Abstract
Bioactive gibberellins (GAs) control many aspects of growth and development in plants. GA1 has been the most frequently found bioactive GA in various tissues of flowering plants, but the enzymes responsible for GA1 biosynthesis have not been fully elucidated due to the enzymes catalyzing the 13-hydroxylation step not being identified. Because of the lack of mutants defective in this enzyme, biological significance of GA 13-hydroxylation has been unknown. Here, we report that two cytochrome P450 genes, CYP714B1 and CYP714B2, encode GA 13-oxidase in rice. Transgenic Arabidopsis plants that overexpress CYP714B1 or CYP714B2 show semidwarfism. There was a trend that the levels of 13-OH GAs including GA1 were increased in these transgenic plants. Functional analysis using yeast or insect cells shows that recombinant CYP714B1 and CYP714B2 proteins can convert GA12 into GA53 (13-OH GA12) in vitro. Moreover, the levels of 13-OH GAs including GA1 were decreased, whereas those of 13-H GAs including GA4 (which is more active than GA1) were increased, in the rice cyp714b1 cyp714b2 double mutant. These results indicate that CYP714B1 and CYP714B2 play a predominant role in GA 13-hydroxylation in rice. The double mutant plants appear phenotypically normal until heading, but show elongated uppermost internode at the heading stage. Moreover, CYP714B1 and CYP714B2 expression was up-regulated by exogenous application of bioactive GAs. Our results suggest that GA 13-oxidases play a role in fine-tuning plant growth by decreasing GA bioactivity in rice and that they also participate in GA homeostasis.
Keywords: biosynthesis, plant hormones
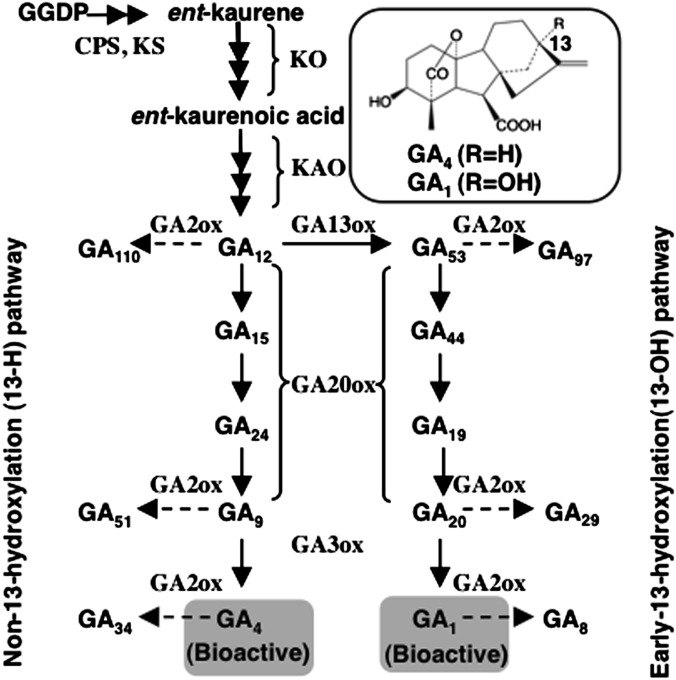
The phytohormone gibberellins (GAs) are a class of diterpenoids that promote growth in various stages of the plant life cycle: seed germination, stem elongation, leaf expansion, flowering, and flower development (1). Bioactive GAs (such as GA1 and GA4) are biosynthesized from geranylgeranyl diphosphate (GGDP) via a multistep process, which involves three classes of enzymes: plastid-localized terpene cyclases, membrane-bound cytochrome P450 monooxygenases (P450s), and soluble 2-oxoglutarate–dependent dioxygenases (2ODDs) (Fig. 1) (reviewed by ref. 2). Endogenous GA levels are also regulated by deactivation. Gibberellin 2-oxidase (GA2ox) encodes a 2ODD and converts bioactive and intermediate forms of GAs to their inactive forms by 2β-hydroxylation (Fig. 1) (3). Recently, CYP714D1/EUI, a cytochrome P450 monooxygenase from rice, has been shown to deactivate 13-H GAs through 16α, 17-epoxidation (4). More recently, reverse genetic studies suggested a potential role of CYP714A1 and CYP714A2 in deactivating GAs in Arabidopsis (5). Some mutants defective in GA deactivation are taller than WT plants, including cyp714d1/eui of rice (4), slender of pea (Psga2ox1) (6, 7), and an Arabidopsis ga2ox quintuple mutant (8).
Fig. 1.
GA biosynthesis and deactivation pathways in flowering plants. Solid and dashed lines indicate GA biosynthesis and deactivation (2β-hydroxylation) pathways, respectively. CPS, ent-copalyl diphosphate synthase; GA2ox, GA 2-oxidase; GA3ox, GA 3-oxidase; GA13ox, GA 13-oxidase; GA20ox, GA 20-oxidase; GGDP, geranylgeranyl diphosphate; KAO, ent-kaurenoic acid oxidase; KO, ent-kaurene oxidase; KS, ent-kaurene synthase.
In flowering plants including Arabidopsis and rice, both 13-OH and 13-H GAs frequently coexist in the same tissue. It has been reported that bioactivity of GA1 (a 13-OH GA) is lower than GA4 (a 13-H GA) in both Arabidopsis (9–11) and rice (12), and this difference presumably attributed to their binding affinity to the GA receptor GIBBERELLIN INSENSITIVE1 (GID1) (13, 14). In Arabidopsis, GA4, which is synthesized through the non–13-hydroxylation pathway, is the predominant bioactive GA in most tissues (Fig. 1); GA1, which is synthesized through the early–13-hydroxylation pathway, accumulates at relatively high levels in siliques (15). In contrast, in rice plants, GA1 is the predominant bioactive form. Levels of GA4 are low in vegetative tissues, whereas they are extremely high in anthers (16, 17). However, how GA 13-hydroxylation affects GA bioactivity and why GA4 and GA1 occasionally accumulate in particular plant organs remain unknown.
GA 13-oxidase activity determines the ratio of GA4 and GA1 (Fig. 1). No gene encoding this enzyme has been identified thus far. The occurrence of GA 13-hydroxylation activity has been found in a microsome fraction of pea (Pisum sativum) embryo (18) and also in a soluble fraction of spinach (Spinacia oleracea) leaves (19). These results suggested that there might be GA 13-oxidases with different properties.
In this study, we report that CYP714B1 and CYP714B2, the rice CYP714 gene family members, encode GA 13-oxidase. We also show that the cyp714b1 cyp714b2 double mutant has a longer uppermost internode. Moreover, both genes are up-regulated by exogenous application of bioactive GAs in WT. Our results suggest that GA 13-oxidases negatively regulate growth and participate in GA homeostasis in rice.
Results
Overexpression of CYP714B1 or CYP714B2 Causes Semidwarfism and Increases 13-OH GAs in Arabidopsis Plants.
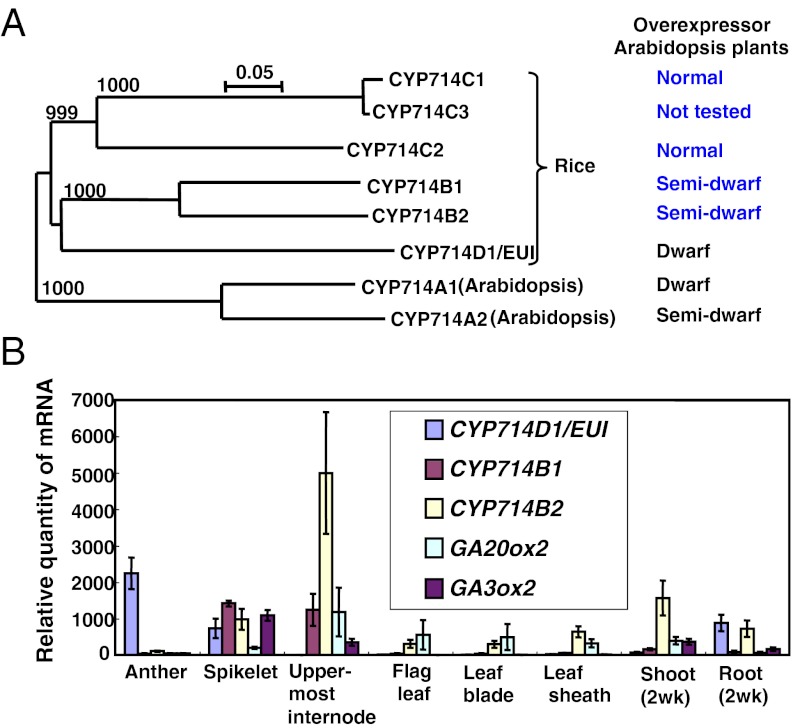
We have previously shown that rice CYP714D1 is a GA 16α,17-epoxidase that deactivates 13-H GAs through epoxidation. Whereas CYP714D1 is the sole member of the CYP714D subfamily, rice has additional CYP714 subfamilies, including CYP714Bs and CYP714Cs (20) (Fig. 2A). To deduce whether these CYP714 family members might also be involved in GA metabolism, we generated transgenic Arabidopsis plants that overexpress each CYP714 gene. Among CYP714B1 (Os07g0681300), B2 (Os03g0332100), C1 (Os12g0118900), and C2 (Os12g0119000), only CYP714B1-overexpressing plants (CYP714B1-atOE) and CYP714B2-overexpressing plants (CYP714B2-atOE) showed semidwarfism (Fig. 2A; Fig. S1A). We did not test CYP714C3 (Os11g0119200), because it is almost identical (95%) to CYP714C1 in the amino acid sequence. The semidwarfism of CYP714B1-atOE and CYP714B2-atOE was rescued when the plants were grown on media containing bioactive GA (0.1 μM GA3; Fig. S1B), suggesting that GA deficiency caused the phenotype. GA analysis showed that there is a trend that CYP714B1-atOE plants have decreased levels of 13-H GAs, whereas levels of 13-OH GAs including GA1 were increased (Table S1). In CYP714B2-atOE plants, levels of some 13-OH GAs were also increased, although many 13-H GAs could not be quantified due to low abundance or to comigrating impurities on LC-MS/MS analysis (Table S1). These results suggested that CYP714B1 and CYP714B2 might encode GA 13-oxidase.
Fig. 2.
Arabidopsis plants that overexpress CYP714B1 or CYP714B2 show semidwarf phenotypes. (A) Phylogenetic tree of the CYP714 family in Arabidopsis and rice with bootstrap values and the gross phenotype of Arabidopsis transgenic plants overexpressing each family member revealed by previous (black) (6) and current (blue) studies. (B) Transcript levels of CYP714Bs in various tissues of WT rice (Nipponbare). Relative levels of each transcript/106 copies of 18S rRNA were determined by quantitative RT-PCR. Data represent means of three biological replicates ± SEM.
We examined the expression profiles of the CYP714B1 and CYP714B2 genes during rice development. Quantitative RT-PCR analysis showed that both genes are expressed in all plant parts examined, with relatively high expression in spikelet and uppermost internode in adult plants (Fig. 2B). The CYP714B2 gene is also highly expressed in the shoot of seedlings. CYP714D1/EUI is highly expressed in anthers as reported previously (17).
CYP714Bs Encode a GA 13-Oxidase.
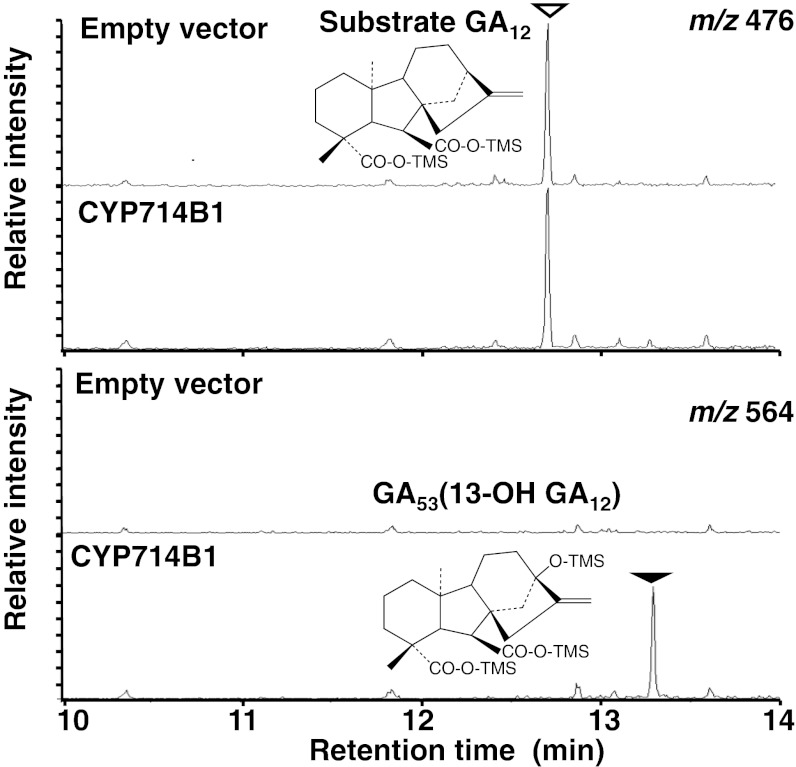
To clarify whether CYP714B1 and CYP714B2 possess GA 13-oxidase activity, recombinant proteins were prepared as a fusion protein with a C-terminal His-tag using a Pichia pastoris yeast expression system (21). We incubated a microsomal fraction containing CYP714B1 protein with various GAs and GA intermediates (ent-kaurenoic acid, GA12, GA9, and GA4) as the putative substrate. As a result, we detected the conversion of GA12 into GA53 (13-OH GA12) (Fig. 3; Table S2), but did not detect any conversion of other substrates. We tried to express CYP714B2 protein using the same system, but did not detect the expected size of CYP714B2 recombinant protein by immunoblot analysis using anti-His-tag antibody (Fig. S2A). Therefore, as an alternative way to produce recombinant CYP714B2, we used a baculovirus-insect cell expression system (Fig. S2B). We carried out the enzyme assay using a microsomal fraction of sf9 cells containing CYP714B2 protein. As a result, we detected the conversion of GA12 into GA53 (Table S2), but did not detect any conversion of other substrates described above.
Fig. 3.
Recombinant CYP714B1 protein has GA 13-hydroxylation activity. Shown are mass chromatograms of GA12 (upper, trimethylsilyl ester-derivative, shown by an open triangle; m/z 476) and GA53 (lower, trimethylsilyl ester-trimethylsilyl ether derivative, shown by a closed triangle; m/z 564) after incubation of GA12 with a microsome fraction purified from control (empty vector) or CYP714B1-producing P. pastoris.
GA 13-Hydroxylation Is Blocked in the cyp714b1 cyp714b2 Double Mutant.
To clarify whether CYP714Bs play a role in GA13-hydroxylation in planta, a cyp714b1 T-DNA insertion line, 2A-20177 [cultivar Hwayoung (HY)], and a cyp714b2 Tos17 retrotransposon insertion line, NG2481 [cultivar Nipponbare (NB)], were obtained and analyzed (Fig. S3A). Despite the disruption of the second exon by the insertion of a T-DNA, CYP714B1 transcripts (probably an aberrant form) were highly expressed in the heterozygous and homozygous cyp714b1 mutants compared with WT (Fig. S3B). This result is probably due to an activation-tagging effect of the enhancer sequence of the inserted T-DNA (22). We found no significant change in endogenous GA levels in homozygous cyp714b1 plants (see below); thus, a possible effect on GA metabolism by the overexpression of the aberrant transcripts is likely to be small. The Tos17 retrotransposon is inserted in the third exon of CYP714B2 in the cyp714b2 mutant in which its transcript levels were significantly decreased (Fig. S3 A and B). We crossed these single mutants and obtained cyp714b1 cyp714b2 double mutants in the F2 generation. We first analyzed endogenous GA profiles using F3 seedlings of the double mutant (lines 32 and 39) and compared them with those of segregated WT plants (lines 33 and 36) and the parental WTs (NB and HY) (Fig. 4). We found that the levels of 13-OH GAs including GA1 were decreased, whereas those of 13-H GAs including GA4 were increased in the double mutant. GA measurements of cyp714b1 and cyp714b2 single mutant seedlings showed that no clear change in the levels of 13-OH GAs in comparison with those in WTs (Table S3), indicating that neither single mutation is sufficient to inhibit the GA 13-hydroxylation pathway. These results indicate that CYP714B1 and CYP714B2 play a major role in GA 13-hydroxylation in rice. However, the levels of some 13-H GAs, including GA12, GA15, and GA24, in the cyp714b2 mutant, but not in the cyp714b1 mutant, were repeatedly higher than those in the WT control. These results suggest that CYP714B2 in comparison with CYP714B1 might play a dominant role in GA 13-hydroxylation at the seedling stage.
Fig. 4.
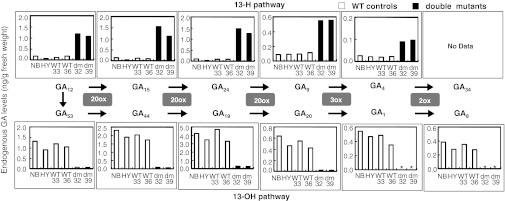
Endogenous GA levels in the cyp714b1 cyp714b2 double mutant. GA levels in 3-wk-old seedlings of the cyp714b1 cyp714b2 double mutant (dm F3 lines 32 and 39; black bar) and WT controls (HY, Hwayoung; NB, Nipponbare; and isolated WT F3 lines 33 and 36; open bar) plants. GAs are shown according to the order in the biosynthesis pathway (Fig. 1). Note that the y axis scale for each GA is arbitrary for clarity. *Undetectable.
cyp714b1 cyp714b2 Double Mutant Has a Longer Uppermost Internode.
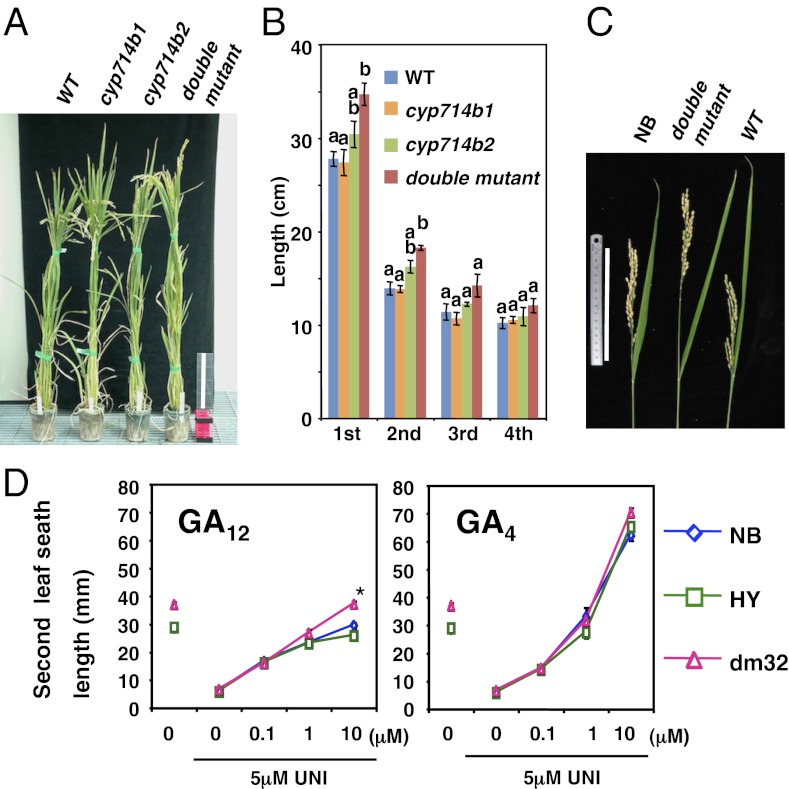
We observed the phenotype of the single and double mutants in the F2 and later generations. Both cyp714b1 and cyp714b2 single mutants were morphologically similar to segregated WTs throughout their lifetime (Fig. 5A). The double mutant was also morphologically normal compared with the segregated WTs at the seedlings stage, but it was taller than WTs at the heading stage (Fig. 5A). In the double mutant, the uppermost internode and, to a lesser extent, the second internode, were significantly longer than those of WT (Fig. 5B). Because of the longer internode, the length of the exposed internode from the flag leaf sheath was much longer in the double mutant compared with the WTs (Fig. 5C). The tall phenotype of the double mutant was clear in the segregants. However, their final height was comparable to that of the Nipponbare WT (Fig. S4A). This observed growth retardation might be due to inheritance of a shorter height trait of the HY cultivar (Fig. S4A) or an unidentified second mutation (23).
Fig. 5.
Phenotypes of cyp714b1 cyp714b2 double mutant plants. (A) Twenty-two-week-old plants of F2 WT, cyp714b1, and cyp714b2 single and the double mutant. (Scale bar, 20 cm.) (B) Internode length of F3 WT (line 57), cyp714b1 (line 1), cyp714b2 (line 4), and the double mutant (line 32). Mean ± SEM, n = 5–6. Letters above bars indicate statistically significant differences between samples by one-way ANOVA with Tukey-Kramer multiple comparison test (P < 0.01). (C) Panicle exertion (heading performance) of Nipponbare (NB), double mutant, and F3 WT. (Scale bar, 15 cm.) (D) Response to exogenous GA12 or GA4. WTs (NB and HY) and double mutants (F5 line 32) grown on half-strength Murashige-Skoog medium containing various concentrations of GAs and/or uniconazole (UNI). Mean ± SEM, n = 6–11. *Significant difference between dm32 and WTs (Student t test with Bonferroni corrections, P < 0.001).
To verify the double mutant phenotype, we performed a genetic complementation test. We used the CYP714B1 gene but not the CYP714B2 gene for this experiment because the latter was too large to subclone (Fig. S3A). An 11-kb genomic DNA fragment containing the coding region of CYP714B1 was introduced into the double mutant by Agrobacterium-mediated transformation. We confirmed that in independent transgenic lines the uppermost internode phenotype of the double mutant was rescued (Fig. S4B). We further checked the genotype–phenotype correlation using F3 progenies. We harvested the F3 seeds from an F2 plant with a cyp714b1−/− (homozygous mutant) cyp714b2+/− (heterozygous mutant) genotype. We then compared the phenotype of the F3 progenies (n = 36). As a result, the homozygous double mutant progenies (cyp714b1−/− cyp714b2−/−) showed a tall phenotype with longer uppermost internodes (Fig. S4C). These two results indicate that the elongated uppermost internode is a loss of function phenotype of the cyp714b1 cyp714b2 double mutant.
To further characterize the cyp714b1 cyp714b2 double mutant, we compared their response to exogenous GA12 and GA4 in the presence of a GA biosynthesis inhibitor, uniconazole (UNI, inhibitor of ent-kaurene oxidase; Fig. 1). The effect of 5 μM UNI was reversed by 1 μM GA4 in the double mutant and two WTs (NB and HY) (Fig. 5D). When the plants were treated with GA4, the dose–response curves of the double mutant and the WTs were nearly overlapped. In contrast, in GA12 treatments, the double mutant was more responsive to GA12 at 10 μM than the WTs (Fig. 5D). Given that bioactivity of GA4 is higher than GA1 (12), the elevated responsiveness to GA12 of the double mutant suggests that GA12, but not GA4, is a substrate for CYP714Bs in planta.
CYP714B Expression Is Up-Regulated by GA.
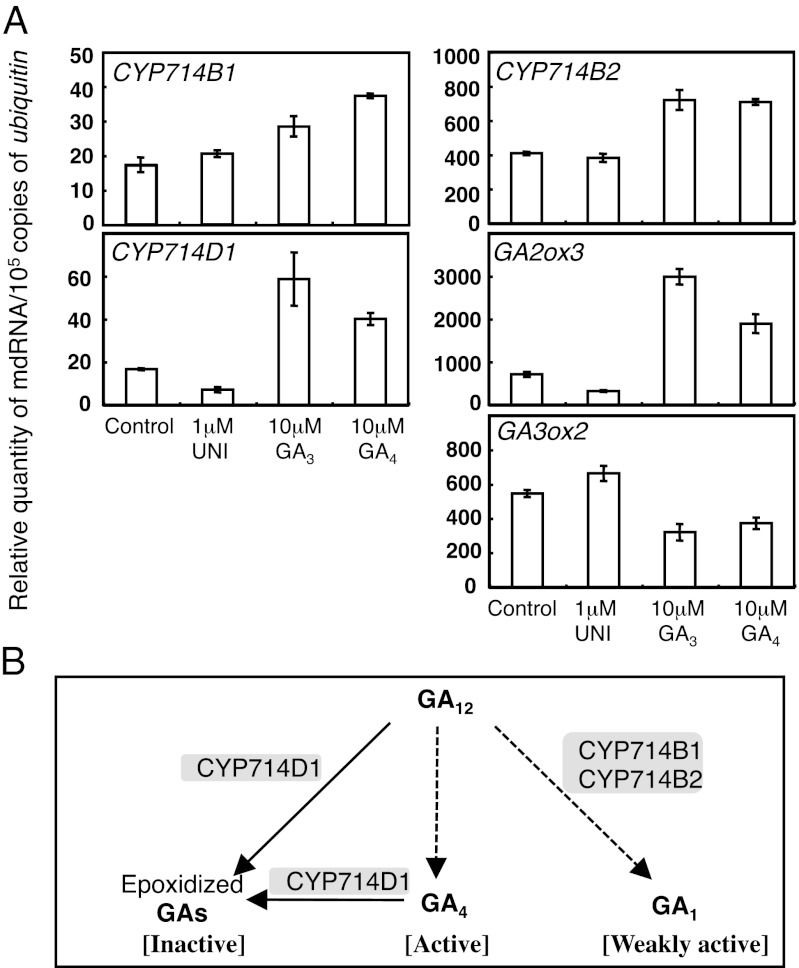
To understand the role of CYP714Bs in GA homeostasis, we examined the effect of exogenous GA or UNI treatment on the levels of CYP714B transcripts. Both CYP714B1 and CYP714B2 transcripts were up-regulated by bioactive GAs: GA3 and GA4 (Fig. 6A). These responses were similar to those of GA inactivation genes, GA2ox3 and CYP714D1 (Fig. 6A), as reported previously (24, 25). Given that bioactivity of GA1 is lower than that of GA4 in rice (12), our results suggests that CYP714Bs may participate in GA4 homeostasis in rice.
Fig. 6.
Physiological role of CYP714B1 and CYP714B2 genes. (A) CYP714B1 and CYP714B2 genes are responsive to bioactive GAs. Transcript levels of CYP714Bs and known GA metabolism genes in aerial part of WT rice plants (Nipponbare). Rice seeds were imbibed in water for 24 h and then planted on half-strength Murashige-Skoog medium containing GA3, GA4, or uniconazole (UNI), and then were grown for 5 d. Relative quantity of mRNA/105 copy of ubiquitin was determined by quantitative RT-PCR. Data represent means of three biological replicates ± SEM. (B) Hypothetical model for a role of GA 13-hydroxylation in rice. Arrows indicate a direct(solid) or indirect (dotted) contribution to the synthesis of respective GAs. GA4 (strongly active), which is produced via the non–13-hydroxylation pathway from GA12, would be useful to induce strong GA signal output. GA1 (weakly active), which is produced via the early–13-hydroxylation pathway through 13-hydroxylation of GA12 by CYP714Bs, would be useful for controlling a small change in GA signal output. GA4 and GA12, but not GA1, are deactivated by CYP714D1. A combination of GA4 and GA1 would allow plants to achieve fine- and flexible-tuning of GA-dependent growth.
Discussion
Here, by using a reverse genetic approach, we demonstrated that CYP714B1 and CYP714B2 encode rice GA 13-oxidases. Functional analysis of recombinant CYP714B1 and CYP714B2 proteins and GA analyses of the single and the double mutants indicated that these two cytochrome P450s play a major role in GA 13-hydroxylation in rice. We also found that the cyp714b1 cyp714b2 double mutant shows an elongated uppermost internode phenotype. Moreover, both genes are up-regulated by bioactive GAs. From these findings, we propose that the rice GA 13-oxidase genes negatively regulate growth and participate in GA homeostasis.
Cytochrome P450s share the same CYP number when the sequence identity is typically >40%; they are further grouped into subfamilies (indicated by letters) based on when the sequence identity is >55% (26). The CYP714B subfamily was found in the maize genome (CYP714B3, ACG27951), but not in Arabidopsis (CYP714A1 and A2), papaya (CYP714A7), poplar (CYP714A3, E2, E4, E5, E6, and F1), or grape (CYP714A8, E8, E12, F2, G5, and G6) (20). It is possible that some other CYP714 subfamilies or other P450 families act as GA 13-oxidase in these plant species. Whereas P450 is generally a membrane-bound protein, GA 13-hydroxylation activity was previously also found in a soluble fraction of spinach leaves (19). It was also reported that recombinant TaGA3ox2 protein (2ODD of wheat) exhibited a weak GA 13-oxidase activity, besides GA 3-oxidase activity in vitro (27). More recently, it was shown that recombinant MmGA3ox2 protein, another 2ODD from the southern wild cucumber Marah macrocarpus, was a multifunctional enzyme that could catalyze GA 13-hydroxylation (28). In addition, we still detected small amounts of 13-OH GAs in the cyp714b1 cyp714b2 double mutant (Fig. 4). These facts suggest that another class of oxidases other than cytochrome P450 might also participate in GA 13-hydroxylation, and they might play a role in plant development.
The cyp714b1 cyp714b2 double mutant showed a tall phenotype compared with the WT controls at the heading stage, and this is mainly attributed to elongated uppermost internodes (Fig. 5 A and B). Consistent with this observation, quantitative RT-PCR analysis showed that both genes are highly expressed in the uppermost internode in WT (Fig. 2B). Because we had to cross single mutants in different genetic backgrounds (Nipponbare and Hwayoung) to make the double mutant, we needed to be careful about the effect of transgressive segregation. However, we believe that the observed phenotype is attributable to the disruption of the two CYP714B genes for the following reasons. First, the genetic complementation test using the CYP714B1 gene rescued the uppermost internode phenotype of the double mutant (Fig. S4B). Second, experiments using F3 progenies showed that there is a correlation between the homozygous double mutation of the CYP714B genes and a tall phenotype with longer uppermost internodes (Fig. S4C). Third, GA measurements showed that GA 13-hydroxylation is blocked in the double mutant (Fig. 4). Because bioactivity of GA4 (13-H GA) is stronger (approximately fourfold) than that of GA1 (13-OH GA) in rice (12), accumulation of GA4 by the disruption of GA 13-hydroxylation is probably a cause of the phenotype of the double mutant. Previously, it was reported that transgenic tobacco plants that overexpress citrus GA 20-oxidase 1 exhibit a tall phenotype due to the production of more GA4 but less GA1 than WT, but the mechanism by which the transgenic plants show such GA profiles has yet to be investigated (29).
Although the elongation of uppermost internodes of the cyp714b1 cyp714b2 double mutant (Fig. 5 B and C) is not as severe as those of the cyp714d1/eui mutant (4, 30), their phenotypes are similar. This fact suggests that CYP714D1 and CYP714Bs cooperatively suppress GA-dependent growth of this tissue. Meanwhile, functional analysis of recombinant CYP714D1 in our previous study indicated that this enzyme specifically deactivates 13-H GAs including GA12 by 16α,17-epoxidization (4), suggesting that CYP714D1 could compete with CYP714Bs for their substrate, GA12. These facts suggest that drastic deactivation of 13-H GAs by CYP714D1 and the production of less active GA (GA1) through 13-hydroxylation by CYP714Bs participate in the regulation of uppermost internode elongation and panicle heading. In other words, one of the roles of the rice GA 13-oxidases in this tissue might be to contribute to weak deactivation of 13-H GAs and to alleviate drastic deactivation 13-H GAs by CYP714D1. Control of not only the levels of bioactive GAs but also GA activity by these enzymes would optimize the uppermost internode elongation.
Arabidopsis CYP714B1- and CYP714B2-atOE plants showed semidwarf phenotypes (Fig. S1A), whereas GA1 levels were increased ∼10-fold in both overexpressor lines compared with controls (Table S1). In Arabidopsis, GA4, which is synthesized through the non–13-hydroxylation pathway, is predominant bioactive GA in most tissues (Fig. 1). Because bioactivity of GA4 (13-H GA) is greater than that of GA1 (13-OH GA) in Arabidopsis (9–11), depletion of 13-H GAs including GA4 due to overexpression of GA 13-oxidase is likely a cause of the semidwarfism in both overexpressor lines, although most 13-H GAs could not be quantified due to their low abundance or comigrating impurities in CYP714B2-atOE plants (Table S1). Restoration of their growth by 0.1 μM GA3 (Fig. S1B) supports this idea. However, we cannot rule out the possibility that their semidwarf phenotypes might be due to unidentified minor enzyme activities of CYP714Bs when overexpressed in planta, besides a reduction in GA4 levels by 13-hydroxylation activity. To address this possibility, more detailed functional analysis of CYP714B proteins would be needed in the future.
In rice plants, GA1 is a predominant bioactive form in vegetative tissues, but in anthers, GA4 levels are much higher than GA1 levels (16, 17). As described above, the cyp714b1 cyp714b2 double mutant in which GA4 predominates appeared normal except for the elongated uppermost internode phenotype at the heading stage (Fig. 5). This result indicated that lower levels of GA4 substituted for WT levels of GA1 in most of the tissues in the double mutant (Fig. 4). Therefore, rice seems to use the less-bioactive GA normally except in anthers. Furthermore, we found that both GA 13-oxidases are up-regulated by exogenous application of bioactive GAs (GA3 and GA4) like some of GA-deactivating genes (Fig. 6A). This result also supports the idea that a role of GA 13-hydroxylation is to synthesize less active GA. Why would plants produce not only strongly active GAs but also weakly active GAs? Accumulating evidence indicates that bioactive GA levels are tightly regulated by developmental and environmental cues (31). We speculate that weakly active GAs might be advantageous when a small change in GA-dependent growth is required. By contrast, to induce strong GA signal output, e.g., in a specific tissue or temporally during development, strongly active GA would be useful. Thus, the combinational use of GA molecules with strong and weak activity would allow plants to fine- and flexible-tune GA-dependent growth (Fig. 6B).
Materials and Methods
The cyp714b1 mutant (2A-20177) was obtained from the Rice T-DNA Insertion Sequence Database (www.postech.ac.kr/life/pfg/risd) (22, 32). The cyp714b2 mutant (NG2481) was obtained from Tos17 insertion lines (33). The cyp714b1 cyp714b2 double mutant was obtained by crossing the single mutants. Specific DNA primers and probes used in this study are described in Table S4. Details of experimental procedures are described in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Tsuyoshi Nakagawa for providing the pGWB8 vector; Kan Wang for the pTF101.1 vector; Gynheung An for the 2A-20177 line; the Rice Genome Resource Center, Japan, for the NG2481 line; and Mitsue Miyao-Tokutomi for advice on the generation of rice transgenic plants. We also thank Ms. Kyoko Koinuma, Ms. Kaoru Fujiwara, and Ms. Yoko Sato for dedicated technical assistance.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. P.H. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1215788110/-/DCSupplemental.
References
- 1.Fleet CM, Sun TP. A DELLAcate balance: the role of gibberellin in plant morphogenesis. Curr Opin Plant Biol. 2005;8(1):77–85. doi: 10.1016/j.pbi.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 2.Yamaguchi S. Gibberellin metabolism and its regulation. Annu Rev Plant Biol. 2008;59:225–251. doi: 10.1146/annurev.arplant.59.032607.092804. [DOI] [PubMed] [Google Scholar]
- 3.Thomas SG, Phillips AL, Hedden P. Molecular cloning and functional expression of gibberellin 2-oxidases, multifunctional enzymes involved in gibberellin deactivation. Proc Natl Acad Sci USA. 1999;96(8):4698–4703. doi: 10.1073/pnas.96.8.4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu Y, et al. ELONGATED UPPERMOST INTERNODE encodes a cytochrome P450 monooxygenase that epoxidizes gibberellins in a novel deactivation reaction in rice. Plant Cell. 2006;18(2):442–456. doi: 10.1105/tpc.105.038455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang YY, et al. Two Arabidopsis cytochrome P450 monooxygenases, CYP714A1 and CYP714A2, function redundantly in plant development through gibberellin deactivation. Plant J. 2011;67(2):342–353. doi: 10.1111/j.1365-313X.2011.04596.x. [DOI] [PubMed] [Google Scholar]
- 6.Lester DR, Ross JJ, Smith JJ, Elliott RC, Reid JB. Gibberellin 2-oxidation and the SLN gene of Pisum sativum. Plant J. 1999;19(1):65–73. doi: 10.1046/j.1365-313x.1999.00501.x. [DOI] [PubMed] [Google Scholar]
- 7.Martin DN, Proebsting WM, Hedden P. The SLENDER gene of pea encodes a gibberellin 2-oxidase. Plant Physiol. 1999;121(3):775–781. doi: 10.1104/pp.121.3.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rieu I, et al. Genetic analysis reveals that C19-GA 2-oxidation is a major gibberellin inactivation pathway in Arabidopsis. Plant Cell. 2008;20(9):2420–2436. doi: 10.1105/tpc.108.058818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cowling RJ, Kamiya Y, Seto H, Harberd NP. Gibberellin dose-response regulation of GA4 gene transcript levels in Arabidopsis. Plant Physiol. 1998;117(4):1195–1203. doi: 10.1104/pp.117.4.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang YY, et al. Effects of gibberellins on seed germination of phytochrome-deficient mutants of Arabidopsis thaliana. Plant Cell Physiol. 1995;36(7):1205–1211. [PubMed] [Google Scholar]
- 11.Talon M, Koornneef M, Zeevaart JA. Endogenous gibberellins in Arabidopsis thaliana and possible steps blocked in the biosynthetic pathways of the semidwarf ga4 and ga5 mutants. Proc Natl Acad Sci USA. 1990;87(20):7983–7987. doi: 10.1073/pnas.87.20.7983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ueguchi-Tanaka M, et al. Molecular interactions of a soluble gibberellin receptor, GID1, with a rice DELLA protein, SLR1, and gibberellin. Plant Cell. 2007;19(7):2140–2155. doi: 10.1105/tpc.106.043729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ueguchi-Tanaka M, et al. GIBBERELLIN INSENSITIVE DWARF1 encodes a soluble receptor for gibberellin. Nature. 2005;437(7059):693–698. doi: 10.1038/nature04028. [DOI] [PubMed] [Google Scholar]
- 14.Nakajima M, et al. Identification and characterization of Arabidopsis gibberellin receptors. Plant J. 2006;46(5):880–889. doi: 10.1111/j.1365-313X.2006.02748.x. [DOI] [PubMed] [Google Scholar]
- 15.Varbanova M, et al. Methylation of gibberellins by Arabidopsis GAMT1 and GAMT2. Plant Cell. 2007;19(1):32–45. doi: 10.1105/tpc.106.044602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kobayashi M, et al. Fluctuation and localization of endogenous gibberellins in rice. Agric Biol Chem. 1988;52(5):1189–1194. [Google Scholar]
- 17.Hirano K, et al. Comprehensive transcriptome analysis of phytohormone biosynthesis and signaling genes in microspore/pollen and tapetum of rice. Plant Cell Physiol. 2008;49(10):1429–1450. doi: 10.1093/pcp/pcn123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamiya Y, Graebe JE. The biosynthesis of all major pea gibberellins in a cell-free system from Pisum sativum. Phytochemistry. 1983;22(3):681–689. [Google Scholar]
- 19.Gilmour SJ, Zeevaart JAD, Schwenen L, Graebe JE. Gibberellin metabolism in cell-free extracts from spinach leaves in relation to photoperiod. Plant Physiol. 1986;82(1):190–195. doi: 10.1104/pp.82.1.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nelson DR, Ming R, Alam M, Schuler MA. Comparison of cytochrome P450 genes from six plant genomes. Trop Plant Biol. 2008;1(3-4):216–235. [Google Scholar]
- 21.Katsumata T, et al. Arabidopsis CYP85A2 catalyzes lactonization reactions in the biosynthesis of 2-deoxy-7-oxalactone brassinosteroids. Biosci Biotechnol Biochem. 2008;72(8):2110–2117. doi: 10.1271/bbb.80192. [DOI] [PubMed] [Google Scholar]
- 22.Jeong DH, et al. T-DNA insertional mutagenesis for activation tagging in rice. Plant Physiol. 2002;130(4):1636–1644. doi: 10.1104/pp.014357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hirochika H. Insertional mutagenesis with Tos17 for functional analysis of rice genes. Breed Sci. 2010;60(5):486–492. [Google Scholar]
- 24.Sakai M, et al. Expression of novel rice gibberellin 2-oxidase gene is under homeostatic regulation by biologically active gibberellins. J Plant Res. 2003;116(2):161–164. doi: 10.1007/s10265-003-0080-z. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y, et al. Gibberellin homeostasis and plant height control by EUI and a role for gibberellin in root gravity responses in rice. Cell Res. 2008;18(3):412–421. doi: 10.1038/cr.2008.28. [DOI] [PubMed] [Google Scholar]
- 26.Schuler MA, Werck-Reichhart D. Functional genomics of P450s. Annu Rev Plant Biol. 2003;54:629–667. doi: 10.1146/annurev.arplant.54.031902.134840. [DOI] [PubMed] [Google Scholar]
- 27.Appleford NE, et al. Function and transcript analysis of gibberellin-biosynthetic enzymes in wheat. Planta. 2006;223(3):568–582. doi: 10.1007/s00425-005-0104-0. [DOI] [PubMed] [Google Scholar]
- 28.Ward DA, MacMillan J, Gong F, Phillips AL, Hedden P. Gibberellin 3-oxidases in developing embryos of the southern wild cucumber, Marah macrocarpus. Phytochemistry. 2010;71(17–18):2010–2018. doi: 10.1016/j.phytochem.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 29.Vidal AM, et al. The ectopic overexpression of a citrus gibberellin 20-oxidase enhances the non-13-hydroxylation pathway of gibberellin biosynthesis and induces an extremely elongated phenotype in tobacco. Physiol Plant. 2001;112(2):251–260. doi: 10.1034/j.1399-3054.2001.1120214.x. [DOI] [PubMed] [Google Scholar]
- 30.Luo AD, et al. EUI1, encoding a putative cytochrome P450 monooxygenase, regulates internode elongation by modulating gibberellin responses in rice. Plant Cell Physiol. 2006;47(2):181–191. doi: 10.1093/pcp/pci233. [DOI] [PubMed] [Google Scholar]
- 31.Sun TP. The molecular mechanism and evolution of the GA-GID1-DELLA signaling module in plants. Curr Biol. 2011;21(9):R338–R345. doi: 10.1016/j.cub.2011.02.036. [DOI] [PubMed] [Google Scholar]
- 32.Jeon JS, et al. T-DNA insertional mutagenesis for functional genomics in rice. Plant J. 2000;22(6):561–570. doi: 10.1046/j.1365-313x.2000.00767.x. [DOI] [PubMed] [Google Scholar]
- 33.Miyao A, et al. Target site specificity of the Tos17 retrotransposon shows a preference for insertion within genes and against insertion in retrotransposon-rich regions of the genome. Plant Cell. 2003;15(8):1771–1780. doi: 10.1105/tpc.012559. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.