Summary
Background and objectives
In the FSGS Clinical Trial, 22 cyclosporine-treated and 20 mycophenolate/dexamethasone-treated patients experienced a complete or partial remission after 26 weeks, completed 52 weeks of treatment, and were studied through 78 weeks. Herein, changes in the urine protein/creatinine ratio (UP/C) and estimated GFR (eGFR) throughout the entire study period are defined.
Design, setting, participants, and measurements
The FSGS Clinical Trial, which was conducted from November 2004 to January 2010, enrolled patients aged 2–40 years, with eGFR ≥40 ml/min per 1.73 m2 and UP/C >1 mg/mg after ≥4 weeks of corticosteroid therapy. Both groups received lisinopril or losartan throughout the study. UP/C and eGFR were measured at 0, 26, 52, and 78 weeks.
Results
The median UP/C in the cyclosporine- and mycophenolate/dexamethasone-responsive patients fell by 89.8% and 82.7% at 52 weeks; the fall was largely sustained at 78 weeks (74.7% and 80.3%, respectively). The mean eGFR fell by 19.4% in the cyclosporine group and rose by 7.0% in the mycophenolate mofetil/dexamethasone group at 52 weeks, but subsequently rose by 16.4% and fell by 2.6%, respectively, in the two groups from 52 to 78 weeks.
Conclusions
In this subset of responding FSGS patients, the improvement in UP/C after cyclosporine or mycophenolate/dexamethasone treatment was largely sustained for 6 months after therapy. Reduction in eGFR in the cyclosporine group was improved 6 months after cyclosporine was stopped although the levels were lower than baseline in seven patients who entered the study with decreased eGFR.
Introduction
Many reports have suggested that decreasing the level of proteinuria in patients with FSGS reduces the long-term effect of this disease on GFR (1–3), but it is unclear whether all methods of reducing proteinuria will have the same potential. Other concerns include the apparent lack of persistent effect on proteinuria when therapies such as cyclosporin A (CsA) are discontinued (4–8), and the potential for nephrotoxicity when CsA is continued over many years. This has led to considerable variability in the duration of treatment, ranging from 6 months to ≥2 years.
The National Institutes of Health (NIH)–sponsored clinical trial of CsA versus mycophenolate mofetil (MMF) and dexamethasone (DEX) in patients with FSGS was recently published (9). Our results showed that 39 patients (54.2%) in the CsA group and 26 patients (39.4%) in the MMF/DEX group achieved a complete or partial remission after 6 months of therapy and went on to complete a 12-month course. In the primary outcome analysis of this trial, we noted a greater reduction in proteinuria after 26 weeks of treatment with CsA compared with MMF/DEX (26 week/baseline UP/C median ratios of 0.24 versus 0.46, respectively; P=0.04). However, there was also a greater reduction in the 26-week/baseline GFR ratio in the CsA group (0.73) compared with the MMF/DEX group (0.95) over the same period of follow-up (P=0.001). Although the GFR rose during the 26-week period after CsA treatment was discontinued, this improvement did not return the GFR to the original level in this group (9). This report further delineates the time course and relationship between changes in UP/C and estimated GFR (eGFR) during and 6 months after 12 months of treatment with CsA or MMF/DEX in patients who showed a complete or partial remission at 26 weeks.
Materials and Methods
The FSGS Clinical Trial was a 78-week multi-center, prospective, open label, randomized controlled trial (RCT) that compared CsA in the control arm versus DEX in combination with MMF (MMF/DEX) as the experimental therapy. Patients were eligible to enter this trial if they had primary FSGS confirmed by central pathologists, were aged between 2 and 40 years, had an eGFR ≥40 ml/min per 1.73 m2, and had a UP/C >1 g/g after a minimum of 4 weeks of corticosteroid therapy. Details of the inclusion and exclusion criteria were provided in a prior publication (10).
Monitoring of Laboratory Studies
All laboratory assays were conducted in a central laboratory. Two first-morning urine samples were obtained at baseline and after the patients had been treated for 26, 52, and 78 weeks and the results were expressed as the mean of the two measurements. If the two UP/C values differed by >50%, a third urine sample was collected and the two results closest to one another were used to determine the mean UP/C. GFR was estimated by the Schwartz formula in patients aged <18 years (11) and by the Cockroft–Gault formula (adjusted for body surface area) in patients aged ≥18 years (12).
Treatment Regimens
All patients were given 0.3 mg/kg of prednisone or prednisolone (maximum 15 mg) every other day for the first 6 months of the trial. An angiotensin converting enzyme (ACE) inhibitor, lisinopril, was given to all patients who tolerated this drug for the entire 18 months of the trial. Dosages of lisinopril were initiated at approximately 0.1 mg/kg per day (25% of the target dose) and increased every 2 weeks as tolerated until the target dose of 0.4 mg/kg (40 mg maximum daily) was reached. Losartan (maximum dosage 100 mg/d) was provided to ACE inhibitor–intolerant patients. Management of edema and control of BP were left to the discretion of the participating investigators.
In the CsA arm, an initial dosage of 5–6 mg/kg per day (maximum 250 mg/d) was given in two equal doses. The subsequent doses were adjusted to achieve 12-hour trough level concentrations of 100–250 ng/ml, or in response to prespecified toxicities (reductions were applied in 30% decrements).
In the MMF/DEX arm, an initial dosage of MMF (25–36 mg/kg per day, maximum 2 g/d), was given in two equal daily doses, and DEX (0.9 mg/kg per dose, maximum 40 mg) was given on two consecutive days at the start of weeks 1–8, 10, 12, 14, 16, 18, 20, 22, 24, 26, 30, 34, 38, 42, 46, and 50. MMF and DEX dose adjustments were made in 30% decrements for prespecified toxicities. Treatment with CsA or MMF/DEX was given for up to 52 weeks, depending on response, whereas lisinopril or losartan was given for 78 weeks.
Treatment Failure
Treatment failure in this trial was defined as failure to achieve at least a partial remission by week 26, failure to achieve a partial or complete remission by week 52, or if a patient reached a protocol-defined stop point. Complete remission was defined as UP/C <0.2; partial remission was defined as UP/C <2.0 plus 50% decrease compared with baseline. Study stop points included a 50% decline from baseline in the eGFR, onset of dialysis, or prespecified severe medication related toxicity. After the confirmation of treatment failure, all study medications were discontinued. Thus, only patients who had at least a partial remission at week 26 received 12 months of treatment with CsA or MMF/DEX.
Entry Criteria for Inclusion in This Post Hoc Analysis
The cohort of patients that forms the basis of this post hoc analysis of the FSGS Clinical Trial consists of patients with complete data who experienced a partial remission or complete remission at 26 weeks in the study, completed 52 weeks of CsA or MMF/DEX treatment, were seen and had laboratory studies done at the 78-week visit (after the primary study drugs had been withdrawn for 26 weeks), and did not receive any immunosuppressive agents between weeks 52 and 78 of the study. All of the patients continued to receive lisinopril or losartan from 52 to 78 weeks at the same doses they received during the first 52 weeks of the study.
Ethical Considerations
This study was registered with ClinicalTrials.gov (identifier NCT00135811) and was monitored by a data and safety monitoring board that was established by the NIH. The study protocol was reviewed and approved by the institutional review board at each participating site.
Statistical Analyses
In this report, we consider changes in eGFR and UP/C over the follow-up period conditional on achievement of remission of proteinuria at week 26 and other conditions related to the maintenance of the patient’s randomly assigned treatment regimen and data completeness. Because inclusion of patients in the analysis is dependent on their outcome, patients who were retained for analyses may not be strictly comparable between the treatment groups. Therefore, statistical analyses are limited to assessments of change within each of the two treatment groups, and we present no formal statistical comparisons of the treatment groups to each other.
Baseline characteristics of the patients are summarized as frequencies and percentages for categorical variables, and as medians with 10th–90th percentile ranges for most of the numeric variables. The eGFR data are shown as mean ± 1 SD. For each of the two treatment groups, baseline summaries are provided for all randomized patients and separately for those meeting the criteria for inclusion in the analyses and for those not meeting the inclusion criteria. Fisher exact tests, t tests, or Wilcoxon rank-sum tests were used as appropriate to compare these two subgroups within each of the two randomized treatment groups.
Levels of eGFR and UP/C were summarized at the baseline and follow-up week 26, 52, and 78 visits and expressed as mean ± 1 SD for eGFR and median plus 10th–90th percentile ranges for UP/C. Changes from baseline to 26, 52, and 78 weeks, and from week 52 to week 78 were summarized as mean ± 1 SD for eGFR and as median percentage changes plus 10th–90th percentiles for UP/C. The statistical significance of the changes in eGFR and in UP/C was evaluated separately for each treatment group. The data analysis for this paper was generated using SAS/STAT software, version 9.3 of the SAS System for UNIX.
Results
Patient Baseline Characteristics
Tables 1 and 2 show the baseline characteristics of the 42 patients who fulfilled the basis for this subanalysis, the 96 patients who did not fulfill the entry criteria and the entire cohort of patients (N=138). The numbers of patients fulfilling all of the above-listed criteria were 22 in the CsA group (Table 1) and 20 in the MMF/DEX group (Table 2). This group of patients completed 12 months of CsA or MMF/DEX, was maintained on ACE inhibitors/angiotensin receptor blockers throughout the 18-month trial, and had no missing data. They tended to have lower baseline levels of UP/C and serum cholesterol, and higher serum albumin levels than the patients who were not included in this subanalysis. This resulted in part from the fact that, by definition, they all had at least a partial remission, which required a fall in UP/C to <2.0 in addition to a 50% reduction. Patients were more likely to achieve a partial remission, as defined here, and be eligible to complete the 12 months of study drugs if their UP/C was lower at baseline. Although 39 patients in the CsA group and 26 patients in the MMF/DEX group had a partial or complete remission at 26 weeks, 17 and 6 patients, respectively, in the two groups did not fulfill all of the criteria and are not included in this analysis. The reasons for their exclusion are listed in Table 3. Note that failure to maintain a complete or partial remission at the 52-week visit did not constitute a reason for exclusion from this analysis.
Table 1.
Baseline characteristics of patients randomized to CsA
| Baseline Factor | All Randomized(n = 72) | Patients in This Substudy (A) (n = 22) | Patients not in This Substudy (B) (n = 50) | P Value (A) versus (B) |
| Age at Enrollment (yr) | 15 (4–34) | 15 (7–34) | 15 (2.5–32) | 0.52 |
| White race | 40 (55.6) | 15 (68.2) | 25 (50.0) | 0.47 |
| Male sex | 40 (55.6) | 13 (59.1) | 27 (54.0) | 0.80 |
| Baseline GFR (ml/min per 1.73m2) | 136.6 ± 79.0 | 126.8 ± 50.5 | 140.9 ± 88.8 | 0.49 |
| UP/C (mg/mg) | 4.4 (1.2–13.3) | 2.7 (1.1–5.1) | 5.4 (1.3–15.4) | 0.002 |
| Serum Albumin (g/d) | 3.0 (1.7–4.1) | 3.5 (2.6–4.2) | 2.6 (1.6–4.0) | 0.003 |
| Serum Cholesterol (mg/dl) | 283 (203–571) | 259 (163–354) | 318 (233–597) | 0.01 |
| Hypertensive | 41 (56.9) | 13 (59.1) | 28 (56.0) | 1.00 |
Data shown as median (10th–90th percentile), n (%), or mean ± SD. CsA, cyclosporin A; UP/C, urinary protein/creatinine ratio.
Table 2.
Baseline characteristics of patients randomized to MMF/DEX
| Baseline Factor | All Randomized(n = 66) | Patients in This Substudy (C) (n = 20) | Patients not in This Substudy (D) (n = 46) | P Value (C) versus (D) |
| Age at Enrollment (yr) | 14.5 (6–35) | 14 (9.5–34.5) | 15 (5–35) | 0.68 |
| White race | 38 (57.6) | 13 (65.0) | 25 (54.3) | 0.71 |
| Male sex | 33 (50) | 12 (60.0) | 21 (45.6) | 0.42 |
| Baseline GFR (ml/min per 1.73m2) | 132.0 ± 73.6 | 122.6 ± 50.7 | 136.2 ± 81.7 | 0.49 |
| UP/C (mg/mg) | 3.7 (1.2–13.1) | 3.6 (1.1–9.6) | 4.0 (1.5–13.6) | 0.17 |
| Serum albumin (g/d) | 2.7 (1.6–4.0) | 3.2 (1.9–4.0) | 2.6 (1.5–4.0) | 0.10 |
| Serum cholesterol (mg/dl) | 312 (221–569) | 271.5 (187–480) | 365 (232–589) | 0.02 |
| Hypertensive | 39 (59.1) | 13 (65.0) | 26 (56.5) | 0.59 |
Data shown as median (10th–90th percentile), n (%), or mean ± SD. MMF/DEX, mycophenolate mofetil/dexamethasone; UP/C, urinary protein/creatinine ratio.
Table 3.
Reasons for exclusion from this analysis of patients who achieved partial or complete remission at week 26
| CsA Group (n) | MMF/DEX Group (n) | |
|---|---|---|
| Missing eGFR, UP/C, or medication information at week 52 or week 78 | 10 | 2 |
| Not on all prescribed study medications at week 52 | 2 | 4 |
| Nonstudy immunosuppressive drug (e.g., CsA, tacrolimus) given between week 52 and week 78 | 5 | 0 |
| Total | 17 | 6 |
CsA, cyclosporin A; MMF/DEX, mycophenolate mofetil/dexamethasone; eGFR, estimated GFR; UP/C, urinary protein/creatinine ratio.
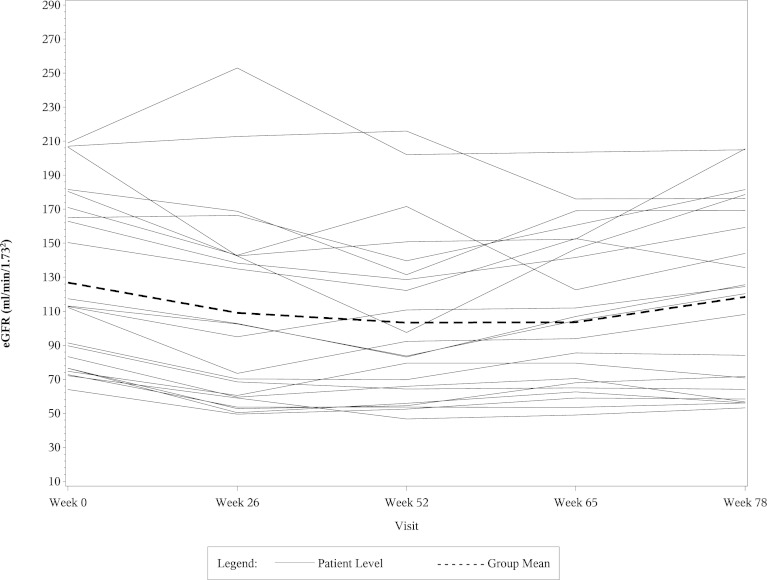
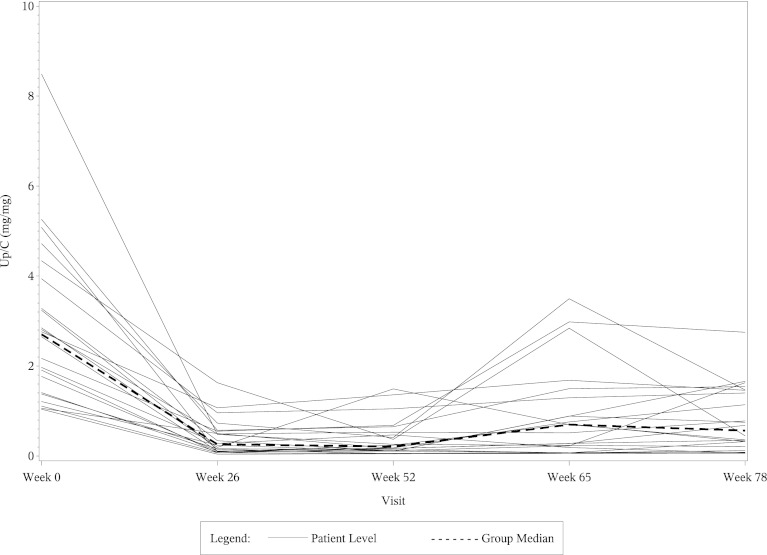
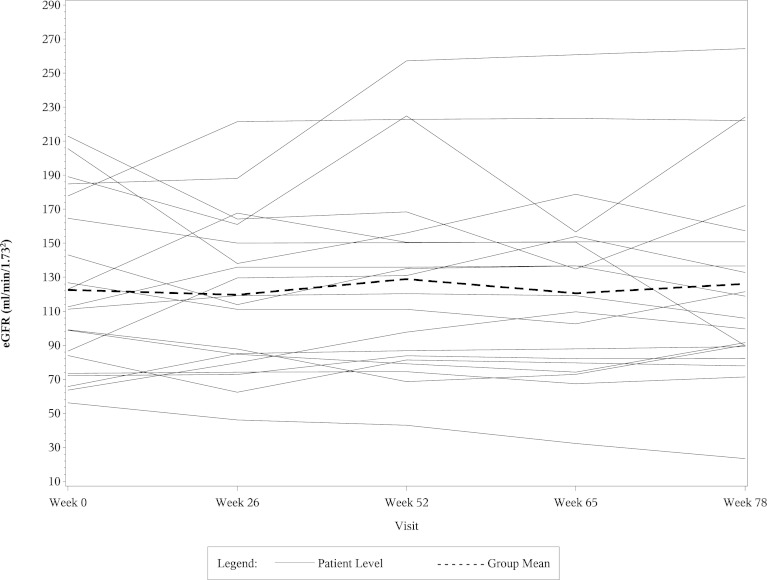
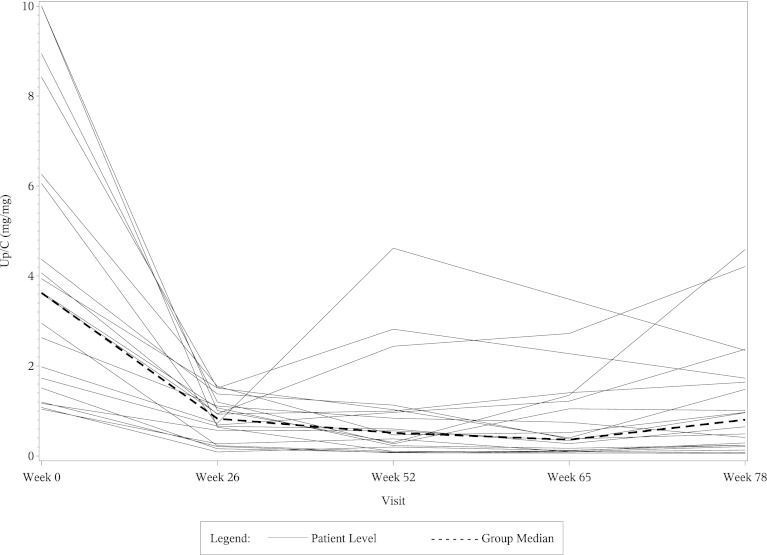
The left side of Table 4 shows the mean eGFR and median UP/C values at weeks 0, 26, 52, and 78 in the 22 CsA responsive patients who fulfilled entry criteria for this post hoc study. The right side of Table 4 shows percentage changes in the mean eGFR and median UP/C ratios in these patients at weeks 26, 52, and 78 compared with baseline. The eGFR values fell significantly in the CsA group at weeks 26 and 52 compared with baseline, but the levels returned toward baseline in most of the patients by the week 78 visit. As shown in the table, most of the decrease in both eGFR and UP/C in the CsA group occurred in the first 6 months of therapy. When analyzed separately, the mean eGFR and median UP/C levels in the CsA group at week 26 were not significantly different from those at week 52. Table 5 shows comparable data in the 20 MMF/DEX responsive patients. The eGFR levels in the MMF/DEX group of patients remained stable throughout the study. As with the CsA group, most of the decrease in UP/C levels in the MMF/DEX group occurred in the first 6 months and the decrease from 26 weeks to 52 weeks was not statistically significant. Figures 1, 2, 3, and 4 show the timeline of individual eGFR and UP/C values in the two groups of patients.
Table 4.
eGFR and UP/C levels at weeks 0, 26, 52, and 78 in 22 patients responding to CsA
| Week | eGFR and UP/C Level | Change (wk) | % Change in eGFR and UP/C | ||||
|---|---|---|---|---|---|---|---|
| eGFR mean ± SD (ml/min per 1.73 m2) | UP/C median (10th–90th percentiles) (mg/mg) | eGFR mean ±SD (%) | P Value | UP/C median (10th–90th percentiles) (%) | P Value | ||
| 0 | 126.8±50.5 | 2.7 (1.1–5.1) | 0–26 | −17.0±13.3 | <0.001 | −89.4 (−96.2 to −62.4) | <0.001 |
| 26 | 109.1±57.1 | 0.3 (0.1–1.0) | 0–52 | −19.4±13.9 | <0.001 | −89.8 (−96.9 to −68.7) | <0.001 |
| 52 | 103.3±49.3 | 0.2 (0.1–1.1) | 0–78 | −8.4±13.3 | 0.01 | −74.7 (−96.4 to −35.6) | <0.001 |
| 78 | 118.4±53.2 | 0.6 (0.1–1.6) | 52–78 | +16.4±24.3 | 0.004 | +40.3 (−27.3 to +581.8) | 0.001 |
CsA, cyclosporin A; eGFR, estimated GFR; UP/C, urinary protein/creatinine.
Table 5.
eGFR and UP/C levels at weeks 0, 26, 52, and 78 in 20 patients responding to MMF/DEX
| Week | eGFR and UP/C Level | Change (wk) | % Change in eGFR and UP/C | ||||
|---|---|---|---|---|---|---|---|
| eGFR mean ± SD (ml/min per 1.73 m2) | UP/C median (10th–90th percentiles) (mg/mg) | eGFR mean ± SD (%) | P Value | UP/C median (10th–90th percentiles) (%) | P Value | ||
| 0 | 122.6±50.7 | 3.6 (1.1–9.6) | 0–26 | +0.8±23.1 | 0.88 | −76.2 (−91.9 to −59.7) | <0.001 |
| 26 | 119.7±46.3 | 0.8 (0.2–1.5) | 0–52 | +7.0±25.7 | 0.24 | −82.7 (−97.0 to −30.4) | <0.001 |
| 52 | 128.9±57.0 | 0.5 (0.1–2.6) | 0–78 | +3.8±28.5 | 0.56 | −80.3 (−94.5 to −0.7) | <0.001 |
| 78 | 126.1±58.9 | 0.8 (0.1–3.3) | 52–78 | −2.6±16.6 | 0.50 | +25.0 (−50.2 to 268.3) | 0.06 |
MMF/DEX, mycophenolate mofetil/dexamethasone; eGFR, estimated GFR; UP/C, urinary protein/creatinine.
Figure 1.
Line diagram of individual eGFR results and the mean eGFR from week 0 to week 78 in CsA-responsive patients. eGFR, estimated GFR; CsA, cyclosporin A.
Figure 2.
Line diagram of individual UP/C ratios and the median UP/C from week 0 to week 78 in CsA-responsive patients. UP/C, urinary protein/creatinine ratio; CsA, cyclosporin A.
Figure 3.
Line diagram of individual eGFR results and the mean eGFR from week 0 to week 78 in MMF/DEX-responsive patients. eGFR, estimated GFR; MMF/DEX, mycophenolate mofetil/dexamethasone.
Figure 4.
Line diagram of individual UP/C ratios and the median UP/C from week 0 to week 78 in MMF/DEX-responsive patients. UP/C, urinary protein/creatinine ratio; MMF/DEX, mycophenolate mofetil/dexamethasone.
Subtyping of FSGS Lesions
The distribution of lesions in the CsA group was as follows: collapsing (n=2), tip (n=2), peri-hilar (n=4), and not otherwise specified (n=14). The lesions in the MMF/DEX group were collapsing (n=2), tip (n=4), cellular (n=1), and not otherwise specified (n=13). The number of patients with each of these lesions was insufficient for analysis to predict whether the changes in eGFR or UP/C were related to the histology.
Discussion
The results of this post hoc analysis show that most of our patients with FSGS who had a good response to a 12-month course of CsA or MMF/DEX were able to maintain this response over the subsequent 6 months. These results are in conflict with some previous statements regarding the risk of increasing levels of proteinuria when CsA therapy is discontinued in patients with FSGS who show a complete or partial remission. For example, Burgess concluded that “relapse after reducing or stopping CsA is very common” in these patients (4). This was based on evidence that was available in 1999 (4). Burgess rated the evidence for this statement as grade B-level 2 (RCT with surrogate end point). However, the trial used to provide this evidence, by Ponticelli et al. in 1993, included only 14 patients who were given CsA for 6 months, after which patients with complete or partial remission received a tapering dose over the next 6 months (5). Eight of the patients received the full 12 months of therapy: five of these patients were in relapse at the end of 12 months and two were in complete or partial remission.
Additional information was provided by Cattran et al. for the North American Nephrotic Syndrome Study Group (6). They conducted an RCT comparing 6 months of therapy with CsA in 26 FSGS patients versus placebo in 23 patients. The mean dosage of CsA over the treatment period was 4.2 mg/kg per day. ACE inhibitors were not permitted during the treatment period but were allowed, albeit not required, in the follow-up period. Partial remission (n=15) or complete remission (n=3) occurred in 18 CsA-treated patients (69% of total). Eight of these 18 patients (44%) relapsed within 6 months of stopping CsA and an additional three patients (17%) relapsed in the subsequent 6 months. Cattran et al. proposed that future trials should consider longer CsA treatment periods.
A high rate of relapse in CsA-responsive adult FSGS patients was reported by Heering et al. in 2004 (7). CsA treatment was given as initial therapy for varying periods of time in 34 patients. All patients received a minimum of 6 months of treatment with CsA and the total duration of CsA therapy was 23±16 months. Complete or partial remission was seen in 21 of 34 patients, for an overall response rate of 62%. A relapse occurred in 14 of 18 patients in whom withdrawal was attempted (78%).
The only controlled trial of CsA in children with FSGS was conducted by Lieberman et al., who reported the effect of high-dose CsA in 12 patients who completed 6 months of therapy (8). The target whole blood level of CsA was 300–500 mg/ml by polyclonal RIA. All 12 patients in the CsA group showed reduction in their level of proteinuria. Unfortunately, there is no information on the rate of relapse in this group of children. It is interesting to note, however, that their complete remission/partial remission response rate was superior to that seen in other trials, perhaps due to the higher doses of CsA that were used.
It is not clear why the increase in proteinuria after immunosuppression in our patients was less than those described previously. One possibility is that all patients in our complete remission/partial remission group received 12 months of therapy with CsA, whereas other studies required only 6 months of treatment, with optional tapering doses subsequently. A second possibility is that our study may have selected “better responders” by requiring patients to achieve a first-morning UP/C level <2.0 as well as a 50% reduction, which is common to most studies, to be defined as partial remission, whereas other studies only required the 24-hour urine protein excretion to be <3.5 g/d (13). A third possibility is the benefit of continued use of ACE inhibitors after CsA or MMF/DEX was stopped. This is consistent with the recent report from Kangovi et al. that described a better outcome in children with FSGS who received ACE inhibitor or angiotensin receptor blocker therapy as first-line treatment rather than immunosuppressive drugs (14). The results in our study and the Kangovi trial (14) raise questions as to the relative benefit versus risk of these different forms of therapy, especially in patients with relatively low UP/C ratios. We are unable to answer this question on the basis of our results.
Our study was also able to examine changes in eGFR. There was a significant fall in eGFR in the CsA patients while the therapy was being administered, but this effect was reversed significantly when the treatment was discontinued (Table 3). This was despite ongoing therapy with lisinopril or losartan. It must be acknowledged that our analysis was confined to patients who responded well to CsA therapy and it is not possible to extrapolate the results to all patients with FSGS who are treated with CsA. It should also be noted that the eGFR did not return to baseline in any of the seven patients who entered the study with eGFR <90 ml/min per 1.73 m2. The mean eGFR fell from 74 at baseline to 60 ml/min per 1.73 m2 at the 78-week visit in these seven patients.
Although MMF has been reported to be of some benefit when given separately to patients with FSGS (15–18), response to the combined use of this agent with DEX and the subsequent clinical course of the patients after therapy was stopped have not been described. Our MMF/DEX-responsive patients had eGFR and proteinuria measurements that were relatively stable throughout the course of therapy and beyond. In this subset of responding FSGS patients, the decrease in UP/C in response to CsA or MMF/DEX was largely sustained for 6 months after therapy. Reduction in eGFR in the CsA group was much improved 6 months after CsA was stopped.
The FSGS Clinical Trial study design included some similar features to previous reports in the literature but differed in a number of ways that should be noted. We opted to provide the immunosuppressive medications for 12 months, but only if a patient showed a least a partial remission after 6 months and all of our patients received ACE inhibitors after the immunosuppression was discontinued. Previous studies of CsA that used a 6-month course or 6 months plus a tapering dose for an additional 6 months were more often associated with increased levels of proteinuria after therapy. We hypothesize that a longer course might produce a more sustained response, especially when the patients continue to receive an ACE inhibitor.
Disclosures
None.
Acknowledgments
We express our thanks to all of the site investigators and coordinators who assisted with patient identification, enrollment, treatment, and follow-up. A full listing of the participating investigators was provided in a previous publication (10). We also thank Mrs. Gena Garcia and Mrs. Gina Du Par for their assistance in the production and submission of this manuscript.
This study was sponsored by the NIH/NIDDK Grants U01- DK063385, DK063490, DK063455, and DK063549 and was supported by many Clinical and Translational Science Award/NIH-funded institutions for the conduct of study visits, and the use of nursing, laboratory, and outpatient research facilities throughout the trial.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.08330812/-/DCSupplemental.
References
- 1.Rydel JJ, Korbet SM, Borok RZ, Schwartz MM: Focal segmental glomerular sclerosis in adults: Presentation, course, and response to treatment. Am J Kidney Dis 25: 534–542, 1995 [DOI] [PubMed] [Google Scholar]
- 2.Troyanov S, Wall CA, Miller JA, Scholey JW, Cattran DC, Toronto Glomerulonephritis Registry Group : Focal and segmental glomerulosclerosis: Definition and relevance of a partial remission. J Am Soc Nephrol 16: 1061–1068, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Gipson DS, Chin H, Presler TP, Jennette C, Ferris ME, Massengill S, Gibson K, Thomas DB: Differential risk of remission and ESRD in childhood FSGS. Pediatr Nephrol 21: 344–349, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Burgess E: Management of focal segmental glomerulosclerosis: Evidence-based recommendations. Kidney Int Suppl 70: S26–S32, 1999 [DOI] [PubMed] [Google Scholar]
- 5.Ponticelli C, Rizzoni G, Edefonti A, Altieri P, Rivolta E, Rinaldi S, Ghio L, Lusvarghi E, Gusmano R, Locatelli F, Pasquali S, Castellani A, Della Casa-Alberighi O: A randomized trial of cyclosporine in steroid-resistant idiopathic nephrotic syndrome. Kidney Int 43: 1377–1384, 1993 [DOI] [PubMed] [Google Scholar]
- 6.Cattran DC, Appel GB, Hebert LA, Hunsicker LG, Pohl MA, Hoy WE, Maxwell DR, Kunis CL, North America Nephrotic Syndrome Study Group : A randomized trial of cyclosporine in patients with steroid-resistant focal segmental glomerulosclerosis. Kidney Int 56: 2220–2226, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Heering P, Braun N, Müllejans R, Ivens K, Zäuner I, Fünfstück R, Keller F, Krämer BK, Schollmeyer P, Risler T, Grabensee B, German Collaborative Glomerulonephritis Study Group : Cyclosporine A and chlorambucil in the treatment of idiopathic focal segmental glomerulosclerosis. Am J Kidney Dis 43: 10–18, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Lieberman KV, Tejani A: A randomized double-blind placebo-controlled trial of cyclosporine in steroid-resistant idiopathic focal segmental glomerulosclerosis in children. J Am Soc Nephrol 7: 56–63, 1996 [DOI] [PubMed] [Google Scholar]
- 9.Gipson DS, Trachtman H, Kaskel FJ, Greene TH, Radeva MK, Gassman JJ, Moxey-Mims MM, Hogg RJ, Watkins SL, Fine RN, Hogan SL, Middleton JP, Vehaskari VM, Flynn PA, Powell LM, Vento SM, McMahan JL, Siegel N, D’Agati VD, Friedman AL: Clinical trial of focal segmental glomerulosclerosis in children and young adults. Kidney Int 80: 868–878, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gipson DS, Trachtman H, Kaskel FJ, Radeva MK, Gassman J, Greene TH, Moxey-Mims MM, Hogg RJ, Watkins SL, Fine RN, Middleton JP, Vehaskari VM, Hogan SL, Vento S, Flynn PA, Powell LM, McMahan JL, Siegel N, Friedman AL: Clinical trials treating focal segmental glomerulosclerosis should measure patient quality of life. Kidney Int 79: 678–685, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwartz GJ, Haycock GB, Edelmann CM, Jr, Spitzer A: A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics 58: 259–263, 1976 [PubMed] [Google Scholar]
- 12.Cockcroft DW, Gault MH: Prediction of creatinine clearance from serum creatinine. Nephron 16: 31–41, 1976 [DOI] [PubMed] [Google Scholar]
- 13.Deegens JK, Wetzels JF: Immunosuppressive treatment of focal segmental glomerulosclerosis: Lessons from a randomized controlled trial. Kidney Int 80: 798–801, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Kangovi S, Edwards M, Woloszynek S, Mitra N, Feldman H, Kaplan BS, Meyers KE: Renin-angiotensin-aldosterone system inhibitors in pediatric focal segmental glomerulosclerosis. Pediatr Nephrol 27: 813–819, 2012 [DOI] [PubMed] [Google Scholar]
- 15.Day CJ, Cockwell P, Lipkin GW, Savage CO, Howie AJ, Adu D: Mycophenolate mofetil in the treatment of resistant idiopathic nephrotic syndrome. Nephrol Dial Transplant 17: 2011–2013, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Cattran DC, Wang MM, Appel G, Matalon A, Briggs W: Mycophenolate mofetil in the treatment of focal segmental glomerulosclerosis. Clin Nephrol 62: 405–411, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Senthil Nayagam L, Ganguli A, Rathi M, Kohli HS, Gupta KL, Joshi K, Sakhuja V, Jha V: Mycophenolate mofetil or standard therapy for membranous nephropathy and focal segmental glomerulosclerosis: A pilot study. Nephrol Dial Transplant 23: 1926–1930, 2008 [DOI] [PubMed] [Google Scholar]
- 18.de Mello VR, Rodrigues MT, Mastrocinque TH, Martins SPL, de Andrade OV, Guidoni EBM, Scheffer DK, Martini Filho D, Toporovski J, Benini V: Mycophenolate mofetil in children with steroid/cyclophosphamide-resistant nephrotic syndrome. Pediatr Nephrol 25: 453–460, 2010 [DOI] [PubMed] [Google Scholar]