Abstract
Acidocalcisomes are acidic calcium stores rich in polyphosphate and found in a diverse range of organisms. The mechanism of Ca2+ release from these organelles was unknown. Here we present evidence that Trypanosoma brucei acidocalcisomes possess an inositol 1,4,5-trisphosphate receptor (TbIP3R) for Ca2+ release. Localization studies in cell lines expressing TbIP3R in its endogenous locus fused to an epitope tag revealed its partial colocalization with the vacuolar proton pyrophosphatase, a marker of acidocalcisomes. IP3 was able to stimulate Ca2+ release from a chicken B-lymphocyte cell line in which the genes for all three vertebrate IP3Rs have been stably ablated (DT40-3KO) and that were stably expressing TbIP3R, providing evidence of its function. IP3 was also able to release Ca2+ from permeabilized trypanosomes or isolated acidocalcisomes and photolytic release of IP3 in intact trypanosomes loaded with Fluo-4 elicited a transient Ca2+ increase in their cytosol. Ablation of TbIP3R by RNA interference caused a significant reduction of IP3-mediated Ca2+ release in trypanosomes and resulted in defects in growth in culture and infectivity in mice. Taken together, the data provide evidence of the presence of a functional IP3R as a Ca2+ release channel in acidocalcisomes of trypanosomes and suggest that a Ca2+ signaling pathway that involves acidocalcisomes is required for growth and establishment of infection.
Intracellular Ca2+ serves as a second messenger for a variety of cell functions, including secretion, contraction, cell division, and differentiation (1). Cells use two sources of Ca2+ for generating signals: Ca2+ release from intracellular stores and Ca2+ entry across the plasma membrane. Ca2+ release from intracellular stores of mammalian cells is controlled by at least three groups of channels: ryanodine receptors, located in the endoplasmic reticulum (ER) and stimulated by cyclic ADP ribose (cADPR) (2); inositol 1,4,5-trisphosphate receptors (IP3R), also mainly located in the ER and stimulated by IP3 (3); and two pore channels, preferentially located in acidic calcium stores and stimulated by NAADP (4, 5). IP3 is generated by hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP2) catalyzed by phosphoinositide-specific phospholipases C (PI-PLCs) (6), which also generate diacylglyerol, whereas ribosyl cyclases catalyze the generation of both cyclic ADP ribose and NAADP from NAD (7). Orthologs to genes encoding some of these enzymes and channels are present in protists (8, 9), suggesting an early appearance of Ca2+ signaling during evolution.
Cytosolic Ca2+ in trypanosomatids such as Trypanosoma cruzi, the etiologic agent of Chagas disease, and Trypanosoma brucei, which belongs to the group of parasites that causes African trypanosomiasis or sleeping sickness, is maintained through the concerted operation of distinct Ca2+ transporting systems located in the plasma membrane, ER, mitochondria, and the acidic calcium stores known as acidocalcisomes (10). The IP3/diacylglycerol pathway was described in T. cruzi more than 20 y ago (11), whereas IP3 was also detected in T. brucei (12). T. cruzi PI-PLC has been studied in more detail and was shown to be rather unusual in that it does not have a pleckstrin homology domain to bind to the plasma membrane, has a highly charged region between the catalytic X and Y domains, and is N-myristolyated and palmitoylated (13). This lipid modification is important for plasma membrane localization and for stimulation of differentiation of the infective trypomastigotes into amastigotes (14, 15). A gene ortholog to that encoding TcPI-PLC has been found in T. brucei, and the protein product appears to have similar domains to those of the T. cruzi enzyme. Despite the presence of a PI-PLC and the detection of IP3 in both T. cruzi and T. brucei, early attempts to detect Ca2+ release by IP3 in permeabilized cells were unsuccessful (12, 16).
With the completion of several genome projects, it was possible to use bioinformatic methods to identify genes encoding putative IP3 receptors (8, 17–19) and other channels (9) in protists. Interestingly, apart from one study that described a functional IP3 receptor in Paramecium tetraurelia (18), biochemical evidence that the other genes identified encode for functional IP3 receptors is still lacking. Proteomic data from enriched contractile vacuole fractions of T. cruzi (20) included spectra from the putative IP3Rs, suggesting that these proteins are expressed in subcellular fractions (20).
In this work, we report the characterization of the IP3R from T. brucei, which localizes to their acidocalcisomes. IP3 was able to release Ca2+ from permeabilized cells and isolated acidocalcisomes, and UV light photolysis of caged IP3 produced a significant increase in intracellular Ca2+ in live trypanosomes. Knockdown of the TbIP3R gene by RNA interference (RNAi) in bloodstream form (BSF) trypanosomes led to growth defects and reduced infectivity in vivo underscoring the relevance of this channel in the parasite.
Results
Characterization of TbIP3R.
One gene (Tb927.8.2770) encoding for a putative IP3R was found in the T. brucei genome at the TriTryp database (http://tritrypdb.org/tritrypdb/) and named TbIP3R. The full-length cDNA of TbIP3R was cloned by PCR amplification and confirmed by sequencing as described in SI Materials and Methods. The orthologs identified in T. cruzi (TcCLB.509461.90) and Leishmania major (LmjF.16.0280) shared 41% and 19.3% amino acid identity, respectively, to TbIP3R. Structural analysis (ELM and TMHMM servers) predicted five transmembrane domains in the C-terminal region between amino acids 2615–2637, 2649–2671, 2730–2752, 2771–2795, and 2862–2884. The ORF predicts a 3,099-amino-acid protein with an apparent molecular weight of 345 kDa. TbIP3R possesses a series of conserved domains including putative suppressor domain-like (SD), ryanodine receptor IP3R homology (RIH), and RIH-associated (RIAD) domains (9). A motif for a Ca2+-specific selectivity filter (GVGD) (21) is present in the putative intraluminal loop between transmembrane domains at the C-terminal region. Of the 10 residues that have been proposed to form a basic pocket that binds IP3 (22, 23), 4 are conserved in TbIP3R. Other features of trypanosomatid putative IP3Rs have been described previously (9).
TbIP3R Localization in T. brucei.
To investigate the localization of T. brucei IP3R, the C terminus was tagged in procyclic (PCF) trypomastigotes with an HA tag using homologous recombination with the endogenous gene locus. Western blot analysis confirmed expression of proteins of the expected size (Fig. S1A). The TbIP3R partially localized to acidocalcisomes, as demonstrated by colocalization with antibodies against T. brucei acidocalcisomal marker vacuolar proton pyrophosphatase (TbVP1) (24) (Fig. 1A). An additional punctate staining of TbVP1 that did not colocalize with TbIP3R was also detected (Fig. 1A) and could correspond to trafficking vesicles. In this regard, it has been described that the adaptor protein-3 (AP-3) complex is involved in sorting proteins, like TbVP1, to acidocalcisomes from the Golgi or from endosomes in both L. major (25) and T. brucei (26). No colocalization with TbBiP, an ER marker (27) with a clear reticular labeling (Fig. 1B), was detected, ruling out ER localization of the TbIP3R.
Fig. 1.
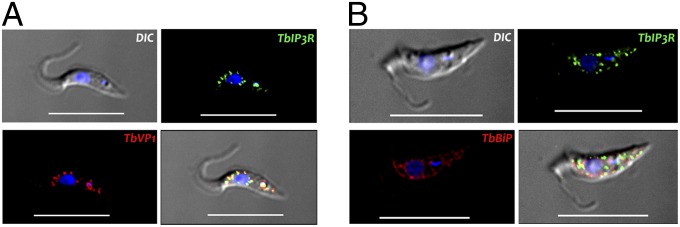
Localization of TbIP3R in PCF trypanosomes. (A) TbIP3R partially colocalizes with TbVP1 in acidocalcisomes (Pearson’s correlation coefficient of 0.874), as shown by immunofluorescence microscopy analysis. The merge images show the colocalization in yellow. (B) Lack of colocalization of TbIP3R with TbBiP in the endoplasmic reticulm (Pearson’s correlation coefficient of 0.156). (Scale bars, 10 µm.) DIC, differential interference contrast.
TbIP3R Expression in DT40 Cells and Response to IP3.
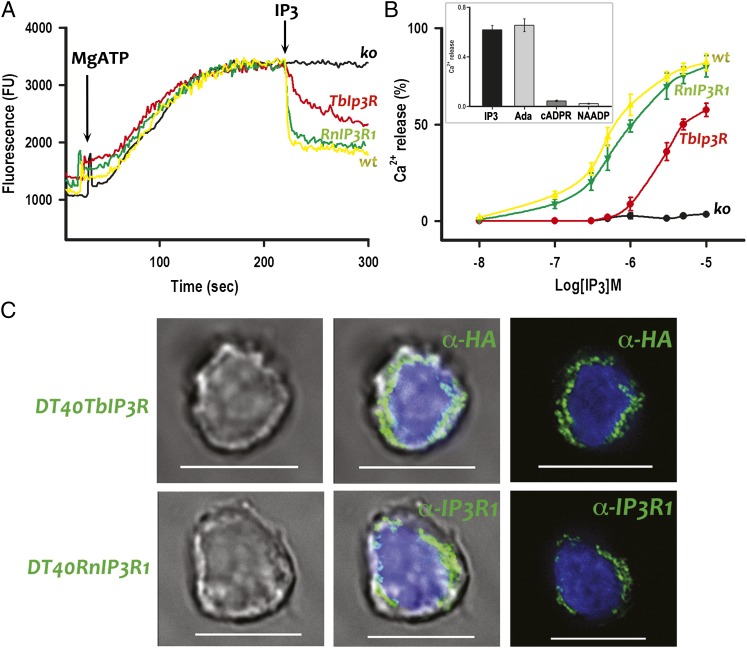
To study whether Ca2+ is released on stimulation of the TbIP3R by IP3, we stably transfected the gene encoding this receptor and that of the rat IP3R1 (RnIP3R1), used as a positive control, in a chicken B-lymphocyte cell line (DT40) in which the genes for all three vertebrate IP3Rs were stably ablated (DT40-3KO) (28). DT40-3KO cells were used as negative controls. Heterologous expression of the TbIP3R and RnIP3R1 was confirmed by immunocytochemistry (Fig. 2C) and by Western blot analyses (Fig. S1 B and C). The results shown in Fig. 2C are consistent with the localization of both TbIP3R and RnIP3R1 to the ER of DT40 cells. A low-affinity Ca2+ indicator (Mag-Fluo-4) was trapped within the ER to measure luminal free [Ca2+] in saponin-permeabilized DT40 cells. Addition of MgATP (1.5 mM) stimulated Ca2+ uptake until a steady-state Ca2+ loading was reached (Fig. 2A). The time course of Ca2+ loading was similar for DT40-3KO, DT40-WT, DT40-3KO(RnIP3R1), and DT40-3KO(TbIP3R) cells, but only the last three responded to IP3 with a decrease in Mag-Fluo-4 fluorescence. In DT40-3KO(RnIP3R1) and DT40-WT cells, a maximal concentration of IP3 (10 µM) caused the fluorescence to decrease by 81.2 ± 4.9%, whereas the same IP3 concentration caused the fluorescence of DT40-3KO(TbIP3R) cells to decrease slowly and by 61.7 ± 3.6%. There was a concentration-dependent release of Ca2+ by IP3 in all cases (Fig. 2B), and TbIP3R was considerably less sensitive to IP3 than RnIP3R1 (Fig. 2B). In addition, the IP3 agonist adenophostin A (0.5 µM) showed comparable Ca2+ release to 10 µM IP3 in the TbIP3R-expressing DT40-3KO cells (Fig. 2B, Inset). In contrast, addition of 10 µM cADPR or 1 µM NAADP did not stimulate Ca2+ release (Fig. 2B, Inset).
Fig. 2.
IP3-evoked Ca2+ release in permeabilized DT40 cells. (A) Fluoresence changes evoked by addition of MgATP (1.5 mM) and IP3 (10 µM) to DT40-WT (wt), DT40-3KO (ko), DT40-3KO(TbIP3R) (TbIP3R), and DT40-3KO(RnIP3R1) (RnIP3R) cells. An increase in fluorescence indicates decreasing medium Ca2+ or increasing vesicular Ca2+. (B) Effect of different concentrations of IP3 on Ca2+ release by cells described in A. Inset shows Ca2+ release by IP3 (10 µM), adenophostin A (Ada, 0.5 µM), cADPR (10 µM), or NAADP (1 µM). Values in B and Inset are means ± SD of three independent experiments. (C) TbIP3R (DT40TbIP3R)- and RnIP3R1 (DT40RnIP3R)-transfected DT40-3KO cells were stained with anti-HA (1:250) and anti-rat IP3R1 (1:200), respectively, and analyzed by immunofluorescence microscopy. (Scale bar, 10 µm.) Left images are by DIC.
IP3 Releases Ca2+ from Permeabilized T. brucei Cells and Isolated Acidocalcisomes.
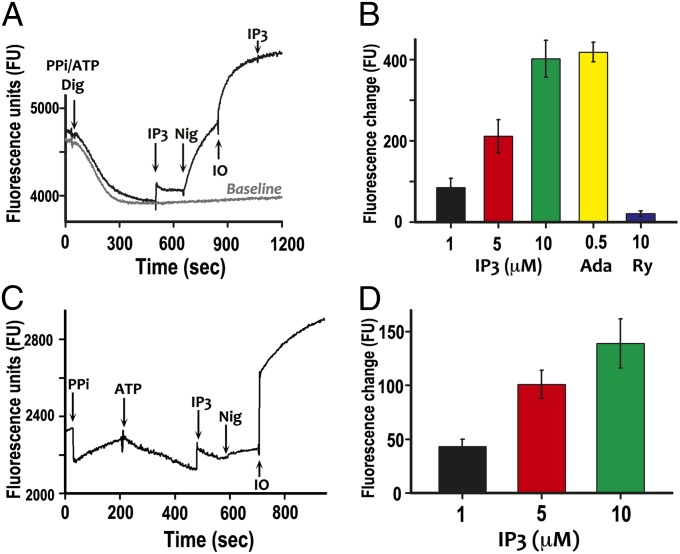
To demonstrate the response of TbIP3R in T. brucei, we used permeabilized PCF previously incubated with 0.1 mM PPi (to acidify acidocalcisomes) and 1 mM ATP (to stimulate Ca2+ uptake by the Ca2+/H+ countertransporting ATPase of acidocalcisomes (29). Uptake and release of Ca2+ were measured with Calcium Green-5N. Digitonin addition caused a drop in Ca2+ due to ATP-driven Ca2+ uptake until a steady state was reached. Under these conditions, Ca2+ release was detected on addition of IP3 (Fig. 3A) and was concentration dependent (Fig. 3B). In the absence of PPi, no significant IP3-evoked Ca2+ release was detected, as reported previously (12), suggesting that acidification of acidocalcisomes by TbVP1 was required for Ca2+ uptake by the Ca2+-ATPase located in the organelles, as reported before (29). Adenophostin A (Ada, 0.5 µM) had a comparable effect to IP3 (10 µM), whereas ryanodine (Ry, 10 µM) had no effect of Ca2+ release (Fig. 3B). Fig. 3A shows that addition of nigericin, which is a K+/H+ exchanger that alkalinizes acidocalcisomes, released the amount of Ca2+ taken up in response to ATP addition, whereas further addition of ionomycin, which is a Ca2+ ionophore that releases Ca2+ from alkaline or neutral compartments, was able to release additional Ca2+, probably present in acidocalcisomes and the ER. A similar experiment was done with isolated acidocalcisome fractions of PCF. H+ transport activity by the H+-PPase was detectable in the purified acidocalcisome fraction using acridine orange, indicating that the organelles were intact (Fig. S2). In this assay, acridine orange accumulates and dimerizes in vesicles as they acidify, leading to changes in its fluorescence properties (30). As reported before (29), Ca2+ uptake using ATP was more efficiently detected after previous addition of PPi to acidify the organelles (Fig. 3C). When 0.1 mM PPi was added, there was an initial sharp drop in fluorescence due to Ca2+ chelation and then a steady-state rise as pyrophosphate was hydrolyzed and chelated Ca2+ was released. Subtraction of this rising background allowed the detection of Ca2+ uptake when ATP was added. Addition of 10 µM IP3 led to a rise in the medium Ca2+, followed by return to a similar rate of Ca2+ uptake to that before IP3 addition. The effect of IP3 on Ca2+ release was concentration dependent (Fig. 3D). Addition of nigericin plus ionomycin released the Ca2+ taken up and additional Ca2+ stored in acidocalcisomes (Fig. 3C). It has been shown before that previous alkalinization with nigericin is needed for ionomycin to be able to release Ca2+ from acidocalcisomes (29).
Fig. 3.
Ca2+ uptake and release by digitonin-permeabilized T. brucei procyclic trypomastigotes and by isolated acidocalcisomes. (A) The reaction medium contained 125 mM sucrose, 65 mM KCl, 10 mM Hepes-KOH buffer, pH 7.2, 1 mM MgCl2, 2.5 mM potassium phosphate (reaction buffer), 2 mM succinate, 0.1 mM PPi, 1 mM ATP, and 1 µM Calcium Green-5N. Cells (5 × 107) were added to the reaction medium, and the reaction was started adding 50 µM digitonin. IP3 (5 µM), nigericin (Nig, 2 µM), and ionomycin (IO, 1 µM) were added where indicated. A decrease in fluorescence indicates decreasing medium Ca2+ or increasing vesicular Ca2+. (B) Dose–response effect of IP3 and effect of adenophostin A (Ada, 0.5 µM) or ryanodine (Ry, 10 µM) on Ca2+ release. The results are means ± SD of data from three independent experiments. (C) Assay mixtures contained reaction buffer, 1 µM Calcium Green 5-N, and acidocalcisome fraction (50 µg protein). Data after ATP addition were corrected for steadily rising background by subtraction of data points for control assays without ATP addition, using Microsoft Excel. PPi (0.1 mM), ATP (1 mM), IP3 (10 µM), nigericin (Nig, 2 µM), and ionomycin (IO, 1 µM) were added at the points indicated. (D) Effect of different concentrations of IP3 on Ca2+ release from isolated acidocalcisomes. The results are means ± SD of two independent experiments, each one with five replicates.
TbIP3R Is Required for Optimal Growth In Vitro.
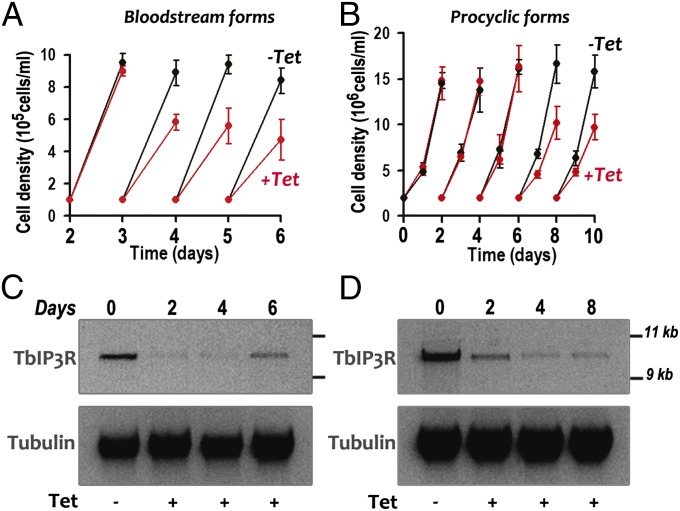
Knockdown of TbIP3R by induction of double-stranded RNA resulted in growth defects in both BSF (Fig. 4A) and PCF trypanosomes (Fig. 4B). The effects were most pronounced with BSF trypanosomes, with up to a 30–43% reduction in the number of cells. Northern blot and ImageJ analyses showed that the mRNA was down-regulated by 49.3–79.6% after 2–8 d of tetracycline addition to PCF trypanosomes (Fig. 4D). Similar results were observed after RNAi of BSF trypanosomes (Fig. 4C), although the mRNA recovered from 20.9% to 44% after 6 d. All further phenotypic analyses were performed on day 4 of RNAi induction for both PCF and BSF trypanosomes, unless indicated otherwise.
Fig. 4.
Effect of inhibition of TbIP3R expression by tetracycline-inducible RNAi on cell growth. A and B show the growth of BSF and PCF trypanosomes in the absence (black circles) or presence (red circles) of 1 µg/mL tetracycline for the indicated number of days. Values are mean ± SD (n = 4). (C and D) Northern blot analysis of TbIP3R RNAi of BSF and PCF trypanosomes grown in the absence (Tet−) and presence (Tet+) of tetracycline. Total RNA was subjected to gel electrophoreses before transfer to a nylon membrane and then hybridized with the 32P-labeled probe corresponding to the TbIP3R coding sequence (Upper). Tubulin is shown as a loading control (Lower). Molecular markers are shown on Right. The transcript of TbIP3R, including the 5′- and 3′-UTR was ∼10 kb in length.
Ca2+ Release by IP3 Is Decreased in Mutants Deficient in TbIP3R.
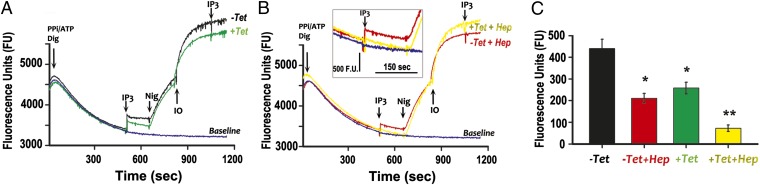
Knockdown of the expression of TbIP3R in PCF by RNAi reduced the ability of IP3 to release Ca2+ from permeabilized cells (Fig. 5 A and C). Ca2+ release by IP3 was inhibited by heparin (Fig. 5 B and C), a commonly used inhibitor of the IP3 receptors of mammalian cells (31). The combination of TbIP3R RNAi induction and heparin almost completely blocked IP3-induced Ca2+ release.
Fig. 5.
Deficient Ca2+ release by IP3 in permeabilized PCF after RNAi knockdown and heparin inhibition of TbIP3R. (A and B) Ca2+ uptake and release and additions were as described in Fig. 3. The blue line was without further additions after digitonin. (A) Ca2+ release by IP3 addition (10 µM) in PCF grown in the presence (+Tet) or absence (−Tet) of tetracycline to induce RNAi and (B) in the presence (+Hep) or absence (−Hep) of heparin (100 µg/mL). Other aditions as in Fig. 3. (C) Results are means ± SD of data from three independent experiments. *,**Differences are statistically significant compared with respective controls, P < 0.001 (Student t test).
IP3 Releases Ca2+ from Intracellular Stores in Live Trypanosomes.
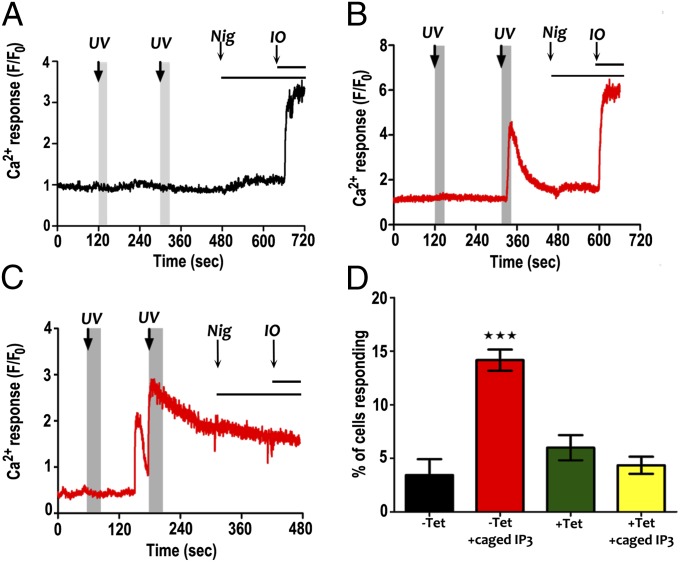
To investigate Ca2+ release by IP3 in live trypanosomes, we loaded TbIP3R RNAi-induced and uninduced PCF trypanosomes with Fluo4-AM with or without caged IP3 and recorded changes in cytosolic Ca2+. IP3 was released from caged IP3 by UV light. Fig. 6 A and B shows representative traces of Ca2+ responses to UV flashes (three pulses at 120 s and then six pulses at 300 s) in uninduced cells in the absence and presence of caged IP3, respectively. In cells loaded with caged IP3, there were rapid Ca2+ increases after the first (lower level) or second (higher level) flash to release free IP3 (Fig. 6B), with a few cells responding to both UV flashes. However, the data analysis was complicated by spontaneous Ca2+ transients that occurred in some cells even in the absence of caged IP3 or the UV flash (Fig. S3). Fig. 6C shows an example of a spontaneous Ca2+ response occurring before the IP3-dependent Ca2+ release elicited by IP3 uncaging. Spontaneous Ca2+ transients have also been reported in other protists (18, 32, 33). Because spontaneous Ca2+ transients were observed at similar rates in both uninduced and TbIP3R RNAi-induced cells (Fig. S3), the response to IP3 uncaging was analyzed by quantitating the Ca2+ responses occurring within 30 s after the UV flash (Fig. 6 A–C, shaded gray area). Data shown in Fig. S4 demonstrate that this encompasses the Ca2+ signals elicited by IP3 uncaging. Fig. 6D shows the percentage of cells in which a Ca2+ transient occurred within 30 s of a UV flash. A significantly higher proportion of cells responded in the uninduced group loaded with caged IP3 than in the TbIP3R RNAi-induced group or either group without caged IP3 (data for each experimental group are from >500 cells from four independent experiments).
Fig. 6.
Caged IP3-dependent Ca2+ release in T. brucei. (A and B) Representative traces of Ca2+ responses to UV flash in control cells in the absence (A) and presence (B) of caged IP3, respectively (first flash, three pulses; second flash, six pulses). Nigericin (Nig; 5 μM) and Ionomycin (IO; 5μM) were added where indicated. (C) Ca2+ response to UV flash in a control cell loaded with caged IP3 in which a spontaneous transient occurred before IP3-dependent Ca2+ release. (D) Percentage of cells in which a Ca2+ transient occurred within 30 s of a UV flash (indicated by gray shaded areas). Data for each experimental group are means ± SEM from >500 cells from four independent experiments. ***Differences are statistically significant, P < 0.001 (ANOVA with Bonferroni posttest).
TbIP3R Is Essential for Infectivity of T. brucei in Mice.
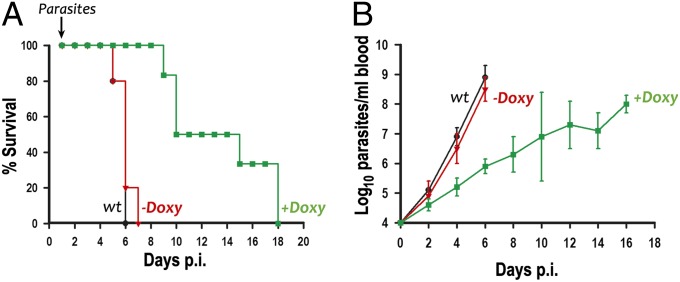
To investigate whether TbIP3R is essential for the establishment of an infection in animals, we inoculated groups of five to six mice with WT and RNAi transgenic BSF trypanosomes for TbIP3R. Induction of RNAi was obtained by feeding mice with water containing doxycycline. Mice infected with the WT and noninduced cells died 5–7 d after infection with high parasitemias (Fig. 7 A and B). This result was in contrast to the doxycycline-fed mice, where most mice survived more than 10 d. By 18 d, all doxycycline-fed mice died, suggesting that a small number of parasites (RNAi escape mutants) are able to survive in the presence of doxycycline and subsequently outgrow and kill the mice. In agreement with this suggestion, tetracycline addition in vitro reduced but did not completely eliminate the expression of TbIP3R (Fig. 4C). Doxycycline alone is known to have no effects on mice survival under the conditions used (26).
Fig. 7.
Effect of silencing of TbIP3R expression on virulence in mice. (A) Groups of five mice were infected with WT T. brucei (wt) or trypanosomes transfected with the construct for RNAi of TbIP3R. Doxycycline [Doxy(+); 200 µg/mL] was given in the drinking water throughout a 18-d period. (B) Parasitemia levels were monitored at 2-d intervals after infection.
Discussion
Our work clearly establishes the presence of a functional IP3 receptor in T. brucei and, together with our previous reports (11–16), the function of a complete IP3/diacylglycerol pathway in trypanosomes. Our results support the early emergence of this Ca2+ signaling pathway, preceding the origin of animals. Of the six supergroups in which eukaryotes have been divided (34), orthologs to putative IP3Rs have been reported in protists of five supergroups [Amoebozoa (17), Archaeplastida (35), Ophistokonta (8), Chromoalveolate (18), and Excavata (9, 19, 20), and this work], although their functional roles in Ca2+ release have been studied only in P. tetraurelia (18) and in T. brucei (this work). As discussed before (18), putative IP3R from protists differ significantly from mammalian IP3 receptors. Only 4 of the 10 conserved amino acids that have been reported to be important for IP3 binding to mouse IP3R1 are conserved in TbIP3R, which in addition, has only five instead of six transmembrane domains. However, the pore region is well conserved. Interestingly, the functional IP3R of P. tetraurelia is located in the contractile vacuole complex of this Chromoalveolata (18), whereas we found that in T. brucei the receptor is localized to acidocalcisomes. Proteomic analyses of subcellular fractions have also suggested the localization of the IP3R in the contractile vacuole (20) of T. cruzi.
Most IP3Rs reside in ER membranes, although it has been reported that IP3 also stimulates Ca2+ release from the Golgi complex (36), from the nucleus (37), and from secretory granules (38) of mammalian cells. In some mammalian cells, IP3Rs are also targeted to the plasma membrane, where they contribute to Ca2+ entry (39). Secretory granules of chromaffin cells (40), pancreatic β-cells (41), acinar (42) cells, secretory granules of airway goblet cells (43), and mast cells (44) have been shown to possess IP3Rs, although some of these results have been disputed (as discussed in ref. 45). However, it is interesting to note that some of these secretory granules, such as the mast cells granules, have similarities with acidocalcisomes (46). Although acidocalcisomes were initially described more than 18 y ago in T. brucei (47), the mechanism for Ca2+ release from these organelles was unknown until now. Our results are consistent with previous work using aequorin targeted to the mitochondrion of T. brucei (48), which showed that the intramitochondrial Ca2+ concentration can reach values much higher than cytosolic Ca2+ rises when Ca2+ release from acidocalcisomes is stimulated, suggesting a close proximity of these organelles and the presence of microdomains of high Ca2+ concentration in the vicinity of acidocalcisomes (49).
The sensitivity of TbIP3R to IP3 was lower than that of rat IP3R1. One possible explanation was that TbIP3R lacked 6 of the 10 basic residues required for high-affinity binding of IP3 in rat IP3R1. Another was that TbIP3R might respond to a different intracellular messenger (such as cADPR, NAADP, and ryanodine). However, no response to those signaling molecules was detected in TbIP3R-expressing DT40-3KO cells or permeabilized trypanosomes, excluding the latter possibility. The mammalian type III IP3R also has low affinity and is poorly activated by IP3 (50). However, it has been shown that the low affinity for IP3 does not necessarily dictate the concentration at which the type III IP3R opens (50). Rather, the IP3 concentration needed to initiate Ca2+ release from intracellular stores is determined by the concentration of both Ca2+ and IP3 because Ca2+ increases the sensitivity of the type III IP3R to IP3 (50). Interestingly, type III IP3R has been localized to insulin and somatostatin secretory granules (41), as well as in the region containing secretory granules of salivary gland and pancreatic acinar cells (50), which has been called the trigger zone. This name is because Ca2+ signals originate in this region in each of these cell types (50).
Although Ca2+ signaling appears to be important for several functions in T. cruzi such as host cell invasion (51), multiplication and differentiation (52), osmoregulation (53), and programmed cell death (54), there is much less information on the role of Ca2+ in T. brucei. Based on the use of calcium ionophores, roles for Ca2+ in the release of the bloodstream stage surface coat (55) and in the maintenance of the cytoskeleton (56) have been proposed. Changes in cytosolic Ca2+ levels have also been reported during differentiation from bloodstream to procyclic stages of T. brucei (57). Our results indicate that Ca2+ signaling through the TbIP3R has roles in growth in vitro and in vivo.
In conclusion, we identified and characterized at the molecular and biochemical levels, an IP3 receptor in T. brucei, which is essential for growth and establishment of an efficient animal infection. The localization of this receptor in acidocalcisomes provides the long sought mechanism for Ca2+ release from these organelles.
Materials and Methods
Functional Analyses of IP3Rs in DT40 Cells.
Uptake of Ca2+ into intracellular stores of permeabilized DT40 cells and its release by IP3 were measured using a low-affinity Ca2+ indicator (Mag-Fluo-4) trapped within the ER as described previously (31) with some modifications. Confluent cells (50 mL, 2 × 106 cells/mL) were collected by centrifugation at 180 × g for 10 min and suspended in 3 mL Hepes-buffered saline (HBS) containing 1 mg/mL BSA, 0.02% (wt/vol) Pluronic F127, and 20 µM Mag-Fluo-4 AM as described (31). After incubation at 20 °C for 1 h in the dark with gentle shaking, cells were centrifuged and resuspended in 10 mL Ca2+-free cytosol-like medium (CLM) (31) (140 mM KCl, 20 mM NaCl, 1 mM EGTA, 2 mM MgCl2, and 20 mM Pipes, pH 7.0) containing 50 µg/mL saponin (Sigma). Cells were incubated with shaking at 37 °C for 4 min. Cells (20 µL) were sampled to confirm permeabilization of cells by using 0.1% Trypan Blue, and the cells were centrifuged and gently resuspended in 2.5 mL Mg2+-free CLM supplemented with 375 µM CaCl2 and 10 µM FCCP. Ca2+ uptake was initiated by adding 250 µL of permeabilized cells into a cuvette containing 2.25 mL Mg2+-free CLM supplemented with 375 µM CaCl2 and 1.5 mM Mg2+-ATP. Various concentrations (1–10 µM) of IP3 were tested. The free Ca2+ concentration of the solution was monitored continuously under constant agitation at room temperature in a F-4500 Fluorescence Spectrophotometer (Hitachi), with excitation at 490 nm and emission at 525 nm.
IP3-Mediated Ca2+ Release from Isolated Acidocalcisomes.
Acidocalcisomes were isolated and purified from PCF of T. brucei Lister strain 427 by using iodixanol gradient centrifugation as previously described (58). After gradient centrifugation, the acidocalcisome pellet was collected and washed twice with isolation buffer (125 mM sucrose, 50 mM KCl, 4 mM MgCl2, 0.5 mM EDTA, 3 mM DTT, and 20 mM Hepes-KOH, pH 7.2) containing protease inhibitor mixture (Roche) and then resuspended in 1 mL of reaction buffer (125 mM sucrose, 65 mM KCl, 2 mM MgCl2, 2.5 mM potassium phosphate, and 20 mM Hepes-KOH, pH 7.2). The protein concentration was determined by using Pierce BCA protein assay kit with a SpectraMax. The isolated acidocalcisomes were acidified by addition of 0.1 mM PPi, and Ca2+ uptake was initiated by adding 50 µg of isolated acidocalcisomes into a cuvette containing 1.9 mL reaction buffer, 1 µM Calcium Green-5N, and 1 mM Mg2+-ATP. Various concentrations (1–10 µM) of IP3 were tested. Fluorescence changes were monitored in a fluorometer, with excitation at 506 nm and emission at 531 nm.
IP3-Evoked Ca2+ Release by Digitonin-Permeabilized T. brucei.
The uptake and release of Ca2+ from intracellular stores by permeabilized T. brucei PCF were assayed by fluorescence measurements using Calcium Green-5N as described above with some modifications. Midlog phase PCF of T. brucei was collected by centrifugation at 1,000 × g for 7 min and washed twice with cold buffer A, which contained 116 mM NaCl, 5.4 mM KCl, 0.8 mM MgSO4, 5.5 mM d-glucose, and 50 mM Hepes at pH 7.0. Cells were resuspended to a final density of 1 × 109 cells/mL in buffer A and kept on ice. A 50-µL aliquot (5 × 107 cells) of the cell suspension was added to the reaction buffer (2.45 mL) containing 1 µM Calcium Green-5N, 0.1 mM PPi, 1 mM ATP, and 2 mM succinate. Ca2+ uptake by the cells was initiated by the addition of 50 µM digitonin, and Ca2+ release was stimulated by the sequential additions of IP3, nigericin, and ionomycin.
Photorelease of Caged IP3 and Ca2+ Imaging.
WT- or TbIP3R RNAi–treated T. brucei PCFs were washed in Ringer buffer [10 mM Hepes, 155 mM NaCl, 3.0 mM KCl, 3.0 mM KH2PO4, 1.0 mM MgCl2, 2.0 mM CaCl2, 10 mM glucose, 40 µM probenecid, and 0.25% (wt/vol) fatty acid-free BSA, pH 7.4] and loaded, in suspension, with caged IP3 (10 µM; 40 min) followed by Fluo4-AM (10 µM; 20 min), and then plated on CellTak-coated coverslips (10 min) and mounted on the stage of an Axiovert 2000 (Zeiss) spinning disk confocal microscope. Fluo4-AM fluorescence images (excitation, 488 nm; emission, 510 nm) were acquired at 5 Hz with a cooled charge-coupled device CCD camera using the data acquisition software Piper Control (Stanfordphotonics). Photo release of caged IP3 was achieved by light flashes (burst of three to six pulses of 1-ns duration with a wavelength of 337 nm and 1.45 mJ of energy) from a nitrogen-charged UV flash lamp (Photon Technology International) guided through the objective (C-Achromatx40/1.2). Data analysis was performed using ImageJ (NIH). Spontaneous Ca2+ transients observed in some cells were also characterized using Fura-2 AM (5 µM; 20-min dye loading). Fura 2 fluorescence images (excitation, 340 and 380 nm; emission, 420–600 nm) were acquired at 3-s intervals with a wide-field epifluorescent microscope coupled to a CCD camera.
Supplementary Material
Acknowledgments
We thank David I. Yule for the DT40 cells and plasmids; Norbert Bakalara and James Bangs for antibodies; George A. M. Cross, John Donelson, and Thomas Seebeck for reagents; and Thayer King for technical help. This work was supported by National Institutes of Health Grants AI077538 (to R.D.) and AI099277 (to A.P.T.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1216955110/-/DCSupplemental.
References
- 1.Bootman MD, Berridge MJ. The elemental principles of calcium signaling. Cell. 1995;83(5):675–678. doi: 10.1016/0092-8674(95)90179-5. [DOI] [PubMed] [Google Scholar]
- 2.Mészáros LG, Bak J, Chu A. Cyclic ADP-ribose as an endogenous regulator of the non-skeletal type ryanodine receptor Ca2+ channel. Nature. 1993;364(6432):76–79. doi: 10.1038/364076a0. [DOI] [PubMed] [Google Scholar]
- 3.Streb H, Irvine RF, Berridge MJ, Schulz I. Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature. 1983;306(5938):67–69. doi: 10.1038/306067a0. [DOI] [PubMed] [Google Scholar]
- 4.Calcraft PJ, et al. NAADP mobilizes calcium from acidic organelles through two-pore channels. Nature. 2009;459(7246):596–600. doi: 10.1038/nature08030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brailoiu E, et al. Essential requirement for two-pore channel 1 in NAADP-mediated calcium signaling. J Cell Biol. 2009;186(2):201–209. doi: 10.1083/jcb.200904073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berridge MJ. Inositol trisphosphate and diacylglycerol: Two interacting second messengers. Annu Rev Biochem. 1987;56:159–193. doi: 10.1146/annurev.bi.56.070187.001111. [DOI] [PubMed] [Google Scholar]
- 7.Lee HC. Physiological functions of cyclic ADP-ribose and NAADP as calcium messengers. Annu Rev Pharmacol Toxicol. 2001;41:317–345. doi: 10.1146/annurev.pharmtox.41.1.317. [DOI] [PubMed] [Google Scholar]
- 8.Cai X. Unicellular Ca2+ signaling ‘toolkit’ at the origin of metazoa. Mol Biol Evol. 2008;25(7):1357–1361. doi: 10.1093/molbev/msn077. [DOI] [PubMed] [Google Scholar]
- 9.Prole DL, Taylor CW. Identification of intracellular and plasma membrane calcium channel homologues in pathogenic parasites. PLoS ONE. 2011;6(10):e26218. doi: 10.1371/journal.pone.0026218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moreno SN, Docampo R. Calcium regulation in protozoan parasites. Curr Opin Microbiol. 2003;6(4):359–364. doi: 10.1016/s1369-5274(03)00091-2. [DOI] [PubMed] [Google Scholar]
- 11.Docampo R, Pignataro OP. The inositol phosphate/diacylglycerol signalling pathway in Trypanosoma cruzi. Biochem J. 1991;275(Pt 2):407–411. doi: 10.1042/bj2750407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moreno SN, Docampo R, Vercesi AE. Calcium homeostasis in procyclic and bloodstream forms of Trypanosoma brucei. Lack of inositol 1,4,5-trisphosphate-sensitive Ca2+ release. J Biol Chem. 1992;267(9):6020–6026. [PubMed] [Google Scholar]
- 13.Furuya T, Kashuba C, Docampo R, Moreno SN. A novel phosphatidylinositol-phospholipase C of Trypanosoma cruzi that is lipid modified and activated during trypomastigote to amastigote differentiation. J Biol Chem. 2000;275(9):6428–6438. doi: 10.1074/jbc.275.9.6428. [DOI] [PubMed] [Google Scholar]
- 14.Okura M, et al. A lipid-modified phosphoinositide-specific phospholipase C (TcPI-PLC) is involved in differentiation of trypomastigotes to amastigotes of Trypanosoma cruzi. J Biol Chem. 2005;280(16):16235–16243. doi: 10.1074/jbc.M414535200. [DOI] [PubMed] [Google Scholar]
- 15.de Paulo Martins V, et al. Acylation-dependent export of Trypanosoma cruzi phosphoinositide-specific phospholipase C to the outer surface of amastigotes. J Biol Chem. 2010;285(40):30906–30917. doi: 10.1074/jbc.M110.142190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moreno SN, Vercesi AE, Pignataro OP, Docampo R. Calcium homeostasis in Trypanosoma cruzi amastigotes: Presence of inositol phosphates and lack of an inositol 1,4,5-trisphosphate-sensitive calcium pool. Mol Biochem Parasitol. 1992;52(2):251–261. doi: 10.1016/0166-6851(92)90057-q. [DOI] [PubMed] [Google Scholar]
- 17.Traynor D, Milne JL, Insall RH, Kay RR. Ca(2+) signalling is not required for chemotaxis in Dictyostelium. EMBO J. 2000;19(17):4846–4854. doi: 10.1093/emboj/19.17.4846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ladenburger EM, Korn I, Kasielke N, Wassmer T, Plattner H. An Ins(1,4,5)P3 receptor in Paramecium is associated with the osmoregulatory system. J Cell Sci. 2006;119(Pt 17):3705–3717. doi: 10.1242/jcs.03075. [DOI] [PubMed] [Google Scholar]
- 19.Ulrich PN, Cintron R, Docampo R. Calcium homeostasis and acidocalcisomes in Trypanosoma cruzi. Microbiol. Monographs. 2010;17:299–317. [Google Scholar]
- 20.Ulrich PN, et al. Identification of contractile vacuole proteins in Trypanosoma cruzi. PLoS ONE. 2011;6(3):e18013. doi: 10.1371/journal.pone.0018013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boehning D, Mak DO, Foskett JK, Joseph SK. Molecular determinants of ion permeation and selectivity in inositol 1,4,5-trisphosphate receptor Ca2+ channels. J Biol Chem. 2001;276(17):13509–13512. doi: 10.1074/jbc.C100094200. [DOI] [PubMed] [Google Scholar]
- 22.Yoshikawa F, et al. Mutational analysis of the ligand binding site of the inositol 1,4,5-trisphosphate receptor. J Biol Chem. 1996;271(30):18277–18284. doi: 10.1074/jbc.271.30.18277. [DOI] [PubMed] [Google Scholar]
- 23.Bosanac I, et al. Structure of the inositol 1,4,5-trisphosphate receptor binding core in complex with its ligand. Nature. 2002;420(6916):696–700. doi: 10.1038/nature01268. [DOI] [PubMed] [Google Scholar]
- 24.Lemercier G, et al. A pyrophosphatase regulating polyphosphate metabolism in acidocalcisomes is essential for Trypanosoma brucei virulence in mice. J Biol Chem. 2004;279(5):3420–3425. doi: 10.1074/jbc.M309974200. [DOI] [PubMed] [Google Scholar]
- 25.Besteiro S, Tonn D, Tetley L, Coombs GH, Mottram JC. The AP3 adaptor is involved in the transport of membrane proteins to acidocalcisomes of Leishmania. J Cell Sci. 2008;121(Pt 5):561–570. doi: 10.1242/jcs.022574. [DOI] [PubMed] [Google Scholar]
- 26.Huang G, et al. Adaptor protein-3 (AP-3) complex mediates the biogenesis of acidocalcisomes and is essential for growth and virulence of Trypanosoma brucei. J Biol Chem. 2011;286(42):36619–36630. doi: 10.1074/jbc.M111.284661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bangs JD, Uyetake L, Brickman MJ, Balber AE, Boothroyd JC. Molecular cloning and cellular localization of a BiP homologue in Trypanosoma brucei. Divergent ER retention signals in a lower eukaryote. J Cell Sci. 1993;105(Pt 4):1101–1113. doi: 10.1242/jcs.105.4.1101. [DOI] [PubMed] [Google Scholar]
- 28.Miyakawa T, et al. Encoding of Ca2+ signals by differential expression of IP3 receptor subtypes. EMBO J. 1999;18(5):1303–1308. doi: 10.1093/emboj/18.5.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scott DA, Docampo R. Characterization of isolated acidocalcisomes of Trypanosoma cruzi. J Biol Chem. 2000;275(31):24215–24221. doi: 10.1074/jbc.M002454200. [DOI] [PubMed] [Google Scholar]
- 30.Palmgren MG. Acridine orange as a probe for measuring pH gradients across membranes: Mechanism and limitations. Anal Biochem. 1991;192(2):316–321. doi: 10.1016/0003-2697(91)90542-2. [DOI] [PubMed] [Google Scholar]
- 31.Laude AJ, et al. Rapid functional assays of recombinant IP3 receptors. Cell Calcium. 2005;38(1):45–51. doi: 10.1016/j.ceca.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 32.Enomoto M, et al. Blockage of spontaneous Ca2+ oscillation causes cell death in intraerythrocitic Plasmodium falciparum. PLoS ONE. 2012;7(7):e39499. doi: 10.1371/journal.pone.0039499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lovett JL, Sibley LD. Intracellular calcium stores in Toxoplasma gondii govern invasion of host cells. J Cell Sci. 2003;116(Pt 14):3009–3016. doi: 10.1242/jcs.00596. [DOI] [PubMed] [Google Scholar]
- 34.Simpson AG, Roger AJ. The real ‘kingdoms’ of eukaryotes. Curr Biol. 2004;14(17):R693–R696. doi: 10.1016/j.cub.2004.08.038. [DOI] [PubMed] [Google Scholar]
- 35.Ladenburger EM, Sehring IM, Korn I, Plattner H. Novel types of Ca2+ release channels participate in the secretory cycle of Paramecium cells. Mol Cell Biol. 2009;29(13):3605–3622. doi: 10.1128/MCB.01592-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pinton P, Pozzan T, Rizzuto R. The Golgi apparatus is an inositol 1,4,5-trisphosphate-sensitive Ca2+ store, with functional properties distinct from those of the endoplasmic reticulum. EMBO J. 1998;17(18):5298–5308. doi: 10.1093/emboj/17.18.5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Echevarría W, Leite MF, Guerra MT, Zipfel WR, Nathanson MH. Regulation of calcium signals in the nucleus by a nucleoplasmic reticulum. Nat Cell Biol. 2003;5(5):440–446. doi: 10.1038/ncb980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yoo SH. Secretory granules in inositol 1,4,5-trisphosphate-dependent Ca2+ signaling in the cytoplasm of neuroendocrine cells. FASEB J. 2010;24(3):653–664. doi: 10.1096/fj.09-132456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dellis O, et al. Ca2+ entry through plasma membrane IP3 receptors. Science. 2006;313(5784):229–233. doi: 10.1126/science.1125203. [DOI] [PubMed] [Google Scholar]
- 40.Yoo SH. pH-dependent interaction of chromogranin A with integral membrane proteins of secretory vesicle including 260-kDa protein reactive to inositol 1,4,5-triphosphate receptor antibody. J Biol Chem. 1994;269(16):12001–12006. [PubMed] [Google Scholar]
- 41.Blondel O, et al. Localization of inositol trisphosphate receptor subtype 3 to insulin and somatostatin secretory granules and regulation of expression in islets and insulinoma cells. Proc Natl Acad Sci USA. 1994;91(16):7777–7781. doi: 10.1073/pnas.91.16.7777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gerasimenko OV, Gerasimenko JV, Belan PV, Petersen OH. Inositol trisphosphate and cyclic ADP-ribose-mediated release of Ca2+ from single isolated pancreatic zymogen granules. Cell. 1996;84(3):473–480. doi: 10.1016/s0092-8674(00)81292-1. [DOI] [PubMed] [Google Scholar]
- 43.Nguyen T, Chin WC, Verdugo P. Role of Ca2+/K+ ion exchange in intracellular storage and release of Ca2+ Nature. 1998;395(6705):908–912. doi: 10.1038/27686. [DOI] [PubMed] [Google Scholar]
- 44.Quesada I, Chin WC, Steed J, Campos-Bedolla P, Verdugo P. Mouse mast cell secretory granules can function as intracellular ionic oscillators. Biophys J. 2001;80(5):2133–2139. doi: 10.1016/S0006-3495(01)76186-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alvarez J. Calcium dynamics in the secretory granules of neuroendocrine cells. Cell Calcium. 2012;51(3-4):331–337. doi: 10.1016/j.ceca.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 46.Moreno-Sanchez D, Hernandez-Ruiz L, Ruiz FA, Docampo R. Polyphosphate is a novel pro-inflammatory regulator of mast cells and is located in acidocalcisomes. J Biol Chem. 2012;287(34):28435–28444. doi: 10.1074/jbc.M112.385823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vercesi AE, Moreno SN, Docampo R. Ca2+/H+ exchange in acidic vacuoles of Trypanosoma brucei. Biochem J. 1994;304(Pt 1):227–233. doi: 10.1042/bj3040227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xiong ZH, Ridgley EL, Enis D, Olness F, Ruben L. Selective transfer of calcium from an acidic compartment to the mitochondrion of Trypanosoma brucei. Measurements with targeted aequorins. J Biol Chem. 1997;272(49):31022–31028. doi: 10.1074/jbc.272.49.31022. [DOI] [PubMed] [Google Scholar]
- 49.Docampo R, Lukeš J. Trypanosomes and the solution to a 50-year mitochondrial calcium mystery. Trends Parasitol. 2012;28(1):31–37. doi: 10.1016/j.pt.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.O’Neill AF, Hagar RE, Zipfel WR, Nathanson MH, Ehrlich BE. Regulation of the type III InsP(3) receptor by InsP(3) and calcium. Biochem Biophys Res Commun. 2002;294(3):719–725. doi: 10.1016/S0006-291X(02)00524-7. [DOI] [PubMed] [Google Scholar]
- 51.Moreno SN, Silva J, Vercesi AE, Docampo R. Cytosolic-free calcium elevation in Trypanosoma cruzi is required for cell invasion. J Exp Med. 1994;180(4):1535–1540. doi: 10.1084/jem.180.4.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lammel EM, Barbieri MA, Wilkowsky SE, Bertini F, Isola EL. Trypanosoma cruzi: Involvement of intracellular calcium in multiplication and differentiation. Exp Parasitol. 1996;83(2):240–249. doi: 10.1006/expr.1996.0070. [DOI] [PubMed] [Google Scholar]
- 53.Rohloff P, Rodrigues CO, Docampo R. Regulatory volume decrease in Trypanosoma cruzi involves amino acid efflux and changes in intracellular calcium. Mol Biochem Parasitol. 2003;126(2):219–230. doi: 10.1016/s0166-6851(02)00277-3. [DOI] [PubMed] [Google Scholar]
- 54.Irigoín F, et al. Mitochondrial calcium overload triggers complement-dependent superoxide-mediated programmed cell death in Trypanosoma cruzi. Biochem J. 2009;418(3):595–604. doi: 10.1042/BJ20081981. [DOI] [PubMed] [Google Scholar]
- 55.Bowles DJ, Voorheis HP. Release of the surface coat from the plasma membrane of intact bloodstream forms of Trypanosoma brucei requires Ca2+ FEBS Lett. 1982;139(1):17–21. doi: 10.1016/0014-5793(82)80477-8. [DOI] [PubMed] [Google Scholar]
- 56.Selzer PM, Webster P, Duszenko M. Influence of Ca2+ depletion on cytoskeleton and nucleolus morphology in Trypanosoma brucei. Eur J Cell Biol. 1991;56(1):104–112. [PubMed] [Google Scholar]
- 57.Stojdl DF, Clarke MW. Trypanosoma brucei: Analysis of cytoplasmic Ca2+ during differentiation of bloodstream stages in vitro. Exp Parasitol. 1996;83(1):134–146. doi: 10.1006/expr.1996.0057. [DOI] [PubMed] [Google Scholar]
- 58.Salto ML, Kuhlenschmidt T, Kuhlenschmidt M, de Lederkremer RM, Docampo R. Phospholipid and glycolipid composition of acidocalcisomes of Trypanosoma cruzi. Mol Biochem Parasitol. 2008;158(2):120–130. doi: 10.1016/j.molbiopara.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.