Abstract
Each year, more than 700,000 people undergo cancer surgery in the United States. However, more than 40% of those patients develop recurrences and have a poor outcome. Traditionally, the medical community has assumed that recurrent tumors arise from selected tumor clones that are refractory to therapy. However, we found that tumor cells have few phenotypical differences after surgery. Thus, we propose an alternative explanation for the resistance of recurrent tumors. Surgery promotes inhibitory factors that allow lingering immunosuppressive cells to repopulate small pockets of residual disease quickly. Recurrent tumors and draining lymph nodes are infiltrated with M2 (CD11b+F4/80hiCD206hi and CD11b+F4/80hiCD124hi) macrophages and CD4+Foxp3+ regulatory T cells. This complex network of immunosuppression in the surrounding tumor microenvironment explains the resistance of tumor recurrences to conventional cancer vaccines despite small tumor size, an intact antitumor immune response, and unaltered cancer cells. Therapeutic strategies coupling antitumor agents with inhibition of immunosuppressive cells potentially could impact the outcomes of more than 250,000 people each year.
Keywords: immunology, tumor macrophages, T regulatory cells
Solid cancers are the leading cause of death in the United States and affect 1.4 million people each year (1). Surgical resection is the most effective therapy for patients with solid tumors, and half of all cancer patients undergo surgery with curative intent (2, 3). Unfortunately, disease recurs within 5 y in up to 40% of those patients. These patients have a 10-fold worse prognosis than patients who do not develop recurrences (3). They tend not to respond to conventional therapies. Traditionally, the medical community has assumed that recurrent tumors arise from transformed neoplastic clones that are more resistant to chemotherapy, immunotherapy, and radiation (4). We hypothesized that primary and recurrent tumors of equal size have fundamentally different microenvironments that explain their response to therapies.
Cancer vaccines have been proposed to treat patients after surgery to prevent relapses by augmenting endogenous antitumor immune responses (5, 6). This strategy has theoretical benefits such as low toxicity, tumor specificity, and long-lasting immunity. Most importantly, cancer vaccines are maximally effective for limited disease burden; thus postoperative administration is appealing because of the presence of minimal residual disease (4, 6, 7, 8).
Accordingly, the treatment of minimal disease with cancer vaccines has been tested in hundreds of preclinical studies in animal models with small amounts of tumor burden (6, 9–13). These preclinical studies have translated to several trials of cancer vaccines to prevent recurrences in patients after surgery (6, 12). To date, responses have been infrequent despite the generation of antigen-specific effector T lymphocytes (5, 6, 14). One potential explanation for the discordance between preclinical studies and postoperative clinical trials is that most evidence was derived from xenograft or transgenic mouse models with primary tumors (12, 15). We propose that these studies have not considered the changes in a recurrent tumor.
We hypothesized that, although a primary and a recurrent tumor may have the same size, fundamental differences in their microenvironments allow cancer vaccines to inhibit a primary tumor but to fail to inhibit a recurrent tumor. In comparing the biology of small primary and small recurrent tumors, we observed that recurrent tumors have an immunosuppressive milieu marked by expanded populations of tumor-associated macrophages (TAMs), regulatory T cells (Tregs), and protumor cytokines that inhibit cytotoxic CD8 T lymphocytes. In contrast, small primary tumors had a healthy population of antitumor effector CD8 T lymphocytes. Furthermore, in animal models, recurrent tumors were refractory to several cancer vaccines that typically would eliminate primary tumors. Disrupting these immunosuppressive pathways restored the efficacy of tumor vaccine in recurrent tumors.
Results
Tumor Vaccines Lack Efficacy After Surgery Despite Generating Antigen-Specific CD8 T Lymphocytes.
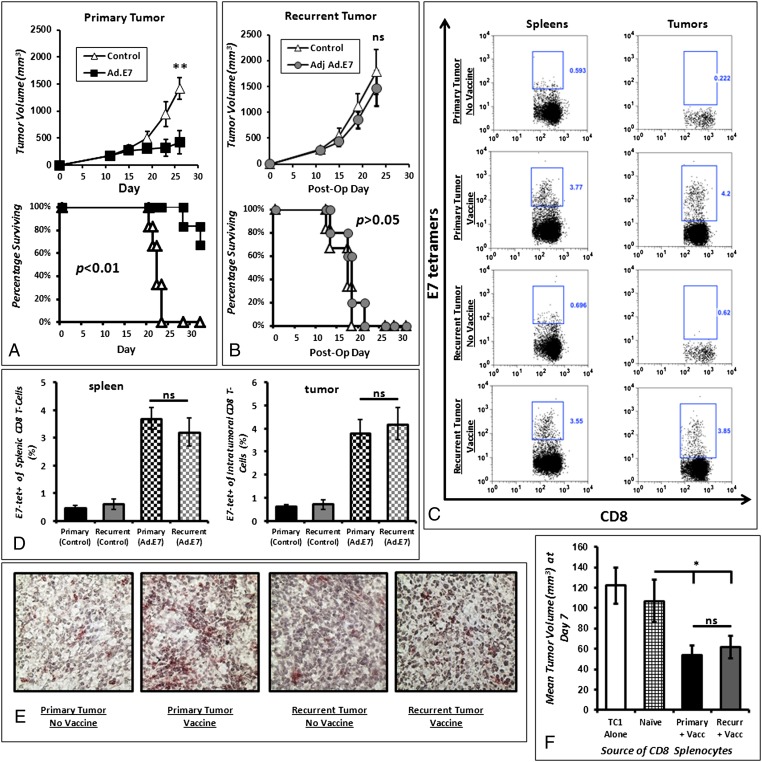
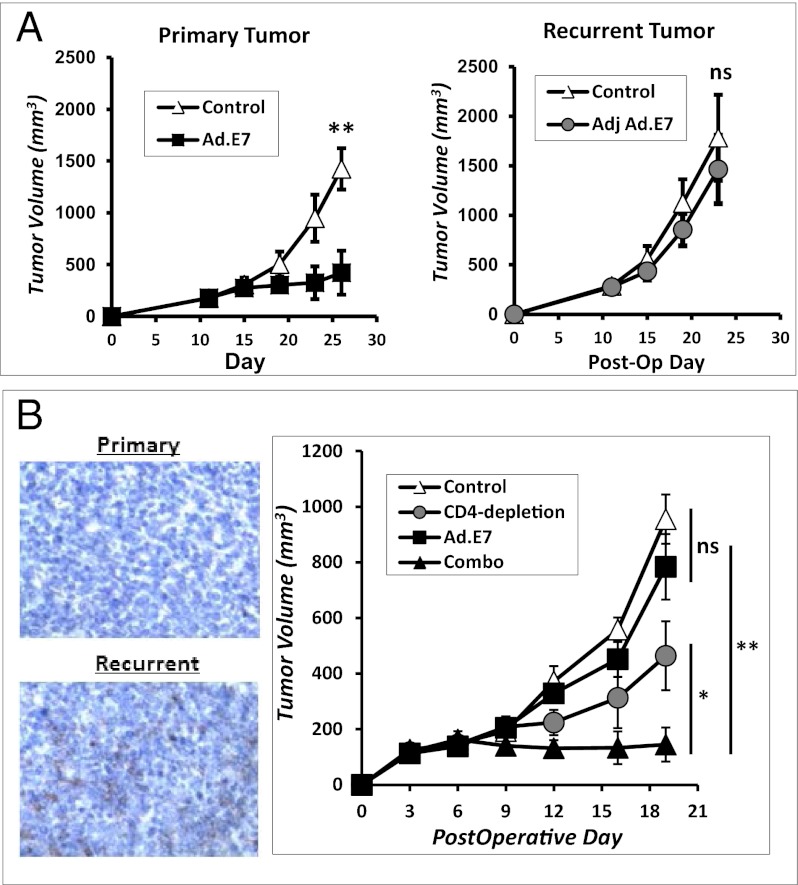
We hypothesized that tumor vaccines may function differently on primary versus recurrent cancers despite equal tumor burden, as recently described in another vaccine model (16). Mice bearing 200-mm3 primary or 200-mm3 recurrent TC1 flank tumors were vaccinated with an adenoviral vaccine to a known tumor antigen (Ad.E7). We observed significant decreases in disease burden in mice with primary flank tumors (Fig. 1A). In other mice, the vaccine was used to treat recurrent tumors of equivalent size; however, there was no survival benefit (Fig. 1B). We repeated this strategy in several vaccine models, including a poly(I:C)-ova vaccine to treat LLC.ova tumors and a Listeria.mesothelin vaccine to treat AE17 mesothelioma tumors (Fig. S1). We consistently observed that vaccines were effective on 200-mm3 primary tumors but failed on 200-mm3 recurrent tumors.
Fig. 1.
Vaccination of primary tumors and adjuvant vaccination of recurrent tumors yields differential efficacy. (A) Ad.E7 vaccination of mice bearing primary TC1 tumors (at day 7 and 14) resulted in decreased tumor growth and increased overall survival. (B) Vaccination of mice bearing recurrent TC1 tumors (at postoperative day 4 and 11) did not correlate with decreased growth of recurrent tumors or survival benefits. (C) Flow cytometry of representative spleen and tumors of mice undergoing Ad.E7 vaccination demonstrates that epitope-specific T cells are generated equally in primary and recurrent disease scenarios. (D) Bar graph representation of the number of E7-specific CD8 T cells in spleens (Left) and tumors (Right) of mice bearing primary and recurrent tumors. (E) Immunohistochemistry of representative tumors reveals that recurrent tumors have less CD8 T-cell trafficking than primary tumors. (F) Tumor neutralization assay demonstrating that splenic CD8 T cells harvested from vaccinated mice with primary or recurrent tumors are equally capable of neutralizing in vivo TC1 tumor cells. As a control, TC1 tumor cells were injected alone or with CD8 T cells harvested from spleens of tumor-naive mice. *P < 0.05; **P < 0.01.
To determine if Ad.E7 vaccinations were generating similar levels of antigen-specific antitumor T cells, spleens and tumors of animals with primary and recurrent tumors were harvested 3 d after vaccination. We found equivalent quantities of E7-specific CD8 T cells after vaccination in the spleen (3.86 vs. 3.46%; P = 0.34) and the tumor (3.92 vs. 4.11%; P = 0.63) (Fig. 1 C and D). However, there were fewer infiltrating CD8 T cells in recurrent tumors than in primary tumors (Fig. 1E).
To determine if the CD8 T cells were cytotoxic after Ad.E7 vaccination, we performed an in vivo neutralization assay. Spleens of animals with primary and recurrent tumors were harvested 3 d after the second vaccination. CD8 T cells were isolated from the splenocytes, mixed with naive TC1 tumor cells in a 3:1 (CD8 T cell:tumor cell) ratio, and reinjected into naive C57bl/6 mice. After 7 d, the CD8 T lymphocytes from vaccine-treated mice with both primary and recurrent tumors showed equivalent efficacy in eliminating TC1 cells in tumor-naive syngeneic mice (Fig. 1F).
Together, these data show that the lack of vaccine efficacy in recurrent tumors is caused by a non–CD8 T-cell mechanism that inhibits the efficacy of antigen-specific CD8 T lymphocytes in recurrent tumors.
Peripheral Leukocyte Populations Are Similar in Primary and Recurrent Tumors.
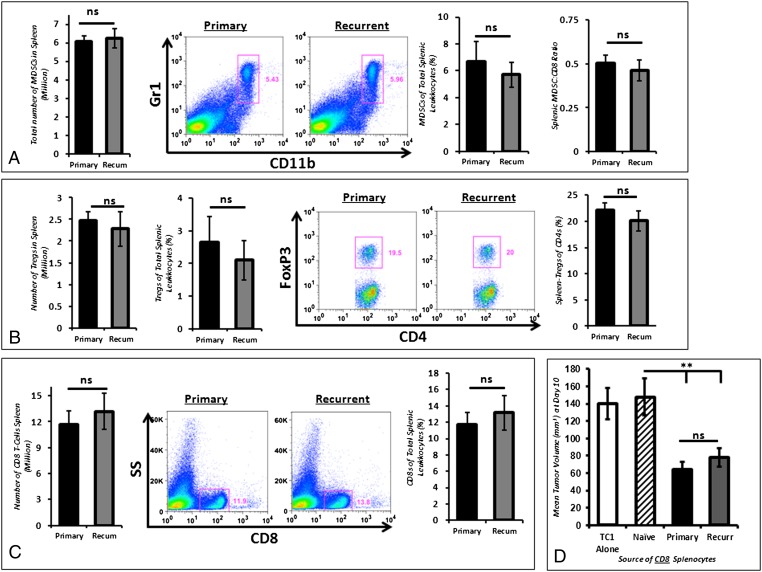
To explain the blunting of CD8 T cells in recurrent tumors, we examined the immunologic differences in mice with primary vs. recurrent tumors. Mice bearing primary or recurrent TC1 tumors were killed when tumors measured 200 mm3 (Fig. S2A), and we characterized the systemic CD45+ subpopulations. B-cell populations accounted for ∼35% of the splenic leukocytes in both groups (P > 0.05) (Fig. S2B). There also were no differences in the absolute number of CD11b+Gr1hi myeloid-suppressor cells (MDSCs) or in the proportion of MDSCs in the spleen or alterations of the MDSC:CD8 T cell ratio (Fig. 2A). In both cohorts, more than 70% of splenic MDSCs had a granulocytic phenotype (CD11b+Gr1hiLy6ClowLyG+). Depletion of granulocytic MDSCs using the Ly6G-specific antibody, 1A8, before surgical resection did not alter recurrent tumor growth kinetics in either group (Fig. S3).
Fig. 2.
Splenic immunocytes are similar in primary and recurrent tumor states. (A) By flow cytometry, the number of MDSCs (CD11b+Gr+) was comparable in mice bearing primary and recurrent tumors. Furthermore, the MDSC percentage of total splenic leukocytes (CD45+) was similar in primary and recurrent tumors. The ratio of splenic MDSCs to CD8+ T cells was not statistically different in primary and recurrent tumors. (B) A similar number of Tregs (CD4+Foxp3+) was found in spleens of mice bearing primary and recurrent TC1 tumors. Foxp3+ Tregs accounted for a similar proportion of total splenic leukocytes and total CD4 T cells. (C) The absolute number of splenic CD8 T cells and the percentage of CD8 T cells of splenic leukocytes were not statistically different in mice bearing primary and recurrent tumors. (D) In vivo tumor neutralization assay demonstrating that splenic CD8 T cells obtained from mice bearing primary and recurrent tumors are equally capable of inhibiting the growth of TC1 tumors in naive mice. As a control, TC1 tumor cells were injected alone or with CD8 T cells harvested from the spleens of tumor-naive mice. **P < 0.01.
We assessed systemic (splenic) Foxp3+CD4+ Tregs in primary and recurrent tumors by implanting TC1 cells in transgenic B6.Cg-Foxp3tm2Tch/J mice (17). There was no inhibition of tumor growth in this strain compared with wild-type mice. There were no differences in the total number or percentage of Foxp3+ Tregs of total splenic leukocytes in mice bearing primary and recurrent 200-mm3 tumors (Fig. 2B). The ratios of Foxp3+:total CD4 T lymphocytes were similar (Fig. 2B).
We also assessed the quantitative and functional differences of CD8 T cells in mice with primary vs. recurrent tumors. Wild-type mice were killed when tumors reached 200 mm3. There were similar quantities of splenic CD8 T cells (11.7 vs. 13.2 million, P = 0.43) and percentages of splenic leukocytes expressing CD8 (11.8 vs. 12.9%, P = 0.37) (Fig. 2C). CD8 T cells were harvested from the spleens of mice bearing primary and recurrent TC1 tumors. These cells were mixed with fresh TC1 tumor cells at a CD8-T cell:tumor cell ratio of 3:1. This mixture then was injected into the flanks of tumor-naive mice. After 10 d of growth, we observed that CD8 T cells obtained from tumor-bearing mice, regardless of primary or recurrent tumor state, had endogenous antitumor activity and were capable of slowing tumor growth (Fig. 2D).
Together, these findings demonstrate that primary and recurrent tumors have equivalent numbers of splenic B cells, immunosuppressive MDSCs, and Tregs. Furthermore, there are equivalent numbers and function of splenic CD8 T cells.
Tumor Cells from Primary and Recurrent Cancer Nodules Show No Phenotypic or Functional Difference.
We then postulated that an intrinsic difference in the tumor cells in primary and recurrent cancer nodules because of immunoediting and selection may explain the resistance of recurrent tumors to cancer vaccines.
To determine if tumor cells in primary and recurrent tumor cells had different growth potential in vivo, we isolated tumor cells from primary and recurrent cancer nodules. Primary and recurrent flank tumors (250 mm3) were harvested and digested with collagenase/DNase, and tumor cells were isolated by negative selection using CD45+ isolation beads. Isolated tumor cells then were reinjected into the flanks of naive C57bl/6 mice. Control animals were injected with freshly cultured tumor cells that never had been passaged in animals. We found no difference in tumor growth kinetics of fresh control tumor cells, tumor cells from primary cancer nodules, and tumor cells from recurrent cancer nodules (Fig. S4A). In addition, control tumor cells, primary tumor cells and recurrent tumor cells were analyzed by flow cytometry before reinjection into naive mice. We found no difference in the expression of MHC I, PD-L1, and FAS-L (P < 0.01) (Fig. S4B).
Another potential explanation for the resistance of recurrent tumors to vaccines might be the selection over time of cancer cells that no longer respond to antitumor effector CD8 T lymphocytes. To examine this hypothesis in vivo, we took advantage of the fact that antitumor CD8 T cells are induced in the spleens of mice 7 d after the injection of TC1 tumors. These splenic CD8 T cells can be mixed with tumors cells and used in “Winn assays” where they inhibit the growth of the tumor cells when coinjected into the flanks of naive mice. We again isolated tumor cells from 250-mm3 primary cancer nodules (“primary TC1 cells”) and 250-mm3 recurrent cancer nodules (“recurrent TC1 cells”). Control tumor cells were taken directly from culture. CD8 T cells were isolated from the spleens of naive mice (“control CD8 T cells”) and mice that had primary flank tumors for 7 d (“primed CD8 T cells”). Different combinations of CD8 T cells and tumor cells were mixed in a 3:1 T cell:tumor cell ratio and injected into naive immunocompetent mice. We tested four experimental groups (n = 24): control TC1 tumor cells and control CD8 T cells (group 1); control TC1 tumor cells and primed CD8 T cells (group 2); primary TC1 cells and primed CD8 T cells (group 3); and recurrent TC1 tumor cells and primed CD8 T cells (group 4) (Fig. S4C). At 10 d, control tumor cells mixed with naive T cells (group 1) grew to a size of about 130 mm3, the size of control TC1 tumor cells alone. As expected, primed CD8 T cells (group 2) markedly slowed the growth of naive TC1 tumor cells to about 40 mm3. Importantly, the primed CD8 T cells had an equivalent inhibitory effect on TC1 tumor cells isolated from primary (group 3) and recurrent (group 4) cancer nodules. Thus, the tumor cells from recurrent tumor were not intrinsically more resistant to CD8 T-cell killing.
In conclusion, these data suggest that tumor cells in primary and recurrent cancer are phenotypically and biologically comparable when isolated from their respective microenvironments.
Recurrent Tumors Are Heavily Infiltrated with Suppressive Macrophages.
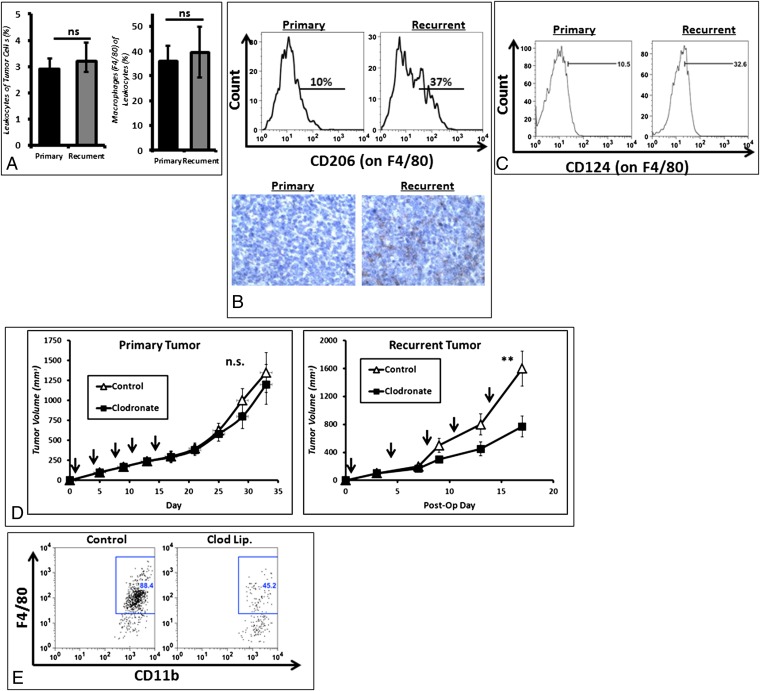
Given these results, we hypothesized that the differences between size-matched primary and recurrent tumors may reside in the local (intratumoral) and regional (draining lymph node; DLN) microenvironments. To examine these differences, we analyzed the immune cell composition of flank tumors. There were equivalent proportions of CD45+ leukocytes infiltrating primary and recurrent tumors (2.89 vs. 3.23%, P = 0.54) (Fig. 3A). Macrophages were the most abundant infiltrating immune cell in both primary and recurrent tumors (36.1 vs. 39.7%, P = 0.56) (Fig. 3A).
Fig. 3.
Immunosuppressive TAMs infiltrate recurrent tumors. (A) Flow cytometry shows that similar absolute numbers of leukocytes (CD45+) infiltrate primary and recurrent tumors; the majority are macrophages (CD11b+Ly6G-F4/80+). (B) Macrophages express higher levels of CD206 in recurrent tumors than in primary tumors. (C) Macrophages also express higher levels CD124 in recurrent tumors than in primary tumors. (D) Selective depletion of macrophages using liposomal encapsulated clodronate decreases growth in recurrent tumors but has no significant effect on primary tumors. Arrows indicate time of clodronate administration. **P < 0.01. (E) Flow cytometry confirmed depletion of F4/80 macrophages.
To evaluate the phenotype of the TAMs, we harvested tumors and DLNs and analyzed them by flow cytometry. In recurrent tumors, macrophages had higher expression levels of protumor M2 phenotypical markers, including: the mannose receptor CD206 (Fig. 3B), which was nearly fourfold higher (P = 0.01), and CD124 (IL-4 Rα) (Fig. 3C), which was threefold elevated (P = 0.04). We depleted macrophages using systemically delivered liposomal clodronate in mice with primary and recurrent TC1 flank tumors. When clodronate was given to small primary tumors, there was no statistically significant difference in tumor growth compared with controls. However, macrophage depletion in recurrent tumors markedly inhibited the growth of flank tumors (P < 0.001) (Fig. 3D). Macrophage depletion was confirmed by flow cytometry (Fig. 3E).
These findings demonstrate that recurrent tumors are infiltrated with a higher percentage of suppressive M2 TAM.
Foxp3+ Tregs Are Increased in the DLNs of Recurrent Tumors.
Like TAMs, the presence of Foxp3+ CD4 Tregs in tumors has been associated with a negative prognosis after cancer diagnosis and blunted vaccine efficacy. Therefore we examined the presence of Tregs in equivalent-sized primary and recurrent tumors. As described above, TC1 tumors were implanted into the flanks of transgenic B6.Cg-Foxp3tm2Tch/J mice.
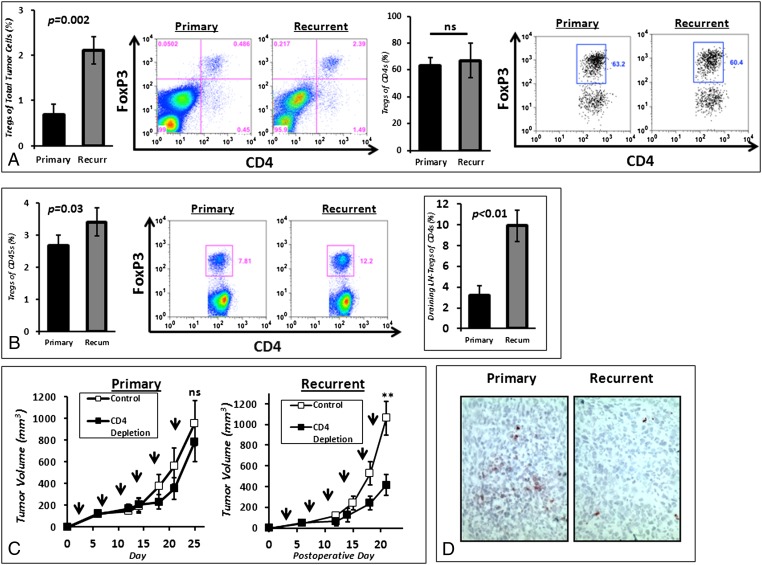
Within the tumor bed of TC1 flank tumors, the percentage of Tregs was significantly higher in recurrent tumors than in primary tumors (2.11 vs. 0.78%; P = 0.002) (Fig. 4A), but there was no difference in the ratio of Foxp3+ CD4 cells to total CD4 lymphocytes (P = 0.74) (Fig. 4A), suggesting increased infiltration of both types of CD4 cells. The proportion of Tregs to total leukocytes was higher within the DLNs of recurrent tumors than in the DLNs of primary tumors (3.40 vs. 2.67%; P = 0.03) (Fig. 4B). However, unlike the tumor, in the DLNs there was a threefold increase in the ratio of Foxp3+ CD4 cells to total CD4 T lymphocytes (9.89 vs. 3.20%; P < 0.01) (Fig. 4B).
Fig. 4.
Tregs infiltrate recurrent tumors and are increasingly prevalent in lymph nodes draining recurrent tumor. (A) Tregs (CD4+Foxp3+) accounted for a larger percentage of total tumor cells in recurrent tumor states. However, the percentage of Tregs of intratumoral CD4+ T cells remained constant in mice bearing primary and recurrent tumors. (B) Tregs accounted for a larger percentage of total leukocytes in lymph nodes draining recurrent tumors than in lymph nodes draining primary tumors. Furthermore, the ratio of Tregs to total CD4+ T cells was markedly increased in the lymph nodes draining recurrent tumors. (C) Selective depletion of CD4 T cells using anti-CD4 antibodies did not affect the growth of primary tumors but significantly inhibited the growth of recurrent tumors. Arrows indicate time of administration of anti-CD4 antibody. **P < 0.01. (D) Using immunohistochemistry, we confirmed that there were fewer CD8 T cells trafficking into recurrent tumors than into primary tumors.
We then performed two experiments using the TC1 flank tumor model: (i) mice bearing primary tumors were randomized to control or anti-CD4 antibody, and (ii) mice with recurrent tumors were randomized to control or anti-CD4 antibody. Eliminating CD4 T lymphocytes in animals with primary tumors had relatively minor impact (Fig. 4C). However, depletion of CD4 T cells in animals with recurrent tumors led to a significant (P < 0.05) reduction in tumor growth (Fig. 4C).
Together, these results show that the locoregional environment of recurrent tumor is associated with an increased population of inhibitory Foxp3+ CD4 Tregs.
CD8 T Lymphocytes Traffic Poorly into Recurrent Tumors After Surgery.
Given that there are more suppressive intratumoral TAMs and regional Tregs in animals with recurrent tumors than in animals with primary tumors of equal size, we hypothesized that this difference may explain the impaired CD8 T lymphocyte infiltration of the tumor microenvironment (Fig. 1E). There were fivefold fewer CD8 T cells in recurrent tumors than in primary tumors (2.7 vs. 10%; P = 0.007) (Fig. 4D) and significantly reduced IFN-γ levels in tumor lysates (66 vs. 119 pg/mL; P = 0.01).
Surgery Drives the Development of Tumor Immunosuppression in Recurrent Tumors.
It is well established that “wounding” generates a robust inflammatory response that is essential for healing (16, 18, 19). We hypothesized that “surgical wounding” may generate an inflammatory response in the residual tumor that ultimately promotes the development of M2 macrophages and Tregs in the recurrent local-regional tumor microenvironment. At various time points (0, 2, 6, 24, and 48 h) after surgery, residual tumors were harvested, and lysates were analyzed for cytokine changes. There were significant increases in several potent protumor cytokines, including VEGF, IL-1β, IL-6, IL-10, MCP-1, and TGF-β (total and active forms) (Fig. 5A), and significant decreases in IFN-γ after resection (Fig. 5A). These results demonstrate that surgery generates a strongly immunosuppressive milieu which may be responsible for rapid recurrent tumor growth and the associated immunosuppressive cellular changes.
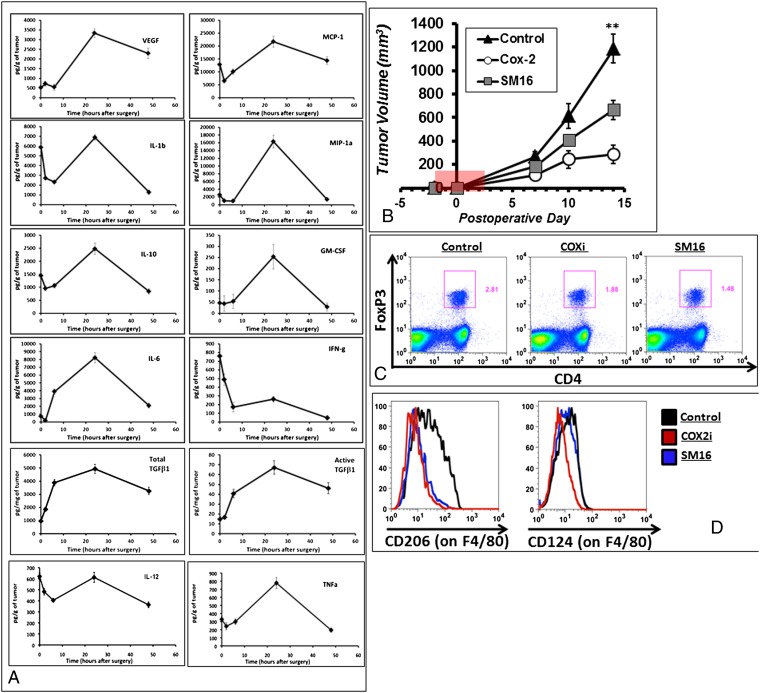
Fig. 5.
Recurrent tumors have a pronounced change in the intratumoral cytokine milieu. (A) TC1 flank tumors were harvested at 0, 2, 6, 24, and 48 h, and lysates were analyzed by Luminex and ELISA. (B) SM16 and rofecoxib were administered in the chow for 5 d perioperatively (area highlighted in red) to mice bearing flank TC1 tumors. Flow cytometry of (C) FoxP3 and (D) CD206 and CD124 was done on harvested flank tumors after inhibition of TGF-β or COX2.
Two key factors were identified as being present in high levels in the wound after surgery: TGF-β and COX-2. Thus, tumor-bearing mice randomized to partial resection were fed with a control chow, a chow containing a TGF-β inhibitor (SM16), or a chow containing a COX-2 inhibitor (Celecoxib). Treatments were very limited and were initiated 2 d before surgery and continued for 3 d after the resection. Impressively, even with only a short time of drug delivery, there were substantial decreases in recurrent tumor volumes up to postoperative day 14 (P < 0.01 for both treatment groups) (Fig. 5B).
Three mice from each group were killed at postoperative day 6 (3 d after the end of therapy). Within the DLN, the percentage of Tregs to total leukocytes was lower in mice treated with the TGF-β inhibitor than in controls (1.56 vs. 2.65%; P = 0.006) and in mice treated with the COX-2 inhibitor than in controls (1.92 vs. 2.65%; P = 0.03) (Fig. 5C).
Within the tumor, there were fewer M2 macrophages in mice randomized to the TGF-β– and COX-2–inhibiting diets. More specifically, there were fewer macrophages expressing CD206 in mice receiving TGF-β– and COX-2–inhibiting chow (12.2 and 14.4%, respectively) than in mice on the control diet (28.1%) (P = 0.01 and P = 0.02, respectively) (Fig. 5D). Similarly, fewer macrophages expressed CD124 in mice on the TGF-β– (22.4%) and COX-2–inhibiting chow (22.4 and 7.2%, respectively) than in mice on the control diet (34.5%) (P = 0.01 and P = 0.02, respectively) (Fig. 5D).
Blocking TAMs and Tregs After Surgery Restores the Efficacy of Tumor Vaccines.
These observations in animals bearing primary or recurrent tumors suggested that the presence of immunosuppressive TAMs and Tregs likely blunts the endogenous tumor-neutralizing cytotoxic CD8 T cells (Fig. 1 and Fig. S1) and thus negatively impacts antitumor vaccines.
To test this possibility, Treg depletion was combined with cancer vaccine strategies. Mice bearing recurrent TC1 tumors were randomized to four treatment protocols: (i) control, (ii) postoperative Ad.E7 vaccination, (iii) CD4 depletion, or (iv) the combination of postoperative Ad.E7 and CD4 depletion. CD4 depletion was begun 2 d before surgical resection and continued throughout recurrent tumor growth. Vaccination took place at postoperative days 6 and 13. As shown earlier (Figs. 1 and 4), the Ad.E7 vaccine alone had little effect on recurrent tumors, and depletion of CD4 T cells slowed tumor growth significantly. However, the combination of CD4 depletion and vaccination dramatically slowed the growth of recurrent tumors (Fig. 6A). Combining vaccination with Treg depletion also resulted in significantly increased tetramer-positive CD8 T cells in both the spleen and tumor (Fig. 6 B and C). Furthermore, the combination resulted in increased IFN-γ–secreting CD8 T cells (Fig. 6C). Eliminating CD4 cells resulted in greater trafficking of CD8 T cells into recurrent tumors; presumably because of the elimination of Treg-related immunosuppression (Fig. 6D).
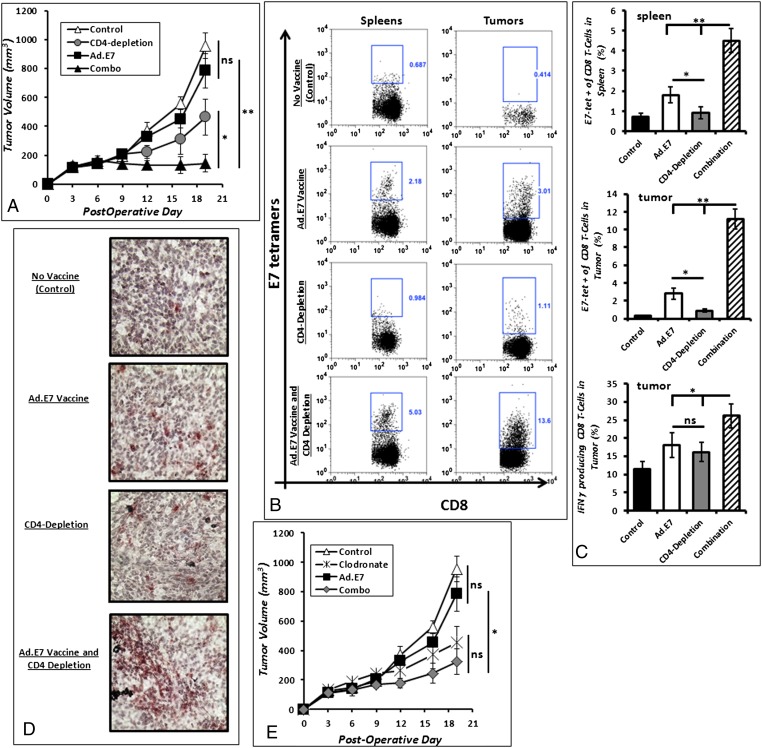
Fig. 6.
Elimination of Tregs and macrophages restores adjuvant vaccine efficacy. (A) The growth of recurrent TC1 tumors was decreased significantly in mice that were randomized to Ad.E7 vaccination with CD4 depletion (Combo) as compared with mice receiving either Ad.E7 or CD4-depleting antibodies alone. (B) By flow cytometry, the combination of Ad.E7 vaccination with CD4 depletion generated an increase of CD8 T cells specific to the E7 antigen in the spleen and tumor. (C) Combining vaccination with CD4 T cell depletion increased the percentage of CD8 T cells secreting IFN-γ after 6 h of in vitro stimulation with PMA/ionomycin (Bottom). (D) Immunohistochemistry of representative recurrent tumors show that Ad.E7 combined with CD4-depleting antibodies also increased the number of intratumoral CD8 T cells in recurrent lesions. *P < 0.05; **P < 0.01. (E) Mice bearing recurrent TC1 tumors that were randomized to Ad.E7 vaccination with macrophage depletion (liposomal clodronate) had significantly decreased recurrent tumor growth compared with mice receiving monotherapy.
We repeated the previous experiments combining vaccination with TAM depletion using liposomal clodronate. Again, mice bearing recurrent TC1 tumors were randomized to four treatment protocols: (i) control, (ii) postoperative Ad.E7 vaccination, (iii) clodronate, or (iv) the combination of postoperative Ad.E7 and clodronate. Macrophage depletion was begun 2 d before surgical resection and continued throughout recurrent tumor growth. Vaccination took place at postoperative days 6 and 13. As above (Fig. 3), macrophage depletion significantly slowed the growth of recurrent tumors. Although not quite reaching statistical significance, there appeared to be a trend towards a reduction in postoperative tumor volumes with vaccination and macrophage depletion (P = 0.06) (Fig. 6E).
Discussion
Cancer vaccines have been shown to have potent antitumor effects in preclinical models of primary de novo cancers (12, 15); however, these results have translated poorly to clinical trials to prevent recurrences after surgery (6). Our studies demonstrate strong vaccine efficacy when treating small (200 mm3) primary tumors. However, the same treatment regimen is not useful for size-matched recurrent tumors. Tumor vaccines generated equivalent numbers of cytotoxic antigen-specific CD8 T cells systemically in both situations, but local immunosuppressive forces rapidly induced tumor resistance in small pockets of recurrent disease as they begin to repopulate with cancer cells. Systemically, there are minimal changes in suppressive populations after surgery. As previously reported (20), there is a transient reduction in splenic myeloid suppressor cells after surgery, however, these cells quickly repopulate as the tumor recurs.
One source of immunosuppression appeared to be TAMs. We observed large populations of intratumoral macrophages in recurrent tumors that are considered phenotypically immunosuppressive “M2” (F4/80hiCD206hi and F4/80hiCD124+) (21). Depletion of intratumoral macrophages slowed the growth of recurrent tumors more effectively than in primary tumors and demonstrated a strong trend toward augmenting the potency of tumor vaccines; these findings further support the harmful role of M2 macrophages in recurrent tumors.
Our second major observation was a dramatic increase in Tregs in the DLNs and in recurrent tumors. When these Tregs were eliminated by depleting CD4 cells, recurrent tumors grew significantly more slowly, and vaccines were more efficacious. Of note, a transgenic B6.Cg-Foxp3tm2Tch/J mouse model was useful in localizing intratumoral Treg populations because the tumor digestion protocols (which use collagenases) cleave T-cell surface markers, thus making Treg detection inconsistent.
Third, there was a significant paucity of infiltrating CD8 T cells trafficking into recurrent tumors as compared with primary tumors. This reduced CD8 T-cell trafficking correlated with less IFN-γ and an increase in immunosuppressive cytokines such as IL-6, IL-10, and VEGF. Decreased migration of T cells into tumors would limit the efficacy of immunotherapy.
Finally, we did not find significant tumor-cell editing after surgery. Both primary and recurrent tumor cells had similar growth kinetics, expression of transmembrane immune proteins, and response to primed cytotoxic T lymphocytes.
The mechanisms underlying the immunologic changes after surgery remain to be elucidated. One possibility is that surgery generates a sequence of events meant to induce wound healing, characterized by the release of VEGF, PDGF, prostaglandins, TGF-β, clotting factors, and complement (19). Although useful for wound healing, in the context of recurrent tumors, these mediators contribute to a rapid expansion of Tregs, MDSCs (18, 19), and angiogenic factors. Another explanation for the differences in the macrophages and Tregs in primary and recurrent tumors could be related to the amount of time the immune system has been exposed to the implantation of the first tumor cells (reflecting the concept of cancer as an “unhealing wound”). Early in the course of the tumor history, there may be an equal number of antitumor and protumor immune cells. However, by the time recurrent tumors develop (after the immune system has been exposed to tumor antigens for longer period), there is an exhaustion of antitumor T lymphocytes, and a proliferation of protumor cells (especially Tregs and M2 macrophages), perhaps leading to tumor escape.
There are some potential limitations to this study. First, the immune system of the flank is different from orthotopic sites because of the presence of Langerhans cells and increased vascularity. However, because of the need for a cytoreductive procedure, lung tumor models were not technically possible. Second, we were limited to anti-CD4 antibodies as a way to eliminate the Tregs. Although alternative agents exist [i.e., anti–glucocorticoid-induced tumor necrosis factor receptor (GITR) and anti-CD25 antibodies], we found these agents have other unfavorable effects, including the elimination of active CD8 T cells which transiently up-regulate CD25 (the IL-2 receptor) and GITR, or lack strong efficacy. Despite the depletion of Foxp3 Tregs and antitumor effector CD4 T cells, the effects of the anti-CD4 antibody remain impressive and likely will be more pronounced if a specific Treg inhibitor is discovered.
In summary, despite these caveats, these findings provide a potential explanation for the suboptimal results associated with clinical trials evaluating postoperative immunotherapy and offer some potential solutions. The future success of immunotherapy in preventing recurrences will depend on developing biocompatible inhibitors of TAMs and Tregs that will not disrupt the wound healing that follows surgery.
Materials and Methods
Animals.
Female C57BL/6 mice (B6, Thy1.2) were purchased from Charles River Laboratories. Male mice expressing GFP driven from the FoxP3 promoter (B6.Cg-Foxp3tm2Tch/J) (17) were purchased from Jackson Laboratory. All mice were maintained in pathogen-free conditions and used for experiments at age 8 wk or older. Recognized principles of laboratory animal care (22) were followed, and the Animal Use Committees of the Children’s Hospital of Philadelphia, the Wistar Institute, and the University of Pennsylvania approved all protocols.
Cell Lines and Tumor Models.
The murine lung cancer cell line, TC1, was derived from mouse lung epithelial cells immortalized with HPV-16 E6 and E7 and transformed with the c-Ha-ras oncogene (23). The murine lung cancer cell line (LLC) expressing ovalbumin (Ova) was provided by Richard G. Vile (Molecular Medicine, Mayo Clinic). The murine AE17 mesothelioma cell line (24) was engineered to express human mesothelin. The cells were grown in vitro in Roswell Park Memorial Institute medium, 10% (vol/vol) FBS, 2 mmol/L glutamine, and 1% (vol/vol) penicillin/streptomycin, were tested regularly and maintained negative for Mycoplasma spp. Tumor cells for s.c. injections were suspended in 100 μL PBS. Tumor volume was calculated using the equation (3.14 × long axis × short axis2)/6.
Surgery and Recurrent Tumor Model.
Surgery was performed on mice bearing flank tumors using an established partial resection model (25). Briefly, mice bearing flank tumors (500 mm3) were anesthetized using i.m. ketamine (80 mg/kg) and xylazine (10 mg/kg) and then were shaved with hair clippers. A 1- to 2-cm incision was made immediately adjacent to the tumor. Resections were completed using standard blunt dissection. To mimic a positive margin, ∼10% of the tumor burden was left at the tumor margin. Skin closure was performed using sterile silk 4–0 sutures. Buprenorphine (0.2 mg/kg) was administered at the time of surgery and 6 h after as postoperative analgesia. Preoperative treatment was unknown to the investigator performing surgery and making tumor measurements.
Cancer Vaccination.
An adenoviral vector expressing the HPV-E7 protein under control of a cytomegalovirus promoter was provided by Hildegund Ertl (Wistar Institute, Philadelphia, PA) (26) and subsequently produced in the University of Pennsylvania Viral Core Facility. Mice were vaccinated s.c. in the left flank (contralateral to the tumor) with 1 × 109 pfu of Ad.E7 vector. Seven days after the initial vaccination, mice received a booster vaccine of 1 × 109 pfu of Ad.E7. Control animals received the same doses of a control virus (Ad.LacZ). Poly(I:C) (P0913) and OVA protein (A6075) was purchased from Sigma Aldrich. The vaccine was administered as a poly(I:C) (50 mg/mouse) and OVA protein (2–11 nmol/mouse) mixture as previously described (27). A Listeria monocytogenes-based vaccine engineered to express human mesothelin (CRS-207) was obtained from Anza Therapeutics (28).
In Vivo Depletion of CD8 T Cells, CD4 T Cells, Neutrophils, and Macrophages.
To deplete specific T-cell populations in our model, mice were injected i.p. with mAbs purified from the anti-CD4 hybridoma GK1.5 or the anti-CD8 hybridoma 53–6.7 (American Type Tissue Culture Collection) (29). Mice were given 300 μg of purified antibody i.p. dissolved in 200 μL of PBS for CD4+ and CD8+ antibodies. To deplete neutrophils, mice were injected i.p. with purified anti-Ly6G (IA8) mAbs (BioXCell). Mice received 150 μg of purified antibody i.p. dissolved in 200 μL of saline. Antibodies against CD4, CD8, and Ly6G were administered twice weekly for 3 wk. Depletions were confirmed by flow cytometry of splenic suspension. To deplete macrophages, liposome-encapsulated clodronate was used as previously described (30). For recurrent tumors, mice bearing flank TC1 tumors were randomized to macrophage depletion with liposomal clodronate or a PBS-packaged control 2 d before surgery. Macrophage depletion was confirmed by intratumoral flow cytometry.
Immunohistochemical Studies.
Tumors were harvested and frozen in Tissue-Tek OCT compound (Sakura Finetek) to be stored at −80 °C. Five-micrometer sections were cut. mAbs against CD8 T cells, CD206 (macrophage mannose receptor), and CD4 T cells were obtained (BD Biosciences), and immunohistochemical staining was performed according to established protocols. Tumor cell infiltrate was quantified by counting the number of positively staining cells in four high-powered fields (magnification, 400×). Five slides for each specimen were analyzed.
Measurement of Intratumoral, Intracellular, and Systemic Cytokine Levels.
TC-1 tumors were harvested at various time points. Tumors were weighed, and lysis buffer [PBS with 1% Triton-X (Fisher Biotech), 1 mmol/L phenylmethanesulfonyl fluoride (catalog no. P7626-5G; Sigma-Aldrich) plus other protease inhibitors (Complete Mini Protease inhibitors; catalog no. 13999600; Roche Diagnostic)] was added according to the tumor weight (1 mL of lysis buffer per 100 mg tumor). The samples then were homogenized and centrifuged. Cytokine levels of the supernatants were analyzed using Mouse Cytokine 20-Plex Panel (catalog no. LMC0006; Invitrogen).
For intracellular cytokine staining studies, cells were suspended in medium before being plated in 24-well plates and incubated for 18 h in 5% CO2/humidified air at 37 °C. Then 10 μg of brefeldin A (Sigma) was added to each well to immobilize the interleukins in the Golgi apparatus. Cells were pretreated with DMSO (Sigma) vehicle control for 2 h. Then 50 ng/mL of phorbol myristate acetate (PMA) and 0.5 μg/mL of ionomycin were added for 4 h. Cells then were fixed with 2% paraformaldehyde and incubated at 37 °C for 15 min in the dark. Cells were washed and suspended in 0.5% saponin and incubated for 30 min on ice in the dark to permeabilize the cells. After incubation, cells were washed, suspended in 0.5% saponin, and stained with IFN-γ (BD Biosciences).
TGF-β Inhibition.
Small-molecule inhibitors of TGF-β type I receptor (ALK5) kinase have been previously described (31). SM16 (Oncology Cell Signaling, Biogen Idec, Cambridge, MA) is an ALK5/ALK4 kinase inhibitor with a molecular weight of 430. Briefly, SM16 binds ALK5 (Ki,10 nmol/L) and ALK4 (Ki,10 nmol/L) with high affinity at the ATP-binding site. In these studies, mice were fed standard chow ad libitum. When tumors grew to designated size, the mice were fed SM16 at 0.5g SM16 per kg of chow (Research Diets, New Brunswick, NJ).
COX-2 Inhibition.
Specific COX inhibition was achieved using the COX-2 inhibitor celecoxib purchased from the Hospital of the University of Pennsylvania pharmacy. Celecoxib was incorporated into mouse chow by Test Diet at a concentration of 0.1%. Mice were fed this chow for 2 wk.
Flow Cytometric Analysis of Tumors.
For flow cytometric analysis, tumors were removed from euthanized mice and minced into fine pieces in digestion buffer containing 0.1 mg/mL DNase I and 2.0 mg/mL collagenase type IV (Sigma). Samples were incubated in digestion buffer at 37 °C for 30 min, filtered through a 70-μm filter, and washed twice with R10. Fc receptors were blocked with anti-mouse CD16/CD32 antibodies (BD Biosciences). After one wash with PBS plus 2% FBS (staining buffer), cells were incubated for 30 min at 4 °C with appropriate antibodies [CD45, CD4, CD8, B220, CD11b, GR1, CD25, Foxp3, Ly6G, Ly6C, F4/80, CD206, TNFα, IL4-Rα (CD124), MHCI, PD-L1, FAS-L (BD Biosciences and eBiosciences)] or allophycocyanin-labeled H-2Db tetramers (1:500 dilution) loaded with E7 peptide (RAHYNIVTF) that were obtained from the National Institute for Allergy and Infections Diseases Tetramer Core Facility. Samples then were washed and resuspended in staining buffer or fixed in 2% paraformaldehyde. Flow cytometry was completed using a Becton Dickinson FACSCalibur flow cytometer and analyzed using FlowJo software.
Detection of Tumor-Neutralizing CD8 T cells (Winn Assay).
Splenocytes (three mice per group) or splenic CD8 T cells from tumor-bearing mice at various time points or control mice were used. CD8 T cells were isolated using the MACS isolation system [CD8a (Ly-2) mouse MicroBeads; Miltenyi Biotec]. More than 90% of the isolated cells were CD8 T cells. Fresh TC1 cells were mixed with CD8 T cells in a ratio of three CD8 T cells to one TC1 tumor cell (as described in ref. 32). The resulting mixture (1.5 × 106 CD8 T cells to 0.5 × 106 TC1 tumor cells) was injected s.c. into the flanks of five naive C57BL/6 mice. A control group of mice was injected in an analogous fashion with TC1 cells alone (0.5 × 106 cells) or tumor cells mixed with CD8 T lymphocytes from tumor-naive mice. Tumor size was assessed over the next 10 d.
Statistical Analyses.
For flow cytometry, immunohistochemistry, and flank tumor volume studies comparing differences between two groups, we used unpaired Student’s t tests. For studies comparing more than two groups, ANOVA with appropriate post hoc testing was implemented. Kaplan–Meier curves were used to determine postoperative median survival. Survivals were defined as the time from flank tumor injection (primary tumors) or from the time of surgery (recurrent tumors) to the time at which flank tumor volume reached 1,500 mm3. Treatment groups were compared using the log-rank statistic. Differences were considered significant when P < 0.05. Data are presented as mean ± SEM unless otherwise noted.
Supplementary Material
Acknowledgments
We thank Theresa Busch and Cory Rice for their assistance in performing TGF-β assays. S.S. is the recipient of a National Institutes of Health Paul Calabresi Scholarship. Further funding came from the Lavin Family Foundation (S.S.), the American Foundation for Cancer Research (S.S.), and the National Lung Cancer Partnership (S.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. G.K. is a guest editor invited by the Editorial Board.
See Author Summary on page 1582 (volume 110, number 5).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1211850110/-/DCSupplemental.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2. National Cancer Institute (2009) SEER Stat Database. (NCI, Bethesda)
- 3.Aliperti LA, Predina JD, Vachani A, Singhal S. Local and systemic recurrence is the Achilles heel of cancer surgery. Ann Surg Oncol. 2011;18(3):603–607. doi: 10.1245/s10434-010-1442-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCarter MD, Fong Y. Role for surgical cytoreduction in multimodality treatments for cancer. Ann Surg Oncol. 2001;8(1):38–43. doi: 10.1007/s10434-001-0038-0. [DOI] [PubMed] [Google Scholar]
- 5.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: Moving beyond current vaccines. Nat Med. 2004;10(9):909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tucker ZC, Laguna BA, Moon E, Singhal S. Adjuvant immunotherapy for non-small cell lung cancer. Cancer Treat Rev. 2012;38(6):650–661. doi: 10.1016/j.ctrv.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 7.Mocellin S, Rossi CR, Lise M, Marincola FM. Adjuvant immunotherapy for solid tumors: From promise to clinical application. Cancer Immunol Immunother. 2002;51(11-12):583–595. doi: 10.1007/s00262-002-0308-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Predina JD, et al. Neoadjuvant in situ gene-mediated cytotoxic immunotherapy improves postoperative outcomes in novel syngeneic esophageal carcinoma models. Cancer Gene Ther. 2011;18(12):871–883. doi: 10.1038/cgt.2011.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haas AR, et al. Cycloxygenase-2 inhibition augments the efficacy of a cancer vaccine. Clin Cancer Res. 2006;12(1):214–222. doi: 10.1158/1078-0432.CCR-05-1178. [DOI] [PubMed] [Google Scholar]
- 10.Kinoshita Y, et al. 2001. Antitumor effect on murine renal cell carcinoma by autologous tumor vaccines genetically modified with granulocyte-macrophage colony-stimulating factor and interleukin-6 cells J Immunother 24:205–211.
- 11.Rosenberg SA. Progress in human tumour immunology and immunotherapy. Nature. 2001;411(6835):380–384. doi: 10.1038/35077246. [DOI] [PubMed] [Google Scholar]
- 12.Schreiber K, Rowley DA, Riethmüller G, Schreiber H. Cancer immunotherapy and preclinical studies: Why we are not wasting our time with animal experiments. Hematol Oncol Clin North Am. 2006;20(3):567–584. doi: 10.1016/j.hoc.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 13.Ledford H. A shot in the arm for cancer vaccines? Nature. 2010;464(7292):1110–1111. doi: 10.1038/4641110a. [DOI] [PubMed] [Google Scholar]
- 14.Henderson RA, Mossman S, Nairn N, Cheever MA. Cancer vaccines and immunotherapies: Emerging perspectives. Vaccine. 2005;23(17-18):2359–2362. doi: 10.1016/j.vaccine.2005.01.082. [DOI] [PubMed] [Google Scholar]
- 15.Block MS, Nevala WK, Leontovich AA, Markovic SN. Differential response of human and mouse dendritic cells to VEGF determines interspecies discrepancies in tumor-mediated TH1/TH2 polarity shift. Clin Cancer Res. 2011;17(7):1776–1783. doi: 10.1158/1078-0432.CCR-10-2836. [DOI] [PubMed] [Google Scholar]
- 16.Grinshtein N, Bridle B, Wan Y, Bramson JL. Neoadjuvant vaccination provides superior protection against tumor relapse following surgery compared with adjuvant vaccination. Cancer Res. 2009;69(9):3979–3985. doi: 10.1158/0008-5472.CAN-08-3385. [DOI] [PubMed] [Google Scholar]
- 17.Lin PP, et al. Chemotherapy response is an important predictor of local recurrence in Ewing sarcoma. Cancer. 2007;109(3):603–611. doi: 10.1002/cncr.22412. [DOI] [PubMed] [Google Scholar]
- 18.Roh JL, et al. Celecoxib can prevent tumor growth and distant metastasis in postoperative setting. Cancer Res. 2004;64(9):3230–3235. doi: 10.1158/0008-5472.can-03-3050. [DOI] [PubMed] [Google Scholar]
- 19.Shakhar G, Ben-Eliyahu S. Potential prophylactic measures against postoperative immunosuppression: Could they reduce recurrence rates in oncological patients? Ann Surg Oncol. 2003;10(8):972–992. doi: 10.1245/aso.2003.02.007. [DOI] [PubMed] [Google Scholar]
- 20.Sinha P, Clements VK, Ostrand-Rosenberg S. Reduction of myeloid-derived suppressor cells and induction of M1 macrophages facilitate the rejection of established metastatic disease. J Immunol. 2005;174(2):636–645. doi: 10.4049/jimmunol.174.2.636. [DOI] [PubMed] [Google Scholar]
- 21.Sica A, Bronte V. Altered macrophage differentiation and immune dysfunction in tumor development. J Clin Invest. 2007;117(5):1155–1166. doi: 10.1172/JCI31422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.NIH 1985. Guide for the Care and Use of Laboratory Animals (National Institutes of Health, Bethesda, MD). NIH Publication No. 85-23, revised 1985.
- 23.Lin KY, et al. Treatment of established tumors with a novel vaccine that enhances major histocompatibility class II presentation of tumor antigen. Cancer Res. 1996;56(1):21–26. [PubMed] [Google Scholar]
- 24.Jackaman C, et al. IL-2 intratumoral immunotherapy enhances CD8+ T cells that mediate destruction of tumor cells and tumor-associated vasculature: A novel mechanism for IL-2. J Immunol. 2003;171(10):5051–5063. doi: 10.4049/jimmunol.171.10.5051. [DOI] [PubMed] [Google Scholar]
- 25.Predina JD, et al. A positive-margin resection model recreates the postsurgical tumor microenvironment and is a reliable model for adjuvant therapy evaluation. Cancer Biol Ther. 2012;13(9):745–755. doi: 10.4161/cbt.20557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He Z, et al. Viral recombinant vaccines to the E6 and E7 antigens of HPV-16. Virology. 2000;270(1):146–161. doi: 10.1006/viro.2000.0271. [DOI] [PubMed] [Google Scholar]
- 27.Aranda F, et al. Adjuvant combination and antigen targeting as a strategy to induce polyfunctional and high-avidity T cell responses against poorly immunogenic tumors. Cancer Res. 2011;71(9):3214–3224. doi: 10.1158/0008-5472.CAN-10-3259. [DOI] [PubMed] [Google Scholar]
- 28.Le DT, et al. A live-attenuated Listeria vaccine (ANZ-100) and a live-attenuated Listeria vaccine expressing mesothelin (CRS-207) for advanced cancers: Phase I studies of safety and immune induction. Clin Cancer Res. 2012;18(3):858–868. doi: 10.1158/1078-0432.CCR-11-2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Judy BF, et al. Vascular endothelial-targeted therapy combined with cytotoxic chemotherapy induces inflammatory intratumoral infiltrates and inhibits tumor relapses after surgery. Neoplasia. 2012;14(4):352–359. doi: 10.1593/neo.12208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Rooijen N, Sanders A. Liposome mediated depletion of macrophages: Mechanism of action, preparation of liposomes and applications. J Immunol Methods. 1994;174(1-2):83–93. doi: 10.1016/0022-1759(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 31.Suzuki E, et al. A novel small-molecule inhibitor of transforming growth factor beta type I receptor kinase (SM16) inhibits murine mesothelioma tumor growth in vivo and prevents tumor recurrence after surgical resection. Cancer Res. 2007;67(5):2351–2359. doi: 10.1158/0008-5472.CAN-06-2389. [DOI] [PubMed] [Google Scholar]
- 32.Predina JD, et al. Cytoreduction surgery reduces systemic myeloid suppressor cell populations and restores intratumoral immunotherapy effectiveness. J Hematol Oncol. 2012;5:34–45. doi: 10.1186/1756-8722-5-34. [DOI] [PMC free article] [PubMed] [Google Scholar]