As the world’s largest reservoir of exchangeable carbon on millennial timescales, the oceans play a dominant role in global change. Indeed, the oceans currently act as a major sink for anthropogenic carbon (1), partially moderating increases in atmospheric CO2 at the expense of a more acidic ocean. One of the great challenges for the next century is understanding how this shift in seawater chemistry will affect marine systems. Serving as a vivid microcosm for the ocean as whole, coral reefs display the beauty, diversity, and complexity of the ocean, while also exhibiting the ocean’s sensitivity to environmental perturbations. Built from a framework of CaCO3 skeletons, coral reefs are particularly sensitive to ocean acidification because acidified seawater tends to slow skeletal growth (2). Despite the threat posed by ocean acidification to reef health, the detailed mechanisms responsible for this sensitivity are still poorly understood. In PNAS, Venn et al. (3) address a key component of this problem, providing a detailed view of the chemical-scale changes that link coral skeletal growth and ocean acidification.
Seawater is primarily buffered by dissolved inorganic carbon thorough the equilibria among dissolved CO2, HCO3−, and CO32−, intimately linking the acid–base chemistry of the ocean with carbon dynamics. Ocean acidification is a direct result of this intimate connection; subject to mass balance and charge balance constraints, adding more carbon to seawater shifts the buffer state of the ocean toward lower pH. At a smaller scale, many marine calcifiers appear to exploit the connection between pH and inorganic carbon. Skeletal growth in coral occurs in an extracellular region termed the calcifying space, which allows coral to control local chemistry (4). By increasing the pH of the calcifying fluid in this space, coral shift dissolved inorganic carbon from predominantly HCO3− toward higher [CO32−]. This biological manipulation favors skeletal growth, as the thermodynamic driving force for CaCO3 mineralization, the saturation state (Ω), depends in large part on [CO32−]. Thus, pH is a useful indicator for the energetics of CaCO3 biomineralization, with higher pH roughly indicating more favorable growth conditions, even though several additional parameters ultimately control the Ω of a solution. Following this schema, ocean acidification reduces the potential for skeletal growth, whereas, conversely, organisms can encourage growth through pH elevation. The balance between these two forces modulates skeletal growth. Furthermore, this balance is expressed in the pH of the calcifying fluid. Thus, it is vitally important to measure how calcifying fluid pH is affected by ocean acidification and to understand what mechanisms regulate this response.
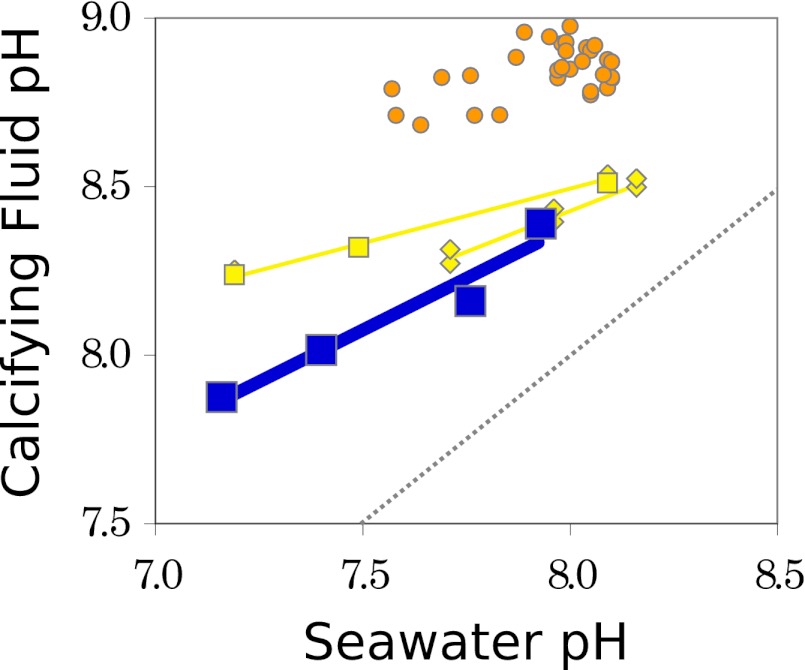
Most current estimates of calcifying fluid pH rely on an indirect geochemical technique, the boron isotope pH proxy (5). Boron isotope measurements from the skeletons of cultured tropical coral (6–8) suggest that calcifying fluid pH is elevated with respect to seawater, but that this pH systematically decreases with seawater pH (Fig. 1). Thus, calcifying fluid pH is impacted by ocean acidification. Furthermore, different species of coral seem to elevate calcifying fluid pH by different amounts (Fig. 1). In particular, deep-sea coral show higher calcifying fluid pH values and lower pH sensitivity than tropical species (9, 10). These results could explain divergent sensitivities to ocean acidification, showing the utility of calcifying fluid pH data (10, 11).
Fig. 1.
Coral calcifying fluid pH decreases with ocean acidification, as shown by Venn et al. (3) from direct pH measurements in the tropical coral S. pistillata (blue boxes), and as inferred from previous boron isotope measurements (6–10). Boron isotope data from S. pistillata (yellow boxes) and other tropical species (yellow diamonds) roughly agree with direct measurements, whereas deep-sea coral (orange circles) are characterized by higher calcifying fluid pH values and lower sensitivity to seawater pH. Compared with seawater (dashed line), tropical coral calcifying fluid pH is more offset under more acidified conditions. Calcifying fluid pH calculated from boron isotope data using an δ11B of total boron of 39.16 ‰, a fractionation of 1.0272, and with pKB set by temperature and salinity. Total pH scale is used throughout.
Although the boron isotope results provide a rich picture of calcifying fluid pH, a number of questions remain regarding the interpretation of these data. Specifically, it is unknown whether boron isotopes indeed probe calcifying fluid pH or whether physiological effects related to coprecipitation, ion transport, or other processes act to distort the pH signal. Furthermore, there are questions regarding which fractionation factor should be used to generate pH estimates from boron data. Depending on the choice of this factor, calcifying fluid pH is either offset from seawater, as plotted in Fig. 1, or, with the use of an alternative fractionation factor, no pH difference is found between the calcifying fluid and seawater. Direct measurements of calcifying fluid pH could help resolve these issues, validating the boron isotope paleoproxy while also establishing the role of calcifying fluid pH in coral’s response to ocean acidification.
Early and groundbreaking attempts to directly measure calcifying fluid pH in coral relied on microelectrodes (12), but extensive use of this technique has been limited by the complexity of the method. As an alternative approach to microelectrodes, confocal microscopy combined with fluorescent probes can directly characterize the calcifying fluid in coral. This technique relies on a unique coral culture method (13) whereby a thin sheet of skeleton is grown across glass slides, simplifying skeletal geometry and allowing optical access to the site of calcification. This approach was first used with bulky fluorescent dyes to demonstrate direct transport between seawater and the site of calcification (14, 15). Exploiting this seawater pathway, Venn et al. (16) loaded the calcifying fluid of cultured coral with a pH-sensitive fluorescent indicator, demonstrating that they could directly probe local pH.
Following up on their earlier work, Venn et al. (3) have now definitively shown that calcifying fluid pH decreases with ocean acidification (Fig. 1) through direct measurements in the cultured tropical coral Stylophora pistillata. Compared with boron isotope data from the same species, directly measured pH exhibits a somewhat steeper sensitivity to acidification, but this slope is reasonably similar to boron-isotope–derived slopes from other tropical coral. Although only one species of coral has been measured by the fluorescence method thus far, the rough agreement between the two techniques is exciting: direct pH measurements appear to affirm that boron isotopes measure calcifying fluid pH in coral. If this interpretation is correct, the boron isotope proxy must respond to pH indirectly: the signal is first filtered through the physiology of biomineralization. Clearly, further experiments across a range of different coral will be necessary to confirm this picture—but the path forward is promising.
At a more fundamental level, we need to understand why calcifying fluid varies with external pH and what modulates this sensitivity. One key observation, first noted by using boron isotopes (10, 11) and now confirmed through direct measurements (3), is that the pH offset between calcifying fluid and seawater increases with acidification; coral build a stronger pH gradient at lower pH. Given that coral actively alter calcifying fluid pH, with an even stronger biological effect under harsher conditions, why does seawater chemistry impact calcifying fluid pH at all? This question is at the heart of the ocean acidification problem and is also key for understanding what sets the sensitivity of the boron isotope proxy.
Venn et al. have now definitively shown that calcifying fluid pH decreases with ocean acidification.
Local pH elevation is thought to be controlled through alkalinity pumping, as supported by geochemical arguments (17) and by the presence of a biological pump in coral that is capable of exchanging 2H+ for Ca2+ across cell membranes (18). If the rate of this alkalinity pumping is insensitive to seawater pH, calcification rates and calcifying fluid pH would decrease with ocean acidification. This model can explain compositional patterns and growth rate sensitivity in some culture experiments (19). However, Venn et al. (3) use their pH results together with growth rate data to show that a threshold-like decrease in calcification rate is inconsistent with constant alkalinity pumping. Furthermore, a constant alkalinity-pumping model cannot explain compositional patterns found in coral cultured at higher than ambient pH (20). In these nonacidified conditions, coral may regulate alkalinity pumping to reach a target calcifying fluid pH. This alternative hypothesis predicts that calcifying fluid pH should remain relatively constant at higher than ambient seawater pH values, a pattern that may be followed in deep-sea coral (Fig. 1) and that is a clear target for future experiments in tropical coral. Alternatively, McCulloch et al. (10) propose that calcifying fluid pH and skeletal growth rates are ultimately limited by the energy required to maintain a particular pH gradient. Indeed, the metabolic cost of pH regulation and the effect of this process on an organism-scale energy balance is likely to play a major role in modulating the impact of ocean acidification on marine calcifiers. Probing the mechanism of coral biomineralization is clearly an area of intensely active research, but, by using the technique demonstrated by Venn et al. (3), as well as a growing set of biological and geochemical tools, we are finally poised to understand at a chemical scale how and why coral calcification feels ocean acidification.
Footnotes
The author declares no conflict of interest.
See companion article on page 1634.
References
- 1.Sabine CL, et al. The oceanic sink for anthropogenic CO2. Science. 2004;305(5682):367–371. doi: 10.1126/science.1097403. [DOI] [PubMed] [Google Scholar]
- 2.Gattuso J-P, Hansson L. Ocean Acidification. Oxford: Oxford Univ Press; 2011. [Google Scholar]
- 3.Venn AV, et al. Impact of seawater acidification on pH at the tissue–skeleton interface and calcification in reef corals. Proc Natl Acad Sci USA. 2013;110:1634–1639. doi: 10.1073/pnas.1216153110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnston I. The ultra structure of skeletogenesis in hermatypic corals. Int Rev Cytol. 1980;67:171–214. [Google Scholar]
- 5.Hemming N, Hanson G. Boron isotopic composition and concentration in modern marine carbonates. Geochim Cosmochim Acta. 1992;56:537–543. [Google Scholar]
- 6.Hönisch B, et al. Assessing scleractinian corals as recorders for paleo-pH: Empirical calibration and vital effects. Geochim Cosmochim Acta. 2004;68:3675–3685. [Google Scholar]
- 7.Reynaud S, Hemming N, Jullet-Leclerc A, Gattuso J-P. Effect of pCO2 and temperature on the boron isotopic composition of the zooxanthellate coral Acropora sp. Coral Reefs. 2004;23:539–546. [Google Scholar]
- 8.Krief S, et al. Physiological and isotopic responses of scleractinian corals to ocean acidification. Geochim Cosmochim Acta. 2010;74:4988–5001. [Google Scholar]
- 9.Trotter J, et al. Quantifying the pH ‘vital effect’ in the temperate zooxanthellate coral Cladocora caespitosa: Validation of the boron seawater pH proxy. Earth Planet Sci Lett. 2011;303:163–173. [Google Scholar]
- 10.McCulloch M, et al. Resilience of cold-water scleractinian corals to ocean acidification: Boron isotopic systematics of pH and saturation state up-regulation. Geochim Cosmochim Acta. 2012;87:21–34. [Google Scholar]
- 11.Anagnostou E, Huang K-F, You C-F, Sikes E, Sherrell R. Evaluation of boron isotope ratio as a pH proxy in the deep sea coral Desmophyllum dianthus: Evidence of physiological pH adjustment. Earth Planet Sci Lett. 2012;349-350:251–260. [Google Scholar]
- 12.Al-Horani F, Al-Moghrabi S, de Beer D. The mechanism of calcification and its relation to photosynthesis and respiration in the scleractinian coral Galaxea fascicularis. Mar Biol. 2003;142:419–426. [Google Scholar]
- 13.Reynaud-Vaganay S, Gattuso J-P, Cuif J-P, Jaubert J, Jullet-Leclerc A. A novel culture technique for scleractinian corals: Application to investigate changes in skeletal delta δ18O as a function of temperature. Mar Ecol Prog Ser. 1999;180:121–130. [Google Scholar]
- 14.Erez J, Braun A. Calcification in hermatypic corals is based on direct seawater supply to the biomineralization site. Geochim Cosmochim Acta. 2007;71:A260. [Google Scholar]
- 15.Tambutté E, et al. Calcein labeling and electrophysiology: Insights on coral tissue permeability and calcification. Proc Royal Chem Soc B. 2011;279:19–27. doi: 10.1098/rspb.2011.0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Venn A, Tambutté E, Holcomb M, Allemand D, Tambutté S. Live tissue imaging shows reef corals elevate pH under their calcifying tissue relative to seawater. PLoS ONE. 2011;6(5):e20013. doi: 10.1371/journal.pone.0020013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McConnaughey T. 13C and 18O isotopic disequilibrium in biological carbonates. 2. In vitro simulation of kinetic isotope effects. Geochim Cosmochim Acta. 1989;53:163–171. [Google Scholar]
- 18.Zoccola D, et al. Molecular cloning and localization of a PMCA P-type calcium ATPase from the coral Stylophora pistillata. Biochim Biophys Acta. 2004;1663(1-2):117–126. doi: 10.1016/j.bbamem.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 19.Cohen A, McCorkle D, de Putron S, Gaetani G, Rose K. Morphological and compositional changes in the skeletons of new coral recruits reared in acidified seawater: Insights into the biomineralization response to ocean acidification. Geochem Geophys Geosyst. 2009;10:Q07005. [Google Scholar]
- 20.Gagnon A, Adkins J, Erez J, Eiler J, Guan Y. Sr/Ca sensitivity to aragonite saturation state in cultured subsamples from a single colony of coral: Mechanism of biomineralization during ocean acidification. Geochim Cosmochim Acta. 2013;105:240–254. [Google Scholar]