Abstract
A 66-year-old woman presented with new onset generalised tonic-clonic seizures following her first dose of chemotherapy comprising Rituximab, Cyclophosphamide, Hydroxydaunorubicin, Oncovin and Prednisolone (R-CHOP) 10 days earlier for non-Hodgkin’s lymphoma. On admission, computed tomography (CT) scan of the cranium showed no abnormality. The CT was repeated within 48 hours as the patient developed status epilepticus and papilledema; the repeat scan showed characteristics of posterior reversible encephalopathy syndrome (PRES). Association of rituximab with this condition was suspected as there was no recurrence of PRES after receiving two more cycles of CHOP without rituximab. Contrary to previously published case reports, this patient had a delayed clinical presentation.
Keywords: Posterior reversible encephalopathy syndrome, chemotherapy, seizures
Background
PRES was first described by Hinchey et al. in 19961 and the pathophysiology of the syndrome remains unclear. It presents with headaches, seizures, loss of vision, and altered mental status1,2 with radiologic findings of focal reversible vasogenic oedema. The syndrome is commonly found to be associated with acute hypertension, preeclampsia or eclampsia, renal disease, sepsis, and exposure to immunosuppressive agents. 3
Three theories for a possible underlying mechanism have been suggested: hypertension causes a breakdown in cerebral auto-regulation which leads to hyperperfusion and protein and fluid extravasation producing vasogenic oedema4; vasospasm leads to ischemia5; endothelial dysfunction develops in patients with pre-eclampsia, eclampsia and septis.6
If undetected, PRES may lead to permanent neurologic damage or death.2 Secondary complications such as status epilepticus (SE), intracranial haemorrhage, and ischemic infarction can cause substantial morbidity and mortality, 8 therefore early recognition and correct treatment of PRES are important.
Case details
A 66-year-old woman was admitted after a series of seizures. She reported a seizure-like episode at home, and then suffered a generalised tonic-clonic seizure in the accident and emergency department which was terminated by intravenous lorazepam. She had a prolonged post-ictal phase of six hours during which her Glasgow Coma Scale (GCS) fluctuated between 5 to 9 (eyes score 2, verbal score 2, motor score 5). Her daughter reported that the patient complained of a moderate intensity occipital headache for two days prior to the first seizure, and also reported reduced vision for approximately six hours prior to the onset of seizures. She neither had a known seizure disorder nor had a seizure prior to this admission. Non-Hodgkin’s lymphoma (diffuse large B cell lymphoma) was diagnosed following a cervical biopsy and bone marrow examination two months prior. The patient did not have any other past medical history of significance, and had received her first dose of chemotherapy 10 days prior to the onset of the seizures. The chemotherapy consisted of R-CHOP.
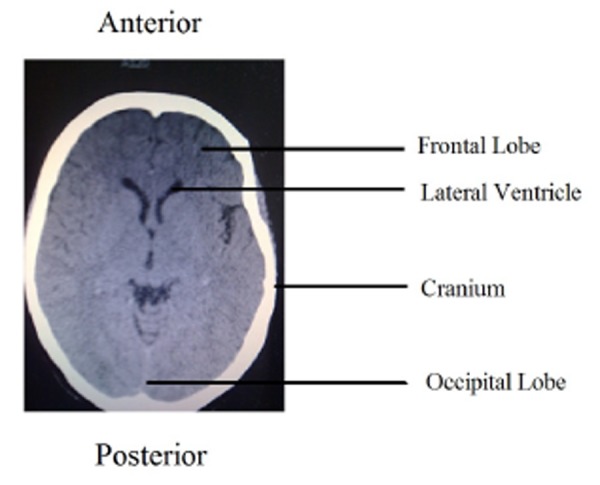
There were no signs of meningeal irritation on admission; however detailed clinical examination was limited by the effects of the lorazepam. Examination of the chest, abdomen and cardiovascular system did not reveal any abnormality. Blood pressure (BP) was 197/114 mmHg. A cranial CT was reported as normal (Figure 1). Blood results for electrolytes (sodium, potassium, calcium, phosphate and magnesium), renal and liver functions were all normal, and C-reactive protein was 7mg/L (range 0-10mg/L). Full blood count revealed normal platelet count but severe neutropenia 0.2x10*9/L. Cephalosporin and acyclovir were commenced empirically in the emergency department on discovering neutropenia.
Figure 1. CT scan of brain on admission. Normal appearance of occipital lobe.
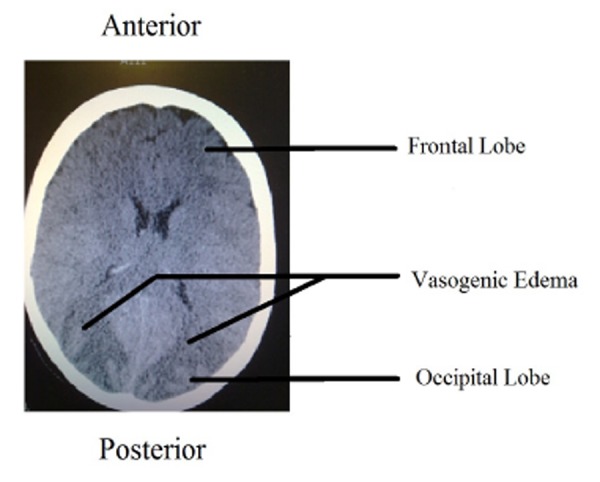
After approximately six hours her GCS improved to 13 (spontaneous eye opening, normal motor function and confused words) and the patient reported blurred vision. No signs of meningeal irritation could be elicited but detailed examination was again limited due to her confusion. Lumbar puncture was planned to be performed. However, before lumbar puncture could be performed, status epilepticus ensued and the patient was transferred to the intensive care unit, received a loading dose of phenytoin, and was then intubated and ventilated. Fundoscopy revealed papilledema and a subsequent cranial CT scan (second CT scan) showed oedema in the occipital lobe consistent with a diagnosis of PRES (Figure 2). Post diagnosis cephalosporin and acyclovir were immediately discontinued and phenytoin 100mg three times a day was continued. Her neutrophil count increased to above 1x 10*9/L over the following three days and she was weaned off ventilation in 48 hours. She had three more seizures while being removed from the ventilator, all of which responded to lorazepam. Her phenytoin was discontinued once her GCS improved to 15 and she remained seizure free for more than 48 hours on day four of ITU admission. She received physiotherapy and made a complete recovery in two weeks without any residual neurological deficit.
Figure 2. CT scan following status epilepticus. Note the oedema in occipital lobe.
It was difficult to be sure about the chemotherapeutic agent which led to development of PRES. Rituximab is a relatively newer agent in this regimen of R-CHOP. None of the patients had developed PRES in our experience locally who received CHOP only. A literature search also suggested rituximab a more likely agent than any other agent of this chemotherapy regimen. These facts were discussed with the patient. The patient agreed to receive her subsequent treatments of chemotherapy without rituximab, and has since received two doses of CHOP without recurrence of PRES. It therefore appears rituximab was linked to the observed symptoms and subsequent diagnosis of PRES.
Discussion
PRES is a radiological diagnosis and the condition does not have pathognomonic clinical features. The diagnosis can only be confirmed following a CT or magnetic resonance (MR) scan of the brain with typical findings of PRES. Headache, visual problems and seizures in a postchemotherapy patient with malignant disease has a broad differential diagnosis such as subarachnoid haemorrhage, primary or secondary malignant deposits in brain, bacterial, viral or fungal infections, stroke, side effects of chemotherapeutic agents and PRES. Intervention for PRES is minimal, requiring only repeated brain imaging and symptomatic treatment with complete recovery in most of cases within two weeks of onset of symptoms.2
Rituximab (anti CD 20 monoclonal antibody) is used in the treatment of patients with non-Hodgkin’s lymphoma in addition to its use in conditions such as lupus-related renal failure9 and sarcoidosis. There have been a handful of reports detailing cases of patients developing PRES after receiving rituximab.9,10 Patients in these reports developed symptoms early, even within a few hours of receiving rituximab, and symptoms lasted for a day or two before disappearing without leaving any neurological deficit.
This patient’s course of disease was similar to these previously reported cases; however symptom onset was much more delayed. The onset of symptoms 10 days after receiving chemotherapy makes intracranial haemorrhage due to thrombocytopenia, infection due to neutropenia, or metabolic disorder due to tumour lyses syndrome also possible diagnoses in this patient in addition to PRES except, the onset of symptoms was delayed.
High BP is mostly a non-specific finding in most acute medical admissions, and this patient did have very high BP on admission. Most of the patients reported to have PRES were associated with hypertension as well.7 This is one of the possible explanations for development of PRES, however it is difficult to be certain if very high BP leads to PRES or PRES leads to very high BP, especially when another case report of PRES did not have very high BP.8 Immunosuppression has also been found to be associated with PRES in a few case reports (e.g. gemcitabine, cisplatin).11-13 This patient developed symptoms of PRES once her neutrophil count dropped very low, possibly because of a combination of immunosuppression and hypertension which leads to development of PRES.
This case study describes a late presentation of PRES after a first dose of rituximab, a combination which has not previously been reported. PRES should not be considered a first-line diagnosis in every immunocompromised patient who presents to the emergency department with seizures and high BP as the condition is rare and there are no specific clinical signs. However, it is worth considering if a definitive diagnosis cannot be reached in a patient with similar presentation or if a patient does not show improvement with initial treatment. On the basis of this case PRES should be considered in patients who present late even after their first dose of rituximab.
ACKNOWLEDGEMENTS
Dr Gavin Campbell. Consultant Haematologist. Colchester General Hospital.
Footnotes
CONFLICTS OF INTEREST
The author has no competing interests.
FUNDING
The author did not receive funding for this case report.
Please cite this paper as: Siddiqi AI. Rituximab as a possible cause of posterior reversible encephalopathy syndrome. AMJ 2011, 4, 9, 513-515. http//dx.doi.org/10.4066/AMJ.2011.627
References
- 1.Hinchey J, Chaves C, Appignani B, Pao L, Wang A, Pessin MS, Lamy C, Mas JL, Caplan LR. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996;334(8):494–500. doi: 10.1056/NEJM199602223340803. [DOI] [PubMed] [Google Scholar]
- 2.Kwon J, Kim S, Kim K. et al. A case of gemcitabine and cisplatin associated posterior reversible encephalopathy syndrome.. Cancer Treat Res. 2009 Mar;41(1):53–55. doi: 10.4143/crt.2009.41.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hauser RA, Lacey DM, Knight MR. Hypertensive encephalopathy: magnetic resonance imaging demonstration of reversible cortical and white matter lesions. Arch Neurol. 1988;45(10):1078–1083. doi: 10.1001/archneur.1988.00520340032007. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz RB, Jones KM, Kalina P, Bajakian RL, Mantello MT, Garada B, Holman BL. Hypertensive encephalopathy: findings on CT MR imaging, and SPECT imaging in 14 cases. AJR Am J Roentgenol. 1992;159(2):379–383. doi: 10.2214/ajr.159.2.1632361. [DOI] [PubMed] [Google Scholar]
- 5.Trommer BL, Homer D, Mikhael MA. Cerebral vasospasm and eclampsia. Stroke. 1988;19(3):326–329. doi: 10.1161/01.str.19.3.326. [DOI] [PubMed] [Google Scholar]
- 6.Dekker GA, Sibai BM. Etiology and pathogenesis of preeclampsia: current concepts. Am J Obstet Gynecol. 1998;179(5):1359–1375. doi: 10.1016/s0002-9378(98)70160-7. [DOI] [PubMed] [Google Scholar]
- 7.Abenza-Abildua MJ, Fuentes B, Diaz D, Royo A, Olea T, Aguilar-Amat MJ, Diez-Tejedor E. Cyclophosphamide induced reversible posterior leukoencephalopathy syndrome. BMJ Case Rep. 2009 doi: 10.1136/bcr.07.2008.0467. pii: bcr07.2008.0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ay H, Buonanno FS, Schaefer PW, Le DA, Wang B, Gonzalez RG, Koroshetz WJ. Posterior leukoencephalopathy without severe hypertension: utility of diffusion-weighted MRI. Neurology. 1998;51(6):1369–1376. doi: 10.1212/wnl.51.5.1369. [DOI] [PubMed] [Google Scholar]
- 9.Mavragani CP, Vlachoyiannopoulos PG, Kosmas N, Boletis I, Tzioufas AG, Voulgarelis M. A case of reversible posterior leucoencephalopathy syndrome after rituximab infusion. Rheumatology (Oxford) 2004 Nov;43(11):1450–1. doi: 10.1093/rheumatology/keh305. [DOI] [PubMed] [Google Scholar]
- 10.Sánchez-Carteyron A, Alarcia R, Ara JR, Martin J. Posterior reversible encephalopathy syndrome after rituximab infusion in neuromyelitis optica. Neurology. 2010 May 4;74(18):1471–3. doi: 10.1212/WNL.0b013e3181dc1af3. [DOI] [PubMed] [Google Scholar]
- 11.Russell MT, Nassif AS, Cacayorin ED, Awwad E, Perman W, Dunphy F. Gemcitabine-associated posterior reversible encephalopathy syndrome: MR imaging and MR spectroscopy findings. Magn Reson Imaging. 2001;19:129–132. doi: 10.1016/s0730-725x(01)00217-x. [DOI] [PubMed] [Google Scholar]
- 12.Rajasekhar A, George TJ. Jr Gemcitabine-induced reversible posterior leukoencephalopathy syndrome: A case report and review of literature. Oncologist. 2007;12:1332–1335. doi: 10.1634/theoncologist.12-11-1332. [DOI] [PubMed] [Google Scholar]
- 13.Ito Y, Arahata Y, Goto Y, Hirayama M, Nagamutsu M, Yasuda T, Yanaqi T, Sobue G. Cisplatin neurotoxicity presenting as reversible posterior leukoencephalopathy syndrome. AJNR Am J Neuroradiol. 1998;19:415–417. [PMC free article] [PubMed] [Google Scholar]


