Abstract
OBJECTIVE
We previously demonstrated that short-term treatment with a standardized kudzu extract (NPI-031) reduced alcohol drinking by men and women in a natural setting. The present study was conducted in non treatment-seeking heavy drinkers to assess the safety and efficacy of four weeks of kudzu extract in an outpatient setting.
METHOD
This randomized between-subject, double-blind, placebo-controlled study involved two weeks of baseline, four weeks of treatment and two weeks of follow-up. Seventeen men (21–33 years) who reported drinking 27.6 ± 6.5 drinks/week with a diagnosis of alcohol abuse/dependence took either kudzu extract (250 mg isoflavones, t.i.d.) or matched placebo on a daily basis. They reported alcohol consumption and desire to use alcohol using a wrist actigraphy device; twice weekly laboratory visits were scheduled to monitor medication adherence and adverse events.
RESULTS
Medication adherence was excellent and there were no adverse events, changes in vital signs, blood chemistry, renal or liver function. There was no effect on alcohol craving, but kudzu extract significantly reduced the number of drinks consumed each week by 34–57%, reduced the number of heavy drinking days and significantly increased the percent of days abstinent and the number of consecutive days of abstinence.
CONCLUSIONS
A standardized formulation of kudzu extract produced minimal side effects, was well-tolerated and resulted in a modest reduction in alcohol consumption in young non treatment-seeking heavy drinkers. Additional studies using treatment-seeking alcohol-dependent persons will be necessary to determine the usefulness of this herbal preparation in reducing alcohol use in other populations.
1. Introduction
Fifty-one percent of adults over the age of 18 and 56% of those age 18–44, are current regular drinkers (Schiller et al., 2012). Thirty percent of current drinkers report drinking excessively (Naimi et al., 2003) and 92% of U.S. adults who drink excessively report binge drinking in the past 30 days (Town et al., 2006). Current use was defined as at least one drink in the past 30 days while binge use includes drinking five or more drinks per day.
Because prescription medications are not universally used or have a perceived modest effect (Krystal et al., 2001; Mark et al., 2003), providing heavy drinkers with any type of intervention that yields even a modest reduction in drinking is desirable. Furthermore, there is a need to develop efficacious medicines from natural products that have a low incidence of side effects or toxicity (Xu et al., 2005). The ultimate role that natural preparations play will be complementary and might be useful in treating drug withdrawal and possibly relapse (Lu et al., 2009).
One Chinese herbal medicine, XJL (NPI-028), has long been used to reduce the inebriation that results from alcohol consumption. NPI-028 contains the extracts of several plants including Puerariae lobata (kudzu) and Citrus reticulata, which have long been used to lower intoxication (Sun, 600). It is difficult to speculate on the mechanism of action of kudzu extract, but biochemical studies with the three major isoflavones, puerarin, daidzin and daidzein have concluded that they may reduce alcohol consumption by altering either mitochondrial aldehyde dehydrogenase (ALDH2) or monoamine oxidase-acetaldehyde pathways (Keung, 2002, 2003). Rooke et al., (2000) has suggested that puerarin may block biogenic amine metabolic pathways, resulting in an alteration in central reward pathways. In our most recent study (Penetar et al., 2011) we provided evidence that kudzu extract may alter blood ethanol levels after an acute drinking episode. We posited that the observed more rapid increase in blood alcohol levels may result in a faster entry of alcohol to the CNS and thus increase either the intensity or duration of the rewarding effects of the first drink resulting in a delay in the time to a subsequent drink. In fact, we reported that kudzu extract did just that and interrupted binge drinking (Lukas et al., 2005). Recently, Shen et al., (2012) reported that another flavonoid, dihydromyricetin, reduced alcohol consumption in rats, antagonized the acute effects of alcohol, counteracted withdrawal signs and the mechanism was postulated to be due to interactions with the benzodiazepine site on GABAA receptors and increased expression of the GABAA subunit in hippocampus. This profile differs somewhat from that of kudzu extract and may represent a novel herbal compound that may be a therapeutic candidate.
Extracts of the kudzu plant are best known for their ability to suppress alcohol intake or alter alcohol effects by laboratory animals (Heyman et al., 1996; Keung and Vallee, 1993b; Keung, 2003; Overstreet et al., 1996; Rezvani et al., 2003; Benlhabib et al., 2004). The degree of reduction is often as high as 50% and the effects appeared within one to two days of treatment. Puerarin, daidzin and daidzein are the active isoflavones in kudzu. Regardless of the actual mechanism of action, it is widely accepted that the isoflavones in kudzu are effective in reducing alcohol intake in a number of mammalian species.
We developed a standardized kudzu extract that preserved the ratio of the major isoflavones found in the raw root, except that the concentration of isoflavones was increased to 25%. We previously demonstrated that a 7-day treatment with this extract significantly reduced alcohol consumption by heavy drinkers in a natural setting laboratory (Lukas et al., 2005) and that this effect is not due to a kudzu-induced increase in alcohol’s intoxicating effects (Penetar et al., 2011). The present randomized, double-blind, placebo controlled study was designed to determine if four weeks of treatment with kudzu extract would reduce alcohol consumption in male non treatment-seeking heavy drinkers in an outpatient setting and to document the safety and side effect profile of this herbal preparation.
2. Methods
2.1 Participants
A total of 21 adult males (17 Caucasian, 1 African American, 2 Hispanic, 1 Middle Eastern; mean age 23.8 ± 3.46 years, range 21–33) were recruited through advertisements in local and college newspapers and flyers posted in the Boston area. Because one of the isoflavones in the kudzu plant (daidzein) has weak estrogenic activity in animal models (c.f., Amer et al., 2010) and one of the aims of this study was to extend the safety assessments of kudzu extract to a 4-week period, only male subjects were recruited; this restriction was recommended by our local IRB. Persons who responded to these advertisements were given a brief telephone screen, and invited to the laboratory for further evaluations. After providing written informed consent, a psychiatric evaluation (Structured Clinical Interview for DSM-IV disorders (First, 2002)) was performed and a physical exam including electrocardiogram, vital signs, full hematology, blood chemistry and urinalysis tests was conducted. Persons of Asian descent were excluded because of their known increased flushing reaction to alcohol. Individuals with a body mass index (BMI) outside the range of 18–25 kg/m2 were also excluded.
Only individuals who smoked less than 10 tobacco cigarettes per day, drank less than 2 cups of coffee per day, smoked less than four marihuana cigarettes per month and used other illicit drugs less than 20 times in their life were recruited. Participants could meet DSM-IV criteria for alcohol abuse or dependence but not dependence on any other drug in the past three years. They could not be taking psychotropic medication. Individuals who had maternal alcoholism were also excluded to avoid potential sub clinical fetal alcohol syndrome. The demographic profiles of the participants in the two groups are provided in Table 1; there were no significant differences among any of these variables (p values ranged between 0.137 and 0.943, df=15). The study was approved by the McLean Hospital Institutional Review Board and individuals were paid for their participation.
Table 1.
Demographic profile at Baseline of individuals who completed 3 weeks of treatment (means ± sd). All variable were non significant between the two groups (df=15).
| Variable | Kudzu Extract | Placebo | t |
|---|---|---|---|
| N (all male) | 10 | 7 | |
| Age (years) | 24.60 ± 4.33 | 23.14 ± 2.34 | 0.807 |
| BMI* | 23.31 ± 1.11 | 23.40 ± 2.65 | 0.088 |
| Weight (kg) | 77.11 ± 7.97 | 76.85 ± 6.16 | 0.073 |
| Height (cm) | 181.48 ± 6.99 | 180.52 ± 7.27 | 0.275 |
| Race (C/A/H)** | 8/1/1 | 7/0/0 | |
| Alcohol Dependent/ | |||
| Abuse | 8/2 | 5/2 | |
| Current alcohol† | |||
| (# drinks/week) | 26.85 ± 6.75 | 27.71 ± 5.49 | 0.280 |
| Current Alcohol† | |||
| (range) | 20.0 – 40.0 | 21.0 – 36.0 | |
| Age first tried | |||
| alcohol (years) | 15.25 ± 2.88 | 12.83 ± 3.13 | 1.576 |
| Age of regular | |||
| Alcohol Use‡ (years) | 17.50 ± 1.78 | 17.71 ± 1.70 | 0.248 |
| Number of years | |||
| as regular drinker | 7.10 ± 3.75 | 5.43 ± 1.51 | 1.108 |
| Tobacco use | |||
| (Number of smokers) | 7 | 3 | |
| (cigarettes/day) | 4.59 ± 5.68 | 2.55 ± 3.42 | 0.977 |
| Marihuana use | |||
| (cigarettes/week) | 0.25 ± 0.27 | 0.23 ± 0.35 | 0.179 |
| Caffeine use | |||
| (# cups/day) | 0.92 ± 1.38 | 1.51 ± 1.68 | 0.834 |
BMI- Body Mass Index (kg/m2)
C-Caucasian, A-African American, H-Hispanic
Self-reported number of drinks per week obtained during screening
Defined as age at which subjects began drinking at least once a week
2.2 Measures
2.2.1 Blood Alcohol Levels
Estimates of blood alcohol levels were obtained during each study visit using a breathalyzer device (AlcoSensor®, Intoximeter, St Louis, MO). They were required to come to the lab twice a week to provide urine, breath and blood samples to monitor drug use, alcohol drinking and liver function, respectively. A total of 227 breath samples were possible from all subject visits during the trial and compliance was equally distributed between the two groups; the kudzu extract group provided 131 of a possible 136 samples and the placebo group provided 84 of a possible 91 samples. Only one breath sample was positive for alcohol during all three phases of the study.
2.2.2 Drinking Behavior
Drinking data were collected using a small wristwatch-like device (ActiWatch® Score, MiniMitter Co., OR) and daily diaries. The ActiWatch has a small button and digital LED faceplate and participants wore the device 24 hours a day for the entire 8-week study and were asked to record all drug and alcohol use by pressing the button to enter the proper code. Participants were provided with a small card that identified unique codes to record use of alcohol, cigarettes, caffeine, and other drugs. Participants were instructed to report when they consumed each drink, which was defined as a 12 oz can of beer, 5 oz glass of wine or 1.5 oz distilled spirits. (97% reported drinking beer).
In addition, the wrist actigraphy device was programmed to provide an audible "beep" every 3 hours ± 20 minutes to which the participant was required to enter a number between 0 (no desire) to 10 (greatest desire ever) to record his desire to drink alcohol AT THAT TIME. The beep was not loud enough to wake the participant from sleep. The ActiWatch® Score device contains an accelerometer that was used to record sleep/wake activity. We have previously reported on the sleep/wake patterns of the participants in this study (Bracken et al., 2011).
Participants filled out a daily diary every morning as soon as possible upon awakening. The Daily Diary was made up of a total of 14 questions of which 9 were Likert-type scales to assess subjective effects on mood, appetite, anxiety, ability to concentrate, irritability, desire to drink alcohol, amount of sleep, physical tension and physical symptoms in the past 24 hours. Three questions asked them to report how many alcoholic drinks, tobacco cigarettes and caffeinated beverages they consumed and two questions asked about bedtime and wake time.
Participants were assessed with the Beck Depression Inventory or BDI (1996) and the Beck Anxiety Inventory or BAI (1988) four times during the study: twice during baseline, once at the end of the medication phase (week 6), and once at the end of follow-up (week 8).
2.3 Experimental Procedure
The study consisted of a 2-week baseline period, 4 weeks of medication (subjects were randomized to either kudzu extract or placebo treatments) and a 2-week follow up period. Participants recorded their drug use and desire to use alcohol via the wrist actigraphy and daily diaries, and reported to the lab twice per week for the first six weeks and then once per week for weeks seven and eight for data collection, urine screening, safety testing and periodic blood samples to assess puerarin levels using a recent assay that we developed (Ma et al., 2005).
2.4 Safety, Side Effects and Adverse Event Reporting
The safety of kudzu extract was monitored via a number of methods including twice weekly assessments of vital signs (resting pulse, blood pressure), collecting daily reports of side effects using a daily diary and twice weekly assessments of adverse events by study staff. An industry standard adverse event reporting form was used to collect information on potential adverse and serious adverse events during each laboratory visit. Side/adverse effects also were tracked on a daily basis by entries in their diaries; items addressed a wide range of somatic complaints.
Participants also provided a blood sample during weeks 2 through 8 for laboratory testing including: hematology (white blood cell, red blood cell, platelets, hemoglobin, hematocrit and mean corpuscular volume), blood chemistry (phosphorus, calcium, cholesterol, triglycerides and iron), renal function (blood urea nitrogen (BUN), uric acid, sodium and potassium), and liver function (bilirubin [total, direct and indirect], alkaline phosphatase (ALP), lactate dehydrogenase (LDH), alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyl transferase (GGT), total protein, albumin and globulin). In addition, participants were required to have an ECG performed at the screening visit and at the end of treatment and follow up phases.
2.5 Medication Adherence Assessment
Kudzu extract (Alkontrol-Herbal™; NPI-031; Natural Pharmacia International, Inc., Burlington, MA) was prepared under Good Laboratory Manufacturing (GLM) procedures. Each 500 mg capsule of kudzu extract contained sugar beet filler and 125 mg of isoflavones (19% puerarin, 4% daidzin and 2% daidzein). Quality control measures including chemical stability measures were performed on the capsules to verify that activity did not change over the course of the study (Ma et al, 2005). Participants took two 500 mg capsules, t.i.d. (morning: 6:00 a.m. to 8:30 a.m.; afternoon: 2:00 p.m. to 4:30 p.m.; evening: 9:00 p.m. to 11:30 p.m.) for a total daily dose of 750 mg of isoflavones. Commercially available gelatin capsules (650 mg/capsule, Nature’s Bounty®) served as the placebo treatment.
To preserve the double-blind, kudzu and placebo capsules were repacked into #00 opaque capsules (Apothecary Products, Minneapolis, MN). Morning and evening sets of capsules also contained a 25 mg tablet of riboflavin (vitamin B2) and the afternoon sets contained an additional placebo capsule, in addition to the two kudzu extract capsules, in order to maintain a uniform number (3) of capsules. Capsule sets were individually packaged in small envelopes and marked on the outside with date and time of day to be consumed. Medication adherence was measured using ultraviolet (UV) light to detect riboflavin in centrifuged urine samples (Del Boca et al., 1996) that were collected twice a week. Endogenous levels of riboflavin are too low to fluoresce and participants were instructed to avoid multivitamin complexes while participating in the study. Because riboflavin is cleared from the body quickly, fluorescing urine indicated compliance in the past 18–24 hours. In addition, participants reported pill taking by checking time-of-day boxes on daily diaries and by entries on the wrist actigraphy watches.
Urine samples were qualitatively analyzed by HPLC for puerarin levels on a weekly basis starting on the second week of baseline and continuing through the two follow-up visits. Although blood samples were collected from all participants to examine the safety of kudzu, only the last 12 subjects had blood samples collected for quantitative analysis of puerarin levels.
2.6 Data Analysis
Data from the actiwatch device was the primary source for all analyses while the daily diary served as a back up to verify daily totals and in case of equipment failure. In addition, the diaries permitted the participants to enter additional data that could not be recorded on the watch.
The primary efficacy endpoint was the number of drinks per week and the number of heavy drinking days (5 drinks or more) per week. Other measured variables included: the percentage of days without drinking, and maximal number of consecutive days of no drinking.
Sporadic missing data from the actiwatches were filled in from the back up daily diary data. Data due to dropouts were handled as missing data and no values were entered. Missing data points within the treatment weeks in non dropout participants were Missing Completely at Random and so were imputed using multiple regression. A total of 42 out of 532 data points in the kudzu extract group and 26 out of 357 data points in the placebo group were handled this way, corresponding to 7.9% and 7.3% of the data, respectively.
Separate general linear model ANOVAs for data with repeated measurements were used to analyze the number of drinks per week and the number of heavy drinking days per week. The two treatments (kudzu and placebo) were analyzed as fixed effects and time (weeks since randomization) as a repeated-measurements effect. In all analyses, the baseline drinking level was modeled as a covariate of the respective dependent variable (e.g., the number of drinks per day in the baseline phase was modeled as a covariate when the dependent variable was the number of drinks during the treatment phase). Main effects were followed by Bonferroni post hoc tests. Effect size is reported using the variance-accounted for statistic Partial Eta-Squared (η2Partial). All tests were two-sided with significance level of 0.05 and were performed using SPSS software (version 19.0, IBM SPSS, Inc, Chicago, IL).
3. Results
3.1 Participant Flow Through Study
Of the 117 individuals who responded to advertisements and called the laboratory for a telephone screen, 34 were eligible to receive further screening. They were invited to the laboratory, signed informed consent and the full screening procedure was initiated. Thirteen individuals were either not eligible because of medical reasons, could not be contacted after the screening visit or declined to participate. A total of 21 individuals were randomized to receive kudzu extract (n=11) or placebo (n=10). Our a priori target for inclusion for data analysis was completing three weeks of treatment—10 and 7 participants met these criteria for the kudzu extract/placebo groups, respectively. One placebo-treated subject dropped out of the study during the baseline phase of the study, two more were removed during week three due to non compliance with study procedures (e.g., refused to wear the wrist activity device, fill out daily diaries, etc.) and one dropped out after four weeks because of scheduling problems with his job. In the kudzu extract group, one individual withdrew during week three and one withdrew at week five (both for job-related scheduling issues and not for side effects).
3.2 Safety
There were no changes in any vital signs, hematology, blood chemistry, renal or liver function tests during the three phases of the study. The maximal possible observations were based on the number of side effect categories that were recorded, multiplied by the number of study days in each phase, which was then multiplied by the number of subjects participating in each study phase. The overall incidence of side effects (e.g., headaches, shakes, chills, nausea, etc.) in both groups ranged between 1.7 and 3%. None of the study participants reported any insomnia, sedation, dizziness, blurred vision, tinnitus, or altered libido.
The BDI and BAI scores remained unchanged during the course of treatment with both kudzu extract and placebo. The BDI for the kudzu extract-treated participants was 1.78 ± 2.11 and 2.63 ± 2.50 at baseline and during treatment, respectively. The BDI measures of the placebo-treated participants were 2.0 ± 2.58 and 1.67 ± 1.37 at baseline and during treatment, respectively. The BAI scores were equally stable and ranged from 0.33 to 1.71 during treatment.
3.3 Medication Adherence
Subjects were told that they would be removed from the study if they accumulated three riboflavin negative urine specimens. Of the urine samples collected from the kudzu-treated participants (N=72), 99% were positive for riboflavin while 100% of the samples from the placebo-treated participants (N=44) were positive. The number of positive puerarin samples in the kudzu-treatment group was 98.5% (N=64/65) and 97.2% (N=35/36) for urine and blood samples, respectively. Both negative samples were from the same subject during the first week of kudzu extract treatment; all of his subsequent samples were positive. Finally, participants did not report any missing capsules and did not arrive at the laboratory with extra capsules.
3.4 Alcohol Consumption—Baseline Phase
The major dependent variable was self-reported alcohol consumption as measured via the wrist actigraphy device. Using the continuous measure of alcohol consumption, drinking was quantified using a number of different variables as noted above. In addition, responses to the audible prompts multiple times a day were used to assess desire to consume alcohol.
During the two-week baseline phase, the average number of drinks/day was 4.96 ± 1.63 (range 2–8) for the kudzu-treated group and 4.05 ± 2.52 (range 1–8) for the placebo-treated group; these values were not statistically different (p > 0.4).
3.5 Alcohol Consumption—Treatment Phase
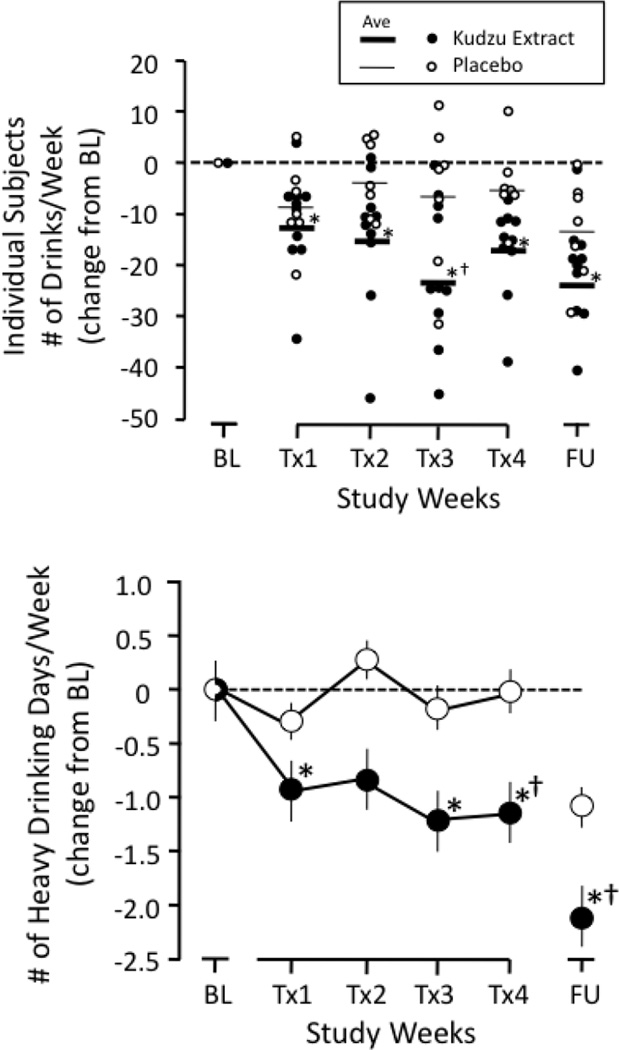
Figure 1 (top) shows the effects of kudzu extract or placebo treatment on weekly drinking, both as individual data points and as the average. There was an overall main effect for time on number of drinks per week [F(5,75) = 9.49, p< 0.001, η2 Partial = 0.388] and there was an interaction for all kudzu extract treatment weeks [F(5,75) = 2.56, p = 0.034, η2 Partial = 0.146]. Both kudzu extract- and placebo-treated individuals reduced their drinking somewhat during the first treatment week (Tx1), but these changes were significant only for the kudzu treated group. Significant reductions from baseline drinking continued for the kudzu treated group for treatment weeks 2, 3, and 4. There was a trend towards a significant difference between placebo and kudzu at Tx3 (p = 0.055). Figure 1 (bottom) shows the effects of treatment on the number of heavy drinking days per week. An overall main effect was observed for both time ([F(5,75) = 3.684, p<0.005, η2 Partial = 0.197 ] and for drug [F(1,15) = 9.428, p = 0.008, η2 Partial = 0.386]. There were no significant changes in the placebo-treated group, while significant reductions were observed in treatment weeks 1, 3, and 4 for the kudzu-treated group. Additionally, there was a significant difference between the two drug treatments during treatment week 4.
Figure 1.
Top Panel-Kudzu extract and Placebo effects on weekly alcohol consumption among heavy drinkers in an outpatient setting. Subjects reported drinking via ActiWatch-Score wrist actigraphy and daily diaries. Individual data are plotted as symbols and the average value as a thick (kudzu extract) or thin (placebo) line. Bottom Panel-Kudzu extract and Placebo effects on number of heavy drinking days per week. BL-Baseline; Tx1-Treatment Week 1; Tx2-Treatment Week 2; Tx3-Treatment Week 3; Tx4-Treatment Week 4; FU-Follow-up. *Indicates significantly different from baseline; †indicates significantly different from placebo.
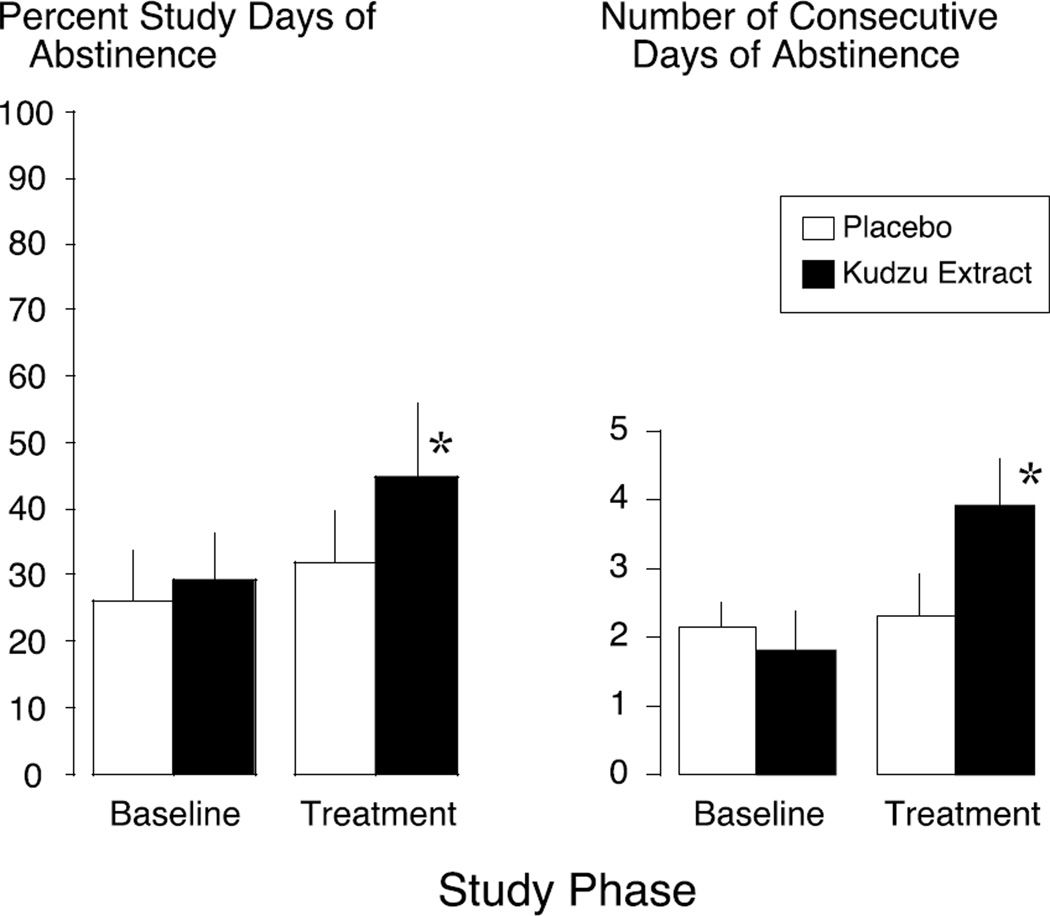
The percentage of abstinent days and consecutive days of abstinence are additional ways of assessing alcohol consumption that have been shown to be sensitive outcome measures (Anton et al., 2002; Maisto et al., 2003; O’Malley et al., 2007); both of these metrics were differentially affected by kudzu extract. Treatment with kudzu extract increased the percentage of abstinent days by 16% as compared to placebo [t(14) = −2.24, p = 0.042] (Figure 2-left). During the baseline phase of the study, both groups averaged a similar number of consecutive days of abstinence with 2.29 ± 0.64 days in the placebo group and 1.80 ± 0.29 in the kudzu extract group; these were not significantly different. During kudzu extract treatment, the number of consecutive days of abstinence increased to 3.90 ± 0.60 compared to placebo, which had no effect on this measure (2.14 ± 0.59 days) (Figure 2-right). This difference between treatments was statistically significant [F(1,14) = 7.30, p = 0.017]. It is noteworthy that these participants were daily drinkers and only one of the placebo-treated participants and two of the kudzu-treated participants achieved 3 or more consecutive days of abstinence during baseline phase. However, during the treatment phase, 9 out of 10 of the kudzu-treated and only 3 out of 7 of the placebo-treated participants achieved 3 or more consecutive days of abstinence.
Figure 2.
Effects of Kudzu Extract and Placebo on the percentage of study days in which participants were abstinent (left) and the number of consecutive days of abstinence (right) from drinking during weeks 3–6 of the study. *Indicates significantly different from placebo treatment using baseline drinking as a covariate at p < 0.05.
3.6 Alcohol Consumption—Follow-up Phase
Medication was terminated and blinded assessments continued to: 1) to evaluate any medical issues associated with abrupt termination of treatment, and 2) to determine whether any treatment-related effects persisted. As depicted in Figure 1, both consumption and heavy drinking days during the follow-up week in the kudzu group were significantly different from baseline. The placebo follow-up week was not significantly different from its baseline on either measure. For heavy drinking days, the post hoc analyses from the overall ANOVAs reported above showed that there was a group (placebo vs. kudzu) difference in the follow-up week comparison with the kudzu group being significantly lower than the placebo group.
3.7 Desire for Alcohol
In spite of observing statistically significant reductions in alcohol consumption, we found no indication that kudzu extract altered the desire for alcohol during any phase of the study. This was evident from a global analysis of the Daily Diary data during all phases of the study. On a 0–10 point Likert scale, the participants consistently rated their desire for alcohol in the 4–5 point range. For the placebo group, their average craving scores were 4.8 ± 1.5 during baseline, 5.2 ± 1.2 during treatment, and 4.3 ± 2.4 during the follow-up period. For the kudzu-treated group, their average craving scores were 5.0 ± 1.1 during baseline, 4.4 ± 1.0 during treatment, and 4.3 ± 0.8 during the follow-up period. There were no significant differences due to medication treatment [F(2,43) = 0.255, p=0.616] or phase [F(1,43) = 0.974, [=0.386], and no interaction between the main factors [F(2,43) = 0.571, p=0.569].
3.8 Other Effects
Because tobacco and alcohol are so often consumed together, we collected data on cigarette use in order to determine if tobacco intake paralleled the changes in alcohol consumption or alternatively, if there was a compensatory increase in smoking. As noted in Table 1, only 7 and 3 subjects in the kudzu- and placebo-treatment groups, respectively, smoked tobacco during the baseline phase of the study (4.59 ± 5.68 cigarettes/day for kudzu extract treated and 2.55 ± 3.42 cigarettes/day for placebo-treated subjects). During treatment, the number of cigarettes smoked per day decreased to 2.3 ± 3.26 and 2.0 ± 2.07 for the kudzu- and placebo-treated subjects, respectively. Even though there was a slight reduction in tobacco intake in the kudzu-treated individuals, this was not significant based on a Fixed Effects analysis for Drug F(1,48) = 0.867, p = 0.356; Week F(5,48) = 0.599, p = 0.701; or interaction F(5,48) = 0.091, p = 0.993]. However, the study was not designed to test this hypothesis as individuals were purposely screened for low tobacco intake.
4. Discussion
The most important finding of the present study was that 4-weeks of treatment with a standardized kudzu extract reduced ad libitum alcohol consumption in a group of non treatment-seeking heavy drinkers. We previously demonstrated that this kudzu extract significantly reduced alcohol drinking in a group of heavy drinkers during one night of access to preferred brand of beer in a simulated natural environment (Lukas et al., 2005). The present study revealed that kudzu extract is also effective in heavy drinkers in their home, work and/or school environment.
As drinking behavior was measured using a wrist actigraphy device, we were able to monitor alcohol consumption continuously, 24 hours a day and seven days a week. The reductions in drinking during kudzu extract treatment were modest, but were equivalent to a 34–57% reduction over the treatment weeks; reductions in drinking during placebo treatment ranged from 5.8–36%. The reductions in drinking by kudzu extract were observed by the second week of treatment and persisted through the 4th week of treatment. In addition, the two treatments greatly differed in the percent of days abstinent as well as the number of consecutive days of abstinence. Increased consecutive days of abstinence is a desirable outcome of an alcohol treatment program, especially during the initial phases of treatment (Washton and Zweben, 2006). The fact that kudzu extract nearly doubled the number of consecutive days that individuals maintained abstinence suggests that it may be a useful adjunct during the early weeks of treatment.
The finding that alcohol intake was reduced without affecting desire to use alcohol at first seemed counter-intuitive. However, it is important to recognize that one of the major weaknesses of the measure that we used to record desire to drink alcohol was that it was unidimensional. In reality, drug and alcohol craving is a multifaceted behavior (Potgieter et al., 1999; Singleton and Gorelick, 1998), and while the simplicity of the ActiWatch® Score device certainly helped maintain compliance and clearly offered the participants an opportunity to quickly report on the most basic aspect of their desire to use alcohol in real time, it came at the expense of obtaining a more multidimensional assessment of desire or craving for alcohol. It is quite likely that the desire to use alcohol in an outpatient setting cannot be captured with a single question; we also observed an absence of an effect on desire for alcohol in our previous study (Lukas et al., 2005).
The rates of alcohol drinking during follow-up phase were also reduced compared to baseline in both the kudzu- and placebo-treated groups. We had not expected that any beneficial effects would remain after kudzu extract had cleared the body based on our pharmacokinetic results (Penetar et al., 2006). The reduced drinking by both groups during the follow-up phase may have been due to the debriefing protocol that was implemented after treatment ended as participants were reminded that they met criteria for heavy alcohol use and that their drinking was discussed and then they were offered a referral for conventional treatment.
A prior study of kudzu to treat alcohol use in an outpatient setting reported that kudzu had no effect on maintaining sobriety or altering alcohol craving (Shebek and Rindone, 2000). However, these authors noted that there was a large dropout rate, subjects were not supervised while they completed the questionnaires, and there was only a single assessment each month. In addition, there were no measures of compliance collected and no information on the origin, quality or content of the kudzu preparation provided (we analyzed a variety of commercial preparations and found them to vary widely in their isoflavone concentration from 0–50% of claimed amounts). All of the above factors may have contributed to the negative findings of this study.
We employed two different biochemical methods (urinary riboflavin and plasma puerarin levels), to monitor medication adherence. The lack of side effects after four weeks of treatment with both placebo and kudzu extract is likely one of the major reasons for the high rate of medication adherence. Vital signs, measures of blood chemistry, liver function tests and urinalysis assessments were all normal. One animal study in particular demonstrated that kudzu extract and its major components are very safe, even after relatively large doses (2 g/day) over a 3-month treatment period (Keyler et al., (2002).
It is important to place the magnitude of the effects of kudzu extract on alcohol drinking in context. Alcohol drinking was not completely eliminated by kudzu extract in the present study, but was reduced from baseline drinking by an average of 45% over the four weeks of treatment. From a harm reduction perspective, kudzu extract results in a desirable outcome for a population of heavy drinkers, especially when one considers the other important finding of the present study—kudzu extract was without any adverse events and minimal side effects. We recently demonstrated that kudzu extract does not potentiate the intoxicating effects of alcohol (Penetar et al., 2010) and it does not interfere with sleep wake activity (Bracken et al., 2011). These findings, coupled with the present report support the notion that kudzu extract might be useful as an adjunct to a comprehensive treatment program and combined with other medications such that the dose of the other (stronger) medication might be lowered and thus reduce the incidence of side effects. In addition, this was not a treatment clinical trial, so the effects on alcohol intake might have been more robust in treatment-seeking individuals.
The limitations of this study include: 1) a relatively small sample size; 2) short (4-week) treatment interval; 3) only young male beer drinkers were included; 4) only visual inspection of the fluorescence for riboflavin was performed; 5) the three times a day dosing interval may not be optimal; and 6) a unidimensional metric of “desire for alcohol” was used. Despite these limitations, statistical significance was observed in a number of primary measures of drinking and because the participants were not seeking treatment, a longer treatment protocol would have had many more dropouts. Future studies are planned to include older subjects who have been drinking longer, both male and females and individuals who are actively seeking treatment and a spectophotometer will be used to quantify riboflavin levels and a more comprehensive method of assessing “craving” will be used.
In conclusion, the results of the present study demonstrate that four weeks of treatment with a kudzu extract results in modest reductions in alcohol consumption among heavy drinkers who are not seeking treatment. The advantages of kudzu over other medications may be: 1) the public is embracing the role that complementary/ alternative or nutraceutical products play in managing health, so there is a clear interest in trying novel therapies; 2) the low incidence of side effects helps to maintain medication adherence; and 3) kudzu extract appears to work rather quickly (within 2–3 weeks) so the likelihood that a person might “give up” because the medication “is not working” may be reduced. Additional research is clearly warranted to determine exactly how this preparation might fit into current alcohol treatment programs.
Acknowledgements
Supported by Grant R01-AA10536 (SEL) from the National Institute on Alcohol Abuse and Alcoholism, Grant K05-DA00343 (SEL) from the National Institute on Drug Abuse, and P01-AT002038 (DYWL) from the National Center for Complementary and Alternative Medicine. The authors thank Carol Buchanan, Ronna Shostak and Barbara Beake for administrative support during the conduct of this study.
Footnotes
Conflict of Interest
Based on data presented in this study, Drs. Lukas and Lee applied for, and were granted, a patent for kudzu extract to treat alcohol abuse and dependence. McLean Hospital has licensed the production of kudzu extract (NPI-031) to Natural Pharmacie International (NPI), Inc. and they are marketing it as Alkontrol-Herbal®. Dr. Lee has a financial interest in NPI, Inc. All other authors declare that they have no actual or potential conflict of interest that could inappropriately influence, or be perceived to influence, this work.
References
- Amer DA, Kretzschmar G, Müller N, Stanke N, Lindemann D, Vollmer G. Activation of transgenic estrogen receptor-beta by selected phytoestrogens in a stably transduced rat serotonergic cell line. J Steroid Biochem Mol Biol. 2010 Jun;120(4–5):208–217. doi: 10.1016/j.jsbmb.2010.04.018. Epub 2010 Apr 28. [DOI] [PubMed] [Google Scholar]
- Anton RF, Myrick H, Baros AM, Latham PK, Randall PK, Wright TM, Stewart SH, Waid R, Malcolm R. Efficacy of a combination of flumazenil and gabapentin in the treatment of alcohol dependence: relationship to alcohol withdrawal symptoms. J Clin Psychopharmacol. 2009;29(4):334–342. doi: 10.1097/JCP.0b013e3181aba6a4. [DOI] [PubMed] [Google Scholar]
- Beck AT, Epstein N, Brown GJ. An inventory for measuring clinical anxiety. J. Consult. Clin. Psychol. 1988;56:893–987. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory. 2nd ed. San Antonio, TX: The Psychological Corporation; 1996. [Google Scholar]
- Benlhabib E, Baker JI, Keyler DE, Singh AK. Kudzu root extract suppresses voluntary alcohol intake and alcohol withdrawal symptoms in P rats receiving free access to water and alcohol. J. Med. Food. 2004;7:168–179. doi: 10.1089/1096620041224210. [DOI] [PubMed] [Google Scholar]
- Bracken BK, Penetar DM, Maclean RR, Lukas SE. Kudzu root extract does not perturb sleep/wake cycle of moderate drinkers. J Altern. Comp. Med. 2011;17:1–6. doi: 10.1089/acm.2010.0540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Boca FK, Kranzler HR, Brown J, Korner PF. Assessment of medication compliance in alcoholics through UV light detection of a riboflavin tracer. Alcohol: Clin. Exp. Res. 1996;20:1412–1417. doi: 10.1111/j.1530-0277.1996.tb01142.x. [DOI] [PubMed] [Google Scholar]
- Heyman GM, Keung W-W, Vallee BL. Daidzin decreases ethanol consumption in rats. Alcohol: Clin. Exp. Res. 1996;20:1083–1087. doi: 10.1111/j.1530-0277.1996.tb01950.x. [DOI] [PubMed] [Google Scholar]
- Hufford MR, Shields AL, Shiffman S, Paty J, Balabanis M. Reactivity to ecological momentary assessment: An example using undergraduate problem drinkers. Psychol. Addict. Behav. 2002;16:205–211. [PubMed] [Google Scholar]
- Keung WM. Preclinical studies of Kudzu (Pueraria lobata) as a treatment for alcohol abuse. In: Keung WM, editor. Pueraria: The Genus Pueraria. New York: Taylor & Francis; 2002. pp. 144–158. [Google Scholar]
- Keung WM. Anti-dipsotropic isoflavones: the potential therapeutic agents for alcohol dependence. Med. Res. Rev. 2003;23:669–696. doi: 10.1002/med.10049. [DOI] [PubMed] [Google Scholar]
- Keung W-M, Vallee BL. Daidzin and daidzen suppress free-choice ethanol intake by Syrian Golden hamsters. Proc. Natl. Acad. Sci. U.S.A. 1993b;90:10008–10012. doi: 10.1073/pnas.90.21.10008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyler DE, Baker JI, Lee DY, DH O, Boucher TA, Lenz SK. Toxicity study of an antidipsotropic Chinese herbal mixture in rats: NPI-028. J. Altern. Complement. Med. 2002;8:175–183. doi: 10.1089/107555302317371460. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Cramer JA, Krol WF, Kirk GF, Rosenheck RA. Naltrexone in the treatment of alcohol dependence. N. Engl. J. Med. 2001;345:1734–1739. doi: 10.1056/NEJMoa011127. [DOI] [PubMed] [Google Scholar]
- Li SC. Ben Cho Gang Mu, 1590-1596 AD [Google Scholar]
- Lin RC, Li TK. Effects of isoflavones on alcohol pharmacokinetics and alcoholdrinking behavior in rats. Am. J. Clin. Nutr. 1998;68:1512S–1515S. doi: 10.1093/ajcn/68.6.1512S. [DOI] [PubMed] [Google Scholar]
- Litt MD, Morse P, Cooney NL. Ecological momentary assessment (EMA) with treated alcoholics: Methodological problems and potential solutions. Health Psychol. 1998;17:48–52. doi: 10.1037//0278-6133.17.1.48. [DOI] [PubMed] [Google Scholar]
- Lu L, Liu Y, Shi J, Liu Y, Ling W, Kosten TR. Traditional medicine in the treatment of drug addiction. Am. J. Drug Alcohol Abuse. 2009;35:1–11. doi: 10.1080/00952990802455469. [DOI] [PubMed] [Google Scholar]
- Lukas SE, Daniels SL, Lundahl LH, Kern BJ, Wines J. Kudzu, a Chinese herb, attenuates ethanol’s subjective effects in women; Paper presented to the American College of Neuropsychopharmacology (ACNP); 1997. Dec, [Google Scholar]
- Lukas SE, Penetar D, Berko J, Vicens L, Palmer C, Mallya G, Macklin EA, Lee DYW. An extract of the Chinese herbal root kudzu reduces alcohol drinking by heavy drinkers in a naturalistic setting. Alcohol: Clin. Exp. Res. 2005;29:756–762. doi: 10.1097/01.alc.0000163499.64347.92. [DOI] [PubMed] [Google Scholar]
- Ma Z, Wu Q, Lee DYW, Tracy M, Lukas SE. Determination of puerarin in human plasma by high performance liquid chromatography. J Chromatogr B Biomed Appl, J Chromatogr B Analyt Technol Biomed Life Sci. 2005;823:108–114. doi: 10.1016/j.jchromb.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Maisto SA, Pollock NK, Cornelius JR, Lynch KG, Martin CS. Alcohol relapse as a function of relapse definition in a clinical sample of adolescents. Addict Behav. 2003;28(3):449–459. doi: 10.1016/s0306-4603(01)00267-2. [DOI] [PubMed] [Google Scholar]
- Mark TL, Kranzler HR, Song X, Bransberger P, Poole VH, Crosse S. Physician's opinions about medications to treat alcoholism. Addiction. 2003;98:617–626. doi: 10.1046/j.1360-0443.2003.00377.x. [DOI] [PubMed] [Google Scholar]
- Naimi TS, Brewer RD, Mokdad A, Denny C, Serdula MK, Marks JS. Binge drinking among US adults. J. Am. Med. Assoc. 2003;289:70–75. doi: 10.1001/jama.289.1.70. [DOI] [PubMed] [Google Scholar]
- Nelson RO. Assessment and therapeutic functions of self-monitoring. In: Hersen M, Eisler RM, Miller P, editors. Progress in Behavioral Modification. New York: Academic Press; 1977. pp. 263–308. [Google Scholar]
- NIAAA Eighth Special Report to US Congress on Alcohol and Health. Washington, D.C.: U.S. Department of Health and Human Services; 1993. p. 261. [Google Scholar]
- O'Malley SS, Garbutt JC, Gastfriend DR, Dong Q, Kranzler HR. Efficacy of extended-release naltrexone in alcohol-dependent patients who are abstinent before treatment. J Clin Psychopharmacol. 2007;27(5):507–512. doi: 10.1097/jcp.0b013e31814ce50d. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Lee YW, Rezvani AH, Pei YH, Criswell HE, Janowsky DS. Suppression of alcohol intake after administration of the Chinese herbal medicine, NPI-028, and its derivatives. Alcohol: Clin. Exp. Res. 1996;20:221–227. doi: 10.1111/j.1530-0277.1996.tb01633.x. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Yee Y-W, Chen YT, Rezvani AH. The Chinese herbal medicine NPI-028 suppresses alcohol intake in alcohol-preferring rats and monkeys without inducing taste aversion. J. Perfusion. 1998;11:381–389. [Google Scholar]
- Penetar DM, MacLean RR, McNeil JF, Lukas SE. Kudzu extract does not increase the intoxicating effects of acute alcohol in human volunteers. Alcoholism: Clin Exp Res. 2011;35:726–734. doi: 10.1111/j.1530-0277.2010.01390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penetar D, Teter C, Ma Z, Tracy M, David Y-W, Lee’ DYW, Lukas SE. Pharmacokinetic Profile of the Isoflavone Puerarin After Acute and Repeated Administration of a Novel Kudzu Extract to Human Volunteers. J Altern Complement Med. 2006;12:543–548. doi: 10.1089/acm.2006.12.543. [DOI] [PubMed] [Google Scholar]
- Potgieter AS, Deckers F, Geerlings PA. Craving and relapse measurement in alcoholism. Alcohol Alcohol. 1999;34:254–260. doi: 10.1093/alcalc/34.2.254. [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Overstreet DH, Perfumi M, Massi M. Plant derivatives in the treatment of alcohol dependency. Pharmacol. Biochem. Behav. 2003;75:593–606. doi: 10.1016/s0091-3057(03)00124-2. [DOI] [PubMed] [Google Scholar]
- Rooke N, Li DJ, Li J, Keung WM. The mitochondrial monoamine oxidase-aldehyde dehydrogenase pathway: a potential site of action of daidzin. J. Med. Chem. 2000;43:4169–4179. doi: 10.1021/jm990614i. [DOI] [PubMed] [Google Scholar]
- SAMSHA, Substance Abuse and Mental Health Services Administration. Results from the 2007 National Survey on Drug Use and Health: National Findings. Rockville, MD: 2008. (Office of Applied Studies, NSDUH Series H-34, DHHS Publication No. SMA 08-4343) [Google Scholar]
- Schiller JS, Lucas JW, Ward BW, Peregoy JA. Summary health statistics for U.S. adults: National Health Interview Survey 2010. National Center for Health Statistics. Vital Health Stat. 2012;10(252) [PubMed] [Google Scholar]
- Shebek J, Rindone JP. A pilot study exploring the effect of kudzu root on the drinking habits of patients with chronic alcoholism. J. Altern. Complement. Med. 2000;6:45–48. doi: 10.1089/acm.2000.6.45. [DOI] [PubMed] [Google Scholar]
- Shen Y, Lindemeyer AK, Gonzalez C, Shao XM, Spigelman I, Olsen RW, Liang J. Dihydromyricetin as a novel anti-alcohol intoxication medication. J Neurosci. 2012;32:390–401. doi: 10.1523/JNEUROSCI.4639-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton EG, Gorelick DA. Mechanisms of alcohol craving and their clinical implications. In: Galanter M, editor. Recent Developments in Alcoholism, Vol. 14: The Consequences of Alcoholism. New York: Plenum Press; 1998. pp. 177–195. [DOI] [PubMed] [Google Scholar]
- Stone AA, Shiffman S. Ecological momentary assessment (EMA) in behavioral medicine. Ann. Behav. Med. 1994;16:203–209. [Google Scholar]
- Sun S-M. Beiji-Quianjin-Yaofang, circa 600 AD [Google Scholar]
- Town M, Naimi TS, Mokdad AH, Brewer RD. Health care access among U.S. adults who drink alcohol excessively: missed opportunities for prevention. [Accessed January 22, 2012];Prev Chronic Dis. 2006 Apr; [serial online]. Available from: URL: http://www.cdc.gov/pcd/issues/2006/apr/05_0182.htm. [PMC free article] [PubMed]
- US Department of Health and Human Services Healthy People 2010. 2nd ed. Vol 1. Washington, DC: US Gov Print Office; 2000. [Google Scholar]
- Washton AM, Zweben JE. Treating Alcohol and Drug Problems in Psychotherapy Practice. New York: The Guilford Press; 2006. [Google Scholar]
- Xu BJ, Zheng YN, Sung CK. Natural medicines for alcoholism treatment: A review. Drug Alcohol Rev. 2005;24:525–536. doi: 10.1080/09595230500293795. [DOI] [PubMed] [Google Scholar]