Summary
Treatment of uremia by hemodialysis has become widespread over the last 40 years and has improved substantially over that time. However, people treated with this modality continue to suffer from multiple disabilities. Retention of organic solutes, especially those poorly removed by hemodialysis, likely contributes to these disabilities. Certain classes of solutes are removed less well than urea by hemodialysis and by the normal kidney. These include protein-bound solutes, relatively large solutes, sequestered compounds, and substances removed at rates higher than urea by the normal kidney. Several strategies could be used to discover the solutes responsible for residual morbidities in standardly dialyzed people. Rather than continue to focus only on urea removal as an index for dialysis adequacy, finding additional approaches for removing toxic solutes with characteristics different from urea (and the similar small solutes it represents) is a desirable and feasible goal.
Residual Syndrome in Uremia
For several decades, virtually all uremic patients in the developed countries have received treatment aimed at replacing kidney functions, mostly some form of dialysis. In the United States, this broad provision of a life-sustaining therapy began 40 years ago with the extension of Medicare coverage to people with ESRD. Chronic dialysis treatment has expanded from highly selective experimental care in a few academic centers to industrial delivery, largely by for-profit companies. The social, economic, and medical forces that have made dialysis widely available are inspiring. However, the growth in dialysis care has not been accompanied by an equally impressive improvement in dialysis methods. Without doubt, dialysis as practiced even in 1972 was effective at sustaining life and awakening patients from coma. However, despite some important incremental advances in ESRD care, people living on dialysis today suffer a large number of still largely unaddressed disabilities (Table 1) (1). It is important to note that many also suffer from the complications of their primary disease process, particularly diabetes and hypertension, and even restoration of normal kidney function does not reverse these. However, the general success of renal transplantation does indicate that improvements in hemodialysis that provided a closer approximation of normal renal function would improve the lives of even those with chronic diabetic and hypertensive complications. The set of signs and symptoms still present in people receiving dialysis currently defined as adequate have been described by Thomas Depner, who wrote the following: “Patients who would have died from uremia but survive because of dialysis suffer from a previously non-existent life-threatening disease that is labeled here for lack of a better term the ‘residual syndrome’” (2). As he described, this syndrome likely has complex origins. We assume that at least some of these residual morbidities are due to retained organic solutes that are poorly removed by dialysis.
Table 1.
Selected signs and symptoms in residual syndrome
| Hypothermia | Reduced muscle membrane potential |
| Poor appetite/malnutrition | Delayed nerve conduction |
| Diminished smell | Na/K ATPase inhibition |
| Diminished taste | Insulin resistance |
| Pruritus | Shortened red blood cell life span |
| Restless legs | Platelet dysfunction |
| Cramps | Immune dysfunction |
| Bone disease | Inflammation |
| Accelerated cardiovascular disease | Impaired vascular reactivity |
| Impaired cognition | Low-grade serositis |
| Loss of focus and ambition | Increased susceptibility to infection |
| Sleep disturbance/fatigue | Nausea/vomiting |
Dialysis as practiced does not faithfully reproduce normal renal function or the endocrine functions of the kidney. Conventional hemodialysis treatment aims at removing about two-thirds of the accumulated total body urea content during each of three weekly sessions. Urea itself seems to have little toxicity (3). Conventional hemodialysis also removes other unidentified toxic solutes but removes them as well as urea only if they too are small, unbound, and traffic readily across capillaries and cell membranes. Removal of other compounds may be limited due to large molecular size, protein binding, or sequestration within body compartments. Consequently, conventional dialysis has widely different effects on uremic solute levels owing to their different chemical characteristics. A list compiled >10 years ago by the European Uremic Toxin Work Group identified >90 compounds that accumulate in patients on dialysis (4). The application of new analytic methods is lengthening this list (5). Thus, identification of the chemicals responsible for the residual syndrome is a formidable task. A rational approach is to first consider those general classes of compounds that are poorly removed by dialysis compared with urea.
Properties of Poorly Dialyzed Solutes
Large Molecule Solutes
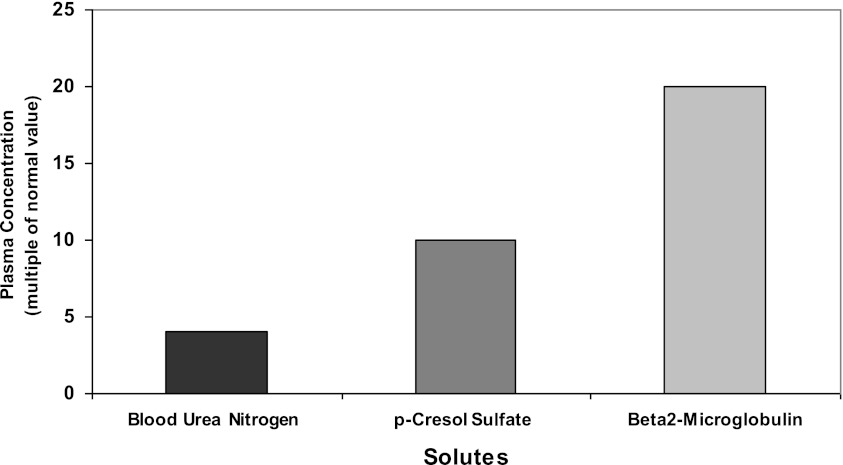
The early dialysis membranes provided diminishing clearance of solutes larger than urea, which has a molecular mass of 60 D, and afforded very little clearance for solutes >1000 D (6). Development of more permeable, high-flux membranes allowed the removal of the larger molecule β-2 microglobulin (12 kD), which had been associated with acquired amyloidosis. However, uremic solutes that are relatively large compared with urea, including β-2 microglobulin, are poorly cleared by even high-flux dialyzers compared with the normal kidney. For example, the clearance of β-2 microglobulin by the normally functioning kidney is close to the GFR or about 100 ml/min, whereas the clearance of β-2 microglobulin by a high-flux dialyzer is about 35 ml/min. Summed over a week, the normal kidneys thus provide about 1000 L of clearance, whereas high-flux dialysis provides about 20 L. Even with high-flux membranes, levels of β-2 microglobulin in dialysis patients are therefore >20-fold higher than normal and would indeed rise even higher if there were not some nonrenal clearance of this solute (7,8). By comparison, the typical time-averaged urea levels are usually about three-fold normal because the dialysis provides about one-third the weekly total urea clearance of normal kidneys (Figure 1). Examples of potentially toxic large molecule compounds in addition to β-2 microglobulin include complement factor D, various advanced glycosylation end products, and fragments of parathyroid hormone, with the application of proteomic techniques adding steadily to this list (4).
Figure 1.
Time-averaged plasma solute levels in patients undergoing conventional thrice-weekly hemodialysis. Uremic solutes that are relatively large compared to urea are poorly cleared by even high flux dialyzers. The increase in the level of p-cresol sulfate would be of even greater magnitude if free rather than total plasma solutes levels were compared.
Protein-Bound Solutes
The protein-bound uremic solutes have a low clearance because only the unbound fraction is available for diffusion across the dialysis membrane (9). In the normally functioning kidney, the protein-bound solutes are removed through the active process of tubular secretion, which efficiently transports the free moiety across the tubule cells into the lumen. This process transpires with such efficiency that for many such solutes a large fraction of the incoming bound portion is removed in a single pass. Dialysis does not replicate secretion, however, and the weekly dialytic clearance of many of these compounds is less than one-tenth that provided by the normal kidneys. As a result, their levels in patients on dialysis are typically >10 times their concentrations in normal serum (10). The increase in plasma levels is of even greater magnitude if free rather than total solute concentrations are compared. Furthermore, recent experimental and clinical studies have suggested that the two most studied protein-bound solutes, indoxyl sulfate and p-cresol sulfate, have toxic effects both in experimental and clinical studies (9,11–16). The time-averaged clearance of protein-bound solutes provided by peritoneal dialysis is even lower than that provided by hemodialysis. However, plasma levels of p-cresol sulfate and indoxyl sulfate do not rise higher in peritoneal dialysis patients than in hemodialysis patients. The reasons for this are not completely understood, but appear to include reduced production of the solutes as residual function is lost in peritoneal dialysis patients (17,18).
Sequestered Solutes
A third category of toxic solutes that are difficult to remove through conventional dialysis are those sequestered in compartments in which their concentration does not equilibrate rapidly with that of the plasma. Dialysis rapidly reduces the plasma concentration of such solutes but clears only a limited proportion of the total body solute content if they do not readily transfer out of intracellular water or other reservoirs. The best known example of a sequestered solute is phosphate; however, some organic solutes, including guanidinoacetic acid, guanidine, and methylguanidine, appear to behave in this manner (5,6,19). Similar to protein-bound solutes, sequestered molecules respond differently from urea to changes in the dialysis prescription.
Solute movement in and out of erythrocytes deserves additional consideration. Urea has selective membrane transporters that facilitate its diffusion in and out of red blood cells (20). Therefore, with adequate dialysate flow and dialysis membrane size, urea is removed from both plasma and erythrocytes as blood transits the dialyzer. For this reason, changes in hematocrit have little effect on urea clearance by hemodialysis (21). Molecules without facilitated transport in and out of cells, like creatinine, cannot have a dialytic clearance exceeding plasma flow. Therefore, their dialytic clearances are lower than the dialytic urea clearance and, unlike urea, they may be dependent on hematocrit. Some other molecules with higher intra-red blood cell concentration compared with plasma include methylamine and methylguanidine (22,23). They also appear to be sequestered in other cellular compartments as they have volumes of distribution greater than the body water. For these reasons their fractional removal by dialysis is less than that of urea and they display considerably more postdialysis rebound as cellular compartments release them into the extracellular fluid after cessation of treatment.
Other Solutes with Very High Clearances by Native Kidneys
Urea and solutes like it are cleared at the highest rate by hemodialysis. All other solutes are cleared at lower rates. This is not true for the normal kidney. With normal renal function, urea clearance is only one-half of creatinine clearance. Other endogenous compounds, such as hippurate, may be cleared at rates ≥2-fold the rate of GFR or about five times the clearance of urea. Of course, these high clearances result from active tubular secretion, whereas dialytic clearance depends largely on diffusion. As a result of the much greater relative clearance by the native kidneys, patients on hemodialysis have hippurate levels 20–40 times those in normal participants (24). Hippurate does not seem to be particularly toxic, but other undiscovered compounds that also enjoy large secretory clearances by the normal kidney may be toxic and would also be expected to circulate in equally large multiples in dialysis patients. In 1939, the early renal physiologist James Shannon, who was also the first director of the National Institutes of Health, asked rhetorically whether loss of renal secretion might contribute to the symptoms of CKD. After raising this possibility, he wrote: “The answer to this question must await the further identification of those substances which make up the unknown portion of the urinary constituents” (25). We have yet to exploit this insight at least in part because we still have compiled only a limited catalog of secreted toxic metabolites.
Removal of Useful Vitamins and Minerals with Dialysis
Dialysis removes some important vitamins, minerals, and trace elements. Water-soluble vitamins are filtered through the dialysis membrane and require supplementation if intake is low. Vitamin C is one such compound. Dialysis removes 80–280 mg of vitamin C with each treatment (26). Vitamin C deficiency is exacerbated by the restricted diets required for ESRD patients that prohibit potassium-rich foods that provide the major portion of dietary vitamin C. Another hydrosoluble vitamin, folic acid, was shown to be significantly cleared or lost during high-efficiency hemodialysis (27). Furthermore, minerals like zinc are also removed by usual dialysis and may require supplementation. Loss of amino acids during dialysis has been documented and likely contributes to malnutrition in hemodialysis patients (28). Because there are presumably unknown toxic molecules that accumulate in dialysis patients, there may also be some unknown valuable molecules that are inappropriately removed by hemodialysis.
How to Test for Uremic Solutes
Better Endpoints Are Needed
Even with classification of candidate solutes into identifiable categories of those removed less well than urea, final identification of solutes responsible for the residual syndrome will require better assessment of clinical endpoints. The HEMO study of dialysis efficacy, which was focused on interventions designed to enhance urea and β-2 microglobulin removal, used death as a primary outcome (29). The emphasis on mortality as an outcome is in some ways compelling. However, one feels obliged to ask, as Joanne Bargman did in the editorial, “Is there more to living than not dying?” (30). Indeed, components of the residual syndrome (e.g., poor appetite, sleep disturbances, restless legs, pruritus, cognitive impairment, etc.) may substantially diminish the quality independent of the length of life. As shown by Blake and O’Meara (1), subtle neurophysiologic deficits afflict even young highly functioning dialysis patients. A dialytic intervention that could lengthen life by reducing cardiovascular events should therefore not be our only goal in a population of individuals who are largely aged >60 years, and three-quarters of whom have had decades of diabetes and hypertension. Even if achievable, a few months of extra life may mean little to a person encumbered by multiple disabilities, especially if improved life expectancy were obtained at the cost of more hours of hemodialysis treatment. Better quantification of any of the onerous facets of the residual syndrome (Table 1) should provide us with better endpoints for exploring the contribution of retained solutes to uremic illness.
Epidemiologic Association of Solutes with Outcomes
Several candidate solutes have been tested for their relation to outcomes in dialysis patients. These tests have focused almost entirely on mortality and cardiovascular disease because outcomes and the relations of solute levels to more subtle morbidities have not been much assessed. Higher levels of p-cresol sulfate have been associated with increased mortality (16,28,31,32). Indoxyl sulfate levels were reported to be associated with mortality and cardiovascular disease across a range of GFRs (33). In addition, greater concentrations of asymmetric dimethyl arginine and symmetrical dimethyl arginine, whose plasma level rises in CKD, predict death in ESRD patients (34,35).
In a small study, no relation could be found between four retention solutes (monomethylamine, ethylamine, indoxyl sulfate, and p-cresol sulfate) and either olfactory discrimination or global assessment of nutrition (36). All of these studies have used assays targeted for specified solutes. By contrast, untargeted analysis by mass spectroscopy has begun to achieve success in other areas of risk prediction. For example, Wang et al. identified three metabolites of the dietary lipid phosphatidylcholine (choline, trimethylamine N-oxide, and betaine) and linked them to risk of subsequent cardiovascular events in a cohort of participants undergoing elective cardiac evaluations (37). Coupling such methods of untargeted solute analysis with quantifiable components of the residual syndrome would seem a promising approach for the future.
In Vitro and In Vivo Approaches
By analogy to Koch’s postulates for proving the causal role of a microbe in infectious disease, infusion of a test solute into an experimental animal with reproduction of some component of the residual syndrome would be a satisfying step in assessing candidate toxins. There are only limited examples of this approach. For example, feeding indoxyl sulfate to hypertensive rats was shown to exacerbate vascular calcification (14). There are several explanations for the paucity of such studies. First, most of the disabilities in Table 1 have not been well quantitated in patients, much less modeled in animals. Furthermore, firm or even suggestive associations between particular solutes and most of the morbidities experienced by patients with ESRD are few. Therefore, the choice of solute for infusion would itself be almost capricious. A related deficiency in this area is the lack of an animal model of ESRD treated by dialysis. Because of the high metabolic rates of small mammals, such a model would need to be created in larger animals such as pigs or dogs. This approach would potentially be very useful but also costly.
Application of candidate uremic toxins to cell culture in vitro is another tactic and there are more examples of such work than that of in vivo studies of uremic toxicology. Both indoxyl sulfate and p-cresol sulfate can provoke abnormalities in cultured endothelial cells and the vasculature of experimental animals that are reminiscent of in vivo human pathology (14,38). The study by Meijers et al. with p-cresol sulfate is particularly attractive because the in vitro effect, release of microparticles, mirrored the in vivo association of p-cresol sulfate levels and levels of microparticles (38). More sophisticated approaches such as using gene expression patterns in response to uremic fluid, fractions thereof, or specific candidate toxins have not been reported.
Selective Reduction of Solutes
The strongest test of a solute’s role in producing a sign or symptom would be to selectively lower its levels in human participants with ESRD and show a diminution in that sign or symptom. This approach has not been applied to any specific solute. The aggregate contribution of large solutes to uremic illness, however, has been tested first by the use of high-flux membranes and later high ultrafiltration rates to selectively increase their clearance. Results thus far have unfortunately not been strongly positive. Recent studies have identified means to utilize a similar approach for protein-bound solutes. Increases in dialyzer surface area and dialysate flow rates can double the clearance of bound solutes (39). With constant production, this should reduce their plasma levels by almost half. The increase in bound solute clearances can be obtained without altering urea clearance so that urea levels can be held constant while reducing the levels of bound solutes. A key element in exploring the role of selected solutes using such a design will, as we have emphasized, be the careful choice of endpoints.
An alternative to improving solute removal is to reduce solute production. A problem is that the metabolic sources for many potential uremic toxins are unknown. However, the microbial population of the colon has emerged as a source for a large number of retained solutes (40,41). When ESRD patients who had undergone colectomy were compared with other ESRD patients with intact gastrointestinal tracts, p-cresol sulfate and indoxyl sulfate were nearly absent in patients lacking a colon (41). More than 30 other compounds, most of which were not yet chemically identified, also emanated from the gut. Of course, it is still uncertain which, if any, are toxic. However, colonic production of retained solutes stands as an example of a potentially modifiable production site (42). Niwa pioneered the use of an oral sorbent to reduce levels of indoxyl sulfate and other gut-derived uremic solutes (43). The production of such solutes may also be reduced by alteration of the diet (40,44).
Progress in Identifying Uremic Toxins
Hemodialysis has not only become widely available but has undoubtedly improved over the last 40 years. However, morbidities still persist and attempts to understand their chemical etiologies have lagged behind other major advances in the field such as more accurate ultrafiltration, bone and mineral therapy, and mitigation of anemia. Over the last decade, a few solutes have begun to receive serious attention as potential toxins but progress has been slow. The reasons for this slow pace certainly include the chemical complexity of the uremic milieu and the multiplicity of clinical disturbances within the standardly dialyzed population. However, improving techniques for chemical analysis and the prospect of clinically meaningful quantifiable endpoints should propel more investigation. That one solute or even one class will account for all aspects of the residual syndrome seems unlikely. Undoubtedly other components of chronic hemodialysis therapy beyond retained solutes, such as episodic volume removal, also contribute morbidities.
Effective therapy of most chronic conditions requires targeting multiple mechanisms. Adequate treatment of diabetes encompasses not only glycemic control but also BP and lipid therapies. Unraveling the complexity of the residual syndrome is daunting but that does not mean that it is insoluble. However, it is certainly insoluble if never attempted. Efforts to resolve the residual syndrome by utilizing yet more intensive dialysis based on urea removal may have some benefits. However, such efforts will likely have only a modest effect on some important solutes while significantly increasing the burden on patients and providers. We need to know which solutes or at least which classes of solutes are generating which disabilities and where these solutes come from if chronic dialysis care is to advance rationally. We must do better than simply doing more and more of what was done 40 years ago.
Disclosures
None.
Acknowledgments
This work was supported in part by grants from the National Institutes of Health to M.D. (5T32DK007470), T.W.M. (5R01DK080123), and T.H.H. (5R01DK080123).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Blake C, O’Meara YM: Subjective and objective physical limitations in high-functioning renal dialysis patients. Nephrol Dial Transplant 19: 3124–3129, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Depner TA: Uremic toxicity: Urea and beyond. Semin Dial 14: 246–251, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Johnson WJ, Hagge WW, Wagoner RD, Dinapoli RP, Rosevear JW: Effects of urea loading in patients with far-advanced renal failure. Mayo Clin Proc 47: 21–29, 1972 [PubMed] [Google Scholar]
- 4.Vanholder R, De Smet R, Glorieux G, Argilés A, Baurmeister U, Brunet P, Clark W, Cohen G, De Deyn PP, Deppisch R, Descamps-Latscha B, Henle T, Jörres A, Lemke HD, Massy ZA, Passlick-Deetjen J, Rodriguez M, Stegmayr B, Stenvinkel P, Tetta C, Wanner C, Zidek W, European Uremic Toxin Work Group (EUTox) : Review on uremic toxins: Classification, concentration, and interindividual variability. Kidney Int 63: 1934–1943, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Rhee EP, Souza A, Farrell L, Pollak MR, Lewis GD, Steele DJ, Thadhani R, Clish CB, Greka A, Gerszten RE: Metabolite profiling identifies markers of uremia. J Am Soc Nephrol 21: 1041–1051, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meyer TW, Sirich TL, Hostetter TH: Dialysis cannot be dosed. Semin Dial 24: 471–479, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheung AK, Rocco MV, Yan G, Leypoldt JK, Levin NW, Greene T, Agodoa L, Bailey J, Beck GJ, Clark W, Levey AS, Ornt DB, Schulman G, Schwab S, Teehan B, Eknoyan G: Serum beta-2 microglobulin levels predict mortality in dialysis patients: Results of the HEMO study. J Am Soc Nephrol 17: 546–555, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Ward RA, Greene T, Hartmann B, Samtleben W: Resistance to intercompartmental mass transfer limits beta2-microglobulin removal by post-dilution hemodiafiltration. Kidney Int 69: 1431–1437, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Vanholder R, Schepers E, Pletinck A, Neirynck N, Glorieux G: An update on protein-bound uremic retention solutes. J Ren Nutr 22: 90–94, 2012 [DOI] [PubMed] [Google Scholar]
- 10.Martinez AW, Recht NS, Hostetter TH, Meyer TW: Removal of P-cresol sulfate by hemodialysis. J Am Soc Nephrol 16: 3430–3436, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Niwa T: Indoxyl sulfate is a nephro-vascular toxin. J Ren Nutr 20[Suppl]: S2–S6, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Vanholder R, De Smet R, Lameire N: Protein-bound uremic solutes: The forgotten toxins. Kidney Int Suppl 78: S266–S270, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Yu M, Kim YJ, Kang DH: Indoxyl sulfate-induced endothelial dysfunction in patients with chronic kidney disease via an induction of oxidative stress. Clin J Am Soc Nephrol 6: 30–39, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adijiang A, Goto S, Uramoto S, Nishijima F, Niwa T: Indoxyl sulphate promotes aortic calcification with expression of osteoblast-specific proteins in hypertensive rats. Nephrol Dial Transplant 23: 1892–1901, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Jourde-Chiche N, Dou L, Cerini C, Dignat-George F, Brunet P: Vascular incompetence in dialysis patients—protein-bound uremic toxins and endothelial dysfunction. Semin Dial 24: 327–337, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Meijers BK, Bammens B, De Moor B, Verbeke K, Vanrenterghem Y, Evenepoel P: Free p-cresol is associated with cardiovascular disease in hemodialysis patients. Kidney Int 73: 1174–1180, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Bammens B, Evenepoel P, Verbeke K, Vanrenterghem Y: Removal of middle molecules and protein-bound solutes by peritoneal dialysis and relation with uremic symptoms. Kidney Int 64: 2238–2243, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Pham NM, Recht NS, Hostetter TH, Meyer TW: Removal of the protein-bound solutes indican and p-cresol sulfate by peritoneal dialysis. Clin J Am Soc Nephrol 3: 85–90, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eloot S, Torremans A, De Smet R, Marescau B, De Wachter D, De Deyn PP, Lameire N, Verdonck P, Vanholder R: Kinetic behavior of urea is different from that of other water-soluble compounds: The case of the guanidino compounds. Kidney Int 67: 1566–1575, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Cheung AK, Alford MF, Wilson MM, Leypoldt JK, Henderson LW: Urea movement across erythrocyte membrane during artificial kidney treatment. Kidney Int 23: 866–869, 1983 [DOI] [PubMed] [Google Scholar]
- 21.Lim VS, Flanigan MJ, Fangman J: Effect of hematocrit on solute removal during high efficiency hemodialysis. Kidney Int 37: 1557–1562, 1990 [DOI] [PubMed] [Google Scholar]
- 22.Ponda MP, Quan Z, Melamed ML, Raff A, Meyer TW, Hostetter TH: Methylamine clearance by haemodialysis is low. Nephrol Dial Transplant 25: 1608–1613, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eloot S, Torremans A, De Smet R, Marescau B, De Deyn PP, Verdonck P, Vanholder R: Complex compartmental behavior of small water-soluble uremic retention solutes: Evaluation by direct measurements in plasma and erythrocytes. Am J Kidney Dis 50: 279–288, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Duranton F, Cohen G, De Smet R, Rodriguez M, Jankowski J, Vanholder R, Argiles A, on behalf of the European Uremic Toxin Work Group : Normal and pathologic concentrations of uremic toxins. J Am Soc Nephrol 23: 1258–1270, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shannon JA: Renal tubular excretion. Physiol Rev 19: 63–93, 1939 [Google Scholar]
- 26.Sullivan JF, Eisenstein AB: Ascorbic acid depletion in patients undergoing chronic hemodialysis. Am J Clin Nutr 23: 1339–1346, 1970 [DOI] [PubMed] [Google Scholar]
- 27.Leblanc M, Pichette V, Geadah D, Ouimet D: Folic acid and pyridoxal-5′-phosphate losses during high-efficiency hemodialysis in patients without hydrosoluble vitamin supplementation. J Ren Nutr 10: 196–201, 2000 [DOI] [PubMed] [Google Scholar]
- 28.Ikizler TA, Flakoll PJ, Parker RA, Hakim RM: Amino acid and albumin losses during hemodialysis. Kidney Int 46: 830–837, 1994 [DOI] [PubMed] [Google Scholar]
- 29.Eknoyan G, Beck GJ, Cheung AK, Daugirdas JT, Greene T, Kusek JW, Allon M, Bailey J, Delmez JA, Depner TA, Dwyer JT, Levey AS, Levin NW, Milford E, Ornt DB, Rocco MV, Schulman G, Schwab SJ, Teehan BP, Toto R, Hemodialysis (HEMO) Study Group Effect of dialysis dose and membrane flux in maintenance hemodialysis. N Engl J Med 347: 2010–2019, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Bargman JM: Is there more to living than not dying? A reflection on survival studies in dialysis. Semin Dial 20: 50–52, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Liabeuf S, Barreto DV, Barreto FC, Meert N, Glorieux G, Schepers E, Temmar M, Choukroun G, Vanholder R, Massy ZA, European Uraemic Toxin Work Group (EUTox) : Free p-cresylsulphate is a predictor of mortality in patients at different stages of chronic kidney disease. Nephrol Dial Transplant 25: 1183–1191, 2010 [DOI] [PubMed] [Google Scholar]
- 32.Bammens B, Evenepoel P, Keuleers H, Verbeke K, Vanrenterghem Y: Free serum concentrations of the protein-bound retention solute p-cresol predict mortality in hemodialysis patients. Kidney Int 69: 1081–1087, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Barreto FC, Barreto DV, Liabeuf S, Meert N, Glorieux G, Temmar M, Choukroun G, Vanholder R, Massy ZA, European Uremic Toxin Work Group (EUTox) : Serum indoxyl sulfate is associated with vascular disease and mortality in chronic kidney disease patients. Clin J Am Soc Nephrol 4: 1551–1558, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mallamaci F, Tripepi G, Cutrupi S, Malatino LS, Zoccali C: Prognostic value of combined use of biomarkers of inflammation, endothelial dysfunction, and myocardiopathy in patients with ESRD. Kidney Int 67: 2330–2337, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Meinitzer A, Kielstein JT, Pilz S, Drechsler C, Ritz E, Boehm BO, Winkelmann BR, März W: Symmetrical and asymmetrical dimethylarginine as predictors for mortality in patients referred for coronary angiography: The Ludwigshafen Risk and Cardiovascular Health study. Clin Chem 57: 112–121, 2011 [DOI] [PubMed] [Google Scholar]
- 36.Raff AC, Lieu S, Melamed ML, Quan Z, Ponda M, Meyer TW, Hostetter TH: Relationship of impaired olfactory function in ESRD to malnutrition and retained uremic molecules. Am J Kidney Dis 52: 102–110, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, Wu Y, Schauer P, Smith JD, Allayee H, Tang WH, DiDonato JA, Lusis AJ, Hazen SL: Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 472: 57–63, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meijers BK, Van Kerckhoven S, Verbeke K, Dehaen W, Vanrenterghem Y, Hoylaerts MF, Evenepoel P: The uremic retention solute p-cresyl sulfate and markers of endothelial damage. Am J Kidney Dis 54: 891–901, 2009 [DOI] [PubMed] [Google Scholar]
- 39.Sirich TL, Luo FJ, Plummer NS, Hostetter TH, Meyer TW: Selectively increasing the clearance of protein-bound uremic solutes. Nephrol Dial Transplant 27: 1574–1579, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Evenepoel P, Meijers BK, Bammens BR, Verbeke K: Uremic toxins originating from colonic microbial metabolism. Kidney Int Suppl 114: S12–S19, 2009 [DOI] [PubMed] [Google Scholar]
- 41.Schepers E, Glorieux G, Vanholder R: The gut: The forgotten organ in uremia? Blood Purif 29: 130–136, 2010 [DOI] [PubMed] [Google Scholar]
- 42.Aronov PA, Luo FJ, Plummer NS, Quan Z, Holmes S, Hostetter TH, Meyer TW: Colonic contribution to uremic solutes. J Am Soc Nephrol 22: 1769–1776, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Niwa T: Role of indoxyl sulfate in the progression of chronic kidney disease and cardiovascular disease: Experimental and clinical effects of oral sorbent AST-120. Ther Apher Dial 15: 120–124, 2011 [DOI] [PubMed] [Google Scholar]
- 44.Patel KP, Luo FJ, Plummer NS, Hostetter TH, Meyer TW: The production of p-cresol sulfate and indoxyl sulfate in vegetarians versus omnivores. Clin J Am Soc Nephrol 7: 982–988, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]