Epidemiologic research examines relationships between exposures and outcomes, with the ultimate goal of improving patient outcomes. To affect a change and improve outcomes, the relationships described must not only be causal, but modifiable (1). Numerous studies have described independent associations between AKI and subsequent CKD and mortality (2). The majority of these studies focused on one dimension of AKI, “severity,” via the magnitude of peak rise in serum creatinine (2–4). Recently, some investigations have examined long-term outcomes associated with other dimensions of AKI including duration (5) and kidney function after peak (i.e., recovery) (6–8).
Pannu and colleagues performed a retrospective analysis of 190,962 Albertans to assess the modifying effect of renal recovery on the relationship between AKI and long-term outcomes (9). They found that participants who did not recover to within 25% of baseline GFR within 90 days of AKI were at higher risk for doubling of creatinine and ESRD, and for all-cause mortality. It is a well done study with several strengths. First, this is a large population-based study with appropriate inclusion/exclusion criteria, which limited selection bias. Participants were required to have serum creatinine measurement within 180 days before, ≥1 measurement during, and at least one measurement after hospitalization; only those who survived at least 90 days post-AKI were included in the analyses. Second, the AKI definition used for the primary exposure was appropriate (Kidney Disease Improving Global Outcomes stage 2). Third, the recovery definition was valid and sound (post-AKI creatinine value within 25% of baseline within 90 days, and sensitivity analyses performed using 10% increments to find the threshold for the association between recovery and outcomes). Finally, the dataset provided sufficient outcomes for robust analyses and multivariable adjustment, and there was adequate length of follow-up (median 3 years).
Unlike other studies that have examined the association between reversible AKI and long-term outcomes (6,7), Pannu and colleagues treated reversible AKI (instead of the non-AKI group) as the referent group for their analyses. In the follow-up period, 27% of those who did not experience AKI recovery developed doubling of serum creatinine/ESRD compared with 7.5% in those that recovered (adjusted hazard ratio of approximately 4). These data certainly quantify the public health effect of irreversible AKI in a broad, nonselected population. Because nonrecovery from AKI results in a substantial healthcare burden, these data can help clinicians make plans for follow-up with patients, their primary care providers, and nephrologists after discharge (10).
The authors did not make any claims about causality in their paper. Looking forward, however, it is important to determine whether we can affect and improve these outcomes. Reiterating the above, the relationship between the exposure and outcome must be causal in order to modify risk. Are the relationships between AKI and long-term outcomes causal? Pannu et al. adjusted for several demographic characteristics, comorbidities, primary admission diagnoses, and resource intensity weights. Nevertheless, the “independent associations” described in the fully adjusted models may still represent a biased estimate of the true independent and biologically based relative risks. First, those with nonrecovery from AKI had a large “head-start” toward reaching the composite renal endpoint. An appropriate sensitivity analysis could have examined the relative risk of doubling of serum creatinine from this new baseline at 90 days post-AKI. Second, nonrecovery from AKI may be due to several nonrenal factors, such as congestive heart failure, chronic infection, and so forth. The re-assessment of kidney function 90 days after the hospitalization, without re-assessment of comorbidities, physiologic variables, and laboratory variables reflecting the severity of dysfunction of other organs (i.e., time-updated covariates), biases toward exaggerating the effect of the kidney on the outcomes, even if the kidneys are simply serving as a surrogate for the overall health status of the patient (see number 2 in Figure 1).
Figure 1.
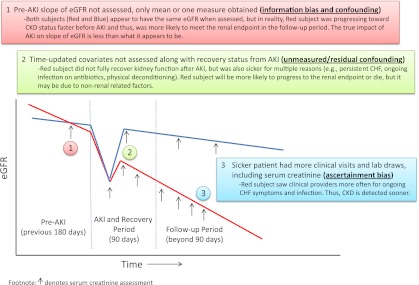
Possible sources of bias and confounding in observational studies as demonstrated using two hypothetical subjects with AKI. eGFR, estimated GFR; CHF, congestive heart failure.
Furthermore, examination of recovery versus nonrecovery from AKI does not answer whether it was AKI per se that was responsible for the increased risk of poor outcomes. Instead, those with reversible AKI versus those without AKI need to be compared. In this regard, several animal models suggest that one isolated episode of AKI that is completely reversible leads to CKD and renal fibrosis (11). In this study, after full adjustment, the AKI-recovered group had 30%–40% higher risk for CKD and for death versus the no-AKI group (see Supplemental Table 2 in the article by Pannu et al.) (9). This association is potentially confounded; patients that recovered from AKI versus no-AKI patients were sicker at baseline (older, more cardiovascular disease, heart failure, diabetes, malignancy, lower baseline GFR, higher Charlson Comorbidity Index score, and higher resource intensity weight). These variables, however, still do not completely capture burden of illness, because physiologic and laboratory variables were not included. Data on proteinuria, a potent risk factor for both AKI (12) and CKD progression (13), were lacking. Moreover, severity of illness is underestimated using diagnostic codes (14), and cardiovascular disease prediction is improved by using variables such as hemoglobin A1C rather than diabetes status alone (15). As evidence of the importance of granular data, it has been demonstrated that in patients with congestive heart failure, AKI that was induced by physician treatment (via aggressive diuresis, decongestion, hemoconcentration) was actually associated with improved outcomes (16,17), and those that experienced prompt improvement in kidney function (i.e., recovery from AKI) actually had worse clinical outcomes (18,19). The reason for these paradoxical associations may be due to the copious amounts of physiologic and laboratory data to identify different phenotypes of AKI in the studies by Testani et al. (16–19) The bottom line is that the associations between acute change in kidney function and clinical outcomes most likely depend on factors that drive that change in GFR more than the change itself.
In observational studies using clinically available laboratory data, there is the possibility of differential misclassification of outcome due to ascertainment bias. Those with AKI had nearly twice the mean number of assessments of serum creatinine in the follow-up period (n=25 in the group AKI versus n=14 in the no-AKI group) (9). The more something is tested for, the more likely it is that it will be detected (see number 3 in Figure 1). The composite renal outcome was subject to ascertainment bias because it was driven by doubling of serum creatinine (10%) and not ESRD (2%) (9).
Moreover, distinguishing progressive CKD with intercurrent episodes of AKI versus AKI-induced CKD is difficult in observational studies. CKD is the most important prognostic factor for an AKI episode (12,20). Unless there is a prolonged ischemic event (e.g., cardiac arrest) with obvious acute tubular necrosis, teasing out the contribution of the episode of AKI toward further renal injury is nearly impossible. Underlying glomerulosclerosis and tubulointerstitial fibrosis heighten susceptibility to AKI from any stressor. Thus, causal directionality and temporality become intricately intertwined. Elucidation that AKI actually changed pre-AKI slope of declining GFR may provide insight into this enigma (21). Few studies have determined the pre-AKI slope, and most instead adjust for the static pre-AKI GFR (see number 1 in Figure 1). One study that identified four different trajectories of kidney function decline before ESRD showed that AKI was more common in those with fast progression to ESRD, yet precipitants such as “hematologic malignancy,” “other,” and “unknown” were also much more common in fast progressors (22).
The death outcome should be more valid than CKD, because death is not subject to ascertainment bias. The fully adjusted risk for death with no AKI was 0.69 (versus AKI-recovered), and it was also 0.65 in patients that had AKI with “recovery status unknown” (9). A naive epidemiologist would say, “Eureka! AKI-induced death can be ameliorated by not checking serum creatinine!” Clearly this is a flawed argument; instead, these data indicate that clinician intuition provided the strongest risk discrimination for poor outcomes among all the clinical variables captured. In another study of patients with reversible AKI, by far, the strongest predictor of post-AKI CKD was the variable “serum albumin test ordered during the 12 months before index admission date” (adjusted hazard ratio, 4.27; 95% confidence interval, 2.38–7.67) (6). Given that ordering a test (creatinine and albumin, respectively) was overwhelmingly the strongest predictor of CKD and death in these studies demonstrates the degree of residual confounding that is not captured by variables in datasets. Remarkably, two other studies demonstrated that the association between reversible AKI and long-term mortality was abolished by using propensity-score based matching (7,8). Thus, the only way to definitively overcome these biases and residual confounding in observational datasets is to examine the association between AKI recovery and hard outcomes in randomized controlled trials (RCTs). Regrettably, a renal-specific therapy that effectively ameliorates AKI does not exist. Interestingly, chloride-poor intravenous fluids reduced AKI by nearly 50% in one study, yet there was no difference in mortality, length of stay, or ESRD (23).
Although the notion that AKI will be a modifiable risk factor for long-term outcomes needs to be tested, many recent trials have generally indicated that moving the surrogate marker in the desired direction has not translated into consistent improvements in clinical outcomes. For example, although it has been dogma in the nephrology community that baseline proteinuria and treatment-induced changes in proteinuria are strongly associated with risk for CKD progression, recent large RCTs have not demonstrated improved clinical outcomes commensurate with reductions in proteinuria (24–26). Moreover, years ago, nobody would have believed that raising hemoglobin would not have translated into improved clinical outcomes in CKD (27). Dissociation between surrogate and hard outcomes have emerged for BP in patients with diabetes (28), GFR in CKD (29), HDL for cardiovascular disease (30), and fluid-resuscitation for children with infection and poor perfusion (31). Because we do not know for certain whether the kidneys are the cause or the consequence of the “badness,” we must acknowledge the uncomfortable possibility that prevention of an acute rise in serum creatinine and/or reduction in kidney injury via a kidney-specific therapy may or may not translate into meaningful improvement in clinical outcomes. If the kidneys are largely serving as an exquisitely sensitive barometer of systemic dysfunction, it is possible that expecting an AKI treatment to improve long-term outcomes is akin to expecting application of nail polish on the yellow fingernails of smokers to reduce risk of lung cancer.
In summary, the associations observed between AKI and CKD/mortality may be influenced by missing data on important confounders (pre-AKI GFR slope, physiologic and laboratory variables during the acute illness), data on comorbidities and organ function after the initial hospitalization (time-updated variables), and ascertainment bias (sicker patients have more assessments on follow-up). This is supported by the fact that physician judgment to assess serum creatinine in the follow-up period was the variable with the strongest association with long-term survival (9). Thus, this important study by Pannu and colleagues allows us to better understand the burden of CKD after AKI that does not “appear” to recover. RCTs will be needed, however, to determine whether facilitating and enabling AKI recovery will translate into improved outcomes.
Disclosures
None.
Acknowledgments
S.G.C. is supported by a Career Development Award from the National Institutes of Health (NIH) (K23DK080132) to study long-term outcomes after AKI, and is also a member of the NIH-sponsored ASSESS AKI Consortium (Assessment, Serial Evaluation, and Subsequent Sequelae of Acute Kidney Injury) (U01DK082185).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related article, “Association between AKI, Recovery of Renal Function, and Long-Term Outcomes after Hospital Discharge,” on pages 194–202.
References
- 1.Grimes DA, Schulz KF: Bias and causal associations in observational research. Lancet 359: 248–252, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Coca SG, Singanamala S, Parikh CR: Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int 81: 442–448, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chawla LS, Amdur RL, Amodeo S, Kimmel PL, Palant CE: The severity of acute kidney injury predicts progression to chronic kidney disease. Kidney Int 79: 1361–1369, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ishani A, Nelson D, Clothier B, Schult T, Nugent S, Greer N, Slinin Y, Ensrud KE: The magnitude of acute serum creatinine increase after cardiac surgery and the risk of chronic kidney disease, progression of kidney disease, and death. Arch Intern Med 171: 226–233, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Coca SG, King JT, Jr, Rosenthal RA, Perkal MF, Parikh CR: The duration of postoperative acute kidney injury is an additional parameter predicting long-term survival in diabetic veterans. Kidney Int 78: 926–933, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bucaloiu ID, Kirchner HL, Norfolk ER, Hartle JE, 2nd, Perkins RM: Increased risk of death and de novo chronic kidney disease following reversible acute kidney injury. Kidney Int 81: 477–485, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Jones J, Holmen J, De Graauw J, Jovanovich A, Thornton S, Chonchol M: Association of complete recovery from acute kidney injury with incident CKD stage 3 and all-cause mortality. Am J Kidney Dis 60: 402–408, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wald R, Quinn RR, Luo J, Li P, Scales DC, Mamdani MM, Ray JG; University of Toronto Acute Kidney Injury Research Group: Chronic dialysis and death among survivors of acute kidney injury requiring dialysis. JAMA 302: 1179–1185, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Pannu N, James M, Hemmelgarn B, Klarenbach S; for the Alberta Kidney Disease Network: Association between AKI, recovery of renal function, and long-term outcomes after hospital discharge. Clin J Am Soc Nephrol 8: 194–202, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siew ED, Peterson JF, Eden SK, Hung AM, Speroff T, Ikizler TA, Matheny ME: Outpatient nephrology referral rates after acute kidney injury. J Am Soc Nephrol 23: 305–312, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Basile DP, Donohoe D, Roethe K, Osborn JL: Renal ischemic injury results in permanent damage to peritubular capillaries and influences long-term function. Am J Physiol Renal Physiol 281: F887–F899, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Grams ME, Astor BC, Bash LD, Matsushita K, Wang Y, Coresh J: Albuminuria and estimated glomerular filtration rate independently associate with acute kidney injury. J Am Soc Nephrol 21: 1757–1764, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tonelli M, Muntner P, Lloyd A, Manns BJ, James MT, Klarenbach S, Quinn RR, Wiebe N, Hemmelgarn BR; Alberta Kidney Disease Network: Using proteinuria and estimated glomerular filtration rate to classify risk in patients with chronic kidney disease: A cohort study. Ann Intern Med 154: 12–21, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Quan H, Parsons GA, Ghali WA: Validity of information on comorbidity derived from ICD-9-CCM administrative data. Med Care 40: 675–685, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Paynter NP, Mazer NA, Pradhan AD, Gaziano JM, Ridker PM, Cook NR: Cardiovascular risk prediction in diabetic men and women using hemoglobin A1c vs diabetes as a high-risk equivalent. Arch Intern Med 171: 1712–1718, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Testani JM, Kimmel SE, Dries DL, Coca SG: Prognostic importance of early worsening renal function after initiation of angiotensin-converting enzyme inhibitor therapy in patients with cardiac dysfunction. Circ Heart Fail 4: 685–691, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Testani JM, Chen J, McCauley BD, Kimmel SE, Shannon RP: Potential effects of aggressive decongestion during the treatment of decompensated heart failure on renal function and survival. Circulation 122: 265–272, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Testani JM, McCauley BD, Chen J, Coca SG, Cappola TP, Kimmel SE: Clinical characteristics and outcomes of patients with improvement in renal function during the treatment of decompensated heart failure. J Card Fail 17: 993–1000, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Testani JM, McCauley BD, Kimmel SE, Shannon RP: Characteristics of patients with improvement or worsening in renal function during treatment of acute decompensated heart failure. Am J Cardiol 106: 1763–1769, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsu CY, Ordoñez JD, Chertow GM, Fan D, McCulloch CE, Go AS: The risk of acute renal failure in patients with chronic kidney disease. Kidney Int 74: 101–107, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu KD, Lo L, Hsu CY: Some methodological issues in studying the long-term renal sequelae of acute kidney injury. Curr Opin Nephrol Hypertens 18: 241–245, 2009 [DOI] [PubMed] [Google Scholar]
- 22.O’Hare AM, Batten A, Burrows NR, Pavkov ME, Taylor L, Gupta I, Todd-Stenberg J, Maynard C, Rodriguez RA, Murtagh FE, Larson EB, Williams DE: Trajectories of kidney function decline in the 2 years before initiation of long-term dialysis. Am J Kidney Dis 59: 513–522, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yunos NM, Bellomo R, Hegarty C, Story D, Ho L, Bailey M: Association between a chloride-liberal vs chloride-restrictive intravenous fluid administration strategy and kidney injury in critically ill adults. JAMA 308: 1566–1572, 2012 [DOI] [PubMed] [Google Scholar]
- 24.Parving HH, Brenner BM, McMurray JJ, de Zeeuw D, Haffner SM, Solomon SD, Chaturvedi N, Persson F, Desai AS, Nicolaides M, Richard A, Xiang Z, Brunel P, Pfeffer MA; ALTITUDE Investigators: Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N Engl J Med 367: 2204–2213, 2012 [DOI] [PubMed] [Google Scholar]
- 25.Mann JF, Schmieder RE, McQueen M, Dyal L, Schumacher H, Pogue J, Wang X, Maggioni A, Budaj A, Chaithiraphan S, Dickstein K, Keltai M, Metsärinne K, Oto A, Parkhomenko A, Piegas LS, Svendsen TL, Teo KK, Yusuf S; ONTARGET investigators: Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): A multicentre, randomised, double-blind, controlled trial. Lancet 372: 547–553, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Coca SG, Ismail-Beigi F, Haq N, Krumholz HM, Parikh CR: Role of intensive glucose control in development of renal end points in type 2 diabetes mellitus: Systematic review and meta-analysis intensive glucose control in type 2 diabetes. Arch Intern Med 172: 761–769, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pfeffer MA, Burdmann EA, Chen CY, Cooper ME, de Zeeuw D, Eckardt KU, Feyzi JM, Ivanovich P, Kewalramani R, Levey AS, Lewis EF, McGill JB, McMurray JJ, Parfrey P, Parving HH, Remuzzi G, Singh AK, Solomon SD, Toto R; TREAT Investigators: A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med 361: 2019–2032, 2009 [DOI] [PubMed] [Google Scholar]
- 28.Cushman WC, Evans GW, Byington RP, Goff DC, Jr, Grimm RH, Jr, Cutler JA, Simons-Morton DG, Basile JN, Corson MA, Probstfield JL, Katz L, Peterson KA, Friedewald WT, Buse JB, Bigger JT, Gerstein HC, Ismail-Beigi F; ACCORD Study Group: Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 362: 1575–1585, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pergola PE, Raskin P, Toto RD, Meyer CJ, Huff JW, Grossman EB, Krauth M, Ruiz S, Audhya P, Christ-Schmidt H, Wittes J, Warnock DG; BEAM Study Investigators: Bardoxolone methyl and kidney function in CKD with type 2 diabetes. N Engl J Med 365: 327–336, 2011 [DOI] [PubMed] [Google Scholar]
- 30.Schwartz GG, Olsson AG, Abt M, Ballantyne CM, Barter PJ, Brumm J, Chaitman BR, Holme IM, Kallend D, Leiter LA, Leitersdorf E, McMurray JJ, Mundl H, Nicholls SJ, Shah PK, Tardif JC, Wright RS; dal-OUTCOMES Investigators: Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med 367: 2089–2099, 2012 [DOI] [PubMed] [Google Scholar]
- 31.Maitland K, Kiguli S, Opoka RO, Engoru C, Olupot-Olupot P, Akech SO, Nyeko R, Mtove G, Reyburn H, Lang T, Brent B, Evans JA, Tibenderana JK, Crawley J, Russell EC, Levin M, Babiker AG, Gibb DM; FEAST Trial Group: Mortality after fluid bolus in African children with severe infection. N Engl J Med 364: 2483–2495, 2011 [DOI] [PubMed] [Google Scholar]


