Abstract
Although inflammation plays a central role in the pathogenesis of acute lung injury, the molecular mechanisms underlying inflammatory responses in acute lung injury are poorly understood, and therapeutic options remain limited. CCAAT/enhancer-binding proteins, C/EBPβ and C/EBPδ, are expressed in the lung and have been implicated in the regulation of inflammatory mediators. However, their functions in lung pathobiological characteristics are not well characterized. Herein, we show that C/EBPβ and C/EBPδ are activated in mouse lung after intrapulmonary deposition of lipopolysaccharide (LPS). Mice carrying a targeted deletion of the C/EBPδ gene displayed significant attenuation of the lung permeability index (lung vascular leak of albumin), lung neutrophil accumulation (myeloperoxidase activity), and neutrophils in bronchial alveolar lavage fluids compared with wild-type mice. These phenotypes were consistent with morphological evaluation of lung, which showed reduced inflammatory cell influx and minimal intra-alveolar hemorrhage. Moreover, mutant mice expressed considerably less tumor necrosis factor-α, IL-6, and macrophage inflammatory protein-2 in bronchial alveolar lavage fluids in LPS-injured lung compared with wild-type mice. In contrast, C/EBPβ deficiency had no effect on LPS-induced lung injury. By using small-interfering RNA–mediated knockdown for C/EBPδ, we demonstrate, for the first time to our knowledge, that C/EBPδ plays a critical role for the tumor necrosis factor-α, IL-6, and macrophage inflammatory protein-2 production in LPS-stimulated alveolar macrophages. These findings demonstrate that C/EBPδ, but not C/EBPβ, plays an important role in LPS-induced lung inflammatory responses and injury.
Acute lung injury affects approximately 190,000 patients annually in the United States.1 Despite the recent development of protective lung ventilation strategies, acute lung injury and its severe form, acute respiratory distress syndrome, remain leading factors of morbidity and mortality in critically ill patients.1–4 One of the major cellular responses in lung initiated by various direct and indirect stimuli is the activation of genes encoding cytokines and other mediators that promote inflammation. The resulting levels of pro- and anti-inflammatory mediators and their balance determine the magnitude of lung injury and outcome. Growing evidence indicates that the expression of these inflammatory mediators in the lung is regulated by a highly intricate network of transcription factors, such as NF-κB and STAT3.5–9 However, their exact contributions to lung pathogenesis during acute lung injury remain largely undetermined, because most studies have been either correlative or indirect (ie, based on various inhibitors that may have effects on divergent signaling pathways). Thus, one of the major roadblocks in developing treatments for acute lung injury is the lack of basic knowledge of the transcriptional mechanisms underlying lung inflammation.
The CCAAT/enhancer-binding proteins (C/EBPα, C/EBPβ, C/EBPδ, C/EBPε, C/EBPγ, and C/EBPζ) compose a family of basic region-leucine zipper (bZIP) transcription factors that dimerize through a leucine zipper and bind to DNA through an adjacent basic region.10–12 C/EBP proteins can generally form homodimers and heterodimers with other family members. The variety of C/EBP isoforms and their potential for heterodimer formation could provide a large repertoire of transcription factors with complex in vivo regulatory features. C/EBPβ and C/EBPδ are regulators of pro-inflammatory cytokines and other gene products of the acute-phase response.11,13–19 C/EBPβ and C/EBPδ are structurally similar in their DNA-binding and dimerization domains, but differ in their transactivation domains, implying that they may have unique functions in response to different stimuli. Both C/EBPβ and C/EBPδ are expressed in the lung.20–22 C/EBPβ and C/EBPδ are not vital to baseline lung function and development, as suggested by the finding that mice lacking both C/EBPβ and C/EBPδ exhibit no histological abnormalities of the lung.23 Levels of both C/EBPβ and C/EBPδ are increased in the lung after systemic endotoxin administration.24,25 By using a gene expression profiling approach, a recent study identified C/EBPδ as a potential candidate regulator of endotoxin-induced disseminated intravascular coagulation.26 However, the functions of C/EBPβ and C/EBPδ in acute lung inflammation remain poorly understood. C/EBPβ was recently shown to mediate inflammatory and innate immune responses in lung to cigarette smoke.27 C/EBPβ also reportedly played an essential role in bleomycin-induced pulmonary fibrosis.28 The role of C/EBPδ in lung inflammation and injury remains unknown. Herein, we identify C/EBPδ, but not C/EBPβ, as a critical regulator of inflammatory responses in lipopolysaccharide (LPS)–induced acute lung injury.
Materials and Methods
Cells and Reagents
Mouse alveolar macrophage–derived cell line, MH-S, was obtained from ATCC (Manassas, VA); cultured in RPMI 1640 medium, supplemented with 10% fetal bovine serum, 2 mmol/L l-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin, and 0.01 mol/L HEPES; and maintained in a humidified incubator at 37°C with 5% CO2. Enzyme-linked immunosorbent assay (ELISA) kits for mouse IL-6, tumor necrosis factor (TNF)-α, macrophage inflammatory protein (MIP)-2, and keratinocyte cell–derived chemokine (KC) were obtained from R&D Systems (Minneapolis, MN). p38 Mitogen-activated protein kinase (MAPK) inhibitor VIII and p44/p42 inhibitor, U0126, were obtained from EMD Biosciences (Gibbstown, NJ).
LPS-Induced Acute Lung Injury
All procedures involving mice were approved by the Animal Care and Use Committee of Harvard Medical School (Boston, MA). Specific pathogen-free male C57BL/6 mice, aged 8 to 12 weeks, were obtained from Jackson Laboratories (Bar Harbor, ME). Mice were anesthetized i.p. with 100 mg/kg ketamine HCl, followed by intratracheal instillation of 50 μL of LPS (1 mg/mL serotype 0111.B4; Sigma-Aldrich, St. Louis, MO) dissolved in PBS during inspiration. Negative control mice received 50 μL of PBS intratracheally. Unless otherwise indicated, 18 hours after LPS deposition, mice were exsanguinated and the pulmonary circulation was flushed with 1 mL of PBS via the pulmonary artery. The lungs were surgically dissected and immediately frozen in liquid nitrogen. The generation of C/EBPβ−/− and C/EBPδ−/− mice by homologous recombination was previously described.29,30 Cebpb−/− mice and wild-type (WT) littermates were on a C57BL/6:Sv129 F1 hybrid background (to circumvent low mutant viability on each pure strain background), and Cebpd−/− animals and WT controls were on a C57Bl/6 background.
MPO Activity
Mice were sacrificed, and the lungs were perfused via the right ventricle with 3 mL of PBS. To measure myeloperoxidase (MPO) activity, whole lungs were homogenized in 50 mmol/L potassium phosphate buffer containing 0.5% hexadecyltrimethylammonium bromide and 5 mmol/L EDTA. The samples were sonicated for 1 minute and centrifuged at 9600 × g for 10 minutes. A total of 10 μL of the recovered supernatants was added to a 96-well plate, followed by the addition of 100 mmol/L potassium phosphate buffer containing 1.5 mol/L H2O2 and 167 μg/mL o-dianisidine dihydrochloride. The enzyme activity was determined by measuring the change in OD at 450 nm over 4.5 minutes using a 96-well plate reader.
Histological Assay
At 18 hours after LPS deposition, 1 mL of 10% buffered (pH 7.2) formalin was instilled into the lung via the trachea. The lungs were then surgically removed and further fixed in 10% buffered formalin solution for morphological assay by tissue sectioning and staining with H&E.
BAL Fluid Collection, Differential White Blood Cell Counts, and Albumin and Chemokine/Cytokine ELISAs
At 18 hours after initiation of the acute lung injury, the thorax was opened and 0.8 mL of ice-cold, sterile PBS was instilled into the lung via a tracheal incision. The recovered bronchial alveolar lavage (BAL) fluid was centrifuged at 450 × g for 6 minutes, and the cell-free supernatants were stored at −20°C. Cell pellets were resuspended in 1 mL of HBSS containing 0.5% bovine serum albumin, and differential cell analyses were performed by Diff-Quik–stained cytospin preparations (Dade, Düdingen, Switzerland), counting 300 cells per slide in randomly selected high-powered fields (original magnification, ×1000). The supernatant was used for chemokine and cytokine measurements by sandwich ELISA. Mouse albumin levels in BAL fluid were measured using a mouse albumin ELISA kit obtained from Bethyl Laboratories (Montgomery, TX).
Alveolar Macrophage Depletion
Mice were anesthetized with 100 mg/kg i.p. ketamine HCl. A suspension of dichloromethylene diphosphonate (Cl2MDP) liposomes in PBS (10 μL of the liposome stock in a total volume of 50 μL) was administered intratracheally during inspiration. As a control, PBS liposomes were administered in a similar manner. All subsequent interventions were performed 24 hours after liposome instillation. Liposome-encapsulated clodronate was prepared as previously described.31 Mice receiving Cl2MDP liposomes showed >75% depletion of alveolar macrophages compared with mice receiving PBS liposomes. The administration of PBS liposomes did not reduce the number of alveolar macrophages.
Assessment of C/EBP Activation by EMSA
Nuclear extracts of whole lung tissues were prepared as previously described.21 Briefly, frozen lungs were homogenized in 0.6% (v/v) Nonidet P-40, 150 mmol/L NaCl, 10 mmol/L HEPES (pH 7.9), 1 mmol/L EDTA, 0.5 mmol/L phenylmethylsulfonyl fluoride, 2.5 μg/mL leupeptin, 5 μg/mL antipain, and 5 μg/mL aprotinin. The homogenate was incubated on ice for 5 minutes and then centrifuged for 5 minutes at 5000 × g at 4°C. Proteins were extracted from the pelleted nuclei by incubation at 4°C with solution B [420 mmol/L NaCl, 20 mmol/L HEPES (pH 7.9), 1.2 mmol/L MgCl2, 0.2 mmol/L EDTA, 25% (v/v) glycerol, 0.5 mmol/L dithiothreitol, 0.5 mmol/L phenylmethylsulfonyl fluoride, 2.5 μg/mL leupeptin, 5 μg/mL antipain, and 5 μg/mL aprotinin]. Nuclear debris was pelleted by centrifugation at 13,000 × g for 30 minutes at 4°C, and the supernatant extract was collected and stored at −80°C. Protein concentrations were determined by a Bio Rad protein assay using bovine serum albumin as a reference standard (Pierce Co, Rockford, IL). The electrophoretic mobility shift assay (EMSA) probes were double-stranded oligonucleotides containing a C/EBP consensus oligonucleotide (5′-TGCAGATTGCGCAATCTGCA-3′; Santa Cruz Biotechnology, Santa Cruz, CA). C/EBP probes were labeled with [32P] ATP (3000 Ci/mmol at 10 mCi/mL) (Amersham Biosciences Corp, Sunnyvale, CA). DNA-binding reactions were performed at room temperature in a 25-μL reaction mixture containing 6 μL of nuclear extract (1 mg/mL in buffer C or solution B) and 5 μL of five times binding buffer [20% (w/v) Ficoll, 50 mmol/L HEPES (pH 7.9), 5 mmol/L EDTA, and 5 mmol/L dithiothreitol]. The remainder of the reaction mixture contained KCl at a final concentration of 50 mmol/L, Nonidet P-40 at a final concentration of 0.1%, 1 μg of poly(dI-dC), 200 pg of probe (unless otherwise noted), bromphenol blue at a final concentration of 0.06% (w/v), and water to a volume of 25 μL. Samples were electrophoresed through 5.5% polyacrylamide gels in one times TBE (90 mmol/L Tris base, 90 mmol/L boric acid, and 0.5 mmol/L EDTA) at 190 V for approximately 3.5 hours, dried under a vacuum, and exposed to X-ray film. For supershifts, nuclear extracts were pre-incubated with antibodies (1 to 2 μg) for 0.5 hours at 4°C before the binding reaction. The following antibodies were obtained from Santa Cruz Biotechnology, Inc.: C/EBPα, C/EBPβ, C/EBPδ, C/EBPε, C/EBPγ, and normal rabbit IgG.
siRNA Transfection
Transient small-interfering RNA (siRNA) transfections were performed by transfecting 1 × 106 to 2 × 106 MH-S cells with control siRNA or C/EBPδ siRNA (Santa Cruz Biotechnology) using Amaxa nucleofector kit V. After 12 hours, MH-S cells were treated with or without 100 ng/mL LPS (Sigma-Aldrich) for 6 hours. RNAs were harvested for RT-PCR to analyze down-regulation of C/EBPδ expression, or supernatants were collected for ELISAs.
Luciferase Assay
MH-S cells were transfected with the indicated reporter plasmids by using the Fugene6 Transfection Reagent (Roche, Indianapolis, IN). At 48 hours after transfection, cells were treated with or without 100 ng/mL LPS. After 4 hours, cells were lysed in Passive Lysis 5X Buffer (Promega, Madison, WI), and luciferase activity was measured. The mouse IL-6 promoter-reporter, the TNF-α promoter-reporter, and the C/EBPδ expression plasmid have been described in our previous publications.32,33
RNA Isolation and Detection of mRNA by RT-PCR
Total RNAs were extracted from lungs with TRIzol (Invitrogen, Carlsbad, CA), according to the manufacturer’s procedure. After isolation, total cellular RNA was incubated with RQ1 RNase-free DNase (Promega) to remove contaminating DNA. A total of 2 μg of total RNA was submitted to reverse transcription by using the Superscript II RNase H-Reverse Transcriptase (Invitrogen). PCR was performed with primers for the following: C/EBPä, 5′-CGCAGACAGTGGTGAGCTT-3′ (forward) and 5′-CTTCTGCTGCATCTCCTGGT-3′ (reverse); and glyceraldehyde-3-phosphate dehydrogenase (GAPDH), 5′-GCCTCGTCTCATAGACAAGATG-3′ (forward) and 5′-CAGTAGACTCCACGACATAC-3′ (reverse). After a hot start for 5 minutes at 94°C, 28 to 33 cycles were used for amplification, with a melting temperature of 94°C, an annealing temperature of 60°C, and an extending temperature of 72°C, each for 1 minute, followed by a final extension at 72°C for 8 minutes. PCR was performed using different cycle numbers for all primers, to ensure that DNA was detected within the linear part of the amplifying curves for both primers.
Western Blot Analysis
MH-S cells were lysed in radioimmune precipitation assay buffer. Samples containing 80 μg of protein were electrophoresed in a 12% polyacrylamide gel and then transferred to a polyvinylidene difluoride membrane. Membranes were incubated with rabbit anti-C/EBPδ antibody (Santa Cruz Biotechnology), rabbit anti–phospho-p38 MAPK antibody (Cell Signaling, Danvers, MA), rabbit anti–phospho-p44/42 MAPK antibody (Cell Signaling), rabbit anti-p38 MAPK antibody (Cell Signaling), rabbit anti-p44/42 antibody (Cell Signaling), and rabbit anti–GAPDH antibody (Cell Signaling). After three washes in Tris-buffered saline with Tween, the membranes were incubated with a 1:5000 dilution of horseradish peroxidase–conjugated donkey anti-rabbit IgG (GE Healthcare, Woburn, MA). The membrane was developed by the enhanced chemiluminescence technique, according to the manufacturer’s protocol (Thermo Fisher Scientific, Rockford, IL).
Statistical Analysis
All values were expressed as the mean ± SEM. Data sets were analyzed using the Student’s t-test or a one-way analysis of variance, with individual group means being compared with the Student-Newman-Keuls multiple comparison test.
Results
Activation of C/EBPs during LPS-Induced Acute Lung Injury
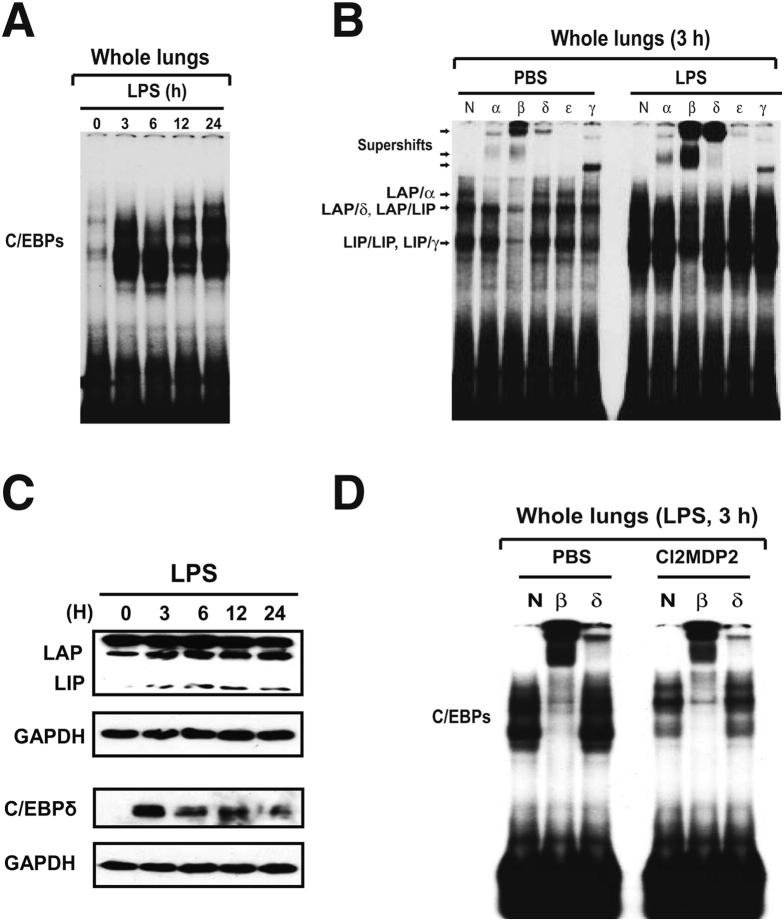
The time course of C/EBP activation during LPS-induced lung injury was evaluated by EMSA, using nuclear extracts from whole lung obtained at various time points after the onset of lung injury. As shown in Figure 1A, at time 0, low levels of constitutive C/EBP DNA-binding species in whole lung nuclear extracts were observed. Increased C/EBP DNA binding was evident and strong by 3 to 24 hours. Antibody supershift assays were used to identify individual C/EBP family members present in the EMSA complexes. There are three DNA-binding species in the nuclear proteins of control-treated lungs (PBS): a low, but detectable, level of C/EBPα/β heterodimers, heterodimers between C/EBPβ liver-enriched activator protein and its short isoform, liver-enriched inhibitory protein, which is translated from an alternative start site in the same mRNA,34 and liver-enriched inhibiting protein/liver-enriched inhibiting protein homodimers (Figure 1B). However, little C/EBPδ DNA binding was evident. In contrast, the binding activities of both C/EBPβ and C/EBPδ were significantly induced in LPS-injured lungs (Figure 1B). We next evaluated whether LPS induced expression of C/EBPβ and C/EBPδ at the protein level. Western blot analysis of lysates harvested over a time course after LPS deposition revealed increased abundance of both C/EBPβ and C/EBPδ during the 24-hour period (Figure 1C and data not shown). Thus, increased expression of the C/EBPβ and C/EBPδ proteins coincided with their enhanced DNA-binding activity in the lung. To localize the expression of C/EBPδ protein, we performed immunohistochemical staining in lung sections after LPS administration. We found strong staining for C/EBPδ protein in LPS-injured lung, especially in bronchiolar and alveolar epithelium (data not shown). We further performed immunocytostaining experiments using cells in BAL fluids harvested from lungs after LPS administration. Strong C/EBPδ expression was detectable in alveolar macrophages (data not shown).
Figure 1.
Lung C/EBP activation during LPS-induced alveolitis. A: Time course for C/EBP activation in LPS-injured lungs. Nuclear extracts from whole lung tissues were subjected to EMSA analysis using a labeled canonical C/EBP site probe. B: Nuclear proteins extracted from whole lung at 0 and 3 hours after LPS deposition were subjected to supershift. The following antibodies were used: normal rabbit IgG (N), anti-C/EBPα antibody (α), anti-C/EBPβ antibody (β), anti-C/EBPδ antibody (δ), anti-C/EBPε antibody (ε), and anti-C/EBPγ antibody (γ). Supershifted species and C/EBP dimers are indicated. C: Western blot analysis of C/EBPβ and C/EBPδ during LPS-induced lung injury. D: Effects of alveolar macrophage depletion on C/EBP activation during LPS-induced lung injury. Mice received PBS-liposome or Cl2MDP-liposomes 24 hours before intratracheal challenge with either PBS or LPS. At 3 hours after PBS or LPS challenge, lungs were harvested. C/EBP binding activity in whole lung nuclear extracts was assessed by EMSA. LAP, liver-enriched activator protein; LIP, liver-enriched inhibitory protein.
A previous study demonstrated that alveolar macrophage was a critical component for initiation of the LPS-induced innate immune response in the lung.35 We sought to determine the role of macrophages in regulating C/EBP activation in whole lung tissues in response to LPS. Nuclear extracts from whole lungs harvested 3 hours after the onset of injury were analyzed by EMSA. As shown in Figure 1D, strong LPS-induced C/EBP activation in the lung occurred in mice pretreated with PBS liposomes. In contrast, mice pretreated with Cl2MDP liposomes showed significantly reduced lung C/EBP activation after LPS instillation (Figure 1D), suggesting that alveolar macrophages played a critical role in lung C/EBP activation induced by LPS.
Effects of C/EBPδ Deficiency on LPS-Induced Lung Injury
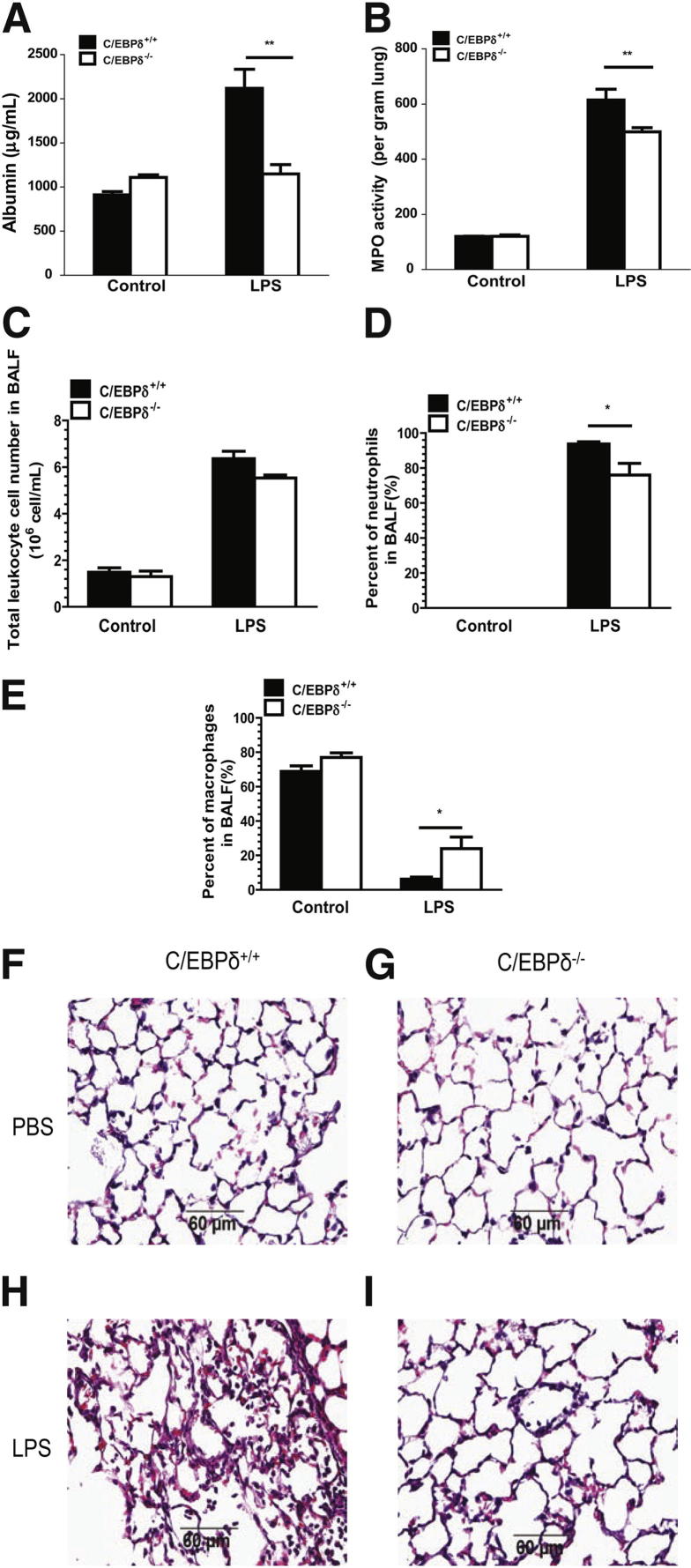
By using systems biology approaches, Litvak et al36 recently reported that C/EBPδ played a critical role in a regulatory circuit that discriminated between transient and persistent Toll-like receptor (TLR) 4–induced signals. Nevertheless, the role of C/EBPδ in acute lung injury remained largely unknown. To examine whether C/EBPδ was involved in lung injury after LPS deposition, we first measured leakage of mouse albumin into the lungs of wild-type and C/EBPδ-deficient mice. Endogenous mouse albumin levels in BAL fluids were determined by ELISA.37 As shown in Figure 2A, albumin levels in BAL fluids were significantly increased in WT mice that received intratracheal administration of LPS. However, the BAL fluid albumin levels in C/EBPδ-deficient mice remained at nearly background levels (Figure 2A). We also examined MPO content to evaluate neutrophil accumulation in the lung. As shown in Figure 2B, C/EBPδ deficiency alone did not cause a change in MPO activity in the lung. LPS administration led to increased MPO activity in WT mice, whereas MPO content in LPS-treated C/EBPδ-deficient mice was modestly, but significantly (P < 0.05), reduced relative to WT mice (Figure 2B).
Figure 2.
Effects of C/EBPδ deficiency on LPS-induced acute lung injury. At 18 hours after LPS deposition, BAL fluids (BALFs) and whole lungs were harvested. A: Mouse albumin content in BAL fluids was determined using ELISA as an index of lung microvascular permeability. B: Changes in lung MPO activity were measured as an index of lung neutrophil accumulation. C–E: Leukocytes were quantitated in BAL fluids. The total numbers of leukocytes (C), neutrophils (D), and macrophages (E) are shown. The data are expressed as mean ± SEM; n = 3 for control-treated groups, and n = 5 for injured groups. F–I: Sections from lungs harvested 18 hours after PBS or LPS deposition in WT and C/EBP-deficient mice were stained with H&E. Representative sections for each condition are shown. Lung sections shown include the following: C/EBPδ+/+ + PBS (F), C/EBPδ−/− + PBS (G), C/EBPδ+/+ + LPS (H), and C/EBPδ−/− + LPS (I). Original magnification, ×200. *P < 0.05, **P < 0.01.
We next evaluated the effects of C/EBPδ deficiency on leukocyte content in BAL fluids from LPS-injured lung. As shown in Figure 2, C and D, injured lungs from WT and C/EBPδ-deficient mice contained mostly neutrophils (93.8% and 76%, respectively), followed by macrophages (6% and 24%, respectively). Other cell types were present in insignificant numbers. More important, C/EBPδ-deficient mice displayed fewer neutrophils (P < 0.05, Figure 2D) compared with WT mice. Moreover, in the lung injury groups, C/EBPδ-deficient mice displayed a significant increase in BAL macrophages relative to WT mice (P < 0.05) (Figure 2E).
We further evaluated whether C/EBPδ-deficient mice exhibited reduced lung injury, as judged by histological criteria. As shown in Figure 2, F and G, WT and C/EBPδ-deficient mice receiving PBS alone exhibited normal lung architecture. As expected, LPS instillation led to a strong lung hemorrhage and inflammatory cell influx in WT mouse lung (Figure 2H). In contrast, lungs from C/EBPδ-deficient mice showed significantly decreased neutrophil accumulation and negligible intra-alveolar hemorrhage after LPS instillation (Figure 2I). Collectively, the data of Figures 2, 3, and 4 showed that C/EBPδ plays a critical role in LPS-induced acute lung injury.
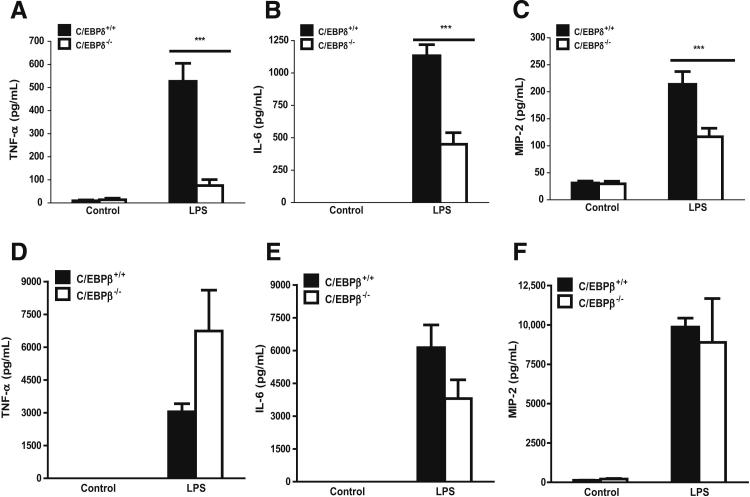
Figure 3.
Effects of C/EBPδ and C/EBPβ deficiency on LPS-induced production of cytokines and chemokines in BAL fluids. BAL fluids were harvested 18 hours after onset of LPS-induced lung injury in WT, C/EBPδ-deficient mice (A–C) and C/EBPβ-deficient mice (D–F). ELISAs were performed to determine the levels of TNF-α (A and D), IL-6 (B and E), and MIP-2 (C and F) in BAL fluids. Results are the mean ± SEM for three (control group) or five (LPS-challenged group) mice. ***P < 0.001.
Figure 4.
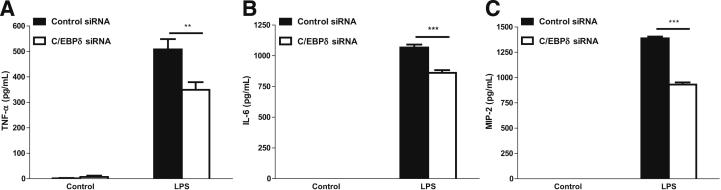
Effects of C/EBPδ knockdown on LPS-induced production of cytokines and chemokines from alveolar macrophage cells. MH-S cells were transiently transfected with 40 nmol/L control siRNA or C/EBPδ siRNA. At 12 hours after transfection, the cells were incubated with 100 ng/mL LPS for 6 hours. Supernatants were harvested, and an ELISA was performed to determine the expressions of TNF-α (A), IL-6 (B), and MIP-2 (C). The data are expressed as mean ± SEM (n = 9 to 10). **P < 0.01, ***P < 0.001.
Production of Cytokines and Chemokines in Lung after LPS Deposition in WT, C/EBPδ-Deficient Mice
We examined whether the decreases in BAL neutrophils and reduced lung injury in C/EBPδ-deficient mice were associated with altered levels of inflammatory mediators in lung. The contents of TNF-α, IL-6, and MIP-2 in BAL fluids were determined. As expected, WT mice subjected to LPS deposition showed dramatically increased levels of TNF-α, IL-6, and MIP-2 compared with controls (Figure 3, A–C). In the lung injury groups, the levels of these inflammatory mediators in C/EBPδ-deficient mice were significantly decreased by 85% (TNF-α, P < 0.01) (Figure 3A), 60% (IL-6, P < 0.001) (Figure 3B), and 45% (MIP-2, P < 0.001) (Figure 3C), relative to WT animals.
C/EBPβ Deficiency Does Not Affect LPS-Induced Lung Injury
Because C/EBPβ DNA-binding activity was also significantly induced by LPS in the lung (Figure 1B), we evaluated whether C/EBPβ was involved in LPS-induced lung inflammation. WT and C/EBPβ-deficient mice receiving PBS alone exhibited normal lung architecture. In contrast to C/EBPδ-deficient mice, LPS-injured lungs from C/EBPβ-deficient mice showed neither significantly decreased neutrophil accumulation nor reduced intra-alveolar hemorrhage when compared with WT injured mice (see Supplemental Figure S1). Consistently, BAL fluids from LPS-injured lungs of C/EBPβ-deficient mice showed no significant decrease in IL-6 and MIP-2 levels when compared with WT mice (Figure 3, E and F). Interestingly, C/EBPβ deficiency led to an increase in TNF-α production in BAL fluids of injured lung when compared with WT mice (Figure 3D), suggesting that C/EBPβ might have an inhibitory effect on TNF-α expression in this setting. Together, our data suggested that, among the C/EBP proteins, C/EBPδ specifically mediated LPS-induced inflammation and injury in the lung.
Effects of C/EBPδ Knockdown on Cytokine and Chemokine Protein Production in LPS-Stimulated Alveolar Macrophages
C/EBPδ activation might play a critical role in LPS-induced inflammatory response in the alveolar macrophages. To further address this possibility, we determined the effects of C/EBPδ knockdown on LPS-stimulated production of inflammatory mediators in the alveolar macrophage cell line, MH-S. C/EBPδ expression was efficiently down-regulated by siRNA specific for C/EBPδ in MH-S cells, but the same siRNA had no inhibitory effect on C/EBPβ expression (data not shown). Notably, there was a significant reduction in LPS-induced cytokine and chemokine production after C/EBPδ ablation in MH-S cells: TNF-α decreased by 62%, IL-6 decreased by 77%, and IL-6 and MIP-2 decreased by 48% (Figure 4). These results suggested that C/EBPδ in alveolar macrophages significantly contributed to the LPS-elicited inflammatory responses in the lung.
Effects of C/EBPδ Overexpression on LPS-Induced TNF-α and IL-6 Expression in Alveolar Macrophages
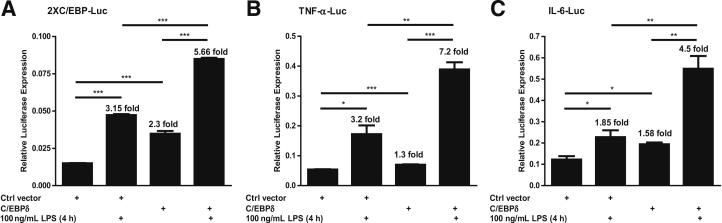
We next evaluated the LPS-induced C/EBP activation in transient transfection assays (Promega) using 2XC/EBP-Luc, a promoter-reporter that contains two copies of a C/EBP binding site, and an expression vector for C/EBPδ. These transfections were performed in MH-S cells, with and without LPS treatment. Consistent with the results from EMSA (Figure 1), LPS stimulation alone (without transfection of a C/EBPδ expression vector) induced a 3.15-fold increase in luciferase activity compared with untreated cells (Figure 5A). The C/EBPδ vector alone, in the absence of LPS stimulation, also elevated transcription from the reporter (2.3-fold), whereas LPS treatment of C/EBPδ-transfected cells induced luciferase expression 5.66-fold over the reporter alone. Thus, LPS or ectopic C/EBPδ expression could stimulate a C/EBP-dependent promoter in alveolar macrophages.
Figure 5.
Effects of C/EBPδ expression on LPS-induced activity of the C/EBP-luciferase (A), TNF-α–luciferase (B), and IL-6–luciferase (C) in alveolar macrophage cells. MH-S cells were transiently transfected with a total of 0.5 μg of the indicated DNAs. At 24 hours after transfection, the cells were challenged with the indicated stimulus for 4 hours. Cell lysates were used for luciferase activity assays. Luminometer values were normalized for expression from a cotransfected thymidine kinase reporter gene. The data are expressed as mean ± SEM of three experiments (n = 3). Ctrl, control. *P < 0.05, **P < 0.01, and ***P < 0.001.
We also assessed the ability of C/EBPδ to regulate LPS-induced TNF-α and IL-6 expression in transient transfections using TNF-α-Luc and IL-6-Luc, two promoter-reporters that contain C/EBP binding sites, and a C/EBPδ expression vector. These transfections were performed with and without LPS treatment. LPS alone significantly increased luciferase activity (3.2-fold for TNF-α and 1.85-fold for IL-6) compared with controls (Figure 5, B and C). Moreover, C/EBPδ overexpression, in the absence of LPS stimulation, resulted in 1.3-fold (TNF-α) and 1.58-fold (IL-6) induction of luciferase activity. Importantly, LPS treatment of C/EBPδ transfectants induced TNF-α and IL-6 luciferase expression by 7.2-fold and 4.5-fold, respectively, over the control values. Because the effects of LPS and C/EBPδ together on these promoters were more than additive, LPS might stimulate C/EBPδ activity and its expression in alveolar macrophages.
Extracellular Signal–Regulated Kinase 1/2 and p38 MAPK Are Involved in LPS-Induced C/EBPδ Expression and Cytokine/Chemokine Production in Alveolar Macrophages
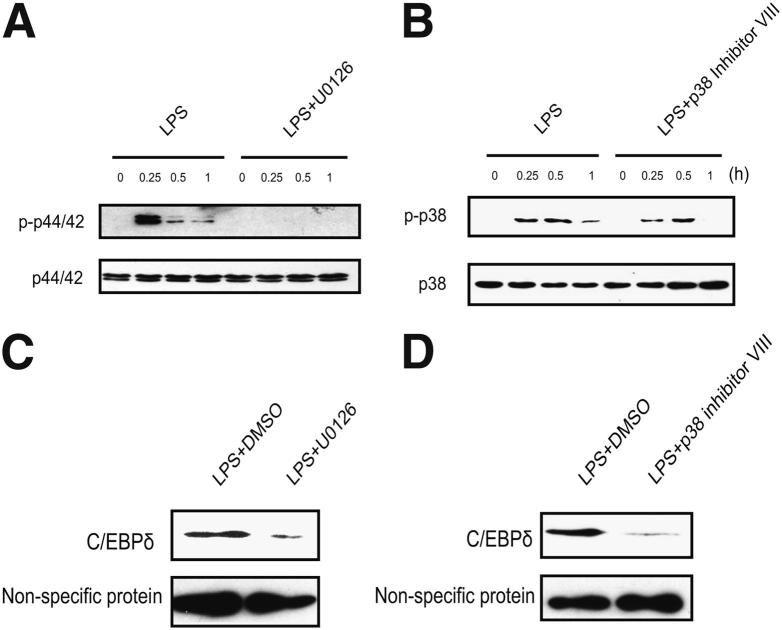
We evaluated the MAPK pathways in LPS-stimulated MH-S cells. As shown in Figure 6, A and B, LPS treatment led to the phosphorylation of both p44/42 and p38 MAPK in a time-dependent manner. We determined the influence of the MAPK phosphorylation on nucleus C/EBPδ expression by using specific pharmacological inhibitors for p44/42 and p38 MAPK. Phosphorylation of p44/42 and p38 MAPK was significantly inhibited by U0126 and p38 MAPK inhibitor VIII, respectively (Figure 6, A and B). Furthermore, U0126 and p38 MAPK inhibitor VIII markedly suppressed the nuclear C/EBPδ level induced by LPS (Figure 6, C and D).
Figure 6.
Both p44/p421 and p38 MAPK are involved in LPS-induced C/EBPδ expression in alveolar macrophages. A and B: MH-S cells were treated with 100 ng/mL LPS in the presence or absence of p44/42 MAPK inhibitor, U0126 (10 μmol/L) (A), or p38 MAPK inhibitor VII (10 μmol/L) (B) for the indicated time periods. Total proteins were subjected to Western blot analysis by using rabbit anti–phospho-p44/42 (p-p44/42) antibody, rabbit anti-p44/42 antibody, rabbit anti–phospho-p38 antibody (p-p38), and rabbit anti-p38 antibody. C and D: MH-S cells were treated with 100 ng/mL LPS, LPS plus p44/42 inhibitor, U0126 (10 μmol/L), or LPS plus p38 MAPK inhibitor VII (10 μmol/L) for 4 hours. The nuclear proteins were extracted to perform Western blot analysis. Non-specific bands were shown at the bottom as the loading control. DMSO, dimethyl sulfoxide.
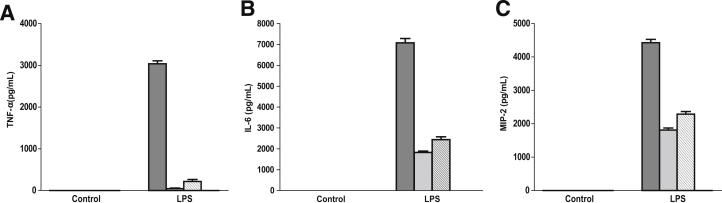
We further determined the effect of U0126 and p38 MAPK inhibitor VIII on TNF-α, IL-6, and MIP-2 production in MH-S cells. U0126 and p38 MAPK inhibitor VIII significantly inhibited LPS-stimulated TNF-α, IL-6, and MIP-2 production (Figure 7, A–C). Furthermore, when MH-S cells were treated with p44/42 and p38 inhibitors together, the production of TNF-α, IL-6, and MIP-2 was at undetectable levels (Figure 7, A–C), suggesting that p44/42 and p38 MAPK acted in concert to mediate the LPS signal in alveolar macrophages.
Figure 7.
Both p44/p42 and p38 MAPK are involved in LPS-induced cytokine/chemokine production in alveolar macrophages. A–C: MH-S cells were treated with 100 ng/mL LPS in the presence or absence of p44/42 MAPK inhibitor, U0126 (10 μmol/L), p38 MAPK inhibitor VII (10 μmol/L), or both for 8 hours. Supernatants were subjected to ELISA analysis for TNF-α (A), IL-6 (B), and MIP-2 (C) production. Data are presented as means ± SEM (n = 4 to 6). DMSO, dimethyl sulfoxide.
Discussion
LPS is the major outer surface membrane component present in Gram-negative bacteria and is strong stimulator of innate immunity.38 LPS has been administered to humans via the intratracheal route, which resulted in an early phase reaction characterized by increases in BAL fluid neutrophils, albumin, and pro-inflammatory mediators (eg, TNF-α, IL-6, and MIPs) and a later phase reaction (24 to 48 hours after instillation) characterized by normalization of the BAL fluid cytokine concentrations.39 In vivo animal studies of LPS-induced lung injury are widely used as experimental approaches to investigate the mechanisms of acute lung inflammatory injury. This animal model displays key features of microvascular injury, as seen in patients with acute respiratory distress syndrome.40 Furthermore, LPS is a potent activator of the innate immune responses via TLR4 pathways, and the use of LPS provides information about the effects of host inflammatory responses, which occur during bacterial infections.41 Despite its wide use, the underlying pathophysiological mechanisms for the LPS model have not been comprehensively described. In the current study, we show that C/EBPδ deficiency significantly inhibits LPS-induced acute lung injury, as defined by reduced albumin leakage into lung, MPO content, and histological change in the lung, suggesting that C/EBPδ is a critical mediator of LPS-induced acute lung injury and inflammatory response in alveolar macrophages. Importantly, we show that the role of C/EBPδ in acute lung injury is specific, because C/EBPβ, a closely related family member whose expression is also increased in the lung by LPS stimulation (Figure 1), does not affect acute lung inflammation in vivo.
Both C/EBPβ and C/EBPδ are involved in the regulation of the acute phase and inflammatory responses.11,12 The roles of C/EBPβ and C/EBPδ in regulating inflammation have also been studied using knockout mice. LPS stimulation of peritoneal macrophages from C/EBPβ-deficient mice led to normal induction of several inflammatory cytokines, including IL-6 and TNF-α.42–45 Similarly, C/EBPδ-deficient macrophages did not show significant defects in IL-6 and TNF-α production in response to several TLR ligands.46 In contrast, the absence of both C/EBPβ and C/EBPδ results in a significant decrease in the TLR ligand–induced production of IL-6 and TNF-α.46 Thus, either C/EBPβ or C/EBPδ is sufficient to support the induction of these pro-inflammatory cytokines. Indeed, C/EBPδ has bound to the same DNA sequence as C/EBPβ and acted as a homodimer or heterodimer with C/EBPβ.47 Although C/EBP protein function has been studied extensively in a variety of tissues, such as liver and adipose, much less is known about the role of these transcription factors in modulating acute inflammatory response and injury in the lung. Both C/EBPβ and C/EBPδ are strongly induced in LPS-injured lung (Figure 1). Furthermore, we show that C/EBPδ, but not C/EBPβ, plays an important regulatory role in LPS-induced lung inflammatory responses and injury. These data suggest that, in the lung, C/EBPβ and C/EBPδ may have differential roles in regulating the inflammatory responses to stimuli. Indeed, our recent findings implicate C/EBPβ as a critical regulator of IgG immune complex–induced inflammatory responses and injury in the lung, whereas C/EBPδ deficiency had no effect on IgG immune complex–induced lung injury (Yan C., unpublished data). These data, together with the current findings, suggest that C/EBPβ and C/EBPδ differentially regulate FcγR- and TLR-mediated inflammatory responses in the lung.
Molecular regulation of lung inflammatory response to LPS is complex and involves a variety of relatively ubiquitous transcription factors.9 NF-κB played a key role in the transcriptional up-regulation of many inflammation-associated genes induced by LPS. In the lung, the NF-κB pathway in airway epithelial cells may be critical for the generation of pulmonary inflammation and injury in response to local and systemic LPS stimuli.48 NF-κB interacts with many heterologous transcription factors, and these interactions can select for specific NF-κB subunits and, thereby, lead to greater transcriptional selectivity.9 For example, we have previously shown that the C/EBP bZIP domain can mediate LPS induction of IL-6 and MCP-1 in B cells.49 Furthermore, the ability of the C/EBPβ bZIP region to activate the IL-6 promoter is completely dependent on an intact NF-κB binding site, supporting a model in which the bZIP protein primarily functions to augment the activity of NF-κB.49 This is consistent with a study by Stein and Baldwin,50 showing a direct physical association of the bZIP region of C/EBP with the Rel homology domain of NF-κB. Therefore, in the future, it will be interesting to investigate the molecular mechanisms by which NF-κB subunits act in concert with members of the C/EBP family to regulate inflammatory gene expression during acute lung injury.
Several possible mechanisms may explain the impact of the C/EBPδ pathway on neutrophil recruitment into the alveolar compartment and lung interstitium (Figures 2B and 3A) during LPS-induced lung injury. One mechanism is that of the modulation of inflammatory cytokines. Another possible mechanism of C/EBPδ involvement in neutrophil infiltration is its regulation of chemokine production. Our current finding that C/EBPδ deficiency led to a significant decrease in the contents of TNF-α, IL-6, and MIP-2 (Figure 5) supports both hypotheses. Conversely, C/EBPβ deficiency has no significant effect on the production of BAL cytokines and chemokines (Figure 7). This observation further implicates the critical and specific role of C/EBPδ in LPS-induced lung inflammation.
Activated alveolar macrophages constitute a critical modulator of the lung inflammatory response through the production of various inflammation-associated mediators. The interaction of the lipid A moiety of LPS with alveolar macrophages in the lung appears to be especially important because subsequent cellular activation results in the release of inflammatory mediators and phenotypic changes.51 By using dichloromethylene diphosphonate (clodronate)–mediated macrophage depletion, Koay et al35 demonstrated that alveolar macrophages are a critical component of the innate immune response in the lungs that regulate the development of neutrophilic lung inflammation, lung NF-κB activation, and production of cytokines and chemokines after administration of LPS. In the current study, we show that siRNA-mediated knockdown of C/EBPδ led to a significant reduction in TNF-α, IL-6, and MIP-2 production in alveolar macrophage cells on LPS stimulation (Figure 6). These results suggest that C/EBPδ in alveolar macrophages plays an important regulatory role for the LPS-induced production of inflammatory mediators. It is possible that other lung cells, such as alveolar epithelial cells, might also contribute to C/EBPδ-mediated cytokine production in the LPS-injured lung. Increasing studies suggest that alveolar type II epithelial cells play a critical role in regulating local lung inflammatory response. For example, previous studies52–55 have suggested that alveolar type II epithelial cells may play special roles in counteracting microbes by releasing cytokines and chemokines that recruit both dendritic cells and alveolar macrophages to the site of infection. By using inducible transgenic mice that express an activator or dominant inhibitor of the NF-κB pathway in airway epithelial cells, Cheng et al48 recently showed that activation of NF-κB in airway epithelial cells is sufficient for generating acute lung injury, and inhibition of NF-κB activation in airway epithelium abrogates lung inflammation and injury induced by LPS. Future studies using lung epithelial cell–specific C/EBPδ knockout mice may allow us to determine whether C/EBPδ activation in airway epithelial cells is involved in the parenchymal lung inflammation and injury via production of mediators, such as TNF-α and IL-6.
Acknowledgments
We thank Karen Saylor and Nancy Martin for assistance with mouse breeding and genotyping.
Footnotes
Supported by NIH grants 5R01HL092905-04 and 3R01HL092905-02S1 (H.G.), NIH grant ES014690, Flight Attendant Medical Research Institute grant 103007 (M.W.), and the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research (P.F.J.).
Supplemental Data
H&E-stained paraffin-embedded lung sections from WT C/EBPβ-deficient mice. At 18 hours after LPS deposition in the lungs of WT and C/EBPβ-deficient mice, whole lungs were harvested. Paraffin-embedded lung sections were stained with H&E. Representative sections for each condition are shown. Lung sections shown include the following: C/EBPβ+/+ + PBS (A), C/EBPβ−/− + PBS (B), C/EBPβ+/+ + LPS (C), and C/EBPβ−/− + LPS (D). Original magnification, ×200.
References
- 1.Tsushima K., King L.S., Aggarwal N.R., De Gorordo A., D’Alessio F.R., Kubo K. Acute lung injury review. Intern Med. 2009;48:621–630. doi: 10.2169/internalmedicine.48.1741. [DOI] [PubMed] [Google Scholar]
- 2.Chopra M., Reuben J.S., Sharma A.C. Acute lung injury: apoptosis and signaling mechanisms. Exp Biol Med (Maywood) 2009;234:361–371. doi: 10.3181/0811-MR-318. [DOI] [PubMed] [Google Scholar]
- 3.Erickson S.E., Martin G.S., Davis J.L., Matthay M.A., Eisner M.D. Recent trends in acute lung injury mortality: 1996-2005. Crit Care Med. 2009;37:1574–1579. doi: 10.1097/CCM.0b013e31819fefdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rubenfeld G.D., Caldwell E., Peabody E., Weaver J., Martin D.P., Neff M., Stern E.J., Hudson L.D. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353:1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 5.Gao H., Guo R.F., Speyer C.L., Reuben J., Neff T.A., Hoesel L.M., Riedemann N.C., McClintock S.D., Sarma J.V., Van Rooijen N., Zetoune F.S., Ward P.A. Stat3 activation in acute lung injury. J Immunol. 2004;172:7703–7712. doi: 10.4049/jimmunol.172.12.7703. [DOI] [PubMed] [Google Scholar]
- 6.Guo R.F., Lentsch A.B., Sarma J.V., Sun L., Riedemann N.C., McClintock S.D., McGuire S.R., Van Rooijen N., Ward P.A. Activator protein-1 activation in acute lung injury. Am J Pathol. 2002;161:275–282. doi: 10.1016/S0002-9440(10)64179-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lentsch A.B., Czermak B.J., Bless N.M., Ward P.A. NF-kappaB activation during IgG immune complex-induced lung injury: requirements for TNF-alpha and IL-1beta but not complement. Am J Pathol. 1998;152:1327–1336. [PMC free article] [PubMed] [Google Scholar]
- 8.Lentsch A.B., Shanley T.P., Sarma V., Ward P.A. In vivo suppression of NF-kappa B and preservation of I kappa B alpha by interleukin-10 and interleukin-13. J Clin Invest. 1997;100:2443–2448. doi: 10.1172/JCI119786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fan J., Ye R.D., Malik A.B. Transcriptional mechanisms of acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2001;281:L1037–L1050. doi: 10.1152/ajplung.2001.281.5.L1037. [DOI] [PubMed] [Google Scholar]
- 10.Lekstrom-Himes J., Xanthopoulos K.G. Biological role of the CCAAT/enhancer-binding protein family of transcription factors. J Biol Chem. 1998;273:28545–28548. doi: 10.1074/jbc.273.44.28545. [DOI] [PubMed] [Google Scholar]
- 11.Poli V. The role of C/EBP isoforms in the control of inflammatory and native immunity functions. J Biol Chem. 1998;273:29279–29282. doi: 10.1074/jbc.273.45.29279. [DOI] [PubMed] [Google Scholar]
- 12.Tsukada J., Yoshida Y., Kominato Y., Auron P.E. The CCAAT/enhancer (C/EBP) family of basic-leucine zipper (bZIP) transcription factors is a multifaceted highly-regulated system for gene regulation. Cytokine. 2011;54:6–19. doi: 10.1016/j.cyto.2010.12.019. [DOI] [PubMed] [Google Scholar]
- 13.Cappelletti M., Alonzi T., Fattori E., Libert C., Poli V. C/EBPbeta is required for the late phases of acute phase genes induction in the liver and for tumour necrosis factor-alpha, but not interleukin-6, regulation. Cell Death Differ. 1996;3:29–35. [PubMed] [Google Scholar]
- 14.Gorgoni B., Maritano D., Marthyn P., Righi M., Poli V. C/EBP beta gene inactivation causes both impaired and enhanced gene expression and inverse regulation of IL-12 p40 and p35 mRNAs in macrophages. J Immunol. 2002;168:4055–4062. doi: 10.4049/jimmunol.168.8.4055. [DOI] [PubMed] [Google Scholar]
- 15.Kozawa O., Otsuka T., Uematsu T. Leukemia inhibitory factor enhances bFGF-induced IL-6 synthesis in osteoblasts: involvement of JAK2/STAT3. Cell Signal. 2002;14:311–315. doi: 10.1016/s0898-6568(01)00248-0. [DOI] [PubMed] [Google Scholar]
- 16.Adams T.E., Hansen J.A., Starr R., Nicola N.A., Hilton D.J., Billestrup N. Growth hormone preferentially induces the rapid, transient expression of SOCS-3, a novel inhibitor of cytokine receptor signaling. J Biol Chem. 1998;273:1285–1287. doi: 10.1074/jbc.273.3.1285. [DOI] [PubMed] [Google Scholar]
- 17.Caivano M., Gorgoni B., Cohen P., Poli V. The induction of cyclooxygenase-2 mRNA in macrophages is biphasic and requires both CCAAT enhancer-binding protein beta (C/EBP beta) and C/EBP delta transcription factors. J Biol Chem. 2001;276:48693–48701. doi: 10.1074/jbc.M108282200. [DOI] [PubMed] [Google Scholar]
- 18.Albina J.E., Mahoney E.J., Daley J.M., Wesche D.E., Morris S.M., Jr., Reichner J.S. Macrophage arginase regulation by CCAAT/enhancer-binding protein beta. Shock. 2005;23:168–172. doi: 10.1097/01.shk.0000148054.74268.e2. [DOI] [PubMed] [Google Scholar]
- 19.Howden B.P., Ward P.B., Johnson P.D., Charles P.G., Grayson M.L. Low-level vancomycin resistance in Staphylococcus aureus: an Australian perspective. Eur J Clin Microbiol Infect Dis. 2005;24:100–108. doi: 10.1007/s10096-004-1261-y. [DOI] [PubMed] [Google Scholar]
- 20.Fong Y., Shen K.H., Chen L.J., Cheng J.T. Changes of CCAAT enhancer-binding proteins (CEBPs) in the lung of streptozotocin-induced diabetic rats. Horm Metab Res. 2011;43:261–267. doi: 10.1055/s-0030-1270452. [DOI] [PubMed] [Google Scholar]
- 21.Browder W., Ha T., Chuanfu L., Kalbfleisch J.H., Ferguson D.A., Jr., Williams D.L. Early activation of pulmonary nuclear factor kappaB and nuclear factor interleukin-6 in polymicrobial sepsis. J Trauma. 1999;46:590–596. doi: 10.1097/00005373-199904000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Miglino N., Roth M., Lardinois D., Sadowski C., Tamm M., Borger P. Cigarette smoke inhibits lung fibroblast proliferation by translational mechanisms. Eur Respir J. 2012;39:705–711. doi: 10.1183/09031936.00174310. [DOI] [PubMed] [Google Scholar]
- 23.Tanaka T., Yoshida N., Kishimoto T., Akira S. Defective adipocyte differentiation in mice lacking the C/EBPbeta and/or C/EBPdelta gene. EMBO J. 1997;16:7432–7443. doi: 10.1093/emboj/16.24.7432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burgess-Beusse B.L., Darlington G.J. C/EBPalpha is critical for the neonatal acute-phase response to inflammation. Mol Cell Biol. 1998;18:7269–7277. doi: 10.1128/mcb.18.12.7269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alam T., An M.R., Papaconstantinou J. Differential expression of three C/EBP isoforms in multiple tissues during the acute phase response. J Biol Chem. 1992;267:5021–5024. [PubMed] [Google Scholar]
- 26.Slofstra S.H., Groot A.P., Obdeijn M.H., Reitsma P.H., ten Cate H., Spek C.A. Gene expression profiling identifies C/EBPdelta as a candidate regulator of endotoxin-induced disseminated intravascular coagulation. Am J Respir Crit Care Med. 2007;176:602–609. doi: 10.1164/rccm.200609-1250OC. [DOI] [PubMed] [Google Scholar]
- 27.Didon L., Barton J.L., Roos A.B., Gaschler G.J., Bauer C.M., Berg T., Stampfli M.R., Nord M. Lung epithelial CCAAT/enhancer-binding protein-β is necessary for the integrity of inflammatory responses to cigarette smoke. Am J Respir Crit Care Med. 2011;184:233–242. doi: 10.1164/rccm.201007-1113OC. [DOI] [PubMed] [Google Scholar]
- 28.Hu B., Ullenbruch M.R., Jin H., Gharaee-Kermani M., Phan S.H. An essential role for CCAAT/enhancer binding protein beta in bleomycin-induced pulmonary fibrosis. J Pathol. 2007;211:455–462. doi: 10.1002/path.2119. [DOI] [PubMed] [Google Scholar]
- 29.Sterneck E., Tessarollo L., Johnson P.F. An essential role for C/EBPbeta in female reproduction. Genes Dev. 1997;11:2153–2162. doi: 10.1101/gad.11.17.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sterneck E., Paylor R., Jackson-Lewis V., Libbey M., Przedborski S., Tessarollo L., Crawley J.N., Johnson P.F. Selectively enhanced contextual fear conditioning in mice lacking the transcriptional regulator CCAAT/enhancer binding protein delta. Proc Natl Acad Sci U S A. 1998;95:10908–10913. doi: 10.1073/pnas.95.18.10908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Rooijen N., Sanders A. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J Immunol Methods. 1994;174:83–93. doi: 10.1016/0022-1759(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 32.Gao H., Parkin S., Johnson P.F., Schwartz R.C. C/EBP gamma has a stimulatory role on the IL-6 and IL-8 promoters. J Biol Chem. 2002;277:38827–38837. doi: 10.1074/jbc.M206224200. [DOI] [PubMed] [Google Scholar]
- 33.Hu H.M., Baer M., Williams S.C., Johnson P.F., Schwartz R.C. Redundancy of C/EBP alpha, -beta, and -delta in supporting the lipopolysaccharide-induced transcription of IL-6 and monocyte chemoattractant protein-1. J Immunol. 1998;160:2334–2342. [PubMed] [Google Scholar]
- 34.Descombes P., Schibler U. A liver-enriched transcriptional activator protein, LAP, and a transcriptional inhibitory protein LIP, are translated from the same mRNA. Cell. 1991;67:569–579. doi: 10.1016/0092-8674(91)90531-3. [DOI] [PubMed] [Google Scholar]
- 35.Koay M.A., Gao X., Washington M.K., Parman K.S., Sadikot R.T., Blackwell T.S., Christman J.W. Macrophages are necessary for maximal nuclear factor-kappa B activation in response to endotoxin. Am J Respir Cell Mol Biol. 2002;26:572–578. doi: 10.1165/ajrcmb.26.5.4748. [DOI] [PubMed] [Google Scholar]
- 36.Litvak V., Ramsey S.A., Rust A.G., Zak D.E., Kennedy K.A., Lampano A.E., Nykter M., Shmulevich I., Aderem A. Function of C/EBPdelta in a regulatory circuit that discriminates between transient and persistent TLR4-induced signals. Nat Immunol. 2009;10:437–443. doi: 10.1038/ni.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun L., Guo R.F., Gao H., Sarma J.V., Zetoune F.S., Ward P.A. Attenuation of IgG immune complex-induced acute lung injury by silencing C5aR in lung epithelial cells. FASEB J. 2009;23:3808–3818. doi: 10.1096/fj.09-133694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rietschel E.T., Kirikae T., Schade F.U., Mamat U., Schmidt G., Loppnow H., Ulmer A.J., Zähringer U., Seydel U., Di Padova F., Schreier M., Brade H. Bacterial endotoxin: molecular relationships of structure to activity and function. FASEB J. 1994;8:217–225. doi: 10.1096/fasebj.8.2.8119492. [DOI] [PubMed] [Google Scholar]
- 39.O’Grady N.P., Preas H.L., Pugin J., Fiuza C., Tropea M., Reda D., Banks S.M., Suffredini A.F. Local inflammatory responses following bronchial endotoxin instillation in humans. Am J Respir Crit Care Med. 2001;163:1591–1598. doi: 10.1164/ajrccm.163.7.2009111. [DOI] [PubMed] [Google Scholar]
- 40.Kabir K., Gelinas J.P., Chen M., Chen D., Zhang D., Luo X., Yang J.H., Carter D., Rabinovici R. Characterization of a murine model of endotoxin-induced acute lung injury. Shock. 2002;17:300–303. doi: 10.1097/00024382-200204000-00010. [DOI] [PubMed] [Google Scholar]
- 41.Matute-Bello G., Frevert C.W., Martin T.R. Animal models of acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2008;295:L379–L399. doi: 10.1152/ajplung.00010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tanaka T., Akira S., Yoshida K., Umemoto M., Yoneda Y., Shirafuji N., Fujiwara H., Suematsu S., Yoshida N., Kishimoto T. Targeted disruption of the NF-IL6 gene discloses its essential role in bacteria killing and tumor cytotoxicity by macrophages. Cell. 1995;80:353–361. doi: 10.1016/0092-8674(95)90418-2. [DOI] [PubMed] [Google Scholar]
- 43.Uematsu S., Kaisho T., Tanaka T., Matsumoto M., Yamakami M., Omori H., Yamamoto M., Yoshimori T., Akira S. The C/EBP beta isoform 34-kDa LAP is responsible for NF-IL-6-mediated gene induction in activated macrophages, but is not essential for intracellular bacteria killing. J Immunol. 2007;179:5378–5386. doi: 10.4049/jimmunol.179.8.5378. [DOI] [PubMed] [Google Scholar]
- 44.Matsumoto M., Tanaka T., Kaisho T., Sanjo H., Copeland N.G., Gilbert D.J., Jenkins N.A., Akira S. A novel LPS-inducible C-type lectin is a transcriptional target of NF-IL6 in macrophages. J Immunol. 1999;163:5039–5048. [PubMed] [Google Scholar]
- 45.Uematsu S., Matsumoto M., Takeda K., Akira S. Lipopolysaccharide-dependent prostaglandin E(2) production is regulated by the glutathione-dependent prostaglandin E(2) synthase gene induced by the Toll-like receptor 4/MyD88/NF-IL6 pathway. J Immunol. 2002;168:5811–5816. doi: 10.4049/jimmunol.168.11.5811. [DOI] [PubMed] [Google Scholar]
- 46.Lu Y.C., Kim I., Lye E., Shen F., Suzuki N., Suzuki S., Gerondakis S., Akira S., Gaffen S.L., Yeh W.C., Ohashi P.S. Differential role for c-Rel and C/EBPbeta/delta in TLR-mediated induction of proinflammatory cytokines. J Immunol. 2009;182:7212–7221. doi: 10.4049/jimmunol.0802971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kinoshita S., Akira S., Kishimoto T. A member of the C/EBP family, NF-IL6 beta, forms a heterodimer and transcriptionally synergizes with NF-IL6. Proc Natl Acad Sci U S A. 1992;89:1473–1476. doi: 10.1073/pnas.89.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cheng D.S., Han W., Chen S.M., Sherrill T.P., Chont M., Park G.Y., Sheller J.R., Polosukhin V.V., Christman J.W., Yull F.E., Blackwell T.S. Airway epithelium controls lung inflammation and injury through the NF-kappa B pathway. J Immunol. 2007;178:6504–6513. doi: 10.4049/jimmunol.178.10.6504. [DOI] [PubMed] [Google Scholar]
- 49.Hu H.M., Tian Q., Baer M., Spooner C.J., Williams S.C., Johnson P.F., Schwartz R.C. The C/EBP bZIP domain can mediate lipopolysaccharide induction of the proinflammatory cytokines interleukin-6 and monocyte chemoattractant protein-1. J Biol Chem. 2000;275:16373–16381. doi: 10.1074/jbc.M910269199. [DOI] [PubMed] [Google Scholar]
- 50.Stein B., Baldwin A.S., Jr. Distinct mechanisms for regulation of the interleukin-8 gene involve synergism and cooperativity between C/EBP and NF-kappa B. Mol Cell Biol. 1993;13:7191–7198. doi: 10.1128/mcb.13.11.7191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hashimoto N., Kawabe T., Imaizumi K., Hara T., Okamoto M., Kojima K., Shimokata K., Hasegawa Y. CD40 plays a crucial role in lipopolysaccharide-induced acute lung injury. Am J Respir Cell Mol Biol. 2004;30:808–815. doi: 10.1165/rcmb.2003-0197OC. [DOI] [PubMed] [Google Scholar]
- 52.Kannan S., Huang H., Seeger D., Audet A., Chen Y., Huang C., Gao H., Li S., Wu M. Alveolar epithelial type II cells activate alveolar macrophages and mitigate P. aeruginosa infection. PLoS One. 2009;4:e4891. doi: 10.1371/journal.pone.0004891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vanderbilt J.N., Mager E.M., Allen L., Sawa T., Wiener-Kronish J., Gonzalez R., Dobbs L.G. CXC chemokines and their receptors are expressed in type II cells and upregulated following lung injury. Am J Respir Cell Mol Biol. 2003;29:661–668. doi: 10.1165/rcmb.2002-0227OC. [DOI] [PubMed] [Google Scholar]
- 54.Thorley A.J., Goldstraw P., Young A., Tetley T.D. Primary human alveolar type II epithelial cell CCL20 (macrophage inflammatory protein-3alpha)-induced dendritic cell migration. Am J Respir Cell Mol Biol. 2005;32:262–267. doi: 10.1165/rcmb.2004-0196OC. [DOI] [PubMed] [Google Scholar]
- 55.Sato K., Tomioka H., Shimizu T., Gonda T., Ota F., Sano C. Type II alveolar cells play roles in macrophage-mediated host innate resistance to pulmonary mycobacterial infections by producing proinflammatory cytokines. J Infect Dis. 2002;185:1139–1147. doi: 10.1086/340040. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
H&E-stained paraffin-embedded lung sections from WT C/EBPβ-deficient mice. At 18 hours after LPS deposition in the lungs of WT and C/EBPβ-deficient mice, whole lungs were harvested. Paraffin-embedded lung sections were stained with H&E. Representative sections for each condition are shown. Lung sections shown include the following: C/EBPβ+/+ + PBS (A), C/EBPβ−/− + PBS (B), C/EBPβ+/+ + LPS (C), and C/EBPβ−/− + LPS (D). Original magnification, ×200.