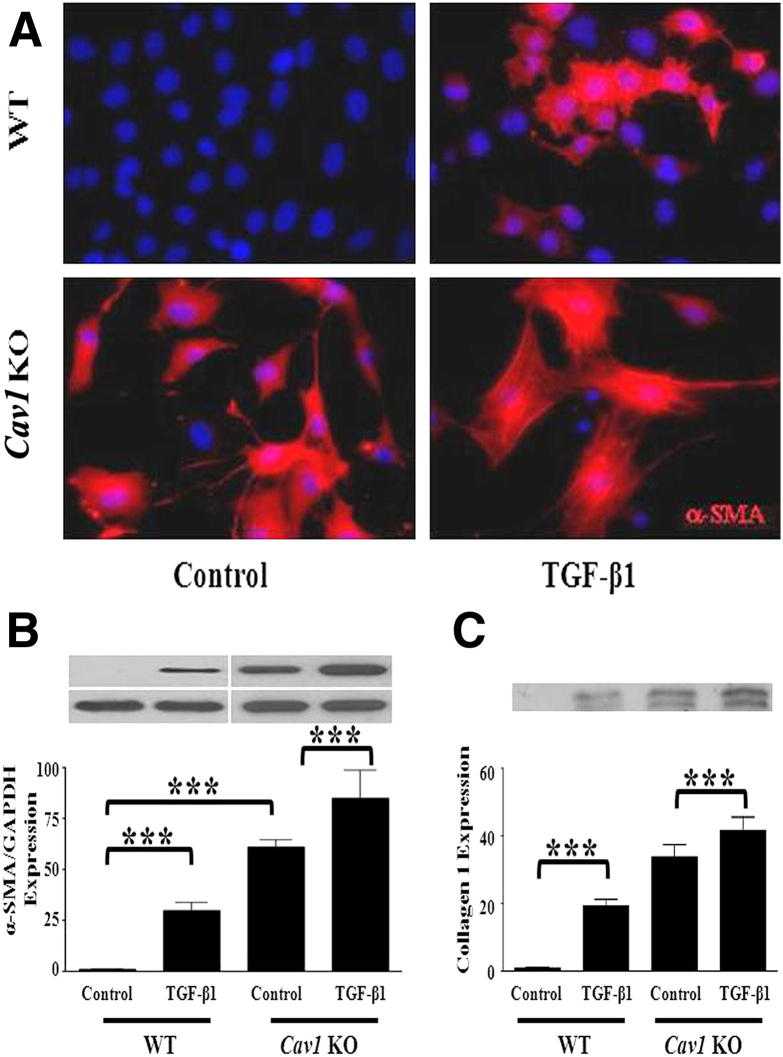
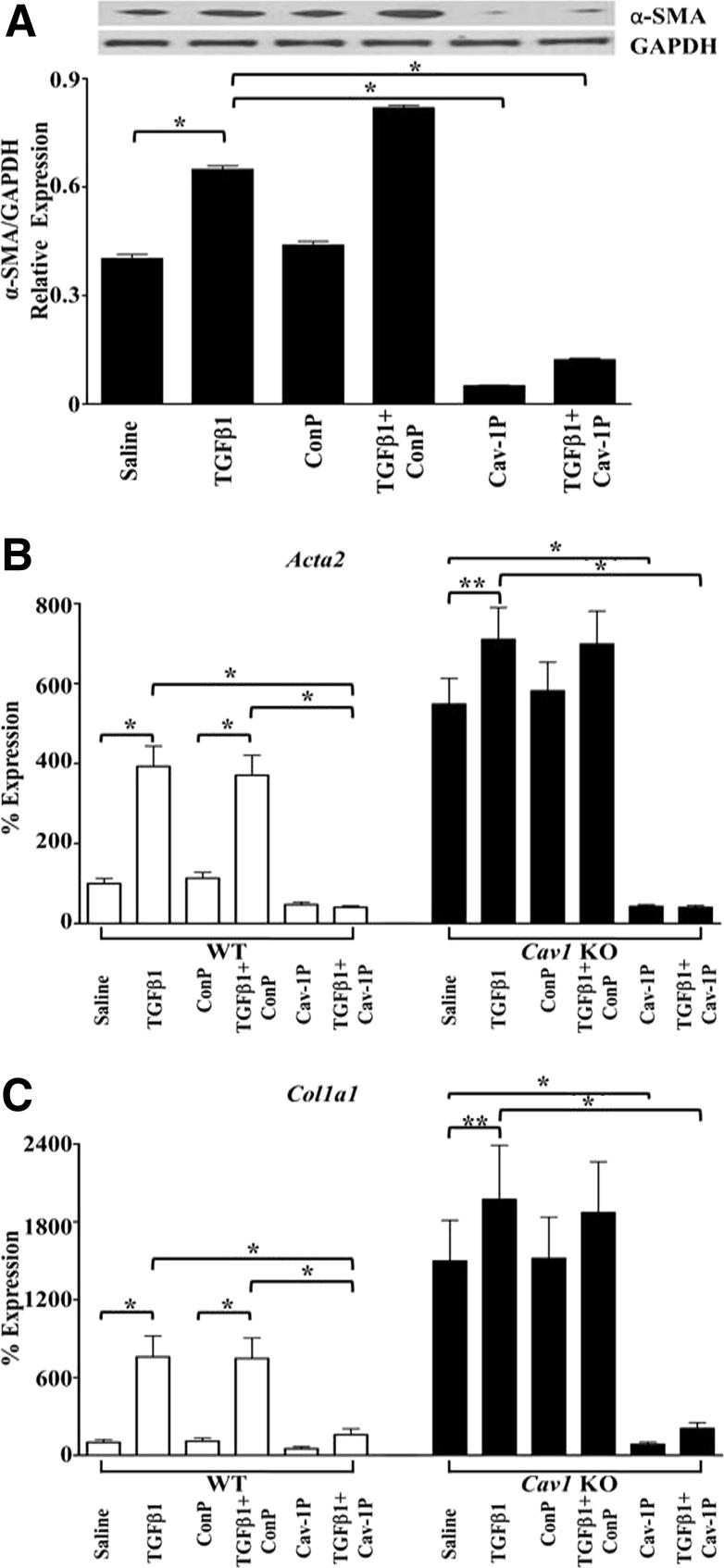
Herein, we describe studies examining the participation of CAV1 in the EndoMT process using pulmonary ECs from WT and Cav1 KO mice. Isolation of pulmonary ECs used a sequential immunomagnetic purification of enzymatically dissociated lung cells with anti-CD31 followed by anti-C102 antibodies as described previously.22 This procedure yields an essentially pure (95% to 98%) EC population as identified by their cellular morphology and their uptake of DiI-labeled acetylated low-density lipoprotein, as described in Materials and Methods. The results with pulmonary ECs derived from two Cav1 KO mice showed high and spontaneous constitutive expression of α-SMA by immunofluorescence staining. Furthermore, CAV1 deficiency increased α-SMA expression induced by treatment with TGF-β1 (Figure 1A). These results were confirmed by Western blot assays that showed very high spontaneous expression of α-SMA in Cav1 KO cells and a further increase in these levels after TGF-β treatment (Figure 1B). Western blot analysis of culture media for type I collagen, a characteristic mesenchymal cell product, showed high levels in the culture media of untreated Cav1 KO cells, with a further increase after exposure to TGF-β (Figure 1C). A cell-permeable CAV1 scaffolding domain peptide previously shown to cause selective restoration of functional CAV1 domains20,29 caused a potent reduction in spontaneous and TGF-β–stimulated α-SMA protein expression, whereas a control peptide lacking the CAV1 sequence had no such effects (Figure 2A). These effects on Acta2 expression were confirmed by quantitative RT-PCR on total EC RNA isolated from an additional three WT and three Cav1 KO mice (Figure 2B). Expression of the Col1a1 gene was also induced in these cells by TGF-β, and this increased expression was reversed by preincubation with the CAV1 scaffolding domain peptide (Figure 2C).
Figure 1.
Spontaneous and TGF-β1–stimulated EndoMT in Cav1−/− primary pulmonary ECs. A: Pulmonary ECs from two WT mice and two Cav1 KO mice were treated with TGF-β1 for 72 hours and were immunostained for α-SMA (red fluorescence). B: Pulmonary ECs from two WT and two Cav1 KO mice were cultured in media alone (Control) or with 10 ng/mL of TGF-β1 for 72 hours, and protein from cell lysates was electrophoresed and probed in Western blot analysis for α-SMA. GAPDH was used as loading control. C: Aliquots of culture media from the same samples shown in B underwent Western blot analysis for type I collagen. The bars show quantitative densitometry analysis of α-SMA and type I collagen using ImageJ software. The values shown are the mean ± SE of two separate experiments, each performed in triplicate. Significance was determined by the two-tailed t-test. ***P < 0.001.
Figure 2.
Effects of a cell-permeable CAV1 scaffolding domain peptide (cav-1P) on α-SMA expression in ECs from Cav1 KO mice. Pulmonary ECs from two Cav1 KO mice were treated with TGF-β1 with or without 5 μmol/L cav-1P or 5 μmol/L control peptide (ConP) for 72 hours. A: Cell lysates were probed with α-SMA. GAPDH was used as loading control. The bars show quantitative densitometry of α-SMA analyzed using ImageJ software. The experiment was performed in triplicate. The values shown are the mean ± SE of the triplicates. B and C: Expression levels of Acta2 (B) and Col1a1 (C) in WT and Cav1 KO mice as determined by semiquantitative RT-PCR. Values represent the mean ± SD expression levels of three replicates of two separate experiments with ECs isolated from three WT and three Cav1 KO mice. CT values were normalized with Gapdh. The saline control levels were arbitrarily set at 100% expression. Values for other samples are expressed relative to the saline control. Significance was determined by the two-tailed t-test. *P < 0.1, **P < 0.01.
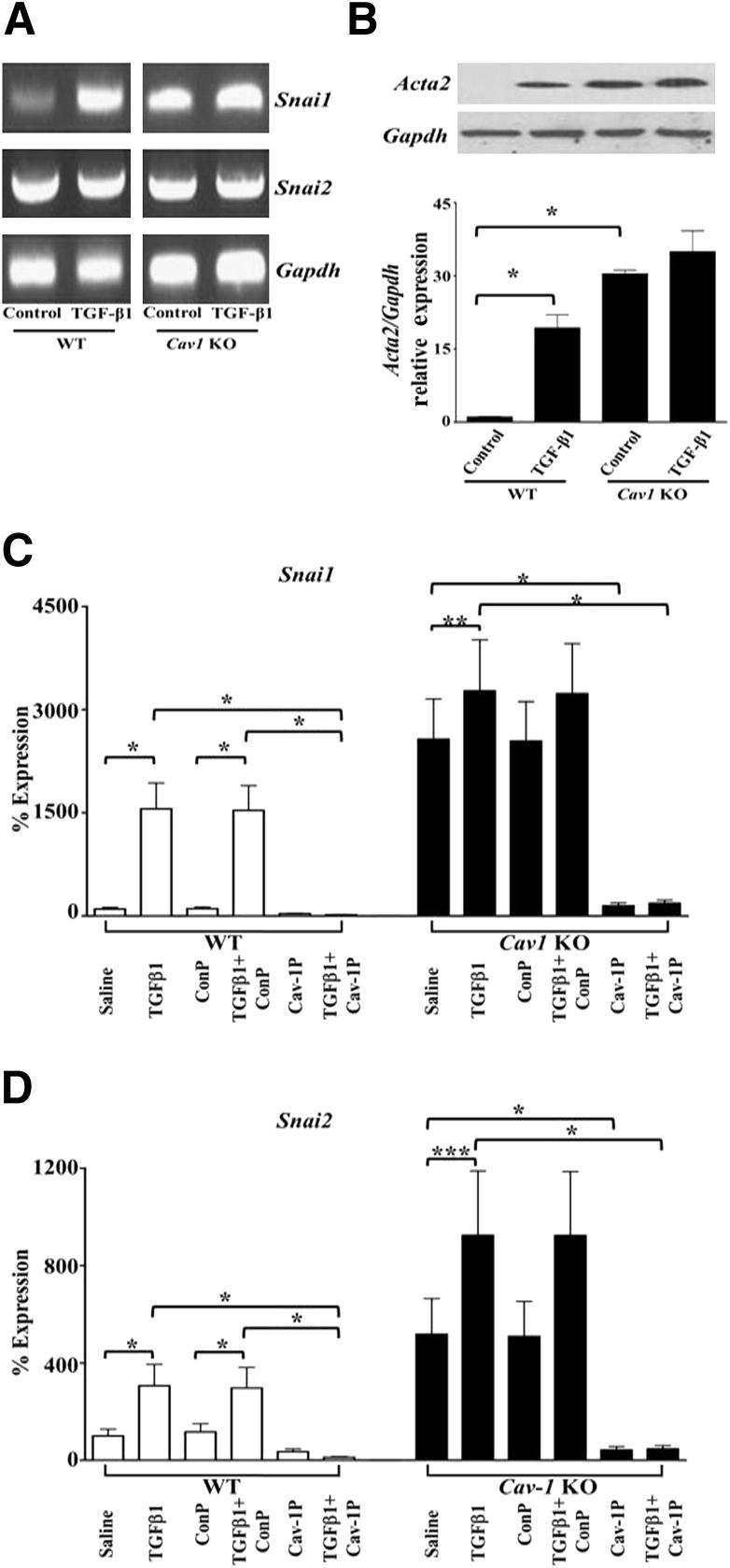
The molecular mechanisms involved in EndoMT have not been completely elucidated, although current evidence indicates that the transcriptional repressor SNAIL1 is involved.22–25 SNAIL1 is a zinc finger transcription factor that regulates transcription of the E-cadherin gene in cultured cells24 and plays a crucial role in the process of epithelial-to-mesenchymal transition.24,25 SNAIL2 has also been implicated in epithelial-to-mesenchymal transition.31 Herein, we observed remarkable spontaneous increased expression of Snai1 and Snai2 in pulmonary ECs by standard RT-PCR from Cav1 KO mice that was further increased after treatment with TGF-β1 (Figure 3A). A similar pattern of changes in SNAIL1 protein levels was observed in cell lysates by Western blot analysis (Figure 3B). Analysis of cells from an additional three WT and three Cav1 KO mice by quantitative RT-PCR showed similarly striking TGF-β–dependent up-regulation of Snai1 (Figure 3C) and Snai2 (Figure 3D) expression and that preincubation with the cav-1P peptide completely abrogated the increased expression levels of both genes (Figure 3, C and D).
Figure 3.
Increased Snai1 expression and protein levels in Cav1−/− pulmonary ECs. Pulmonary ECs isolated from two WT and two Cav1 KO mice were treated with TGF-β1 for 72 hours. A: RNA was purified, reverse transcribed, and assessed by PCR for Snai1, Snai2, and Gapdh expression. The figure shows representative results from three separate experiments. B: Cell lysates from one of the experiments underwent Western blot analysis for SNAIL1 levels. The bars show quantitative densitometry using ImageJ software. The values are the mean ± SE of two different experiments. C and D: Expression levels of Snai1 (C) and Snai2 (D) in WT and Cav1 KO mice as determined by semiquantitative RT-PCR. Values represent the mean ± SD expression levels of three replicates of two separate experiments with ECs isolated from three WT and three Cav1 KO mice. CT values were normalized with Gapdh. The saline control levels were arbitrarily set at 100% expression. Values for other samples are expressed relative to the saline control. Significance was determined by the two-tailed t-test. *P < 0.1, **P < 0.01, and ***P < 0.001.
Discussion
EndoMT is a process whereby ECs transdifferentiate into mesenchymal cells, losing their EC-specific functions and initiating the expression of α-SMA, E-cadherin, and interstitial collagens, such as type I collagen.9,10 EndoMT has recently been reported in experimentally induced cardiac, pulmonary, and renal fibrosis (reviewed by Piera-Velazquez et al11,12).
CAV1 is the main protein component of 50- to 100-nm flask-shaped invaginations found on the plasma membrane known as caveolae (reviewed in other studies13,14). CAV1 plays an important role in the internalization, trafficking, and degradation of TGF-β receptors15; therefore, it plays a crucial role in the regulation of TGF-β signaling and TGF-β–mediated fibrotic responses.16 CAV1 has been shown to be down-regulated in affected tissues from patients with SSc and idiopathic pulmonary fibrosis,17,18 and restoration of CAV-1 functional domains or adenoviral-mediated expression of CAV1 abrogated bleomycin-induced pulmonary fibrosis in mice.19 Previous studies of Cav1 KO mice are in support of this notion.17,27 Although the earlier description of these mice27 was not focused on the development of tissue fibrosis, the presence of markedly abnormal alveolar spaces that appeared constricted by thickened alveolar septa and hypercellularity, and thickening of the alveolar basement membrane including accumulation and disorganization of reticulin fibers were noted. The subsequent study17 was focused on the fibrotic and connective tissue abnormalities in Cav1 KO mice. The results showed a marked increase in interstitial collagen evidenced by trichrome staining and a >2.5-fold increase in lung hydroxyproline content, a direct and quantitative reflection of the extent of tissue fibrosis.
The results described herein demonstrate the spontaneous occurrence of EndoMT in Cav1−/− pulmonary ECs as evidenced by the high constitutive expression of α-SMA, the high levels of the mesenchymal cell product type I collagen, and the high expression of the transcriptional repressors Snai1 and Snai2, molecules previously shown to be up-regulated in TGF-β1–induced EndoMT in normal murine pulmonary ECs. These observations support the concept that EndoMT may occur spontaneously in Cav1-deficient mice in vivo and may play a crucial role in the fibrotic process previously described in these mice.17,26 The results described herein suggest that caveolin deficiency–mediated EndoMT may participate in the development of proliferative vasculopathy in diseases such as SSc and idiopathic pulmonary fibrosis.
Acknowledgment
We thank Melissa Bateman for assistance in the preparation of the manuscript.
Footnotes
Supported by NIH grant RO1 AR055660 (S.A.J.).
A guest editor acted as editor-in-chief for this manuscript. No person at Thomas Jefferson University was involved in the final disposition of this article.
Current address of M.P.L., Breakthrough Breast Cancer Research Unit, The University of Manchester, Manchester, UK.
References
- 1.Hinz B., Phan S.H., Thannickal V.J., Galli A., Bochaton-Piallat M.L., Gabbiani G. The myofibroblast: one function, multiple origins. Am J Pathol. 2007;170:1807–1816. doi: 10.2353/ajpath.2007.070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kis K., Liu X., Hagood J.S. Myofibroblast differentiation and survival in fibrotic disease. Expert Rev Mol Med. 2011;13:e27. doi: 10.1017/S1462399411001967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hinz B., Phan S.H., Thannickal V.J., Prunotto M., Desmouliere A., Varga J., De Wever O., Mareel M., Gabbiani G. Recent developments in myofibroblast biology: paradigms for connective tissue remodeling. Am J Pathol. 2012;180:1340–1355. doi: 10.1016/j.ajpath.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Watsky M.A., Weber K.T., Sun Y., Postlethwaite A. New insights into the mechanism of fibroblast to myofibroblast transformation and associated pathologies. Int Rev Cell Mol Biol. 2010;282:165–192. doi: 10.1016/S1937-6448(10)82004-0. [DOI] [PubMed] [Google Scholar]
- 5.Desmouliere A., Guyot C., Gabbiani G. The stroma reaction myofibroblast: a key player in the control of tumor cell behavior. Int J Dev Biol. 2004;48:509–517. doi: 10.1387/ijdb.041802ad. [DOI] [PubMed] [Google Scholar]
- 6.Räsänen K., Vaheri A. Activation of fibroblasts in cancer stroma. Exp Cell Res. 2010;316:2713–2722. doi: 10.1016/j.yexcr.2010.04.032. [DOI] [PubMed] [Google Scholar]
- 7.Xing F., Saidou J., Watabe K. Cancer associated fibroblasts (CAFs) in tumor microenvironment. Front Biosci. 2010;15:166–179. doi: 10.2741/3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kalluri R., Neilson E.G. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest. 2003;112:1776–1784. doi: 10.1172/JCI20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arciniegas E., Neves C.Y., Carrillo L.M., Zambrano E.A., Ramírez R. Endothelial-mesenchymal transition occurs during embryonic pulmonary artery development. Endothelium. 2005;12:193–200. doi: 10.1080/10623320500227283. [DOI] [PubMed] [Google Scholar]
- 10.van Meeteren L.A., Ten Dijke P. Regulation of endothelial cell plasticity by TGF-β. Cell Tissue Res. 2012;347:177–186. doi: 10.1007/s00441-011-1222-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Piera-Velazquez S., Li Z., Jimenez S.A. Role of endothelial-mesenchymal transition (EndoMT) in the pathogenesis of fibrotic disorders. Am J Pathol. 2011;179:1074–1080. doi: 10.1016/j.ajpath.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Piera-Velazquez S., Jimenez S.A. Molecular mechanisms of endothelial to mesenchymal cell transition (EndoMT) in experimentally induced fibrotic diseases. Fibrogenesis Tissue Repair. 2012;5(Suppl 1):S7. doi: 10.1186/1755-1536-5-S1-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Razani B., Lisanti M.P. Caveolins and caveolae: molecular and functional relationships. Exp Cell Res. 2001;271:36–44. doi: 10.1006/excr.2001.5372. [DOI] [PubMed] [Google Scholar]
- 14.Chidlow J.H., Jr., Sessa W.C. Caveolae, caveolins, and cavins: complex control of cellular signaling and inflammation. Cardiovasc Res. 2010;86:219–225. doi: 10.1093/cvr/cvq075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Razani B., Zhang X.L., Bitzer M., von Gersdorff G., Bottinger E.P., Lisanti M.P. Caveolin-1 regulates transforming growth factor (TGF)-β/SMAD signaling through an interaction with the TGF-β type I receptor. J Biol Chem. 2001;276:6727–6738. doi: 10.1074/jbc.M008340200. [DOI] [PubMed] [Google Scholar]
- 16.Pike L.J. Growth factor receptors, lipid rafts and caveolae: an evolving story. Biochim Biophys Acta. 2005;1746:260–273. doi: 10.1016/j.bbamcr.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 17.Del Galdo F., Sotgia F., de Almeida C.J., Jasmin J.F., Musick M., Lisanti M.P., Jimenez S.A. Decreased expression of caveolin 1 in patients with systemic sclerosis: crucial role in the pathogenesis of tissue fibrosis. Arthritis Rheum. 2008;58:2854–2865. doi: 10.1002/art.23791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Del Galdo F., Lisanti M.P., Jimenez S.A. Caveolin-1, transforming growth factor-β receptor internalization, and the pathogenesis of systemic sclerosis. Curr Opin Rheumatol. 2008;20:713–719. doi: 10.1097/bor.0b013e3283103d27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang X.M., Zhang Y., Kim H.P., Zhou Z., Feghali-Bostwick C.A., Liu F., Ifedigbo E., Xu X., Oury T.D., Kaminski N., Choi A.M. Caveolin-1: a critical regulator of lung fibrosis in idiopathic pulmonary fibrosis. J Exp Med. 2006;203:2895–2906. doi: 10.1084/jem.20061536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jasmin J.F., Mercier I., Dupuis J., Tanowitz H.B., Lisanti M.P. Short-term administration of a cell-permeable caveolin-1 peptide prevents the development of monocrotaline-induced pulmonary hypertension and right ventricular hypertrophy. Circulation. 2006;114:912–920. doi: 10.1161/CIRCULATIONAHA.106.634709. [DOI] [PubMed] [Google Scholar]
- 21.Tourkina E., Richard M., Gööz P., Bonner M., Pannu J., Harley R., Bernatchez P.N., Sessa W.C., Silver R.M., Hoffman S. Antifibrotic properties of caveolin-1 scaffolding domain in vitro and in vivo. Am J Physiol Lung Cell Mol Physiol. 2008;294:L843–L861. doi: 10.1152/ajplung.00295.2007. [DOI] [PubMed] [Google Scholar]
- 22.Li Z., Jimenez S.A. Protein kinase Cδ and c-Abl kinase are required for transforming growth factor β induction of endothelial-mesenchymal transition in vitro. Arthritis Rheum. 2011;63:2473–2483. doi: 10.1002/art.30317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kokudo T., Suzuki Y., Yoshimatsu Y., Yamazaki T., Watabe T., Miyazono K. Snail is required for TGFβ-induced endothelial-mesenchymal transition of embryonic stem cell-derived endothelial cells. J Cell Sci. 2008;121:3317–3324. doi: 10.1242/jcs.028282. [DOI] [PubMed] [Google Scholar]
- 24.Cano A., Perez-Moreno M.A., Rodrigo I., Locascio A., Blanco M.J., del Barrio M.G., Portillo F., Nieto M.A. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2:76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- 25.Vincent T., Neve E.P., Johnson J.R., Kukalev A., Rojo F., Albanell J., Pietras K., Virtanen I., Philipson L., Leopold P.L., Crystal R.G., de Herreros A.G., Moustakas A., Pettersson R.F., Fuxe J. A SNAIL1-SMAD3/4 transcriptional repressor complex promotes TGF-β mediated epithelial-mesenchymal transition. Nat Cell Biol. 2009;11:943–950. doi: 10.1038/ncb1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Razani B., Engelman J.A., Wang X.B., Schubert W., Zhang X.L., Marks C.B., Macaluso F., Russell R.G., Li M., Pestell R.G., Di Vizio D., Hou H., Jr., Kneitz B., Lagaud G., Christ G.J., Edelmann W., Lisanti M.P. Caveolin-1 null mice are viable but show evidence of hyperproliferative and vascular abnormalities. J Biol Chem. 2001;276:38121–38138. doi: 10.1074/jbc.M105408200. [DOI] [PubMed] [Google Scholar]
- 27.Razani B., Lisanti M.P. Caveolin-deficient mice: insights into caveolar function human disease. J Clin Invest. 2001;108:1553–1561. doi: 10.1172/JCI14611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marelli-Berg F.M., Peek E., Lidington E.A., Stauss H.J., Lechler R.I. Isolation of endothelial cells from murine tissue. J Immunol Methods. 2000;244:205–215. doi: 10.1016/s0022-1759(00)00258-1. [DOI] [PubMed] [Google Scholar]
- 29.Schlegel A., Schwab R.B., Scherer P.E., Lisanti M.P. A role for the caveolin scaffolding domain in mediating the membrane attachment of caveolin-1: the caveolin scaffolding domain is both necessary and sufficient for membrane binding in vitro. J Biol Chem. 1999;274:22660–22667. doi: 10.1074/jbc.274.32.22660. [DOI] [PubMed] [Google Scholar]
- 30.Batlle E., Sancho E., Franci C., Dominguez D., Monfar M., Baulida J., Garcia De Herreros A. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol. 2000;2:84–89. doi: 10.1038/35000034. [DOI] [PubMed] [Google Scholar]
- 31.Casas E., Kim J., Bendesky A., Ohno-Machado L., Wolfe C.J., Yang J. Snail2 is an essential mediator of Twist1-induced epithelial mesenchymal transition and metastasis. Cancer Res. 2011;71:245–254. doi: 10.1158/0008-5472.CAN-10-2330. [DOI] [PMC free article] [PubMed] [Google Scholar]