Abstract
Aspergillus spp. often colonise the respiratory tract of critically ill patients in intensive care units and subsequently cause invasive disease. The risk of developing invasive disease is more in immunocompromised patients. Here we report a case of fatal invasive pulmonary aspergillosis caused by Aspergillus versicolor in a post-operative patient on mechanical ventilation, who did not respond to intravenous itraconazole. We then discuss the challenges involved in the accurate diagnosis of this condition and appropriate management.
Keywords: Aspergillosis, Aspergillus versicolor, itraconazole, ventilator
Introduction
Fungal infections are emerging as a worldwide healthcare problem. The spores of Aspergillus spp. are ubiquitously found in the hospital environment and ventilation systems.1 Therefore, the Aspergillus spp. often colonise the respiratory tract of critically ill patients in intensive care units and subsequently cause invasive disease. Although the risk of developing invasive disease is more in immunocompromised patients, there are also reports of invasive aspergillosis in apparently immunocompetent hosts with certain predisposing factors, such as underlying lung disease and systemic illness.1 The early diagnosis of invasive aspergillosis is important as it is generally associated with a poor prognosis. The majority of these isolates are susceptible to itraconazole and amphotericin B. However, there are recent reports of increasing resistance to these drugs and treatment failures.1-3 Here we report a case of fatal invasive pulmonary aspergillosis caused by Aspergillus versicolor in a post-operative patient on mechanical ventilation, who did not respond to intravenous itraconazole.
Case history
A 45-year-old male with duodenal ulcer perforation underwent emergency laparotomy and simple closure of the perforation using omental patch. Post-operatively the patient was kept nil orally and connected to a ventilator for respiratory support. The patient was administered piperacillin/tazobactam and metronidazole intravenously. On post-operative day 1, the patient was semi-conscious and drowsy, afebrile (37°C), transcutaneous O2 saturation was 100%, heart rate was 98/min and blood pressure was 100/50 mm Hg. On chest auscultation the air entry was equal on both the sides. Culture of endotracheal aspirate was sterile. He was not a diabetic or hypertensive and was not treated with immunosuppressive agents or corticosteroids. His serological investigation was non-reactive for HIV.
Over the next five days the patient maintained normal O2 saturation, blood pressure was 120/60 mmHg, he showed equal air entry on both the sides and the endotracheal secretions were minimal. However, there were no spontaneous respiratory efforts and so he was continuously on ventilator support. On post-operative day 6, the patient showed febrile spikes (38°C), the air entry was reduced on the right side with basal crepitations and he had thick endotracheal secretions. The culture of the endotracheal aspirate grew Acinetobacter baumannii (susceptible to amikacin, ciprofloxacin and imipenem) and Aspergillus spp. The Aspergillus spp. was considered to be a coloniser, and the patient was administered ciprofloxacin for treatment of Acinetobacter baumannii infection.
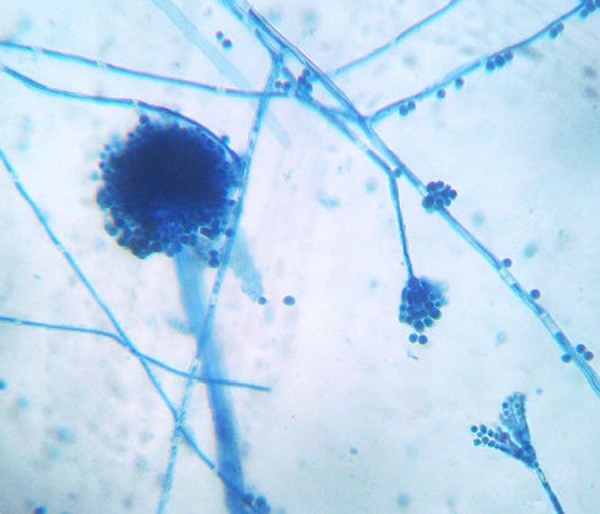
On post-operative day 8, the patient continued to be febrile (38°C) with appearance of infiltrates on the right side in chest radiograph and the clinical pulmonary infection score4 was eight suggestive of ventilator-associated pneumonia. Direct examination of the endotracheal aspirate collected on day 8 revealed fungal hyphae. The culture of endotracheal aspirate on Sabouraud’s dextrose agar showed green-coloured colonies with a powdery texture and no pigment on the reverse. The lactophenol cotton blue (LPCB) examination of the fungus revealed hyaline, septate hyphae with dichotomous branching, and conidophores bearing vesicles almost completely covered by metulae and phialides (Figure 1). Reduced penicillium-like fruiting structures and globose Hulle cells were also observed in the LPCB mount. Based on these findings the fungus was finally identified as Aspergillus versicolor.
Figure 1. Lactophenol cotton blue mount showing a completely covered vesicle and reduced penicillium-like structure of Aspergillus versicolor.
The patient was diagnosed to have invasive pulmonary aspergillosis due to Aspergillus versicolor and intravenous itraconazole (200mg twice daily) was started on day 10. Despite administration of itraconazole, the patient had febrile spikes and his pulmonary condition deteriorated and he died on day 12. The endotracheal secretions collected on day 12 before the death of the patient grew Aspergillus versicolor, suggesting treatment failure.
Discussion
Ventilator associated pneumonia (VAP) is a common intensive-care unit acquired infection. VAP is usually caused by bacterial agents and fungal agents are only rarely involved. Aspergillus fumigatus is the most common mold causing VAP in critically ill mechanically ventilated patients.1 In our patient, Aspergillus versicolor was repeatedly isolated from the endotracheal secretions, suggesting its role in the causation of VAP. A. versicolor is a cosmopolitan fungus often found in damp indoor environments.2,5 It has been commonly reported as an agent of onychomycosis, otomycosis, cutaneous disease, osteomyelitis and ocular disease.2 In addition, it has been frequently observed as a coloniser of the respiratory tract, however there are only a few reports of invasive pulmonary disease caused by this fungus.6
Aspergillus spp. usually cause pulmonary infection in individuals with certain predisposing factors such as prolonged neutropenia, chronic administration of corticosteroids, chronic debilitating disease, immunosuppressive treatment, organ transplantation, diabetes mellitus, insertion of prosthetic devices and tissue damage due to prior infection or trauma.1,6 However, Aspergillus spp. can cause invasive pulmonary disease even in apparently immunocompetent individuals.1 Our patient also was immunocompetent and did not have any of the above-mentioned predisposing factors except the presence of an endotracheal tube. The endotracheal intubation could have favoured the colonisation by A. versicolor, which has subsequently resulted in invasive infection.
The diagnosis of invasive pulmonary aspergillosis is difficult and often delayed as isolation of the fungus from respiratory secretions is usually regarded as colonisation.1 Even in this patient, the invasive disease caused by A. versicolor was initially disregarded as colonisation and was diagnosed later when it was repeatedly isolated from culture. Invasive pulmonary aspergillosis should therefore be suspected in patients with lung infiltrates who do not respond to conventional treatment with antibiotic chemotherapy.1
The correct identification of A. versicolor is also challenging because of the frequent presence of reduced penicillium-like structures on LPCB examination, resulting in misidentification of this fungus as Penicillium spp.5 Moreover, the simultaneous occurrence of the typical vesicles of Aspergillus spp. and the penicillium-like structures may be mistaken for a mixed growth of contaminant fungi. The varied morphology may contribute to under-reporting of this fungus in patients with invasive disease. Therefore, A. versicolor should be suspected, when the characteristic biseriate vesicles and the reduced penicillium-like structures are present in a fungus. 5 The presence of globose Hulle cells can be an additional clue for identification of this fungus.5
Amphotericin B is often used for treatment of invasive infections by Aspergillus spp. including Aspergillus versicolor. 1 Although, amphotericin B is generally considered as the “gold standard” in the treatment of invasive aspergillosis caused by A. fumigatus, high rates of resistance to amphotericin B have been reported among the clinical isolates of A. terreus.7 Recently A. versicolor isolated from patients with onychomycosis has been shown to be resistant to amphotericin B by in-vitro tests and there are also reports of treatment failure.2 However, there is no data regarding the amphotericin B resistance in A. versicolor isolated from invasive aspergillosis. Intravenous itraconazole has good activity against A. versicolor and other Aspergillus spp. 2,5 However, its effectiveness can be limited by reduced bioavailability. A child with invasive pulmonary and CNS aspergillosis has been reported to be refractory to treatment with intravenous itraconazole.3 Similarly, our patient also did not respond to intravenous itraconazole. Recently, in a randomised controlled trial, voriconazole, a new second-generation triazole, with a very good bioavailability, has been demonstrated to be more effective than amphotericin B in the treatment of invasive aspergillosis.8 Therefore, the current guidelines of the Infectious Disease Society of America (IDSA) recommends voriconazole for the primary treatment of invasive aspergillosis.9
Invasive pulmonary infection caused by Aspergillus spp. is associated with a very high mortality.3 The death of our patient may be attributed to the delay in the initiation of the anti-fungal therapy or resistance to itraconazole. So, early diagnosis and treatment with potent anti-fungals such as voriconazole is necessary to improve the outcome of invasive pulmonary aspergillosis.
Footnotes
PEER REVIEW
CONFLICTS OF INTEREST
The authors declare that they have no competing interests.
FUNDING
Nil
Please cite this paper as: Charles MVP, Joseph NM, Easow JM, Ravishankar M. Invasive pulmonary aspergillosis caused by Aspergillus versicolor in a patient on mechanical ventilation. AMJ 2011, 4, 11, 632-634 http//dx.doi.org/10.4066/AMJ.2011.905
References
- 1.Kristan SS, Kern I, Music E. Invasive pulmonary aspergillosis. Respiration. 2002;69:521–5. doi: 10.1159/000066470. [DOI] [PubMed] [Google Scholar]
- 2.Torres-Rodriguez JM, Madrenys-Brunet N, Siddat M, Lopez-Jodra O, Jimenez T. Aspergillus versicolor as cause of onychomycosis: report of 12 cases and susceptibility testing to antifungal drugs. J Eur Acad Dermatol Venereol. 1998;11:25–31. [PubMed] [Google Scholar]
- 3.Leroy P, Smismans A, Seute T. Invasive pulmonary and central nervous system aspergillosis after near-drowning of a child: case report and review of the literature. Pediatrics. 2006;118:e509–e513. doi: 10.1542/peds.2005-2901. [DOI] [PubMed] [Google Scholar]
- 4.Fartoukh M, Maitre B, Honore S, Cerf C, Zahar JR, Brun-Buisson C. Diagnosing pneumonia during mechanical ventilation: the clinical pulmonary infection score revisited. Am J Respir Crit Care Med. 2003;168:173–179. doi: 10.1164/rccm.200212-1449OC. [DOI] [PubMed] [Google Scholar]
- 5.Patterson TF, Sutton DA. Advances in the diagnosis and treatment of invasive aspergillosis. Infectious Disease Special Edition. 2004;7:1–6. [Google Scholar]
- 6.Cahill BC, Hibbs JR, Savik K, Juni BA, Dosland BM, Edin-Stibbe C, Hertz MI. Aspergillus airway colonization and invasive disease after lung transplantation. Chest. 1997;112:1160–4. doi: 10.1378/chest.112.5.1160. [DOI] [PubMed] [Google Scholar]
- 7.Sutton DA, Sanche SE, Revankar SG, Fothergill AW, Rinaldi MG. In vitro amphotericin B resistance in clinical isolates of Aspergillus terreus, with a head-to-head comparison to voriconazole. J Clin Microbiol. 1999;37:2343–5. doi: 10.1128/jcm.37.7.2343-2345.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herbrecht R, Denning DW, Patterson TF, Bennett JE, Greene RE, Oestmann JW, Kern WV, Marr KA, Ribaud P, Lortholary O, Sylvester R, Rubin RH, Wingard JR, Stark P, Durand C, Caillot D, Thiel E, Chandrasekar PH, Hodges MR, Schlamm HT, Troke PF, Pauw BD. Voriconazole versus amphotericin B for primary therapy if invasive aspergillosis. N Engl J Med. 2002;347:408–415. doi: 10.1056/NEJMoa020191. [DOI] [PubMed] [Google Scholar]
- 9.Walsh TJ, Anaissie EJ, Denning DW, Herbrecht R, Kontoyiannis DP, Marr KA, Morrison VA, Segal BH, Steinbach WJ, Stevens DA, Burik JV, Wingard JR, Patterson TF. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis. 2008;46:327–60. doi: 10.1086/525258. [DOI] [PubMed] [Google Scholar]


