Summary
Background and objectives
Most reports of pregnancy outcome in women with kidney transplants are single-center, retrospective, and include small numbers and few are compared with controls. The aim of this study was to collect information about pregnancy outcomes among all kidney transplant recipients in the United Kingdom, managed with current antenatal and nephrologic care, and to compare these data with a contemporaneous control group.
Design, setting, participants, & measurements
Pregnant women with a kidney transplant were identified through the UK Obstetric Surveillance System (UKOSS) between January 1, 2007 and December 31, 2009. Data on a comparison cohort were obtained from the UKOSS database, containing information on comparison women identified in previous studies. Outcomes were also compared with national data.
Results
There were 105 pregnancies identified in 101 recipients. Median prepregnancy creatinine was 118 μmol/L. Preeclampsia developed in 24% compared with 4% of the comparison group. Median gestation at delivery was 36 weeks, with 52% of women delivering at <37 weeks, significantly higher than the national rate of 8%. Twenty-four infants (24%) were small for gestational age (<10th centile). There were two (2%) cases of acute rejection. Potential predictive factors for poor pregnancy outcome included >1 previous kidney transplant (P=0.03), first trimester serum creatinine >125 μmol/L (P=0.001), and diastolic BP >90 mmHg in the second (P=0.002) and third trimesters (P=0.05).
Conclusions
Most pregnancies in the United Kingdom in women with kidney transplants are successful but rates of maternal and neonatal complications remain high.
Introduction
Women with CKD have markedly reduced fertility with increasing severity of renal dysfunction (1). A kidney transplant rapidly restores the ability to conceive within a few months (2), and approximately 2% of women with a kidney transplant of child-bearing age become pregnant (3). Over 14,000 births to women with transplanted organs have been reported (3). Most studies reporting pregnancy outcome in women with kidney transplants are single-center–based and retrospective, may span ≥2 decades, and the majority include <50 pregnancies (4,5). Four voluntary registers have collected data (6–9) in larger numbers of women, but are reliant on self-reporting.
A recent meta-analysis assessed adverse obstetric events in women with kidney transplants, including timing of conception after transplantation (10). Although this study provides information to guide clinicians, the authors acknowledge inconsistencies in diagnostic criteria of complications, lack of adjustment for baseline differences in medical care, and underlying socioeconomic factors between countries and other unmeasured confounding issues. Furthermore, studies examining pregnancy outcome to date are likely to be restricted by reporting bias, and not all are compared with a contemporaneous control group.
The aim of this study was to collect information about pregnancy outcomes, using consistent diagnostic criteria, among renal transplant recipients in the United Kingdom, managed with current antenatal and nephrologic care over a short time period and to compare these data with a contemporaneous control group.
Materials and Methods
Data Sources and Definitions
We identified pregnant women who had previously undergone a renal transplant through the UK Obstetric Surveillance System (UKOSS) between January 1, 2007 and December 31, 2009.
The UKOSS methodology has been described in detail elsewhere (11). In brief, nominated clinicians in each consultant-led maternity unit in the United Kingdom were sent a case notification card each month and asked to report all cases. They were also asked to return cards indicating a “nil report” in order to distinguish a nil response from a lack of response. Reporting clinicians were then asked to complete data collection forms to provide additional information about patients. All data collected were anonymous. The entire cohort of UK maternities in this study period was covered.
Data on a comparison cohort were obtained from the UKOSS database, containing information on comparison women identified in previous studies. These women were identified as the two women delivering in the same hospital immediately before a case in previous UKOSS studies (antenatal pulmonary embolism, eclampsia, and peripartum hysterectomy) (12–14), and have been shown to be representative of the general pregnancy population (15). The medical and pregnancy histories for all of the women were checked; any woman having a renal transplant history was excluded from this comparison group. For all women, data on maternal demographic characteristics and pregnancy complications, as well as maternal and infant outcomes, were collected. For the transplant cohort, we collected additional data on serum creatinine (SCr), as well as systolic and diastolic BP before the pregnancy and during each trimester. Immunosuppressive medications taken before and during pregnancy were also recorded.
National outcome data for all births in the United Kingdom were compared with the transplant cohort, using the birth statistics in 2008 (16–18) as a proxy for the period from 2007 to 2010. Where national data were unavailable, we used data from maternity hospital episode statistics for England or the British Isles Network of Congenital Anomalies Registers (BINOCAR) (19,20).
Predefined poor pregnancy outcomes were stillbirth, first or second trimester loss, termination due to a woman’s medical condition, very preterm delivery (<32 weeks), congenital anomaly, or neonatal death (death up to 1 month after live birth). Small for gestational age was calculated by comparing birth weight to British 1990 growth references (21,22). Significant proteinuria was defined as >300 mg/24 h, >30 mg/mmol creatinine, or ≥2+ on standard urinalysis.
Statistical Analyses
Continuous variables were summarized as means (SDs), or medians (interquartile or entire ranges) for skewed data, and were compared by t test or Wilcoxon rank-sum test, respectively. Categorical variables were summarized as frequencies (percentages). Associations between categorical variables were assessed using logistic regression, chi-squared tests, or Fisher’s exact tests where appropriate. Where comparable outcome data were available for women with a kidney transplant and the comparison cohort, odds ratios were adjusted for potential confounding using logistic regression. To allow for the nonindependence of multiple pregnancies from the same women, robust SEMs were calculated in the logistic regression to take into account within cluster correlation.
Highest SCr levels before birth, and in each trimester, were recorded for each woman with a kidney transplant. To estimate the changes in SCr levels during pregnancy and assess the significance of the difference in SCr levels between women with good and poor pregnancy outcomes, a generalized estimating equation approach was used to fit population-averaged panel-data models. To account for correlations in repeated measurements in SCr within each woman, we used robust (Huber–White) variance–covariance estimates at the individual level, and an autoregressive correlation structure of order 1 within women over time. SCr levels were not normally distributed, so were transformed to log-scale before entered into the model. Geometric means and 95% confidence intervals of SCr were estimated using Wald test statistics. Reduction in renal function was defined as a rise in SCr of ≥20% from the lowest level recorded during pregnancy.
Statistical analyses were carried out using STATA 11 software (StataCorp LP, College Station, TX).
Results
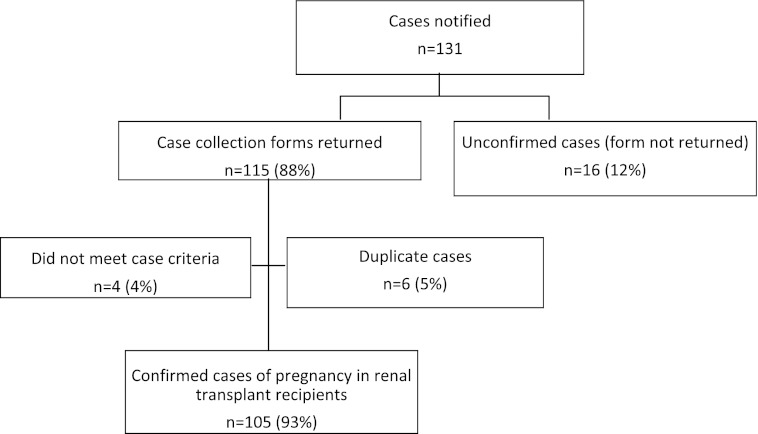
All 226 hospitals in the United Kingdom with consultant-led maternity units participated in the study (100%). Data collection and case ascertainment are presented in Figure 1.
Figure 1.
One hundred five cases were identified and unconfirmed and duplicate cases excluded.
A total of 105 pregnancies in 101 kidney transplant recipients were identified between January 2007 and December 2009, including four women who had two pregnancies each. Maternal characteristics are presented in Table 1. Mean age of kidney transplant recipients at pregnancy was 32 years (range, 18–44 years). Women with kidney transplants were more likely to be aged ≥35 years compared with the comparison group, and women in the comparison group were more likely to have ≥2 previous pregnancies.
Table 1.
Characteristics of women with a renal transplant and the comparison cohort
| Demographic Characteristic | Renal Transplant Recipients (n=105) | Comparison Cohort (n=1360) | Unadjusted OR | P Value |
|---|---|---|---|---|
| Maternal age (yr) | <0.001 | |||
| <20 | 2 (2) | 69 (5) | 1 | |
| 20–34 | 60 (57) | 995 (73) | 2.08 (0.50–8.71) | |
| ≥35 | 43 (41) | 294 (22) | 5.05 (1.19–21.4) | |
| Ethnic groupa | 0.25 | |||
| White | 87 (86) | 1080 (82) | 1 | |
| Nonwhite | 14 (14) | 244 (18) | 0.68 (0.38–1.22) | |
| Socioeconomic statusb | 0.80 | |||
| Managerial/professional | 27 (30) | 367 (30) | 1 | |
| Other employed | 51 (58) | 685 (56) | 1.03 (0.64–1.68) | |
| Unemployed | 11 (12) | 169 (14) | 0.85 (0.41–1.77) | |
| Parity | 0.003 | |||
| 0 | 62 (60) | 587 (43) | 1 | |
| 1 | 37 (36) | 430 (32) | 0.55 (0.35–0.87) | |
| 2+ | 5 (5) | 339 (25) | 0.47 (0.27–0.83) | |
| Smoking status during pregnancy | 0.01 | |||
| Did not smoke | 89 (87) | 985 (74) | 1 | |
| Smoked | 13 (13) | 340 (26) | 0.42 (0.22–0.80) | |
| Body mass index | 0.43 | |||
| Normal (<25) | 43 (47) | 626 (53) | 1 | |
| Overweight (25–29.9) | 31 (34) | 340 (29) | 1.33 (0.82–2.16) | |
| Obese (≥30) | 18 (20) | 225 (19) | 1.16 (0.66–2.07) | |
| Multiple pregnancy | 0.13 | |||
| Yes | 3 (3) | 15 (1) | 2.64 (0.75–9.27) | |
| No | 102 (97) | 1345 (99) | 1 |
Data are shown as n (%) or odds ratios (95% confidence intervals).
Reported for the 101 renal transplant women, rather than 105 pregnancies, because these characteristics did not change between pregnancies.
Percentages of those with data.
Median interval from transplantation to pregnancy was 5 years (range, 2 months to 28 years; interquartile range, 2–10 years). The most common indications for transplant were reflux nephropathy (including recurrent urinary tract infections), 34 (34%), GN, 15 (15%), adult polycystic kidney disease 7 (7%) and diabetes 6 (7%). Five women (5%) also had a pancreas transplant. Over half of women (n=57, 56%) conceived while being treated with ≥1 antihypertensives (including three patients with angiotensin converting enzyme inhibitors).
Management
Sixteen different combinations of immunosuppressive therapies were reported (Table 2). Forty-two women (40%) took both tacrolimus and prednisolone, and 29 women (28%) took tacrolimus, azathioprine, and prednisolone before and during pregnancy. Only one woman was taking mycophenolate mofetil at conception.
Table 2.
Drugs taken before and during pregnancy
| Drug | Total, n (%) |
|---|---|
| Azathioprine | 61 (60) |
| Cyclosporine | 22 (21) |
| Prednisolone | 56 (53) |
| Mycophenolate mofetil | 4 (4) |
| Tacrolimus | 65 (62) |
| Rapamycin | 2 (2) |
| Aspirin | 32 (30) |
| Calcium supplements | 10 (10) |
| Erythropoietin | 3 (3) |
| Anticoagulants | 3 (3) |
| Folic acid (at conception) | 62 (64) |
Maternal Complications and Outcomes
Maternal complications and outcomes are shown in Table 3. Fourteen women (13%) had proteinuria at the start of pregnancy and 32 (30%) subsequently developed proteinuria, none of whom were diagnosed with preeclampsia. There were no significant differences in maternal characteristics between the women who developed proteinuria without preeclampsia and those who did not (data not shown).
Table 3.
Pregnancy complications in women with a renal transplant and an ongoing pregnancy in the third trimester compared with the comparison cohort
| Outcome | Renal Transplant Recipients (n=95)a | Comparison Cohort (n=1360) | Unadjusted Odds Ratio | Adjusted Odds Ratiob |
|---|---|---|---|---|
| Preeclampsia in this pregnancyc | n=477 | |||
| Yes | 23 (24) | 17 (4) | 7.59 (3.87–14.9) | 6.31 (2.97–13.4) |
| No | 72 (76) | 460 (96) | 1 | 1 |
| Gestational diabetes in this pregnancy | ||||
| Yes | 3 (3) | 26 (2) | 1.5 (0.45–5.04) | 1.21 (0.35–4.25) |
| No | 91 (97) | 1324 (98) | 1 | 1 |
| Induced delivery | ||||
| Yes | 42 (44) | 300 (22) | 2.79 (1.82–4.29) | 2.67 (1.73–4.13) |
| No | 53 (56) | 1057 (78) | 1 | 1 |
| Delivery by caesarean section | ||||
| Yes | 61 (64) | 326 (24) | 5.69 (3.64–8.89) | 4.57 (2.83–7.35) |
| No | 34 (36) | 1034 (76) | 1 | 1 |
Data are shown as n (%) or odds ratios (95% confidence intervals).
Ten women whose pregnancies did not continue into the third trimester are excluded from this table.
Adjusted for woman’s age, parity, and smoking status. Woman’s age and parity are treated as continuous linear terms in the model.
Including only a subset of the comparison group with data about preeclampsia.
There was no difference in risk of preeclampsia between women who had taken aspirin (n=7 of 32; 22%) compared with those who did not (n=16 of 73; 22%). However, a significantly higher proportion of those with a diastolic BP of ≥90 mmHg immediately before pregnancy were prescribed aspirin (n=5 of 7; 71%) compared with those who had a lower BP (n=13 of 45; 29%) (P=0.04). Seventeen women (16%) who were not receiving antihypertensives at conception were subsequently prescribed them during pregnancy. Only three women developed gestational diabetes, all of whom were taking prednisolone and/or tacrolimus. Just over half of caesareans in transplant recipients (n=35; 57%) were of grade 1 or 2 urgency (immediate threat to life of the woman or fetus or maternal or fetal compromise not immediately life threatening) with the remaining 43% classified as grade 3 or 4 (n=26). The most commonly reported indications for caesarean delivery before onset of labor were concern about fetal wellbeing (n=10; 23%), previous caesarean section (n=8; 19%), and deteriorating renal function (n=7; 16%). The most common indication for in-labor caesarean was fetal distress (n=12; 67%). Two women (3%) were reported to have been delivered by caesarean section solely because they had a kidney transplant, in the absence of any other clinical reason. There were no maternal deaths; however, 20% of the women (n=21) were admitted to high dependency or intensive care units compared with 1% (n=14) of the comparison group.
Allograft Function
The median SCr fell from 118 µmol/L prepregnancy to 104 µmol/L in the first and second trimesters, followed by a rise to 123 µmol/L in the third trimester. However, 49% of women (n=51) did not show a fall in SCr in the second trimester. Reduction in renal function, defined as a rise in SCr of ≥20% from the lowest level recorded during pregnancy, was reported in 38% (n=40 of 105); over three-quarters had just one episode (n=31; 79%), 15% had two to three episodes (n=6), and 5% had four or more episodes (n=2), with details for one woman missing. The cause for increased creatinine was reported in approximately half of cases (n=19; 48%); 12 were due to preeclampsia (63%) and 2 due to acute rejection (11%).
Neonatal Outcomes
Neonatal outcomes are reported in Table 4. Ten women had a first or second trimester loss or had a termination due to deteriorating renal function.
Table 4.
Pregnancy outcomes comparing to comparison cohort and national data
| Outcome | Renal Transplant Cohort (n=108)a | Comparison Cohort (n=1375)a | Unadjusted Odds Ratio | Adjusted Odds Ratiob | National Data | Unadjusted Odds Ratio |
|---|---|---|---|---|---|---|
| Pregnancy outcome | ||||||
| First or second trimester loss or termination | 10 (9) | NA | NA | NA | NA | NA |
| Live birth | 98 (91) | 1366 (99) | NA | NA | ||
| Premature birth (<37 wk) | NA | |||||
| Yes | 51 (52) | 114 (8) | 11.7 (7.57–18.3) | 12.7 (8.05–20.1) | 36,558 (8)c | 12.57 (8.48–18.6) |
| No | 47 (48) | 1235 (92) | 1 | 1 | 423,475 (92) | |
| Very preterm birth (<32 wk) | ||||||
| Yes | 9 (9) | 27 (2) | 4.95 (2.26–10.9) | 6.64 (2.88–15.3) | 10,932 (2)c | 4.15 (2.12–8.14) |
| No | 89 (91) | 1322 (98) | 1 | 1 | 449,101 (98) | |
| Low birthweight (<2.5 kg) | ||||||
| Yes | 47 (48) | 109 (8) | 10.48 (6.73–16.3) | 12.11 (7.60–19.3) | 57,072 (7) | 11.52 (7.77–17.1) |
| No | 51 (52) | 1239 (92) | 1 | 1 | 713,201 (93) | |
| Very low birthweight (<1.5 kg) | ||||||
| Yes | 9 (9) | 24 (2) | 5.58 (2.52–12.4) | 7.76 (3.29–18.3) | 10,955 (1) | 7.01 (3.58–13.7) |
| No | 89 (91) | 1324 (98) | 1 | 1 | 759,318 (99) | |
| Small for gestational age | ||||||
| Yes | 24 (24) | 99 (7) | 4.07 (2.46–6.73) | 4.87 (2.87–8.26) | 10% (assumed) | 2.92 (1.85–4.61) |
| No | 74 (76) | 1242 (93) | 1 | 1 | ||
| Congenital anomaly | ||||||
| Yes | 4 (5) | NA | NA | NA | 4308 (2) | 2.46 (0.94–6.44) |
| No | 94 (95) | NA | 248,644 (100) | |||
| Perinatal mortality | ||||||
| Yes | 1 (1) | 10 (1) | 1.38 (0.17–10.86) | 1.57 (0.19–12.9) | 6025 (1) | 1.36 (0.00–7.74) |
| No | 97 (99) | 1335 (99) | 1 | 1 | 793,022 (99) | |
| Neonatal unit admission | ||||||
| Yes | 37 (38) | NA | NA | NA | NA | NA |
| No | 61 (62) | NA | NA | NA |
Includes all fetuses/infants (N=108). Data are shown as n (%) or odds ratios (95% confidence intervals). NA, not available.
Includes 3 pairs of twins in the renal transplant cohort and 15 in the comparison cohort.
Adjusted for woman’s age and smoking status. Woman’s age was treated as a continuous linear term in the model. Parity was dropped from the model due to multicollinearity.
Data from hospital episode statistics 2008–2009 (England and Wales only).
Median gestation at delivery was 36 weeks (interquartile range, 27–43 weeks). A quarter (n=13; 25%) of preterm deliveries (<37 weeks) followed induction of labor. Over three-quarters were by caesarean section (n=42; 82%), including 8 (16%) after induction of labor. The most common indications for early iatrogenic delivery were suspected renal compromise (n=18; 39%) and preeclampsia or worsening hypertension (n=11; 23%).
There were no stillbirths; however, 16 infants had at least one significant morbidity, including 10 infants with complications related to prematurity (including 2 with intraventricular hemorrhages) and a further 4 with jaundice.
Prognostic Factors for Pregnancy Outcome in Renal Transplant Recipients
About three-quarters of the transplant recipient cohort (77%) had a good pregnancy outcome, defined as any pregnancy that resulted in a live birth at >32 weeks gestation. The remaining 23% were classed as having a poor outcome (first or second trimester loss, stillbirth, neonatal death, very preterm birth <32 weeks, or a congenital anomaly). Women with ≥2 previous kidney transplants (n=20) were more likely to have poor pregnancy outcomes (P=0.03). There were no significant differences in maternal age, ethnicity, socioeconomic status, parity, smoking, body mass index, simultaneous pancreas transplant, etiology of underlying renal disease, live or cadaveric kidney donor, timing of transplantation to conception, or diastolic BP before pregnancy according to pregnancy outcomes.
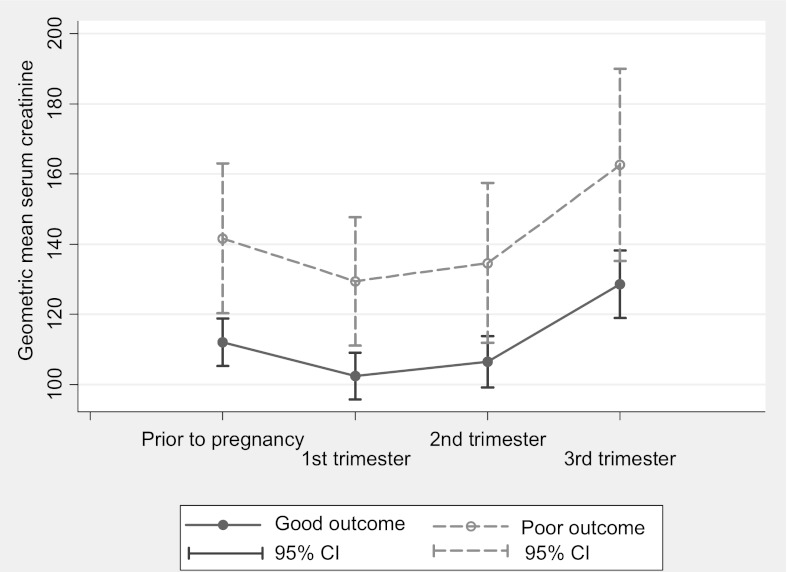
Overall, women with a poor pregnancy outcome had significantly higher median creatinine, both prepregnancy and in each trimester compared with women with a good pregnancy outcome (Table 5). Taking into account the correlations in the repeated measurements in SCr within women, the overall estimated SCr levels were significantly higher in women with a poor outcome (P=0.01) (Figure 2).
Table 5.
Association of pregnancy outcomes with clinical parameters during pregnancy
| Characteristic | Good Pregnancy Outcome (n=81) | Poor Pregnancy Outcome (n=24)a | Unadjusted Odds Ratio | P Value |
|---|---|---|---|---|
| Preeclampsia in this pregnancyb | ||||
| No | 61 (75) | 11 (79) | 1 | 0.79 |
| Yes | 20 (25) | 3 (21) | 0.44 (0.12–1.62) | |
| Gestational diabetes in this pregnancya | ||||
| No | 78 (96) | 13 (100) | — | 1.00 |
| Yes | 3 (4) | 0 (0) | — | |
| Renal dysfunction during pregnancy | ||||
| No | 48 (59) | 15 (68) | 1 | 0.46 |
| Yes | 33 (41) | 7 (32) | 0.68 (0.25–1.85) | |
| SCr (µmol/L), median (IQR) | 113 (99–127) | 148 (118–174) | — | 0.01 |
| First trimester SCr (µmol/L)b | ||||
| ≤125 | 60 (82) | 7 (41) | 1 | 0.001 |
| >125 | 13 (18) | 10 (59) | 6.59 (2.12–20.55) | |
| Highest proteinuria at any point in pregnancy (g/24 h) | ||||
| ≤0.3 | 14 (27) | 2 (14) | 1 | 0.49 |
| >0.3 | 38 (73) | 12 (86) | 2.21 (0.44–11.14) | |
| Lowest hemoglobin at any point in pregnancy (g/L) | ||||
| <80 | 8 (10) | 2 (10) | 1 | 1. 00 |
| ≥80 | 72 (90) | 18 (90) | 1 (0.20–5.12) | |
| Diastolic BP during pregnancy (mmHg) | ||||
| Highest diastolic BP during first trimester | ||||
| ≤90 | 69 (92) | 17 (81) | 1 | 0.16 |
| >90 | 6 (8) | 4 (19) | 2.71 (0.69–10.67) | |
| Highest diastolic BP during second trimester | ||||
| ≤90 | 70 (88) | 9 (53) | 1 | 0.002 |
| >90 | 10 (13) | 8 (47) | 6.22 (1.95–19.85) | |
| Highest diastolic BP during third trimester | ||||
| ≤90 | 52 (64) | 2 (25) | 1 | 0.05 |
| >90 | 29 (36) | 6 (75) | 5.38 (1.02–28.39) |
Data are shown as n (%) or odds ratios (95% confidence intervals), unless otherwise specified. SCr, serum creatinine; IQR, interquartile range.
Good pregnancy outcome: Live birth at >32 weeks’ gestation. Poor pregnancy outcome: First or second trimester loss, stillbirth, neonatal death, very preterm birth (<32 weeks), or a congenital anomaly.
Using the subset of women who had an ongoing pregnancy in the third trimester.
Figure 2.
Estimated mean serum creatinine and 95% confidence interval (CI) before pregnancy and in each trimester by pregnancy outcomes. Women with poor pregnancy outcome had higher serum estimated mean creatinine prior to pregnancy and in each trimester.
However, there were no significant differences in pregnancy outcomes between women whose SCr showed the expected fall in the second trimester, compared with women whose SCr remained stable or rose in the second trimester. There was no evidence of an association between pregnancy outcome and systolic BP or use of antihypertensive treatment. Pregnancy outcome was not significantly associated with episodes of changes in renal function.
Discussion
Our data suggest that the majority of women with kidney transplants will have successful pregnancies although adverse events are common. A quarter of women have serious pregnancy complications, defined as at least one of very preterm delivery (<32 weeks’ gestation) first or second trimester loss, stillbirth, neonatal death, or congenital abnormalities. Rates of preeclampsia, induction, and caesarean section were significantly higher than a comparison cohort. Rates of preterm delivery and small for gestational age infants were high compared with national data and 21% of mothers and 38% of neonates required intensive care. Previous studies have been mostly small, with data collected usually from single centers over a long time period, which may be influenced by changes in perinatal care, and protocol changes in immunosuppression regimens. Our study reflects current perinatal and nephrologic practice, and, through its national prospective design, attempts to reduce reporting bias, which is inherent in retrospective study designs. Furthermore, data analysis from 226 UK centers allowed all pregnancies in women with kidney transplants from the United Kingdom to be assessed, and therefore is unlikely to be affected by local demographic variations and individual clinicians’ management of women with kidney transplants.
A recent meta-analysis of pregnancy outcome in 4706 pregnancies in 3570 women with kidney transplants (10), which predominantly included women from single centers, with deliveries before 2001 has provided important references for rates of complications, but inconsistencies in definitions for adverse events, including hypertension, preeclampsia, and preterm delivery, require these data to be interpreted with caution. Our results regarding neonatal outcome, mode of delivery, and predictive factors of pregnancy complications are comparable, adding validity to both data sets.
Our study confirms the relatively high rates of obstetric complications in women with kidney transplants, and highlights the significantly increased risk of serious adverse events. The percentage of infants born before 32 weeks’ gestation was four times that expected from national data, and a quarter were small for gestational age. Previous studies with comparable outcomes included women managed more than 20 years ago (4); our data suggest little improvement in neonatal outcomes over this time.
A quarter of women developed preeclampsia compared with 4% in the comparison cohort, consistent with the findings of other reports (4). Aspirin has been shown to reduce the risk of preeclampsia in high-risk populations (23), but has never specifically been studied in women with kidney transplants. Relatively few women were prescribed aspirin, despite current UK guidelines suggesting that all women with CKD should be advised to take aspirin in pregnancy (24,25), and for all individuals post-transplantation for cardiovascular protection.
Caesarean section rates in the kidney transplants recipients were more than double the rate in the comparison group, the majority being for fetal distress with grade 1 or 2 urgency (i.e., immediate threat to life of woman or fetus or maternal or fetal compromise not immediately life threatening). Many were performed preterm, and 3% were performed simply because of the presence of the graft.
Gestational diabetes mellitus (GDM) thought to be due to diabetogenic drugs, particularly tacrolimus and prednisolone, has been reported more frequently in kidney transplant recipients (6,26). The pooled incidence of GDM in women with kidney transplants in a meta-analysis of 16 studies was 8% (7% in Europe) (10). In our study, only 3% (a similar proportion to the comparison group) of women developed GDM despite over half taking tacrolimus. Others have also reported low rates of GDM in transplant recipients (4,9), which may be a consequence of different diagnostic blood glucose levels between studies, or the ethnicity of the populations included, but is otherwise unexplained.
Unfortunately, long-term assessment of graft function was not possible using UKOSS methodology, which focuses on perinatal outcomes; however, we were able to assess the proportion of women who experienced changes in renal function during pregnancy. The expected fall in GFR with pregnancy did not occur in almost half of our cohort. Nevertheless, this was not associated with a statistically significant difference in poor pregnancy outcomes compared with women whose SCr decreased. Women with more severe renal impairment (Cr ≥125 μmol/L) were more likely to have poor pregnancy outcome compared with women with Cr <125 μmol/L, which is in keeping with other reports (4,27). A reduction in renal function in transplant recipients may be multifactorial, including preeclampsia, drug-induced, obstruction, rejection, and infection related or a physiologic return to prepregnancy values. Although 41% of women experienced at least one episode of a >20% rise in SCr during pregnancy, it did not appear to influence pregnancy outcomes, a finding comparable with other reports (28). Similarly, nearly a third of women developed new-onset proteinuria during pregnancy that was not associated with preeclampsia.
The UK Pregnancy Transplant Registry (UKTPR) reported that 69% of women had hypertension prepregnancy (7). We found that 56% of women conceived while taking antihypertensives, and 16% required the introduction of antihypertensives in pregnancy compared with 6% in the UKTPR report (7). Others have reported preexisting hypertension to be a predictor of pregnancy complications in kidney transplant recipients (7); however, we found no relationship between prepregnancy diastolic BP and adverse events.
We have no data regarding whether pregnancies in these women were planned; only 64% were taking folic acid at conception, suggesting either that the remainder were unplanned or that they received poor prenatal advice. Several studies have attempted to address the ideal time for conception in relation to transplantation. An interval of 12–24 months has been suggested (29); however, more recently, American Society of Transplantation Guidelines recommend that pregnancy may be considered after 1 year in women who are at low risk of complications (30) on the basis of favorable outcomes 12 months after transplant (31,32). We found no relationship between time from transplantation to conception and pregnancy outcome; however, small numbers limit the conclusions, and similar to other authors, we found no relationship between cadaveric and live organ donation and pregnancy outcome (33).
Studies reporting pregnancies in women on dialysis suggest that length of time on dialysis before pregnancy is predictive of adverse events (34), hence it might be expected that women with >1 kidney transplant (i.e., those likely to have suffered longer periods with severe renal impairment and consequent vascular damage) would have poorer outcomes. Our study is the first to demonstrate an association between number of previous kidney transplants and poor pregnancy outcome.
Limitations
We are unable to comment on long-term graft function because this is beyond the scope of the UKOSS. The live birth rate was high (91%); however, this may reflect underreporting of early losses, for example, the 16 cases for which collection forms were not returned may represent early losses. Despite being a national study over 3 years, the number of women in the study was small, which limits the statistical power of comparisons, particularly where event rates are low and it is likely that some further real differences between the groups may have been missed.
The majority of pregnancies in the United Kingdom in women with kidney transplants are successful but rates of preeclampsia, preterm delivery, and caesarean section remain high despite improvements in obstetric, perinatal, and nephrologic care. Prescribing of aspirin in women with kidney transplants is low and nearly half of women with kidney transplants experience at least a temporary deterioration in graft function during their pregnancy. Predictive factors for poor pregnancy outcome include ≥2 previous kidney transplants, first trimester SCr >125 μmol/L, and diastolic BP >90 mmHg in the second and third trimesters.
Disclosures
None.
Acknowledgments
This study would not have been possible without the contribution and enthusiasm of the UKOSS reporting clinicians who notified patients and completed the data collection forms.
This paper reports on an independent study that was part-funded by the Policy Research Programme in the Department of Health. The salary of K.B. is provided by the UK National Institutes of Health Research. M.K. was funded by a postdoctoral award from the National Institute for Health Research. The views expressed in this publication are those of the authors and not necessarily those of the NHS, the National Institute for Health Research, or the Department of Health. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Pregnancy in Renal Transplant Recipients: More Questions Answered, Still More Asked,” on pages 182–183.
References
- 1.Hou S: Pregnancy in chronic renal insufficiency and end-stage renal disease. Am J Kidney Dis 33: 235–252, 1999 [DOI] [PubMed] [Google Scholar]
- 2.Davison JM, Bailey DJ: Pregnancy following renal transplantation. J Obstet Gynaecol Res 29: 227–233, 2003 [DOI] [PubMed] [Google Scholar]
- 3.McKay DB, Josephson MA: Pregnancy in recipients of solid organs—effects on mother and child. N Engl J Med 354: 1281–1293, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Thompson BC, Kingdon EJ, Tuck SM, Fernando ON, Sweny P: Pregnancy in renal transplant recipients: The Royal Free Hospital experience. QJM 96: 837–844, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Yassaee F, Moshiri F: Pregnancy outcome in kidney transplant patients. Urol J 4: 14–17, 2007 [PubMed] [Google Scholar]
- 6.Coscia LA, Constantinescu S, Moritz MJ, Frank A, Ramirez CB, Maley WL, Doria C, McGrory CH, Armenti VT: Report from the National Transplantation Pregnancy Registry (NTPR): Outcomes of pregnancy after transplantation. Clin Transpl 103–122, 2009 [PubMed] [Google Scholar]
- 7.Sibanda N, Briggs JD, Davison JM, Johnson RJ, Rudge CJ: Pregnancy after organ transplantation: A report from the UK Transplant pregnancy registry. Transplantation 83: 1301–1307, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Rizzoni G, Ehrich JH, Broyer M, Brunner FP, Brynger H, Fassbinder W, Geerlings W, Selwood NH, Tufveson G, Wing AJ: Successful pregnancies in women on renal replacement therapy: Report from the EDTA Registry. Nephrol Dial Transplant 7: 279–287, 1992 [DOI] [PubMed] [Google Scholar]
- 9.Levidiotis V, Chang S, McDonald S: Pregnancy and maternal outcomes among kidney transplant recipients. J Am Soc Nephrol 20: 2433–2440, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deshpande NA, James NT, Kucirka LM, Boyarsky BJ, Garonzik-Wang JM, Montgomery RA, Segev DL: Pregnancy outcomes in kidney transplant recipients: A systematic review and meta-analysis. Am J Transplant 11: 2388–2404, 2011 [DOI] [PubMed] [Google Scholar]
- 11.Knight M, Kurinczuk JJ, Tuffnell D, Brocklehurst P: The UK Obstetric Surveillance System for rare disorders of pregnancy. BJOG 112: 263–265, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Knight M, UKOSS : Peripartum hysterectomy in the UK: Management and outcomes of the associated haemorrhage. BJOG 114: 1380–1387, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Knight M, UKOSS : Eclampsia in the United Kingdom 2005. BJOG 114: 1072–1078, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Knight M, UKOSS : Antenatal pulmonary embolism: Risk factors, management and outcomes. BJOG 115: 453–461, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Knight M, Tuffnell D, Brocklehurst P, Spark P, Kurinczuk JJ, UK Obstetric Surveillance System : Incidence and risk factors for amniotic-fluid embolism. Obstet Gynecol 115: 910–917, 2010 [DOI] [PubMed] [Google Scholar]
- 16.Office for National Statistics: Birth Statistics 2008 in Series FM1, No. 37, Newport, UK, Office for National Statistics, 2010
- 17.Northern Ireland Statistics and Research Agency : Registrar General Annual Report 2008, Belfast, Ireland, Northern Ireland Statistics and Research Agency, 2009 [Google Scholar]
- 18.Information Services Division Scotland: Births in Scottish Hospitals, 2010. Available at: http://www.isdscotland.org/isd/1022.html Accessed August 2011
- 19.BINOCAR: British Isles Network of Congenital Anomaly Registers. Available at: http://www.binocar.org/ Accessed August 2011
- 20.NHS Information Centre: Hospital Episode Statistics Maternity data archive, 2010
- 21.Cole TJ, Freeman JV, Preece MA: British 1990 growth reference centiles for weight, height, body mass index and head circumference fitted by maximum penalized likelihood. Stat Med 17: 407–429, 1998 [PubMed] [Google Scholar]
- 22.Freeman JV, Cole TJ, Chinn S, Jones PR, White EM, Preece MA: Cross sectional stature and weight reference curves for the UK, 1990. Arch Dis Child 73: 17–24, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duley L, Henderson-Smart DJ, Meher S, King JF: Antiplatelet agents for preventing pre-eclampsia and its complications. Cochrane Database Syst Rev (2): CD004659, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Davison JM, Nelson-Piercy C: Consensus Views on Renal Disease in Pregnancy, edited by Kehoe S, Baker P, London, Royal College Obstetrics and Gynaecology Press, 2008, p 250 [Google Scholar]
- 25.National Institute for Clinical Excellence: Hypertension in pregnancy: The management of hypertensive disorders during pregnancy (clinical guideline 107), 2010. Available from: http://guidance.nice.org.uk/CG107 Accessed August 2011
- 26.Al-Khader AA, Basri N, Al-Ghamdi, Shaheen, Hejaili, Flaiw, Qureshi J: Pregnancies in renal transplant recipients—with a focus on babies. Ann Transplant 9: 65–67, 2004 [PubMed] [Google Scholar]
- 27.Stratta P, Canavese C, Giacchino F, Mesiano P, Quaglia M, Rossetti M: Pregnancy in kidney transplantation: Satisfactory outcomes and harsh realities. J Nephrol 16: 792–806, 2003 [PubMed] [Google Scholar]
- 28.Aivazoglou L, Sass N, Silva HT, Jr, Sato JL, Medina-Pestana JO, De Oliveira LG: Pregnancy after renal transplantation: An evaluation of the graft function. Eur J Obstet Gynecol Reprod Biol 155: 129–131, 2011 [DOI] [PubMed] [Google Scholar]
- 29.EBPG Expert Group on Renal Transplantation: European best practice guidelines for renal transplantation. Section IV: Long-term management of the transplant recipient. IV.10. Pregnancy in renal transplant. Nephrol Dial Transplant 17[Suppl 4]: 50–55, 2002 [PubMed] [Google Scholar]
- 30.McKay DB, Josephson MA, Armenti VT, August P, Coscia LA, Davis CL, Davison JM, Easterling T, Friedman JE, Hou S, Karlix J, Lake KD, Lindheimer M, Matas AJ, Moritz MJ, Riely CA, Ross LF, Scott JR, Wagoner LE, Wrenshall L, Adams PL, Bumgardner GL, Fine RN, Goral S, Krams SM, Martinez OM, Tolkoff-Rubin N, Pavlakis M, Scantlebury V, Women’s Health Committee of the American Society of Transplantation : Reproduction and transplantation: Report on the AST Consensus Conference on Reproductive Issues and Transplantation. Am J Transplant 5: 1592–1599, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Fischer T, Neumayer HH, Fischer R, Barenbrock M, Schobel HP, Lattrell BC, Jacobs VR, Paepke S, von Steinburg SP, Schmalfeldt B, Schneider KT, Budde K: Effect of pregnancy on long-term kidney function in renal transplant recipients treated with cyclosporine and with azathioprine. Am J Transplant 5: 2732–2739, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Kim HW, Seok HJ, Kim TH, Han DJ, Yang WS, Park SK: The experience of pregnancy after renal transplantation: Pregnancies even within postoperative 1 year may be tolerable. Transplantation 85: 1412–1419, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Gill JS, Zalunardo N, Rose C, Tonelli M: The pregnancy rate and live birth rate in kidney transplant recipients. Am J Transplant 9: 1541–1549, 2009 [DOI] [PubMed] [Google Scholar]
- 34.Luders C, Castro MC, Titan SM, De Castro I, Elias RM, Abensur H, Romão JE, Jr: Obstetric outcome in pregnant women on long-term dialysis: A case series. Am J Kidney Dis 56: 77–85, 2010 [DOI] [PubMed] [Google Scholar]