Abstract
The conservation of endangered fish is of critical importance. Cryobanking could provide an effective backup measure for use in conjunction with the conservation of natural populations; however, methodology for cryopreservation of fish eggs and embryos has not yet been developed. The present study established a methodology capable of deriving functional eggs and sperm from frozen type A spermatogonia (ASGs). Whole testes taken from rainbow trout were slowly frozen in a cryomedium, and the viability of ASGs within these testes did not decrease over a 728-d freezing period. Frozen-thawed ASGs that were intraperitoneally transplanted into sterile triploid hatchlings migrated toward, and were incorporated into, recipient genital ridges. Transplantability of ASGs did not decrease after as much as 939 d of cryopreservation. Nearly half of triploid recipients produced functional eggs or sperm derived from the frozen ASGs and displayed high fecundity. Fertilization of resultant gametes resulted in the successful production of normal, frozen ASG-derived offspring. Feasibility and simplicity of this methodology will call for an immediate application for real conservation of endangered wild salmonids.
Keywords: fish genetic resources, testes cryopreservation, germ cell transplantation, stem cell, triploid trout
Because of the environmental effects of human activities, a growing number of fish species have become threatened or are already extinct (1). Salmonid species are particularly vulnerable to habitat destruction and the effects of climate change as they occupy a unique ecological niche resulting from their long-range migration between freshwater and marine habitats (2, 3). Conservation strategies for these threatened fish must therefore be developed and implemented with all possible haste. Complicating the conservation of threatened salmonid populations are the risks associated with captive breeding and translocation of live fish, such as facility accidents, pathogen infections, genetic drift, and reduced fitness of individuals within natural habitats (4, 5).
Cryobanking of fish gametes to semipermanently (6) store genetic resources could serve as an alternative approach to traditional conservation methods (7); however, past attempts at cryopreservation of fish embryos and mature oocytes have been unsuccessful (8), as a result of their large size, high yolk content, and low membrane permeability (9, 10). Although androgenesis performed by using frozen sperm and γ-ray–inactivated xenogenic eggs can regenerate live fish, their survival rate is extremely low, and resulting offspring become nuclear–cytoplasmic hybrids (11). The loss of maternally inherited materials including mitochondrial DNA makes this method impractical, as it also does for the transfer of nuclei from cryopreserved somatic cells into xenogenic oocytes (12, 13). In zebrafish, in vitro maturation of immature oocytes subsequent to cryopreservation is also impossible. Although techniques for cryopreservation of stage I and II oocytes (diameter, 90–350 μm) (14) and in vitro maturation of late stage III oocytes (diameter, 650–690 μm) (15) have been established, the development of methodology for cryopreservation of late stage III oocytes is still in its experimental stages (16) because of the dramatic changes in cell size and vitellogenic material that are associated with that stage.
Recently, a technique was developed (17) that was capable of producing induced pluripotent stem cells (iPSCs) from frozen somatic cells in several highly endangered mammalian species; however, protocols for generation of functional oocytes from frozen iPSCs have not yet been developed in any animal species. Furthermore, fish iPSCs are not currently available. Use of primordial germ cells (PGCs), which are known to possess sexual plasticity and high transplantability, could serve as an alternative to the use of iPSCs (18–20); however, for endangered species, whose gametes and larvae are not easily obtainable as a result of their decreased effective population size and lack of established breeding techniques, PGCs are unavailable because they can only be obtained from early-stage larvae that are typically produced via artificial propagation.
The authors of the present study previously outlined a surrogate broodstock technology (21) used to produce donor-derived eggs and sperm by transplanting germ cells into sterile triploid recipients in salmonids. Intraperitoneally transplanted spermatogonial stem cells (SSCs) migrated toward, and were eventually incorporated into, recipient gonads. The transplanted SSCs resumed spermatogenesis and oogenesis in male and female recipients, respectively, and ultimately differentiated into functional sperm or eggs within the recipient gonads (22, 23). The present study attempted to produce functional eggs and sperm from cryopreserved whole testes through i.p. transplantation of frozen testicular cells.
Results
Optimization of Cryopreservation Conditions for Trout Whole Testes.
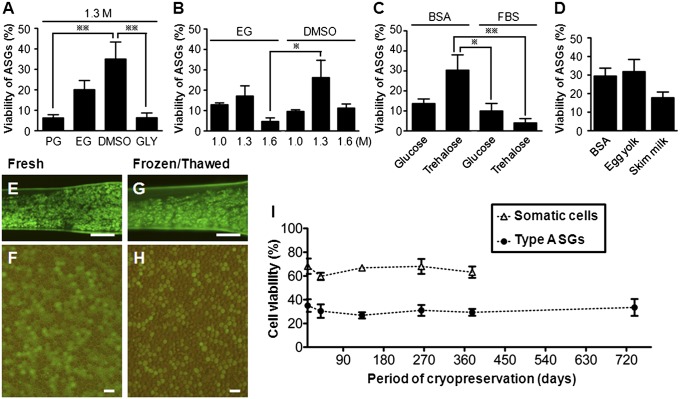
The viability of type A spermatogonia (ASGs) frozen with cryomedium containing 1.3 M DMSO was significantly higher than for those frozen with cryomedium containing 1.3 M propylene glycol (PG) or 1.3 M glycerol (GLY; Fig. 1A). Of the testes cryopreserved with cryomedium containing ethylene glycol (EG) or DMSO at various concentrations (1.0 M, 1.3 M, or 1.6 M), ASGs derived from those frozen with 1.3 M DMSO tended to exhibit the highest rate of survival (Fig. 1B). The effects of nonpermeating cryoprotectant agents dissolved in cryomedium containing 1.3 M DMSO and 35.2% (vol/vol) extender on ASG survival were also assessed. The viability of ASGs obtained from testes whose cryomedium contained 0.1 M trehalose and 1.5% (wt/vol) BSA was significantly higher than for those obtained from testes whose cryomedium contained 0.1 M glucose and 1.5% (vol/vol) FBS or 0.1 M trehalose and 1.5% (vol/vol) FBS (Fig. 1C). Finally, the effects of 1.5% (wt/vol) BSA, 10% (vol/vol) egg yolk, and 1.5% (wt/vol) skim milk dissolved in a cryo-medium containing 1.3 M DMSO, 0.1 M trehalose, and 35.2% (vol/vol) extender on survival of ASGs were elucidated and compared. It was determined that ASGs obtained from testes whose cryomedium contained 10% (vol/vol) egg yolk displayed the highest rates of survival (Fig. 1D).
Fig. 1.
Optimization of cryopreservation conditions for trout testes. (A) Viability of ASGs in frozen-thawed testes with medium containing 1.3 M PG, EG, DMSO, or GLY (**P < 0.01; n = 4) cryoprotectants. (B) Viability of ASGs with medium containing EG or DMSO at various concentrations (1.0 M, 1.3 M, or 1.6 M; *P < 0.05; n = 3–4). (C) Viability of ASGs with medium containing 0.1 M glucose or trehalose cryoprotectants with BSA or FBS (*P < 0.05 and **P < 0.01; n = 6). (D) Viability of ASGs with BSA, egg yolk, or skim milk with medium containing 1.3 M DMSO and 0.1 M trehalose (n = 5–14). Fresh testis (E) in donor trout and dissociated testicular cells (F). Frozen-thawed testis (G) by optimized freezing method and dissociated testicular cells (H). (I) Viability of ASGs and gonadal somatic cells in testes cryopreserved for 1 to 728 d. There were no significant differences of cell viability among different cryopreservation periods (n = 3–5). Data are shown as mean ± SEM. (Scale bars: E and G, 1 mm; F and H, 20 μm.)
The viability of ASGs cryopreserved with a cryomedium (pH 7.8, 0.296 Osm/kg) containing 1.3 M DMSO, 0.1 M trehalose, 10% (vol/vol) egg yolk, and 35.2% (vol/vol) extender for periods of 1, 30, 125, 238, 367, and 728 d was assessed through a combination of flow cytometry and trypan blue (TB) staining (Fig. 1 E–H). The viabilities of both ASGs [viability at 1 d (35.1 ± 5.3%) and at 728 d (33.5 ± 7.1%)] and testicular somatic cells did not exhibit significant changes with increasing durations of cryopreservation (Fig. 1I).
Transplantability of Cryopreserved Testicular Cells.
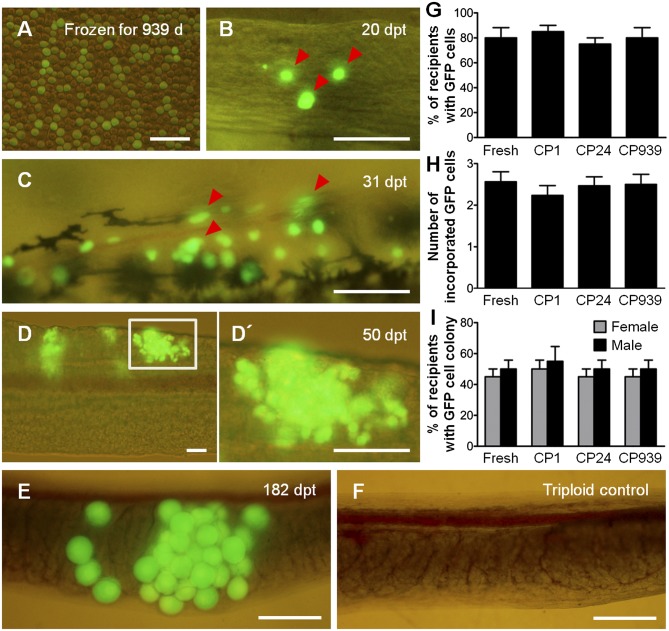
To determine whether testicular cells (Fig. 2A) prepared from long-term–frozen testes maintained their transplantability, the transplantation efficiencies of testicular cells frozen for 1, 24, and 939 d were compared with those of freshly prepared testicular cells. Allogenic recipients were dissected at 20, 31, 50, and 182 d posttransplantation (pt), and the behavior of donor-derived ASGs was observed. By 20 d pt, intraperitoneally transplanted GFP-labeled donor ASGs that had been frozen for 939 d had migrated toward, and were incorporated into, the genital ridges in 16 of 20 recipients examined (Fig. 2 B and G). The mean number of incorporated ASGs was 2.5 ± 0.2 (Fig. 2H) in the 939-d-frozen group. Between 31 and 50 d pt, ASGs frozen for 939 d began to proliferate (Fig. 2C) and formed colonies within the recipient gonads in 18 of 40 female recipients and 20 of 40 male recipients (Fig. 2 D, D′, and I). At 182 d pt, GFP-positive oocytes, which were derived from frozen donor ASGs, were found within the recipient ovary (Fig. 2E). Colonization (Fig. 2G) and proliferation efficiencies (Fig. 2I) of donor-derived ASGs within recipient gonads as well as numbers of incorporated ASGs (Fig. 2H) were not significantly different among ASGs cryopreserved for periods of 1, 24, and 939 d and freshly prepared ASGs.
Fig. 2.
Transplantability of long-term cryopreserved ASGs. (A) Testicular cells dissociated from testis cryopreserved for 939 d. (B) Donor-derived ASGs (arrowheads) showing green fluorescence were incorporated into recipient gonads. (C and D) Incorporated ASGs started to proliferate (arrowheads, C) and made colonies within the recipient gonads (D). (D′) Boxed area in D shown at high magnification. (E and F) Donor-derived GFP-labeled ASGs differentiated into oocytes within a female recipient (E) and the ovary of a nontransplanted triploid control (F). (G–I) The percentage of recipients that contained donor ASGs within their gonads (n = 20; G), number of ASGs incorporated into the recipient gonad (n = 20; H), and percentage of female (gray bars) and male (black bars) recipients having colonies with proliferated donor ASGs (n = 80; I) were not significantly different among ASGs cryopreserved for 1 d (CP 1), 24 d (CP 24), and 939 d (CP 939), as well as freshly prepared ASGs (fresh). Data are shown as mean ± SEM. (Scale bars: A, 50 μm; B–F, 100 μm.)
Production of Functional Eggs Derived from Frozen ASGs.
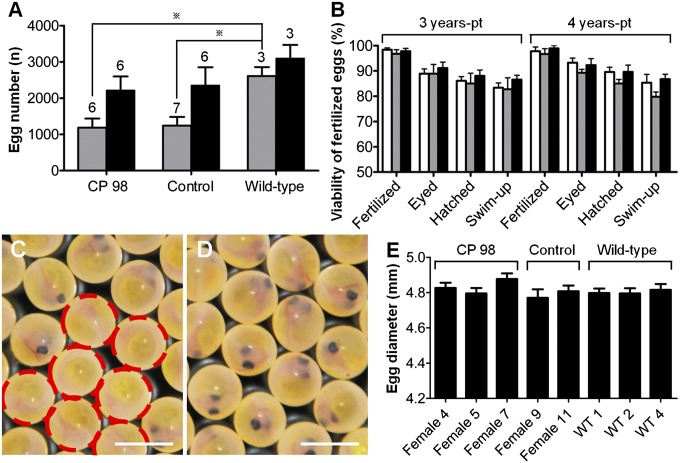
To confirm the functionality of eggs derived from frozen ASGs, progeny tests were performed by using triploid female recipients that had received frozen testicular cells (CP 98; i.e., cryopreservation for 98 d) from dominant orange-colored mutant (heterozygous, orange/WT) vasa-GFP transgenic (hemizygous, vasa-GFP/−) trout. Although none of the triploid females that had not received testicular cell transplantation matured, six of 14 CP 98 females (42.9%) reached sexual maturity at 3 y pt whereas six of 13 CP 98 females (46.2%) reached sexual maturity at 4 y pt (Table 1). These rates were similar to those obtained for control recipients that had received freshly prepared testicular cells (Table 1). The number of eggs per fish in CP 98 females at 3 and 4 y pt (1,183 ± 254 and 2,216 ± 385, respectively) did not significantly differ from the number of eggs obtained from control recipients of the same ages (1,243 ± 240 and 2,351 ± 500, respectively; Fig. 3A). The mean number of eggs in 3-y-old CP 98 females was significantly lower than that of WT trout of the same age (Fig. 3A); however, at 4 y pt, the mean number of eggs per fish in CP 98 females was not significantly different from that of WT trout (Fig. 3A).
Table 1.
Production of gametes in triploid recipients through transplantation of testicular cells taken from cryopreserved whole testes
| Group | Duration in LN2, d | No. of fish | GFP (+) germ cells transplanted per fish | No. of fish matured/survived (%) |
|||||
| 2 y pt |
3 y pt |
4 y pt |
|||||||
| F | M | F | M | F | M | ||||
| CP 98* | 98 | 70 | 5,000 | 0/20 | 4/21 (19.0) | 6/14 (42.9) | 8/17 (47.1) | 6/13 (46.2) | 9/16 (56.3) |
| Control† | — | 100 | 5,000 | 0/26 | 6/27 (22.2) | 7/18 (38.9) | 11/24 (45.8) | 6/16 (37.5) | 11/20 (55.0) |
| WT‡ | — | 35 | 0 | 2/6 (33.3) | 4/7 (57.1) | 3/4 (75.0) | 4/4 (100) | 3/4 (75.0) | 4/4 (100) |
| Triploid§ | — | 63 | 0 | NE | NE | 0/24 | 0/25 | 0/21 | 4/23 (17.4)¶ |
NE, not examined.
*Recipients received testicular cells taken from whole testes cryopreserved for 98 d.
†Recipients received freshly prepared testicular cells.
‡WT diploid trout that did not undergo transplantation.
§WT triploid trout that did not undergo transplantation.
¶Males produced small amount of aneuploid sperm.
Fig. 3.
Production of functional eggs derived from cryopreserved ASGs. (A) Number of eggs produced by female recipients that received ASGs frozen for 98 d (CP 98), recipients with freshly prepared ASGs (control), and WT trout at 3 y (gray bars) and 4 y pt (black bars). Numbers above each bar indicate the number of mature trout (*P < 0.05 at 3 y pt). There were no significant differences among groups at 4 y pt. (B) Developmental performances of eggs obtained from CP 98 (white bars), control (gray bars), and WT females (black bars). Eggs were fertilized with milt obtained from WT males at 3 and 4 y pt. There were no significant differences within developmental stages at the same age (n = 3–7). (C) Eyed-stage eggs derived from a female CP 98. Nearly half of the eggs displayed the orange-color donor-derived phenotype (dashed circles). (D) Eyed-stage eggs of WT trout as a control of C. (E) Diameter of eggs obtained from CP 98, control, and WT females at 4 y pt (n = 50). Data are shown as mean ± SEM. (Scale bars: C and D, 5 mm.)
To determine whether eggs obtained from CP 98 females possessed normal developmental potency, the eggs were fertilized with milt obtained from WT males. Developmental performances [including fertilization rates (eggs from 3-y-old CP 98, 98.4 ± 0.7%; eggs from 4-y-old CP 98, 97.8 ± 1.6%) and swim-up rates (fry from 3-y-old CP 98, 83.3 ± 1.9%; fry from 4-y-old CP 98, 85.3 ± 3.3%)] of eggs obtained from female recipients were not significantly different from those obtained from control recipients and WT females (Fig. 3B). Nearly half of all eyed-stage eggs displayed the donor-derived phenotype [i.e., eggs possessed orange body color (Fig. 3C, dashed circles) and were vasa-GFP transgenic]. Furthermore, the diameter of eggs produced by CP 98 females was not significantly different from the diameters in control recipients and WT females (Fig. 3E).
Production of Functional Sperm by Using Surrogate Triploid Recipients.
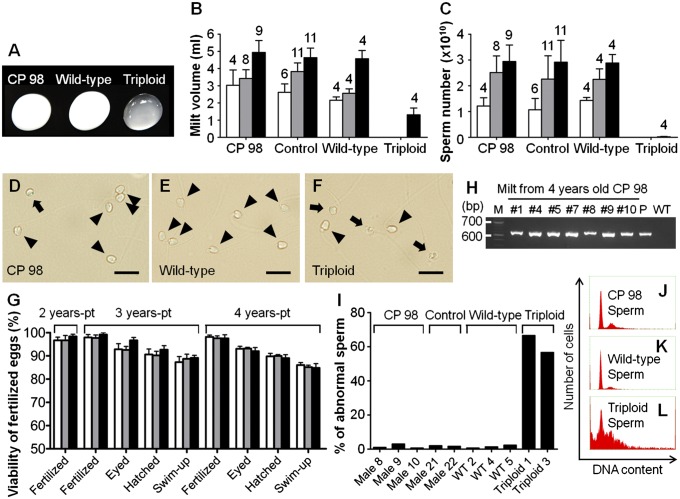
Although the majority of triploid males that had not received testicular cell transplantation did not produce milt, a small number did produce milt containing aneuploid sperm (Fig. 4 A–C, F, and I). Four of 21 (19.0%), eight of 17 (47.1%), and nine of 16 CP 98 males (56.3%) reached sexual maturity at 2, 3, and 4 y pt, respectively (Table 1), after which they all produced milt that was white in color and was indistinguishable from that of WT trout (Fig. 4A). Milt volumes (3.0 ± 0.9 mL, 3.4 ± 0.5 mL, and 4.9 ± 0.7 mL, respectively) and sperm counts (1.2 ± 0.3 × 1010, 2.5 ± 0.7 × 1010, and 2.9 ± 0.7 × 1010, respectively) obtained from CP 98 males did not significantly differ from those of control recipients and WT diploid trout of the same age (Fig. 4 B and C). Furthermore, morphologies of the sperm obtained from CP 98 males (Fig. 4D) appeared similar to those of WT males (Fig. 4E) when observed through an optical microscope.
Fig. 4.
Production of functional sperm derived from cryopreserved ASGs. (A) Milt obtained from a male recipient that received ASGs frozen for 98 d (CP 98), WT diploid, and triploid trout. Milt volume (B) and sperm number (C) produced by CP 98, recipients that received freshly prepared ASGs (control), WT diploid, and triploid trout. Numbers above each bar indicate the number of mature trout. No significant differences were found among groups of the same ages excluding triploid (P < 0.05). (D–F) Sperm obtained from a CP 98 (D), WT diploid (E), and triploid trout (F). Arrowheads indicate morphologically normal sperm; arrows indicate abnormal sperm. (G) Viability of eggs produced with milt obtained from CP 98 (white bars), control (gray bars), and WT (black bars) males. No significant differences within developmental stages were found among specimens of the same age (P < 0.05; n = 4–11). (H) PCR analysis of CP 98 milts with GFP-specific primers. Lanes were labeled as follows: M, MW marker, no. 1, 4, 5, 7, 8, 9, 10: milt obtained from CP 98, P, GFP-plasmid; WT, milt of WT trout. Milt of no. 2 and no. 6 was used only for fertilization as a result of its smaller volume. No. 3 was dead after maturation at 3 y of age. (I) Percentage of abnormal sperm obtained from CP 98, control, WT, and triploid trout (n = 300–302). (J–L) DNA contents of sperm obtained from a CP 98 (J), WT diploid (K), and triploid trout (L). Data are shown as mean ± SEM. (Scale bars: D–F, 10 μm.)
To determine whether sperm produced from CP 98 males were functional, the milt produced was used to fertilize eggs obtained from female WT trout. Developmental performances of eggs generated from CP 98 males were not significantly different from those generated from control recipients and WT males (Fig. 4G). The genetic origins of recipient sperm were examined by using PCR with GFP-specific primers. All milt obtained from CP 98 males tested positive for the presence of GFP gene (Fig. 4H). Although triploid males that did not undergo transplantation also reached sexual maturity at the age of 4 y, the amount of sperm they produced was extremely small (Fig. 4C) and the frequency of morphological abnormalities was high (37 and 43 times the rates observed in transplant recipients and WT sperm, respectively; Fig. 4I). Furthermore, DNA content analyses with flow cytometry revealed that the sperm of triploid males that did not undergo transplantation showed high levels of aneuploidy (Fig. 4L), whereas the sperm of CP 98 males (Fig. 4J) and WT trout (Fig. 4K) did not. To further analyze the sperm produced by triploid trout that had not received transplants, the milt obtained from these trout were used to fertilize eggs obtained from WT trout. Of the 381 eggs inseminated, only one embryo hatched, and the resulting hatchling died within 35 d postfertilization (Fig. S1 A–C and Table S1).
Inheritance of Donor-Derived Haplotype to F1 Generation.
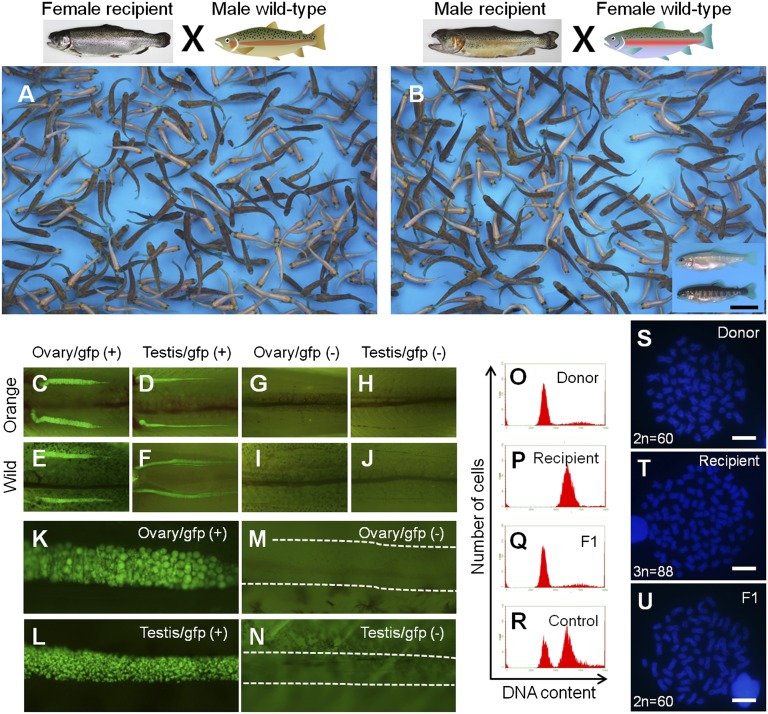
To clarify whether the donor-derived haplotype was transmitted to the F1 generation, body color and green fluorescence of F1 juveniles was examined. In the F1 juveniles produced by CP 98 females at 3 and 4 y pt, the ratios of orange-colored fish [48.5 ± 0.8% (3 y pt) and 49.2 ± 0.5% (4 y pt)] and the ratios of vasa-GFP (+) fish [47.7 ± 0.8% (3 y pt) and 47.9 ± 1.6% (4 y pt)] were nearly 1:1 (Fig. 5 A and C–N and Table S2). In the F1 juveniles produced by CP 98 males at 2, 3, and 4 y pt, the donor-derived haplotypes of orange color and vasa-GFP (+) were also transmitted following Mendelian inheritance (Fig. 5 B–N and Table S3). Phenotypic ratios of F1 juveniles generated from control recipients that had received fresh testicular cells were also close to 1:1, and the F1 progeny of WT trout that had not received transplants were completely devoid of orange-colored and vasa-GFP individuals (Tables S2 and S3). DNA content analyses with flow cytometry was conducted on 20 randomly selected F1 juveniles from the offspring of CP 98 females and a further 20 F1 juveniles selected from the offspring of CP 98 males. Results of these analyses revealed that all 40 F1 juveniles were diploid and that none of them showed any signs of aneuploidy (Fig. 5Q). All mature recipients were identified as triploids through flow cytometry analyses (Fig. 5P), with the exception of one female and two male recipients that failed triploidy induction and were not used in progeny tests. Polymorphism of karyotype in rainbow trout (24) was further used to identify the genetic origin of offspring. Chromosome numbers of the donor strain and triploid recipients were 60 and 88, respectively (Fig. 5 S and T). Karyotype analyses of 10 randomly selected F1 juveniles produced by transplant recipients (CP 98 females, nos. 4, 5, and 7; and CP 98 males, nos. 4 and 9) revealed that each juvenile possessed a normal karyotype consisting of 60 chromosomes with 104 arm numbers (Fig. 5U). These results, when considered alongside the facts that ∼50% of offspring showed donor trout-derived phenotypes (GFP-positive and orange-colored) and that all F1 juveniles showed cytogenetic characteristics (DNA content and karyotype) identical to those of the donor strain, indicated that the F1 offspring obtained from triploid transplant recipients were all derived from donor-frozen testes.
Fig. 5.
Germ-line transmission of frozen testis-derived haplotype to F1 generation. (A and B) Trout juveniles generated from a female CP 98 (A) and a male CP 98 (B) in conjunction with gametes obtained from a male and female WT trout, respectively. The boxed area in B shows the two phenotypic colors of F1 progeny. Donor-derived orange-color trout were observed in the F1 generation along with WT trout. (C–J) Gonadal appearance of donor-derived vasa-GFP transgenic trout in the F1 generation. Ovary of an orange-colored transgenic (C), testis of an orange-colored transgenic (D), ovary of a WT transgenic (E), testis of a WT transgenic (F), ovary of an orange-colored nontransgenic (G), testis of an orange-colored nontransgenic (H), ovary of a WT nontransgenic (I), and testis of a WT nontransgenic (J) trout. (K–N) Gonads of F1 juveniles at high magnification. Ovary of a transgenic (K), testis of a transgenic (L), ovary of a nontransgenic (M), and testis of a nontransgenic (N) trout. Dashed lines indicate gonads. (O–R) DNA contents of a donor (O), triploid recipient (P), F1 juvenile (Q), and mixture of diploid and triploid WT controls (R). (S–U) Karyotype of a donor (2n = 60; S), triploid recipient (3n = 88; T), and F1 juvenile (2n = 60; U). F1 juveniles possessed the same karyotype as that of a donor trout (2n = 60). (Scale bars: B, 1 cm; S–U, 10 μm.)
Discussion
Functional eggs and sperm were successfully derived from frozen testicular germ cells through the i.p. transplantation of those germ cells into sterile triploid hatchlings. It is worth noting that the viability and transplantability of ASGs obtained from frozen testes did not vary with changes to the duration of the cryopreservation period. Furthermore, the fecundity of recipients receiving frozen testicular cells was at a level that would allow for practical application of the new technology for mass production of offspring from the germ cell donors. All triploid trout that did not undergo transplantation were sterile before the age of 4 y, at which point males were capable of producing small amounts of aneuploid sperm, which suggested that all haploid gametes produced by triploid transplant recipients were derived from donor testicular cells. Offspring produced by transplant recipients were not only developmentally and morphologically normal, but were genotypically identical to the donor trout as well. A noteworthy advantage of the methodology outlined in the present study is that the freezing procedure used was quite simple. Indeed, the only requirement for cryopreservation of whole testes is a location equipped with a deep freezer (e.g., most trout hatcheries). The applicability of the methodology outlined here is further enhanced by the fact that frozen whole testes of any developmental stage could allow for the mass isolation of SSCs, thereby producing sufficient material for transplantation into sterile recipients. These results demonstrated that this methodology could be immediately applied on-site to the conservation of endangered wild salmonids.
For the thousands of fish species that are currently threatened with extinction (1), the rapid development of practical conservation strategies is of crucial importance. The use of captive breeding programs is currently the most widely used method, and it carries with it several risks related to problems in rearing systems (e.g., accidental fatalities and pathogen infections). For salmonids in particular, the time required to introduce organisms reared in captivity into their natural habitats is significant. This is the result of associated processes that must be undertaken alongside captive rearing, such as dam decommissioning, elimination of introgressed populations, and improvement of water quality. Therefore, during the resultant prolonged periods of captive breeding, fish can potentially lose genetic diversity and fitness for their natural habitats (4, 5). Cryobanking allows for the long-term preservation (6) of fish species and their genetic diversity, and could therefore be a valid alternative to, or represent an enhancement of, captive breeding programs. Despite its potential applicability, cryopreservation methods for eggs and embryos have not previously been developed for any fish species (8). In the present study, it was demonstrated that ASGs taken from frozen testes possessed the ability to differentiate into oocytes when transplanted into female recipients, which suggested that at least some frozen ASGs possessed high levels of sexual plasticity. These findings that functional eggs can be produced from frozen testicular material offers a solution to the problem of lack of techniques for fish egg or embryo cryopreservation. Another important finding of the present study was that triploid recipients were capable of producing large amounts of frozen ASG-derived gametes for at least three consecutive spawning seasons. These results indicated that frozen ASGs incorporated into recipient gonads, behaved as SSCs capable of differentiating into functional gametes and possessed a high, or even unlimited, ability to self-renew.
The authors of the present study and other research groups had previously developed cryopreservation techniques for fish PGCs (19, 20); however, these techniques were applicable to only PGCs derived from early-stage larvae, which would likely be extremely difficult to obtain from natural environments if the target species were endangered. Therefore, the use of ASGs, which can be harvested in large quantities from male fish of any age, makes the methodology outlined in the present study suitable for use in endangered salmonids. In fact, the ASGs collected from one frozen testis (∼0.014 g) provides sufficient material for transplantation into >150 recipients. Moreover, under field conditions, cryopreservation of the whole testes would be more practical than attempts at cryopreservation of dissociated testicular cells or purified spermatogonia.
The method of i.p. transplantation of cryopreserved ASGs into hatched larvae established in the present study is currently the only available method for long-term, or even semipermanent, preservation of fish genetic resources. The simplicity and feasibility of this methodology are what enable it to pave the way toward effective, reliable, and practical conservation of endangered salmonid species and locally endangered salmonid populations, such as bull trout (Salvelinus confluentus), gila trout (Oncorhynchus gilae), sockeye salmon (Oncorhynchus nerka; ref. 1, 25) and kunimasu trout (Oncorhynchus kawamurae) (26). As long as whole testes have been cryopreserved, even offspring derived from extinct species could be regenerated by using a closely related species as a surrogate and a combination of the techniques established here, as well as the technique for interspecies transplantation of ASGs (21) previously developed by the authors of the present study.
Materials and Methods
Fish and Testes Preparation.
All experiments were carried out in accordance with the Guidelines for the Care and Use of Laboratory Animals of Tokyo University of Marine Science and Technology. Rainbow trout (Oncorhynchus mykiss) used in the present study were maintained at the Oizumi Station of the Field Science Center of Tokyo University of Marine Science and Technology (Yamanashi, Japan). Immature whole testes [testis weight, 0.014 ± 0.001 g; gonad-somatic index (gonad weight divided by body weight × 100), 0.091 ± 0.004%] were obtained from 8- to 11-mo-old dominant orange-colored (heterozygous, OR/WT) (27) vasa-GFP transgenic (hemizygous, vasa-GFP/−) (28, 29) rainbow trout donors (standard length, 11.4 ± 0.2 cm), and ASGs were labeled by using the GFP gene (30). Collected testes were maintained in Eagle minimum essential medium (EMEM; Nissui) supplemented with 5% (vol/vol) FBS (Gibco), 25 mM Hepes (Sigma-Aldrich), and 2 mM L-glutamine (Sigma-Aldrich), and kept on ice before use. WT triploid rainbow trout were used as recipients for germ-cell transplantation. The recipients were made by mating females of the Okutama strain (chromosome number, 2n = 58) originally established in Okutama Branch of Tokyo Fisheries Experimental Station (Tokyo, Japan) and males of the Oizumi strain (chromosome number, 2n = 60), as described earlier. Triploids were induced through heat shock of fertilized eggs at 28 °C for 10 min subsequent to a 15-min postfertilization treatment at 10 °C (31).
Cryopreservation of Whole Testes.
Whole testes obtained from vasa-GFP trout were transferred into TPP CryoTubes (1.2 mL) containing 500 μL of cryomedium composed of permeating cryoprotectants (PG, EG, DMSO, or GLY [all from Wako] with concentrations of 1.0 M, 1.3 M, or 1.6 M), nonpermeating cryoprotectants [0.1 M D-glucose (Sigma-Aldrich) or 0.1 M D-(+)-trehalose dihydrate (Sigma-Aldrich) and 1.5% (wt/vol) BSA (Sigma-Aldrich), 1.5% (vol/vol) FBS, 10% (vol/vol) fresh egg yolk, or 1.5% (wt/vol) skim milk powder (Sigma-Aldrich)], and 35.2% (vol/vol) extender [100% extender; 55.27 mM Hepes, 375.48 mM NaCl (Wako), 7.28 mM KCl (Wako), 23.10 mM KH2PO4 (Wako), 3.82 mM Na2HPO4 (Wako), 3.64 mM sodium pyruvate (Sigma-Aldrich), 2.6 mM CaCl2·2H2O (Wako) and 1.4 mM MgCl2·6H2O (Wako), pH 7.8; n = 3–14] before being equilibrated on ice for 60 min. CryoTubes were then frozen at a rate of −1 °C/min for a period of 90 min by using a Bicell plastic freezing container (Nihon Freezer) located in a deep freezer (−80 °C) before being plunged into liquid nitrogen. After at least 1 d of cryopreservation, the tubes were thawed in a 10 °C water bath for 1 min. Cryomedium attached to testes samples was gently removed by using Kimwipes (S-200; Kimberly-Clark), and samples were rehydrated in three changes of EMEM supplemented with 5% (vol/vol) FBS, 25 mM Hepes, and 2 mM L-glutamine. Osmolality of cryomedium was determined by using an OSMOMAT 030 (Gonotec).
Assessment of Cell Viability.
Fresh (Fig. 1E) and frozen-thawed testes (Fig. 1G) were minced and dissociated by using trypsin in accordance with methodology previously developed by Okutsu et al. (22). The resultant cell suspension was filtered through a 42-μm-pore nylon screen (NBC Industries) to eliminate nondissociated cell clumps, and subsequently observed under a fluorescent microscope (BX-51-34FL; Olympus). Cryoinjury of ASGs (presumably membrane damage) resulted in the loss of GFP gene expression, whereas living ASGs exhibited green fluorescence. Therefore, green fluorescence of ASGs was used as an indicator of viability (32). The majority of cryoinjured cells were lysed by protease activity during the dissociation procedure. As the total numbers of ASGs within the right and left testes of a given individual were almost identical, the numbers of GFP (+) ASGs in frozen (for 1, 30, 125, 238, 367, and 728 d) testes and fresh testes were compared with determine the cell viability of cryopreserved ASGs (n = 3–5). Furthermore, it was determined by TB assay (Gibco) that some GFP (+) ASGs that had dissociated from thawed testes were nonviable. Viability of ASGs (as a percentage) was calculated by using the following formula: number of GFP (+) and TB (−) ASGs in frozen-thawed testis divided by number of GFP (+) ASGs in fresh testis multiplied by 100. Viability of testicular somatic cells (as a percentage) was calculated by using the following formula: number of GFP (−) and TB (−) ASGs in frozen-thawed testis divided by number of GFP (−) ASGs in fresh testis multiplied by 100. The total number of ASGs (i.e., GFP-positive cells) and testicular somatic cells (i.e., GFP-negative cells) was counted by using the Guava PCA-96 flow cytometry system (Millipore).
Transplantation of Testicular Cells Prepared from Frozen Whole Testes.
Approximately 5,000 ASGs taken from whole testes cryopreserved for 1, 24, 98 (i.e., CP 98), and 939 d, along with freshly prepared control ASGs, were transplanted into the peritoneal cavity of WT triploid hatchlings. i.p. transplantation of testicular cells was carried out in accordance with methodology previously described by Takeuchi et al. (33). To determine incorporation (n = 20) and proliferation efficiencies (n = 80) of ASGs in the recipient gonads, recipients were dissected at 20 or 50 d pt and observed under a fluorescent microscope (BX-51-34FL; Olympus). The total number of ASGs incorporated into both genital ridges in each recipient at 20 d pt was also determined (n = 20).
Progeny Tests.
Female and male recipients matured at the ages of 3 to 4 y and 2 to 4 y, respectively. Eggs and milts produced by recipients were obtained by using abdominal pressure. Eggs and milts were examined under a light microscope (BX-51; Olympus) for morphological observation, as well as determination of diameter (eggs) and sperm count (milt). To determine the production of spermatozoa derived from donor frozen ASGs, total genomic DNA was extracted from the semen of recipients and WT diploid trouts and subjected to PCR analyses with the use of GFP-specific primers (34). Sperm number was calculated by using the following formula: whole milt volume (in milliliters) multiplied by spermatozoa density (number per milliliter) multiplied by 100. The number of eggs was also determined. To determine the production of offspring by gametes derived from donor frozen ASGs, eggs and sperm obtained from triploid recipients were fertilized in vitro with sperm and eggs obtained from WT trout. As donor testes were obtained from dominant orange-colored (heterozygous, OR/WT) vasa-GFP transgenic (hemizygous, vasa-GFP/−) trout, F1 larvae would be expected to exhibit a 50% ratio of donor phenotypes (OR/WT and GFP/−) following Mendelian inheritance if they were donor-derived.
Flow Cytometry.
Erythrocytes and spermatozoa collected from recipients were filtered through a 42-μm-pore nylon screen. Cells were fixed in 70% (vol/vol) ethanol and incubated for a period of 30 min at 20 °C in PBS (pH 7.8) that contained RNase A (10 μg/mL; Sigma) and propidium iodide (200 μg/mL; Sigma). DNA content analysis was performed by using a Guava PCA-96 flow cytometry system (Millipore).
Karyotype Analysis.
Chromosome preparations were obtained from the anterior kidneys of recipients and F1 offspring. Kidney cells were incubated using 0.4% (wt/vol) colchicine (Gibco) in EMEM supplemented with 10% (vol/vol) FBS, 25 mM Hepes, and 2 mM L-glutamine for a period of 5 h at 10 °C, treated with hypotonic 0.075 M KCl (Gibco) for a period of 30 min at 20 °C, and fixed in methanol/acetic acid (vol/vol; 3:1). The resulting cell suspension was then dropped and spread on microscope slides. Chromosomes were stained in Vectashield mounting medium (Vector) containing DAPI (1.5 μg/mL; Invitrogen) for 10 min at room temperature. A number of well-spread chromosomes were then observed under a fluorescent microscope (BX-51-34FL; Olympus), with at least 20 metaphase spreads being observed in each preparation.
Statistical Analysis.
All data are presented as mean values ± SEM. Statistical significance was determined by using one-way ANOVA followed by Tukey multiple comparisons test using a statistical significance level of P < 0.05. All analyses were carried out by using GraphPad Prism version 5.0 (GraphPad).
Supplementary Material
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1218468110/-/DCSupplemental.
References
- 1.International Union for Conservation of Nature . IUCN Red List of Threatened Species. Gland, Switzerland: IUCN Species Survival Commission; 2012. Available at www.iucnredlist.org. Accessed October 25, 2012. [Google Scholar]
- 2.Pereira HM, et al. Scenarios for global biodiversity in the 21st century. Science. 2010;330(6010):1496–1501. doi: 10.1126/science.1196624. [DOI] [PubMed] [Google Scholar]
- 3.Battin J, et al. Projected impacts of climate change on salmon habitat restoration. Proc Natl Acad Sci USA. 2007;104(16):6720–6725. doi: 10.1073/pnas.0701685104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Christie MR, Marine ML, French RA, Blouin MS. Genetic adaptation to captivity can occur in a single generation. Proc Natl Acad Sci USA. 2012;109(1):238–242. doi: 10.1073/pnas.1111073109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Araki H, Cooper B, Blouin MS. Genetic effects of captive breeding cause a rapid, cumulative fitness decline in the wild. Science. 2007;318(5847):100–103. doi: 10.1126/science.1145621. [DOI] [PubMed] [Google Scholar]
- 6.Glenister PH, Whittingham DG, Lyon MF. Further studies on the effect of radiation during the storage of frozen 8-cell mouse embryos at -196 ° C. J Reprod Fertil. 1984;70(1):229–234. doi: 10.1530/jrf.0.0700229. [DOI] [PubMed] [Google Scholar]
- 7.Williams SE, Hoffman EA. Minimizing genetic adaptation in captive breeding programs: A review. Biol Conserv. 2009;142:2388–2400. [Google Scholar]
- 8.Mazur P, Leibo SP, Seidel GE., Jr Cryopreservation of the germplasm of animals used in biological and medical research: Importance, impact, status, and future directions. Biol Reprod. 2008;78(1):2–12. doi: 10.1095/biolreprod.107.064113. [DOI] [PubMed] [Google Scholar]
- 9.Hagedorn M, Kleinhans FW, Artemov D, Pilatus U. Characterization of a major permeability barrier in the zebrafish embryo. Biol Reprod. 1998;59(5):1240–1250. doi: 10.1095/biolreprod59.5.1240. [DOI] [PubMed] [Google Scholar]
- 10.Hagedorn M, et al. Magnetic resonance microscopy and spectroscopy reveal kinetics of cryoprotectant permeation in a multicompartmental biological system. Proc Natl Acad Sci USA. 1996;93(15):7454–7459. doi: 10.1073/pnas.93.15.7454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Babiak I, et al. Androgenesis in rainbow trout using cryopreserved spermatozoa: the effect of processing and biological factors. Theriogenology. 2002;57(4):1229–1249. doi: 10.1016/s0093-691x(02)00631-3. [DOI] [PubMed] [Google Scholar]
- 12.Folch J, et al. First birth of an animal from an extinct subspecies (Capra pyrenaica pyrenaica) by cloning. Theriogenology. 2009;71(6):1026–1034. doi: 10.1016/j.theriogenology.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 13.Loi P, et al. Genetic rescue of an endangered mammal by cross-species nuclear transfer using post-mortem somatic cells. Nat Biotechnol. 2001;19(10):962–964. doi: 10.1038/nbt1001-962. [DOI] [PubMed] [Google Scholar]
- 14.Tsai S, Rawson DM, Zhang T. Development of cryopreservation protocols for early stage zebrafish (Danio rerio) ovarian follicles using controlled slow cooling. Theriogenology. 2009;71(8):1226–1233. doi: 10.1016/j.theriogenology.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 15.Seki S, et al. Development of a reliable in vitro maturation system for zebrafish oocytes. Reproduction. 2008;135(3):285–292. doi: 10.1530/REP-07-0416. [DOI] [PubMed] [Google Scholar]
- 16.Seki S, et al. Cryobiological properties of immature zebrafish oocytes assessed by their ability to be fertilized and develop into hatching embryos. Cryobiology. 2011;62(1):8–14. doi: 10.1016/j.cryobiol.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 17.Ben-Nun IF, et al. Induced pluripotent stem cells from highly endangered species. Nat Methods. 2011;8(10):829–831. doi: 10.1038/nmeth.1706. [DOI] [PubMed] [Google Scholar]
- 18.Takeuchi Y, Yoshizaki G, Takeuchi T. Biotechnology: Surrogate broodstock produces salmonids. Nature. 2004;430(7000):629–630. doi: 10.1038/430629a. [DOI] [PubMed] [Google Scholar]
- 19.Kobayashi T, Takeuchi Y, Takeuchi T, Yoshizaki G. Generation of viable fish from cryopreserved primordial germ cells. Mol Reprod Dev. 2007;74(2):207–213. doi: 10.1002/mrd.20577. [DOI] [PubMed] [Google Scholar]
- 20.Higaki S, et al. Production of fertile zebrafish (Danio rerio) possessing germ cells (gametes) originated from primordial germ cells recovered from vitrified embryos. Reproduction. 2010;139(4):733–740. doi: 10.1530/REP-09-0549. [DOI] [PubMed] [Google Scholar]
- 21.Okutsu T, Shikina S, Kanno M, Takeuchi Y, Yoshizaki G. Production of trout offspring from triploid salmon parents. Science. 2007;317(5844):1517. doi: 10.1126/science.1145626. [DOI] [PubMed] [Google Scholar]
- 22.Okutsu T, Suzuki K, Takeuchi Y, Takeuchi T, Yoshizaki G. Testicular germ cells can colonize sexually undifferentiated embryonic gonad and produce functional eggs in fish. Proc Natl Acad Sci USA. 2006;103(8):2725–2729. doi: 10.1073/pnas.0509218103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoshizaki G, et al. Spermatogonial transplantation in fish: A novel method for the preservation of genetic resources. Comp Biochem Physiol Part D Genomics Proteomics. 2011;6(1):55–61. doi: 10.1016/j.cbd.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 24.Thorgaard GH. Chromosomal differences among rainbow-trout populations. Copeia. 1983;3:650–662. [Google Scholar]
- 25.US Fish and Wildlife Service Endangered Species Program. Available at www.fws.gov/endangered. Accessed October 25, 2012.
- 26.Nakabo T, Nakayama K, Muto N, Miyazawa M. Oncorhynchus kawamurae “Kunimasu,” a deepwater trout, discovered in Lake Saiko, 70 years after extinction in the original habitat, Lake Tazawa, Japan. Ichthyol Res. 2011;58:180–183. [Google Scholar]
- 27.Boonanuntanasarn S, Yoshizaki G, Iwai K, Takeuchi T. Molecular cloning, gene expression in albino mutants and gene knockdown studies of tyrosinase mRNA in rainbow trout. Pigment Cell Res. 2004;17(4):413–421. doi: 10.1111/j.1600-0749.2004.00166.x. [DOI] [PubMed] [Google Scholar]
- 28.Yoshizaki G, Sakatani S, Tominaga H, Takeuchi T. Cloning and characterization of a vasa-like gene in rainbow trout and its expression in the germ cell lineage. Mol Reprod Dev. 2000;55(4):364–371. doi: 10.1002/(SICI)1098-2795(200004)55:4<364::AID-MRD2>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 29.Takeuchi Y, Yoshizaki G, Kobayashi T, Takeuchi T. Mass isolation of primordial germ cells from transgenic rainbow trout carrying the green fluorescent protein gene driven by the vasa gene promoter. Biol Reprod. 2002;67(4):1087–1092. doi: 10.1095/biolreprod67.4.1087. [DOI] [PubMed] [Google Scholar]
- 30.Yano A, Suzuki K, Yoshizaki G. Flow-cytometric isolation of testicular germ cells from rainbow trout (Oncorhynchus mykiss) carrying the green fluorescent protein gene driven by trout vasa regulatory regions. Biol Reprod. 2008;78(1):151–158. doi: 10.1095/biolreprod.107.064667. [DOI] [PubMed] [Google Scholar]
- 31.Thorgaard GH, Jazwin ME, Stier AR. Polyploidy induced by heat shock in rainbow trout. Trans Am Fish Soc. 1981;110:546–550. [Google Scholar]
- 32.Elliott G, McGrath J, Crockett-Torabi E. Green fluorescent protein: A novel viability assay for cryobiological applications. Cryobiology. 2000;40(4):360–369. doi: 10.1006/cryo.2000.2258. [DOI] [PubMed] [Google Scholar]
- 33.Takeuchi Y, Yoshizaki G, Takeuchi T. Generation of live fry from intraperitoneally transplanted primordial germ cells in rainbow trout. Biol Reprod. 2003;69(4):1142–1149. doi: 10.1095/biolreprod.103.017624. [DOI] [PubMed] [Google Scholar]
- 34.Takeuchi Y, Yoshizaki G, Takeuchi T. Green fluorescent protein as a cell-labeling tool and a reporter of gene expression in transgenic rainbow trout. Mar Biotechnol (NY) 1999;1(5):448–0457. doi: 10.1007/pl00011801. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.