Summary
Background
Biomarkers of AKI that can predict which patients will develop severe renal disease at the time of diagnosis will facilitate timely intervention in populations at risk of adverse outcomes.
Design, setting, participants, & measurements
Liquid chromatography/tandem mass spectrometry was used to identify 30 potential prognostic urinary biomarkers of severe AKI in a group of patients that developed AKI after cardiac surgery. Angiotensinogen had the best discriminative characteristics. Urinary angiotensinogen was subsequently measured by ELISA and its prognostic predictive power was verified in 97 patients who underwent cardiac surgery between August 1, 2008 and October 6, 2011.
Results
The urine angiotensinogen/creatinine ratio (uAnCR) predicted worsening of AKI, Acute Kidney Injury Network (AKIN) stage 3, need for renal replacement therapy, discharge >7 days from sample collection, and composite outcomes of AKIN stage 2 or 3, AKIN stage 3 or death, and renal replacement therapy or death. The prognostic predictive power of uAnCR was improved when only patients classified as AKIN stage 1 at the time of urine sample collection (n=79) were used in the analysis, among whom it predicted development of stage 3 AKI or death with an area under the curve of 0.81. Finally, category free net reclassification improvement showed that the addition of uAnCR to a clinical model to predict worsening of AKI improved the predictive power.
Conclusions
Elevated uAnCR is associated with adverse outcomes in patients with AKI. These data are the first to demonstrate the utility of angiotensinogen as a prognostic biomarker of AKI after cardiac surgery.
Introduction
One of the most important factors underlying the poor outcomes seen in AKI is our current method of diagnosis, which is based on either an increase in serum creatinine (SCr) or decreased urine output (1,2). SCr and urine output values at the time of diagnosis are of limited prognostic value, making it difficult to discriminate between mild and severe AKI at the time of diagnosis. The need for better biomarkers of AKI has been recognized as a crucial barrier to improvement of the outcomes of AKI patients. Newer biomarkers of AKI include kidney injury molecule 1, neutrophil gelatinase-associated lipocalin (NGAL), IL-18, and cystatin C (3–9). Many of these biomarkers initially appeared capable of early, accurate detection of AKI, but subsequent verification studies have reported lower accuracy (10–17). In addition, the emphasis on early detection has overshadowed investigation of their prognostic predictive power. Available data on the prognostic value of these biomarkers suggest that they are limited predictors of adverse outcomes (18,19). The limitations of biomarkers underscore the need to discover new prognostic biomarkers.
Approximately 20% of patients who undergo cardiac surgery develop AKI. The timing of the injury can be readily determined in these patients (20). We used proteomics to identify prognostic urinary biomarkers of AKI after cardiac surgery. We performed a verification of angiotensinogen in patients who developed AKI after cardiac surgery. This is the first study to demonstrate the utility of angiotensinogen as a prognostic biomarker of AKI.
Materials and Methods
Urine Samples
Urine samples were obtained from 99 consecutively enrolled patients who had cardiac surgery at one of the SAKInet institutions between August 1, 2008 and October 6, 2011. Informed consent was obtained in accordance with the institutional review board–approved protocol at each institution. Samples were collected and stored using a standard operating procedure that included centrifugation, addition of protease inhibitors, and storage at −80°C. Urine samples were collected as early as possible after AKIN serum creatinine criteria were met (2). Inclusion criteria were surgery of the heart or ascending aorta and development of AKI within 2 days of surgery. Participants with baseline SCr >3 mg/dl were excluded. Twelve samples were used in the proteomic studies, 10 of which were also used in a validation set that included samples collected from the remaining 87 participants in the study. Of the samples used in the validation set, 79 were from patients classified as having AKIN stage 1 at the time of sample collection.
Proteomic Analyses
A detailed description of the methods is available in the Supplemental Material. HIV protein gp160 (200 ng; Bioclone Inc) was spiked into each urine sample. Proteins were denatured, alkylated, and digested with trypsin. Samples were prefractionated by solid phase extraction using a Strata-X SPE cartridge (Phenomenex). Sample fractions were reconstituted in mobile phase A (MPA) (98% H20, 0.1% formic acid; 2% acetonitrile). Five microliters of each fraction was injected onto an Acclaim PepMap100 trap column, washed with 100% MPA, and separated on an Acclaim PepMap100 analytical column (75 μm ID × 15 cm, C18, 3 μm, 100 Å; Thermo Scientific) using a 45-minute two-step gradient. Tandem mass spectrometry (MS/MS) was performed using an AB SCIEX Triple TOF 5600 mass spectrometer. Acquired spectra were searched against the 2011_6 release of the Human UniProtKB/Swiss-Prot database (20,127 entries) with addition of common contaminants (112 entries) using the Mascot search engine with trypsin as the specified enzyme. Monoisotopic masses were used, and the error tolerances were 10 ppm and 0.5 Da for peptides and MS/MS fragments, respectively. Mascot search results were loaded into Scaffold (Proteome Software Inc), which used the Peptide Prophet and Protein Prophet algorithms to validate peptide and protein identifications (21,22). The Scaffold quantitative values of identified proteins were normalized to the internal standard HIV protein, and the relative abundance of each protein is reported in normalized spectral counts.
Angiotensinogen ELISA
The Human Total Angiotensinogen Assay Kit (Immuno-Biologic Laboratories Co. Ltd.) was used according to the manufacturer’s protocol. Values for intra- and interassay variability were 2.4% and 9.9%, respectively.
Urine Creatinine Determination
Urine creatinine was measured using the Jaffe assay.
Outcomes
The primary outcome was worsening of AKI, defined as progression to a higher AKIN stage after the time of sample collection. Secondary outcomes were progression to AKIN stage 3, the need for renal replacement therapy (RRT) within 10 days of sample collection, progression to AKIN stage 2 or 3, progression to AKIN stage 3 or death, RRT or death, and discharge >7 days from the time of sample collection or in-hospital mortality. Outcomes were tested using the entire cohort and in the subset of patients classified as AKIN stage 1 at the time of sample collection.
Statistical Analyses
Differential abundance of proteins quantified by LC-MS/MS was tested using the Wilcoxon rank-sum test which has been shown to be a robust test for identification of candidate biomarkers in proteomic studies (23). Candidate biomarkers were selected based upon the combination of P values from the Wilcoxon rank-sum test and mean fold-change between the experimental groups. The relationship between these two measures was visualized by “volcano plot.” In verification studies, count data were analyzed using the chi-squared or Fisher’s exact test as appropriate. Continuous variables were analyzed using the t test or Mann Whitney U test. ANOVA or Kruskal–Wallis ANOVA on ranks test and the post hoc Dunn’s test for pairwise comparison were used to evaluate continuous variables when more than two groups were compared. Odds ratios (ORs) were used to test the association of uAnCR with selected outcomes. Patients were stratified by uAnCR into quartiles. The effect of uAnCR on the risk of developing an outcome was tested by calculating the OR of the upper and lower quartiles and estimating the 95% confidence interval of the OR. Receiver operator characteristic (ROC) curves were constructed to determine the prognostic predictive power of uAnCR. Univariate ROC curves were considered statistically significant if the area under the curve (AUC) differed from 0.5, as determined by the z test. Optimal cut-offs were determined by selecting the data point that minimized the geometric distance from 100% sensitivity and 100% specificity on the ROC curve (24). To visualize the relationship between uAnCR and length of stay, patients were stratified into tertiles by uAnCR. Kaplan–Meier curves with censoring for death were plotted. The log-rank test was used to compare the curves and the Holm-Sidak test was used for post hoc pairwise comparison. Category free net reclassification improvement (cfNRI) was used to determine if addition of uAnCR to a multivariate logistic regression model for prediction of risk increased the ability of the model to predict worsening of AKI (25,26). The risk prediction model consisted of the Cleveland Clinic cardiac surgery risk score and percent change in serum creatinine at the time the urine sample was collected (27). Statistical tests were performed in Matlab or SigmaPlot.
Results
Discovery of Candidate Prognostic AKI Biomarkers
We used LC-MS/MS to compare the urinary proteomic profiles of 12 patients who developed AKI after cardiac surgery, 6 of whom later required RRT and 6 of whom did not. There were no statistically significant differences between the two groups with respect to the demographic characteristics, sample collection time, use of cardiopulmonary bypass, bypass time, type of surgery, preoperative SCr, and SCr at the time of sample collection (Supplemental Table 1). We identified 343 proteins, of which 59 were unique to ≥1 patients who required RRT and 5 were unique to ≥1 patients who did not (Figure 1A). The relative abundance of 30 proteins was statistically different between the two groups (Table 1 and Supplemental Table 2). The abundance of 26 proteins was increased in the urine of patients who required RRT and four were decreased. We selected angiotensinogen as the most promising candidate marker based on the combination of its low P value (P=0.002) and relatively large mean fold-change (9.67-fold) difference between groups (Figure 1B). Relative abundances of angiotensinogen for the individual participants (Figure 1C) demonstrate that urinary angiotensinogen discriminated with 100% accuracy between patients who required RRT and those who did not in this group. On the basis of these data, we attempted to verify the potential of urinary angiotensinogen as a biomarker of severe AKI after cardiac surgery.
Figure 1.
Proteins identified by LC-MS/MS in the urine of patients who developed AKI after cardiac surgery. (A) The Venn diagram shows the number of proteins identified in patients who later developed severe postoperative AKI requiring RRT versus those who did not. (B) The volcano plot shows the significance of the difference in mean abundance between the two groups for each identified protein. It facilitates the selection of candidate biomarkers that have a large magnitude fold-change (positive fold changes indicate elevated protein levels in the RRT group) and highly significant P values. The arrowhead indicates the data point for angiotensinogen. Proteins above the dashed line had a P value <0.05. (C) The scattergram shows the angiotensinogen abundance in each patient in the discovery analysis by group. Angiotensinogen was identified in the urine of two of six AKI patients in the No RRT group and was undetectable in the other four patients. LC-MS/MS, liquid chromatography/tandem mass spectrometry; RRT, renal replacement therapy.
Table 1.
Candidate biomarkers of severe AKI requiring RRT
| Identified Proteins (349) | Uniprot Accession Number | Mean No RRT | Mean RRT | Mean Fold Change | P |
|---|---|---|---|---|---|
| Angiotensinogen | P01019 | 1.69 | 16.33 | 9.67 | 0.002 |
| Serum albumin | P02768 | 653.89 | 2892.67 | 4.42 | 0.002 |
| Apo A-IV | P06727 | 2.36 | 21.45 | 9.09 | 0.01 |
| C3 | P01024 | 8.82 | 50.09 | 5.68 | 0.01 |
| Vitamin D-binding protein | P02774 | 4.51 | 54.58 | 12.11 | 0.01 |
| C4-B | P0C0L5 | 2.71 | 23.25 | 8.58 | 0.01 |
| SOD [Cu-Zn] | P00441 | 10.10 | 23.91 | 2.37 | 0.01 |
| Epididymal secretory protein E1 | P61916 | 3.17 | 10.39 | 3.28 | 0.01 |
| Phosphatidylethanolamine-binding protein 1 | P30086 | 0 | 5.90 | N/C | 0.02 |
| Complement factor D | P00746 | 0 | 5.81 | N/C | 0.02 |
| Coactosin-like protein | Q14019 | 0 | 3.70 | N/C | 0.02 |
| Serotransferrin | P02787 | 72.61 | 472.14 | 6.5 | 0.02 |
| Profilin-1 | P07737 | 5.87 | 24.04 | 4.1 | 0.02 |
| Cystatin-B | P04080 | 0.43 | 4.94 | 11.63 | 0.02 |
| Fibrinogen α chain | P02671 | 7.33 | 35.39 | 4.83 | 0.02 |
| Brain acid soluble protein 1 | P80723 | 0.87 | 5.05 | 5.82 | 0.02 |
| Zinc-α-2-glycoprotein | P25311 | 112.02 | 228.64 | 2.04 | 0.03 |
| α-1-antitrypsin | P01009 | 21.55 | 239.32 | 11.11 | 0.03 |
| α-1-acid glycoprotein 1 | P02763 | 44.94 | 112.82 | 2.51 | 0.03 |
| Hemopexin | P02790 | 7.94 | 30.11 | 3.79 | 0.03 |
| Fibrinogen β chain | P02675 | 3.65 | 14.29 | 3.92 | 0.03 |
| Pigment epithelium-derived factor | P36955 | 2.50 | 22.71 | 9.08 | 0.03 |
| Fatty acid-binding protein, adipocyte | P15090 | 6.25 | 9.99 | 1.6 | 0.03 |
| α-1-acid glycoprotein 2 | P19652 | 18.15 | 47.56 | 2.62 | 0.04 |
| Metallothionein-2 | P02795 | 1.43 | 19.25 | 13.47 | 0.04 |
| Apo A-I | P02647 | 2.39 | 20.36 | 8.53 | 0.04 |
| Keratin, type II cytoskeletal 5 | P13647 | 3.74 | 2.16 | −1.74 | 0.03 |
| Secreted Ly-6/uPAR-related protein 1 | P55000 | 4.49 | 3.18 | −1.41 | 0.03 |
| Nonsecretory ribonuclease | P10153 | 17.15 | 7.72 | −2.22 | 0.04 |
| Keratin, type II cytoskeletal 1 | P04264 | 35.85 | 18.85 | −1.9 | 0.05 |
Relative protein abundance was estimated using normalized spectral counts. RRT, renal replacement therapy. N/C, the value was not calculated because the denominator is zero.
Verification of the Prognostic Ability of Urinary Angiotensinogen
We measured urinary angiotensinogen by ELISA and verified its association with outcomes in patients who had developed AKI after cardiac surgery (n=97). These patients were divided into three groups by maximum AKIN stage: stage 1 (n=59), stage 2 (n=19), and stage 3 (n=19). Seventy-nine patients were classified as AKIN stage 1 at the time of urine sample collection. Ten of these patients progressed to a maximum AKIN stage of 2, 10 progressed to AKIN stage 3 and 59 did not progress. There were no statistically significant differences among the groups with respect to sex, race, age, use of bypass, bypass time, preoperative SCr, and type of surgery (Table 2). In addition, SCr was not statistically different among the groups in the subset of patients who were classified as AKIN stage 1 at collection.
Table 2.
Characteristics of cardiac surgery patients used to verify the potential of urinary angiotensinogen as a biomarker of postoperative AKI
| Maximum AKIN Stage Achieved | AKI of Any Stage at Time of Sample Collection (n=97) | AKIN Stage 1 at Time of Sample Collection (n=79) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| AKIN Stage 1 (n=59) | AKIN Stage 2 (n=19) | AKIN Stage 3 (n=19) | P | AKIN Stage 1 (n=59) | AKIN Stage 2 (n=10) | AKIN Stage 3 (n=10) | P | ||
| uAnCR | 22.6 (13.1–54.0) | 34.1 (11.1–50.4) | 58.8 (20.4–217.1) | 0.01 | 22.6 (13.1–54.0) | 35.3 (22.6–270.3) | 77.0 (30.9–329.4) | 0.01 | |
| Male | 42 (71) | 14 (74) | 13 (68) | 0.94 | 42 (71) | 6 (60) | 7 (70) | 0.78 | |
| Caucasian | 38 (64) | 12 (63) | 15 (79) | 0.47 | 38 (64) | 6 (60) | 8 (80) | 0.58 | |
| Age (yr) | 65.8±10.8 | 64.5±10.0 | 68.5±11.9 | 0.53 | 65.8±10.8 | 68.2±10.8 | 69.0±14.7 | 0.58 | |
| Weight (kg) | 88.2±24.3 | 94.41± 23.8 | 88.9±27.3 | 0.63 | 88.2±24.3 | 84.7±21.7 | 88.1±33.3 | 0.69 | |
| Sample collection time (postoperative h) | 27.9±11.8 | 31.6±14.5 | 36.0±11.0 | 0.04 | 27.9±11.8 | 26.2±15.6 | 35.2±12.2 | 0.23 | |
| Operative variables | |||||||||
| CABG | 36 (61) | 10 (53) | 11 (58) | 0.81 | 36 (61) | 4 (40) | 5 (50) | 0.41 | |
| Valve replacement | 17 (29) | 7 (37) | 4 (21) | 0.56 | 17 (29) | 5 (50) | 2 (20) | 0.30 | |
| CABG + valve replacement | 6 (10) | 2 (11) | 4 (21) | 0.44 | 6 (10) | 1 (10) | 3 (30) | 0.21 | |
| Bypass | 45 (76) | 16 (84) | 14 (74) | 0.71 | 45 (76) | 8 (80) | 7 (70) | 0.88 | |
| Bypass time (min) | 143.2±72.5 | 145.4±75.6 | 118.9±67.3 | 0.31 | 143.2±72.5 | 154.4±74.7 | 146.4±77.8 | 0.95 | |
| SCr (mg/dl) | |||||||||
| Preoperative SCr | 1.2±0.3 | 1.2±0.4 | 1.2±0.5 | 0.19 | 1.2±0.3 | 1.2±0.4 | 1.4±0.5 | 0.74 | |
| SCr at collection | 1.7±0.4 | 2.0±0.7 | 2.5±0.8 | 0.001 | 1.7±0.4 | 1.7±0.6 | 2.3±0.8 | 0.14 | |
| Max SCr | 1.9±0.4 | 2.7±0.8 | 4.0±1.9 | <0.001 | 1.9±0.4 | 2.6±0.8 | 4.3±2.6 | <0.001 | |
| Outcomes | |||||||||
| Days to max SCr (postoperative) | 2.0 (1.0–3.0) | 2.0 (2.0–3.0) | 4.0 (2.0–5.0) | 0.001 | 2.0 (1.0–3.0) | 3.0 (2.0–5.25) | 4.5 (2.7–6.75) | <0.001 | |
| RRT 10 days | 0 | 0 | 9 (47) | <0.001 | 0 | 0 | 8 (80) | <0.001 | |
| Death | 0 | 2 (11) | 6 (32) | <0.001 | 0 | 2 (20) | 3 (30) | <0.001 | |
Data are shown as median (interquartile range), n (%), or mean ± SD. AKIN, Acute Kidney Injury Network; uAnCr, urine angiotensinogen/creatinine ratio; CABG, coronary artery bypass graft; SCr, serum creatinine; Max, maximum; RRT, renal replacement therapy.
Among all patients who had developed AKI of any stage at the time of urine sample collection, uAnCR (nanograms of angiotensinogen per milligrams of creatinine) was correlated with both maximum SCr (r=0.49; P<0.001) and maximum percent change in SCr (r=0.29; P=0.01), and uAnCR increased with maximum AKIN stage achieved in both the whole cohort and the subset of patients classified as AKIN stage 1 at collection (Supplemental Figure 1). Pairwise comparison revealed a statistically significant difference in uAnCR between the patients who developed AKIN stage 3 and those who reached a maximum of stage 1 (Table 2 and Supplemental Figure 1).
Patients with higher uAnCR had increased risk of adverse outcomes (Supplemental Table 3). Comparing patients in the top quartile of uAnCR to those in the bottom quartile, the odds ratio for the primary outcome worsening of AKI was 5.0 (95% CI, 1.2–21.5) in the whole cohort and 4.6 (95% CI, 1.0–21.0) in the subset of patients who were classified as AKIN stage 1 at the time of sample collection. uAnCR discriminated between patients who experienced worsening of AKI after sample collection and those who did not in both the whole cohort (Figure 2A; AUC=0.70) and in the subset classified as AKIN stage 1 at collection (Figure 2B; AUC=0.71). At the optimal cut-off (33.27 ng/mg), the sensitivity and specificity of uAnCR were 70.8% and 66.2%, respectively, in the whole cohort. In patients who were classified as AKIN stage 1 at collection, sensitivity and specificity were 75.0% and 66.1%. ROC curves for the other tested outcomes showed similar results (Supplemental Figures 2 and 3). Notably, the predictive power for most outcomes among those patients classified as AKIN stage 1 at collection appeared to be slightly augmented compared with the analysis of the entire cohort. For example, uAnCR discriminated with high accuracy (AUC=0.81) between patients who later met the outcome of AKIN stage 3 or death and those who did not.
Figure 2.
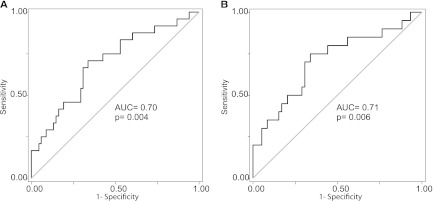
Angiotensinogen is associated with worsening of AKI. ROC curves were used to test the ability of uAnCR to predict worsening of AKI after sample collection among (A) patients who were any stage AKI at the time of collection (n=97) and (B) the subset who were classified as AKIN stage 1 at collection (n=79). ROC, receiver operator characteristic; uAnCr, urine angiotensinogen/creatinine ratio; AUC, area under the curve.
In addition to the prediction of the renal and mortality outcomes, we noted a relationship between uAnCR and length of hospital stay. Among all AKI patients and in the subset of patients classified as AKIN stage 1 at the time of collection, those patients with higher uAnCR concentrations had longer hospital stays (Figure 3, A and B). ROC curve analysis indicated that elevated uAnCR was predictive of longer length of stay defined as discharge >7 days from the time of sample collection or death (Supplemental Figures 2 and 3). Tables 3 and 4 summarize the performance characteristics of uAnCR as a predictor of the tested outcomes in patients who had AKI of any stage at the time of sample collection and those who had not progressed beyond AKIN stage 1 at the time of sample collection.
Figure 3.

Angiotensinogen is associated with increased length of stay in patients with postoperative AKI. Patients who developed AKI after cardiac surgery were stratified into tertiles by uAnCR. Kaplan–Meier survival curves show that (A) among patients with AKI of any severity at the time of collection and (B) among patients with AKIN stage 1 at the time of collection, those who have higher uAnCR have increased length of stay (defined as days to discharge from time of sample collection). *P<0.05 compared with the low uAnCR group. uAnCr, urine angiotensinogen/creatinine ratio.
Table 3.
Performance characteristics of uAnCR as a prognostic AKI biomarker among patients who were any stage AKI at the time of sample collection (n=97)
| Outcome | AUC | Cut-Off (ng/mg) | n (%)a | Sensitivity (%) | Specificity (%) | LR+ | LR− | PPV (%) | NPV (%) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Worsening AKI | 0.70 (0.57–0.82) | Best | >33.27 | 43 (44.3) | 70.8 | 66.2 | 2.09 | 0.44 | 67.7 | 69.4 |
| Max PPV | >392.5 | 6 (6.2) | 16.7 | 98.5 | 11.34 | 0.85 | 91.9 | 54.2 | ||
| Max NPV | >12.55 | 20 (20.6) | 91.7 | 26.5 | 1.25 | 0.31 | 55.5 | 76.1 | ||
| AKIN stage 3 | 0.71 (0.59–0.84) | Best | >34.33 | 40 (41.2) | 68.4 | 65.4 | 1.98 | 0.48 | 66.4 | 67.4 |
| Max PPV | >572.0 | 4 (4.1) | 15.8 | 98.7 | 12.34 | 0.85 | 92.5 | 54.0 | ||
| Max NPV | >14.06 | 25 (25.8) | 94.7 | 30.8 | 1.37 | 0.17 | 57.8 | 85.4 | ||
| RRTb | 0.71 (0.54–0.88) | Best | >58.63 | 26 (26.8) | 66.7 | 77.3 | 2.93 | 0.43 | 74.6 | 69.9 |
| Max PPV | >572.0 | 4 (4.1) | 11.1 | 96.6 | 3.26 | 0.92 | 76.5 | 52.1 | ||
| Max NPV | >20.01 | 37 (38.1) | 88.9 | 40.9 | 1.50 | 0.27 | 60.1 | 78.6 | ||
| AKIN stage 2 or 3 AKI | 0.64 (0.52–0.73) | Best | >34.33 | 40 (41.2) | 57.9 | 69.5 | 1.90 | 0.61 | 65.5 | 62.3 |
| Max PPV | >392.5 | 6 (6.2) | 13.2 | 98.3 | 7.79 | 0.88 | 88.6 | 53.1 | ||
| Max NPV | >6.777 | 7 (7.2) | 97.4 | 10.2 | 1.08 | 0.26 | 52.0 | 79.5 | ||
| AKIN 3 or deathc | 0.75 (0.64–0.87) | Best | >37.36 | 35 (36.1) | 66.7 | 72.4 | 2.41 | 0.46 | 70.7 | 68.5 |
| Max PPV | >392.5 | 6 (6.2) | 23.8 | 98.7 | 18.04 | 0.77 | 94.7 | 56.4 | ||
| Max NPV | >14.06 | 25 (25.8) | 95.2 | 31.6 | 1.39 | 0.15 | 58.2 | 86.9 | ||
| RRT or death | 0.71 (0.55–0.86) | Best | >58.63 | 26 (26.8) | 61.5 | 78.6 | 2.87 | 0.49 | 74.2 | 67.1 |
| Max PPV | >466.6 | 5 (5.2) | 23.1 | 97.6 | 9.70 | 0.79 | 90.7 | 55.9 | ||
| Max NPV | >16.28 | 31 (32) | 92.3 | 35.7 | 1.44 | 0.22 | 58.9 | 82.3 | ||
| Length of stayd | 0.74 (0.64–0.84) | Best | >26.38 | 52 (54.6) | 69.6 | 68.3 | 2.19 | 0.44 | 68.7 | 69.2 |
| Max PPV | >67.97 | 24 (24.7) | 37.5 | 92.7 | 5.12 | 0.67 | 83.7 | 59.7 | ||
| Max NPV | <13.78 | 24 (24.8) | 89.3 | 43.9 | 1.59 | 0.24 | 61.4 | 80.3 | ||
uAnCr, urine angiotensinogen/creatinine ratio; AUC, area under the curve; LR+, positive likelihood ratio; LR−, negative likelihood ratio; PPV, positive predictive value; NPV, negative predictive value; Max, maximum; RRT, renal replacement therapy.
The percentage and number of patients who were above the best and max PPV cut-offs or below the max NPV cut-off.
RRT initiated within 10 days of surgery.
Death defined as 30 day in-hospital mortality.
Length of stay outcome defined as discharge >7 days from sample collection or death before postoperative day 7.
Table 4.
Performance characteristics of uAnCR as a prognostic AKI biomarker among patients who were classified as AKIN stage 1 at the time of sample collection (n=79)
| Outcome | AUC | Cut-Off (ng/mg) | n (%)a | Sensitivity (%) | Specificity (%) | LR+ | LR− | PPV (%) | NPV (%) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Worsening AKI | 0.71 (0.57–0.85) | Best | >33.27 | 35 (44.3) | 75.0 | 66.1 | 2.21 | 0.38 | 68.9 | 72.6 |
| Max PPV | >392.5 | 5 (6.3) | 20.0 | 98.3 | 11.83 | 0.81 | 92.2 | 55.1 | ||
| Max NPV | >19.95 | 29 (36.7) | 85.0 | 44.1 | 1.52 | 0.34 | 60.3 | 74.6 | ||
| AKIN stage 3 AKI | 0.75 (0.58–0.92) | Best | >58.63 | 22 (27.8) | 70.0 | 78.3 | 3.22 | 0.38 | 76.3 | 72.3 |
| Max PPV | >572.0 | 3 (3.8) | 20.0 | 98.6 | 13.79 | 0.81 | 93.2 | 55.2 | ||
| Max NPV | >19.95 | 29 (36.7) | 90.0 | 40.6 | 1.51 | 0.25 | 60.2 | 80.2 | ||
| RRTb | 0.68 (0.49–0.87) | Best | >34.33 | 32 (40.5) | 75.0 | 63.4 | 2.05 | 0.39 | 67.2 | 71.7 |
| Max PPV | >572.0 | 3 (3.8) | 12.5 | 97.2 | 4.43 | 0.90 | 81.6 | 52.6 | ||
| Max NPV | >19.95 | 29 (36.7) | 87.5 | 39.4 | 1.44 | 0.32 | 59.1 | 75.9 | ||
| AKIN 3 or deathc | 0.81 (0.66–0.95) | Best | >58.63 | 22 (27.8) | 75.0 | 80.6 | 3.87 | 0.31 | 79.4 | 76.3 |
| Max PPV | >392.5 | 5 (6.3) | 33.3 | 98.5 | 22.37 | 0.68 | 95.7 | 59.6 | ||
| Max NPV | >19.95 | 29 (36.7) | 91.7 | 41.8 | 1.57 | 0.20 | 61.2 | 83.4 | ||
| RRT or death | 0.76 (0.59–0.93) | Best | >58.63 | 22 (27.8) | 70.0 | 78.3 | 3.22 | 0.38 | 76.3 | 72.3 |
| Max PPV | >466.6 | 4 (5.1) | 30.0 | 98.6 | 20.69 | 0.71 | 95.4 | 58.5 | ||
| Max NPV | >19.95 | 29 (36.7) | 90.0 | 40.6 | 1.51 | 0.25 | 60.2 | 80.2 | ||
| Length of stayd | 0.74 (0.63–0.85) | Best | >26.38 | 43 (54.4) | 72.7 | 68.6 | 2.31 | 0.40 | 69.8 | 71.5 |
| Max PPV | >109.0 | 13 (16.5) | 27.3 | 97.1 | 9.5 | 0.75 | 90.5 | 57.1 | ||
| Max NPV | >13.78 | 59 (74.5) | 89.3 | 43.9 | 1.59 | 0.24 | 61.4 | 80.3 | ||
uAnCr, urine angiotensinogen/creatinine ratio; AKIN, Acute Kidney Injury Network; AUC, area under the curve; LR+, positive likelihood ratio; LR−, negative likelihood ratio; PPV, positive predictive value; NPV, negative predictive value; Max, maximum; RRT, renal replacement therapy.
The percentage and number of patients who were above the best and max PPV cut-offs or below the max NPV cut-off.
RRT initiated within 10 days of surgery.
Death defined as 30 day in-hospital mortality.
Length of stay outcome defined as discharge >7 days from sample collection or death before postoperative day 7.
We determined the ability of uAnCR to improve the prediction of worsening AKI of a clinical risk model in both the entire cohort and in the subset of patients classified as AKIN stage 1 at the time of urine collection. The clinical model was a multivariate logistic regression model consisting of the percent change in SCr from baseline at the time of sample collection and the patient’s Cleveland Clinic score, a perioperative risk score that predicts AKI severity after cardiac surgery (27,28). When uAnCR was added to the clinical model, we found that it predicted worsening of AKI independently of the percent change in SCr and the Cleveland Clinic score (P=0.02). We used cfNRI, which compares each patient’s calculated risk for an outcome using a reference model to a new model, to capture the added benefit of including uAnCR in the model. Addition of uAnCR in the new model improved the ability to predict a patients risk of worsening of AKI in both the entire cohort and the subset of patients who were classified as AKIN stage 1 (cfNRI=45.7% and 42.8%, respectively). To visualize the improvement in prediction, we constructed a risk assessment plot, as proposed by Pickering and Endre (26). This plot compares the sensitivity and 1-specificity of the reference and new models across the spectrum of calculated risk for each model. Figure 4 shows that the addition of uAnCR into the model resulted in patients who met the outcome (events) having a greater calculated risk, and patients who did not meet the outcome (nonevents) had a lower calculated risk. Therefore, both sensitivity and specificity were improved by including uAnCR in the prediction model.
Figure 4.

Risk assessment plots showing the improved prediction of worsening AKI when uAnCR is included in the model. Results from both (A) the entire cohort and (B) the subset of patients who were classified as AKIN stage 1 at the time of sample collection are shown. Of 97 patients in the whole cohort (A), 39 patients met the outcome worsening of AKI after sample collection, whereas in the subset analysis, 20 patients met the outcome. Two multivariate logistic regression models were created to predict risk of worsening of AKI after sample collection. The first model (reference) used percent change in SCr from baseline and Cleveland Clinic score created by Thakar et al. The second model included these variables plus uAnCR. Each patient’s probability (i.e., risk) of meeting the outcome worsening of AKI was calculated with both models. The sensitivity (proportion of events with a calculated risk equal to or above the defined threshold) and 1-specificity (proportion of nonevents with a calculated risk below the defined threshold) was calculated across all possible unique thresholds using both models. uAnCr, urine angiotensinogen/creatinine ratio.
Discussion
We identified candidate biomarkers for the prediction of the development of severe AKI, and the prognostic potential of the most promising candidate, angiotensinogen, was verified in a larger set of patients who developed AKI after cardiac surgery. We found that uAnCR was elevated in patients who developed more severe AKI after sample collection. Elevated uAnCR was associated with worsening of AKI, independent of changes in SCr and Cleveland Clinic score, and it was also associated with several secondary outcomes. The prognostic predictive power of uAnCR was improved when only patients who were classified as AKIN stage 1 at the time of sample collection were used in the analysis, indicating that angiotensinogen could be used to predict adverse outcomes among patients who have not yet developed severe AKI as measured by serum creatinine.
Our data suggest that angiotensinogen could be used at the time of diagnosis with AKI to assess the risk of adverse outcomes. This risk assessment could lead to improved outcomes by identifying high-risk patients in need of therapeutic intervention as was highlighted in the Kidney Disease Improving Global Outcomes (KDIGO) Clinical Practice Guideline for Acute Kidney Injury (29). The guidelines suggest several interventions in patients with stage 2 and 3 AKI that are not recommended for patients with stage 1 AKI, including checking drug dosing, considering RRT, and considering intensive care unit (ICU) admission. Elevation in uAnCR could suggest a population of patients with stage 1 AKI who are likely to worsen and could benefit from more intensive intervention.
Although we did not directly compare the prognostic predictive power of angiotensinogen to that of other biomarkers, our results are comparable with what has been reported in the literature for previously described AKI biomarkers. For example, Hall et al. reported unadjusted AUCs of 0.71, 0.64, and 0.63 for the prediction of the composite outcome of worsening of AKI or death for urine NGAL, kidney injury molecule-1, and IL-18, respectively (18). Koyner recently reported unadjusted AUCs of 0.58, 0.63, and 0.74 for urine NGAL, urine IL-18, and plasma NGAL, respectively, for the outcome of worsening of AKI (19). Thus, uAnCR, alone or in combination with other biomarkers could improve risk classification models in these patients.
Angiotensinogen is the principal substrate of the renin-angiotensin system (RAS), a hormonal cascade that has pleiotropic effects in the kidney, including the regulation of hemodynamics, sodium reabsorption, aquaresis, cellular proliferation and apoptosis, fibrosis, and inflammation (30). Our findings regarding the prognostic utility of angiotensinogen as a biomarker of AKI suggest that the RAS could be mechanistically involved in AKI. This is supported by observational studies that have noted an association between pharmacologic inhibition of the RAS and risk of developing AKI, although there are conflicting reports in the literature (31–34). The ACE II genotype has been associated with increased risk of AKI in the ICU (35). It is unclear whether the elevated levels of urinary angiotensinogen observed in severe AKI reflect cleavage of existing angiotensinogen into angiotensin I and subsequent bioactive molecules in the RAS cascade. The identified portions of angiotensinogen in our proteomic study did not include the proximal domain of angiotensinogen from which angiotensin I is cleaved (Supplemental Figures 4 and 5). Likewise, the ELISA used to quantify angiotensinogen recognizes an epitope distal to the angiotensin I domain, so it is insensitive to the detection of cleavage of angiotensinogen by renin. Also unclear is whether increases in angiotensinogen are systemic or intrarenal in nature. However, others have shown that intrarenal angiotensin II is increased after renal ischemia-reperfusion injury in a rat model (36). In order to understand the relevance of urinary angiotensinogen in the pathobiology of AKI, it will be necessary to determine the status of the renin-angiotensinogen system during AKI.
Disclosures
None.
Acknowledgments
Additional members of the SAKInet consortium (www.sakinet.org) include Juan Carlos Q. Velez, Elizabeth G. Hill, Milos N. Budisavljevic, Rick G. Schnellmann of the Medical University of South Carolina, and Frederick T. (Josh) Billings of Vanderbilt University.
This study was supported by the National Institutes of Health (Grants R01 DK080234 and UL1 RR029882) and by a Merit Review award from the Biomedical Laboratory Research and Development Program of the Department of Veterans Affairs. M.J. was supported by the Nephcure Foundation as a young investigator. The contents do not necessarily represent the views of the Department of Veterans Affairs or the US Government.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.06280612/-/DCSupplemental.
References
- 1.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P, Acute Dialysis Quality Initiative workgroup : Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: The Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 8: R204–R212, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A, Acute Kidney Injury Network : Acute Kidney Injury Network: Report of an initiative to improve outcomes in acute kidney injury. Crit Care 11: R31, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Han WK, Bailly V, Abichandani R, Thadhani R, Bonventre JV: Kidney Injury Molecule-1 (KIM-1): A novel biomarker for human renal proximal tubule injury. Kidney Int 62: 237–244, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Mishra J, Ma Q, Prada A, Mitsnefes M, Zahedi K, Yang J, Barasch J, Devarajan P: Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol 14: 2534–2543, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, Ruff SM, Zahedi K, Shao M, Bean J, Mori K, Barasch J, Devarajan P: Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet 365: 1231–1238, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Melnikov VY, Ecder T, Fantuzzi G, Siegmund B, Lucia MS, Dinarello CA, Schrier RW, Edelstein CL: Impaired IL-18 processing protects caspase-1-deficient mice from ischemic acute renal failure. J Clin Invest 107: 1145–1152, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parikh CR, Jani A, Melnikov VY, Faubel S, Edelstein CL: Urinary interleukin-18 is a marker of human acute tubular necrosis. Am J Kidney Dis 43: 405–414, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Herget-Rosenthal S, Marggraf G, Hüsing J, Göring F, Pietruck F, Janssen O, Philipp T, Kribben A: Early detection of acute renal failure by serum cystatin C. Kidney Int 66: 1115–1122, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Portilla D, Dent C, Sugaya T, Nagothu KK, Kundi I, Moore P, Noiri E, Devarajan P: Liver fatty acid-binding protein as a biomarker of acute kidney injury after cardiac surgery. Kidney Int 73: 465–472, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Liangos O, Tighiouart H, Perianayagam MC, Kolyada A, Han WK, Wald R, Bonventre JV, Jaber BL: Comparative analysis of urinary biomarkers for early detection of acute kidney injury following cardiopulmonary bypass. Biomarkers 14: 423–431, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koyner JL, Vaidya VS, Bennett MR, Ma Q, Worcester E, Akhter SA, Raman J, Jeevanandam V, O’Connor MF, Devarajan P, Bonventre JV, Murray PT: Urinary biomarkers in the clinical prognosis and early detection of acute kidney injury. Clin J Am Soc Nephrol 5: 2154–2165, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wagener G, Gubitosa G, Wang S, Borregaard N, Kim M, Lee HT: Urinary neutrophil gelatinase-associated lipocalin and acute kidney injury after cardiac surgery. Am J Kidney Dis 52: 425–433, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Parikh CR, Mishra J, Thiessen-Philbrook H, Dursun B, Ma Q, Kelly C, Dent C, Devarajan P, Edelstein CL: Urinary IL-18 is an early predictive biomarker of acute kidney injury after cardiac surgery. Kidney Int 70: 199–203, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Haase M, Bellomo R, Story D, Davenport P, Haase-Fielitz A: Urinary interleukin-18 does not predict acute kidney injury after adult cardiac surgery: A prospective observational cohort study. Crit Care 12: R96, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koyner JL, Bennett MR, Worcester EM, Ma Q, Raman J, Jeevanandam V, Kasza KE, O’Connor MF, Konczal DJ, Trevino S, Devarajan P, Murray PT: Urinary cystatin C as an early biomarker of acute kidney injury following adult cardiothoracic surgery. Kidney Int 74: 1059–1069, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parikh CR, Devarajan P, Zappitelli M, Sint K, Thiessen-Philbrook H, Li S, Kim RW, Koyner JL, Coca SG, Edelstein CL, Shlipak MG, Garg AX, Krawczeski CD, TRIBE-AKI Consortium : Postoperative biomarkers predict acute kidney injury and poor outcomes after pediatric cardiac surgery. J Am Soc Nephrol 22: 1737–1747, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parikh CR, Coca SG, Thiessen-Philbrook H, Shlipak MG, Koyner JL, Wang Z, Edelstein CL, Devarajan P, Patel UD, Zappitelli M, Krawczeski CD, Passik CS, Swaminathan M, Garg AX, TRIBE-AKI Consortium : Postoperative biomarkers predict acute kidney injury and poor outcomes after adult cardiac surgery. J Am Soc Nephrol 22: 1748–1757, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hall IE, Coca SG, Perazella MA, Eko UU, Luciano RL, Peter PR, Han WK, Parikh CR: Risk of poor outcomes with novel and traditional biomarkers at clinical AKI diagnosis. Clin J Am Soc Nephrol 6: 2740–2749, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koyner JL, Garg AX, Coca SG, Sint K, Thiessen-Philbrook H, Patel UD, Shlipak MG, Parikh CR, TRIBE-AKI Consortium : Biomarkers predict progression of acute kidney injury after cardiac surgery. J Am Soc Nephrol 23: 905–914, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Englberger L, Suri RM, Li Z, Casey ET, Daly RC, Dearani JA, Schaff HV: Clinical accuracy of RIFLE and Acute Kidney Injury Network (AKIN) criteria for acute kidney injury in patients undergoing cardiac surgery. Crit Care 15: R16, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keller A, Nesvizhskii AI, Kolker E, Aebersold R: Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal Chem 74: 5383–5392, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Nesvizhskii AI, Keller A, Kolker E, Aebersold R: A statistical model for identifying proteins by tandem mass spectrometry. Anal Chem 75: 4646–4658, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Dakna M, Harris K, Kalousis A, Carpentier S, Kolch W, Schanstra JP, Haubitz M, Vlahou A, Mischak H, Girolami M: Addressing the challenge of defining valid proteomic biomarkers and classifiers. BMC Bioinformatics 11: 594, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pepe M: The Statistical Evaluation of Medical Tests for Classification and Prediction, New York, Oxford University Press, 2004 [Google Scholar]
- 25.Pencina MJ, D'Agostino RB Sr, D'Agostino RB Jr, Vasan RS: Evaluating the added predictive ability of a new marker: From area under the ROC curve to reclassification and beyond. Stat Med 27: 157–172, discussion 207–212, 2008 [DOI] [PubMed]
- 26.Pickering JW, Endre ZH: New metrics for assessing diagnostic potential of candidate biomarkers. Clin J Am Soc Nephrol 7: 1355–1364, 2012 [DOI] [PubMed] [Google Scholar]
- 27.Thakar CV, Arrigain S, Worley S, Yared JP, Paganini EP: A clinical score to predict acute renal failure after cardiac surgery. J Am Soc Nephrol 16: 162–168, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Englberger L, Suri RM, Li Z, Dearani JA, Park SJ, Sundt TM, 3rd, Schaff HV: Validation of clinical scores predicting severe acute kidney injury after cardiac surgery. Am J Kidney Dis 56: 623–631, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Kidney Disease. Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group : Clinical practice guideline for acute kidney injury. Kidney Int Suppl 2: 1–138, 2012 [Google Scholar]
- 30.Velez JC: The importance of the intrarenal renin-angiotensin system. Nat Clin Pract Nephrol 5: 89–100, 2009 [DOI] [PubMed] [Google Scholar]
- 31.Arora P, Rajagopalam S, Ranjan R, Kolli H, Singh M, Venuto R, Lohr J: Preoperative use of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers is associated with increased risk for acute kidney injury after cardiovascular surgery. Clin J Am Soc Nephrol 3: 1266–1273, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Benedetto U, Sciarretta S, Roscitano A, Fiorani B, Refice S, Angeloni E, Sinatra R: Preoperative Angiotensin-converting enzyme inhibitors and acute kidney injury after coronary artery bypass grafting. Ann Thorac Surg 86: 1160–1165, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Plataki M, Kashani K, Cabello-Garza J, Maldonado F, Kashyap R, Kor DJ, Gajic O, Cartin-Ceba R: Predictors of acute kidney injury in septic shock patients: An observational cohort study. Clin J Am Soc Nephrol 6: 1744–1751, 2011 [DOI] [PubMed] [Google Scholar]
- 34.Yoo YC, Youn YN, Shim JK, Kim JC, Kim NY, Kwak YL: Effects of renin-angiotensin system inhibitors on the occurrence of acute kidney injury following off-pump coronary artery bypass grafting. Circ J 74: 1852–1858, 2010 [DOI] [PubMed] [Google Scholar]
- 35.du Cheyron D, Fradin S, Ramakers M, Terzi N, Guillotin D, Bouchet B, Daubin C, Charbonneau P: Angiotensin converting enzyme insertion/deletion genetic polymorphism: Its impact on renal function in critically ill patients. Crit Care Med 36: 3178–3183, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Allred AJ, Chappell MC, Ferrario CM, Diz DI: Differential actions of renal ischemic injury on the intrarenal angiotensin system. Am J Physiol Renal Physiol 279: F636–F645, 2000 [DOI] [PubMed] [Google Scholar]



