Summary
Background and objectives
The microvascular circulation plays an important role in bone health. This study examines whether albuminuria, a marker of renal microvascular disease, is associated with incident hip and pelvic fractures.
Design, setting, participants, & measurements
This study reanalyzed data from the Ongoing Telmisartan Alone and in combination with Ramipril Global End Point Trial/Telmisartan Randomized Assessment Study in Angiotensin-Converting Enzyme Intolerant Subjects with Cardiovascular Disease trials, which examined the impact of renin angiotensin system blockade on cardiovascular outcomes (n=28,601). Albuminuria was defined as an albumin-to-creatinine ratio≥30 mg/g (n=4597). Cox proportional hazards models were used to determine the association of albuminuria with fracture risk adjusted for known risk factors for fractures, estimated GFR, and rapid decline in estimated GFR (≥5%/yr).
Results
There were 276 hip and pelvic fractures during a mean of 4.6 years of follow-up. Participants with baseline albuminuria had a significantly increased risk of fracture compared with participants without albuminuria (unadjusted hazard ratio=1.62 [1.22, 2.15], P<0.001; adjusted hazard ratio=1.36 [1.01, 1.84], P=0.05). A dose-dependent relationship was observed, with macroalbuminuria having a large fracture risk (unadjusted hazard ratio=2.01 [1.21, 3.35], P=0.007; adjusted hazard ratio=1.71 [1.007, 2.91], P=0.05) and microalbuminuria associating with borderline or no statistical significance (unadjusted hazard ratio=1.52 [1.10, 2.09], P=0.01; adjusted hazard ratio=1.28 [0.92, 1.78], P=0.15). Estimated GFR was not a predictor of fracture in any model, but rapid loss of estimated GFR over the first 2 years of follow-up predicted subsequent fracture (adjusted hazard ratio=1.47 [1.05, 2.04], P=0.02).
Conclusions
Albuminuria, especially macroalbuminuria, and rapid decline of estimated GFR predict hip and pelvic fractures. These findings support a theoretical model of a relationship between underlying causes of microalbuminuria and bone disease.
Introduction
The microcirculation plays an important role in the maintenance of bone physiology in both embryogenesis, where new blood vessel formation precedes osteogenesis (1), and adult bone formation, where endothelial cells from capillaries at sites of bone remodeling release factors associated with mineralization and the coupling of osteoclast and osteoblast function (2). Bone perfusion is perturbed in avascular necrosis and Paget’s disease, and it is diminished in osteoporotic hip fracture specimens compared with those specimens from people having hip replacement surgery for arthritis (3).
The prevalence of microvascular disease (e.g., retinal arteriolar narrowing and lacunar infarcts) increases with age. Albuminuria—the excessive excretion of albumin (>30 mg/g creatinine) in the urine—is, in many people, a reflection of microvascular disease, affecting ∼15% of people 60–69 years of age and ∼20% of people older than 69 years. In people with diabetes, the prevalence of albuminuria is even higher: ∼37% and ∼42%, respectively (4). Albuminuria is a marker of endothelial dysfunction, and it is associated with increased levels of markers of inflammation (5). There is little previous work on the association of albuminuria with osteoporotic fracture risk. One study identified a relationship between albuminuria and nonvertebral fractures in a general population sample (6). However, no study has examined the association of albuminuria or changes in albuminuria with osteoporotic fracture risk and adjusted the findings for estimated GFR (eGFR) or the effect of changes in eGFR over time.
In the present study, we examine whether albuminuria, eGFR, change in albuminuria, and change in eGFR are associated with an increased risk of hip and pelvic fractures over 4.6 years of follow-up in participants in the Ongoing Telmisartan Alone and in combination with Ramipril Global End Point Trial (ONTARGET) and the Telmisartan Randomized Assessment Study in Angiotensin-Converting Enzyme (ACE) Intolerant Subjects with Cardiovascular Disease (TRANSCEND) studies of people at high risk for vascular disease.
Materials and Methods
We used data prospectively collected in the ONTARGET and TRANSCEND trials. The ONTARGET trial (7) was a double-blind randomized cardiovascular outcome study of 25,620 participants age≥55 years with vascular disease or diabetes mellitus randomly assigned to the angiotensin receptor blocker telmisartan (80 mg/d), the ACE inhibitor ramipril (10 mg/d), or a combination. The two medications led to equivalent cardiovascular and renal outcomes, but their combination gave no additional benefit overall (8). The parallel TRANSCEND trial (9) compared telmisartan with placebo in 5926 people who were intolerant of ACE inhibitors, and it showed no statistically significant effect on the primary outcome (cardiovascular death, myocardial infarction, stroke, or hospitalization for heart failure) but a reduction in the risk of the secondary composite: cardiovascular death, myocardial infarction, and stroke (10). All participants signed informed consent on study entry. Each study site had institutional review board (IRB) approval. Sites without an IRB were covered under the McMaster University IRB. The studies were conducted in accordance with the Declaration of Helsinki.
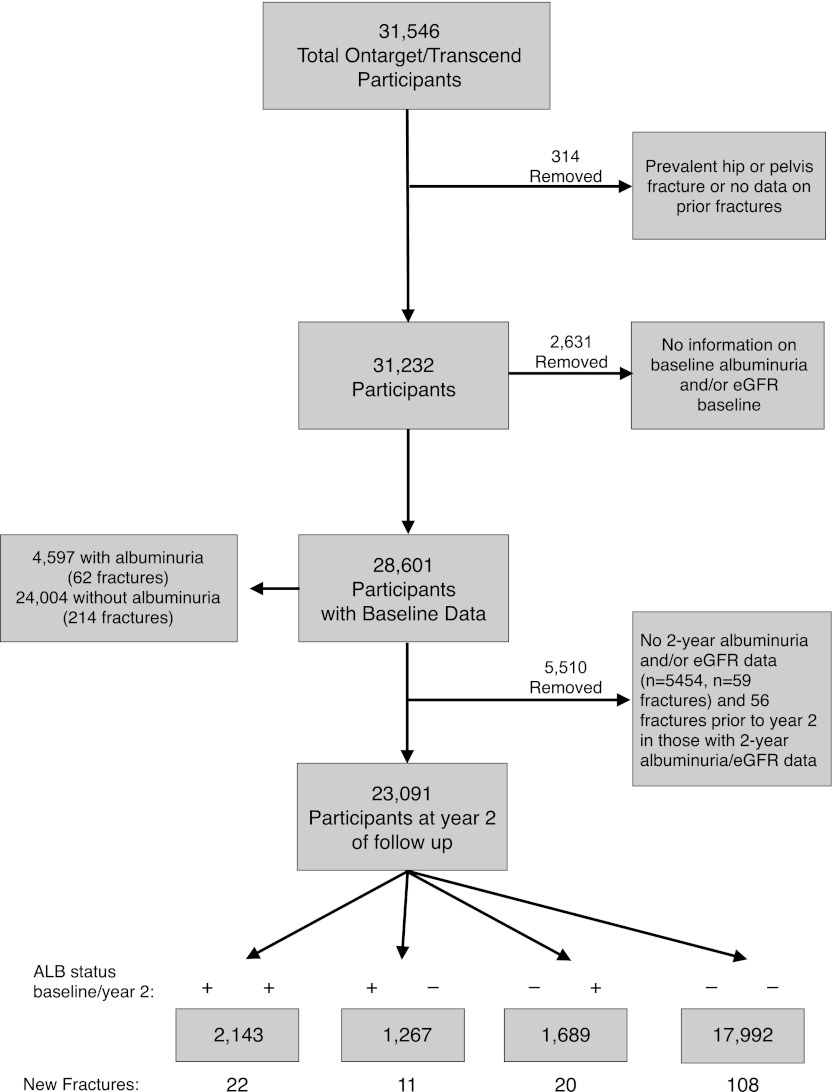
For the current analyses of incident fracture, we excluded 314 participants with a history of hip or pelvic fracture before enrollment or unknown fracture history. An additional 2631 participants were excluded who did not have data on baseline albuminuria or eGFR. These exclusions left 28,601 participants for analysis (Figure 1). Urine albumin and creatinine were measured centrally from first morning urine. eGFR was derived using the Modification of Diet in Renal Disease Equation (11) on uncalibrated, locally measured serum creatinine. Changes in eGFR per year during the study period were estimated using within-patient linear regression. A rapid decline in eGFR was defined as a decrease in eGFR (negative slope) of ≥5% per year (12) over the period baseline to 2 years.
Figure 1.
Flow chart of Ongoing Telmisartan Alone and in combination with Ramipril Global End Point Trial (ONTARGET) and the Telmisartan Randomized Assessment Study in Angiotensin-Converting Enzyme Intolerant Subjects with Cardiovascular Disease (TRANSCEND) participants showing albuminuria (ALB) status and the number of hip and pelvis fractures. ALB is defined as an albumin-to-creatinine ratio≥30 mg/g.
Subjects were asked at baseline whether they had fallen in the preceding year. At baseline, 2-year, and penultimate visits, participants were asked whether they had sustained a fracture in the preceding 2 years (ribs, spine, wrist, hip, and pelvis). Those fractures that required hospitalization were reported on the hospitalization record form. We analyzed the first occurrence of hip or pelvis fracture.
Baseline characteristics categorized by baseline albuminuria status were compared using standard statistical methods. The primary analyses used a time-to-event approach. We examined them for the risk of the first occurrence of hip and pelvic fractures in participants with or without albuminuria at baseline. Albuminuria was further classified as microalbuminuria (30–299 mg/g creatinine) or macroalbuminuria (>299 mg/g creatinine). We also examined the risk of fracture from year 2 for an additional 2.6 years of follow-up among participants with or without albuminuria at baseline who also had an albuminuria and creatinine value at year 2 of follow-up. We examined the risk of fracture in people with persistent albuminuria versus people who regressed, progressed, or remained without albuminuria, and this information also permitted the examination of the effect of rapid decline in eGFR. Risk comparisons are displayed as hazard ratios (HRs) with 95% confidence intervals (CIs). Adjustments were made in nested blocks as follows: Model 1, unadjusted; Model 2 (demographic adjusted), adjusted for age, sex, and ethnicity; and Model 3 (osteoporosis adjusted), adjusted for factors in model 2, factors associated with risk of osteoporosis [prevalent cardiovascular disease (13), smoking, body mass index (BMI), alcohol use, and low level of physical activity], and baseline eGFR. We also included terms for treatment (ACE inhibitor, angiotensin receptor blocker, or both). We tested for interaction between albuminuria and age (categorized as people less than 70 years of age versus people 70 years of age and older), diabetes, and sex. Population attributable risk for this high-risk vascular population was calculated as
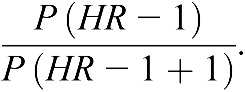 |
P is the prevalence of albuminuria. SAS, version 9.1 was used for analyses.
Results
Baseline characteristics of the participants are shown in Table 1. Of the 28,601 participants who were examined, 4597 (16.1%) had albuminuria. Those participants with albuminuria were older, more likely to be of non-European ancestry, more likely to be current smokers, more likely to have diabetes, more likely to have baseline coronary artery disease, stroke, or peripheral arterial disease, and more likely to have lower eGFR, than those participants without albuminuria. Participants with albuminuria also had a higher proportion with a rapid decline in eGFR (≥5% per year) from baseline to year 2 of follow-up. Mean eGFR in both groups at baseline was >60 ml/min.
Table 1.
Baseline characteristics of ONTARGET and TRANSCEND participants, without a history of hip or pelvic fractures before study entry, stratified by the presence or absence of albuminuria (≥30 mg/g creatinine)
| Overall | Albuminuria− | Albuminuria+ | P value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | n | Percent | N | n | Percent | N | n | Percent | ||
| Patients | 28,601 | 28,601 | 100.0 | 24,004 | 24,004 | 100.0 | 4597 | 4597 | 100.0 | |
| Male | 28,601 | 20,192 | 70.6 | 24,004 | 16,986 | 70.8 | 4597 | 3206 | 69.7 | 0.16 |
| Age<65 yr | 28,601 | 12,116 | 42.4 | 24,004 | 10,396 | 43.3 | 4597 | 1720 | 37.4 | 0.000 |
| Age=65–74 yr | 28,601 | 12,195 | 42.6 | 24,004 | 10,164 | 42.3 | 4597 | 2031 | 44.2 | 0.02 |
| Age≥75 yr | 28,601 | 4290 | 15.0 | 24,004 | 3444 | 14.3 | 4597 | 846 | 18.4 | 0.000 |
| History of diabetes | 28,601 | 10,602 | 37.1 | 24,004 | 7695 | 32.1 | 4597 | 2907 | 63.2 | 0.000 |
| Asian | 28,596 | 4459 | 15.6 | 23,999 | 3628 | 15.1 | 4597 | 831 | 18.1 | 0.000 |
| Arab | 28,596 | 269 | 0.9 | 23,999 | 182 | 0.8 | 4597 | 87 | 1.9 | 0.000 |
| African | 28,596 | 693 | 2.4 | 23,999 | 503 | 2.1 | 4597 | 190 | 4.1 | 0.000 |
| European | 28,596 | 20,401 | 71.3 | 23,999 | 17,413 | 72.6 | 4597 | 2988 | 65.0 | 0.000 |
| Native/aboriginal | 28,596 | 2514 | 8.8 | 23,999 | 2062 | 8.6 | 4597 | 452 | 9.8 | 0.007 |
| Other | 28,596 | 260 | 0.9 | 23,999 | 211 | 0.9 | 4597 | 49 | 1.1 | 0.22 |
| Never smoked | 28,565 | 10,606 | 37.1 | 23,973 | 8884 | 37.1 | 4592 | 1722 | 37.5 | 0.57 |
| Former smoker | 28,565 | 14,488 | 50.7 | 23,973 | 12,236 | 51.0 | 4592 | 2252 | 49.0 | 0.01 |
| Current smoker | 28,565 | 3471 | 12.2 | 23,973 | 2853 | 11.9 | 4592 | 618 | 13.5 | 0.003 |
| BL eGFR<30 | 28,601 | 177 | 0.6 | 24,004 | 81 | 0.3 | 4597 | 96 | 2.1 | 0.000 |
| BL eGFR≥30 and <45 | 28,601 | 1436 | 5.0 | 24,004 | 917 | 3.8 | 4597 | 519 | 11.3 | 0.000 |
| BL eGFR≥45 and <60 | 28,601 | 5425 | 19.0 | 24,004 | 4305 | 17.9 | 4597 | 1120 | 24.4 | 0.000 |
| BL eGFR≥60 | 28,601 | 21,563 | 75.4 | 24,004 | 18,701 | 77.9 | 4597 | 2862 | 62.3 | 0.000 |
| eGFR decrease from baseline to year 2≥5%/yr | 25,301 | 9310 | 36.8 | 21,478 | 7582 | 35.3 | 3823 | 1728 | 45.2 | 0.000 |
| CVD baseline | 28,601 | 26,138 | 91.4 | 24,004 | 22,275 | 92.8 | 4597 | 3863 | 84.0 | 0.000 |
All participants had baseline urinary albumin levels and baseline and 2-year follow-up estimated GFR values. Mean age (years): albuminuria−, 66.34±7.16; albuminuria+, 67.40±7.43, P<0.001. Overall=66.51±7.21. Baseline eGFR (ml/min): albuminuria−, 74.11±18.86; albuminuria+, 68.68±22.71, P<0.001. Overall=73.24±19.63. BMI (kg/m2): albuminuria−, 27.98±4.46; albuminuria+, 28.73±4.90, P<0.001. Overall=28.10±4.54. Percent decrease in eGFR (ml/min per 1.73m2) over 2 years: albuminuria−, 1%±23%; albuminuria+, 6%±24% (P<0.001 compared with albuminuria−). Overall=2%±23%. ONTARGET, Ongoing Telmisartan Alone and in combination with Ramipril Global End Point Trial; TRANSCEND, Telmisartan Randomized Assessment Study in ACE Intolerant Subjects with Cardiovascular Disease; BL, baseline; eGFR, estimated GFR; CVD, coronary artery disease, stroke, or peripheral arterial disease; BMI, body mass index.
Over 4.6 years of follow-up, 276 participants (124 men and 152 women) experienced a first hip or pelvic fracture. Among those participants with albuminuria (62 fractures), the rate of first fracture was 0.31/100 person-years, whereas among those participants without albuminuria (214 fractures), the rate was 0.19/100 person-years. The unadjusted HR for the association between albuminuria and fracture was 1.62 (95% CI=1.22, 2.15; P<0.001) (Table 2). Adjustment for age, sex, and ethnicity somewhat attenuated the risk (1.48 [1.12, 1.97]; P=0.006). With additional adjustment for BMI, history of coronary artery disease, stroke, or peripheral arterial disease, smoking status, baseline eGFR, alcohol use, and physical activity, the risk estimate was attenuated but remained statistically significant (1.35 [1.00, 1.82]; P=0.05). Increasing age, female sex, a history of diabetes, and a BMI less than 20 were also associated with fracture risk in the models. Low eGFR, at any level, was not associated with fracture risk in any model (all P values≥0.22). There were also no differences by race/ethnicity or treatment type.
Table 2.
Sequentially adjusted models of the association of albuminuria (>30 mg/g creatinine) with incident risk of hip and pelvic fracture among ONTARGET and TRANSCEND participants without a history of hip or pelvic fractures before study entry stratified by the absence or presence of albuminuria at baseline
| Variables | M1: HR (95% CI) | M2: HR (95% CI) | M3: HR (95% CI) |
|---|---|---|---|
| BL ALB+ versus BL ALB− | 1.62 (1.22–2.15), P<0.001 | 1.48 (1.12–1.97), P=0.006 | 1.35 (1.00–1.82), P=0.05 |
| Age (yr) | 1.10 (1.08–1.11), P≤0.001 | 1.09 (1.07–1.11), P≤0.001 | |
| Female versus male | 2.62 (2.06–3.33), P≤0.001 | 2.63 (2.01–3.45), P≤0.001 | |
| Asian versus European | 1.23 (0.89–1.69), P=0.22 | 1.07 (0.76–1.49), P=0.71 | |
| Other versus European | 0.89 (0.62–1.28), P=0.54 | 0.89 (0.62–1.28), P=0.53 | |
| BMI<20 versus BMI≥30 | 3.55 (2.00–6.30), P≤0.001 | ||
| BMI=20 to <30 versus BMI≥30 | 1.39 (1.03–1.88), P=0.03 | ||
| History of diabetes | 1.34 (1.03–1.75), P=0.03 | ||
| History of CVD | 1.19 (0.77–1.83), P=0.44 | ||
| Current versus never smoker | 1.14 (0.74–1.77), P=0.55 | ||
| Former versus never smoker | 0.99 (0.75–1.31), P=0.94 | ||
| BL eGFR MDRD<30 versus ≥60 | 1.65 (0.67–4.08), P=0.28 | ||
| BL eGFR MDRD=30 and <45 versus ≥60 | 1.30 (0.85–1.99), P=0.22 | ||
| BL eGFR MDRD≥45 and <60 versus ≥60 | 1.14 (0.86–1.51), P=0.36 | ||
| Alcohol ≥3 drinks/d (21 drinks/wk) | 0.99 (0.36–2.68), P=0.98 | ||
| Physical activity less than once a week | 0.97 (0.76–1.25), P=0.82 | ||
| Ramipril (OT) versus placebo (TR) | 1.15 (0.72–1.84), P=0.56 | ||
| Telmisartan (OT + TR) versus placebo (TR) | 1.23 (0.79–1.93), P=0.36 | ||
| Combination (OT) versus placebo (TR) | 1.48 (0.93–2.33), P=0.10 |
Participants were followed for 4.6 years. All participants had baseline albuminuria and eGFR levels and 2-year eGFR values. eGFR is ml/min per 1.73m2 ONTARGET, Ongoing Telmisartan Alone and in combination with Ramipril Global End Point Trial; TRANSCEND, Telmisartan Randomized Assessment Study in ACE Intolerant Subjects with Cardiovascular Disease; M, model; HR, hazard ration; CI, confidence interval; BL, baseline; ALB, albuminuria; BMI, body mass index; CVD, coronary artery disease, stroke, or peripheral arterial disease; eGFR, estimated GFR; MDRD, Modification of Diet in Renal Disease; OT, ONTARGET; TR, TRANSCEND.
A dose-dependent relationship was observed when albuminuria was dichotomized. There were 46 first fractures among 3583 participants with microalbuminuria (0.29/100 person-years) and 16 fractures among 1014 participants (0.39/100 person-years) with macroalbuminuria (Table 3). In multivariate analysis, compared with participants without albuminuria, macroalbuminuria was associated with a statistically significant fracture risk (unadjusted HR=2.01 [1.21, 3.35], P=0.007; adjusted for osteoporosis risks HR=1.71 [1.007, 2.91], P=0.05), whereas microalbuminuria was associated with tendencies to increased risk that were of borderline or no statistical significance. The estimated population attributable risk indicated that, of 100 fracture events occurring in the population, an estimated 2.6% (95% CI=0.07%–6.50%) would be prevented if every person were free of macroalbuminuria.
Table 3.
Hazard ratios for the association of baseline microalbuminuria (30–299 mg/g creatinine) and macroalbuminuria (>299 mg/g creatinine) with incident hip and pelvic fractures among ONTARGET and TRANSCEND participants without a history of hip or pelvic fractures
| Variables | M1: HR (95% CI) | M2: HR (95% CI) | M3: HR (95% CI) |
|---|---|---|---|
| BL micro versus normal | 1.52 (1.10–2.09), P=0.01 | 1.36 (0.99–1.87), P=0.06 | 1.27 (0.910–1.76), P=0.16 |
| BL macro versus normal | 2.01 (1.21–3.35), P=0.007 | 2.02 (1.21–3.37), P=0.007 | 1.71 (1.007–2.91), P=0.05 |
All other results are similar to Table 2. ONTARGET, Ongoing Telmisartan Alone and in combination with Ramipril Global End Point Trial; TRANSCEND, Telmisartan Randomized Assessment Study in Angiotensin-Converting Enzyme Intolerant Subjects with Cardiovascular Disease; M, model; HR, hazard ration; CI, confidence interval; BL, baseline.
Interaction terms of albuminuria with fracture risk by age, diabetes status, and sex were not statistically significant (all P>0.21) (Supplemental Table 1).
To gain additional insight into the temporal relationship of the progression or regression of albuminuria with fracture risk, we examined the rate of first hip and pelvic fractures from year 2 to the last visit 2.6 years later in participants with albuminuria and eGFR values both at baseline and year 2 of follow-up (n=23,091 participants) (Table 4 and Supplemental Table 2). There was no statistically significant difference in fracture risk between participants who never had albuminuria (−/−) and participants who regressed (+/−; unadjusted HR=1.48 [0.80, 2.76], P=0.21; osteoporosis risk-adjusted HR=1.24 [0.66, 2.33], P=0.50). Participants without albuminuria at baseline who progressed to albuminuria at year 2 and patients with persistent baseline and 2-year albuminuria had tendencies to higher subsequent fracture risk than those patients who were persistently normal (progression, unadjusted HR=2.06 [1.28, 3.32], P=0.003; osteoporosis risk-adjusted HR=1.61 [0.99, 2.62], P=0.05; persistent unadjusted HR=1.79 [1.13, 2.83], P=0.01; osteoporosis risk-adjusted HR=1.52 [0.93, 2.47], P=0.09). In these models, there was a tendency for eGFR<30 ml/min per 1.73 m2 to predict fractures, but this finding did not reach formal significance (osteoporosis risk-adjusted HR=1.96 [0.91, 4.24], P=0.09). However, rapid (≥5%) decline in eGFR was a statistically significant risk factor for fracture risk over 2.5 years of follow-up (osteoporosis risk-adjusted HR=1.47 [1.05, 2.04], P=0.02) in the fully adjusted model, which accounted for baseline albuminuria and change in albuminuria over the first 2 years of the study.
Table 4.
Relative risks of incident hip and pelvic fractures among ONTARGET and TRANSCEND participants from year 2 to the last visit at 4.2 years with baseline albuminuria status that progressed (− → +), regressed (+ → −), or stayed the same at 2 years of follow-up compared with participants with no albuminuria at baseline or year 2 of follow-up
| Variables | M1: HR (95% CI) | M2: HR (95% CI) | M3: HR (95% CI) |
|---|---|---|---|
| ALB +/− versus −/− | 1.48 (0.80–2.76), P=0.21 | 1.31 (0.70–2.44), P=0.39 | 1.24 (0.66–2.33), P=0.50 |
| ALB −/+ versus −/− | 2.06 (1.28–3.32), P=0.003 | 1.70 (1.05–2.75), P=0.03 | 1.61 (0.99–2.62), P=0.05 |
| ALB +/+ versus −/− | 1.79 (1.13–2.83), P=0.01 | 1.70 (1.07–2.70), P=0.02 | 1.52 (0.93–2.47), P=0.09 |
| Age (yr) | 1.09 (1.07–1.12), P≤0.001 | 1.09 (1.07–1.11), P≤0.001 | |
| Female versus male | 2.40 (1.75–3.27), P≤0.001 | 2.41 (1.70–3.43), P≤0.001 | |
| Asian versus European | 0.98 (0.63–1.52), P=0.92 | 0.87 (0.55–1.37), P=0.54 | |
| Other versus European | 0.88 (0.55–1.40), P=0.58 | 0.87 (0.54–1.39), P=0.55 | |
| BMI<20 versus ≥30 | 2.42 (0.99–5.89), P=0.05 | ||
| BMI=20 to <30 versus BMI≥30 | 1.43 (0.97–2.11), P=0.07 | ||
| History of diabetes | 1.25 (0.88–1.78), P=0.21 | ||
| History of CVD | 1.09 (0.62–1.91), P=0.76 | ||
| Current versus never smoker | 1.28 (0.74–2.22), P=0.38 | ||
| Former versus never smoker | 0.93 (0.65–1.34), P=0.69 | ||
| Year 2 eGFR MDRD<30 versus ≥60 | 1.96 (0.91–4.24), P=0.09 | ||
| Year 2 eGFR MDRD=30 and <45 versus ≥60 | 0.98 (0.58–1.67), P=0.95 | ||
| Year 2 eGFR MDRD≥45 and <60 versus ≥60 | 0.88 (0.60–1.30), P=0.53 | ||
| Alcohol ≥3 drinks/d (21 drinks/wk) | 1.16 (0.37–3.68), P=0.80 | ||
| Physical activity less than once a week | 0.92 (0.65–1.28), P=0.61 | ||
| Rapid decline in eGFR≥5%/yr from baseline to year 2 | 1.47 (1.05–2.04), P=0.02 |
eGFR is ml/min per 1.73m2. ONTARGET, Ongoing Telmisartan Alone and in combination with Ramipril Global End Point Trial; TRANSCEND, Telmisartan Randomized Assessment Study in Angiotensin-Converting Enzyme Intolerant Subjects with Cardiovascular Disease; M, model; HR, hazard ration; CI, confidence interval; ALB, albuminuria; BMI, body mass index; CVD, coronary artery disease, stroke, or peripheral arterial disease; eGFR, estimated GFR; MDRD, Modification of Diet in Renal Disease.
Discussion
In this study, we examined data from two studies of similar participants (ONTARGET and TRANSCEND) to obtain insights into the relationship of albuminuria, a marker of microvascular disease, with the risk of hip and pelvic fracture. A statistically significant association was found between baseline albuminuria and fracture risk over a 4.6-year period of follow-up (1.36 [1.01, 1.84], P=0.04). The association did not differ between those participants older and younger than 70 years of age, between men and women, or by diabetic status. Risk was graded, higher for macroalbuminuria than for microalbuminuria. Progression to albuminuria during the study increased the risk of subsequent hip and pelvis fractures, and outcomes in those participants with regression of albuminuria were not statistically different from those outcomes in participants who were persistently negative.
Two hypotheses may be proposed to explain the association of albuminuria with fracture risk that invoke causal pathways between endothelial dysfunction or inflammation and bone fracture. One explanation is that albuminuria is associated with reduced bone blood flow and endothelial dysfunction, resulting in a decreased rate of bone remodeling and loss of bone mineral density (14,15). Histologic (14) and radiologic (16–18) studies of osteoporotic bone have shown decreased interosseous blood flow. Ovariectomized rats develop reduced bone marrow blood flow together with reduced bone mineral density and evidence of endothelial dysfunction (19). In people with type 1 diabetes with microvascular complications, serum osteocalcin levels (a protein involved in bone mineralization) are lower than in those people without albuminuria (20,21). Also, in people with type 1 diabetes, higher urinary albumin excretion is associated with lower hip bone mineral density (22). In the general population Tromsø study, higher albuminuria was associated with lower bone mineral density in the distal radius in both men and women, although the finding was statistically significant only in men (6).
An alternative or complementary hypothesis is that albuminuria is associated with decreased bone quality. Albuminuria is associated with inflammation and oxidative stress (23), and bones with osteoporosis are characterized by the presence of inflammatory markers and mediators (24). In the setting of diabetes, albuminuria is also associated with increased serum levels of advanced glycation end products (25). Accumulation of advanced glycation end products in bone collagen leads to inflammation, weakening of crosslinking between collagen fibrils, and loss of bone plasticity and strength (26,27). In keeping with this hypothesis, in the Tromsø study (6), the statistically significant association between albuminuria and fracture risk in women was unlikely to have been be mediated by bone mineral density, because the effect size and statistical significance of the association changed little when bone mineral density was included in the statistical model. Also in keeping with this hypothesis is the observation that people with diabetes—who have higher bone mineral density than people without diabetes and who have a high prevalence of albuminuria—paradoxically have more fractures (27).
It is also possible that albuminuria predicted fracture risk because of uncontrolled confounding by vitamin D deficiency, hyperparathyroidism, hyperphosphatemia, and acidosis, because these factors are known to be associated with albuminuria independent of GFR (28). These factors may alter bone mineral density. None of them are available in the datasets. However, these factors are more strongly associated with decreased GFR, and if uncontrolled confounding explained the predictive effect of albuminuria on fractures, one would expect that baseline GFR would also strongly predict fractures, which was not the case in our study.
One other point regarding the relationship of albuminuria and fracture risk should be noted. The Tromsø study (6), which was a prospective, observational study of a population-based random sample, showed a statistically significant association between albuminuria and subsequent nonvertebral fractures in women but not men. This finding differs from our findings of no differences between the sexes for hip and pelvis fracture risk. The sex difference in the Tromsø study was based on a prespecified analysis as two subgroups rather than a formal test for interaction. We, however, chose a formal test for interaction, which was not statistically significant.
To our knowledge, this study is the first to examine the effects of albuminuria and eGFR adjusted for each other and examine the association between change in albuminuria and change in eGFR on fracture risk. In our data, baseline eGFR did not predict fractures, but in the models that included eGFR trajectory, rate of eGFR loss was predictive of fracture independent of albuminuria. The importance of dynamic rather than static measures of eGFR lends additional weight to the idea of a common element, which we hypothesize to be microvascular dysfunction that is causal in both pathways.
The above findings are novel observations in a study characterized by prospective data collection, rigorous study design, near-complete data capture, and ethnic diversity. Major known risk factors for osteoporosis were captured at baseline, permitting multivariate adjustment for their effects. Because albuminuria was measured centrally, it is unlikely that it led to ascertainment bias in the fracture outcome. We recognize several limitations. Data on fractures were gathered with a plan to analyze the factors that predicted them, but the specific analysis plan was conceived after the data were collected. The number of outcomes is relatively small, particularly in the analyses of the final 2.6 years of the study, and the follow-up time is limited compared with our understanding of the biologic trajectory and clinical history of the evolution of fracture risk and fractures. The ONTARGET and TRANSCEND studies consisted of people at high risk for cardiovascular disease, and although that makes them well suited to answer questions around microvascular disease and in this case, bone health, results may not be generalizable. All participants in this study were treated with renin-angiotensin blockade, which reduces albuminuria, and this treatment may have biased results, possibly to the null. We had no information on whether fractures were high or low impact. We assumed fractures were low impact and osteoporotic in nature. However, we may have included some fractures that were unrelated to osteoporosis or any other feature of bone quality; this inclusion would be expected to reduce precision but not introduce bias. Last, although the HRs for hip and pelvic fractures were increased in association with albuminuria and particularly, macroalbuminuria, the absolute percentage of fractures attributable to macroalbuminuria was low.
In conclusion, we found associations between albuminuria and hip and pelvic fractures and between rapid loss of eGFR and fractures. The significance of these findings is the possibility that renal microvascular disease or generally, the microvascular environment may play a role in the pathogenesis of some osteoporotic fractures. This finding leads to additional questions around pathogenic mechanisms and provides opportunities for possible treatment and prophylaxis strategies that target these pathways.
Disclosures
None of the authors have any conflict of interest regarding the contents of this paper. J.I.B. has received research funding from the National Institute of Diabetes and Digestive and Kidney Diseases and Boehringer-Ingelheim. P.G. has no funding to declare. C.M.C. has received honoraria or research funding from Pfizer, Leo Pharma, Astellas, Janssen, Amgen, and Boehringer-Ingelheim. A.M. has no funding to declare. J.F.E.M. has received honoraria or research funding from the European Union, Boehringer-Ingelheim, Amgen, Roche, Bayer, and Actelion. P.S. has received honoraria from Novo, Novartis, Abbott, and Boehringer-Ingelheim. S.Y. has received research grants, honoraria, and travel expenses from Boehringer-Ingeleheim, Astra–Zeneaca, Sanofi, Servier, BMS, and Bayer. K.K.T. received research funding from Boehringer-Ingelheim during the conduct of the ONTARGET/TRANSCEND trials.
Acknowledgments
Supported by a grant from Boehringer Ingelheim, the Heart and Stroke Foundation of Ontario, and a Senior Scientist Award from the Canadian Institutes of Health Research (to S.Y.).
J.I.B. takes responsibility for the integrity of the data analysis.
This study is ClinicalTrials.gov number NCT00153101 (ONTARGET/ TRANSCEND).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.06640712/-/DCSupplemental.
References
- 1.Brandi ML, Collin-Osdoby P: Vascular biology and the skeleton. J Bone Miner Res 21: 183–192, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Parfitt AM: The mechanism of coupling: A role for the vasculature. Bone 26: 319–323, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Laroche M, Ludot I, Thiechart M, Arlet J, Pieraggi M, Chiron P, Moulinier L, Cantagrel A, Puget J, Utheza G, Mazieres B: Study of the intraosseous vessels of the femoral head in patients with fractures of the femoral neck or osteoarthritis of the hip. Osteoporos Int 5: 213–217, 1995 [DOI] [PubMed] [Google Scholar]
- 4.Garg AX, Kiberd BA, Clark WF, Haynes RB, Clase CM: Albuminuria and renal insufficiency prevalence guides population screening: Results from the NHANES III. Kidney Int 61: 2165–2175, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Satchell SC, Tooke JE: What is the mechanism of microalbuminuria in diabetes: A role for the glomerular endothelium? Diabetologia 51: 714–725, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jørgensen L, Jenssen T, Ahmed L, Bjørnerem A, Joakimsen R, Jacobsen BK: Albuminuria and risk of nonvertebral fractures. Arch Intern Med 167: 1379–1385, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Teo K, Yusuf S, Sleight P, Anderson C, Mookadam F, Ramos B, Hilbrich L, Pogue J, Schumacher H, ONTARGET/TRANSCEND Investigators : Rationale, design, and baseline characteristics of 2 large, simple, randomized trials evaluating telmisartan, ramipril, and their combination in high-risk patients: The Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial/Telmisartan Randomized Assessment Study in ACE Intolerant Subjects with Cardiovascular Disease (ONTARGET/TRANSCEND) trials. Am Heart J 148: 52–61, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Yusuf S, Teo KK, Pogue J, Dyal L, Copland I, Schumacher H, Dagenais G, Sleight P, Anderson C, ONTARGET Investigators : Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med 358: 1547–1559, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Yusuf S, Teo K, Anderson C, Pogue J, Dyal L, Copland I, Schumacher H, Dagenais G, Sleight P, Telmisartan Randomised AssessmeNt Study in ACE iNtolerant subjects with cardiovascular Disease (TRANSCEND) Investigators : Effects of the angiotensin-receptor blocker telmisartan on cardiovascular events in high-risk patients intolerant to angiotensin-converting enzyme inhibitors: A randomised controlled trial. Lancet 372: 1174–1183, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Yusuf S, Teo K, Anderson C, Pogue J, Dyal L, Copland I, Schumacher H, Dagenais G, Sleight P, Telmisartan Randomised AssessmeNt Study in ACE iNtolerant subjects with cardiovascular Disease (TRANSCEND) Investigators : Effects of the angiotensin-receptor blocker telmisartan on cardiovascular events in high-risk patients intolerant to angiotensin-converting enzyme inhibitors: A randomized controlled trial. Lancet 372: 1174–1183, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Soares AA, Eyff TF, Campani RB, Ritter L, Camargo JL, Silveiro SP: Glomerular filtration rate measurement and prediction equations. Clin Chem Lab Med 47: 1023–1032, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Clark WF, Sontrop JM, Macnab JJ, Suri RS, Moist L, Salvadori M, Garg AX: Urine volume and change in estimated GFR in a community-based cohort study. Clin J Am Soc Nephrol 6: 2634–2641, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eastell R, Newman C, Crossman DC: Cardiovascular disease and bone. Arch Biochem Biophys 503: 78–83, 2010 [DOI] [PubMed] [Google Scholar]
- 14.Burkhardt R, Kettner G, Böhm W, Schmidmeier M, Schlag R, Frisch B, Mallmann B, Eisenmenger W, Gilg T: Changes in trabecular bone, hematopoiesis and bone marrow vessels in aplastic anemia, primary osteoporosis, and old age: A comparative histomorphometric study. Bone 8: 157–164, 1987 [DOI] [PubMed] [Google Scholar]
- 15.Prisby RD, Ramsey MW, Behnke BJ, Dominguez JM, 2nd, Donato AJ, Allen MR, Delp MD: Aging reduces skeletal blood flow, endothelium-dependent vasodilation, and NO bioavailability in rats. J Bone Miner Res 22: 1280–1288, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Griffith JF, Yeung DKW, Antonio GE, Lee FKH, Hong AWL, Wong SYS, Lau EMC, Leung PC: Vertebral bone mineral density, marrow perfusion, and fat content in healthy men and men with osteoporosis: Dynamic contrast-enhanced MR imaging and MR spectroscopy. Radiology 236: 945–951, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Shih TTF, Liu HC, Chang CJ, Wei SY, Shen LC, Yang PC: Correlation of MR lumbar spine bone marrow perfusion with bone mineral density in female subjects. Radiology 233: 121–128, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Griffith JF, Yeung DK, Tsang PH, Choi KC, Kwok TC, Ahuja AT, Leung KS, Leung PC: Compromised bone marrow perfusion in osteoporosis. J Bone Miner Res 23: 1068–1075, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Griffith JF, Wang YX, Zhou H, Kwong WH, Wong WT, Sun YL, Huang Y, Yeung DK, Qin L, Ahuja AT: Reduced bone perfusion in osteoporosis: Likely causes in an ovariectomy rat model. Radiology 254: 739–746, 2010 [DOI] [PubMed] [Google Scholar]
- 20.Pietschmann P, Schernthaner G, Woloszczuk W: Serum osteocalcin levels in diabetes mellitus: Analysis of the type of diabetes and microvascular complications. Diabetologia 31: 892–895, 1988 [DOI] [PubMed] [Google Scholar]
- 21.Mathiassen B, Nielsen S, Johansen JS, Hartwell D, Ditzel J, Rødbro P, Christiansen C: Long-term bone loss in insulin-dependent diabetic patients with microvascular complications. J Diabet Complications 4: 145–149, 1990 [DOI] [PubMed] [Google Scholar]
- 22.Jehle PM, Jehle DR, Mohan S, Böhm BO: Serum levels of insulin-like growth factor system components and relationship to bone metabolism in Type 1 and Type 2 diabetes mellitus patients. J Endocrinol 159: 297–306, 1998 [DOI] [PubMed] [Google Scholar]
- 23.Barzilay JI, Peterson D, Cushman M, Heckbert SR, Cao JJ, Blaum C, Tracy RP, Klein R, Herrington DM: The relationship of cardiovascular risk factors to microalbuminuria in older adults with or without diabetes mellitus or hypertension: The cardiovascular health study. Am J Kidney Dis 44: 25–34, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Syed FA, Ng AC: The pathophysiology of the aging skeleton. Curr Osteoporos Rep 8: 235–240, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Aso Y, Inukai T, Tayama K, Takemura Y: Serum concentrations of advanced glycation endproducts are associated with the development of atherosclerosis as well as diabetic microangiopathy in patients with type 2 diabetes. Acta Diabetol 37: 87–92, 2000 [DOI] [PubMed] [Google Scholar]
- 26.Vashishth D: Advanced glycation end-products and bone fractures. IBMS BoneKEy 6: 268–278, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Merlotti D, Gennari L, Dotta F, Lauro D, Nuti R: Mechanisms of impaired bone strength in type 1 and 2 diabetes. Nutr Metab Cardiovasc Dis 20: 683–690, 2010 [DOI] [PubMed] [Google Scholar]
- 28.Inker LA, Coresh J, Levey AS, Tonelli M, Muntner P: Estimated GFR, albuminuria, and complications of chronic kidney disease. J Am Soc Nephrol 22: 2322–2331, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]