Summary
Background and objectives
Sarcopenia is common in hemodialysis patients. This study examined whether the anabolic steroid oxymetholone improves muscle mass and handgrip strength in hemodialysis patients and possible mechanisms that might engender such changes.
Design, setting, participants, & measurements
Forty-three eligible hemodialysis patients were randomly assigned to ingest oxymetholone or placebo for 24 weeks. Body composition, handgrip strength, and quality of life were measured during the study. Muscle biopsies were performed and analyzed for mRNA levels for myostatin, IGF-I, IGF binding proteins, and myosin heavy chains and protein expression. Muscle fiber types and diameter were assessed by reduced nicotinamide–adenine dinucleotide staining.
Results
There was a significantly greater increase in fat-free mass and handgrip strength and decrease in fat mass in the oxymetholone compared with the placebo group. Moreover, compared with baseline values, patients given oxymetholone exhibited an increase in fat-free mass, handgrip strength, physical functioning scores, and type I muscle fiber cross-sectional area and a decrease in fat mass, whereas patients receiving placebo did not undergo changes. There was a significantly greater increase in muscle mRNA levels for myosin heavy chain 2×, IGF-I, and IGF-II receptor with oxymetholone treatment than placebo. Liver enzyme rose significantly in the oxymetholone group, but the number of values greater than three times the upper limit of normal were not different between these groups.
Conclusions
In hemodialysis patients, ingesting oxymetholone was associated with an increase in fat-free mass, handgrip strength, and muscle mRNA levels for several growth factors and a decrease in fat mass, but it also induced liver injury.
Introduction
Protein-energy wasting is a common adverse consequence of end stage kidney disease, and it is associated with impaired rehabilitation and increased morbidity and mortality (1–4). Individuals with end stage kidney disease are often poorly muscled. Moreover, low skeletal muscle mass in maintenance hemodialysis (MHD) patients is associated with increased mortality (5). Therapies designed to increase muscle mass and strength of dialysis patients might, therefore, be expected to improve their exercise capacity and possibly, their survival.
Anabolic–androgenic steroid therapy seems to be a promising adjunctive therapy for treatment of skeletal muscle wasting in chronic illness (6). Parenterally administered anabolic steroids may increase lean body mass and muscle mass in adults without CKD as well as MHD patients (7–12). Oxymetholone has the advantages that it can be given orally and it seems to exhibit higher anabolic activity and lower androgenic effects than testosterone (13). Several studies have shown increased fat-free mass (FFM) in people without CKD who were taking oxymetholone (6,14,15), but no such studies have been conducted in CKD patients. Oxymetholone also increased anthropometric measures, serum albumin, and lean body mass in continuous ambulatory peritoneal dialysis patients (16). The present study was undertaken to examine whether orally administered oxymetholone may improve protein-energy status and increase skeletal muscle mass in MHD patients and the possible mechanisms that may engender such changes.
Materials and Methods
This 24-week randomized, double-blind, placebo-controlled study was conducted in patients undergoing MHD at the Hemodialysis Unit of The Kidney Foundation of Thailand (ClinicalTrials.gov number ISRCTN41591818). The study was approved by the Institutional Review Boards of the Phramongkutklao Hospital. Recruitment began in June of 2006 and was completed in August of 2007. Treatment protocol patients were randomized by a method of block randomization by a research pharmacist to one of two double-blinded treatment groups. A computer-generated randomization procedure in blocks of four was used, and the protocol was successfully blinded through the end of the study. One group ingested oxymetholone (50 mg tablet two times daily) for 24 weeks. The other group ingested a placebo, which was identical in appearance to the oxymetholone, in the same manner. Treatment allocation was not known to patients, their physicians, or anybody within or outside the study. If serious adverse event was detected during study, the patient was assigned to an open-label phase, during which the patient continued to receive treatment with oxymetholone or placebo according to his/her original treatment assignment. However, if the decision was made that the serious adverse event was likely or possibly caused by the treatment protocol, the treatment was discontinued. Inclusion criteria into the study were age of 20 years or older, treatment with MHD for at least 3 months, a single-pool Kt/V urea of 1.2 or greater per MHD treatment, and no treatment with androgens or glucocorticoids within 6 months before starting the study. Patients with diabetes mellitus, active malignancy, severe heart, lung, or liver disease, strokes, or chronic infection (e.g., tuberculosis) within 1 year of starting the study and any immunologic or inflammatory disorders were excluded from the study. All patients gave informed written consent and typically continued their normal daily activities during treatment with oxymetholone or placebo. They were monitored by the Global Physical Activity Questionnaire (17).
Nutrient Intake, Body Composition, Quality of Life, and Muscle Strength
Every 4 weeks, all patients kept a 3-day food record and underwent dietary interviews by a registered dietitian. Nutrient composition of the diets was analyzed with the Inmucal National Food Database Program. Daily protein intake was determined by the calculated protein equivalent of total nitrogen appearance (18). Body composition was assessed by dual energy X-ray absorptiometry (DEXA) on the day after a hemodialysis treatment before and after the study period. All patients agreed to take part in a self-assessed health-related quality of life test using the short-form health survey with only 36 questions (SF-36) containing eight domains divided into two parts: physical health (physical functioning, role limitations caused by physical health, bodily pain, and general health) and mental health (vitality, social functioning, role limitations caused by emotional problems, and mental health). Hence, in the SF-36 scoring system, the scales are assessed quantitatively, and a physical and mental health SF-36 score between 0 and 100 is then calculated, with a higher score indicating a better state of health. Grip strength was also measured three times on each hand during each visit alternating each hand grip measurement between right and left hands using a handgrip dynamometer.
Skeletal Muscle Biopsy
Muscle biopsies of the right vastus lateralis muscle were performed at baseline and at the end of the study (19). The muscle analyses include (1) identification of mRNA levels by real-time PCR amplification for myostatin, IGF-I, the splice variants IGF-IEa and IGF-IEc, IGF-II, the IGF-I receptor (IGF-IR) and IGF-IIR, IGF binding proteins 2–6 (IGFBPs 2–6), and myosin heavy chains (MyHCs) and (2) measurement of protein concentrations of IGF-I and IGF-II (20). The concentrations of IGF-I and IGF-II protein are expressed as the ratio of IGF protein (nanograms or micrograms) to total protein (milligrams) in the homogenates of muscle tissue. Muscle fiber types were identified by reduced nicotinamide-adenine dinucleotide staining, and cross-sectional areas were examined by a renal pathologist.
Clinical Laboratory Measurements
After a 12-hour overnight fast, blood was collected immediately before a midweek hemodialysis for biochemical measurements, including testosterone, luteinizing hormone, and cortisol at baseline, every 4 weeks and at the end of the trial.
Safety Monitoring
Adverse events that were or were not considered to be related to oxymetholone treatment were monitored every 4 weeks. The patients were questioned in a systematic way about their experiences concerning any adverse events during the previous 4 weeks. Patients also underwent blood drawing for safety tests that included complete blood counts, liver function tests, and prostate-specific antigen.
Statistical Analyses
The key method of analysis was the comparison of the changes, if any, between the baseline and 24-week values in the oxymetholone- and placebo-treated groups. Statistical analyses were performed using the STATA program. Continuous variables between study and control groups were compared with unpaired t tests. Differences between baseline and the end of study for each group of patients were also compared using paired t tests. One-way repeated ANOVA was used to examine the main outcome variable when comparing mean differences within patients and changes between groups. Univariate correlations were evaluated using Pearson correlation analysis. Statistical significance was taken as P<0.05.
Results
Patients
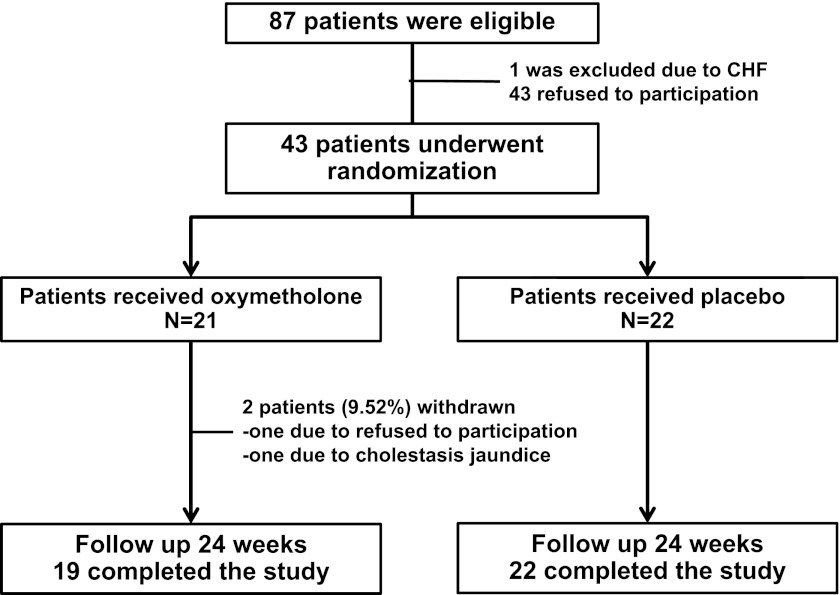
A total of 423 patients in the dialysis unit were screened for possible study enrollment. Eighty-seven patients were eligible according to the entry criteria (Figure 1). One patient was excluded because of congestive heart failure, and 43 patients refused to participate. Thus, 43 patients actually received either oxymetholone or placebo. Twenty-two patients were assigned to the placebo control group, and 21 individuals were assigned to the oxymetholone group. One patient who received oxymetholone therapy decided not to participate in the study after taking the medication for 1 month, and another patient in the oxymetholone-treated group was removed from the study after developing altered liver function. The remaining 41 patients completed the study, and all of these patients were 100% adherent to the oxymetholone or placebo prescription based on pill counts.
Figure 1.
Diagram of patient flow through the clinical trial.
Blood Measurements
Characteristics of the study population are shown in Table 1. There were no significant differences between treatment and placebo groups at baseline (Table 2). At the end of study, there was a significant increase in predialysis serum creatinine concentrations in the oxymetholone group compared with the placebo group by 1.47 mg/dl (95% confidence interval [CI]=0.08–2.86, P<0.01). Serum creatinine also rose significantly by 1.2±2.8 mg/dl (P<0.01) in the oxymetholone group but did not change significantly in the placebo group. Serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), and total and direct bilirubin levels also increased significantly more in the oxymetholone group compared with the placebo group (Table 2). Serum levels of ALT, AST, and direct bilirubin also increased significantly from baseline only in the oxymetholone group. However, abnormal persistent increases in total bilirubin of >2 mg/dl or AST/ALT of more than three times the upper limit of the normal range were not significantly different between the two groups (Table 2).
Table 1.
Baseline characteristics of the study population
| Characteristic | Oxymetholone (n=19) | Placebo (n=22) |
|---|---|---|
| Age (yr) | 41.0±10.5 | 45.1±8.5 |
| Number of males (%) | 10 (52.6) | 15 (68.2) |
| Dry weight (kg) | 54.4±8.8 | 56.8±8.9 |
| Median dialysis vintage (mo; IQR) | 98.0 (61.0–110.0) | 96.0 (59.0–115.7) |
| Body mass index (kg/m2) | 21.6±3.2 | 21.2±3.5 |
| Single-pool Kt/V urea | 1.9±0.3 | 1.8±0.2 |
| Predialysis SBP (mmHg) | 139.9±15.1 | 139.0±10.4 |
| Predialysis DBP (mmHg) | 78.6±6.1 | 79.6±4.3 |
| Causes of ESRD (n [%]) | ||
| Chronic glomerulonephritis | 6 (31.6) | 6 (27.3) |
| Hypertension | 3 (15.8) | 3 (13.6) |
| IgA nephropathy | 1 (5.3) | 3 (13.6) |
| Other causes | 3 (15.8) | 2 (9.1) |
| Unknown | 6 (31.6) | 8 (36.4) |
Data are mean ± SD. IQR, interquartile range; SBP, systolic BP; DBP, diastolic BP.
Table 2.
Changes in predialysis blood or serum measurements
| Parameter | Oxymetholone (n=19) | Placebo (n=22) | Difference between Groups in the Mean Change from Baseline (95% CI) |
|---|---|---|---|
| Hemoglobin (g/dl) | |||
| Baseline | 9.1±1.0 | 10.1±1.1 | |
| Week 24 | 9.3±1.2 | 10.1±1.3 | |
| Change from baseline | 0.1±1.1 | −0.04±1.2 | 1.17 (−0.57 to 0.91) |
| Fasting plasma glucose (mg/dl) | |||
| Baseline | 77.2±6.7 | 77.4±12.9 | |
| Week 24 | 77.3±9.4 | 83.9±26.5 | |
| Change from baseline | 0.1±8.1 | 6.5±20.9 | −6.37 (−16.25 to 3.51) |
| Serum urea nitrogen (mg/dl) | |||
| Baseline | 56.6±12.1 | 62.7±14.1 | |
| Week 24 | 63.0±16.7 | 59.0±15.5 | |
| Change from baseline | 6.4±14.6a | −3.7±14.9 | 10.03 (0.71–19.35)a |
| Serum creatinine (mg/dl) | |||
| Baseline | 11.1±2.7 | 11.5±1.5 | |
| Week 24 | 12.3±2.9b | 11.2±1.9 | |
| Change from baseline | 1.2±2.8c | −0.3±1.0 | 1.47 (0.08–2.86)c |
| Serum albumin (g/dl) | |||
| Baseline | 3.8±0.3 | 4.1±0.2 | |
| Week 24 | 3.9±0.3 | 4.1±0.3 | |
| Change from baseline | 0.1±0.3 | −0.02±0.2 | 0.09 (−0.09 to 0.27) |
| Serum AST (U/L) | |||
| Baseline | 16.2±6.6 | 13.8±4.9 | |
| Week 24 | 47.8±45.5b | 15.3±7.8 | |
| Change from baseline | 31.7±32.5c | 1.5±6.5 | 30.15 (14.27–46.03)c |
| Serum ALT (U/L) | |||
| Baseline | 14.6±6.5 | 13.1±5.0 | |
| Week 24 | 70.1±71.3b | 15.6±7.5 | |
| Change from baseline | 55.5±50.6c | 2.5±6.4 | 53.02 (28.44–77.56)c |
| Serum total bilirubin (mg/dl) | |||
| Baseline | 0.3±0.1 | 0.3±0.1 | |
| Week 24 | 0.8±1.0 | 0.3±0.1 | |
| Change from baseline | 0.5±0.7c | −0.01±0.1 | 0.47 (0.13–0.81)b |
| Serum direct bilirubin (mg/dl) | |||
| Baseline | 0.09±0.02 | 0.10±0.02 | |
| Week 24 | 0.51±0.77d | 0.10±0.06 | |
| Change from baseline | 0.42±0.54a | 0.00±0.04 | 0.42 (0.16–0.68)a |
| Number of patients with | |||
| AST more than three times the ULN | 0 (0.0) | 0 (0.0) | |
| ALT more than three times the ULN | 2 (9.5) | 0 (0.0) | |
| TB>2 mg/dl | 3 (14.3) | 0 (0.0) | — |
Values were all obtained immediately before the onset of a hemodialysis treatment. Data are mean ± SD. CI, confidence interval; AST, aspartate aminotransferase; ALT, alanine aminotransferase; ULN, upper limit of normal; TB, total bilirubin.
Compared with placebo: P<0.05.
Week 24 value compared with baseline: P<0.01.
Compared with placebo: P<0.01.
Week 24 value compared with baseline: P<0.05.
Fat-Free Mass, Fat Mass, Muscle Strength, Quality of Life, and Muscle Fiber Cross-Sectional Area
Body weight, body composition, handgrip strength, quality of life, and muscle fiber cross-sectional area during the study are shown in Table 3. There was a significantly greater increase in FFM (2.59 kg, 95% CI=1.65–3.53) and decrease in fat mass (FM; 1.32 kg, 95% CI=−2.54 to −0.10) in the oxymetholone-treated group compared with the placebo group. With regard to the SF-36 scores, both the physical component (from 55.9±18.1 to 63.0±15.6, P<0.05) and the mental component (from 58.4±16.5 to 63.8±14.3, P<0.05) also increased from baseline in the oxymetholone-treated group, whereas no change was found in the placebo group. The differences in the changes in the SF-36 physical and mental scores between the two groups did not reach statistical significance (Table 3). Compared with baseline values, the oxymetholone-treated group underwent a significant increase in FFM and decrease in FM. The cross-sectional area of type I muscle fibers displayed a significantly greater increase (25.66, 95% CI=7.92–43.40) in the oxymetholone-treated group compared with the placebo group (Table 3). This significantly positive change in cross-sectional area of these type I fibers in the oxymetholone group was largely caused by the significant reduction from baseline in the cross-sectional area in the placebo group. Indeed, in the placebo group, there was a significant decrease from baseline in the cross-sectional area of both type I and type II fibers (Table 3). There was an increase in handgrip strength that was significantly greater in the oxymetholone-treated group than the placebo group (2.61 kg; 95% CI=0.08–5.14).
Table 3.
Changes in body composition, nutrient intake, muscle strength, and muscle fiber cross-sectional area
| Parameter | Oxymetholone (n=19) | Placebo (n=22) | Difference between Groups in the Mean Change from Baseline (95% CI) |
|---|---|---|---|
| Body composition | |||
| Body weight (kg) | |||
| Baseline | 55.0±9.4 | 58.1±8.9 | |
| Week 24 | 55.7±8.3 | 57.5±8.6 | |
| Change from baseline | 0.8±3.0 | −0.5±1.8 | 1.29 (−0.31 to 2.89) |
| Fat-free mass (kg) | |||
| Baseline | 38.0±6.7 | 42.2±7.3 | |
| Week 24 | 41.4±7.7a | 42.8±7.1 | |
| Change from baseline | 3.2±1.7b | 0.7±1.2 | 2.59 (1.65–3.53)b |
| Fat mass (kg) | |||
| Baseline | 13.3±7.4 | 12.5±6.5 | |
| Week 24 | 11.6±6.2c | 12.1±6.5 | |
| Change from baseline | −1.7±2.4b | −0.4±1.1 | −1.32 (−2.54 to −0.10)b |
| Nutrient intake | |||
| Energy intake (kcal/kg per d) | |||
| Baseline | 22.2±6.4 | 19.1±6.1 | |
| Week 24 | 19.8±7.6 | 21.3±8.5 | |
| Change from baseline | −2.4±5.9b | 2.2±6.5 | −4.59 (−8.55 to −0.63)b |
| Protein intake (g/kg per d) | |||
| Baseline | 1.0±0.2 | 1.1±0.2 | |
| Week 24 | 1.2±0.3a | 1.2±0.3a | |
| Change from baseline | 0.2±0.3 | 0.1±0.2 | 0.07 (−0.08 to 0.22) |
| Handgrip strength (kg) | |||
| Baseline | 26.3±8.3 | 31.4±10.8 | |
| Week 24 | 28.6±10.1c | 31.1±9.7 | |
| Change from baseline | 2.3±4.8b | −0.3±3.1 | 2.61 (0.08–5.14)b |
| Quality of life (SF-36) | |||
| Physical component | |||
| Baseline | 55.9±18.1 | 63.9±20.4 | |
| Week 24 | 63.0±15.6c | 69.9±14.6 | |
| Change from baseline | 7.1±10.3 | 6.0±15.1 | 1.11 (−7.21 to 9.43) |
| Mental component | |||
| Baseline | 58.4±16.5 | 66.8±18.2 | |
| Week 24 | 63.8±14.3c | 72.5±12.7 | |
| Change from baseline | 5.3±9.1 | 5.7±14.6 | −0.40 (−8.01 to 7.21) |
| Muscle fiber type (cross-sectional area) | |||
| Type I | |||
| Baseline | 82.6±16.9 | 88.8±22.3 | |
| Week 24 | 93.3±29.4 | 73.9±19.4c | |
| Change from baseline | 10.8±30.6d | −14.9±25.6 | 25.66 (7.92–43.40)b |
| Type II | |||
| Baseline | 72.4±19.1 | 73.6±17.2 | |
| Week 24 | 69.4±32.4 | 61.5±10.6c | |
| Change from baseline | −3.0±33.4 | −12.1±19.6 | 9.06 (−8.80 to 26.92) |
Data are mean ± SD. Measurements were made at the time of dual energy X-ray absorptiometry scanning on the day after a hemodialysis treatment. CI, confidence interval; SF-36, short-form health survey with only 36 questions.
Compared with placebo: P<0.05.
Week 24 value compared with baseline: P<0.01.
Compared with placebo: P<0.01.
Week 24 value compared with baseline: P<0.05.
Nutrient Intake
Estimated daily dietary energy and protein intake are shown in Table 3. There was a significantly greater reduction in energy intake in the oxymetholone-treated patients compared with the placebo group, although neither group displayed a significant difference between the initial and final values for energy intake. There was no difference significantly in the change in protein intake between the groups, and at the 24th week, the protein intake had increased significantly in both groups.
mRNA and Protein Levels in Vastus Lateralis Muscle
There were several changes in muscle mRNA levels in the oxymetholone-treated patients that are consistent with promotion of protein anabolism (Table 4). There was a significantly greater increase in skeletal muscle mRNA levels for MyHC 2×, IGF-IR, and IGF-IIR in the oxymetholone-treated group compared with the placebo group. With oxymetholone treatment, there was also a significant increase from baseline in skeletal muscle mRNA levels for IGF-IEc, MyHC 2×, and IGF-IIR. In the placebo group, the only significant change from baseline was a decrease in IGF-IR mRNA. IGFBPs 1–6 did not change significantly in either group (Table 4).
Table 4.
Changes in mRNA and protein expression of growth factors in vastus lateralis muscle from baseline to week 24
| Muscle mRNA (density unit) | Oxymetholone (n=19) | Placebo (n=22) | Difference between Groups in the Mean Change from Baseline (95% CI) |
|---|---|---|---|
| IGF-IEa mRNA | |||
| Baseline | 0.18±0.08 | 0.21±0.09 | |
| Week 24 | 0.23±0.16 | 0.25±0.10 | |
| Change from baseline | 0.05±0.18 | 0.04±0.09 | 0.01 (−0.08 to 0.10) |
| IGF-IEc × 103 mRNA | |||
| Baseline | 2.71±2.63 | 3.16±3.97 | |
| Week 24 | 5.52±6.40a | 4.27±4.07 | |
| Change from baseline | 2.81±3.18 | 1.11±3.22 | 1.71 (−0.33 to 3.73) |
| IGF-II mRNA | |||
| Baseline | 5.03±2.09 | 5.76±2.61 | |
| Week 24 | 5.19±2.88 | 5.02±2.69 | |
| Change from baseline | 0.16±2.47 | −0.74±2.03 | 0.92 (−0.52 to 2.32) |
| Myostatin mRNA | |||
| Baseline | 1.06±0.61 | 1.14±0.87 | |
| Week 24 | 1.40±1.10 | 1.05±0.79 | |
| Change from baseline | 0.33±0.92 | −0.09±0.87 | 0.42 (−0.15 to 0.99) |
| Myosin heavy-chain 2a mRNA | |||
| Baseline | 29.84±0.76 | 29.83±0.87 | |
| Week 24 | 30.08±0.16 | 29.99±0.19 | |
| Change from baseline | 0.24±0.84 | 0.15±0.86 | 0.09 (−0.45 to 0.63) |
| Myosin heavy-chain 2× mRNA | |||
| Baseline | 25.67±17.57 | 34.20±18.84 | |
| Week 24 | 44.60±61.68a | 29.65±14.12 | |
| Change from baseline | 18.93±31.51b | −4.55±16.94 | 23.48 (6.90–40.06)b |
| IGF-I receptor mRNA | |||
| Baseline | 0.45±0.19 | 0.45±0.15 | |
| Week 24 | 0.48±0.19 | 0.36±0.17b | |
| Change from baseline | 0.05±0.28b | −0.09±0.09 | 0.14 (0.01–0.28)b |
| IGF-II receptor mRNA | |||
| Baseline | 2.43±0.99 | 2.41±0.82 | |
| Week 24 | 3.09±2.68a | 2.12±1.21 | |
| Change from baseline | 0.66±1.08b | −0.29±1.10 | 0.95 (0.26–1.64)a |
| Muscle protein | |||
| IGF-I protein (ng/mg muscle protein) | |||
| Baseline | 21.84±7.03 | 24.87±8.21 | |
| Week 24 | 23.92±7.20 | 22.01±6.50 | |
| Change from baseline | 2.08±9.57 | −2.86±11.44 | 4.94 (−1.79 to 11.67) |
| IGF-II protein (μg/mg muscle protein) | |||
| Baseline | 137.11±47.01 | 131.43±52.67 | |
| Week 24 | 163.84±79.66 | 151.47±47.16 | |
| Change from baseline | 26.73±88.63 | 20.04±68.95 | 6.69 (−43.13 to 56.51) |
Data are mean ± SD. The concentrations of IGF-I and IGF-II protein are expressed as the ratio of IGF protein (nanograms or micrograms) to total protein (milligrams) in the homogenates of muscle tissue. CI, confidence interval.
Compared with placebo: P<0.01.
Compared with placebo: P<0.05.
Hormonal Profiles
In males only, there was a significantly greater decrease in serum total testosterone in the oxymetholone-treated group compared with the placebo group at 24 weeks. Serum total testosterone decreased significantly in the oxymetholone-treated group and rose significantly in placebo group (Table 5). In males, there was a significantly greater decrease in serum prostatic surface antigen in the oxymetholone-treated group compared with the placebo group at 24 weeks (Table 5). Serum prostatic surface antigen rose slightly only in the placebo group.
Table 5.
Changes in serum hormone concentrations
| Parameter | Oxymetholone (n=19) | Placebo (n=22) | Difference between Groups in the Mean Change from Baseline (95% CI) |
|---|---|---|---|
| Serum testosterone (ng/dl) | |||
| Male | |||
| Baseline | 604.9±165.5 | 528.3±176.0 | |
| Week 24 | 247.2±207.8a | 658.1±333.2 | |
| Change from baseline | −357.7±91.3b | 129.8±328.4 | −487.52 (−638.02 to −336.98)b |
| Female | |||
| Baseline | 67.5±40.6 | 95.2±88.3 | |
| Week 24 | 94.2±73.2 | 115.0±116.5 | |
| Change from baseline | 26.7±61.8 | 19.8±30.0 | 6.93 (−25.11 to 38.97) |
| Serum luteinizing hormone (mIU/L) | |||
| Male | |||
| Baseline | 16.4±11.1 | 14.3±9.8 | |
| Week 24 | 10.3±18.5 | 15.6±11.3 | |
| Change from baseline | −6.0±20.4 | 1.3±3.4 | −7.31 (−17.21 to 2.61) |
| Female | |||
| Baseline | 62.4±10.5 | 20.9±23.1 | |
| Week 24 | 74.5±97.0 | 20.9±28.1 | |
| Change from baseline | 12.1±37.1 | 0.0±5.1 | 12.07 (−5.88 to 30.02) |
| Serum prostatic surface antigen in males (ng/ml) | |||
| Baseline | 0.4±0.3 | 0.5±0.3 | |
| Week 24 | 0.3±0.1 | 0.6±0.3a | |
| Change from baseline | −0.1±0.1b | 0.1±0.2 | −0.23 (−0.32 to −0.14)b |
Data are mean ± SD. Normal values for testosterone in men and women are 270–1070 and 6–86 ng/dl, respectively. Normal values for luteinizing hormone in men and women during the reproductive years are 3–15 and 5–22 mIU/L, respectively. CI, confidence interval.
Compared with placebo: P<0.05.
Compared with placebo: P<0.01.
Safety Profile
During the 24-week study period, 2 of 21 patients (9.52%) in the oxymetholone-treated group withdrew from the study because of adverse events. One subject withdrew because of undesired nonedematous weight gain. The other patient was removed from the study because of altered liver function. The serum liver function measurements all returned to normal within 1 month of discontinuing oxymetholone. Other side effects in the oxymetholone-treated patients included acne (52.4%), amenorrhea (23.8%), diminished menses (4.8%), alopecia (4.8%), hirsutism (4.8%), deepening voice (9.5%), and decreased serum HDL cholesterol (14.3%).
Discussion
The present study constitutes the first randomized, placebo-controlled trial of oral oxymetholone in MHD patients. The increase in FFM and handgrip strength and decrease in FM in the oxymetholone-treated group were significantly greater than in the placebo group. In addition, compared with baseline, the oxymetholone-treated group underwent an increase in FFM, handgrip strength, physical functioning scores, and type I muscle fiber cross-sectional area and a decrease in FM. Thus, oxymetholone showed significantly beneficial effects on body composition, muscle metabolism, strength, and mass. However, it also increased the incidence of minor side effects and slightly increased liver dysfunction.
The increase in FFM with oxymetholone treatment was substantial, with an average of 3.24±1.74 kg. Moreover, there was a significantly greater increase in FFM in the oxymetholone-treated group, with an average of 2.59 kg (95% CI=1.65–3.53), compared with the placebo group. Because no patient had sign of edema, much of the FFM gain was presumably of protoplasm. The fact that the fall in FM, although statistically significant, was less, averaging 1.73±2.77 kg, provides additional evidence that the patients receiving oxymetholone treatment gained protoplasm. In addition, there was a significantly greater decrease in FM in the oxymetholone group than in the placebo group, with an average of −1.32 kg (95% CI=−2.54 to −0.10). Other findings support the likelihood that the increase in FFM with oxymetholone was, at least partly, caused by a gain in muscle mass. These findings include the rise in predialysis serum creatinine, the increase mRNA levels for several growth factors in the muscle, and the increase in the cross-sectional area for type I muscle fibers.
The decrease in FM of 1.73±2.77 kg in the patients receiving oxymetholone is also consistent with androgen’s known lipolytic effects (21). There were no significant changes in consumption of daily total calories, and the reported daily physical activity did not change significantly. Protein intake increased to a similar degree in both groups. It is the randomized design and the difference in changes between groups that support the causality of the intervention.
The increase in handgrip strength in the oxymetholone-treated group was consistent with the foregoing findings that suggest an increase in muscle mass. Muscle strength is usually directly correlated with muscle mass (22–24), and therefore, if muscle mass increased, one would expect there to be an increase in strength. Moreover, the oxymetholone-treated patients described an increase in physical performance on their SF-36 forms. Self-report from our patients receiving oxymetholone indicated an increase in physical function, which is consistent with a previous report of MHD patients who received nandrolone decanoate and described an increase in physical performance (12). In addition, resistance exercise training that was safe resulted in a training-specific increase in muscle strength as well as an improvement in self-reported physical functioning. However, one study suggests that both administration of anabolic steroids and exercise training may be necessary to maximally increase muscle mass in MHD patients (25).
The work by Wang et al. (26) previously showed that, in muscle of sedentary MHD patients, there were decreased mRNA levels for a number of proteins involved with the stimulation of protein anabolism and inhibition of protein degradation. Particularly, the mRNA levels for IGF-IEa, IGF-IR, IGF-II, and IGFBP-2 decreased compared with normal patients. In this study, oxymetholone increased muscle mRNA levels for IGF-IR and IGF-IIR. IGF-I is known to stimulate myoblast proliferation and differentiation in vitro as well as muscle protein synthesis (27). It preserves muscle architecture and promotes muscle hypertrophy. The mechanically sensitive isoform IGF-IEc, also called mechano-growth factor, induces myofiber hypertrophy and enhances physical capacity of skeletal muscle.
It is noteworthy that no significant increase was observed in the protein levels for IGF-I and IGF-II in skeletal muscle. The weak correlations of muscle mRNA levels with protein expression for IGF-I and IGF-II could have several causes. The mRNA levels were only measured at baseline and 24 months later at the end of the trial. It is possible that the expression of IGF-I and IGF-II rose transiently, promoted the observed anabolic changes in body composition and increased muscle strength, and then declined to near baseline levels before the second muscle biopsy was performed. It is also possible that one or more post-transcriptional processes could alter the synthesis of these IGF proteins, notwithstanding the rise in their mRNA levels (28). MyHC is the major contractile protein in skeletal muscle, and it is responsible for a number of contractile properties of the different fiber types. Our study is the first to indicate that MyHC 2× increased significantly in MHD patients when they are administered an anabolic agent (in our case, oxymetholone).
Oxymetholone offers several theoretical advantages over many testosterone preparations for the treatment of MHD patients (13). One advantage of oxymetholone is absorption through oral administration. The oral preparations are easier to administer and have a lower incidence of some side effects (13). However, in this clinical trial, oxymetholone treatment is associated with a rather high incidence of liver dysfunction. Two patients receiving oxymetholone developed substantial alterations in liver function tests. We suspect that, unless methods can be found to administer oxymetholone without causing abnormal liver function, the use of this medicine as an anabolic agent for MHD patients should be closely monitored.
The mechanisms for muscle wasting and weakness in MHD patients include decreased synthesis of muscle contractile and mitochondrial proteins (29) in response to circulating levels of hormones anabolic to skeletal muscle. Testosterone supplements are reported to increase muscle protein accretion by elevation fractional muscle protein synthesis, facilitating the reuse of amino acids by the muscle and decreasing muscle protein degradation (30,31). Our study confirms similar effects in MHD patients as those effects reported for testosterone on engendering hypertrophy of skeletal muscle fibers but with the use of another anabolic agent (32). These considerations provide support for a possible role of anabolic steroids in the treatment of sarcopenia in MHD patients.
The limitations of this clinical trial include its relatively small sample size and the fact that most outcome measurements were only obtained at baseline and the end of the study 24 weeks later. The strength of the study includes the rather comprehensive assessment of the patients’ response to oxymetholone. This assessment included measurements of body composition, muscle fiber cross-sectional area, muscle mRNA levels of various growth factors, and protein concentrations of IGF-I and IGF-II, measures of muscle strength, self-assessment of health by the SF-36 scale, and serum measurements of certain relevant hormones.
This study is the first to show that, in MHD patients, ingestion of oxymetholone results in an increase in FFM, muscle cross-sectional area for type I fibers, mRNA values for IGF-I as well as IGF-IIRs, MyHC, handgrip strength, predialysis BUN, and predialysis serum creatinine and a decrease in FM. Oxymetholone, in low doses, seems to have an important anabolic effect in MHD patients, although the potential risk for abnormal liver function is a source of concern.
Disclosures
None.
Acknowledgments
We are grateful for the support from the Ministry of Higher Education, Bangkok, Thailand and The Kidney Foundation of Thailand.
Neither the manuscript nor any significant part of it is under consideration for publication elsewhere or has appeared elsewhere in a manner that could be construed as a prior or duplicate publication of the same or very similar work.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Acchiardo SR, Moore LW, Latour PA: Malnutrition as the main factor in morbidity and mortality of hemodialysis patients. Kidney Int Suppl 16: S199–S203, 1983 [PubMed] [Google Scholar]
- 2.Kalantar-Zadeh K, Supasyndh O, Lehn RS, McAllister CJ, Kopple JD: Normalized protein nitrogen appearance is correlated with hospitalization and mortality in hemodialysis patients with Kt/V greater than 1.20. J Ren Nutr 13: 15–25, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Qureshi AR, Alvestrand A, Divino-Filho JC, Gutierrez A, Heimbürger O, Lindholm B, Bergström J: Inflammation, malnutrition, and cardiac disease as predictors of mortality in hemodialysis patients. J Am Soc Nephrol 13[Suppl 1]: S28–S36, 2002 [PubMed] [Google Scholar]
- 4.Rambod M, Bross R, Zitterkoph J, Benner D, Pithia J, Colman S, Kovesdy CP, Kopple JD, Kalantar-Zadeh K: Association of Malnutrition-Inflammation Score with quality of life and mortality in hemodialysis patients: A 5-year prospective cohort study. Am J Kidney Dis 53: 298–309, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang CX, Tighiouart H, Beddhu S, Cheung AK, Dwyer JT, Eknoyan G, Beck GJ, Levey AS, Sarnak MJ: Both low muscle mass and low fat are associated with higher all-cause mortality in hemodialysis patients. Kidney Int 77: 624–629, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hengge UR, Stocks K, Wiehler H, Faulkner S, Esser S, Lorenz C, Jentzen W, Hengge D, Goos M, Dudley RE, Ringham G: Double-blind, randomized, placebo-controlled phase III trial of oxymetholone for the treatment of HIV wasting. AIDS 17: 699–710, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Ball JH, Lowrie EG, Hampers CL, Merrill JP: Testosterone therapy in hemodialysis patients. Clin Nephrol 4: 91–98, 1975 [PubMed] [Google Scholar]
- 8.Barton Pai A, Chretien C, Lau AH: The effects of nandrolone decanoate on nutritional parameters in hemodialysis patients. Clin Nephrol 58: 38–46, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Bhasin S, Storer TW, Berman N, Callegari C, Clevenger B, Phillips J, Bunnell TJ, Tricker R, Shirazi A, Casaburi R: The effects of supraphysiologic doses of testosterone on muscle size and strength in normal men. N Engl J Med 335: 1–7, 1996 [DOI] [PubMed] [Google Scholar]
- 10.Bhasin S, Storer TW, Berman N, Yarasheski KE, Clevenger B, Phillips J, Lee WP, Bunnell TJ, Casaburi R: Testosterone replacement increases fat-free mass and muscle size in hypogonadal men. J Clin Endocrinol Metab 82: 407–413, 1997 [DOI] [PubMed] [Google Scholar]
- 11.Bhasin S, Woodhouse L, Storer TW: Proof of the effect of testosterone on skeletal muscle. J Endocrinol 170: 27–38, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Johansen KL, Mulligan K, Schambelan M: Anabolic effects of nandrolone decanoate in patients receiving dialysis: A randomized controlled trial. JAMA 281: 1275–1281, 1999 [DOI] [PubMed] [Google Scholar]
- 13.Pavlatos AM, Fultz O, Monberg MJ, Vootkur A, Pharmd : Review of oxymetholone: A 17alpha-alkylated anabolic-androgenic steroid. Clin Ther 23: 789–801, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Schroeder ET, Singh A, Bhasin S, Storer TW, Azen C, Davidson T, Martinez C, Sinha-Hikim I, Jaque SV, Terk M, Sattler FR: Effects of an oral androgen on muscle and metabolism in older, community-dwelling men. Am J Physiol Endocrinol Metab 284: E120–E128, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Hengge UR, Baumann M, Maleba R, Brockmeyer NH, Goos M: Oxymetholone promotes weight gain in patients with advanced human immunodeficiency virus (HIV-1) infection. Br J Nutr 75: 129–138, 1996 [DOI] [PubMed] [Google Scholar]
- 16.Aramwit P, Palapinyo S, Wiwatniwong S, Supasyndh O: The efficacy of oxymetholone in combination with erythropoietin on hematologic parameters and muscle mass in CAPD patients. Int J Clin Pharmacol Ther 48: 803–813, 2010 [DOI] [PubMed] [Google Scholar]
- 17.Myers J, Bader D, Madhavan R, Froelicher V: Validation of a specific activity questionnaire to estimate exercise tolerance in patients referred for exercise testing. Am Heart J 142: 1041–1046, 2001 [DOI] [PubMed] [Google Scholar]
- 18.K/DOQI : Clinical practice guidelines for nutrition in chronic renal failure. K/DOQI, National Kidney Foundation. Am J Kidney Dis 35[Suppl 2]: S1–S140, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Bergström J: Muscle-biopsy needles. Lancet 1: 153, 1979 [DOI] [PubMed] [Google Scholar]
- 20.Kopple JD, Wang H, Casaburi R, Fournier M, Lewis MI, Taylor W, Storer TW: Exercise in maintenance hemodialysis patients induces transcriptional changes in genes favoring anabolic muscle. J Am Soc Nephrol 18: 2975–2986, 2007 [DOI] [PubMed] [Google Scholar]
- 21.De Pergola G: The adipose tissue metabolism: Role of testosterone and dehydroepiandrosterone. Int J Obes Relat Metab Disord 24[Suppl 2]: S59–S63, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Johansen KL, Shubert T, Doyle J, Soher B, Sakkas GK, Kent-Braun JA: Muscle atrophy in patients receiving hemodialysis: Effects on muscle strength, muscle quality, and physical function. Kidney Int 63: 291–297, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Macdonald JH, Phanish MK, Marcora SM, Jibani M, Bloodworth LL, Holly JM, Lemmey AB: Muscle insulin-like growth factor status, body composition, and functional capacity in hemodialysis patients. J Ren Nutr 14: 248–252, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Storer TW, Woodhouse L, Magliano L, Singh AB, Dzekov C, Dzekov J, Bhasin S: Changes in muscle mass, muscle strength, and power but not physical function are related to testosterone dose in healthy older men. J Am Geriatr Soc 56: 1991–1999, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johansen KL, Painter PL, Sakkas GK, Gordon P, Doyle J, Shubert T: Effects of resistance exercise training and nandrolone decanoate on body composition and muscle function among patients who receive hemodialysis: A randomized, controlled trial. J Am Soc Nephrol 17: 2307–2314, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Wang H, Casaburi R, Taylor WE, Aboellail H, Storer TW, Kopple JD: Skeletal muscle mRNA for IGF-IEa, IGF-II, and IGF-I receptor is decreased in sedentary chronic hemodialysis patients. Kidney Int 68: 352–361, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Ding H, Gao XL, Hirschberg R, Vadgama JV, Kopple JD: Impaired actions of insulin-like growth factor 1 on protein Synthesis and degradation in skeletal muscle of rats with chronic renal failure. Evidence for a postreceptor defect. J Clin Invest 97: 1064–1075, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo Y, Xiao P, Lei S, Deng F, Xiao GG, Liu Y, Chen X, Li L, Wu S, Chen Y, Jiang H, Tan L, Xie J, Zhu X, Liang S, Deng H: How is mRNA expression predictive for protein expression? A correlation study on human circulating monocytes. Acta Biochim Biophys Sin (Shanghai) 40: 426–436, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Adey D, Kumar R, McCarthy JT, Nair KS: Reduced synthesis of muscle proteins in chronic renal failure. Am J Physiol Endocrinol Metab 278: E219–E225, 2000 [DOI] [PubMed] [Google Scholar]
- 30.Ferrando AA, Sheffield-Moore M, Paddon-Jones D, Wolfe RR, Urban RJ: Differential anabolic effects of testosterone and amino acid feeding in older men. J Clin Endocrinol Metab 88: 358–362, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Ferrando AA, Sheffield-Moore M, Yeckel CW, Gilkison C, Jiang J, Achacosa A, Lieberman SA, Tipton K, Wolfe RR, Urban RJ: Testosterone administration to older men improves muscle function: Molecular and physiological mechanisms. Am J Physiol Endocrinol Metab 282: E601–E607, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Sinha-Hikim I, Artaza J, Woodhouse L, Gonzalez-Cadavid N, Singh AB, Lee MI, Storer TW, Casaburi R, Shen R, Bhasin S: Testosterone-induced increase in muscle size in healthy young men is associated with muscle fiber hypertrophy. Am J Physiol Endocrinol Metab 283: E154–E164, 2002 [DOI] [PubMed] [Google Scholar]