Summary
Background and objectives
A dose-finding study was undertaken to investigate the efficacy of PA21, a novel polynuclear iron(III)-oxyhydroxide phosphate binder.
Design, setting, participants, & measurements
In a randomized, active-controlled, multicenter, open-label study at 50 clinical sites in Europe and the United States, hemodialysis patients were randomized to PA21 at dosages of 1.25, 5.0, 7.5, 10.0, or 12.5 g/d or sevelamer-HCl 4.8 g/d for 6 weeks. The primary efficacy endpoint was the change in serum phosphorus concentration from baseline.
Results
There were 154 participants who were randomized and received the study drug. All groups except PA21 1.25 g/d showed a significant decrease in serum phosphorus. Mean decreases in serum phosphorus in PA21 10 g/d and 12.5 g/d were −2.00±1.71 mg/dl and −1.69±1.81 mg/dl, respectively. A similar decrease to sevelamer-HCl (−1.06±1.35 mg/dl) was seen with PA21 5.0 g/d (−1.08±2.12 mg/dl) and 7.5 g/d (−1.25±1.21 mg/d). Overall, 60.9% of participants randomized to PA21 and 57.7% randomized to sevelamer-HCl reported ≥1 adverse event. The most frequent adverse events were hypophosphatemia (18.0%) and discolored feces (11.7%) for the pooled PA21 dose groups, and diarrhea, hypophosphatemia, and hypotension (each 11.5%) for sevelamer-HCl. Discontinuation due to adverse events occurred at a similar rate in PA21-treated (21.1%) and sevelamer-HCl–treated (23.1%) participants.
Conclusions
PA21 5–12.5 g/d significantly reduces serum phosphorus in hemodialysis patients. The 5 g/d and 7.5 g/d dosages showed similar efficacy to 4.8 g/d of sevelamer-HCl. The adverse events rate was similar for PA21 and sevelamer-HCl.
Introduction
Hyperphosphatemia is frequently present in advanced CKD. Dietary restriction of phosphate intake is rarely adequate beyond the early stages of renal dysfunction (1) and phosphate binding agents are almost universally required as kidney disease progresses. Although conventional calcium-based phosphate binders are effective and inexpensive, concerns about calcium overload, hypercalcemia, and vascular calcifications (2–4) have led to the development of alternative therapeutic approaches such as a cationic polymer (e.g., sevelamer hydrochloride [HCl], sevelamer carbonate) and lanthanum carbonate. Sevelamer is effective in lowering serum phosphorus levels (5), but often requires a relatively high pill burden, with large nonchewable tablets, and is associated with gastrointestinal problems (6), all of which can potentially compromise patient adherence, thus reducing efficacy. Lanthanum carbonate appears to be better tolerated with a reduced pill burden compared with sevelamer (7,8) and calcium-based binders. However, absorption of low but measurable levels of lanthanum has been observed (9,10), raising potential long-term toxicity concerns.
PA21 is a novel polynuclear iron(III)-oxyhydroxide phosphate binder that has been shown in vitro to have a high phosphate binding capacity over the physiologically relevant pH range that occurs in the gastrointestinal tract of humans (11). In a phase 1 study, 10 g/d of PA21 was administered for 7 days to 16 patients with CKD (stage 3–5) and 8 healthy volunteers. A very low iron uptake from PA21 (median 0.04% in CKD patients) was observed, with a significant reduction in serum phosphorus levels (12). PA21 was well tolerated, with only some reports of mild to moderate diarrhea.
On the basis of these early results, a randomized, multicenter, dose-finding phase 2 study of PA21 was undertaken in patients with CKD on maintenance hemodialysis. The objective of the trial was to investigate the effect of different doses of PA21 on reducing serum phosphorus levels.
Materials and Methods
Study Design and Conduct
This was a randomized, parallel-group, active-controlled, multicenter, open-label, dose-ranging study undertaken at 50 dialysis centers in eight European countries and the United States from January 2009 to October 2009. It consisted of a screening phase and a washout phase of 2 weeks, followed by a 6-week treatment phase and a 2-week run-out. The study was conducted in compliance with the European Guidelines for Good Clinical Practice and the Declaration of Helsinki (revised version of Seoul, Korea, October 2008). All patients provided written informed consent following institutional review board approval at each center. This study is registered with ClinicalTrials.gov (NCT00824460).
Patient Population
Adult patients (≥18 years) were eligible to enter the study if they had been receiving maintenance hemodialysis three times a week for a minimum of 3 months before the screening visit, with stable calcium content in dialysate for at least 1 month before screening. All patients were to remain on a restricted phosphate diet at screening and throughout the study. In addition, all patients had to be on a stable regimen of phosphate binder therapy for at least 1 month before screening. Patients receiving vitamin D or calcimimetics had to be on a constant dose for a least 1 month before screening and during the study. Any patient receiving an erythropoiesis stimulating agent had to be on a stable dose (defined as ± 25% dose adjustments compared with dose at screening) for at least 1 month before screening and throughout the study. Key exclusion criteria were uncontrolled hyperphosphatemia (serum phosphorus >7.7 mg/dl) at screening, hypercalcemia (serum calcium >10 mg/dl) at screening or during washout, hypocalcemia (serum calcium <7.6 mg/dl) at screening or during washout, intact parathyroid hormone (iPTH) >600 ng/L at screening, iron-deficiency anemia (hemoglobin <10 g/dl) in combination with either serum ferritin <100 ng/ml or transferrin saturation <20% at screening, a history of hemochromatosis or other iron storage disorders, use of oral iron preparations within 1 month before screening, and a history of nonresponse to phosphate binders. Intravenous iron preparations were only permitted until end of screening.
After screening (week −3), patients who met all inclusion/exclusion criteria entered the 2-week washout period in which the use of phosphate binders was discontinued (weeks −2 to −1). Patients whose serum phosphorus levels increased to >5.5 mg/dl at the second or third dialysis session during week −1 were randomized to enter the treatment phase.
Study Treatment
Randomization was performed centrally using an interactive voice response system with patients randomized in a 1:1:1:1:1:1 ratio to one of the five PA21 treatment groups or to the sevelamer-HCl control arm, stratified by geographical region (i.e., Europe versus United States). Study treatment had to be taken with the largest meal(s) of a day. In the active treatment arms, patients received chewable tablets containing 1.25 g of PA21 (containing 250 mg elemental iron(III)) at a dosage of 1.25 g/d (one tablet per day, taken with the largest meal of the day), 5.0 g/d (four tablets per day, two with the largest meal and one each with smaller meals), 7.5 g/d (six tablets per day, two with each meal of the day), 10.0 g/d (eight tablets per day, four with the largest meal and two with each smaller meal), or 12.5 g/d (10 tablets per day, four with the largest meal and three with each smaller meal). In the active control arm, patients received sevelamer-HCl, at a total dosage of 4.8 g/d (six tablets per day, two with each meal), a commonly used dosage for phosphate binder therapy with sevelamer-HCl. Treatment duration for all groups was 6 weeks.
Participants were withdrawn from the study if they experienced any of the following events: hyperphosphatemia (defined as serum phosphorus >8.5 mg/dl) at any time after 2 weeks of treatment, hypophosphatemia (defined as serum phosphorus <3.5 mg/dl) at any time after start of treatment, or hypercalcemia (defined as serum calcium >10 mg/dl) at any time after the washout period.
Study Endpoints
The primary efficacy endpoint was the change in serum phosphorus concentration from baseline to the end of treatment. Secondary efficacy endpoints were serum phosphorus levels at each time point and their changes from baseline (except end of treatment), the percentage of participants achieving controlled serum phosphorus levels (defined as 3.5–5.5 mg/dl) at each time point and time to reach the first controlled serum phosphorus level. Further, serum calcium, calcium × phosphorus (Ca×P) product, and iPTH levels at each time point and their changes from baseline. These endpoints were selected based on the Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines at the time of protocol development (13). Safety endpoints comprised treatment-emergent adverse events (i.e., events that started or got worse after initiation of treatment with study drug), laboratory parameters, vital signs, physical examination, and electrocardiography results.
Evaluation
Blood samples were collected before the dialysis session and were analyzed centrally using standard validated methods except for blood pH, which was measured locally. Compliance with study medication was assessed by pill count (% compliance = 100 × number of tablets taken/number of tablets expected to be taken).
Statistical Analyses
The sample size calculation determined that 19 participants per group, each with ≥1 postbaseline efficacy measurement, would have 90% power to detect a 2.0 mg/dl change in serum phosphorus from baseline to end of treatment. This calculation assumed a SD of 2.5 mg/dl using a α level of 0.05 and a two-sided paired t test, independently testing each of the treatment doses from highest to lowest in a hierarchical manner.
The primary endpoint, change in serum phosphorus level from baseline to the end of treatment (or last observation carried forward [LOCF] for missing values), was analyzed with a single sample t test within each treatment group. The proportion of participants with controlled serum phosphorus levels (defined as 3.5–5.5 mg/dl) was analyzed for an increasing dose effect within the five PA21 treatment groups using a two-sided Cochran-Armitage test at a 5% significance level. In a pair-wise fashion, the PA21 dose groups were compared with the lowest PA21 dose group (1.25 g/d) as a reference using a chi-squared test (two-sided, 5% significance level) at each time point. The Dunnett procedure was used to control for the overall type 1 error rate for the pair-wise comparisons versus the lowest PA21 dose group but overall no adjustment for multiple testing was applied to the significance level.
Results
Study Population
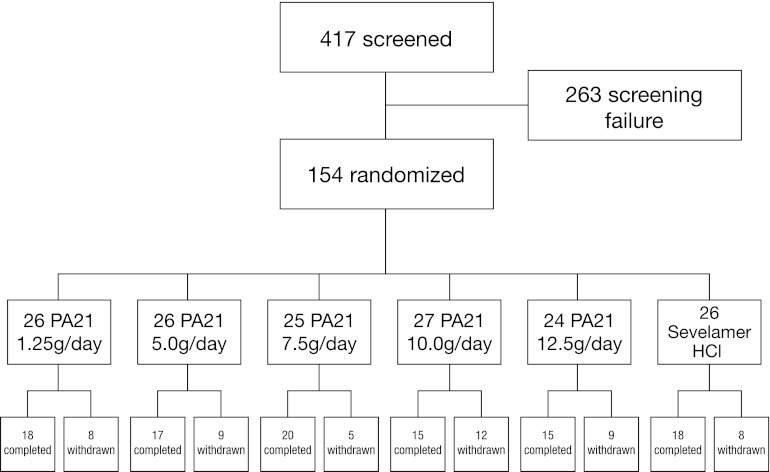
Of 417 patients screened, 154 met the entry criteria and were randomized. All randomized participants received at least one dose of study drug. The safety population thus included 154 participants, 4 of whom provided no postbaseline efficacy assessments such that the efficacy population included 150 participants. Fifty-one participants discontinued the study prematurely; therefore, 103 participants completed the study (Figure 1). The reasons for withdrawal included hypophosphatemia (n=21), hyperphosphatemia (n=10), or hypercalcemia (n=4) (all according to pre-specified withdrawal criteria), or withdrawal of consent (n=5), prohibited concomitant medication (n=2), adverse events (n=4), protocol violation (n=1), death (n=1), and other reasons (n=3). Participants could have more than one reason for withdrawal. Reasons for withdrawal were not related to a particular dose group of PA21 or sevelamer-HCl except for hypophosphatemia, which was more common in the higher PA21 dose groups (10.0 and 12.5 g/d), and hyperphosphatemia and hypercalcemia, which were more common in the lower PA21 dose groups.
Figure 1.
Patient disposition.
All five PA21 treatment groups and the sevelamer-HCl control were similar regarding demographics and cause of CKD (Table 1). The most frequently used phosphate binders before study entry were calcium carbonate (102 of 150, 68.0%) and calcium acetate (31 of 150, 20.7%).
Table 1.
Demographic and baseline characteristics (efficacy population)
| PA21 | Sevelamer-HCl (n=24) | ||||||
|---|---|---|---|---|---|---|---|
| 1.25 g/d (n=26) | 5.0 g/d (n=26) | 7.5 g/d (n=25) | 10.0 g/d (n=25) | 12.5 g/d (n=24) | All Participants (n=126) | ||
| Age (yr) | 60.1±12.3 | 59.7±13.8 | 61.9±13.7 | 60.8±13.2 | 59.3±12.3 | 60.4±12.9 | 61.6±11.2 |
| Male sex | 17 (65.4) | 19 (73.1) | 16 (64.0) | 15 (60.0) | 13 (54.2) | 80 (63.5) | 14 (58.3) |
| White race | 24 (92.3) | 26 (100.0) | 24 (96.0) | 22 (88.0) | 24 (100.0) | 120 (95.2) | 23 (95.8) |
| Concomitant diseasesa | |||||||
| Diabetes mellitus | 8 (30.8) | 7 (26.9) | 9 (36.0) | 7 (28.0) | 9 (37.5) | 40 (31.8) | 9 (37.5) |
| Hypertension | 18 (69.2) | 21 (80.8) | 20 (80.0) | 16 (64.0) | 20 (83.3) | 95 (75.4) | 16 (66.7) |
| Causes of CKDa | |||||||
| Glomerulopathy | 7 (26.9) | 4 (15.4) | 6 (24.0) | 8 (32.0) | 5 (20.8) | 30 (23.8) | 7 (29.2) |
| Vascular nephropathy | 7 (26.9) | 4 (15.4) | 5 (20.0) | 7 (28.0) | 3 (12.5) | 26 (20.6) | 3 (12.5) |
| Interstitial nephropathy | 2 (7.7) | 2 (7.7) | 4 (16.0) | 3 (12.0) | 4 (16.7) | 15 (11.9) | 3 (12.5) |
| Other, not specified | 9 (34.6) | 16 (61.5) | 9 (36.0) | 7 (28.0) | 12 (50.0) | 53 (42.1) | 11 (45.8) |
| Duration since first diagnosis of CKD (mo)b | 40.8 (17.5;68.8) | 47.3 (21.9;107.7) | 63.6 (23.7;114.0) | 48.9 (16.0;119.6) | 74.0 (30.2;131.9) | 54.2 (21.9;107.3) | 65.0 (28.8;126.1) |
| Serum creatinine (mg/dl) | 8.9 (8.4;10.9) | 10.2 (7.6;11.9) | 9.7 (8.5;11.3) | 9.7 (8.0;13.0) | 9.4 (7.8;11.3) | 9.5 (8.1;11.5) | 9.8 (8.4;11.1) |
Values are shown as mean ± SD for normally distributed parameters, n (%), or median (interquartile range) for non-normally distributed parameters.
Several causes per patient possible.
The difference between month and year of screening visit and month and year of the first diagnosis of CKD.
Treatment Compliance
In the efficacy population, median compliance among participants randomized to PA21 was 98% (interquartile range, 95%–100%) compared with 96% (interquartile range, 90%–99%) in the sevelamer-treated participants.
Efficacy
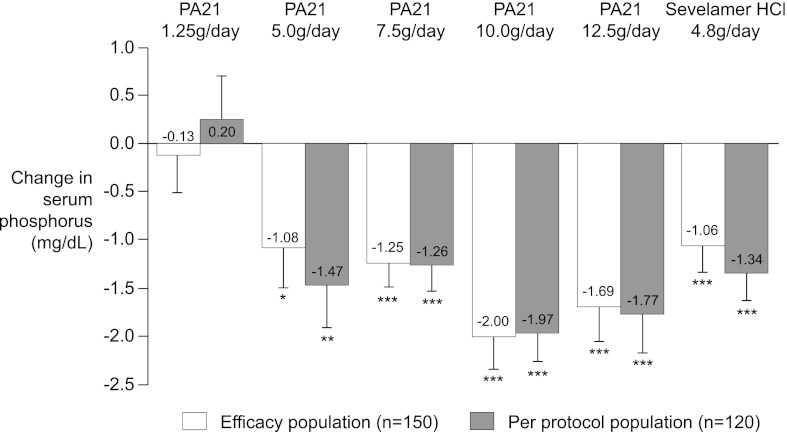
The primary endpoint, change in serum phosphorus level from baseline to end of treatment, showed a significant decrease for all PA21 treatment groups other than 1.25 g/d of PA21 as well as the sevelamer-HCl control arm in the efficacy population (Table 2 and Figure 2). The magnitude of the observed reduction tended to increase with increasing doses of PA21, reaching a maximum for the 10.0 g/d and 12.5 g/ddosages (Table 3 and Figure 2). Pair-wise comparisons (ANCOVA) confirmed that the change in serum phosphorus from baseline to end of treatment was significantly higher in all PA21 dosing groups versus the 1.25 g/d group (5.0 g/d, P=0.003; 7.5 g/d, P=0.01; 10.0 g/d, P<0.001; 12.5 g/d, P<0.001). The change in serum phosphorus in the PA21 5.0 g/d and 7.5 g/d groups was similar to that seen in the sevelamer-HCl control arm (Table 2). When the analysis was repeated for all randomized participants without major protocol violations (the per-protocol population, n=120), the mean decrease in serum phosphorus levels in all treatment groups, except the PA21 10.0 g/d group, was larger than that observed in the efficacy population other than in the 1.25 g/d PA21 cohort, in which the mean serum phosphorus level increased during the study (mean change from baseline: PA21 1.25 g/d, +0.20 mg/dl; PA21 5.0 g/d, −1.47 mg/dl; PA21 7.5 g/d, −1.26 mg/dl; PA21 10.0 g/d, −1.97 mg/dl; PA21 12.5 g/d, −1.77 mg/dl; sevelamer-HCl, −1.34 mg/dl).
Table 2.
Change in laboratory values from baseline to end of treatment according to treatment group (efficacy population)
| PA21 | Sevelamer-HCl (n=24) | |||||
|---|---|---|---|---|---|---|
| 1.25 g/d (n=26) | 5.0 g/d (n=26) | 7.5 g/d (n=25) | 10.0 g/d (n=25) | 12.5 g/d (n=24) | ||
| Serum phosphorus (mg/dl) | ||||||
| Baseline | 6.82±1.64 | 6.61±1.08 | 6.85±1.15 | 6.77±1.75 | 6.47±1.19 | 6.94±1.61 |
| End of treatment | 6.69±2.05 | 5.53±1.94 | 5.60±1.18 | 4.77±1.92 | 4.78±1.67 | 5.88±1.47 |
| Change | −0.13±2.01 | −1.08±2.12 | −1.25±1.21 | −2.00±1.71 | −1.69±1.81 | −1.06±1.35 |
| P valuea (LOCF applied) | 0.75 | 0.02 | <0.001 | <0.001 | <0.001 | <0.001 |
| 95% CI (LOCF applied) | −0.94, 0.68 | −1.93, -0.20 | −1.75, -0.70 | −2.70, -1.20 | −2.46, -0.90 | −1.62, -0.40 |
| Serum calcium (mg/dl) | ||||||
| Baseline | 8.53±0.68 | 8.55±0.71 | 8.63±0.43 | 8.39±0.84 | 8.54±0.56 | 8.57±0.57 |
| End of treatment | 8.30±1.22 | 8.67±0.92 | 8.79±0.59 | 8.49±1.23 | 8.39±1.08 | 8.85±0.57 |
| Change | −0.23±1.22 | 0.12±0.80 | 0.16±0.60 | 0.10±0.96 | −0.15±0.88 | 0.25±0.57 |
| P valuea | 0.35 | 0.46 | 0.19 | 0.62 | 0.40 | 0.04 |
| 95% CI | −0.72, 0.26 | −0.20, 0.44 | −0.09, 0.41 | −0.30, 0.49 | −0.53, 0.22 | 0.01, 0.50 |
| Ca×P (mg2/dl2) | ||||||
| Baseline | 58.13±14.23 | 56.77±11.38 | 59.09±10.29 | 57.31±17.11 | 54.98±9.29 | 59.28±13.38 |
| End of treatment | 55.74±18.70 | 48.27±17.22 | 48.02±9.90 | 40.21±18.23 | 39.99±15.25 | 50.23±12.41 |
| Change | −2.39±22.95 | −8.50±19.20 | −11.07±10.36 | −17.10±16.35 | −14.99±15.97 | −9.71±12.91 |
| P valuea | 0.60 | 0.03 | <0.001 | <0.001 | <0.001 | 0.002 |
| 95% CI | −11.66, 6.88 | −16.26, -0.70 | −15.35, -6.80 | −23.85, -10.30 | −21.73, -8.20 | −15.30, -4.10 |
| Serum iPTH (ng/L) | ||||||
| Baseline | 238±189 | 228±171 | 272±148 | 245±139 | 222±152 | 262±144 |
| End of treatment | 246±191 | 217±165 | 272±197 | 225±156 | 162±93 | 225±137 |
| Change | 7±69 | −11±128 | 0±142 | −20±85 | −61±106 | −39±80 |
| P valuea | 0.59 | 0.67 | 1.00 | 0.25 | <0.01 | <0.03 |
| 95% CI | −20, 35 | −63, 41 | −59, 59 | −55, 15 | −105, -16 | −73, -4 |
Values are shown as mean ± SD. LOCF, last observation carried forward; Ca×P, calcium × phosphorus; CI, confidence interval; iPTH, intact parathyroid hormone.
Two-sided single sample t test.
Figure 2.
Change in serum phosphorus from baseline (week 1) according to treatment group. Data are shown as mean (SEM) change from baseline to end of treatment (primary endpoint) according to treatment group (efficacy and per protocol populations) (last observation carried forward, LOCF). *P=0.02, **P=0.003, ***P<0.001 (all versus baseline).
Table 3.
Serum phosphorus levels and number of patients at each time point according to treatment group (efficacy population)
| Time Point (wk) | PA21 1.25 g/d (1 tablet) | PA21 5.0 g/d (4 tablets) | PA21 7.5 g/d (6 tablets) | PA21 10.0 g/d (8 tablets) | PA21 12.5 g/d (10 tablets) | Sevelamer (HCl) 4.8 g/d (6 tablets) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | n | Mean ± SD | n | Mean ± SD | n | Mean ± SD | n | Mean ± SD | n | Mean ± SD | n | |
| Baseline 1 | 6.82±1.64 | 26 | 6.61±1.08 | 26 | 6.85±1.15 | 25 | 6.77±1.75 | 25 | 6.47±1.19 | 24 | 6.94±1.61 | 24 |
| 2 | 6.72±1.59 | 26 | 5.50±1.26 | 26 | 5.58±1.19 | 25 | 5.29±1.81 | 24 | 5.06±1.13 | 24 | 5.68±1.42 | 24 |
| 3 | 6.88±1.46 | 26 | 5.51±1.65 | 25 | 5.50±1.57 | 24 | 5.13±1.50 | 22 | 4.82±1.05 | 21 | 5.11±1.64 | 22 |
| 4 | 6.78±1.50 | 24 | 5.43±0.98 | 23 | 5.92±1.38 | 22 | 5.07±1.69 | 21 | 5.09±1.28 | 21 | 5.38±1.45 | 22 |
| 5 | 6.67±1.65 | 24 | 5.12±1.66 | 21 | 5.70±1.06 | 21 | 5.12±1.04 | 19 | 4.89±1.48 | 20 | 5.33±1.20 | 20 |
| 6 | 6.61±1.81 | 22 | 5.78±1.54 | 20 | 5.61±1.13 | 20 | 5.10±1.17 | 16 | 5.03±1.51 | 16 | 5.63±1.30 | 18 |
| 7 | 6.76±1.37 | 19 | 5.89±1.70 | 17 | 5.90±1.10 | 20 | 5.04±1.44 | 14 | 5.36±1.48 | 15 | 5.63±1.32 | 19 |
| Follow-up 1 | 6.70±1.17 | 21 | 6.34±2.21 | 19 | 7.00±1.46 | 20 | 7.10±2.01 | 16 | 6.77±1.46 | 16 | 6.63±1.07 | 19 |
| Follow-up 2 | 6.56±1.35 | 19 | 6.53±1.35 | 17 | 6.99±1.40 | 19 | 6.62±1.85 | 14 | 6.52±1.55 | 16 | 6.55±1.45 | 19 |
Values are shown as mean ± SD in mg/dl. Data at follow-up 1 and follow-up 2 were obtained during the 2-week run-out phase when patients stopped taking phosphate binders. If a patient was prematurely withdrawn from the study for any reason after start of treatment, the investigator made every effort to perform the evaluations described for the follow-up 1 visit, as soon as possible after the last study treatment administration, and 1 week later the evaluations as described for the follow-up 2 visit. If it was not possible to carry out both of these early termination visits, at least the evaluations of the follow-up 2 visit were performed.
The proportions of participants achieving a controlled serum phosphorus level (3.5–5.5 mg/dl) after 6 weeks of treatment were 21.1% (4 of 19), 41.2% (7 of 17), 35.0% (7 of 20), 42.9% (6 of 14), and 60.0% (9 of 15) in the PA21 1.25 g/d, 5.0 g/d, 7.5 g/d, 10.0 g/d, and 12.5 g/d groups, respectively (P=0.03, two-sided Cochran-Armitage test for trend over dose groups), and 42.1% (8/19) in the sevelamer-HCl control arm. Median time to achieve controlled serum phosphorus (3.5–5.5 mg/dl) was not different for PA21 5.0–12.5 g/d (1 week) compared with the sevelamer-HCl control arm (2 weeks) (P>0.16).
Mean serum calcium levels were comparable between groups at baseline (Table 2). Changes in serum calcium were small and variable in all PA21 groups and the sevelamer-HCl control arm, with a slight but significant increase in the sevelamer-HCl control arm (P=0.04). The changes in Ca×P product during the study were consistent with the changes in serum phosphorus concentrations (Table 2). Mean serum iPTH varied between groups at baseline, within the range of 222–272 ng/L (Table 2). The greatest mean decrease in iPTH was seen in the PA21 12.5 g/d group; however, there was no observed dose-dependent relationship between the magnitude of iPTH decrease and PA21 dosage in the range of 5.0–10.0 g/d.
Safety and Tolerability
Overall, 60.9% of participants randomized to PA21 and 57.7% randomized to sevelamer-HCl reported one or more adverse events (Table 4). The most frequently occurring adverse event in the pooled PA21 treatment groups was hypophosphatemia (PA21, 18.0%; sevelamer-HCl, 11.5%). Hypotension and diarrhea were the most frequent adverse events in sevelamer-treated participants (both 11.5% versus 0.8% and 5.5%, respectively, in the PA21-treated participants). Muscle spasms occurred in eight PA21-treated participants (6.3%) and no sevelamer-treated participants (P=0.35). In all cases, the principal investigator treating the patient considered muscle spasms to be unrelated to PA21. Gastrointestinal adverse events were reported with no dose-dependent incidence in 22.7% of PA21-treated participants and 26.9% of sevelamer-HCl–treated participants. There were no marked differences in the occurrence of diarrhea, constipation, or vomiting between the PA21 groups and the sevelamer-HCl control arm but, as expected for an iron-based product, discolored feces were only reported in participants receiving PA21 (n=15, 11.7%). No patient reported a severe or serious treatment-related adverse event.
Table 4.
Adverse events according to treatment group (safety population)
| PA21 | Sevelamer-HCl (n=26) | ||||||
|---|---|---|---|---|---|---|---|
| 1.25 g/d (n=26) | 5.0 g/d (n=26) | 7.5 g/d (n=25) | 10.0 g/d (n=27) | 12.5 g/d (n=24) | All (n=128) | ||
| Any adverse event | 14 (53.8) | 16 (61.5) | 13 (52.0) | 18 (66.7) | 17 (70.8) | 78 (60.9) | 15 (57.7) |
| Any severe adverse event | 2 (7.7) | 1 (3.8) | 0 | 1 (3.7) | 0 | 4 (3.1) | 1 (3.8) |
| Any serious adverse event | 2 (7.7) | 2 (7.7) | 1 (4.0) | 1 (3.7) | 2 (8.3) | 8 (6.3) | 2 (7.7) |
| Discontinuation of study or treatment due to adverse event | 5 (19.2) | 5 (19.2) | 4 (16.0) | 8 (29.6) | 5 (20.8) | 27 (21.1) | 6 (23.1) |
| Adverse eventsa | |||||||
| Hypophosphatemia | 2 (7.7) | 4 (15.4) | 2 (8.0) | 8 (29.6) | 7 (29.2) | 23 (18.0) | 3 (11.5) |
| Hyperphosphatemia | 5 (19.2) | 3 (11.5) | 1 (4.0) | 1 (3.7) | 0 | 10 (7.8) | 2 (7.7) |
| Hypercalcemia | 2 (7.7) | 2 (7.7) | 1 (4.0) | 1 (3.7) | 1 (4.2) | 7 (5.5) | 2 (7.7) |
| Discolored feces | 2 (7.7) | 3 (11.5) | 3 (12.0) | 4 (14.8) | 3 (12.5) | 15 (11.7) | 0 |
| Diarrhea | 1 (3.8) | 2 (7.7) | 2 (8.0) | 1 (3.7) | 1 (4.2) | 7 (5.5) | 3 (11.5) |
| Constipation | 0 | 1 (3.8) | 1 (4.0) | 2 (7.4) | 0 | 4 (3.1) | 0 |
| Vomiting | 0 | 2 (7.7) | 0 | 1 (3.7) | 0 | 3 (2.3) | 1 (3.8) |
| Muscle spasms | 1 (3.8) | 1 (3.8) | 2 (8.0) | 1 (3.7) | 3 (12.5) | 8 (6.3) | 0 |
| Pain in extremity | 1 (3.8) | 1 (3.8) | 1 (4.0) | 0 | 0 | 3 (2.3) | 1 (3.8) |
| Hypertension | 1 (3.8) | 0 | 2 (8.0) | 0 | 2 (8.3) | 5 (3.9) | 1 (3.8) |
| Hypotension | 0 | 1 (3.8) | 0 | 0 | 0 | 1 (0.8) | 3 (11.5) |
| Anemia | 0 | 0 | 3 (12.0) | 0 | 0 | 3 (2.3) | 0 |
Values are shown as n (%).
Any adverse event reported by >1 participant in any treatment group or >2 participants in the pooled PA21 group.
In total, 6.3% of the participants (n=8) randomized to PA21 and 7.7% of the participants (n=2) in the sevelamer-HCl control arm experienced a total of 16 serious adverse events. The incidence of adverse events graded severe or serious showed no relevant differences between treatment groups, and none of these events were considered by the investigators treating the affected patients to be related to study treatment. The most frequent causes of discontinuation due to adverse events were hypophosphatemia (PA21, n=13 [10.2%]; sevelamer-HCl, n=2 [7.7%]), hypercalcemia (PA21, n=6 [4.7%]; sevelamer-HCl, n=0 [0%]), and hyperphosphatemia (PA21, n=5 [3.9%]; sevelamer-HCl, n=1 [3.8%]). No other adverse events led to study discontinuation in more than one participant except for diarrhea in two sevelamer-HCl participants. One patient in the PA21 5.0 g/d group died due to gastrointestinal hemorrhage and cardiac arrest, which were not considered by the investigator treating the patient at that center to be related to study drug.
Laboratory assessments, vital signs, electrocardiography results, and physical examinations did not indicate any safety concerns. There were no significant changes from baseline and no trends detected across treatment groups for serum iron parameters (Table 5) or for vitamins (A, E, K, 25(OH)D, and 1.25 (OH)2D) and bone markers (data not shown).
Table 5.
Change in iron parameters from baseline to week 4 according to treatment group (safety population)
| PA21 | Sevelamer-HCl (n=26) | |||||
|---|---|---|---|---|---|---|
| 1.25 g/d (n=26) | 5.0 g/d (n=26) | 7.5 g/d (n=25) | 10.0 g/d (n=27) | 12.5 g/d (n=24) | ||
| Serum ferritin (ng/ml) | ||||||
| Baseline | 242.2 (167.0; 577.6) | 438.7 (293.4; 589.7) | 317.0 (246.3; 543.0) | 247.9 (116.0; 346.4) | 277.9 (128.0; 547.2) | 303.0 (172.6; 542.5) |
| Change from baseline to week 4 | 4.1 (−31.2; 25.6) | −4.4 (−40.5; 24.1) | −5.3 (−26.9; 33.9) | −10.5 (−25.5; 12.4) | 21.0 (−50.2; 34.5) | 7.9 (−42.6; 68.7) |
| Serum iron (µmol/L) | ||||||
| Baseline | 8.2 (6.5; 12.3) | 9.4 (6.6; 10.9) | 9.5 (6.7; 11.4) | 9.6 (7.3; 12.4) | 9.0 (6.7; 10.9) | 8.2 (6.1; 10.4) |
| Change from baseline to week 4 | 0.1 (−1.3; 1.3) | 0.5 (−1.6; 3.2) | −1.3 (−3.0; 1.7) | 0.6 (−1.2; 1.5) | 0.5 (−1.9; 3.6) | −1.2 (−2.0; 2.4) |
| Transferrin (µmol/L) | ||||||
| Baseline | 19.1 (16.3; 23.8) | 18.9 (15.8; 21.8) | 18.5 (16.5; 20.8) | 19.9 (15.5; 22.5) | 19.5 (17.1; 21.7) | 18.4 (17.1; 21.6) |
| Change from baseline to week 4 | −0.1 (−1.1; 0.9) | −0.2 (−0.8; 1.2) | −0.1 (−1.3; 1.6) | 1.0 (−0.5; 2.6) | 0.5 (−1.1; 1.3) | 1.9 (0.3; 2.8) |
| Transferrin saturation (%) | ||||||
| Baseline | 23.7 (18.1; 30.3) | 23.1 (18.4; 28.6) | 25.4 (17.9; 30.6) | 26.2 (18.3; 38.0) | 21.2 (16.0; 28.5) | 21.8 (17.5; 26.0) |
| Change from baseline to week 4 | −0.1 (−4.8; 2.5) | 3.1 (−3.4; 7.1) | −0.3 (−8.2; 5.0) | −0.8 (−4.6; 3.9) | 2.2 (−5.0; 7.3) | −2.9 (−5.7; 4.2) |
Values are shown as median (interquartile range).
Discussion
The results of this randomized dose-finding study demonstrate that PA21 achieves a significant reduction in serum phosphorus levels in maintenance hemodialysis patients at dosages of 5.0 g/d or higher, leading to controlled serum phosphorus levels in >37% of participants within 1 week of starting treatment. PA21 showed a significant reduction in serum phosphorus within the dosage range of 5.0–12.5 g/d. The primary endpoint, change in serum phosphorus level from baseline to end of treatment, was similar between PA21 5.0 g/d or 7.5 g/d and sevelamer-HCl (4.8 g/d). PA21 demonstrated a similar rate of adverse events to sevelamer-HCl. A higher rate of discontinuations due to adverse events in the higher PA21 dose groups (10.0 g/d and 12.5 g/d) was largely due to a cautious predefined criterion for study drug discontinuation at a serum phosphorus level <3.5 mg/dl and was consistent with the dose-dependent effect of the drug.
The most frequent adverse effect in the pooled PA21 treatment group was protocol-specified hypophosphatemia (18.0%), indicating the high phosphate binding potency of PA21. No severe or serious adverse events were reported that were considered by the investigators who treated the affected patients as being related to either PA21 or sevelamer-HCl. Other than contravention of prespecified thresholds for serum phosphorus or calcium levels, no adverse event led to study discontinuation in more than one patient except for treatment-related diarrhea in two participants receiving sevelamer HCI. Importantly, gastrointestinal adverse events showed no dose-dependent association with PA21. Muscle spasms, commonly seen in patients undergoing dialysis, occurred only in PA21-treated participants and were not considered to be related to PA21 by the investigators involved in managing these patients. Iron parameters (serum iron, serum ferritin, transferrin, and transferrin saturation) showed no significant changes from baseline.
This is consistent with a previous investigation demonstrating that iron uptake from PA21 at a dosage of 10 g/d is very low (median 0.04% in CKD patients) (12).
Certain aspects of the study design merit discussion. PA21 was compared with an approved dosage of sevelamer-HCI (two 0.8-g tablets three times a day with meals) which is commonly prescribed in many countries. The magnitude of the serum phosphorus-lowering effect seen with 4.8 g/d of sevelamer-HCl in this study is similar to that observed in previously conducted trials (2,4) and provides evidence of assay sensitivity and clinical relevance of the phosphorus reducing effects of PA21 5.0 g/d to 12.5 g/d. The prespecified criterion that participants were to be discontinued from the study if their serum phosphorus level fell below 3.5 mg/dl may have underestimated the efficacy of PA21 in lowering serum phosphorus levels because the greatest rate of such discontinuations was observed in the PA21 10.0 g/d and 12.5 g/d groups. If a lower serum phosphorus threshold for study discontinuation had been used, a greater mean reduction in serum phosphorus may have been observed. Overall, hypophosphatemia (serum phosphorus <3.5 mg/dl) led to discontinuation in 13 PA21-treated participants (10.2%) versus only two participants (7.7%) randomized to the sevelamer-HCl control arm. As a dose-finding study, the trial was not designed to demonstrate similar efficacy to sevelamer. It should also be noted that the study population was exclusively Caucasian and predominantly male; as such, these findings cannot necessarily be extrapolated to all patient types. However, with respect to diabetes and hypertension, the two most common conditions causing ESRD, >30% of the total study population had diabetes and >70% had hypertension; these prevalences reflect the ESRD population at large.
In conclusion, PA21 at dosages of 5–12.5 g/d was effective in reducing serum phosphorus levels in patients with CKD and hyperphosphatemia undergoing hemodialysis with a similar rate of adverse events to sevelamer-HCl. The results of this study demonstrate that the phosphorus-lowering effect of PA21 is significant, potent, and prompt, providing good phosphorus control while minimizing excessive alterations in other serum electrolytes. Notably, two-thirds of the participants in the 12.5 g/d PA21 dosage group achieved controlled serum phosphorus concentrations (<5.5 mg/dl) at the end of the 6-week treatment phase, despite the fact that 16.7% of the participants in this PA21 dosage group were withdrawn before the end of the treatment phase due to hypophosphatemia.
Disclosures
R.P.W. has received research funding and consulting fees from Genzyme, Shire, and Vifor Pharma. S.G. and E.C. are employees of Vifor Pharma. R.P.W., M.C., A.C., and J.A.T. have served as participants on Vifor Pharma advisory boards.
Acknowledgments
Caroline Dunstall (funded by Vifor Pharma) provided medical writing support, including preparation of the first draft based on detailed directions from the authors, preparation of figures, formatting to meet journal requirements, and incorporation of author amendments. The authors had full access to the study data and made the decision to publish.
The study was funded by Vifor Pharma, Vifor International Inc., St. Gallen, Switzerland, and the company undertook data analysis.
These data have been presented at the 2010 European Renal Association Congress, June 25–28, 2010, in Munich, German; the 2010 American Society of Nephrology Annual Meeting, November 16–21, 2010, in Denver, Colorado; and the 2011 World Congress of Nephrology, April 8–12, 2011, in Vancouver, Canada.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Coladonato JA: Control of hyperphosphatemia among patients with ESRD. J Am Soc Nephrol 16[Suppl 2]: S107–S114, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Bleyer AJ, Burke SK, Dillon M, Garrett B, Kant KS, Lynch D, Rahman SN, Schoenfeld P, Teitelbaum I, Zeig S, Slatopolsky E: A comparison of the calcium-free phosphate binder sevelamer hydrochloride with calcium acetate in the treatment of hyperphosphatemia in hemodialysis patients. Am J Kidney Dis 33: 694–701, 1999 [DOI] [PubMed] [Google Scholar]
- 3.Block GA, Spiegel DM, Ehrlich J, Mehta R, Lindbergh J, Dreisbach A, Raggi P: Effects of sevelamer and calcium on coronary artery calcification in patients new to hemodialysis. Kidney Int 68: 1815–1824, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Chertow GM, Burke SK, Raggi P, Treat to Goal Working Group : Sevelamer attenuates the progression of coronary and aortic calcification in hemodialysis patients. Kidney Int 62: 245–252, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Kidney Disease Improving Global Outcomes (KDIGO): Clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Chapter 3.1: Diagnosis of CKD-MBD: Biochemical abnormalities. Kidney Int Supp 76[Suppl 113]: S22–S49, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Renvela (sevelamer carbonate). Prescribing information. Cambridge, MA, Genzyme Corporation, 2009. Available at: http://www.renvela.com/~/media/Files/RenvelaUS/RV382_Renvela_PI_08.2011.pdf Accessed October 19, 2012
- 7.Fosrenol. Summary of product characteristics. Hampshire, UK, Shire Pharmaceutical Ltd, 2009. Available at: http://emc.medicines.org.uk/document.aspx?documentId=19617
- 8.Mohammed IA, Hutchison AJ: Phosphate binding therapy in dialysis patients: Focus on lanthanum carbonate. Ther Clin Risk Manag 4: 887–893, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pennick M, Dennis K, Damment SJP: Absolute bioavailability and disposition of lanthanum in healthy human subjects administered lanthanum carbonate. J Clin Pharmacol 46: 738–746, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Damment SJP, Pennick M: Clinical pharmacokinetics of the phosphate binder lanthanum carbonate. Clin Pharmacokinet 47: 553–563, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Wilhelm M, Funk F, Gaillard S: PA21, a novel iron-based phosphate binder, has high phosphate binding capacity and low iron release in vitro across a physiological pH range [Abstract]. Poster presentation at Kidney Week 2012, San Diego, CA., October 30–November 4, 2012
- 12.Geisser P, Philipp E: PA21: a novel phosphate binder for the treatment of hyperphosphatemia in chronic kidney disease. Clin Nephrol 74: 4–11, 2010 [DOI] [PubMed] [Google Scholar]
- 13.National Kidney Foundation : K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 42[Suppl 3]: S1–S201, 2003 [PubMed] [Google Scholar]