Abstract
The metabolism of membrane phosphoinositides is critical for a variety of cellular processes. Phosphatidylinositol-3,5-bisphosphate [PtdIns(3,5)P2] controls multiple steps of the intracellular membrane trafficking system in both yeast and mammalian cells. However, other than in neuronal tissues, little is known about the physiological functions of PtdIns(3,5)P2 in mammals. Here, we provide genetic evidence that type III phosphatidylinositol phosphate kinase (PIPKIII), which produces PtdIns(3,5)P2, is essential for the functions of polarized epithelial cells. PIPKIII-null mouse embryos die by embryonic day 8.5 because of a failure of the visceral endoderm to supply the epiblast with maternal nutrients. Similarly, although intestine-specific PIPKIII-deficient mice are born, they fail to thrive and eventually die of malnutrition. At the mechanistic level, we show that PIPKIII regulates the trafficking of proteins to a cell’s apical membrane domain. Importantly, mice with intestine-specific deletion of PIPKIII exhibit diarrhea and bloody stool, and their gut epithelial layers show inflammation and fibrosis, making our mutants an improved model for inflammatory bowel diseases. In summary, our data demonstrate that PIPKIII is required for the structural and functional integrity of two different types of polarized epithelial cells and suggest that PtdIns(3,5)P2 metabolism is an unexpected and critical link between membrane trafficking in intestinal epithelial cells and the pathogenesis of inflammatory bowel disease.
Keywords: embryogenesis, Crohn's disease
Phosphoinositide kinases and their lipid products are involved in signaling events that lead to a variety of cellular responses (1–3). Type III phosphatidylinositol phosphate kinase (PIPKIII; also known as PIKfyve in mammals and Fab1p in yeast) phosphorylates phosphatidylinositol 3-phosphate (PtdIns3P) to produce PtdIns(3,5)P2, the most recently discovered PtdInsP2 isomer in mammals (4–6). Mammalian PIPKIII and yeast Fab1p share a FYVE domain, a chaperonin-like domain, a cysteine-rich domain, and a PIPK catalytic domain (7). The FYVE domain of Fab1p is responsible for the localization of the enzyme at the vacuolar membrane. The chaperonin-like and cysteine-rich domains of Fab1p are involved in its binding to Vac14p and Fig4p, which are two regulatory proteins required for Fab1p’s full kinase activity. Vac14 and Fig4 are conserved in mammals, participate in a trimeric complex with PIPKIII, and are thought to have a similar function in promoting full PIPKIII kinase activity (8, 9).
It has been proposed that PtdIns(3,5)P2 directs the localization and activity of endocytic proteins such as Vps24, Atg18, and TRPML1 and, thereby, regulates certain steps of vesicular trafficking in yeast and mammalian cells (10). Genetic studies of yeast fab1 have shown that deletion of fab1 completely blocks PtdIns(3,5)P2 production under both basal and hyperosmotic stress conditions (11). Fab1p-mediated production of PtdIns(3,5)P2 is thought to have a function: controlling the change in vacuolar size that helps yeast cells to withstand altered tonicity. However, given the plethora of roles proposed for endosomal structures in mammalian cells, PIPKIII-mediated production of PtdIns(3,5)P2 is likely to be involved in numerous complex physiological processes in mammals. This hypothesis has been bolstered by analyses of the phenotypes of mice with germ-line mutations of Vac14 or Fig4 (8, 12, 13). Mice lacking either Vac14 or Fig4 are born but exhibit severe neuronal damage and have a shortened lifespan. The deletion of Vac14 or Fig4 in mice leads to only partial inhibition of PIPKIII activity, so that only limited abnormalities are detected in these mutants. We hypothesized that deletion of PIPKIII itself, which would lead to a complete block in PtdIns(3,5)P2 generation, would expose functions for this lipid kinase and its lipid product in diverse range of vital phenomena in mammalian cells.
In this study, we generated PipkIII-deficient mice and demonstrate the roles of the PIPKIII/PtdIns(3,5)P2 axis in early mammalian postimplantation development and adult intestinal homeostasis, processes that both depend on the polarization of absorptive cells.
Results
PIPKIII Is Indispensable for PtdIns(3,5)P2 Production.
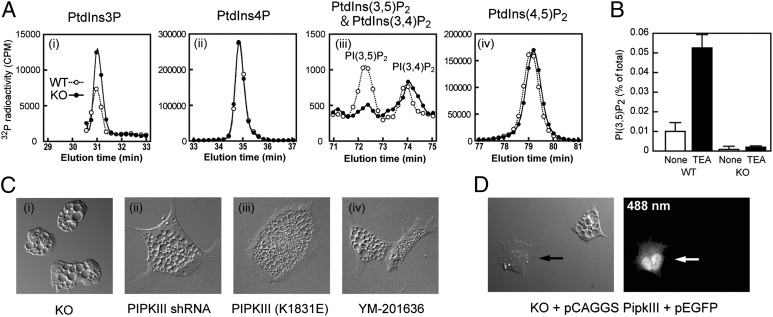
To explore physiological functions of PIPKIII, we used gene targeting to generate PIPKIII null mutant ES cells (PipkIIIneo/neo) that exhibited complete loss of PIPKIII protein (Fig. S1) and PipkIII mRNA expression (Fig. S2). The mRNA expression levels of other PIPKs, and as Vac14 and Fig4, were normal in PipkIIIneo/neo ES cells (Fig. S2). High performance liquid chromatography (HPLC) analyses revealed that PtdIns(3,5)P2 was reduced to a background level in PipkIIIneo/neo ES cells under steady-state conditions (Fig. 1A and Fig. S3). There were no significant differences between PipkIII+/+ (wild-type; WT) and PipkIIIneo/neo cells in levels of PtdIns(3,4)P2, PtdIns(4)P, or PtdIns(4,5)P2 but levels of PtdIns(3)P, the substrate of PIPKIII, were slightly increased in PipkIIIneo/neo cells, suggesting that PIPKIII specifically phosphorylates the 5-hydroxyl of PtdIns(3)P in murine ES cells. It has been reported that PtdIns(3,5)P2 is rapidly produced in yeast and mammalian cells in response to osmotic stresses (4, 5). Although there was no osmolarity-sensitive increase in PtdIns(3,5)P2 in the ES cells, we found that PtdIns(3,5)P2 was produced when the cells were treated with a lysosomotropic agent triethylamine (TEA) (Fig. 1B). TEA-stimulated increase of PtdIns(3,5)P2 was absent in ES cells lacking PIPKIII. These results demonstrate that, as is true for Fab1p in yeast, PIPKIII is the major PtdIns(3,5)P2-producing kinase in mammalian cells, and that disruption of PipkIII leads to a more profound decrease in cellular PtdIns(3,5)P2 levels than does disruption of either Vac14 or Fig4 (8, 12, 13).
Fig. 1.
Depletion of PtdIns(3,5)P2 in PIPKIII-deficient ES cells. (A) Defective PtdIns(3,5)P2 production. PipkIII+/+ (WT) and PipkIIIneo/neo (KO) ES cells were labeled with [3H]-inositol for 48 h, followed by treatment (or not) for 10 min with 10 mM TEA. Lipids were extracted, and PtdIns(3,5)P2 levels were determined by HPLC. PtdIns(3,5)P2 levels are expressed as a percentage of total phosphoinositide levels and are the mean ± SEM (n = 3). (B) Increased PtdIns(3)P and normal PtdIns(4)P, PtdIns(3,4)P2, and PtdIns(4,5)P2. WT and KO ES cells were labeled with [32P]-orthophosphate under steady-state conditions. Lipids were extracted, and the indicated phosphoinositides were analyzed by HPLC and plotted in chromatograms. PtdIns(5)P and PtdIns(3,4,5)P3 were undetectable under the experimental conditions used. Similar results were obtained in five replicate experiments (Fig. S3). (C) Loss of PIPKIII results in vacuolation. Untreated KO ES cells (i) show the same vacuolation phenotype as WT ES cells that were transfected with an shRNA against PipkIII (ii); transfected with a dominant-negative K1831E PIPKIII expression vector (iii); or treated with 800 nM YM-201636 (PIPKIII inhibitor) (iv). Cells were subjected to differential interference contrast (DIC) imaging. (D) PIPKIII expression prevents vacuolation. KO ES cells were cotransfected with a PIPKIII expression vector (pCAGGS PIPKIII) plus an EGFP expression vector (pEGFP). DIC and fluorescent images were captured at 24 h after transfection. Arrows indicate a transfected, PIPKIII-expressing KO ES cell. Results shown are representative of at least three independent experiments.
PipkIIIneo/neo ES cells contained greatly enlarged endosomes that resembled those observed in cells overexpressing kinase-dead PIPKIII, or treated with siRNA against PIPKIII or a PIPKIII inhibitor (14, 15) (Fig. 1C). This vacuolation phenotype was restored to normal by transfection of PipkIIIneo/neo ES cells with PIPKIII cDNA (Fig. 1D). To characterize the abnormal vacuoles, we examined the localization in WT and PipkIIIneo/neo ES cells of GFP fused to either LAMP1 (late endosome/lysosome marker) or the FYVE domain of EEA1 (early endosome marker). In PIPKIII-deficient cells, virtually every large abnormal vacuole was positive for LAMP1-EGFP (Fig. S4A). These structures were also highly acidified and stained positively with neutral red and LysoTracker (Fig. S4B), indicating that they were likely swollen lysosomes. In contrast, every smaller abnormal vacuole in the mutant ES cells was positive for EEA1-FYVE-EGFP (Fig. S4A), suggesting that the swollen lysosomes emanate from early endosomes. No other phenotypic abnormalities were obvious in cultured PipkIIIneo/neo ES cells, and, despite the presence of the abnormal vacuoles, the growth and survival of these cells were normal (Fig. S5).
PipkIII Is Essential for Embryonic Development.
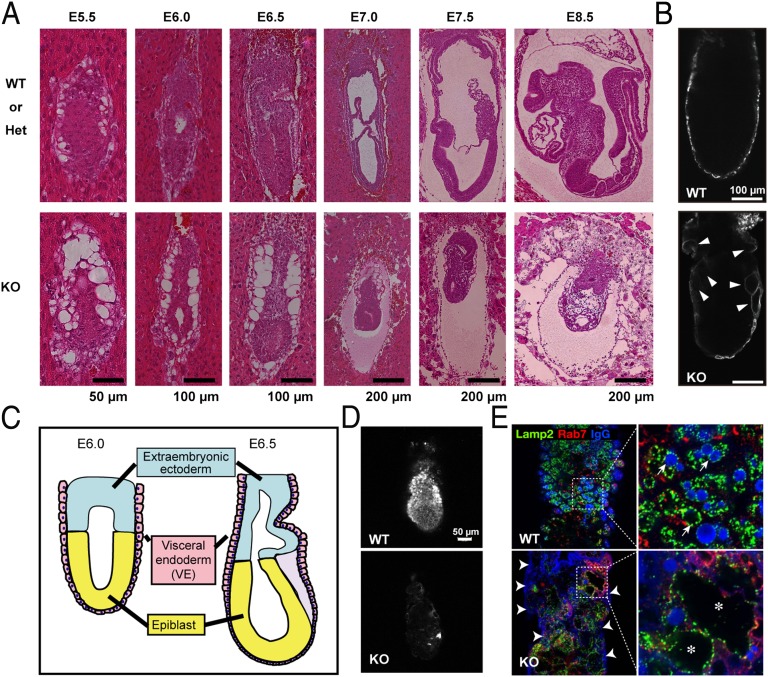
To investigate the physiological roles of PIPKIII in a whole mammal, we generated PipkIIIneo/+ mice and intercrossed them to generate PipkIIIneo/neo progeny. In contrast to Vac14- and Fig4-deficient mice (8, 12, 13), loss of the PipkIII gene was lethal during early embryogenesis (Table 1). Mendelian analysis showed that PipkIIIneo/neo embryos were present at close to the expected frequency at embryonic day (E)6.5 and E7.5 but were visibly abnormal (Fig. S6). Histological analysis revealed that PipkIIIneo/neo embryos were able to form egg cylinders at E5.5 and elongate them at E6.0 (Fig. 2 A and C). However, by the time WT embryos underwent gastrulation at E6.5–7.0, the PipkIIIneo/neo embryos showed significant developmental retardation. In fact, the mutant embryos did not advance to gastrulation but instead remained as egg cylinders. All PipkIIIneo/neo embryos stopped growing after E7.0, were significantly smaller than controls at E7.5, died by E8.5, and were absorbed by E10.5. Most strikingly, although almost normal in size at E6.5, PipkIIIneo/neo embryos showed abnormal organization of the visceral endoderm (VE). The VE is an extraembryonic epithelial cell layer that encases the epiblast (which forms the fetus) and the extraembryonic ectoderm. Notably, VE cells were vacuolated in E5.5–6.5 PipkIIIneo/neo embryos (Fig. 2A and Fig. S7), just as was observed in PIPKIII-deficient ES cells (Fig. 1C). In contrast, the mutant epiblast cells did not show any apparent morphological abnormalities at this stage (Fig. 2A). Because PIPKIII was expressed in both epiblast and VE cells (Fig. S8), the different effect of PIPKIII deficiency could be due to different natures of the two types of cells: Endocytic organelles develop less extensively in the epiblast (16).
Table 1.
Genotypes of offspring from PipkIIIneo/+ intercrosses
| Age | Total | PipkIII+/+ | PipkIII+/neo | PipkIIIneo/neo |
| E3.5 | 47 | 6 | 28 | 13 |
| E6.5 | 268 | 77 | 133 | 58 |
| E7.5 | 83 | 22 | 44 | 17 |
| E8.5 | 42 | 10 | 23 | 9* |
| E9.5 | 69 | 13 | 38 | 18* |
| E10.5 | 48 | 18 | 30 | 0† |
| E14.5–15.5 | 55 | 17 | 38 | 0† |
| Newborn | 157 | 53 | 104 | 0† |
*PIPKIII null mutant mice were clearly nonviable and undergoing tissue autolysis.
†P < 0.001 (χ2 test).
Fig. 2.
Defective embryogenesis in the absence of PIPKIII. (A) Bright-field stereomicroscopic views of H&E-stained longitudinal sections from control PipkIII+/+ (WT) or PipkIII+/neo (Het) and PipkIIIneo/neo (KO) embryos in decidua. VE cells, but not epiblasts, were vacuolated in the mutant embryos at the egg cylinder stage. (B) LAMP1-positive swollen vacuoles (white arrowheads) in the KO VE. Sections of WT and KO E6.5 embryos were subjected to LAMP1 staining to detect lysosomal compartments. (C) Diagram of mouse embryo at E6.0 and E6.5. (D) Reduced endocytosis in the VE. WT and KO E6.2 embryos were isolated from deciduae, freed from the parietal endoderm, labeled with rhodamine-labeled dextran for 15 min, and incubated for 50 min. The stained embryos were fixed and serial optical sections were viewed under a laser scanning confocal microscope. A parasagittal focal plane is shown. (E) Abnormal localization of maternal IgG in VE of KO E6.5 embryos. At low magnification, IgG puncta were reduced in the mutant VE layer compared with the WT, and IgG bound abnormally to the apical surface of KO VE cells (white arrowheads). (E Right) Higher magnification of the Inset areas in Left reveals that most IgG-positive vacuoles in WT VE cells are also positive for LAMP1 (white arrows), whereas the LAMP1-positive, swollen apical vacuoles in KO VE cells (asterisks) contain no IgG. Results shown are representative of at least three independent experiments.
In WT embryos, VE cells develop an extensive vesicular system that enables them to provide maternal nutrients to the epiblasts (17, 18). In VE cells, the vesicles containing extracellular materials are conveyed by a retrograde transport mechanism to the apical vacuoles, which are LAMP1/2-positive endosomes positioned on the apical side of the cell (19, 20). We found that the unusual swollen vacuoles in PipkIIIneo/neo VE cells also stained positively for LAMP1 (Fig. 2B), suggesting that these structures are abnormal apical vacuoles. To investigate whether the endocytosis was intact in PipkIIIneo/neo VE cells, we examined the uptake of the fluid phase marker rhodamine-dextran (RD). When WT embryos were incubated in vitro with RD, RD was properly endocytosed and accumulated in the lumens of apical vacuoles within VE cells (Fig. 2D). In contrast, RD uptake by PipkIIIneo/neo VE cells was significantly reduced and the incorporated RD did not reach the digestive compartment. Similarly, endogenous maternal IgG was readily detected in apical vacuoles in WT VE cells but remained stuck at the apical membrane surface of PipkIIIneo/neo VE cells (Fig. 2E). A small amount of IgG was detected within mutant VE cells but did not accumulate in the enlarged apical vacuoles. These data suggest that PIPKIII is essential for the endocytosis of maternal proteins at the apical plasma membrane and may also be involved in delivery of endosomes to apical vacuoles in the VE cells. We speculate that these defects in the endocytic pathway of VE cell we observe in the absence of PIPKIII may be the primary mechanism underlying the lethality of PIPKIII-deficient embryos, because the mutant epiblasts would not be supplied with nutrients from the maternal environment.
Conditional Mutation of the PipkIII Gene.
To circumvent the lethality of our PipkIIIneo/neo mutants and study PIPKIII functions in adult mice, we generated a PipkIII conditional knockout allele (PipkIIIflox) (Fig. S9 A–E). Upon adenovirus-mediated expression of Cre recombinase, PipkIIIflox/flox murine embryonic fibroblasts (MEFs) lost PIPKIII protein expression (Fig. S9F) and exhibited enlarged vacuoles (Fig. S9G), demonstrating successful conditional targeting of the PipkIII gene. Our objective in this study was to disrupt PipkIII in intestinal epithelial cells (IECs), the cell type in adult mice that is most similar to embryonic VE cells in terms of polarized morphology and absorptive function. To this end, we crossed PipkIIIflox/flox mice with transgenic mice expressing Cre under the control of the villin promoter (VilCre) (21, 22) to generate VilCrePipkIIIflox/+ mice. When VilCrePipkIIIflox/+ mice were crossed to PipkIIIflox/flox mice, VilCrePipkIIIflox/flox mice were recovered at a lower than expected Mendelian frequency (Table S1). Based on our observations of the PipkIIIneo/neo embryos, we suspected that the lethality of the conditional mutant was due to VilCre-mediated disruption of PipkIII in VE cells during embryogenesis (21). To test this hypothesis, we crossed VilCre mice with Rosa26-floxed-stop-EYFP (R26R-EYFP) mice (23) and followed Cre-mediated excision of the floxed gene through detection of the reporter gene product EYFP. Interestingly, we noted a mosaic pattern of Cre expression in the VE cells of VilCre/+; R26R-EYFP/+ embryos at E7.5 (Fig. S10A), which may explain the partial penetrance of the embryonic lethality in VilCrePipkIIIflox/flox embryos. In adult VilCrePipkIIIflox/flox;R26R-EYFP/+ mice, approximately one-half of the IECs expressed Cre. The enterocytes expressing Cre had abnormally large endosomes that were LAMP1-positive (Fig. S10B), just as was observed in the VE cells of PipkIIIneo/neo embryos.
Inflammatory Bowel Disease in VilCrePipkIIIflox/flox Mice.
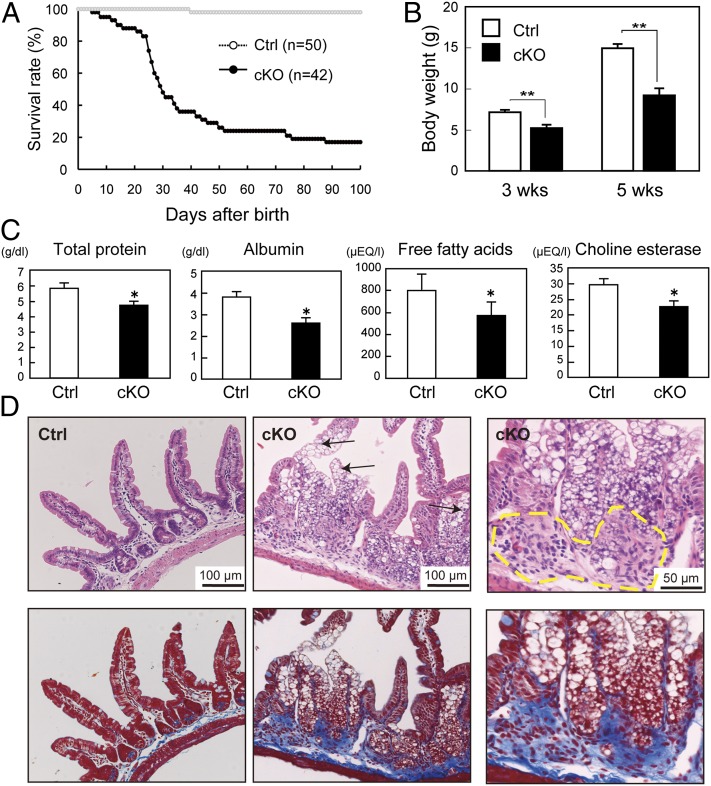
Although the VilCrePipkIIIflox/flox mice that survived to birth appeared normal just after parturition, these mutants had a shortened lifespan in that only 20% of these animals lived for more than 100 d after birth (Fig. 3A). By 5 wk of age, the mutants had body weights that averaged only 60% of the mean littermate control body weight (Fig. 3B). Total protein, albumin, free fatty acids, and choline esterase levels were all significantly decreased in the sera of the mutants compared with controls (Fig. 3C), suggesting that loss of PIPKIII in the intestine results in malnutrition. In addition, VilCrePipkIIIflox/flox mice displayed clinical features of both abdominal and systemic illness, including diarrhea, bloody stool, lethargy, hunched posture, and disheveled fur. Hematoxylin/eosin (H&E) staining of VilCrePipkIIIflox/flox ileum revealed marked vacuolation of IECs (Fig. 3D). Notably, lymphocyte infiltration was prominent in both the mucosa and submucosa in VilCrePipkIIIflox/flox intestines (Fig. 3D). Furthermore, Azan staining of the intestine revealed overt fibrosis and hypertrophy of the muscularis mucosa (Fig. 3D, Lower). This constellation of clinical and pathological features closely matches the hallmarks of human Crohn’s disease.
Fig. 3.
Mortality and intestinal abnormalities of VilCrePipkIIIflox/flox mice. (A) Shortened lifespan. Survival curves of VilCrePipkIIIflox/flox (cKO) and control PipkIIIflox/flox or PipkIIIflox/+ (Ctrl) mice are shown. (B) Reduced body weight. Ctrl and cKO littermates were weighed at 3 wk and 5 wk after birth. Results shown are the mean body weight ± SEM (n = 18–50 mice per genotype). (C) Malnutrition. The indicated nutritional parameters were measured in sera from Ctrl and cKO mice (n = 4 per genotype). (D) Spontaneous inflammation and fibrosis in the ileum. H&E (Upper) and Azan (Lower; blue) staining of consecutive sections of the ileum from 4-wk-old Ctrl and cKO mice. The mutant displays vacuolation of enterocytes (arrows), lymphocyte infiltration (dashed yellow line), and fibrogenic changes (blue) in the lamina propria and muscularis mucosa. For B and C, *P < 0.05; **P < 0.01 (Student t test). Results shown are representative of at least three independent experiments, except D, where results are representative of at least 50 mice examined per genotype.
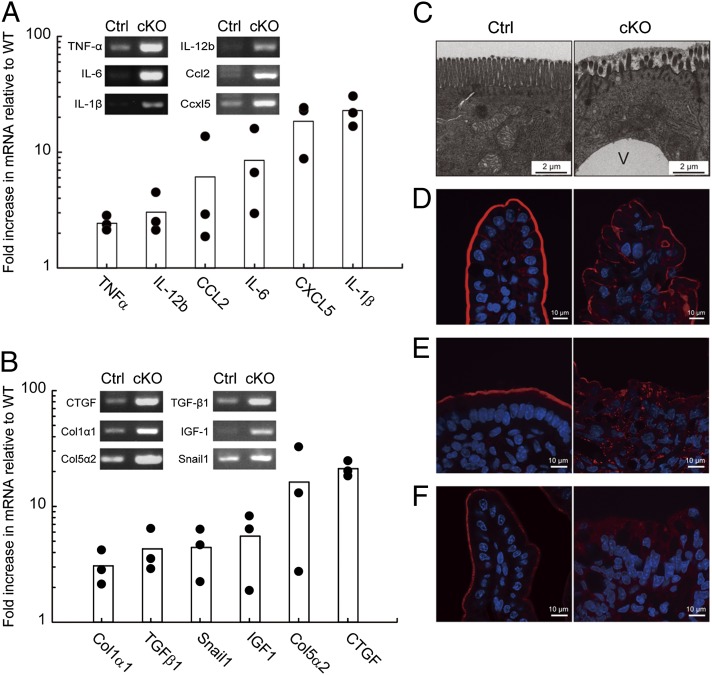
To characterize in more detail the intestinal disorders in VilCrePipkIIIflox/flox mice, we screened genes that exhibited altered expression levels in PIPKIII-deficient intestines with cDNA microarrays. Genes up-regulated in VilCrePipkIIIflox/flox compared with the littermate control PipkIIIflox/flox intestine included those encoding proteins acting on hematopoietic cells, such as TNFα, interleukins (IL-1β, IL-6, IL-12b), and chemokines (Ccl2, Cxcl5) (Fig. 4A). Immunohistochemistry revealed that NFκB, a key regulator of proinflammatory gene expression, was activated in the epithelial cells in the absence of PIPKIII (Fig. S11). In addition to the hematopoietic mediators, mRNAs of profibrotic factors (CTGF, IGF1), collagens (Col1α1, Col5α2), and epithelial-mesenchymal transition-related genes (TGFβ1, Snail1) were also increased in the VilCrePipkIIIflox/flox IECs (Fig. 4B). Up-regulation of these genes related to inflammation and fibrosis were further confirmed by RT-PCR (Fig. 4 A and B, Insets). These data reinforces our contention that loss of PIPKIII in IECs disrupts inflammatory control at the gene expression level.
Fig. 4.
Altered gene expression pattern and compromised apical membrane structure in the small intestine of VilCrePipkIIIflox/flox mice. (A and B) Enhanced expression of genes related to inflammation (A) and fibrosis (B). The mRNA expression of the indicated genes in intestinal mucosa from PipkIIIflox/flox (Ctrl) and VilCrePipkIIIflox/flox (cKO) mice (three pairs from three independent litters; 6-wk-old males) was examined by using cDNA microarray profiling. The mRNA expression level of each gene was normalized to β-actin mRNA. Results are expressed as fold induction in the cKO over the Ctrl. The up-regulation of the genes was also confirmed by RT–PCR (Insets). For A and B, results shown are representative of at least three independent experiments. (C–F) Abnormal intestinal epithelium. Small intestines from Ctrl and cKO mice were examined by electron microscopy (C) and fluorescent imaging (red) plus DAPI counterstaining (blue) (D–F). (C) A cKO enterocyte shows shortened microvilli and an enlarged vacuole (V) compared with a Ctrl enterocyte. (D) Phalloidin staining reveals decreased F-actin underneath the apical plasma membrane of a cKO enterocyte. (E and F) Mislocalization of apical proteins. The ilea of Ctrl and cKO mice were subjected to immunofluorescent microscopy to detect intracellular localization of DPPIV (E) and IAP (F). cKO cells showed abnormal punctate and cytosolic staining of these proteins. For C–F, results shown are representative of at least five mice examined per genotype.
At the ultrastructural level, electron microscopy showed that VilCrePipkIIIflox/flox enterocytes containing enlarged vacuoles were able to establish apical-basolateral polarity but that their microvilli were abnormally short in height and sparse in number compared with the control (Fig. 4C). Consistent with these defects, immunofluorescence microscopy revealed that the filamentous actin normally located beneath the apical membrane was reduced in the mutant IECs (Fig. 4D). A similar defect was found for dipeptidyl peptidase IV (DPPIV) and intestinal alkaline phosphatase (IAP), which are localized specifically at the apical surface in WT IECs (24, 25). Although protein levels of DPPIV and IAP were comparable in extracts of WT and VilCrePipkIIIflox/flox whole small intestine (Fig. S10C), the amounts of DPPIV and IAP localized at the IEC apical membrane were significantly decreased in the absence of PIPKIII (Fig. 4 E and F). Instead, DPPIV and IAP accumulated within VilCrePipkIIIflox/flox IECs in an abnormal punctate pattern, indicating that PIPKIII regulates the vectorial transport of apical proteins. This ectopic localization of DPPIV, along with the drastic reduction in microvillus surface area in VilCrePipkIIIflox/flox mice, may be major contributors to their malnutrition. In a similar vein, the inflammation seen in the VilCrePipkIIIflox/flox small intestine may be due to IAP mislocalization, because IAP has the ability to detoxify lipopolysaccharide and prevent bacterial invasion across the mucosal barrier (25). These results demonstrate that, just as PIPKIII is essential for apical membrane functions in murine embryonic VE cells, it is critical for the biogenesis and normal functions of the apical membrane in murine adult IECs.
Discussion
The biological functions of Fab1p, the yeast counterpart of PIPKIII, have been studied intensely by using genetic approaches. Yeast fab1Δ cells lack PtdIns(3,5)P2 and display defects such as vacuole enlargement and impaired vacuole acidification, implicating Fab1p as the sole PtdIns(3,5)P2-producing enzyme regulating vacuole homeostasis in yeast (11, 26). Our demonstration that disruption of the PipkIII gene in murine ES cells depletes PtdIns(3,5)P2 implies that, although mammals have evolved diverse phosphoinositide kinases, PIPKIII is nonredundant and the principal enzyme responsible for PtdIns(3,5)P2 production in these animals. PIPKIII-deficient ES cells had enlarged endosomes that were positive for LAMP1/2, in agreement with the findings of previous genetic studies of not only fab1Δ yeast cells but also Caenorhabditis elegans (27) and Drosophila (28) mutants lacking PIPKIII orthologs. However, the swollen endosomes in PIPKIII ortholog-deficient C. elegans, Drosophila, and yeast cells are not acidified, unlike the enlarged endosomes in our PipkIIIneo/neo murine ES cells (Fig. S4B). These data suggest that PtdIns(3,5)P2 plays a critical role in controlling the size of endosomal compartments in all eukaryotes but is dispensable for endosomal acidification in mammals.
For this study, we generated PIPKIII-deficient mice by using two different strategies: constitutive gene disruption via insertion of a neomycin resistance cassette (PipkIIIneo/neo) and conditional gene deletion via the Cre-LoxP system (PipkIIIflox/flox). PipkIIIneo/neo embryos were able to implant and form egg cylinders that consists of an outer layer of VE and an inner layer of ectoderm (epiblast). An important clue pointing toward the basis of the lethality of PipkIIIneo/neo embryos was the abnormal morphology of their extraembryonic VE. The mutant VE cells displayed swollen apical vacuoles, deformed microvilli, and impaired endocytosis of extracellular proteins as exemplified by IgG. Thus, it is plausible that PIPKIII is necessary in VE cells to incorporate and digest maternal materials and to secrete nutrients required for the development of the early conceptus. This conclusion is further supported by the fact that deletion of PipkIII specifically in VE cells also caused partially penetrant embryonic lethality during early development.
Ikonomov et al. have reported that systemic disruption of PipkIII leads to lethality before implantation (29). Their finding that mouse embryos lacking PipkIII exon 6 die before the 32- to 64-cell stage stands in contrast to the embryonic phenotypes in our PipkIIIneo/neo mutants, in which the translation-initiating codon of PipkIII is disrupted by the insertion of a neomycin resistance cassette. An explanation for this apparent discrepancy could be differences between the two strains in the levels of maternally inherited PIPKIII protein within the embryos. Ikonomov et al. showed that PIPKIII protein levels in MEFs from their heterozygotes were half of WT levels, whereas the PipkIIIneo/+ heterozygotes in our study expressed normal PIPKIII protein levels. Another possibility is that our PipkIIIneo/neo mice may express a partial PIPKIII protein derived from an as-yet uncharacterized translation start site. Conversely, cell survival is impaired by the small protein that would be created if the N-terminal 159 amino acids of PIPKIII underwent fusion to the 12-aa peptide created by the frameshift mutation in the mutant reported by Ikonomov et al. Despite these discrepancies, we believe that the importance of PIPKIII in murine postimplantation development has been established by the phenotypes of our two different PIPKIII-deficient mutants generated using two different strategies: one by systemic disruption of the first coding exon and the other by conditional deletion of exon 38 encoding the kinase domain.
The importance of PtdIns(3,5)P2 production in vivo has been demonstrated by genetic disruption of Vac14 and Fig4 in mice, which in both cases results in premature death and profound degeneration of the nervous system (8, 12, 13). The results of our study show that PIPKIII is essential for the homeostasis of a nonneuronal mammalian tissue, the murine adult intestinal epithelium. The localization of apical proteins within the VilCrePipkIIIflox/flox IECs was abnormal, and this mislocalization was also found in PIPKIII-deficient VE (Fig. 4 and Fig. S10D), suggesting that a similar mechanism involving PIPKIII operates in the apical transport of proteins in two types of polarized epithelial cells. Dysregulation of PIPKIII’s functions in IECs has serious consequences, including diarrhea, malnutrition, and pathological changes such as blood cell infiltration and fibrosis.
Fibrosis is one of the defining characteristic of the inflammatory bowel disease Crohn’s disease, but most available mouse models of this disorder show intestinal inflammation but not fibrosis (30). A good model for intestinal fibrosis has been awaited to easily study pathophysiology and potential therapeutic strategies. The intestines of our VilCrePipkIIIflox/flox mice show both inflammation and prominent fibrosis. Furthermore, VilCrePipkIIIflox/flox intestinal mucosa had increased expression levels of genes related to inflammation and fibrosis (Fig. 4 A and B). It should be noted that Crohn’s disease is an immunological disorder and its onset is generally in adulthood, whereas our intestine-specific PIPKIII-deficient mice develop colitis as early as 2 wk after birth. Despite these differences, our results clearly show that VilCrePipkIIIflox/flox mice replicate the features of Crohn’s disease including fibrosis better than current models and, thus, may be useful for investigations of the etiology of this disorder. In line with this notion, recent genome-wide association studies in humans have identified a Crohn’s disease-associated mutation in the MTMR3 gene encoding the myotubularin-related protein that dephosphorylates PtdIns(3)P and PtdIns(3,5)P2 (31, 32). Thus, our results bolster the hypothesis that altered metabolism of PtdIns(3,5)P2 may be associated with Crohn’s disease.
In conclusion, our study establishes PIPKIII as a critical regulator of two types of polarized and absorptive cells in mouse embryos and adult mouse intestines. PIPKIII and PtdIns(3,5)P2 therefore play key roles in tissues other than the neuronal system, making the investigation of PIPKIII functions an expanding and important area of study.
Materials and Methods
PIPKIII-Deficient Mice and ES Cells.
Constitutive and conditional PIPKIII-deficient mice were generated by standard gene targeting methods as described (33, 34). To obtain PipkIIIneo/neo ES cells, PipkIIIneo/+ ES cells were cultured for 2 wk in the presence of a high concentration of G418 (2 mg⋅mL−1) (35). Surviving colonies were isolated, expanded, and genotyped by PCR and Southern blotting. All experimental protocols were approved by the Akita University Institutional Committee for Animal Studies.
Phosphoinositide Measurement.
Mouse ES cells were labeled for 4 h with 0.2 mCi⋅mL−1 32Pi (Perkin-Elmer) in Pi-free DMEM containing dialyzed 10% (vol/vol) heat-inactivated FCS. Labeling was quenched and lipids were extracted as described (36). Dried lipids were deacylated and analyzed by HPLC using a Partisphere SAX column (Whatman) as described (37). Briefly, aliquots of deacylated lipids (107 cpm) were applied to the SAX column, and radioactivity eluted by 0–0.2 M (NH4)2HPO4 was assayed in 0.2-mL fractions by a liquid scintillation counter.
Further details of materials and methods can be found in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Y. Kadowaki, R. Itoh, K. Asanuma, R. Nakamura, Y. Sugihara, K. Yanase, M. Itoyama, H. Nakanishi, M. Fukuda, Y. Kanaho, J. Penninger, K. Kofuji and H. Takahashi for technical support. This work was supported by research grants from the Ministry of Education, Culture, Sports and Technology of Japan; the Japan Society for the Promotion of Science; the Kao Foundation for Arts and Sciences; and the Japan Science and Technology Corporation.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1213212110/-/DCSupplemental.
References
- 1.Sasaki T, et al. Mammalian phosphoinositide kinases and phosphatases. Prog Lipid Res. 2009;48(6):307–343. doi: 10.1016/j.plipres.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 2.Wymann MP, Schneiter R. Lipid signalling in disease. Nat Rev Mol Cell Biol. 2008;9(2):162–176. doi: 10.1038/nrm2335. [DOI] [PubMed] [Google Scholar]
- 3.Di Paolo G, De Camilli P. Phosphoinositides in cell regulation and membrane dynamics. Nature. 2006;443(7112):651–657. doi: 10.1038/nature05185. [DOI] [PubMed] [Google Scholar]
- 4.Dove SK, et al. Osmotic stress activates phosphatidylinositol-3,5-bisphosphate synthesis. Nature. 1997;390(6656):187–192. doi: 10.1038/36613. [DOI] [PubMed] [Google Scholar]
- 5.Gary JD, et al. Regulation of Fab1 phosphatidylinositol 3-phosphate 5-kinase pathway by Vac7 protein and Fig4, a polyphosphoinositide phosphatase family member. Mol Biol Cell. 2002;13(4):1238–1251. doi: 10.1091/mbc.01-10-0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shisheva A, Sbrissa D, Ikonomov O. Cloning, characterization, and expression of a novel Zn2+-binding FYVE finger-containing phosphoinositide kinase in insulin-sensitive cells. Mol Cell Biol. 1999;19(1):623–634. doi: 10.1128/mcb.19.1.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Botelho RJ, Efe JA, Teis D, Emr SD. Assembly of a Fab1 phosphoinositide kinase signaling complex requires the Fig4 phosphoinositide phosphatase. Mol Biol Cell. 2008;19(10):4273–4286. doi: 10.1091/mbc.E08-04-0405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jin N, et al. VAC14 nucleates a protein complex essential for the acute interconversion of PI3P and PI(3,5)P(2) in yeast and mouse. EMBO J. 2008;27(24):3221–3234. doi: 10.1038/emboj.2008.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Michell RH, Dove SK. A protein complex that regulates PtdIns(3,5)P2 levels. EMBO J. 2009;28(2):86–87. doi: 10.1038/emboj.2008.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dove SK, Dong K, Kobayashi T, Williams FK, Michell RH. Phosphatidylinositol 3,5-bisphosphate and Fab1p/PIKfyve underPPIn endo-lysosome function. Biochem J. 2009;419(1):1–13. doi: 10.1042/BJ20081950. [DOI] [PubMed] [Google Scholar]
- 11.Gary JD, Wurmser AE, Bonangelino CJ, Weisman LS, Emr SD. Fab1p is essential for PtdIns(3)P 5-kinase activity and the maintenance of vacuolar size and membrane homeostasis. J Cell Biol. 1998;143(1):65–79. doi: 10.1083/jcb.143.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y, et al. Loss of Vac14, a regulator of the signaling lipid phosphatidylinositol 3,5-bisphosphate, results in neurodegeneration in mice. Proc Natl Acad Sci USA. 2007;104(44):17518–17523. doi: 10.1073/pnas.0702275104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chow CY, et al. Mutation of FIG4 causes neurodegeneration in the pale tremor mouse and patients with CMT4J. Nature. 2007;448(7149):68–72. doi: 10.1038/nature05876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jefferies HB, et al. A selective PIKfyve inhibitor blocks PtdIns(3,5)P(2) production and disrupts endomembrane transport and retroviral budding. EMBO Rep. 2008;9(2):164–170. doi: 10.1038/sj.embor.7401155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Lartigue J, et al. PIKfyve regulation of endosome-linked pathways. Traffic. 2009;10(7):883–893. doi: 10.1111/j.1600-0854.2009.00915.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aoyama M, et al. Spatial restriction of bone morphogenetic protein signaling in mouse gastrula through the mVam2-dependent endocytic pathway. Dev Cell. 2012;22(6):1163–1175. doi: 10.1016/j.devcel.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 17.Bielinska M, Narita N, Wilson DB. Distinct roles for visceral endoderm during embryonic mouse development. Int J Dev Biol. 1999;43(3):183–205. [PubMed] [Google Scholar]
- 18.Kawamura N, et al. Delivery of endosomes to lysosomes via microautophagy in the visceral endoderm of mouse embryos. Nat Commun. 2012;3:1071. doi: 10.1038/ncomms2069. [DOI] [PubMed] [Google Scholar]
- 19.Bernard O, Ripoche MA, Bennett D. Distribution of maternal immunoglobulins in the mouse uterus and embryo in the days after implantation. J Exp Med. 1977;145(1):58–75. doi: 10.1084/jem.145.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rachman F, Casimiri V, Bernard O. Maternal immunoglobulins G, A and M in mouse uterus and embryo during the postimplantation period. J Reprod Immunol. 1984;6(1):39–47. doi: 10.1016/0165-0378(84)90040-8. [DOI] [PubMed] [Google Scholar]
- 21.Maunoury R, et al. Villin expression in the visceral endoderm and in the gut anlage during early mouse embryogenesis. EMBO J. 1988;7(11):3321–3329. doi: 10.1002/j.1460-2075.1988.tb03203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.el Marjou F, et al. Tissue-specific and inducible Cre-mediated recombination in the gut epithelium. Genesis. 2004;39(3):186–193. doi: 10.1002/gene.20042. [DOI] [PubMed] [Google Scholar]
- 23.Srinivas S, et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gutschmidt S, Lange U, Riecken EO. “In situ”—measurements of protein contents in the brush border region along rat jejunal villi and their correlations with four enzyme activities. Histochemistry. 1981;72(3):467–479. doi: 10.1007/BF00501789. [DOI] [PubMed] [Google Scholar]
- 25.Goldberg RF, et al. Intestinal alkaline phosphatase is a gut mucosal defense factor maintained by enteral nutrition. Proc Natl Acad Sci USA. 2008;105(9):3551–3556. doi: 10.1073/pnas.0712140105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Odorizzi G, Babst M, Emr SD. Fab1p PtdIns(3)P 5-kinase function essential for protein sorting in the multivesicular body. Cell. 1998;95(6):847–858. doi: 10.1016/s0092-8674(00)81707-9. [DOI] [PubMed] [Google Scholar]
- 27.Nicot AS, et al. The phosphoinositide kinase PIKfyve/Fab1p regulates terminal lysosome maturation in Caenorhabditis elegans. Mol Biol Cell. 2006;17(7):3062–3074. doi: 10.1091/mbc.E05-12-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rusten TE, et al. Fab1 phosphatidylinositol 3-phosphate 5-kinase controls trafficking but not silencing of endocytosed receptors. Mol Biol Cell. 2006;17(9):3989–4001. doi: 10.1091/mbc.E06-03-0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ikonomov OC, et al. The phosphoinositide kinase PIKfyve is vital in early embryonic development: Preimplantation lethality of PIKfyve-/- embryos but normality of PIKfyve+/- mice. J Biol Chem. 2011;286(15):13404–13413. doi: 10.1074/jbc.M111.222364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rieder F, Brenmoehl J, Leeb S, Schölmerich J, Rogler G. Wound healing and fibrosis in intestinal disease. Gut. 2007;56(1):130–139. doi: 10.1136/gut.2006.090456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Imielinski M, et al. Western Regional Alliance for Pediatric IBD International IBD Genetics Consortium NIDDK IBD Genetics Consortium Belgian-French IBD Consortium Wellcome Trust Case Control Consortium Common variants at five new loci associated with early-onset inflammatory bowel disease. Nat Genet. 2009;41(12):1335–1340. doi: 10.1038/ng.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Franke A, et al. Genome-wide meta-analysis increases to 71 the number of confirmed Crohn’s disease susceptibility loci. Nat Genet. 2010;42(12):1118–1125. doi: 10.1038/ng.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sasaki J, et al. The PtdIns(3,4)P(2) phosphatase INPP4A is a suppressor of excitotoxic neuronal death. Nature. 2010;465(7297):497–501. doi: 10.1038/nature09023. [DOI] [PubMed] [Google Scholar]
- 34.Sato T, et al. The Rab8 GTPase regulates apical protein localization in intestinal cells. Nature. 2007;448(7151):366–369. doi: 10.1038/nature05929. [DOI] [PubMed] [Google Scholar]
- 35.Mortensen RM, Conner DA, Chao S, Geisterfer-Lowrance AA, Seidman JG. Production of homozygous mutant ES cells with a single targeting construct. Mol Cell Biol. 1992;12(5):2391–2395. doi: 10.1128/mcb.12.5.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sasaki T, et al. Function of PI3Kgamma in thymocyte development, T cell activation, and neutrophil migration. Science. 2000;287(5455):1040–1046. doi: 10.1126/science.287.5455.1040. [DOI] [PubMed] [Google Scholar]
- 37.Sasaki J, et al. Regulation of anaphylactic responses by phosphatidylinositol phosphate kinase type I alpha. J Exp Med. 2005;201(6):859–870. doi: 10.1084/jem.20041891. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.