Summary
Background and objectives
Despite high mortality and low levels of physical activity (PA) among patients starting dialysis, the link between low PA and mortality has not been carefully evaluated.
Design, setting, participants, & measurements
The Comprehensive Dialysis Study was a prospective cohort study that enrolled patients who started dialysis between June 2005 and June 2007 in a random sample of dialysis facilities in the United States. The Human Activity Profile (HAP) was administered to estimate PA among 1554 ambulatory enrolled patients in the Comprehensive Dialysis Study. Patients were followed until death or September 30, 2009, and the major outcome was all-cause mortality.
Results
The average age was 59.8 (14.2) years; 55% of participants were male, 28% were black, and 56% had diabetes mellitus. The majority (57.3%) had low fitness estimated from the HAP score. The median follow-up was 2.6 (interquartile range, 2.2–3.1) years. The association between PA and mortality was linear across the range of scores (1–94). After multivariable adjustment, lower adjusted activity score on the HAP was associated with higher mortality (hazard ratio, 1.30; 95% confidence interval, 1.23–1.39 per 10 points). Patients in the lowest level of fitness experienced a 3.5-fold (95% confidence interval, 2.54–4.89) increase in risk of death compared with those with average or above fitness.
Conclusions
Low levels of PA are strongly associated with mortality among patients new to dialysis. Interventions aimed to preserve or enhance PA should be prospectively tested.
Introduction
A recent study reported that age-standardized cardiovascular mortality was 8.8-fold higher among patients starting dialysis than in the general population, and noncardiovascular mortality was also substantially elevated (1). Patients on dialysis are extremely inactive compared with individuals with normal kidney function (2–5). However, the extent to which sedentary behavior contributes to mortality in the dialysis population is unknown. O’Hare et al. showed an association between sedentary behavior and mortality based on the response to a single question in the Dialysis Morbidity and Mortality Study, Wave 2 participant survey (6). Other more comprehensive data describing physical activity among patients on dialysis were derived from small, single-center studies (3–5). To obtain detailed information about physical activity within a broader cohort, we administered the Human Activity Profile (HAP) to participants in the Comprehensive Dialysis Study (CDS) (7,8). The HAP collects information on patients’ participation in 94 different activities (9) and has been previously validated against accelerometry in the dialysis population (10).
We hypothesized that lower levels of self-reported physical activity would be associated with higher mortality, even after considering the effects of advanced age and comorbid conditions.
Materials and Methods
Study Design and Participants
Adult patients who started maintenance dialysis within randomly selected centers throughout the United States between June 2005 and June 2007 were eligible to participate (7). The sampling frame of dialysis units was based on the April 7, 2005 version of the Dialysis Facility Compare database, which was merged with the 2003 Centers for Medicare and Medicaid Services (CMS) ESRD Facility Survey containing information about the number of annual incident patients in each unit. The size measure allowed for probability proportional to estimated size sampling. The following types of facilities were eliminated from the sampling frame: Veterans Administration, children only, outside the 50 states and District of Columbia, and transplant only.
The final sampling frame contained 4410 facilities and was sorted by ESRD Network, then by adjacent states within network, and finally within state in a serpentine manner by size, using SAS PROC SURVEYSELECT software (SAS Institute, Cary, NC). A sample of 335 dialysis units was selected using equal probability systematic random sampling. The facility sample matched the facility population closely on size, dialysis chain affiliation, network, and whether peritoneal dialysis was offered.
Patients received information about the study at their dialysis facility or through the mail. Those willing to participate completed and returned a signed consent form or provided verbal consent by telephone. The study was approved by institutional review boards at the location of the US Renal Data System (USRDS) Coordinating Center (University of Minnesota), the USRDS Rehabilitation/Quality of Life Special Studies Center (Emory University), and the USRDS Nutrition Special Studies Center (University of California, San Francisco). For this study, we included all ambulatory CDS participants (able to walk and transfer and without history of amputation) who completed the patient questionnaire, including the HAP.
Self-Reported Physical Activity
CDS participants were asked about physical activity as part of a structured interview administered by professional interviewers using a Computer-Assisted Telephone Interviewing system. Physical activity was assessed using the 94-item HAP, which includes assessment of activities across a broad range of energy requirements (11). Items on the HAP are ranked from lowest (1, getting in and out of chairs or bed without assistance) to highest (94, running or jogging 3 miles in ≤30 minutes) based on energy requirement. For each item, the respondent indicates whether he or she is still doing, has stopped doing, or never did the activity. The maximum activity score (MAS) is the most demanding activity that the respondent still performs. The adjusted activity score (AAS) is determined by subtracting from the MAS the total number of less demanding activities the respondent has stopped doing. The AAS is interpreted as a measure of usual physical activity level (11). The AAS can also be transformed into general fitness categories, including “low,” “fair,” and “average and above” based on extrapolation to maximum oxygen uptake values (9).Maximum oxygen consumption (Vo2max) is estimated from the AAS and then individuals are placed into fitness categories using normative data for their age and sex. For example, individuals aged <30 years would be considered to have low fitness if their estimated Vo2max was <24.5 ml/kg per minute (or 7 metabolic equivalents) and individuals aged >60 years would have low fitness if their Vo2max was <14.5 ml/kg per minute (or 4.1 metabolic equivalents).
In addition to the standard, overall activity scales, the developers of the HAP generated a set of four activity subscales that describe the type of activities, or interest areas, on which respondents focus their efforts. These include self-care activities, personal/household work activities, entertainment/social activities, and independent exercise activities, each of which consists of 8–28 items from the HAP fitting within that specific category.
Covariates and Outcomes
To complement data from the patient questionnaire, we obtained data from the Medical Evidence Form (CMS form 2728), including height, weight, primary cause of kidney disease, comorbidities, and selected laboratory values (i.e., hemoglobin, creatinine, albumin) noted before the initiation of dialysis. We considered the following comorbid conditions: diabetes mellitus, heart failure, and atherosclerotic disease (the latter defined as one or more of the following diagnoses: atherosclerotic heart disease, peripheral arterial disease, or stroke). Date of death was ascertained from the USRDS PATIENTS File.
Statistical Analyses
We described patient characteristics, including demographic information, clinical characteristics, laboratory data, and self-reported physical activity using mean and SD for continuous variables and proportions for categorical variables. Comparisons among groups defined by fitness categories were made by ANOVA and chi-squared testing as appropriate.
We evaluated predictors of mortality using Kaplan–Meier product-limit estimates and the log-rank test for groups. To evaluate the nature and complexity of the relation between physical activity and mortality, we fit a restricted cubic splines model with five knots, and examined results in graphical and in tabular form. We then performed multivariable Cox proportional hazards regression including all covariates and a squared term for body mass index (BMI) because of previous observations of a U-shaped association between BMI and mortality among patients on dialysis. Multiple imputation was used for missing values for serum albumin concentration (24.1%). Cases with missing data on other covariates were excluded, except in the case of hemoglobin, which was not included in the final model because there was no significant association between hemoglobin and mortality (hazard ratio [HR], 1.01 per g/dl; 95% confidence interval [95% CI], 0.94–1.08) or physical activity (2). We included dialysis facility in the models as a random effect to account for clustering. We tested for effect modification by fitting multiplicative interaction terms for physical activity by age, sex, or hemoglobin concentration. We also examined the associations among prespecified fitness categories and mortality, with “average or above” as the referent category. We repeated multivariable Cox regression analyses by fitness category, also including subcategories within the low fitness category (tertiles of estimated Vo2max within age/sex strata of the low fit group), deemed extremely low, very low, and low fitness. Finally, we modeled the self-care, housework, social, and exercise subscales of the HAP using the same multivariable approach as for the main scales. Two-tailed P values of <0.05 were considered statistically significant. We conducted analyses using SAS 9.2 (SAS Institute, Cary, NC) and Stata 12 software (StataCorp, College Station, TX).
Results
A total of 1678 patients from 297 dialysis clinics within 18 ESRD Networks participated in the CDS. Of these, 1643 subsequently completed the telephone interview and 1554 were ambulatory. The average age of the ambulatory participants was 59.8±14.2 years. Fifty-five percent of participants were male, 28% were African American, and 56% had diabetes mellitus (Table 1). Patients with low fitness estimated from the HAP AAS were less likely to be male and to be on peritoneal dialysis. Low fit patients had more comorbidity, higher BMI, and lower serum albumin and creatinine concentrations on average than those with better estimated fitness.
Table 1.
Patient characteristics by fitness categorya
| Characteristic | All (n=1554) | Average or Above (n=347) | Fair (n=317) | Low (n=890) | P Value |
|---|---|---|---|---|---|
| Age, yr | 59.8 (14.2) | 59.6 (12.8) | 60.0 (14.7) | 59.7 (14.6) | 0.89 |
| Sex, % male | 55.3 | 73.8 | 61.8 | 45.8 | <0.001 |
| Race, % black | 28.2 | 22.2 | 30.6 | 29.7 | 0.02 |
| Body mass index, kg/m2 | 29.7 (7.9) | 28.7 (6.9) | 28.8 (7.2) | 30.4 (8.4) | 0.002 |
| Albumin, g/dl | 3.2 (0.7) | 3.3 (0.7) | 3.3 (0.7) | 3.1 (0.7) | <0.001 |
| Hemoglobin, g/dl | 10.1 (1.8) | 10.1 (1.8) | 10.2 (1.7) | 10.1 (1.8) | 0.45 |
| Serum creatinine, mg/dl | 6.9 (3.5) | 8.0 (3.9) | 7.1 (3.6) | 6.4 (3.2) | <0.001 |
| Modality/access, % | |||||
| Peritoneal dialysis | 10.5 | 11.9 | 16.3 | 7.9 | <0.001 |
| Hemodialysis, catheter | 49.7 | 42.0 | 42.2 | 55.4 | |
| Hemodialysis, graft | 10.0 | 9.3 | 9.6 | 10.4 | |
| Hemodialysis, fistula | 29.8 | 36.8 | 31.9 | 26.2 | |
| Comorbidity, % | |||||
| Atherosclerosis | 32.0 | 26.5 | 30.0 | 34.9 | 0.01 |
| Congestive heart failure | 29.8 | 23.6 | 27.1 | 33.1 | 0.002 |
| Diabetes mellitus | 56.2 | 43.8 | 51.7 | 62.7 | <0.001 |
| Current smoker, % | 15.4 | 13.0 | 18.0 | 15.4 | 0.20 |
| Some college education or more, % | 44.8 | 54.4 | 49.7 | 39.3 | <0.001 |
| Maximum activity score | 62 (16) | 77.9 (7.5) | 68.1 (8.5) | 53.1 (13.7) | <0.001 |
| Adjusted activity score | 44 (18) | 67.4 (8.8) | 53.8 (6.0) | 31.3 (11.6) | <0.001 |
Fitness category was estimated based on the adjusted activity score of the Human Activity Profile.
Patients were followed for a median of 2.6 (interquartile range, 2.2–3.1) years; there were 470 deaths (30%). The nature of the association between physical activity and survival was examined using cubic spline analysis with five knots. For AAS, the deviations from linearity were minor, and overall the relation between AAS and survival was remarkably linear. The MAS exhibited a slightly steeper slope at lower levels of physical activity and flattened somewhat at higher levels of activity, but the association did not deviate in a statistically or clinically significant manner from a linear function.
Multivariable analysis confirmed an inverse association between physical activity and mortality after adjustment for demographics, comorbid conditions, and selected laboratory values (HR, 1.30; 95% CI, 1.23–1.39 per 10 points lower for AAS; and HR, 1.23; 95% CI, 1.16–1.32 per 10 points lower for MAS) (Table 2). The association between physical activity and mortality did not differ significantly based on age or sex (both P>0.20 for interactions).
Table 2.
Association between physical activity and mortality in multivariable Cox proportional hazards models
| Variable | Maximum Activity Score | Adjusted Activity Score | ||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Activity, per 10 points lower | 1.23 (1.16–1.32) | <0.001 | 1.30 (1.23–1.39) | <0.001 |
| Age, per 10 yr | 1.37 (1.25–1.50) | <0.001 | 1.37 (1.24–1.50) | <0.001 |
| Female sex | 0.82 (0.68–0.99) | 0.04 | 0.79 (0.65–0.95) | 0.02 |
| Black race | 0.70 (0.56–0.90) | 0.004 | 0.70 (0.55–0.89) | 0.004 |
| BMI, kg/m2 | 0.95 (0.89–1.00) | 0.08 | 0.94 (0.89–1.00) | 0.05 |
| BMI2 | 1.00 (1.00–1.00) | 0.08 | 1.00 (1.00–1.00) | 0.06 |
| Albumin, g/dl | 0.88 (0.75–1.03) | 0.10 | 0.90 (0.77–1.05) | 0.17 |
| Creatinine, mg/dl | 0.97 (0.94–1.01) | 0.15 | 0.99 (0.96–1.02) | 0.50 |
| Modality/access | ||||
| Hemodialysis, catheter (ref) | 1.00 | 1.00 | ||
| Hemodialysis, graft | 0.81 (0.56–1.17) | 0.26 | 0.83 (0.57–1.19) | 0.30 |
| Hemodialysis, fistula | 0.85 (0.68–1.08) | 0.18 | 0.85 (0.68–1.08) | 0.19 |
| Peritoneal dialysis | 1.25 (0.90–1.73) | 0.19 | 1.33 (0.97–1.84) | 0.08 |
| Comorbidity | ||||
| Atherosclerosis | 1.20 (0.98–1.47) | 0.08 | 1.16 (0.95–1.42) | 0.15 |
| Congestive heart failure | 1.18 (0.97–1.43) | 0.10 | 1.17 (0.96–1.42) | 0.12 |
| Diabetes | 1.09 (0.87–1.35) | 0.47 | 1.03 (0.83–1.29) | 0.76 |
| Smoking | 1.59 (1.22–2.07) | 0.001 | 1.57 (1.20–2.05) | 0.001 |
| Education (some college or more) | 0.92 (0.75–1.12) | 0.39 | 0.87 (0.72–1.06) | 0.18 |
There were 1518 patients included in the analyses after exclusion of participants with missing data for covariates. HR, hazard ratio; CI, confidence interval; BMI, body mass index.
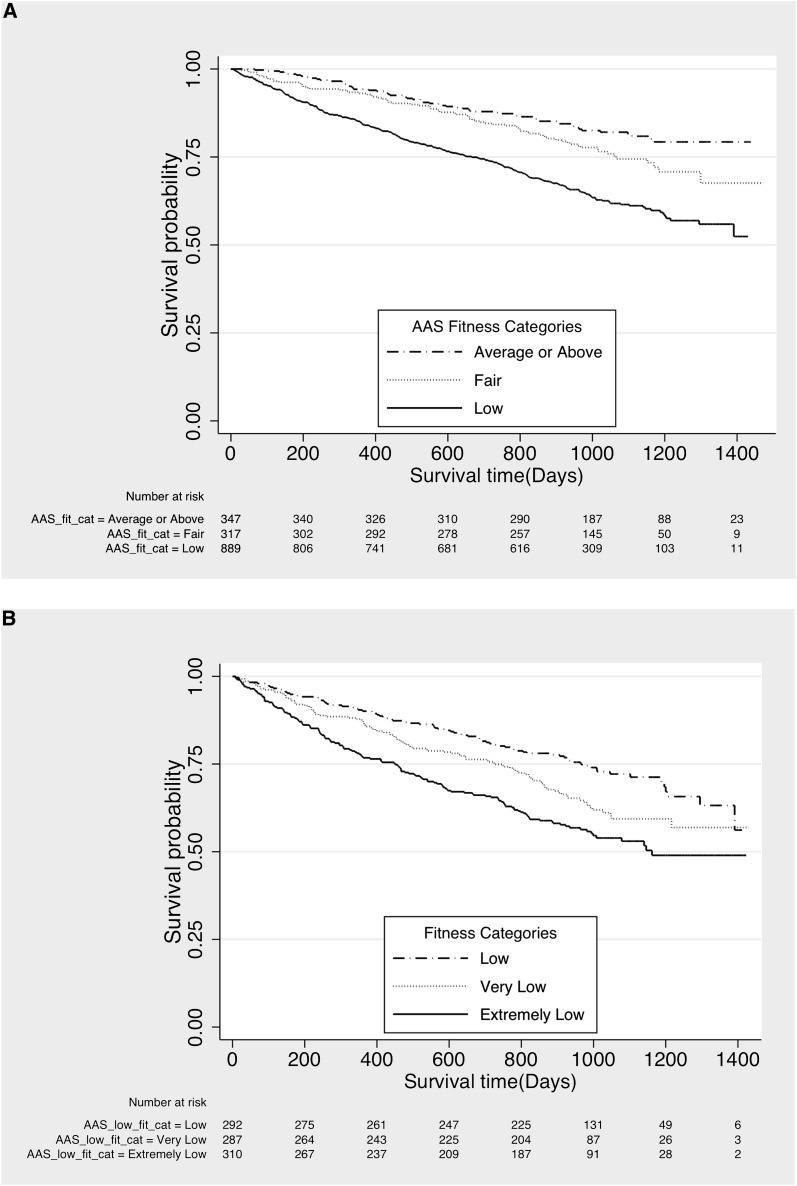
The majority of CDS participants (57.3%) fell into the low fitness category based on their AAS; only 22.3% had fitness that was average or above. Figure 1A shows a graded association between fitness level and survival. Figure 1B shows that even among patients with low fitness, there was a “dose response”—in which the least fit patients experienced the highest mortality rates—3.5-fold higher than the group with average or above fitness based on HAP scores, even after adjustment for age, comorbidities, and other covariates (Table 3).
Figure 1.
Kaplan–Meier plots showing survival. (A) Kaplan–Meier plot showing survival by fitness category, estimated using the adjusted activity scale (AAS) of the Human Activity Profile. The solid line represents the low fitness group, the dotted line the fair fitness group, and the dashed line the group with average or above fitness. (B) Kaplan-Meier plot showing survival by tertiles within the low fitness category. The solid line represents the extremely low tertile, the dotted line the very low tertile, and the dashed line the low tertile.
Table 3.
Association between fitness categories and physical activity subscales and mortality in multivariable Cox proportional hazards models
| Variable | n | HR (95% CI) | P Value |
|---|---|---|---|
| Fitness category | 1517 | ||
| Average or above | 1.00 | Referent | |
| Fair | 1.34 (0.97–1.84) | 0.08 | |
| Low | 1.82 (1.30–2.55) | <0.001 | |
| Very low | 2.56 (1.88–3.49) | <0.001 | |
| Extremely low | 3.53 (2.54–4.89) | <0.001 | |
| Activity subscales, per 1 point lower | |||
| Self-care | 1517 | 1.15 (1.09–1.21) | <0.001 |
| Personal/household work | 1499 | 1.05 (1.03–1.07) | <0.001 |
| Entertainment/social | 1510 | 1.15 (1.11–1.19) | <0.001 |
| Independent exercise | 1469 | 1.14 (1.10–1.19) | <0.001 |
Patients with missing data for covariates or subscales were excluded. Models were adjusted for age, sex, race, BMI, BMI2, serum albumin concentration, serum creatinine concentration, dialysis modality and access type, diagnosis of congestive heart failure, diabetes mellitus, or atherosclerosis (coronary artery disease, peripheral vascular disease, stroke, or transient ischemic attack), smoking, and level of education (some college or more versus no college). HR, hazard ratio; CI, confidence interval; BMI, body mass index.
All of the individual activity subscales were also associated with survival in multivariable analysis, with substantial increase in mortality observed for every one-point decrease in each scale. HRs for the self-care, housework, social, and exercise subscales were 1.15, 1.05, 1.15, and 1.14, respectively (P<0.0001 for all) (Table 3).
Discussion
Low self-reported physical activity was an independent predictor of mortality among CDS participants. For every 10 points lower on the AAS, which is reflective of an individual’s daily activities, mortality was 30% higher after adjustment for other factors. In contrast to the paradoxical associations with mortality that have been observed among patients with ESRD for other traditional cardiovascular risk factors such as hypertension (12), hyperlipidemia (13), and obesity (14), the hazards associated with low physical activity in this study were larger in magnitude than those typically observed in the general population (15). Furthermore, there was no threshold level of activity above or below which greater participation in physical activity was not associated with better survival. These results are consistent with data emerging among healthier individuals suggesting that the association between physical activity and mortality or cardiovascular disease may accrue even at levels of activity below recommended levels. Specifically, although 30 minutes of moderate activity per day is recommended to reduce mortality and cardiovascular risk (16), as few as 10–15 minutes per day is also now recognized to be associated with benefit (15,17,18). In fact, the greatest benefit may be found in moving from no activity to low levels of activity (19), including walking and other moderate-intensity activity performed as part of daily life (15).
The persistence of the linear association between mortality and self-reported physical activity even at extremely low levels may be useful to inform the design and implementation of interventions to increase activity in this population. Our results suggest that it would be worthwhile to evaluate whether even small increases in physical activity can improve survival. The benefits of 30 minutes of moderate activity on most days are well documented in the general population (16,20). Although Kidney Disease Outcomes Quality Initiative (KDOQI) Clinical Practice Guidelines for cardiovascular disease recommend cardiovascular exercise at a moderate intensity for 30 minutes most, if not all, days per week (21), some have suggested that this may be an unrealistic goal for patients on dialysis (22). The possibility that more modest goals could have merit is exciting because it is likely that more modest goals could be achieved more consistently and by more patients. Limited activity targets could be implemented more easily by dialysis providers, who could counsel patients to walk more without extensive training and without the need to refer patients for cardiac screening, which might be necessary before commencing vigorous exercise.
Our study has several strengths, including a relatively large sample size, near complete data collection, and diversity by age, sex, race, geography, comorbid conditions, and dialysis modality. However, there are several important limitations. First, despite the strong association between physical activity and survival, the CDS was an observational study; we are unable to determine whether increasing physical activity would result in reduced mortality. We cannot rule out the possibility that residual confounding by general health status, severity of illness, depression, dementia, or other unmeasured factors contributed to the association between lower self-reported physical activity and mortality. Nevertheless, the HAP appears to provide important, independent prognostic information even if the relation between physical activity and mortality is not a causal one. The magnitude of a 10-point difference on the AAS was similar to that of a 10-year increment in age, and greater than that of a low serum albumin concentration before starting dialysis. In addition to its observational design, the study is limited by the use of the HAP, which does not quantify the amount of physical activity done in a specific time interval. Thus, we could not determine which patients were meeting the levels of physical activity (≥30 minutes of moderate activity on most days) recommended by the KDOQI, Surgeon General, and Centers for Disease Control and Prevention. Nevertheless, we previously validated the HAP against measured physical activity in the dialysis population (10), and the HAP has the advantage of ease of administration and scoring (9,11). Our data also suggest that the shorter activity subscales might offer even more rapid assessment tools; these assessments may also be of value to providers interested in functional and vocational rehabilitation.
In summary, this study demonstrates a strong association between low physical activity and mortality among patients new to dialysis in the United States. Future studies are needed to determine whether increasing physical activity is feasible in this population and whether such changes could result in improved outcomes. The large magnitude of the association suggests that these studies should be given high priority.
Disclosures
K.L.J. has received grant support from Abbott Laboratories Inc and has served on Amgen’s National Nephrology Advisory Board.
Acknowledgments
This study was supported by the National Institutes of Health (Contracts N01-DK-1-2450 to G.M.C. and N01-DK-7-0005 to K.L.J.). The interpretation and reporting of the data presented here are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the US government.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.de Jager DJ, Grootendorst DC, Jager KJ, van Dijk PC, Tomas LM, Ansell D, Collart F, Finne P, Heaf JG, De Meester J, Wetzels JFM, Rosendaal FR, Dekker FW: Cardiovascular and noncardiovascular mortality among patients starting dialysis. JAMA 302: 1782–1789, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Johansen KL, Chertow GM, Kutner NG, Dalrymple LS, Grimes BA, Kaysen GA: Low level of self-reported physical activity in ambulatory patients new to dialysis. Kidney Int 78: 1164–1170, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johansen KL, Chertow GM, Ng AV, Mulligan K, Carey S, Schoenfeld PY, Kent-Braun JA: Physical activity levels in patients on hemodialysis and healthy sedentary controls. Kidney Int 57: 2564–2570, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Cupisti A, Capitanini A, Betti G, D’Alessandro C, Barsotti G: Assessment of habitual physical activity and energy expenditure in dialysis patients and relationships to nutritional parameters. Clin Nephrol 75: 218–225, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Baria F, Kamimura MA, Avesani CM, Lindholm B, Stenvinkel P, Draibe SA, Cuppari L: Activity-related energy expenditure of patients undergoing hemodialysis. J Ren Nutr 21: 226–234, 2011 [DOI] [PubMed] [Google Scholar]
- 6.O’Hare AM, Tawney K, Bacchetti P, Johansen KL: Decreased survival among sedentary patients undergoing dialysis: Results from the dialysis morbidity and mortality study wave 2. Am J Kidney Dis 41: 447–454, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Kutner NG, Johansen KL, Kaysen GA, Pederson S, Chen SC, Agodoa LY, Eggers PW, Chertow GM: The comprehensive dialysis study (CDS): A USRDS special study. Clin J Am Soc Nephrol 4: 645–650, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bao Y, Dalrymple L, Chertow GM, Kaysen GA, Johansen KL: Frailty, dialysis initiation, and mortality in end-stage renal disease. Arch Intern Med 172: 1071–1077, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fix AJ, Daughton DM: Human Activity Profile Professional Manual, Odessa, FL, Psychological Assessment Resources Inc, 1988 [Google Scholar]
- 10.Johansen KL, Painter P, Kent-Braun JA, Ng AV, Carey S, Da Silva M, Chertow GM: Validation of questionnaires to estimate physical activity and functioning in end-stage renal disease. Kidney Int 59: 1121–1127, 2001 [DOI] [PubMed] [Google Scholar]
- 11.Daughton DM, Fix AJ: Human Activity Profile Test Booklet, Odessa, FL, Psychological Assessment Resources Inc, 1986 [Google Scholar]
- 12.Chien CC, Yen CS, Wang JJ, Chen HA, Chou MT, Chu CC, Chio CC, Hwang JC, Wang HY, Lu YH, Kan WC: Reverse epidemiology of hypertension-mortality associations in hemodialysis patients: A long-term population-based study. Am J Hypertens 25: 900–906, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Bowden RG, La Bounty P, Shelmadine B, Beaujean AA, Wilson RL, Hebert S: Reverse epidemiology of lipid-death associations in a cohort of end-stage renal disease patients. Nephron Clin Pract 119: c214–c219, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Kalantar-Zadeh K, Abbott KC, Salahudeen AK, Kilpatrick RD, Horwich TB: Survival advantages of obesity in dialysis patients. Am J Clin Nutr 81: 543–554, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Samitz G, Egger M, Zwahlen M: Domains of physical activity and all-cause mortality: Systematic review and dose-response meta-analysis of cohort studies. Int J Epidemiol 40: 1382–1400, 2011 [DOI] [PubMed] [Google Scholar]
- 16.US Department of Health and Human Services : 2008 Physical Activity Guidelines for Americans, Washington, DC, US Department of Health and Human Services, 2008 [Google Scholar]
- 17.Sattelmair J, Pertman J, Ding EL, Kohl HW, 3rd, Haskell W, Lee IM: Dose response between physical activity and risk of coronary heart disease: A meta-analysis. Circulation 124: 789–795, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wen CP, Wai JP, Tsai MK, Yang YC, Cheng TY, Lee MC, Chan HT, Tsao CK, Tsai SP, Wu X: Minimum amount of physical activity for reduced mortality and extended life expectancy: A prospective cohort study. Lancet 378: 1244–1253, 2011 [DOI] [PubMed] [Google Scholar]
- 19.Woodcock J, Franco OH, Orsini N, Roberts I: Non-vigorous physical activity and all-cause mortality: Systematic review and meta-analysis of cohort studies. Int J Epidemiol 40: 121–138, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Office of the US Surgeon General : Physical Activity and Health: A Report of the Surgeon General, Washington, DC, US Department of Health and Human Services, National Center for Chronic Disease Prevention and Health Promotion, 1996 [Google Scholar]
- 21.K/DOQI Workgroup : K/DOQI Clinical Practice Guidelines: Cardiovascular disease in dialysis patients. Am J Kidney Dis 45: S1–S153, 2005 [PubMed] [Google Scholar]
- 22.Johansen KL, Sakkas GK, Doyle J, Shubert T, Dudley RA: Exercise counseling practices among nephrologists caring for patients on dialysis. Am J Kidney Dis 41: 171–178, 2003 [DOI] [PubMed] [Google Scholar]